Abstract
Neuroligins enhance synapse formation in vitro, but surprisingly are not required for the generation of synapses in vivo. We now show that in cultured neurons, neuroligin-1 overexpression increases excitatory but not inhibitory synaptic responses, and potentiates synaptic NMDA/AMPA-receptor ratios. In contrast, neuroligin-2 overexpression increases inhibitory but not excitatory synaptic responses. Accordingly, deletion of neuroligin-1 in knockout mice depresses the NMDA/AMPA-ratio, whereas deletion of neuroligin-2 selectively depresses inhibitory synaptic responses. Strikingly, chronic inhibition of NMDA-receptors or of CaM-Kinase II that signals downstream of NMDA-receptors suppresses the synapse-boosting activity of neuroligin-1, whereas chronic inhibition of general synaptic activity suppresses the synapse-boosting activity of neuroligin-2. Taken together, these data indicate that neuroligins do not establish, but specify and validate synapses via an activity-dependent mechanism, with different neuroligins acting on distinct types of synapses. This hypothesis reconciles the overexpression and knockout phenotypes, and suggests that neuroligins contribute to the use-dependent formation of neural circuits.
Introduction
Synapse formation and maturation are essential for the normal establishment and remodeling of neuronal circuits in brain, and impairments in synapse formation and maturation are major factors in the pathogenesis of brain disorders such as autism and mental retardation. Neuroligins (NLs) are trans-synaptic cell adhesion molecules that are thought to function in synapse formation and/or specification (Goda and Davis, 2003; Yamagata et al., 2003). NLs were discovered as ligands (or receptors, for that matter) of neurexins, which are synaptic cell-adhesion molecules involved in synapse specification (Ushkaryov et al., 1992; Missler et al., 2003), and bind to neurexins in an interaction that is regulated by alternative splicing of both neurexins and NLs (Ichtchenko et al., 1995 and 1996; Boucard et al., 2005; Chih et al., 2006). Mutations in NL genes are found in patients with familial autistic spectrum disorders (Jamain et al., 2003; Laumonnier et al., 2004; Yan et al., 2005; Zoghbi, 2003), including a point mutation in the neuroligin 3 (NL3) gene (the Arg451 Cys substitution) that causes at least partial retention of NL3 in the endoplasmic reticulum (Chih et al., 2004; Comoletti et al, 2004), suggesting that NLs are critical for normal brain function.
NLs, when expressed in non-neuronal cells, induce formation of synapses by co-cultured neurons, probably via a trans-synaptic interaction with presynaptic α- and β-neurexins (Scheiffele et al., 2000; Chubykin et al., 2005; Boucard et al., 2005; Chih et al., 2006). In support of a central role of neurexins in the synapse-inducing activity of NLs, expression of neurexins in non-neuronal cells elicits formation of postsynaptic specializations by co-cultured neurons onto these cells (Graf et al., 2004; Nam and Chen, 2005) Moreover, overexpression of NLs in neurons increases synapse density as evaluated morphologically, although the functions of these synapses were not studied (Dean et al., 2003; Graf et al., 2004; Chih et al., 2006; Prange et al, 2004; Levinson et al., 2005). Overall, these results established that NLs perform a synaptic function, and gave rise to the hypothesis that NLs function in the initial establishment of synapses (reviewed in Cantallops and Cline, 2000; Hussain and Sheng, 2005; Levinson and El-Husseini, 2005). However, the neuronal culture experiments are also consistent with an alternative hypothesis for the functions of NLs, namely that NLs specify and validate synapses instead of mediating their initial establishment. In this context, we mean synapse specification and validation to refer to the process that instructs synapses to become excitatory or inhibitory, stable or transient, facilitating or depressing – in short, the process that directs the functional development of synapses after establishment of the initial contact. In neuronal cultures, transient synapses are probably constantly formed in a NL-independent manner, and may appear to be increased in numbers by NLs if NLs functionally validate them. Even in the artificial synapse formation assay, non-neuronal cells elicit formation of transient synapses from co-cultured neurons without NLs (Biederer et al., 2002; Boucard et al., 2005; Scheiffele et al, 2000), and may appear to be initiated by NLs even if NLs act only after synapse initiation. Indeed, results from knockout (KO) mice demonstrated that neither NLs nor α-neurexins are required for the initial formation of synapses, but both are essential for synaptic function and mouse survival (Missler et al, 2003; Varoqueaux et al., 2006). An open issue thus is whether NLs function in the initial establishment or the validation/specification of synapses. One of the goals of the present study is to address this issue in cultured neurons by testing whether NLs induce increased synapse numbers by prompting their initial formation, or by acting downstream of synapse initiation at a later, activity-dependent step.
A second open issue – which is related to the question of whether NLs are involved in establishing or in specifying/validating synapses – concerns the differential roles of NL1 and NL2 in excitatory vs. inhibitory synapses. NL1 is predominantly localized to excitatory, and NL2 to inhibitory synapses (Song et al., 1996; Varoqueaux et al., 2004; Graf et al., 2004), and overexpression of NL1 enhances excitatory synapse numbers, whereas overexpression of NL2 enhances inhibitory synapse numbers (Prange et al., 2005; Chih et al., 2005). These observations indicated that expression of distinct NLs may regulate the excitatory/inhibitory balance (Levinson and El-Husseini, 2005). A surprising set of recent data suggested, however, that alternative splicing of NL1 and NL2 may alter their specificity for excitatory vs. inhibitory synapses (Chih et al., 2006). This result would be consistent with the observation that overexpression of NL1 increased the frequency and amplitude of both excitatory and inhibitory spontaneous miniature postsynaptic currents (“minis”) (Chih et al., 2005; Levinson et al., 2005; Nam and Chen, 2005; Prange et al., 2004), and that RNAi-mediated knockdown of NL1 and of NL2 decreased the density of both excitatory and inhibitory synapses in cultured neurons (Chih et al., 2005). Thus there are two conflicting sets of data, the initial localization data suggesting a principal specificity of NL1 and NL2 for excitatory vs. inhibitory synapses, respectively (Song et al., 1998; Graf et al., 2004; Varoqueaux et al., 2004), and the transfection data in cultured neurons suggesting that such specificity arises from alternative splicing of NLs, is not dictated by the principal type of NL expressed (Chih et al., 2005 and 2006). Although analysis of KO mice could potentially have clarified this issue, no comparisons between the effects of different NLs on excitatory vs. inhibitory synaptic transmission were made in these mice (Varoqueaux et al., 2006). Thus, to address this issue in cultured neurons and in KO mice is the second major goal of the present study.
To address these two open issues, we have examined four questions: 1. Does overexpression of NL1 and NL2 in vitro result in a net increase of functional synapses (i.e., synapses capable of evoked neurotransmission), and/or a change in synaptic properties? 2. Do NL1 and NL2 have distinct actions on excitatory vs. inhibitory synapses in vitro and in vivo? 3. Do NL1 and NL2 promote synapse formation independent of activity as a synapse-inducing agent, or do they act downstream of the initiation of synapse formation in an activity-dependent manner? 4. Do NL-deficient neurons in vivo exhibit a phenotype that is at least in part complementary to that of NL-overexpressing neurons in vitro? Our data demonstrate that NL1 specifically increases the number of functional excitatory synapses, whereas NL2 specifically enhances the number of inhibitory synapses. A complementary phenotype was observed in the analysis of NL1 and NL2 KO mice. We show that the effects of both NL1 and NL2 are dependent on synaptic signaling. NL1 action can be blocked by inhibitors of NMDA-receptors or CaM-Kinase IIα, whereas NL2 action can be blocked by general inhibition of synaptic transmission. Moreover, we demonstrate that the effects of NL1 require its extracellular domain, and that introduction of a mutation observed in a patient with an autism-spectrum disorder into NL1 suppresses endogenous excitatory synapse function. These results suggest a model whereby NLs validate transient synapses in an activity-dependent manner that intersects with postsynaptic signaling pathways, a model that accounts for both the NL overexpression and KO phenotypes.
Results
NL1-induced increase in synapse numbers requires NMDA-receptor signaling
To test whether the increase in synapse numbers induced by overexpression of NL1 (Dean et al., 2003; Graf et al., 2004; Chih et al., 2006; Prange et al., 2004; Levinson et al., 2005) is constitutive or involves synaptic signaling, we overexpressed NL1 and control proteins in cultured neurons, and analyzed the number of synapses morphologically. We cultured hippocampal neurons from newborn rats, transfected them at 10 days in vitro (DIV) with NL1 that was expressed either as a EGFP-tagged or untagged protein. and analyzed them at 14-15 DIV. Except when noted differently, we used for the transfections splice variants of NLs that contained all inserts in all splice sites. In these experiments, we incubated neurons from the day of transfection in either control medium or medium containing 50 μM AP5 (a high-affinity NMDA-receptor antagonist) to test whether chronic blockade of NMDA-receptors impairs the ability of NL1 to increase synapse numbers. NMDA-receptor signaling was examined because NMDA-receptor signaling is dispensable for synapse formation as such, but is required for the activity-dependent shaping of synaptic circuits (Feldman et al., 1999; Perez-Otano and Ehlers, 2005; Skuse et al., 1997), and because NMDA-receptors are connected to NL1 in that both bind to PSD-95 (Irie et al., 1997; Kornau et al., 1995; Niethammer et al., 1996).
Transfection of NL1-EGFP but not of EGFP alone caused a ∼100% increase in the spine and synapse density of the transfected neurons, as reported previously (Chih et al., 2004; Boucard et al., 2005), but had no significant effect on synapse size (Figs. 1A). AP5 reversed the increase in synapse density in neurons expressing NL1, but had a much smaller effect on synapse density in control-transfected neurons (Figs. 1B and 1C; see Suppl. Table 2 for a statistical analysis demonstrating that this effect is specific). Chronic AP5 treatment did not alter the expression levels of NL1 or its targeting to postsynaptic spines, suggesting that AP5 treatment directly interferes with the functional action of NL1 on synapses. To ensure that the suppression of the synapse-inducing activity of NL1-EGFP by AP5 was not caused by the EGFP moiety in NL1-EGFP, and that the morphological analysis was not skewed due to a comparison of spines labeled with NL1-EGFP vs. EGFP alone, we also examined neurons that were co-transfected with EGFP and untagged NL1 or untagged SynCAM. Note that the candidate synaptic cell adhesion molecule SynCAM was used as a negative control in these experiments because previous studies showed that overexpression of SynCAM selectively increases the function of nascent synapses in immature neurons without affecting mature synapses (Biederer et al., 2002; Sara et al., 2005). Similar to what we found with EGFP-tagged NL1, we observed a specific, ∼100% increase in the number of spines and synapses in neurons expressing untagged NL1; again, this increase was reversed by chronic treatment with AP5, whereas chronic treatment of AP5 had only a small effect on SynCAM-expressing neurons (Suppl. Figs. 1A-1C).
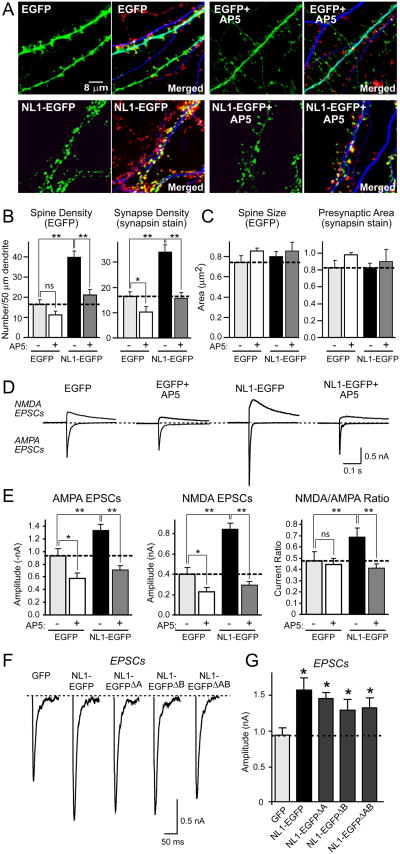
Figure 1. Chronic NMDA-receptor block suppresses NL1-induced increase in the number of functional excitatory synapses.
(A) Representative images of hippocampal neurons transfected with NL1-EGFP or EGFP alone, and cultured in the presence or absence of 50 μM AP5 for four days. Neurons were visualized by EGFP fluorescence (green), and immunolabeling with antibodies to the dendritic marker MAP2 (blue) and the presynaptic marker synapsin (red). For each sample, the EGFP image is shown on the left, whereas the merged image for EGFP, MAP2, and synapsin is shown on the right. (B) and (C) Quantitative analyses of synapse numbers (B) and size (C) in neurons expressing EGFP or EGFP-tagged NL1, and treated with either control medium or AP5. For an analysis of specifically GABAergic synapses, see Suppl. Fig. 2. (D) Representative electrophysiological recordings of evoked NMDA- and AMPA-receptor dependent EPSCs in neurons transfected with EGFP or NL1-EGFP with or without NMDA-receptor blockade by AP5. Recordings were made in the absence of AP5. (E) Amplitudes of AMPA- and NMDA-receptor dependent EPSCs and NMDA/AMPA ratio in neurons transfected with EGFP or NL1-EGFP with and without chronic AP5 treatment. (F) and (G) Representative traces (F) and summary graphs (G) of electrophysiological recordings of AMPA-dependent EPSCs in neurons transfected with control vector or vectors expressing all four alternative splice variants of NL1 (Boucard et al., 2005). Data shown in (B), (C), (E), and (G) are means ± SEMs (n=3 independent experiments with 6-10 neurons/experiment and condition); asterisks indicate statistically significant differences (* = p<0.05; ** = p<0.01; ns = not significant). In all experiments in this and all following figures, the NL splice variant analyzed corresponds to the variant with inserts in all sites of alternative splicing except when indicated otherwise.
NL1 causes an NMDA-receptor dependent increase in excitatory synaptic transmission
To test whether the morphologically observed increase in synapse density corresponds to an increase in synaptic function, we monitored the effects of NL1 on synaptic transmission. Previous studies showed that NL1 overexpression increases the frequency of spontaneous minis, suggesting that the additional synapses induced by overexpressed NL1 may be functional (Chih et al., 2005; Prange et al., 2004). However, synaptic information is normally transferred by evoked transmission whose relationship to spontaneous mini events is complex (e.g., see Pang et al., 2006; Dityatev et al., 2000). To clarify whether NL1-induced synapses are functional, we performed whole-cell voltage-clamp patch recordings of neurons expressing NL1 or control proteins, and monitored excitatory postsynaptic currents (EPSCs) induced by local extracellular stimulation (Maximov and Sudhof, 2005; Maximov et al., 2007). Action potentials in the patched neuron were blocked with QX-314, and recordings were performed with picrotoxin in the bath to abolish GABAA-receptor mediated events. AMPA- and NMDA-receptor mediated EPSCs were measured at -70 mV and +40 mV holding potentials, respectively, in the presence of external Mg2+. In these experiments, AMPA- and NMDA-receptor dependent responses could be reliably resolved because the peak of AMPA receptor-mediated EPSCs occurs 2 ms after stimulation, whereas that of NMDA-receptor-mediated EPSCs occurs 50 ms later (Poncer and Malinow, 2001; Maximov et al., 2007).
NL1-EGFP expression caused a ∼50% increase in AMPA receptor-mediated EPSCs, a ∼100% increase in NMDA-receptor-mediated EPSCs, and a ∼40% increase in the NMDA/AMPA receptor ratio (Figs. 1D and 1E). Chronic blockade of NMDA-receptors with AP5 reversed the action of NL1-EGFP on AMPA- and NMDA-receptor mediated EPSCs and on the NMDA/AMPA receptor ratio, but had little effect on control neurons expressing EGFP alone (Figs. 1D and 1E; again, see Suppl. Table 2 for a detailed statistical analysis of the AP5 effect). Moreover, in neurons expressing untagged NL1 without the EGFP fusion, we observed a similar ∼100% increase of AMPA- and NMDA-receptor dependent responses compared to SynCAM-expressing control neurons, and also detected an increase in the NMDA/AMPA receptor ratio (Suppl. Figs. 1D and 1E). Chronic treatment with AP5 again specifically reversed the effect of NL1. Since it was recently suggested that the relative effects of NLs on excitatory versus inhibitory synapses may be regulated by alternative splicing (Chih et al., 2006), we also tested different splice variants of NL1. We found, however, that all NL1 splice variants lacking inserts in sites A and/or B had similar activities as NL1 containing inserts on EPSCs (Figs. 1F and 1G).
NL1 action is specific for excitatory synapses
Does NL1 boost the numbers and strength of all synapses, or specifically act only on glutamatergic synapses? To address this question, we examined whether NL1 overexpression alters the number of inhibitory synapses (Suppl. Fig. 2) or the size of inhibitory synaptic responses (Fig. 2).
Figure 2. NL1 expression does not alter IPSCs: Effect of chronic NMDA-receptor blockade and alternative splicing.
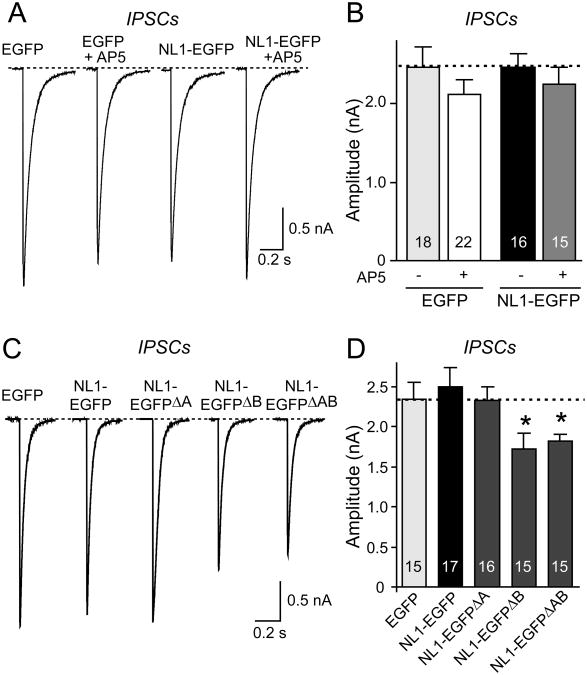
Sample traces (A) and summary graphs (B) of IPSCs recorded from neurons expressing only EGFP or EGFP-tagged NL1 cultured either in control medium or in medium containing 50 μM AP5 for four days prior to the recordings. (C) and (D) Alternative splicing of NL1 does not activate its lack of an effect on IPSCs. IPSCs were monitored in 50 μM AP5 and 10 μM CNQX (means ± SEMs; n=18 cells/3 cultures; asterisks represent statistically significant difference: ** =p<0.01; ns, not significantly different).
We recorded evoked inhibitory postsynaptic currents (IPSCs) in NL1-EGFP and EGFP only expressing neurons (Fig. 2). Overexpression of NL1 did not significantly alter the size of evoked IPSCs, suggesting that consistent with its localization (Song et al., 1999), NL1 specifically acts only on excitatory synapses (Figs. 2A and 2B). Moreover, chronic AP5 treatment did not alter the size of the IPSCs in control EGFP or in NL1-EGFP expressing neurons. The lack of an effect of NL1 on inhibitory synapses was confirmed by staining of NL1-transfected neurons for an inhibitory synapse marker VGAT, the vesicular GABA-transporter (McIntire et al., 1997). This experiment demonstrated that although NL1 induced an increase in total synapse numbers, it had no effect on the number of inhibitory synapses (Suppl. Fig. 2). Finally, we systematically tested all splice variants of NL1 because it was suggested, based on antibody staining, that some splice variants of NL1 may increase inhibitory synapse numbers (Chih et al., 2006). We observed a small but statistically significant decrease in IPSCs with some NL1 splice variants, but failed to detect an increase in IPSCs induced by any splice variant of NL1 (Figs. 2C and 2D).
Effect of chronic blockade of CaM-Kinase II on evoked EPSCs in neurons expressing NL1
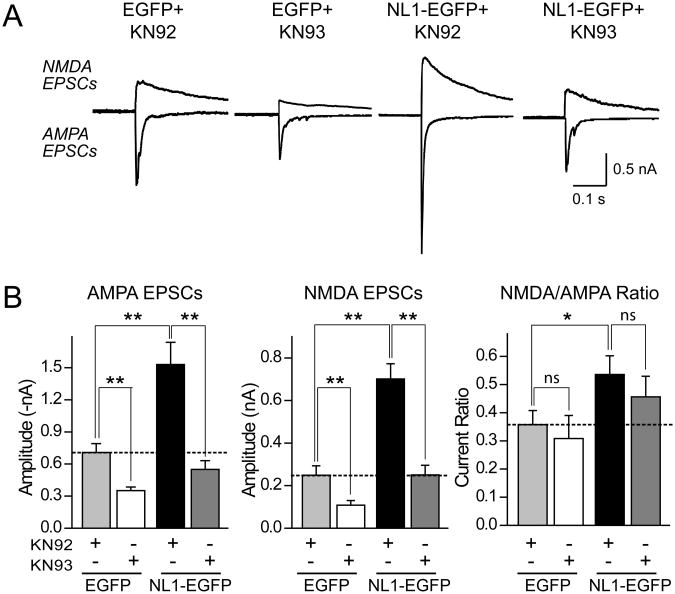
CaM-Kinase II is thought to act downstream of NMDA-receptor activation in synaptic plasticity and in the activity-dependent validation of synaptic connections (Poncer et al., 2002). To probe whether signaling via CaM-Kinase II is essential for NL1-mediated increases in excitatory synaptic transmission, we incubated neurons expressing NL1-EGFP or EGFP only for four days in the presence of 5 μM KN-93 (a CaM-Kinase II inhibitor) or of 5 μM KN-92 (the inactive analog of KN-93), and measured evoked NMDA- and AMPA-receptor dependent EPSCs (Fig. 3A). Chronic blockade of CaM-Kinase II completely reversed the potentiation of both AMPA- and NMDA-receptor dependent EPSCs and of the NMDA/AMPA receptor ratio in NL1-EGFP expressing neurons (Fig. 3B). Thus activation of postsynaptic NMDA-receptors and of downstream signaling CaM-Kinase II are essential for NL1 to enhance synaptic function in neurons.
Figure 3. Chronic blockade of CaM-Kinase IIa mimics the effect of NMDA-receptor blockade on EPSCs in neurons expressing NL1.
Sample traces (A) and summary graphs (B) of EPSCs recorded from neurons expressing only EGFP or EGFP-tagged NL1 cultured either in medium containing 5 μM KN-93 ( CaM-Kinase IIα inhibitor) or in control medium containing 5 μM KN-92 ( inactive analog of KN-93) for four days prior to the recordings. Data shown are means ± SEMs; asterisks represent statistically significant differences (n=18 cells/3 cultures; *=p<0.05, ** =p<0.01; ns, not significantly different).
Deletion of NL1 decreases the ratio of NMDA- to AMPA-receptor mediated EPSCs
The fact that in cultured neurons, chronic blockage of NMDA-receptors or CaM-Kinase II prevents the NL1-induced increase in synapse numbers argues against the notion that NLs simply induce synapses, and suggests that they function in the specification and validation of previously established synaptic contacts. This hypothesis of NL function is consistent with both the KO results showing that NL1 deletion alters synapse function without significantly changing synapse numbers (Varoqueaux et al., 2006), and the overexpression results showing that NL1 can increase the density of synapses in cultured neurons. According to this hypothesis, the transfection results are not an artifact, but reflect a physiological function of NL1 that should be detectable in the NL1 KO mice, i.e. the hypothesis predicts that synapse specification/validation should be altered in the NL1 KO mice.
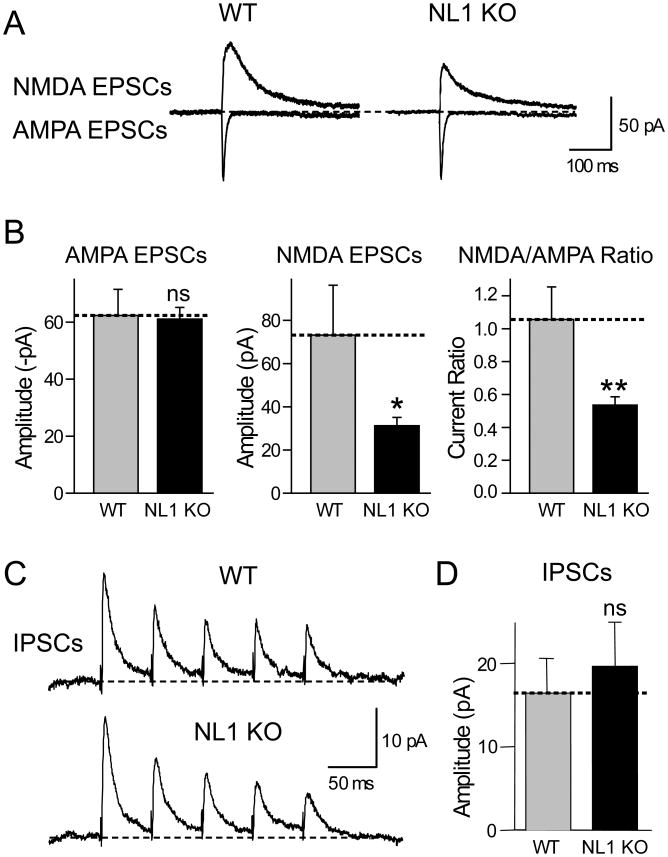
To test this prediction, we performed whole-cell patch-clamp recordings in the CA1 area of acute hippocampal slices from NL1 KO and littermate control mice (Varoqueaux et al., 2006), and measured AMPA- and NMDA-receptor dependent synaptic responses evoked by stimulation of Schaffer collaterals (Fig. 4A). The stimulus strength was adjusted to achieve a similar AMPA-receptor dependent EPSC amplitude (50-100 pA), and the NMDA-receptor dependent EPSC amplitude was then measured in the same neuron with the same stimulus strength. Strikingly, we observed a ∼50% decrease in the mean relative amplitude of NMDA-receptor-mediated EPSCs (Fig. 4B). This relative decrease in NMDA-receptor dependent responses resulted in a highly significant, ∼50% decrease in the NMDA/AMPA receptor ratio.
Figure 4. Deletion of NL1 in KO mice lowers the NMDA/AMPA-receptor ratio without significantly altering the IPSC amplitude.
(A) Representative traces of NMDA- (top) and AMPA-receptor dependent EPSCs (bottom) evoked by local stimulation with a microelectrode and recorded from a pyramidal neuron in the CA1 region of the hippocampus from littermate wild-type and NL1 KO mice. (B) Mean amplitudes of NMDA- and AMPA-receptor dependent EPSCs and mean NMDA/AMPA-receptor dependent EPSC ratio. Stimulation strength was adjusted to yield similar AMPA-receptor dependent EPSC amplitudes, and NMDA-receptor dependent EPSCs were then measured in the same neuron with the same stimulus strength (n=12 for each genotype). (C) and (D) Representative traces (C) and mean amplitudes of IPSCs (D) monitored by paired recordings from adjacent inhibitory and excitatory neurons in the somatosensory cortex (n=12 wild-type and n=10 NL1 KO neurons; data shown are means ± SEMs; asterisks indicate if there is a statistically significant difference between WT and NL1 KO, * = p<0.05; ** = p<0.01).
To ensure that this effect reflects a selective action of NL1 on excitatory synapses, we measured IPSC sizes in NL1 KO mice using paired recordings from acute cortical slices (Fig. 4C). We found no difference in the absolute size of the IPSCs or the failure rate between slices from wild-type and littermate NL1 KO mice, demonstrating that the effect of the NL1 deletion is as selective as the effect of NL1 overexpression in cultured neurons (Figs. 4C and 4D and data not shown). Moreover, the input/output curves of IPSCs in NL1 KO mice failed to uncover a difference (see below). Thus deletion of NL1 depresses NMDA-receptor dependent synaptic responses compared to AMPA-receptor dependent responses, whereas overexpression of NL1 potentiates these responses.
NL2 enhances inhibitory but not excitatory synaptic function
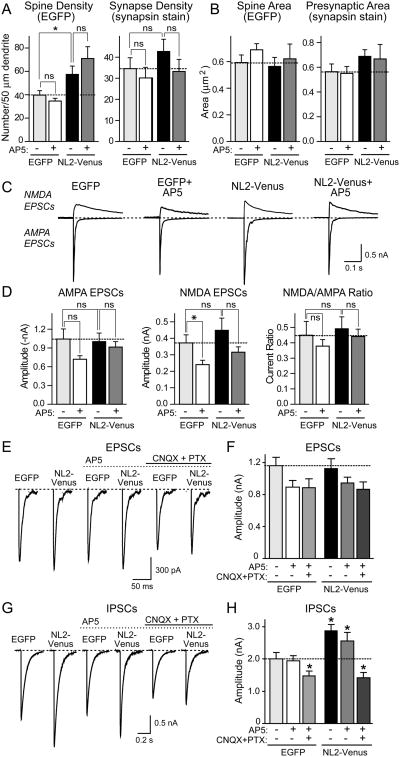
To test whether the differential localizations of NL1 and NL2 to excitatory and inhibitory synapses, respectively, reflect distinct functions, we examined the effects of NL2 on synapse numbers and evoked synaptic responses in transfected neurons (Fig. 5). Overexpression of NL2 caused a moderate increase in synapse numbers mostly on dendritic shafts (Figs. 5A and 5B), and resulted in the increased formation of thin filopodia, many of which lacked associated presynaptic terminals (Suppl. Fig. 3). Strikingly, NL2 had no significant effect on the amplitudes of AMPA-or NMDA-receptor dependent evoked EPSCs (Figs. 5C -5F), but produced a 50% increase in IPSC amplitudes compared to neurons expressing EGFP alone (Figs. 5G and 5H). Thus NL2 selectively enhances inhibitory synaptic function, consistent with its localization.
Figure 5. NL2 selectively enhances inhibitory synaptic function.
Hippocampal neurons were transfected with NL2-Venus or EGFP, and cultured in the presence or absence of 50 μM AP5 for four days. (A) and (B) Summary graphs of the quantitative analysis of synapse numbers (A) and size (B) in neurons expressing EGFP or NL2-Venus, and treated with either control medium or AP5. For representative images, see Suppl. Fig. 3. (C) Representative electrophysiological traces of evoked NMDA- and AMPA-receptor dependent EPSCs in neurons transfected with EGFP or NL2-Venus with or without NMDA-receptor blockade. (D) Amplitudes of AMPA- and NMDA-receptor dependent EPSCs and the NMDA/AMPA ratio in neurons transfected with EGFP and NL2-Venus with and without chronic AP5 treatment. (E) - (H) Effect of chronic treatments with AP5 without and with CNQX and picrotoxin on evoked EPSCs (E and F) and IPSCs (G and H) in NL2-overexpressing neurons. Panels show sample traces (E and G) and summary graphs (F and H). Neurons were transfected at 10 DIV and incubated in 50 μM AP5 with or without 10 μM CNQX and 50 μM picrotoxin for 4 days. IPSCs were monitored in 50 μM AP5 and 10 μM CNQX. Data shown in (A), (B), (D), (F) and (H) are means ± SEMs (n=3 independent experiments with 6-10 neurons/experiment and condition); asterisks indicate statistically significant differences (* = p<0.05; ** = p<0.01; ns - not significant).
Chronic AP5 treatment did not cause a significant change in EPSCs or IPSCs in control- or NL2-transfected neurons (Figs. 5E-5H). However, chronic inhibition of all neuronal network activity by treatments with CNQX (an AMPA-receptor inhibitor) and picrotoxin (a GABAA-receptor inhibitor, included to block chronic hyperpolarization) suppressed the NL2-induced increase in IPSCs. Again, this treatment had only a slight effect on EPSCs (Figs. 5E-5H). Thus, similar to the NL1-induced increase in excitatory synaptic function in cultured neurons, the NL2-induced increase in inhibitory synaptic function is activity-dependent.
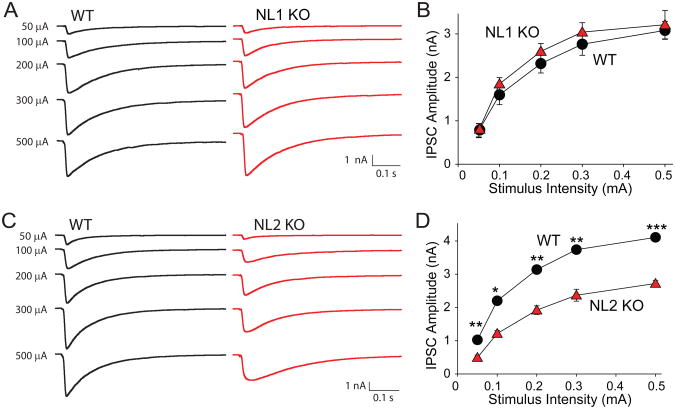
The NL1 and NL2 transfection results strongly suggest that NL1 and NL2 are functionally specialized for excitatory and inhibitory synapses, respectively, but share a similar fundamental requirement for synaptic activity. To confirm these conclusions with in a more physiological preparation, we examined IPSCs and EPSCs in acute cortical slices from NL1 KO (as a control) and NL2 KO mice (Fig. 6 and Suppl. Fig. 4). Input/output curves revealed that IPSCs in NL2-deficient neurons were ∼50% lower than in control neurons, whereas NL1-deficient neurons exhibited no phenotype (Fig. 6). No significant change in EPSC input/output curves was detected, although their interpretation is more difficult due to the recurrent excitatory activity in the slices (Suppl. Fig. 4). Parallel morphological experiments suggested that in the NL2 KO mice, the number of inhibitory synapses is selectively decreased (K. Tabuchi and T.C.S., manuscript in preparation).
Figure 6. Deletion of NL2 but not of NL1 KO depresses IPSC amplitudes in acute cortical slices.
Evoked IPSCs were measured as a function of the stimulus strength in Layer 2/3 of the somatosensory (barrel) cortex in response to extracellular stimulation by a microelectrode positioned nearby. (A) and (B), and (C) and (D) show representative traces (A, C) and summary graph (B, D) for evoked IPSCs from NL1 (A, B) and NL2 KO mice (C,D), respectively, and their wild-type littermate controls (n=4 mouse pairs each). Data shown in (B) and (D) are means ± SEMs; asterisks indicate statistically significant differences (* = p<0.05; ** = p<0.01; *** = p<0.001).
Excitatory synapse function is inhibited by an autism-related mutation of NL1
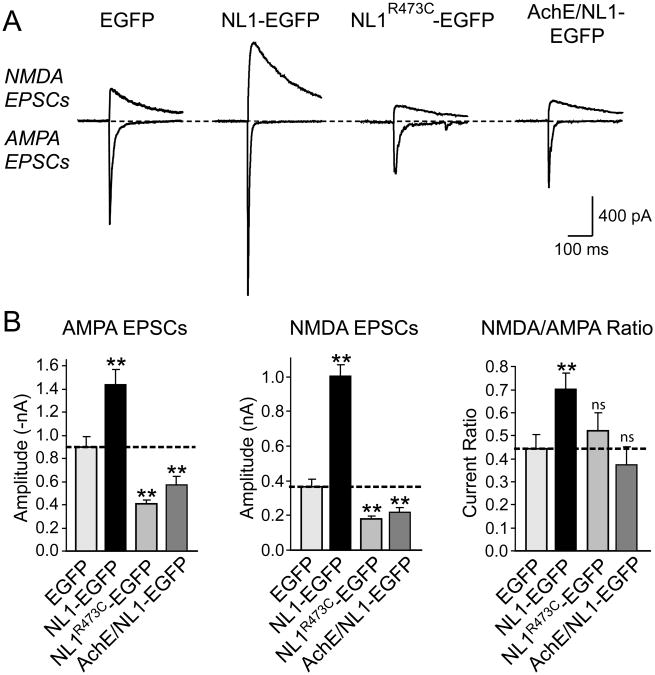
In several patients with autistic-spectrum disorders, mutations in NLs have been observed. As a first step towards understanding how such mutations may alter the synaptic function of NLs, we generated a mutant NL1-EGFP that contains the R473C-substitution which corresponds to a mutation observed in NL3 from an autistic-spectrum patient (NL1R473C-EGFP; Jamain et al., 2003). As a control, we generated a second mutant in which we inserted the acetylcholinesterase esterase domain into NL1 instead of its extracellular esterase-like domain (AchE/NL1-EGFP). We then measured the relative effects of the mutant NLs on synapse density and synaptic transmission.
Compared to control-transfected neurons expressing EGFP, neurons expressing the autism mutant of NL1 exhibited a dramatic decrease in synapse density, whereas neurons expressing wild-type NL1 displayed an enhanced synapse density as expected (Suppl. Fig. 5). Physiologically, the decrease in synapse density manifested as a >2-fold decrease in excitatory synaptic transmission, both for AMPA- and for NMDA-receptor mediated responses (Figs. 7A and 7B). The NL1 mutant in which the entire extracellular esterase domain was replaced by that from acetylcholinesterase was still transported into postsynaptic spines where it had a similar, but less severe effect on synapse numbers and synaptic transmission (Fig. 7 and Suppl. Fig. 5). These results are consistent with the notion that the autism NL mutation does not simply inactivate the extracellular domain of NL, but may act as a dominant negative. Moreover, the effect of the hybrid acetylcholinesterase/NL molecule indicates that coupling of extra- to intracellular domains is important for NL1 function.
Figure 7. Autism mutation of NL1 causes dominant-negative suppression of EPSCs.
(A) Representative traces of NMDA- (top) and AMPA-receptor dependent EPSCs (bottom). For a diagram of the mutants and a morphological analysis of the effect of the mutants on synapse density, see Suppl. Fig. 4. (B) Mean amplitudes of NMDA- and AMPA-receptor dependent EPSCs and mean NMDA/AMPA receptor ratios. Data shown are means ± SEMs (n=18 neurons from 3 cultures); asterisks indicate that a condition exhibits a statistically significant difference from the EGFP-only transfected control condition (* =p<0.05; **=p<0.01).
We investigated this issue further by testing the effects of hybrid molecules in which the extra- and intracellular sequences of SynCAM and NL1 were swapped, resulting in molecules composed of the extracellular NL1 domain fused to the transmembrane region and intracellular sequences of SynCAM (called NL1-SynCAM hybrid), or of the extracellular SynCAM domains fused to the transmembrane region and intracellular sequences of NL1 (called SynCAM-NL1 hybrid; Suppl. Fig. 6A). We then systematically analyzed the effects of these hybrid molecules in a direct comparison with wild-type NL1 and SynCAM on synapse density (Suppl. Fig. 6) and synapse function (Suppl. Fig. 7). The NL1-SynCAM hybrid was as effective in boosting excitatory synapse numbers and function as wild-type NL1, whereas the SynCAM-NL1 hybrid had no significant effect compared to SynCAM alone. These data demonstrate that the extracellular NL1 domain is the major effector in boosting synapse function, and that the dominant-negative action of the autism-mutant NL1 or the acetylcholinesterase-NL1 hybrid are not due to the overexpression of the cytoplasmic tail of NL1.
Discussion
Using a combination of quantitative morphological analyses and electrophysiological measurements, we show that NL1 overexpression increases the number of excitatory synapses, and that these ‘new’ synapses are functional (Fig. 1). NL1 acts selectively, as it does not increase the number of inhibitory synapses (Fig. 2). The effect of NL1 on synapse specificity is not altered by alternative splicing (Figs. 1F and 1G). In contrast to the selective action of NL1 on excitatory synapses, NL2 specifically increased the number of inhibitory synapses (Fig. 5). In view of their high degree of sequence homology, it is surprising that NL1 and NL2 act so selectively in enhancing synapse function. NL1 not only increased excitatory synaptic transmission, but also altered the properties of excitatory synapses since the NMDA/AMPA receptor ratio was potentiated by NL1 (Fig. 1). The relative enhancement of NMDA-receptor dependent responses by NL1 is not an overexpression artifact but is physiologically relevant because deletion of NL1 had the opposite effect: it caused a large relative decrease in NMDA-receptor mediated synaptic responses and in the NMDA/AMPA receptor ratio (Fig. 4). Similarly, deletion of NL2 selectively suppressed the size of IPSCs (Fig. 6 and Suppl. Fig. 4). Moreover, consistent with a role of endogenous NL1 in regulating excitatory synaptic transmission, overexpressed mutant NL1 containing a single amino acid substitution found in autistic-spectrum patients depressed the number of excitatory synapses, and decreased excitatory synaptic strength (Fig. 7). These results extend previous studies showing that NL overexpression increases synapse numbers (Dean et al., 2003; Graf et al., 2004; Chih et al., 2006; Prange et al., 2004; Boucard et al., 2005; Levinson et al., 2005) by demonstrating that the added synapses are actually functional. Importantly, these results resolve the question of the specificity of NL1 and NL2 action by demonstrating that in vitro and in vivo, NL1 and NL2 act in a surprisingly selective manner, independent of alternative splicing, on either only excitatory or only inhibitory synapses, respectively.
The increase in synapse numbers induced by NL overexpression could be explained by at least two principally different hypotheses: (1) NL1 and NL2 selectively induce the formation of new excitatory and inhibitory synapses, respectively; or (2) NL1 and NL2 mediate the activity-dependent specification and validation of initial synaptic transient contacts. To differentiate between these two hypotheses, we investigated the effect of blocking synaptic activity on the synapse-enhancing action of NLs. In what is probably the most important result of our study, we found that the NL1-induced increase in the number of functional excitatory synapses in cultured neurons was blocked by chronic treatment of the neurons with AP5, an NMDA-receptor inhibitor (Fig. 1, Suppl. Fig. 1). Since chronic AP5 treatment also caused a modest depression of excitatory synaptic parameters in control neurons, a potential concern is that the effect of AP5 in NL1-expressing neurons is a simple amplification of the AP5-effect on control neurons. This hypothesis, however, is excluded by a statistical analysis of the various AP5-treatment experiments which demonstrates that AP5 on average induces a 1.3-fold decrease in synaptic parameters in control neurons, but a 2-fold decrease in synaptic parameters in NL1-expressing neurons (Suppl. Table 2). Moreover, the difference between untreated and AP5-treated neurons is rarely significant for control neurons or NL2-expressing neurons, but always significant for NL1-expressing neurons. Finally, the difference between control and NL1 expressing neurons is invariably significant in untreated neurons, but generally not significant in AP5-treated neurons (Suppl. Table 2). In specifically reversing the action of NL1, AP5 had no effect on the expression level or synaptic localization of NL1, and thus interfered with the local action of NL1 in synaptic spines. Chronic inhibition of CaM-Kinase II that is thought a signal downstream of NMDA-receptors in synapses also blocked the NL1-induced increase in excitatory synaptic transmission (Fig. 3). Although the increase in inhibitory synapses by NL2 was not altered by AP5, it was blocked by silencing general synaptic activity using CNQX and picrotoxin (Fig. 5). The reversal of the effects of NL1 or NL2 expression by blockage of synaptic signaling – without altering the expression or localization of NL1 or NL2 – indicate that NLs do not mechanically nucleate synapse formation, but require synaptic activity.
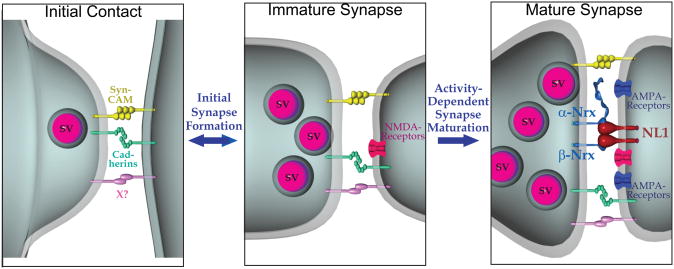
Based on present and previous results, we would like to propose a model suggesting that NLs contribute to the activity-dependent specification and validation of synapses (Fig. 8). In addition to the current data, this suggestion is supported by the finding that NL1 expression in immature neurons (in contrast to mature neurons) does not significantly alter synaptic activity, whereas SynCAM does (Sara et al., 2005). Moreover, consistent with the synapse specification/validation hypothesis, deletions of NLs have only a very small, insignificant effect on synapse numbers (Varoqueaux et al., 2006). It should be noted that consistent with our results, NMDA-receptor activity is not generally required for the normal formation of synapses (Gomperts et al., 2000), but is essential for the validation and specification of synapses similar to the role we propose here for NLs, and is necessary for the synaptic integration of newly generated neurons in the adult dentate gyrus (Tashiro et al., 2006).
Figure 8. Model of the role of NL1 in synapse formation.
The initial synaptic contact between neurons is proposed to involve multiple cell-adhesion molecules, including SynCAM and cadherins which might impart specificity on synaptic contacts. The resulting immature synapses are functional, but are stabilized and further specified in terms of their specific properties (release probability, plasticity, NMDA/AMPA-receptor ratio, and others) by activity-dependent processes. The model suggests that NL1 mediates the activity-dependent stabilization of transient synaptic contacts, but that this function of NL1 depends on the simultaneous activation of NMDA-receptors. In promoting activity-dependent synapse stabilization, postsynaptic NL1 likely transduces a trans-synaptic signal triggered by binding of its extracellular esterase-like domain to presynaptic neurexins. NL2 presumably performs an analogous function in inhibitory synapses.
What is the evidence against this model? The artificial synapse formation assay in which NL1, when expressed in a non-neuronal cell, induces synapse formation by co-cultured neurons (Scheiffele et al., 2000), does not rule out this model because in this assay, a small number of synapses are also formed on control cells (Biederer et al., 2002), suggesting that even in the absence of a neural cell-adhesion molecule, some synapses are transiently formed. Thus the artificial synapse formation assay does not determine whether NL1 induces new synapses or stabilizes transient synapses. Moreover, the synaptic cell-adhesion molecule SynCAM is as active as NL1 in the artificial synapse-formation assay (Biederer et al., 2002), but does not enhance the number or function of excitatory synapses in mature cultured neurons (Suppl. Fig. 2). At first glance, it may seem puzzling that although NL1 and SynCAM have similar effects in the artificial synapse formation assay, they have distinct effects in neurons. However, it appears likely that any postsynaptic molecule capable of activating a presynaptic signal transduction pathway will work in the artificial synapse formation assay, independent of the in vivo function of this molecule. In this view, the artificial synapse formation assay simply reflects the fact that a given protein is a trans-synaptic cell-adhesion molecule, and SynCAM serves as an appropriate control for the actions of NL1 since overexpression of the latter but not the former has significant effects in neurons. The R473C mutant of NL1 may inhibit synaptic function because it heterodimerizes with endogenous NLs, thereby effectively decreasing the neuronal NL concentration. This hypothesis would account for the fact that the R473C mutant of NL1 severely impairs normal synapse formation in neurons despite its continued ability to induce synapse formation in the artificial synapse formation assay (Chubykin et al., 2005). Finally, in RNAi knockdown experiments a decrease in NLs has been correlated with a decrease in synapse numbers (Chih et al., 2004). However, in these experiments NL1 and NL2 exerted similar effects on spontaneous inhibitory vs. excitatory synaptic events, whereas in our in vitro and in vivo experiments their effects are highly specific for excitatory vs. inhibitory synapses. Recent results show that RNAi produces powerful off-target effects on synapses (Alvarez et al., 2006), suggesting that rescue experiments might help to clarify whether RNAi-dependent knockdown of NLs decreases synapse numbers in culture.
How does NL1 act in synapse stabilization? One hypothesis, proposed in the model (Fig. 8), is that the simultaneous postsynaptic activation of NL1 by presynaptic neurexins and of NMDA-receptors by synaptic activity stimulates a signaling cascade involving CaM-Kinase II that triggers synapse maturation (Fig. 3). The coupling between activation of NL1 and NMDA-receptors may be mediated by their common interaction with PSD-95 and other postsynaptic scaffolding molecules (Irie et al., 1997; Kornau et al., 1995; Niethammer et al., 1996). Indeed, it is likely that the cytoplasmic sequences of NLs have a major functional role because the dominant negative action of the acetylcholinesterase/NL fusion protein (which does not heterodimerize with endogenous NLs) suggests an action of the cytoplasmic sequences of NL1 (Fig. 7). Coupling synaptic function (i.e., synaptic transmission) to synaptic structure (i.e., trans-synaptic cell adhesion) would allow coordination of the structural and functional specializations of synapses, with the differential modulation of trans-synaptic neurexin/NL interactions providing a plausible mechanism by which different synaptic properties could be specified.
In terms of neuronal circuits, the activity-dependence of the synapse-boosting actions of NL1 and NL2 raises the question whether synaptic activity is required for NL function in a global ‘permissive’ sense, or whether NLs perform a role as a synapse-specific activity detector. In a global permissive sense, increased activity would promote the actions of both NL1 and NL2 on excitatory and inhibitory synapses, respectively, thereby contributing to the preservation of an excitatory/inhibitory balance. As synapse-specific activity detectors, NL1 and NL2 would contribute to the strengthening of particular synaptic connections dependent on their activity, and thereby contribute to the formation of specific circuits. Independent of which of these hypotheses is correct, it is clear that NLs contribute to determine the excitation/inhibition ratio in a neural circuit. This ratio is crucial for dendritic integration and neuronal computation, and determines whether or not that neuron will fire. Impairments in the overall ratio of excitatory to inhibitory transmission are observed in neurological disorders like autistic spectrum disorders, mental retardation, and epilepsy, where such impairments reflect pathological circuit abnormalities. Moreover, the role of NLs may go beyond setting the excitatory/inhibitory ratio since NLs appear to influence the properties of synapses in addition to enhancing excitatory vs. inhibitory inputs, as indicated in the specific changes observed in the property of excitatory synapses in NL1-overexpressing or NL1-deficient neurons (Figs. 1 and 4).
Experimental Procedures
Constructs
All NL1 expression vectors encode rat NL1 with inserts in splices sites A and B, except when indicated otherwise (Biederer et al., 2002; Sara et al., 2005), and are described in the Suppl. Materials.
Cell culture
Primary hippocampal neuronal cultures were prepared from 1-2 day-old Sprague-Dawley rats (Kavalali et al., 1999), transfected at 10 days in vitro using a calcium phosphate transfection protocol (Xia et al, 1996), and analyzed at 14 days in vitro. Chronic AP5 treatments were performed by adding 50 μM AP5 to the culture medium at 10 to 14 days in vitro; AP5 was washed out before recordings were started.
Electrophysiological analyses of cultured neurons
Whole-cell recordings from pyramidal cells were acquired at room temperature in a modified Tyrode bath solution (150 mM NaCl, 4 mM KCl, 2 mM MgCl2, 10 mM glucose, 10 mM HEPES and 2 mM CaCl2 at pH 7.4, 310 mOsm) with a MultiClamp 700B amplifier and Clampex 8.0 software (Axon Instruments, Union City, CA). Recordings were filtered at 2 kHz and sampled at 200 μs. EPSCs and IPSCs were evoked with a local stimulation electrode (Maximov and Sudhof, 2005). AMPA- and NMDA-receptor mediated EPSCs were recorded in 50 μM picrotoxin at holding potentials of -70 mV and +40 mV, respectively. AMPA receptor-dependent EPSCs and IPSCs were quantified by measuring the amplitude 2 ms after the onset of synaptic responses, NMDA-receptor dependent EPSC amplitudes were measured 50 ms after the EPSC onset. IPSCs were recorded in 10 μM 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX) and 50 μM AP5 at a holding potential of -70 mV. Input and series resistances were monitored (series resistance ∼10 MΩ), and experiments with unstable readings were discarded. Recordings were performed on anonymized samples to avoid observer bias. The recording methods are described in detail in Maximov et al. (2007).
Electrophysiological analyses of NL KO mice
were performed at room temperature (∼22 °C) in standard artificial cerebrospinal fluid in acute hippocampal and cortical slices from littermate mice that were either homozygous NL1 or NL2 KO mice, or contained one or two wild-type NL1 or NL2 alleles (controls). Three types of experiments were performed (see Suppl. Materials for a additional descriptions): 1. Measurements of EPSCs in hippocampal slices. Whole-cell recordings were performed from CA1 pyramidal neurons in slices from mice at P19-21 in 100 μM picrotoxin to prevent disynaptic inhibitory responses, and after removal of the CA3 region to abolish polysynaptic responses. An extracellular stimulating electrode in the stratum radiatum (∼75 μm away from the patched neuron) was used to perform four analysis stages: a. We first tested whether extracellular stimulation produced monosynaptic responses using a holding potential of -70 mV and approximately -30 mV to exclude disynaptic inhibitory responses; b. we then optimized the stimulation strength to elicit AMPA-receptor mediated responses of -50 to s100 pA with current pulses of 50-400 μA; c. using this stimulus strength, we measured AMPA responses to 5-10 stimuli applied at 0.125 Hz with a postsynaptic holding potential of -70 mV; and d. finally, we measured postsynaptic NMDA-receptor mediated responses in the same cell by switching the postsynaptic holding potential to +40 mV. AMPA-mediated responses were monitored as the peak amplitude; NMDA-receptor mediated responses as the amplitude 40 ms after the stimulus. Moreover, in two experiments AP5 was shown to completely block the measured NMDA-receptor dependent currents, validating the measurements. 2. Measurements of IPSCs by paired recordings. These were performed in paired recordings in layer 4 of the somatosensory cortex (within barrel hollows) in acute slices from P14-16 mice. Voltage-clamp whole-cell recordings were established in neighboring presynaptic inhibitory fast-spiking neurons and postsynaptic regular-spiking neurons with -60 mV and -55 mV holding potentials, respectively. Of 24 patched wild-type pairs, 12 had inhibitory connections and 17 had excitatory connections; of 20 patched NL1 KO pairs, 10 had inhibitory connections and 15 excitatory connections, suggesting that the postsynaptic neurons were excitatory stellate neurons, and the presynaptic neurons inhibitory fast-spiking neurons (Gibson et al., 1999). Junction potentials were 9 mV. IPSCs were measured in response to a 20 Hz stimulus train of 8 evoked presynaptic action potentials, and the absolute amplitude and short-term synaptic plasticity of responses were measured (failure rates of unitary IPSCs: 9±3% for wild-type slices (n=12); 6±4% for NL1 KO slices (n=10); means ± SEMs; p=0.57). 3. Measurements of input/output curves in cortical slices. Cortical slices (0.3 mm) were prepared from male littermate mice at P13-P16 according to Agmon and Connors (1991). Mice were anesthetized and decapitated, and the brain was removed and placed into ice cold dissection buffer (in mM: 87 NaCl, 3 KCl, 1.25 NaH2PO4, 7 MgSO4, 26 NaHCO3, 20 d-glucose, 75 sucrose, 1.3 ascorbic acid, and 0.5 CaCl2). The brain was bisected sagitally and the cut surfaces attached to the slicing platform. Slices were made with a Leica Vibratome slicer and incubated at 34°C in artificial CSF (in mM: 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 25 d-glucose, and 2 CaCl2) bubbled with 95%O2/5%CO2. The slices were allowed to recover at 34°C for 1 hour and then were kept at room temperature for the remainder of the experiment. Slices were added to the recording chamber and allowed to equilibrate for 10 minutes prior to recording. The recording chamber was perfused at 1 ml/min with carbogenated ACSF containing either 20 μM CNQX for IPSCs or 2 μM 2-chloradenosine and 50 μM picrotoxin for EPSCs. All recordings were performed in Layer 2/3 pyramidal cells of the somatosensory cortex, identified by their size and single apical dendrite. Evoked synaptic responses were elicited with current injection through an extracellular electrode placed in Layer 2/3 of the cortex 100-150 μm from the post-synaptic cell. Synaptic responses were recorded in a whole-cell mode using a Multiclamp 700A amplifier and the magnitude of the extracellular stimulus was controlled with a Model 2100 Isolated Pulse Stimulator. The whole-cell pipette solution contained, in mM: 145 KCl, 5 NCl, 10 HEPES, 10 EGTA, 0.3 Na2GTP, 4 MgATP, and 10 QX-314. For each cell, the synaptic response was the average of 5 traces at 0.2 Hz after the responses had equilibrated. The responses were sampled at 10 kHz and analyzed using pClamp and Microsoft Excel. Pipettes used for whole-cell recording had a resistance of 3-5 MΩ. Cells were discarded that possessed series resistance greater than 20 MΩ or a leak current greater than 200 pA. Statistical analysis was performed with paired t-test and responses are depicted as absolute values +/- SEM.
Immunocytochemistry, image acquisition and analysis
Neurons were fixed in cold 100% methanol, permeabilized in 0.1% saponin, and incubated with primary and secondary antibodies in PBS with 3% nonfat milk and 0.1% saponin using Alexa Fluor 633 goat anti-rabbit and Alexa Fluor 546 goat anti-mouse antibodies (Molecular Probes) as secondary antibodies (Chubykin et al, 2005; see Suppl Materials). Images were acquired with a Leica TCS2 confocal microscope with identical settings applied to all samples in an experiment, and are presented in three colors: presynaptic terminals (visualized via synapsin staining) in red, dendrites (MAP2 staining) in blue, and spines (EGFP or venus fluorescence, either from transfected tagged NLs or from co-transfected EGFP-tagged β-actin) in green. Stacks of z-section images were coded, converted to maximal projection images, and analyzed blindly with the NIH Image/ImageJ program. Channels corresponding to EGFP and synapsin signals were thresholded to outline spines and presynaptic terminals correspondingly. Area size, fluorescent intensity and density of spines and presynaptic terminals per 50 μm of dendrite were measured using the “Analyze particle” module of the ImageJ program. Each experiment was performed at least three times with 300-1000 spines from 6 to 10 neurons analyzed per condition.
Statistical analysis
All results are expressed as means ± SEMs; significance was determined by Student's t-test.
Supplementary Material
Acknowledgments
We would like to thank Dr. Y. Goda (University College London) and Dr. Antony A. Boucard (UT Southwestern, Dallas) for expression vectors. This study was supported by a grant from the NIMH (R37 MH52804-08 to T.C.S.). ETK is an Established Investigator of the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmon A, Connors BW. Thalamocortical response of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Ridenour DA, Sabatini BL. Retraction of synapses and dendritic spines induced by off-target effects of RNA interference. J Neurosci. 2006;26:7820–7825. doi: 10.1523/JNEUROSCI.1957-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A Splice Code for trans-Synaptic Cell Adhesion Mediated by Binding of NL1 to alpha- and beta-Neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Cantallops I, Cline HT. Synapse formation: if it looks like a duck and quacks like a duck. Curr Biol. 2000;10:R620–623. doi: 10.1016/s0960-9822(00)00663-1. [DOI] [PubMed] [Google Scholar]
- Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of NLs. Hum Mol Genet. 2004;13:1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by NLs. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Sudhof TC. Dissection of synapse induction by NLs: effect of a neuroligin mutation associated with autism. J Biol Chem. 2005;280:22365–22374. doi: 10.1074/jbc.M410723200. [DOI] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Schachner M. Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron. 2000;26:207–217. doi: 10.1016/s0896-6273(00)81151-4. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via NLs. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Sheng M. Neuroscience. Making synapses: a balancing act. Science. 2005;307:1207–1208. doi: 10.1126/science.1110011. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. NL1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Südhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of NLs to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding NLs NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Klingauf J, Tsien RW. Activity-dependent regulation of synaptic clustering in a hippocampal culture system. Proc Natl Acad Sci U S A. 1999;96:12893–12900. doi: 10.1073/pnas.96.22.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA-receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Levinson JN, El-Husseini A. New players tip the scales in the balance between excitatory and inhibitory synapses. Mol Pain. 2005;1:12. doi: 10.1186/1744-8069-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Sudhof TC. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 2005;48:547–554. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Maximov A, Pang Z, Tervo DGR, Südhof TC. Monitoring Synaptic Transmission in Primary Neuronal Cultures Using Local Extracellular Stimulation. J of Neurosci Methods. 2007;161:75–87. doi: 10.1016/j.jneumeth.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. α-Neurexins Couple Ca2+-Channels to Synaptic Vesicle Exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA-receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Sun J, Rizo J, Maximov A, Sudhof TC. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. Embo J. 2006;25:2039–2050. doi: 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA-receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, Malinow R. Postsynaptic conversion of silent synapses during LTP affects synaptic gain and transmission dynamics. Nat Neurosci. 2001;4:989–996. doi: 10.1038/nn719. [DOI] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Sudhof TC, Kavalali ET. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs PA. Evidence from Turner's syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. NL1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Neurexins: Synaptic cell surface proteins related to the α-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins Are Essential for Synapse Function But Not for Initial Synapse Formation. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA-receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sram J, Bockholt A, Jones IR, Craddock N, et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry. 2005;10:329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.