Abstract
In women, ovarian hormone loss associated with menopause has been associated with cognitive decline. Hormone therapy (HT) may ameliorate some of these changes. Understanding the cognitive impact of female steroids, including estrogens, progestogens, and androgens, is key to discovering treatments that promote brain health in women. The preclinical literature has presented elegant and methodical experiments allowing a better understanding of parameters driving the cognitive consequences of ovarian hormone loss and HT. Animal models have been a valuable tool in this regard, and will be vital to future discoveries. Here, we provide an update on the literature evaluating the impact of female steroid hormones on cognition, and the putative mechanisms mediating these effects. We focus on preclinical work that was done with an eye toward clinical realities. Parameters that govern the cognitive efficacy of HT, from what we know thus far, include but are not limited to: type, dose, duration, and route of HT, age at HT initiation, timing of HT relative to ovarian hormone loss, memory type examined, menopause history, and hormone receptor status. Researchers have identified intricate relationships between some of these factors by studying their individual effects on cognition. As of late, there is increased focus on studying interactions between these variables as well as multiple hormone types when administered concomitantly. This is key to translating preclinical data to the clinic, wherein women typically have concurrent exposure to endogenous ovarian hormones as well as exogenous combination HTs, which include both estrogens and progestins. Gains in understanding the parameters of HT effects on cognition provide exciting novel avenues that can inform clinical treatments, eventually expanding the window of opportunity to optimally enhance cognition and brain health in aging women.
Ovarian Hormones and Cognition in the Rodent: Historical Context and Clinical Implications
In 1927, A.S. Parkes published a monograph in The Proceedings of the Royal Society of Medicine entitled “Internal Secretions of the Ovary”, in which it is stated that, “The solution of the type of problem found in studying the internal secretions of the ovary is most satisfactorily sought by experiment, and since the lower mammals have to be used for this type of work, it is on their reactions that our knowledge of ovarian activity mainly depends. At the same time, however, ovarian activity in the human subject must obey similar laws, and with the aid of clinical observations, experimental work on the lower mammals may be made to throw much light on the problems associated with the human species” (Parkes, 1927, page 45). It is notable that even now, some eight decades later, researchers studying “internal secretions of the ovary” employ similar tenets of utilizing rodent models to understand the multiple effects that these hormones have on body systems and functions. Today we typically refer to “internal secretions of the ovary” as ovarian steroid hormones. Early researchers seeking to learn the functions of the internal secretions of the ovary observed, via experimental evaluations, that the ovary is essential for development of accessory reproductive organs, the estrous cycle, sustaining pregnancy, and mammary gland development (Parkes and Bellerby, 1926). Other studies led to the discovery that these secretions are involved in more than reproductive-related morphology and function; they are also involved in reproductive behaviors (Beach, 1947). Subsequent research has now shown that gonadal steroids impact many non-reproductive actions and functions in the brain as well, including learning and memory, and several of its postulated mechanisms. For many researchers, the hope is to translate findings to a genuine clinical impact on women’s health.
In animal models and humans, the female steroids estrogens, progestogens and androgens have each been shown to impact cognition. To evaluate the cognitive effects of female steroid hormones in humans, researchers have been creative in their assessments and have reported effects across menopause transition stages (Luetters et al., 2007), with sex-change operations and concomitant sex hormone treatment (Gomez-Gil et al., 2009), and with hormone therapy before versus after treatment in surgically menopausal women (Sherwin, 2006). Animal models have been used to test the cognitive effects of steroid hormones. In animal models, the traditional procedure is to remove the major source of endogenous synthesis and release, the testes in the male (gonadectomy, or GDX) or the ovaries in the female (ovariectomy, or Ovx), then administer the exogenous steroid of question as a treatment regimen after surgery. Notably, within the last decade, research in both the animal and human literature evaluating the potential influence of gonadal hormones on brain health and function during aging has increased in breadth and depth. Much of this elevated interest is largely because of the recent discussion and debate about whether hormone therapies impact normal aging and/or Alzheimer’s disease (AD). These reports include, but are not limited to: a meta-analysis showing that estrogen-containing hormone therapies decreased the risk of AD by 29% (Yaffe et al., 1998); placebo-controlled studies showing estrogens enhanced memory or improved dementia scores in female AD patients (Asthana et al., 1999; Asthana et al., 2001; Ohkura et al., 1994; Ohkura et al., 1995); a study showing that menopause exacerbated age-related cognitive changes in several domains, including visuospatial abilities (Halbreich et al., 1995); and then more recently, the outcome of the Women’s Health Initiative studies showing null or detrimental effects on cognition or dementia from the most commonly used hormone therapies (for discussion, see Coker et al., 2010; Henderson, 2008; Maki and Henderson, 2012; Sherwin and Henry, 2008).
A major factor propelling research in women’s health is that the life expectancy of women has significantly increased from an average of 54 years, to about 80 years (Singh et al., 1996). Since women are living longer, but age of spontaneous menopause has remained stable, women are now living approximately one-third of their lives in a hypo-estrogenic menopausal state (Amundsen and Diers, 1970; Amundsen and Diers, 1973; Sherwin, 2003). This realization has resulted in an increased interest in understanding the effects of ovarian hormone loss and subsequent hormone therapy administration. Emerging findings are now indicating that multiple parameters impact the extent, and even in some cases the direction, of cognitive effects of hormone loss and administration. Findings show the broad impact of gonadal hormone effects on brain functions such as learning and memory, and underscore the rich effects that ovarian hormones have on brain plasticity.
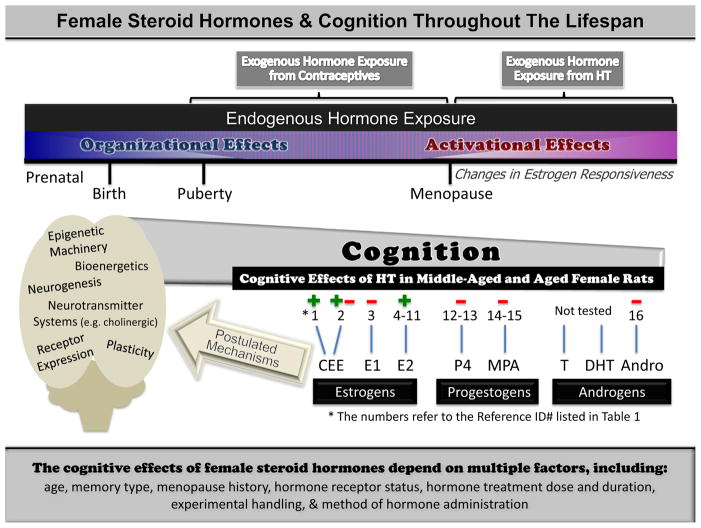
Here, we review recent findings on the impact of gonadal hormones during cognitive aging. We discuss this work in the context of landmark gonadal hormone-related discoveries that have provided the framework to guide a path to healthy cognitive aging. Evidence regarding hormone loss and parameters involving treatment optimization has emerged from both preclinical and clinical realms. Preclinical animal models have been widely used because they allow methodical experimental control of factors likely influencing outcome in clinical studies such as age, duration of hormone loss or treatment, specific type and dose of hormone manipulated, socioeconomic status and education. Here, we review literature focusing on the female, spanning the activational effects of estrogens, progestogens, and androgens. We discuss effects on cognition, brain health, and the neuromechanisms possibly mediating these effects. Further, because spatial cognition has been the focus of the majority of the preclinical research in this area, we will focus on spatial cognition, and review the mnemonic memory types most commonly measured. In this review we will discuss ovarian hormones, as well as synthetic and naturally-derived hormones included in various hormone therapy regimens. Ovarian hormones are endogenous and originating from the ovaries of the organism, and hormones from hormone therapies are exogenous and originating from outside of the organism. Thus, the term “ovarian hormone” does not accurately encompass hormones that are exogenously administered and not released endogenously, such as many of those in hormone therapies. For parsimonious discussion, we refer collectively to both endogenous ovarian-derived hormones from the subject, and administered hormone therapy hormones, as “female steroid hormones”.
Operationally Defining and Testing Memory Effects of Female Steroids in the Preclinical Setting
When studying learning and memory in the rodent model, it is vital to acknowledge the multiple parameters involved in the process of quantifying cognitive scores in order to properly interpret data. Within the specific domain of spatial navigation, rodents learn to navigate through a novel environment so that routes to the target eventually become familiar, and associations are formed from cues in the environment to aid overall navigation. Spatial learning and memory involves the ability to navigate effectively through an environment, acquiring, integrating and retaining environmental features such as landmarks and other prominent cues; spatial abilities depend upon medial temporal lobe structures such as the hippocampus (Barnes, 1998).
Being able to differentiate and test various types of spatial memory is vital to successful translational research. Many experimental tests of rodent spatial memory aim to assess spatial working memory, a form of short-term memory which requires a subject to retain spatial information which must be updated and is useful for only a short period of time (trial-specific information, Baddeley and Hitch, 1974). Working memory requires manipulation of information kept “on-line”; in fact, the late Dr. Patricia Goldman-Rakic cleverly and accurately referred to working memory as “working with memory” (Goldman-Rakic, 1987). Working memory is affected by normal aging, revealing a memory decline that becomes more severe as task difficulty increases (Balota et al., 2000). In animals, age-related spatial deficits become more pronounced as memory demand increases as well. This has been shown for age-associated interference-related deficits (Lebrun et al., 1990) and for memory capacity deficits (Aggleton et al., 1989; Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004b; Bimonte et al., 2003). In general, working memory is distinguished from reference memory, which is necessary to remember information that remains constant over time (task-specific information, for discussion see Olton et al., 1979).
Activational versus Organizational Effects of Hormones: Perspectives For Discussion Herein
Gonadal steroid hormone actions are traditionally referred to as having organizational effects, operationally defined in the traditional sense as occurring early in development and having permanence, or as having activational effects which occur later in development, are transitory, and therefore depend on presence of the hormone at the time of assessment. For example, sex differences in neuroanatomy have been traditionally thought to reflect the permanent organizing effects of steroids present during early development. Activational effects have been thought to activate the underlying organized neural substrate. The expression of many sexually dimorphic behaviors (for example, sexual behaviors in rodents) depend on the presence of circulating hormones; indeed, exogenous administration of sex steroids can induce these behaviors as long as the hormone remains present. Notably, organizational and activational effects work together; as an example, while early hormonal exposure plays a significant role in organizing brain substrates underlying sexually differentiated behaviors, most of these effects are realized only when activational exposure ensues (e.g., Beatty, 1992). Endogenous gonadal hormones or exogenous hormone administration in adulthood can therefore impact behavioral phenomena that were organized early in development. This interactive model demonstrates that activating (or circulating) hormones are acting upon a neural substrate that has been differentiated, and therefore, a “male” versus “female” brain is unlikely to respond to the same circulating hormones in the same way (e.g., Beatty and Beatty, 1970). Accumulating evidence has, however, blurred this traditional organizational/activational dichotomy, with the temporal distinction being called into question. For example, estrogenic activation of sexual behavior in female rat pups has been seen as young as 6 days of age (Williams, 1987), and non-transient changes in brain morphology following post-pubertal hormone manipulations have been noted (Bimonte et al., 2000a; Bimonte et al., 2000b; Bimonte et al., 2000c; Bloch and Gorski, 1988; Pappas et al., 1979; Rodriguez-Sierra, 1986). It has therefore been suggested that the newer organizational/activational distinction should primarily depend on whether the induced effects are permanent or transient, regardless of when they occur during the lifespan (e.g., see Arnold and Breedlove, 1985; Fitch and Denenberg, 1998; Stewart and Kolb, 1988 for discussion).
How do we discuss new findings of ovarian hormone effects on the brain and its function within the context of the organizational/activational dichotomy? While defining these effects as organizational or activational does not necessarily change how we interpret the resulting experimental data per se, it is ideal (and some hormone researchers may say, necessary) to acknowledge the findings within the context of this long-held tenet. There is rich neurophysiological and neurochemical plasticity in the female brain, including brain regions known to subserve learning and memory, suggesting that cognitive brain regions are sensitive to ovarian hormone fluctuation (Becker and Cha, 1989; Frankfurt et al., 1990; Gibbs, 1996; Woolley et al., 1990). These effects are transient and may not necessarily be considered “permanent features,” but nevertheless constitute a part of what makes the female brain distinct from the male brain. Females clearly have an inherent “permanently cyclic” and dynamic neural landscape that modifies along with the reproductive cycle (Fitch and Denenberg, 1998). Moreover, these "permanently cyclic" features have potentially important relevance to cognitive aging since gonadal hormones can impact memory, and gonadal hormone levels change with age (Bimonte-Nelson et al., 2010; Conrad and Bimonte-Nelson, 2010). For the remainder of this review, we focus on the activational effects of female steroid hormones on cognitive function and brain health during aging in females, defining these effects as transient and depending on the presence of hormone.
Menopause Etiology
Studies have shown that ovarian hormone loss negatively impacts cognition in women, and that these effects correspond to the associated estrogen deficiency (Farrag et al., 2002; Phillips and Sherwin, 1992; Sherwin, 1988). Others have shown modest, statistically significant declines in cognitive performance in women after surgical menopause, but express that the effects were small and not likely to be of clinical significance (Kritz-Silverstein and Barrett-Connor, 2002). In numerous studies, cognitive decline has been seen in women after surgical menopause (Farrag et al., 2002; Phillips and Sherwin, 1992; Sherwin, 1988). For example, surgical menopause negatively impacted performance on tests of global cognitive function by three months after surgical ovarian hormone loss, an effect that persisted six months post-surgery (Farrag et al., 2002). Further, surgically menopausal women demonstrated lower memory scores relative to naturally menopausal women, and age of oophorectomy (surgical ovary removal) and greater years since surgery correlated with poorer performance (Nappi et al., 1999). A recent longitudinal study evaluating 1903 women from 2000–2006 found decreased cognitive processing speed in women during late perimenopause, effects not attributed to depression, anxiety, sleep irregularities, or vasomotor symptoms (Greendale et al., 2010). Memory complaints were also associated with poorer memory encoding in women transitioning into menopause, and this was predicted by estrogen levels (Weber and Mapstone, 2009). Moreover, a recent elegantly performed study evaluated cognitive change across time in postmenopausal women, controlling for age and education (Thilers et al., 2010). Results showed a decline in verbal fluency with postmenopause, as well as declines in visuospatial ability and episodic memory measures; the latter effects were greater in women with a body mass index over 25, as compared to those with a lower body mass index considered within the normal range (18.5–25; Thilers et al., 2010). Together, results in women support the hypothesis that decreases in endogenous estrogen levels concordant with postmenopause have a negative influence on cognitive abilities, although this effect is modest in some studies, and may depend on several factors including women’s stage of the menopause transition, time since menopause induction if surgical, and body mass index. Age may be another factor, as increased risk of cognitive impairments was found in surgically menopausal women, and risk increased with younger age at surgery (Rocca et al., 2007).
Preclinical evaluations in rats also provide evidence that ovarian hormone loss impacts cognition. Because these preclinical studies allow methodical manipulation of factors that could impact outcome, the results from these studies can systematically demonstrate that hormone effects depend on many factors, including age (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004b; Bimonte et al., 2003; Markowska and Savonenko, 2002; Savonenko and Markowska, 2003; Talboom et al., 2008). Indeed, cognitive decrements on spatial and non-spatial tasks have been observed in young adult rats in response to surgical menopause (Ovx) (Bimonte and Denenberg, 1999; Daniel et al., 1999; El-Bakri et al., 2004; Feng et al., 2004; Gibbs and Johnson, 2008; Singh et al., 1994; Talboom et al., 2010; Wallace et al., 2006). In contrast, Ovx in aged rats may be beneficial to cognition. Our laboratory showed that Ovx impaired working memory on the water radial arm maze in young animals (Bimonte and Denenberg, 1999), yet, initially to our surprise, years later we found Ovx-induced cognitive enhancements on the same task with aged Ovx animals performing better than ovary-intact age-matched sham animals (Bimonte-Nelson et al., 2003). Moreover, we have replicated this finding multiple times, and our research indicates that the Ovx-induced enhancements are likely due to removal of elevated progesterone levels seen with estropause in the rat (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004b; Braden et al., 2010). At this point then, we know that Ovx in young animals is detrimental, and Ovx in aged animals is beneficial, for water radial arm maze working memory scores. The next question is, “when during aging does Ovx transition from detrimental to beneficial for the rat?”. The cognitive effects of Ovx during middle age are less clear, as only a few studies have evaluated Ovx effects on maze learning and memory in middle-aged rats (Acosta et al., 2009a; Bimonte-Nelson et al., 2006; Markowska and Savonenko, 2002; Savonenko and Markowska, 2003; Talboom et al., 2008). In middle-aged females 12–16 months-old, Ovx did not impact spatial reference memory (Acosta et al., 2009a; Bimonte-Nelson et al., 2006; Markowska and Savonenko, 2002; Talboom et al., 2008) or spatial working memory (Acosta et al., 2009a; Markowska and Savonenko, 2002; Savonenko and Markowska, 2003), as well as tasks on which either a spatial or non-spatial strategy can be used for successful performance (Markowska and Savonenko, 2002; Savonenko and Markowska, 2003). Deficits in spatial working memory were, however, detected in 17 month old Ovx rats following high-demand time delayed memory retention tests (Markowska and Savonenko, 2002). Thus, Ovx-related memory changes during middle-age may become evident when working memory demands are more challenging. In this regard, we previously demonstrated that the cognitive effects of Ovx in both young and old rats were more pronounced as working memory load increased (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004b; Bimonte and Denenberg, 1999; Braden et al., 2010). Hence, increasing the working memory demand, either by extending time delays to challenge retention, or by increasing the number of items to remember, facilitates a broader scope of evaluations to realize Ovx-induced memory changes across the ages. A study methodically testing the effects of Ovx at different ages ranging from young adulthood to old age is necessary to determine the age at which Ovx transitions from detrimental to beneficial. Determining brain changes associated with this transition are not only important for clarifying mechanisms of menopause-related learning and memory alterations, but could also yield valuable information on underlying mechanisms of cognitive aging and potential mechanisms of hormone therapy-induced enhancements.
Preclinical research in rats has yielded much insight regarding the cognitive impact of Ovx effects across the life-span. Of note, the majority of women undergo menopause as a transitional loss of ovarian hormones due to age-related alterations of the hypothalamus, pituitary, and ovary, ultimately resulting in ovarian follicular depletion, rather than an abrupt loss of ovarian hormones via oophorectomy (Quirion et al., 1995). In addition, there are distinct differences in the mechanism and hormone profile of senescent female rats and women that preclude the use of the ovary-intact aging female rat as the sole and optimal model for menopause. In women, menopause occurs at about the fifth decade of life and is characterized by loss of ovarian-derived circulating hormones, including estrogen and progesterone (Quirion et al., 1995). For both women and female rats, neuronal changes in the hypothalamus are hypothesized to initiate the transition into reproductive decline, leading to reproductive senescence (Downs and Wise, 2009). However, the ultimate hormone profile of ovary-intact reproductively senescent female rats and woman differ, limiting the use of the normally aging female rat as an optimal model of human menopause. Nonetheless, the rodent model provides insights into mechanisms of menopause since there are some commonalities in reproductive physiology (Downs and Wise, 2009). In women, as aging ensues, estrogen and progesterone decline due to decreased ovarian follicular reserves (Quirion et al., 1995). Thus, menopause is the cessation of ovarian cyclicity, ultimately caused by ovarian follicular depletion. In contrast, the aging rat undergoes estropause, a persistent estrus state due to chronic anovulation rendering intermediate estrogen levels, or a pseudopregnant/persistent diestrus state characterized by high progesterone levels due to increased ovulation and corpora lutea (Meites and Lu, 1994). These changes in ovarian-derived hormone release in the rat are primarily due to hypothalamic/pituitary axis alterations (Downs and Wise, 2009; Meites and Lu, 1994). Thus, the primary mechanism that ultimately results in reproductive senescence and circulating hormone alterations in the woman is ovarian follicular depletion, while in the rat it is the hypothalamic-pituitary axis.
Transitional hormone loss can be induced in rodents via administration of the industrial chemical 4-vinylcyclohexene diepoxide (VCD) (Mayer et al., 2002; Mayer et al., 2004; Springer et al., 1996). VCD produces follicular depletion by selectively destroying primordial and primary follicles via acceleration of the natural process of atresia, resulting in hormone profiles in rodents that are more similar to naturally menopausal women as compared to Ovx (Mayer et al., 2002; Mayer et al., 2004; Springer et al., 1996; Timaras et al., 1995). We recently showed that VCD-induced transitional menopause impaired learning of a spatial recent memory task (Acosta et al., 2009a). We also demonstrated that rats undergoing transitional menopause before Ovx was better for spatial memory than an abrupt loss of hormones via Ovx without the prior transition (Acosta et al., 2009a). Nappi et al. (1999) showed that in women poorer cognitive scores corresponded with a longer duration of hormone deprivation from oopherectomy. It is of note that in that study, surgically menopausal women were younger at menopause than naturally menopausal women. Therefore, it is plausible that those surgically menopausal women did not undergo an extended period of transitional menopause before surgical menopause, and thus did not have benefits due to a transitional hormone decline prior to oophorectomy. In fact, Rocca et al. (2007) found that women that had undergone oophorectomy prior to menopause onset showed an elevated risk of cognitive impairment as compared to age-matched women without oopherectomy. In addition, risk of cognitive impairment increased as age at oopherectomy decreased, which presumably limited the duration of transitional menopause. Further evaluations of women receiving oopherectomy before, versus after, transitional menopause will better discern whether history of transitional menopause before surgical ovarian removal affects subsequent cognitive change. It is possible that an as yet undetermined duration of transitional menopause before oopherectomy is optimal for cognitive outcome, a tenet supported by the animal literature; indeed, this would obviate the abrupt ovarian hormone loss associated with oopherectomy.
Estrogens
Estrogens are a class of hormones including 17β-estradiol, estrone, and estriol. 17β-estradiol is the most potent naturally-circulating estrogen, followed by estrone and estriol, in order of receptor affinity (Kuhl, 2005; Sitruk-Ware, 2002). Since the first controlled clinical evaluation showing that 17β-estradiol injections enhanced memory in 75 year-old women (Caldwell and Watson, 1952), numerous studies have demonstrated cognitive decline after ovarian hormone loss, and enhancement after treatment with various types of estrogen-containing preparations, in menopausal women (for a review see Sherwin, 2006). Conjugated equine estrogens (CEE; tradename Premarin), is the most commonly prescribed hormone therapy given to women (Hersh et al., 2004). CEE contains the sulfates of at least ten estrogens, is over 50% estrone sulfate, 20–25% equilin sulfate, and has only trace amounts of 17β-estradiol. After CEE is metabolized, the resulting primary circulating hormones are estrone and, after estrone’s conversion, 17β-estradiol, as well as equilin (Bhavnani, 2003; Sitruk-Ware, 2002). It is hypothesized that these three metabolites are primarily responsible for the estrogenic effects of CEE (Sitruk-Ware, 2002), although there are other estrogens and related metabolites that could initiate effects on their own; these hormones include, but are not limited to equilin, Δ8,9 dehydroestrone, dihydroequilin-17β, and equilenin (Kuhl, 2005).
The cognitive effects of CEE are mixed. CEE-containing therapy improves memory in studies of women utilizing self-report (Campbell and Whitehead, 1977), case studies (Ohkura et al., 1995) and randomized psychometric evaluations (Kantor et al., 1973). Yet, findings evaluating global cognitive function in the large placebo-controlled Women’s health Initiative (WHI) Memory Study (WHIMS) demonstrated an increase in probable dementia risk and no effect on mild cognitive impairment in women 65 years or older taking the combination therapy CEE + the progestin, medroxyprogesterone acetate (MPA, Shumaker et al., 2003). CEE alone showed a non-significant increase in incidence of probable dementia and mild cognitive impairment (Espeland et al., 2004; Shumaker et al., 2004). The WHI Study of Cognitive Aging (WHISCA), an ancillary study to the WHI testing more specific cognitive functions, in women 65 and over free of probable dementia, reported that CEE+MPA therapy had a negative effect on verbal memory and a trend for positive effects on figural memory (Resnick et al., 2006). Most recently, the WHIMS-MRI study found that CEE use with or without MPA was associated with small but measurable atrophy in the frontal cortex and hippocampus, brain regions important for cognition (Resnick et al., 2009). Thus, there is currently little consensus in clinical studies regarding the cognitive impact of estrogen-containing therapy. New investigations clarifying the complex effects of ovarian hormone loss and hormone therapies given to women during aging are warranted.
Crucial to advancement of the field is identifying the various administration parameters and components of hormone therapies that impact outcomes on cognition. Detailed hypothesis-driven and methodical assessments using basic science and system approaches are optimal for converging the many findings that seem contradictory. With emerging data and results from these types of studies, it is becoming clear that the cognitive effects of hormone therapy may not be contradictory at all. Findings may, in fact, be dependent on numerous variables not yet taken into account. One way to further our understanding of the impact of gonadal hormone loss and therapeutic interventions on cognition is by using animal models. Rodent models have been invaluable in providing insight into the cognitive effects of hormone therapies administered after hormone loss, while enabling experimental control not possible in human research. Most preclinical studies have utilized 17β-estradiol to test cognitive effects of hormone therapy in Ovx animals. For example, numerous studies have reported beneficial effects of 17β-estradiol on spatial learning and memory in young (Bimonte and Denenberg, 1999; Daniel et al., 1997; Daniel et al., 1999; Dohanich et al., 1994; Galea et al., 2001; Holmes et al., 2002; Luine et al., 1998; Luine et al., 2003; Marriott and Korol, 2003; McLaughlin et al., 2008; Packard and Teather, 1997b; Sandstrom and Williams, 2001) and in middle-aged or older female rats (Bimonte-Nelson et al., 2006; Foster et al., 2003; Gibbs, 2000b; Markham et al., 2002; Markowska and Savonenko, 2002; Savonenko and Markowska, 2003; Talboom et al., 2008; Ziegler and Gallagher, 2005). Effects of 17β-estradiol treatment in young and middle-aged rodents are also observed on the non-spatial visual object recognition task whereby animals are tested on whether they “recognize” a novel object (operationally defined by increased exploration of the novel object; Fernandez et al., 2008; Lewis et al., 2008; Luine et al., 2003).
Recently, our laboratory examined the effects of both tonic and cyclic CEE. In these rodent studies, the doses of CEE administered were relevant to what women take as hormone therapy, with the clinically-used dose only adjusted for body weight. We demonstrated that CEE enhanced spatial working memory and delayed memory retention, and protected against cholinergic challenge on spatial tasks, in Ovx middle-aged rats (Acosta et al., 2009b; Engler-Chiurazzi et al., 2011). Further, a single high dose CEE injection enhanced non-spatial working memory object recognition (Walf and Frye, 2008). However, low CEE doses have been shown to impair spatial working and reference memory performance (Barha and Galea, 2012; Engler-Chiurazzi et al., 2011), an effect we hypothesized to be due to higher circulating levels of estrone relative to 17β-estradiol, resulting after specific doses of CEE administration (Engler-Chiurazzi et al., 2011). Indeed, animals receiving low dose CEE showed increased serum estrone levels, while no increases were found in 17β-estradiol levels, relative to control animals (Enger-Chiurazzi et al., 2011; but see, Barha & Galea, 2012). Moreover, increases in circulating estrone levels were associated with greater working memory errors in rats (Barha and Galea, 2012) and lower cognitive scores in women (Yaffe et al., 1998).
Therefore, our next goal was to methodically evaluate the impact of estrone on learning and memory in middle-aged rats. In contrast to the beneficial effects of CEE in Ovx rats, estrone treatment impaired spatial working memory and memory retention, and did not alter number of choline acetyltransferase (ChAT, the synthesizing enzyme of acetylcholine)-positive basal forebrain neurons, as 17β-estradiol does (Engler-Chiurazzi et al., 2011). Hence, evidence thus far indicates that estrone, the primary active metabolite after CEE administration in rats and women, is detrimental for cognition. To extend evaluations to other CEE components, we tested the cognitive effects of Δ8,9-dehydroestrone and equilin, two CEE components identified to be neuroprotective in vitro (Zhao and Brinton, 2006). We found that chronic Δ8,9-dehydroestrone treatment enhanced memory, whereas equilin had no effect, in Ovx rats (Talboom et al., 2008). Thus, other CEE components may have functional benefits on cognition, providing new treatment opportunities.
Responsiveness to estrogens may depend on many variables other than type of estrogen, including age. Young and middle-aged Ovx rats showed beneficial effects of 17β-estradiol treatment on the spatial reference memory Morris water maze task, and higher circulating 17β-estradiol levels correlated with better performance (Talboom et al., 2008). In contrast, aged Ovx rats were not responsive to the same 17β-estradiol regimen that enhanced spatial reference memory in young and middle-aged Ovx rats (Talboom et al., 2008), concurring with age-related interactions with 17β-estradiol replacement for spatial memory shown by others (Foster et al., 2003). However, some studies have shown that aged female rodents can exhibit cognitive enhancements after 17β-estradiol treatment. For example, 17β-estradiol injections enhanced spatial reference memory in 27–28 month old ovary-intact mice (Frick et al., 2002). The difference in results may relate to the type of 17β-estradiol administration as a cyclic versus tonic regimen. In this regard, for cognition, cyclic 17β-estradiol treatment enhanced responsiveness to tonic 17β-estradiol treatment in older Ovx rats (Markowska and Savonenko, 2002).
In addition to age, estrogenic effects on cognition may be influenced by many other factors including, but not limited to, timing of hormone administration relative to hormone loss, dose, mode of treatment, and whether progestogens are administered concomitantly. Some of the translational questions driving much of the newer preclinical research studying estrogens’ impact on brain health and cognition during aging have been directly spawned from the WHIMS findings. Women participating in the WHIMS were between 65–79 years old, and had experienced ovarian hormone deprivation for a substantial amount of time prior to receiving CEE treatment (Shumaker et al., 1998). These findings beg the question of whether there is a window of opportunity for which hormone therapy can be effective. Indeed, recent preclinical studies found evidence of this. 17β-estradiol given immediately after Ovx enhanced spatial memory performance in middle-aged rats, but had no benefit when given 5 months after Ovx (Daniel et al., 2006). Additionally, 17β-estradiol treatment given immediately after Ovx increased cholineacetyltransferase (ChAT) levels in the hippocampus, an increase not seen when initiated 5 months after Ovx; the opposite pattern was seen in prefrontal cortex, wherein 17β-estradiol treatment given 5 months after Ovx, but not immediately after Ovx, increased prefrontal cortex ChAT levels (Bohacek et al., 2008). Further, 17β-estradiol given immediately or 3 months after Ovx, but not 10 months after Ovx, enhanced delayed-match-to-position performance (Gibbs, 2000b). There may also be a critical window for the well-established findings that 17β-estradiol regulates dendritic spines in the hippocampus (Woolley, 2000); we found that a 10 week delay after Ovx attenuated the effectiveness of 17β-estradiol-induced increases in CA1 apical spine density, as compared to treatment given immediately (McLaughlin et al., 2008). Further, increased cell proliferation was observed in the dentate gyrus when 17β-estradiol was administered 1 week, but not 4 weeks, after Ovx (Tanapat et al., 2005). Thus, clinical and preclinical findings concur that the beneficial effects of estrogens may be dependent on early initiation relative to ovarian hormone loss (Khoo et al., 2010; Sherwin, 2005). However, the specific temporal parameters of these effects have not been discerned, and it remains unclear whether these findings can be extended to estrogens other than 17β-estradiol.
Important to the discussion of parameters contributing to the complexity involved in the cognitive impact of hormone therapy, is the consideration of menopause history (surgical or transitional). Although our laboratory recently found that CEE was beneficial for cognition (Acosta et al., 2009b; Acosta et al., 2010), we observed that these benefits were limited to rats that had undergone surgical menopause (Acosta et al., 2010). In surgically menopausal (Ovx) rats, CEE enhanced reference and working memory (Acosta et al., 2010). In contrast however, VCD-induced transitionally menopausal rats showed the opposite effect after CEE administration, with a CEE-induced increase in errors on these measures (Acosta et al., 2010). Further, CEE-treated Ovx rats showed better memory retention across an 8-hr delay relative to vehicle-treated Ovx rats, while CEE exerted no retention benefit in VCD transitionally menopausal rats (Acosta et al., 2010). Thus, CEE was beneficial after surgical menopause, but detrimental after transitional menopause (Acosta et al., 2010). Accordingly, human studies evaluating surgically menopausal women after hormone treatment show cognitive benefits of hormone therapy (Phillips and Sherwin, 1992; Sherwin, 1988), consistent with our CEE findings in Ovx rats (Acosta et al., 2009b; Acosta et al., 2010; Engler-Chiurazzi et al., 2011). Reports of positive effects of hormone therapy in studies including only oophorectomized women, and the current findings of differential cognitive effects of CEE depending on menopause history in the rodent model, collectively suggest that menopause history is a plausible factor that contributes to cognitive efficacy of hormone therapy.
Progestogens
Progestogens include steroids with a pregnane skeleton, including naturally-occurring progesterone as well as progestins (synthetic progesterones). Inherent to any discussion on the impact of hormone therapy on cognition, and the complexities involved in outcome, is that of combination therapy, which includes estrogen plus a progestogen concomitantly. Investigating combination therapy is crucial since women with a uterus taking estrogens must include a progestogen in their regimen to offset the increased risk of endometrial hyperplasia associated with unopposed estrogen treatment (Smith et al., 1975). Progestins may have a negative impact on cognition in women. For example, findings demonstrated that menopausal women taking CEE alone did not significantly differ from those taking placebo for dementia diagnoses in the WHIMS (Shumaker et al., 2004); in contrast, twice as many women receiving CEE plus the synthetic progestin MPA were diagnosed with dementia as compared to the placebo group in the WHIMS (Shumaker et al., 2003).
Rodent models have been instrumental in deciphering some of the complexities associated with combination therapy. In a rodent study of combination treatment, we showed that natural progesterone abolished 17β-estradiol-induced benefits on the spatial reference memory Morris water maze in middle-aged rats (Bimonte-Nelson et al., 2006). Additionally, while natural progesterone or 17β-estradiol alone did not influence performance on the Morris water maze (Chesler and Juraska, 2000), progesterone plus 17β-estradiol injections did impair performance (Chesler and Juraska, 2000; Lowry et al., 2010). These effects were not seen on the non-spatial Morris water maze, suggesting combination treatment has specific effects within the spatial domain (Chesler and Juraska, 2000). These effects may, however, be specific to reference memory, as long-term progesterone treatment enhanced 17β-estradiol’s effects on the working memory delayed-match-to-position spatial t-maze (Gibbs, 2000b) (but see Chisholm and Juraska, 2012).
In both clinical and preclinical studies, progesterone administration has been associated with cognitive detriment. In pregnant women, there is the reported “maternal amnesia” phenomenon, hypothesized to result from high circulating progesterone levels during late pregnancy (Brett and Baxendale, 2001). Progesterone-induced memory detriments are also seen in healthy women; indeed, healthy women given a high oral progesterone dose showed cognitive impairment (Freeman et al., 1992). High circulating progesterone levels are evident following estropause in the rat (Lu et al., 1979). Many aged female rats enter a pseudopregnant estropause state, whereby progesterone values become significantly elevated but 17β-estradiol levels remain relatively unchanged (Huang et al., 1978; Wise and Ratner, 1980). This pseudopregnant state has been associated with impaired spatial memory (Warren and Juraska, 2000). Similarly, on the spatial Morris water maze, young cycling rats performed best during the estrus phase, when estrogen and progesterone levels are at their lowest, and worst during the proestrus phase, when estrogen and progesterone levels are at their highest (Warren and Juraska, 1997) (but see Berry et al., 1997; Stackman et al., 1997). Further, in contrast to the detrimental effects of Ovx in young animals, in aged female rats Ovx improves cognition (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004b), an effect likely due to the removal of elevated progesterone levels seen with estropause. We have also shown that progesterone administration impairs spatial working and reference memory, and reverses the beneficial effects of Ovx seen in aged rats (Bimonte-Nelson et al., 2004b; Braden et al., 2010).
Since the synthetic progestin, MPA, is the progestin component in the commonly perscribed hormone therapy, Prempro®, it was a goal of our laboratory to examine the impact of MPA on a cognitive battery in a controlled rodent experiment. We found that MPA impaired memory retention after a delay on the water radial arm maze, and exacerbated overnight forgetting on the spatial reference memory Morris water maze task (Braden et al., 2010). MPA treatment also impaired spatial working memory, and detriments were particularly pronounced as the working memory load increased (Braden et al., 2010). Of note, MPA is also the sole hormone in the contraceptive Depo Provera®. While no clinical study has directly evaluated the impact of MPA on cognitive health in younger women using this contraceptive, one case study reported amnesic effects corresponding with Depo Provera® use in young women (Gabriel and Fahim, 2005). To further investigate our findings of MPA-induced cognitive impairments during aging, we conducted a study evaluating the long-term effects of MPA. Results showed that MPA administration in either young adulthood, middle-age, or at both times, impaired working memory when measured at the middle-aged time point (Braden et al., 2011). The deleterious effects of MPA were greater when animals received treatment during both young adulthood and middle-age, as impairments were observed on 2 orthogonal measures of working memory and, in particular, on trials with higher working memory demand (Braden et al., 2011). Further, combination MPA plus 17β-estradiol treatment was detrimental on the spatial reference memory Morris water maze task relative to other hormone-treated groups, including progesterone plus 17β-estradiol treatment (Chisholm and Juraska, 2012). The detrimental effects of MPA in combination with 17β-estradiol may be task and cue-use dependent, as chronic administration of MPA plus 17β-estradiol was beneficial on an alternation t-maze task, a task which can be solved using either a spatial or a non-spatial strategy (Chisholm and Juraska, 2012).
Collectively, evidence spanning clinical and basic science indicates progestogens negatively impact neuronal health, which could result in detrimental effects on brain functions such as learning and memory. Progesterone abolishes the beneficial effects of 17β-estradiol (Bimonte-Nelson et al., 2006; Harburger et al., 2007) (but see Gibbs, 2000b) and attenuates 17β-estradiol’s neurotrophic effects in vivo (Bimonte-Nelson et al., 2004a) and in vitro (Aguirre and Baudry, 2009). Progesterone has been shown to have neuroprotective properties, while MPA does not (Nilsen and Brinton, 2002). Relative to progesterone, MPA causes greater attenuation of 17β-estradiol-induced neurotrophic actions (Nilsen and Brinton, 2002). Thus, while a progestin is necessary as part of hormone therapy in women that have a uterus (Smith et al., 1975; Ziel and Finkle, 1975), accumulating evidence indicates that the addition of a progestin does not result in a positive impact on brain health and function during aging. Progestins are an important and necessary component of hormone therapy for many women and should not be undervalued. A systems and personalized approach, valuing and accounting for multiple aspects of women’s health, is key to optimizing hormone therapy.
Androgens
Androgens are typically thought of as a “male” hormone; a masculinizing hormone which initiates permanent organizational effects on the male brain during a specific critical period in early development, and an activating hormone for male sex behaviors in adulthood. Why study androgens in females as a potential modulator of learning and memory? From our perspective, studying the impact of androgens on cognition in the female rodent model is important for several reasons. First, androgens bind to and activate the androgen receptor, which are expressed in the female brain. In particular, brain areas found to be important for cognition, such as the hippocampus and cerebral cortex, have been shown to express high concentrations of androgen receptors in female rodents (Simerly et al., 1990). Second, activation of these androgen receptors could ultimately impact cognition, as it results in changes in neuronal function via altering gene transcription (McPhaul and Young, 2001). Third, levels of androgen receptors in cognitive brain regions have been found to be modulated by Ovx and androgen administration, showing that androgen receptor levels are dependent upon circulating levels of androgens (Lu et al., 1998). It is therefore clear that androgens play an important role in normal brain functioning in female rodents. How androgens impact cognition in female rodents is still being deciphered, as research in this area is consistently emerging. This portion of the review will focus on the androgens that have been most studied, which are testosterone, dihydrotestosterone (DHT), and androstenedione.
Relations between endogenous testosterone levels and cognition have been noted in younger and older individuals. In general, the strongest associations have been seen in the older population in retrospective and randomized treatment studies. For example, testosterone was related to cognitive performance in young men and women, and spatial ability was related to seasonal changes in accordance with related alterations in testosterone levels (Kimura and Hampson, 1994; Neave et al., 1999; Silverman et al., 1999). Some evidence suggests an inverted U-shaped function for testosterone levels and spatial ability, with moderate levels optimal. An excellent example of this U-shaped function, which also exemplifies the importance of a broad perspective when analyzing the cognitive effects of hormones, is shown via the innovative insight of Dr. Doreen Kimura. For example, in one study, when males and females were both included in analyses, the perspective of an inverted U-shaped function was realized; this was not clearly evident when the sexes were analyzed separately. Specifically, higher relative levels of salivary testosterone were associated with better spatial ability performance in women, and lower relative levels of salivary testosterone were related to better spatial ability performance in men (Gouchie and Kimura, 1991).
Testosterone’s mnemonic effects could be due to either DHT, or to conversion into estrogen; testosterone can be converted to either DHT, which binds to androgen receptors via 5α reductase, or to estrogen via the aromatase enzyme (Becker, 1995). Several studies have been conducted assessing the impact of various types of androgens on cognition in the female rodent model. Bernice and Rader (2009) assessed the impact of androgen supplementation on cognition in the aged (20 to 22 month old) female mouse model by administering either testosterone, testosterone’s metabolite DHT, or vehicle, and testing them on the Morris water maze, novel object recognition, and passive avoidance. Testosterone, but not DHT, improved performance on the Morris water maze compared to vehicle suggesting that testosterone’s conversion to estrogens may relate to the impact of testosterone treatment for spatial reference memory (Benice and Raber, 2009). Enhancements may be specific to spatial navigation, since testosterone did not impact any other measure (Benice and Raber, 2009). In addition, DHT, but not testosterone, impacted passive avoidance scores, and neither DHT nor testosterone affected novel object recognition scores (Benice and Raber, 2009). Androgen-induced enhancements may be of a broader nature in younger animals, as administration of either DHT or testosterone enhanced performance on the Y maze, passive avoidance, and novel object recognition in 3 month old Ovx rats (Frye and Lacey, 2001). In this study, hormones were given post-training, thereby obviating issues of motivational or other non-cognitive factors that could impact interpretation of effects.
We have recently found evidence that the androgen androstenedione negatively impacts cognition in the female rat. This discovery was initially made via a correlational analysis between endogenous androstenedione levels and maze error scores, and then confirmed with experimental manipulation whereby androstenedione was exogenously administered (Acosta et al., 2009a; Acosta et al., 2010; Camp et al., 2012). Initially, we demonstrated that transitionally menopausal (VCD treated) middle-aged female rats exhibited superior cognitive scores across multiple domains, as compared to rats that had undergone surgical menopause. However, this effect was only apparent when the post-VCD follicle-deplete ovary was removed via Ovx. One of the more surprising findings from this study was that higher serum levels of androstenedione, which is released from the follicle-deplete menopausal ovary, correlated with poorer memory scores in VCD rats (Acosta et al., 2009a). In a follow-up study evaluating cognitive effects of CEE hormone therapy in transitionally and surgically menopausal rats, we again found a correlation between higher androstenedione levels and impaired performance in transitionally menopausal rats (Acosta et al., 2010). This correlation was evident for reference memory and two orthogonal working memory measures (Acosta et al., 2010). Importantly, these correlations were shown in ovary intact animals that had VCD treatment, which renders an androgen-rich environment from the postmenopausal ovary (Acosta et al., 2009a; Acosta et al., 2010). If androstenedione is in fact related to poorer memory, impairments should be evident after androstenedione administration to a “blank” ovarian hormone template. We tested this hypothesis in a study in which middle-aged (14 month old) Ovx rats were treated with one of two doses of androstenedione, or vehicle, and tested on a cognitive battery. Androstenedione administration that resulted in blood levels at the higher end of the physiological range in the female rat impaired spatial reference memory on the Morris water maze, impaired performance on the water radial arm maze when the working memory load was at its greatest, and impaired memory retention on a win-stay delay match to sample task, as compared to vehicle treatment (Camp et al., 2012). Thus, a correlation in the rat model showing that higher endogenous androstenedione levels were related to poorer memory performance led to methodical investigation and manipulation in the rat model; results showed that this androgen, released from the follicle-deplete ovary in both women and rats, markedly impairs memory.
Gonadotropins
Although it is well established that the gonadotropins follicle stimulating hormone (FSH) and lutenizing hormone (LH) are involved in regulating reproductive functions via negative and positive feedback loops, increasing evidence is indicating that gonadotropins, directly or indirectly, impact cognitive function as well, including within the spatial domain. Although the links between FSH and cognition do not appear to be realized (e.g., Acosta et al., 2009a; Luetters et al., 2007), there is strong evidence that LH is related to cognition, with the greatest support coming from the neurodegenerative disease literature (Webber et al., 2007). Supporting plausibility of LH effects on the brain and spatial cognition, it is of note that LH can cross the blood-brain-barrier (Lukacs et al., 1995). In addition, the highest density of LH receptors in the brain are found in the hippocampus (Lei et al., 1993; Zhang et al., 1999), a brain region intimately involved in spatial learning and memory, and affected by aging and AD.
We recently evaluated VCD-induced follicular depletion and Ovx effects on cognition in the middle-aged rat, and found a clear inverted U-shaped function for serum LH and number of spatial memory errors, with the highest and lowest LH levels associated with the best performance (Acosta et al., 2009a). A comparable effect was not seen with FSH (Acosta et al., 2009a). This relationship between LH and performance became apparent in scatterplots including all treatment groups, so that the range of values across groups could be noted (conceptually similar to Kimura’s procedure described above; Gouchie and Kimura, 1991). This range of values was broad, because, as expected, Ovx increased LH levels due to a lack of ovarian hormone negative feedback after ovarian hormone loss, while sham control animals showed LH values in a lower relative range. When LH levels ranged from approximately 0-to-2 ng/ml, higher LH levels correlated with worse maze performance thereby revealing a positive relationship with errors. However, when LH levels ranged from approximately 2-to-10 ng/ml, higher LH levels correlated with better maze performance thereby revealing a negative relationship with errors (Acosta et al., 2009a). We noted a striking inverted U-shaped function when the groups were combined into the same scatterplot. This pattern was seen for multiple measures, including both spatial working memory and spatial reference memory. While there are limitations in this study in interpreting this relationship because LH levels were confounded by group membership, this quadratic relationship is nonetheless remarkable. Understanding this relationship between LH and memory can have clinical significance, especially given increasing evidence that LH levels are linked to pathologies associated with neurodegenerative disorders, as well as cognition (Webber et al., 2007). There are other studies reporting that higher LH levels are related to better cognitive performance, similar to the effects seen in our study in the Ovx animals. For example, tonic LH-releasing hormone treatment to elevate LH concentrations to Ovx levels, enhanced performance on visual-discrimination in young rats (Nauton et al., 1992), and enhanced non-spatial working memory in aged rats (Alliot et al., 1993). Relations between higher LH and better memory in these studies are likely related to LH levels being increased to that of Ovx animals. In contrast, corresponding with the Acosta et al. (2009a) findings in ovary-intact animals that higher LH levels correlated with poorer cognitive performance, in aged ovary-intact female mice, experimentally-induced LH attenuation decreased amyloid-β concentrations and enhanced cognition, while LH increases promoted biochemical brain changes consistent with AD; however, none of these studies correlated circulating LH levels with memory scores in individual subjects (Bowen et al., 2004; Casadesus et al., 2006; Casadesus et al., 2007). Other supporting evidence shows that men and women with AD had higher circulating LH levels than controls (Bowen et al., 2004; Short et al., 2001).
The growing literature evaluating the relationship between LH levels and cognitive performance suggests that it may subserve an inverted U-shaped function, with lower and higher levels resulting in optimal learning and memory, and intermediate levels resulting in poorest learning and memory. This is an important area that will require further study, with results possibly revealing important mediators of cognitive function. Effects could explain, in part, how hormone modulation impacts learning and memory.
Female Steroids and Cognition: Landmark and Most Recent Putative Mechanisms of Ovarian Hormone Action
Cholinergic and γ-Aminobutyric acid (GABA)ergic Systems
Abundant evidence suggests that the cholinergic and GABAergic systems are intimately related to the effects of female steroids on cognition. Pharmacological experiments using both peripheral and intracranial infusions show that the cholinergic system may mediate estrogen-induced effects. Much of the landmark work evaluating relations between estrogens and the cholinergic system has been done via the creative and methodical approach of combining hormones and pharmaceutical agents in the laboratory of visionary Dr. Robert Gibbs. In middle-aged Ovx rats, galanthamine, a cholinesterase inhibitor, combined with 17β-estradiol enhanced acquisition of a delayed matching to position (DMP) T-maze task relative to vehicle treatment, while the effects of 17β-estradiol or galanthamine alone did not differ from vehicle (Gibbs et al., 2011a). Donepezil, another cholinesterase inhibitor, combined with 17β-estradiol enhanced acquisition of the DMP task in animals with cholinergic lesions (<50% loss of cholinergic neurons), whereas 17β-estradiol and donepezil alone were ineffective (Gibbs et al., 2011b). 17β-estradiol has also been shown to alter cholinergic markers in the brain. In young adult Ovx rats given 17β-estradiol, maze acquisition correlated with increased ChAT activity in two targets of basal forebrain cholinergic innervation, the hippocampus and frontal cortex (Gibbs, 2002), and 17β-estradiol increased high affinity choline uptake in these regions (O'Malley et al., 1987; Singh et al., 1994). 17β-estradiol increased ChAT in the horizontal limb (Luine and McEwen, 1983; Luine, 1985; Singer et al., 1998) and in projection sites to hippocampus and cortex (Feng et al., 2004; Luine, 1985; Singh et al., 1994), and restored basal forebrain ChAT mRNA expression to levels of ovary-intact animals (Gibbs et al., 1994; McMillan et al., 1996). Lesioning the basal forebrain prevented 17β-estradiol-enhanced learning, indicating the basal forebrain is a key area through which 17β-estradiol cognitive enhancements occur (Gibbs, 2002; Gibbs, 2007). Other estrogens may also alter cholinergic markers as we recently showed that CEE enhanced the number of ChAT immunoreactive neurons in the vertical diagonal band of the basal forebrain. Estrogens may also alter cholinergic neurochemistry, as 17β-estradiol potentiated acetylcholine (ACh) release in the hippocampus during maze learning; this is especially noteworthy since the hippocampus is a primary projection site of the basal forebrain (Marriott and Korol, 2003).
Interestingly, increased release of ACh in the hippocampus may lead to muscarinic receptor M2 activation, which in turn, can suppresses the release of GABA (Birzniece et al., 2006; Daniel and Dohanich, 2001; Daniel et al., 2005). Hence, estrogen-mediated increases in ACh signaling through muscarinic activation may release hippocampal inhibition, and in turn allow for alterations in synaptic plasticity that may ultimately lead to enhanced cognition (Rudick and Woolley, 2001). However, ACh can also stimulate nicotinic receptors and activate GABA interneurons in CA1 (Alkondon et al., 1999) and several other hippocampal lamina (Alkondon and Albuquerque, 2001). Specifically, the α4β2 nicotinic receptor produces the strongest postsynaptic inhibitory current onto CA1 pyramidal neurons as compared to other nicotinic receptors (Alkondon and Albuquerque, 2001). Recently, our laboratory found that the estrogenic CEE component, Δ8,9-dehydroestrone, enhanced memory and decreased hippocampal and entorhinal cortex α4β2 nicotinic receptor expression in Ovx rats, effects which correlated with better memory (Talboom et al., 2010). Hence, an estrogen-mediated reduction in hippocampal and entorhinal cortex α4β2 nicotinic receptor expression may lead to a suppression of GABA-mediated inhibition and subsequent cognitive enhancement. Progesterone has also been shown to enhance high affinity choline uptake in several cognitive brain regions, and it can potentiate 17β-estradiol-mediated increases in ChAT mRNA levels in the basal forebrain of Ovx rats (Gibbs, 1996; Gibbs, 2000a). Progesterone and progestins impact the GABAergic system, which may relate to their effects on cognition (Belelli and Herd, 2003; Braden et al., 2010; Paul and Purdy, 1992; Pazol et al., 2009; Wallis and Luttge, 1980). For example, we found that MPA impaired learning and memory, and altered hippocampal and entorhinal cortex glutamic acid decarboxylase (GAD; the synthesizing enzyme for GABA) concentrations in Ovx rats (Braden et al., 2010). Further, we demonstrated that MPA exposure during either young adulthood, middle-age, or both, produced cognitive detriments, and higher serum levels of MPA were associated with lower levels of GAD in the dorsal hippocampus (Braden et al., 2011). Understanding the possible long-term effects of MPA administration on cognition, and whether there is a subsequent long-term impact on the GABAergic system, is especially relevant as we embark on a new generation of menopausal women who were much more likely to have been prescribed contraceptives when younger, and then hormone therapy when older, as compared to generations before (Diczfalusy 1991).
The mechanism of MPA effects on cognition likely differs from the mechanism of progesterone effects on cognition. There is evidence that MPA’s ring-A reduced metabolites (e.g., dihydroMPA and tetrahydroMPA) do not directly bind to the GABAA receptor (McAuley et al. 1993), as do natural progesterone’s ring-A reduced metabolites (Paul and Purdy 1992). However, MPA might alter progesterone’s metabolic conversions (Penning et al. 1985), which could be related to MPA-induced enhanced synaptic and extrasynaptic GABAA receptor-mediated inhibition (Belelli and Herd 2003) and changes in GAD (Braden et al. 2010). In fact, the same decrease in GAD levels in the dorsal hippocampus after administration of the GABAA agonist diazepam (Raol et al. 2005) indicates that MPA-induced decreases in GAD are a consequence of increased GABAA receptor activation.
Adult Neurogenesis
Adult neurogenesis is the process whereby new-born neurons are produced from progenitor cells populations in the adult central nervous system (Ming and Song, 2011). Whether neurogenesis has a functional impact on cognition has yet to be conclusively determined. However, the literature thus far indicates that female steroids do, in fact, have an effect on many experimental measures and parameters of neurogenesis. Some very recent studies have evaluated the potential effects that estrogens have on cognitive function. For example, 17β-estradiol, but not estrone, increased activation of new neurons in the dentate gyrus of young adult Ovx rats in response to the retrieval of spatial memory (McClure et al., 2012). However, the estrogenic-induced differences in this activation of new neurons do not seem to translate into regulation of cognitive behavior, as there were no differences in spatial memory between the treatment groups. Similarly, tonic low dose CEE treatment increased the number of new neurons in the dentate gyrus (Barha and Galea, 2012); however, these rats showed impaired spatial working and reference memory, again indicating that increases in neurogenesis do not necessarily parallel cognitive outcome. These divergent results between an effect on brain versus an effect on cognition stress the importance of testing functional outcome of brain changes, as brain changes do not always translate to cognitive changes.
Relative to the number of studies testing the effects of estrogens on neurogenesis, the impact of progesterone on neurogenesis is less studied. Results thus far show that progesterone modulated the effects of 17β-estradiol on cell proliferation in vivo (see Pawluski et al., 2009), and the progesterone metabolite, allopregnanolone, enhanced cell proliferation in vitro (Wang et al., 2005). Hence, comprehensive evaluations testing the ability of progesterone alone to concurrently induce markers of neurogenesis and alter cognition using in vivo strategies are warranted. It is noteworthy that while female steroid effects on neurogenesis and its relation to cognitive function is a relatively new area of investigation, such interdisciplinary studies are increasing and may unveil exciting new mechanisms of steroid effects on cognition.
Signaling Cascades
The non-classic effects of ovarian steroids influence numerous signal transduction pathways, or signaling cascades, ultimately leading to modulation of protein kinases (reviewed in Falkenstein et al., 2000). Protein kinases phosphorylate many targets, and the phosphorylation event is usually the last step in a signal transduction cascade that results in a milieu of changes including functional alteration in proteins and modulation of gene transcription (Manning et al., 2002). Some of the kinases influenced by ovarian steroids, and implicated in cognition, are: mitogen-activated protein kinase (MAPK), extracellular-signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K), and the mammalian target of rapamycin (mTOR). MAPK phosphorylates ERK; MAPK and ERK are the final targets of several other kinases including PI3K and Akt (i.e., PKB), and these signaling pathways modulate cognitive processes (e.g., Chen et al., 2005; Fan et al., 2010). For example, ERK activation (i.e., ERK phosphorylation) in the hippocampus is necessary for long-term memory (Kelly et al., 2003). Research has demonstrated that 17β-estradiol enhances memory via dorsal hippocampal ERK activation in Ovx mice (Fernandez et al., 2008). Indeed, dorsal hippocampal infusions of the MAPK inhibitor (U0126) blocked the memory enhancing effects of intracerebroventricular (i.c.) infusion of 17β-estradiol in Ovx mice (Fernandez et al., 2008; Zhao et al., 2010). In addition to 17β-estradiol, Orr and colleagues (2009) recently found that dorsal hippocampal infusions of progesterone increased ERK activation and the mTOR substrate S6K in the dorsal hippocampus of Ovx mice; ERK and mTOR activation were necessary for progesterone to facilitate memory. mTOR is a kinase that has effects on cell growth and proliferation via the regulation of protein synthesis (Hay and Sonenberg, 2004). mTOR is activated by ERK (Kelleher et al., 2004; Mendoza et al., 2011; Tsokas et al., 2005), and phosphorylation of 4E-BP1 and S6 in brain is regulated by the ERK pathway, which is known to play a role in synaptic plasticity (Kelleher et al., 2004). Collectively, ERK and mTOR appear to work together to influence synaptic plasticity (Hay and Sonenberg, 2004). 17β-estradiol has been shown to stimulate MAPK and PI3K via its actions at the G-protein coupled receptor 30 (GPR30) and the subsequent transactivation of epidermal growth factor receptors (Prossnitz et al., 2008). Collectively, these studies suggest that some of the cognitive effects of 17β-estradiol are mediated by GPR30 via signaling through the MAPK and ERK pathways. Furthermore, progesterone appears to enhance object memory via ERK and mTOR, while the mechanism of this effect is still under investigation.
Epigenetics
Interest in mechanisms through which the activational effects of ovarian hormone are realized has led to multi-scale molecular and genetic investigations. Broadly defined, the epigenome is a collection of heritable modifications (e.g., histone modification and DNA methylation) that alters gene transcription via mechanisms other than modification of the underlying genetic sequence (see Russo et al., 1996). Several studies have demonstrated that histone modification and DNA methylation regulate formation of hippocampal-dependent memories (e.g., Levenson et al., 2004; Miller et al., 2008), and that 17β-estradiol can mediate epigenetic change (Zhao et al., 2010; Zhao et al., 2012). Specifically, 17β-estradiol, via MAPK/ERK signaling, increases histone acetylation (i.e., histone H3) in the dorsal hippocampus, and this histone modification is necessary for 17β-estradiol’s memory enhancement in Ovx mice (Zhao et al., 2010; Zhao et al., 2012). However, how estrogen-induced modifications in the epigenome generalize to progesterone-induced and androgen-induced modifications is a question for future research. A recent study has shown that, similar to 17β-estradiol, progesterone activates ERK signaling in the dorsal hippocampus and enhances memory in Ovx mice (Orr et al., 2012), and may therefore similarly modify the epigenome.
Hormone Receptors and the Cognitive Effects of Hormones
The cognitive effects of steroid hormones discussed thus far are in part, mediated by distinct expression of receptors in the brain. Since its discovery in 1966, estrogen receptor-alpha (ERα) was the first, and thought to be the only, member of the nuclear receptor superfamily that exhibites specificity for 17β-estradiol (Toft and Gorski, 1966). It was not until 30 years later that the second ER subtype, ERα, was discovered (Kuiper et al., 1996) The two ERs share a significant degree of sequence homology; however, they also have important differences in their ligand-binding domain and unique distribution patterns in the brain, suggesting divergent roles of these receptors on biological function (Enmark et al., 1997; Matthews and Gustafsson, 2003; Tremblay et al., 1997). Of note, both ERs are found in brain regions thought to regulate cognitive function, including cerebral cortex and hippocampus (Gonzalez et al., 2007; Mitra et al., 2003; Osterlund et al., 2000; Pau et al., 1998; Register et al., 1998; Shughrue et al., 1997).
A few seminal studies have recently brought to light the importance of assessing changes in expression levels of the ERs as a function of age, as it may reflect transitions in 17β-estradiol responsiveness of the brain that ultimately lead to cognitive deficits. As aging ensues, 17β-estradiol responsiveness seems to decline as ERα expression levels decrease in human hippocampus (Ishunina et al., 2007; Tohgi et al., 1995). Similarly, ERα (Adams et al., 2002), but not ERα (Waters et al., 2011), decreases in the hippocampus of aged, compared to young Ovx rats. Some neurological diseases that affect memory, such as Alzheimer’s disease, are also associated with decreased ERα and increased ERα expression in the hippocampus (Ishunina et al., 2007; Perlman et al., 2005; Savaskan et al., 2001). Furthermore, ERα polymorphisms are associated with increased incidence of Alzheimer’s Disease among women (Corbo et al., 2006; Ji and Dani, 2000; Olsen et al., 2006; Yaffe et al., 2002; Yaffe et al., 2009). Collectively, these findings highlight the clinical significance of characterizing the age-related changes in ERs; in particular, decreased ERα/ ERα ratio during aging seems to be associated with cognitive deficits.
These decreases in the ERα/ ERα ratio may be age-dependently reversed by 17β-estradiol. 17β-estradiol treatment increases ERα, but not ERα, in the hippocampus of aged Ovx rats (Adams et al., 2002; Waters et al., 2011), suggesting that ERα retains 17β-estradiol responsiveness while ERα does not. In contrast, 17β-estradiol increases ERα without affecting ERα expression in the hippocampus of middle-aged ovariectomized rats (Bohacek and Daniel, 2009). This 17β-estradiol -induced ERα expression is long lasting, as it is also found in aged Ovx rats that received transient 17β-estradiol treatment discontinued 7 months prior at middle-age (Rodgers et al., 2010). Even more provocative is the finding that this prior 17β-estradiol treatment is also associated with improved memory, suggesting two intriguing tenets. The first is that 17β-estradiol has a long-term effect on ERα expression and cognitive function. Second, these long-term effects do not require 17β-estradiol to be on board at the time of cognitive testing. Significant clinical implication can be drawn by these studies, as these reports illustrate that age-related decreases in ERα/ ERα ratio are associated with cognitive dysfunction, both of which can potentially be enhanced permanently by transient exposure to 17β-estradiol following loss of ovarian function, if initiated in earlier in life.
Delineating the individual roles of the ERs on cognition is a considerable challenge; in this regard, ER knockout (ERKO) mice are an invaluable tool. Studies using this model implicate divergent functions of ERα and ERα on cognition, depending on types of learning. For example, ERα is important for emotional learning, as performance in inhibitory avoidance task is impaired in ERαKO, but not ERαKO mice (Fugger et al., 2000). By contrast, disruption of ERα, but not ERα, impairs spatial learning on the Morris water maze, suggesting that ERα is required for spatial learning (Fugger et al., 2000; Rissman et al., 2002).
An important caveat must be considered, however, when interpreting these findings. Because ER activity is disrupted throughout development of these ERKO mice, it is difficult to distinguish between organizational and activational effects. Thus, moving forward, it becomes increasingly critical to combine other available tools with the ERKO model to circumvent this confound. Two important studies have capitalized on recent advancements in molecular and pharmacological techniques. First, Foster et al (2008) used a viral vector-mediated gene transfer to deliver the ERα gene in the ERαKO mice and found that restoration of ERα in the hippocampus improves spatial learning in Ovx animals. This finding is remarkable, given that this enhanced cognitive function is evident in the absence of 17β-estradiol treatment. This study also implies that learning deficits observed in ERαKO mice are not only due to organizational effects, but also likely to be mediated by hippocampal ERα activity in adulthood. Second, Liu et al (2008) combined the use of the selective estrogen receptor modulators (SERMs) with the ERKO model to show that 17β-estradiol or a selective ERα, but not ERα, agonist improves spatial memory in wildtype and ERαKO, but not ERαKO mice. This result gives further credence to the concept that ERα activation during adulthood is pro-cognitive in the spatial domain.
Mounting evidence from studies using SERMs corroborate the idea put forth by the ERKO model that the two ER subtypes regulate distinct learning types. Acute treatment with an ERα, but not ERα, agonist enhances performance in the spatial water maze and inhibitory avoidance (Rhodes and Frye, 2006), while ERα, not ERα, agonist enhances performance in object placement and object recognition (Frye et al., 2007). However, using similar parameters, Jacome et al (2010) reported opposite findings, implicating ERα in enhanced object placement and object recognition performance. In this case, the difference in the strain of rats used in the two studies (Long Evans versus Sprague Dawley, respectively) may have contributed to the discrepancies, as other conditions were similar. In general, species, age, and duration of treatment may also be important factors, as chronic treatment of either ERα or ERα agonists enhances cognition in young rats (Hammond et al., 2009), impair cognition in middle-aged rats (Neese et al., 2010), and has no cognitive effects in adult monkeys (Lacreuse et al., 2009). These studies underscore the complexity of factors involved in using SERMs and warrant further investigation of potential parameters that impact cognitive outcome.
Prospects of using combined tools to elucidate the distinct functions of ERα and ERα are promising. In recent years, technological advances have flourished in a variety of disciplines, including genetic (i.e., conditional transgenic mice), pharmacological (SERMs), and molecular (gene transfer and antisense oligonucleotide) avenues for region- and time-specific manipulation of the ERs. Thus, we are at a pivotal point where it is now possible to resolve some of the key obstacles that have stymied the field. By embracing these seemingly confounding factors and exploiting all available tools in a multi-disciplined manner, we can advance our knowledge of the roles of these receptors on cognitive function.
General Conclusions
Gonadal hormones have activational effects on cognition. Interest in elucidating the factors that mediate these effects has intensified over the last decade. Notably, this peaked interest is within the realm of both the depth and breadth of the impact that ovarian hormones might exert on cognition. Of these effects, the significance of age-related cognitive decline, and how it may be exacerbated by concurrent gonadal hormone loss, has been an increasingly prominent area of focus in female rodents and in women. Efforts have recently been made to restore balance of the hormonal milieu in an attempt to ameliorate age-related cognitive deficits. The clinical and basic science literature has presented many elegant, methodically controlled, clearly interpretable experiments to better understand the parameters that govern the profound consequences of hormone loss and hormone treatment on cognition and brain health. Animal models have served as a valuable tool in characterizing the parameters that might lead to clinical intervention, and will be key to future discoveries. Researchers have identified the intricate relationships between these factors by understanding their individual contributions on cognition. This type of systematic approach has guided much of the discoveries in the field, including the differential cognitive effects of estrogens, progestogens, and androgens. Indeed, the timing, route, dose and duration of administration are also important factors to consider. Research is now moving to understanding the effects of these variables in concert, as well as interactions between different types of hormones when administered concomitantly. This knowledge is key to translating preclinical data to the clinic, wherein women typically have exposure to endogenous ovarian hormones, and also take combination hormone therapies concurrently. An overview of the existing literature on the impact of hormone therapy on cognition might seem controversial, but when taking these variables into account, we can appreciate the multitude of considerations involved in optimizing treatment for menopause. We are now in an exciting time when technological advances in the field of neuroscience are meeting the demands and enthusiasm of scientists who wish to better understand hormone effects on the brain and cognition. Many interdisciplinary scientists now perform animal studies with a clinically-driven experimental design, which is key to discoveries of a translational nature. In this domain, the holy grail is to use these discoveries to expand the window of opportunity to ultimately enhance cognition in aging women.
Figure 1.
Female steroid hormones can impact the brain and cognition throughout the lifespan. In women, female steroid hormone exposure encompasses both endogenous and exogenous exposures in a lifetime. Endogenous hormone exposure occurs during the prenatal environment, birth, puberty, and menopause, and exogenous hormone exposure occurs from contraceptives during reproductively active years and/or hormone therapy (HT) during menopause. Organizational and activational effects on the brain interact and ultimately impact cognitive outcome. Female steroid-induced effects on cognitive outcome depend on many factors. Putative neurobiological mechanisms underlying hormone-induced alterations in cognition include those listed in the brain schematic in the figure. Regarding research investigating the cognitive effects of various types of HT in middle-aged and aged rats, the plus sign (+) denotes positive effects on cognition and the minus (-) sign denotes detrimental effects on cognition. The numbers under the plus and minus signs refer to the corresponding publication/Reference ID# listed in Table 1.
Table 1.
Effects of female steroid administration on spatial memory in middle-aged and aged female rats. Only hormones given independently are reported here.
| Hormone | Reference ID# | Dose | Regimen | Behavior Examined | Effect on cognition | Reference |
|---|---|---|---|---|---|---|
| CEE | 1 | 10μg injection | Cyclic | MWM, DMS | + | Acosta et al., 2009 |
| 20μg injection | Cyclic | MWM, DMS | ||||
| 30μg injection | Cyclic | MWM, DMS | + | |||
| 2 | 12μg pumps | Tonic | DMS, MWM | − | Engler-Chiurazzi et al., 2011 | |
| 24μg pumps | Tonic | DMS | + | |||
| 36μg pumps | Tonic | DMS, WRAM | + | |||
| E1 | 3 | 8.0μg /day pumps | Tonic | DMS | − | Engler-Chiurazzi et al., 2012 |
| E2 | 4 | 0.25mg/60 day release pellet | Tonic | MWM | + | Talboom et al., 2008 |
| 5 | 10% estradiol benzoate capsule Low- 0.5 cm | Tonic | MWM | + | Foster et al., 2003 | |
| 10% estradiol benzoate capsule High- 1 cm | Tonic | MWM | + | |||
| 6 | 25% E2 implant | Tonic | MWM | + | Markham et al., 2002 | |
| 16.67μg E2/kg in .5cc injections | Acute | MWM | + | |||
| 7* | 25% E2 implant in a 5 mm silastic capsule | Tonic | DMP | + | Gibbs et al., 2000 | |
| 8 | .25mg, 60 day release pellet | Tonic | MWM | + | Bimonte-Nelson et al., 2006 | |
| .50mg, 60 day release pellet | Tonic | MWM | + Only on Day 1 Trial 2 | |||
| 10μg injection | Cyclic | MWM | + | |||
| 9* | 10μg injection + 3mm E2 pellet | Cyclic +Tonic | DNMP, MWM | + | Markowska et al., 2002 | |
| 10* | 3mm E2 pellet | Tonic | T-maze active avoidance | + | Savonenko et al., 2003 | |
| 11 | 47μg/kg/day in drinking water | Tonic (constant, every day) | MWM | + | Lowry et al., 2010 | |
| 47μg/kg/day in drinking water | Cyclic (3 out of every 4 days) | MWM | - (Relative to tonic E2, not vehicle) | |||
| P4 | 12 | (3) P4 pellets of 200mg, 90 day release | Tonic | WRAM | - | Bimonte-Nelson et al., 2004 |
| 13 | 21mg pumps | Tonic | WRAM | − | Braden et al., 2010 | |
| MPA | 14 | 21mg pumps | Tonic | WRAM, MWM | − | Braden et al., 2010 |
| 15 | 3.5mg injection | Cyclic | WRAM, MWM | − | Braden et al., 2011 | |
| Andro | 16 | 8mg/kg | Tonic | WRAM, DMS, MWM | − | Camp et al., 2012 |
Morris water maze (MWM), Water radial arm maze (WRAM), Delayed matched-to-sample plus maze (DMS), Delayed non-match to position (DNMP).
(+) denotes a positive effect on cognition
(-) denotes a negative effect on cognition
indicates that a turn strategy can be used for successful performance; animals are not “encouraged” to use space
17β-estradiol (E2)
Progesterone (P4)
Medroxyprogesterone acetate (MPA)
Androstenedione (Andro)
Table 2.
Effects of combination estrogens + progestogens administration on spatial memory in middle-aged and aged female rats.
| Hormone | Dose | Regimen | Behavior Examined | Effect on cognition | Reference |
|---|---|---|---|---|---|
| E2 + P4 | 10μg injection E2 + 500μg injection P4 | Cyclic E2 + Cyclic P4 | * DMP | + | Gibbs et al., 2000 |
| 0.25mg E2 pellet (60 day release) + Two 200mg P4 pellets (90 day release) | Tonic E2 + Tonic P4 | MWM | − (relative to tonic E2 alone, not vehicle) | Bimonte-Nelson et al., 2006 | |
| 10 μg injection E2+ 2 pellets of 200mg P4, (90 day release) | Cyclic E2+ Tonic P4 | MWM | − (relative to cyclic E2 alone, not vehicle) | ||
| 25% E2+ 75% and 100% P4 implant | Tonic E2+ Tonic P4 | MWM | + | Markham et al., 2002 | |
| 47μg/kg/day E2 in drinking water + 40mm P4 implant | Tonic E2+ Tonic P4 | MWM | + (relative to tonic E2 + MPA, not vehicle) | Lowry et al., 2010 | |
| E2+ MPA | 47μg/kg/day E2 in drinking water + 1.5mg 90 day release MPA pellet | Tonic | MWM | − (relative to cyclic E2, tonic E2, & tonic E2+P4, not vehicle) | Lowry et al., 2010 |
| 47 μg/kg/day E2 in drinking water + 1.5mg 90 day release MPA pellet | Tonic | * T-maze alternation, delayed alternation | + Relative to vehicle, cyclic E2, & tonic E2 | Chisholm et al., 2012 |
Morris water maze (MWM), Water radial arm maze (WRAM), Delayed matched-to-sample plus maze (DMS), Delayed non-match to posotion (DNMP).
(+) denotes a positive effect on cognition (−)
denotes a negative effect on cognition
17β-estradiol (E2)
Progesterone (P4)
Medroxyprogesterone acetate (MPA)
indicates that a turn strategy can be used for successful performance; animals are not “encouraged” to use space
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009a doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009b;55:454–64. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151:3795–804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–14. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Blindt HS, Candy JM. Working memory in aged rats. Behavioral Neuroscience. 1989;103:975–983. doi: 10.1037//0735-7044.103.5.975. [DOI] [PubMed] [Google Scholar]
- Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29:447–54. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–55. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alliot J, Nauton P, Bruhat MA. Administration of LHRH analog can improve working memory in aged female rats. Psychoneuroendocrinology. 1993;18:543–50. doi: 10.1016/0306-4530(93)90031-f. [DOI] [PubMed] [Google Scholar]
- Amundsen DW, Diers CJ. The age of menopause in classical Greece and Rome. Human Biology. 1970;42:79–86. [PubMed] [Google Scholar]
- Amundsen DW, Diers CJ. The age of menopause in medieval Europe. Human Biology. 1973;45:605–612. [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer's disease: results of a placebo–controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–77. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working Memory. In: Bower GA, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 8. Academic Press; New York, NY: 1974. pp. 47–90. [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy young and older adults. In: Tulving E, Craik FIM, editors. Handbook of Memory. Oxford University Press; 2000. pp. 395–410. [Google Scholar]
- Barha CK, Galea LA. The hormone therapy, Premarin, impairs hippocampus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory changes during normal aging: Neurobiological correlates. In: Martinez JA Jr, Kesner RP, editors. Neurobiology of Learning and Memory. Academic Press; San Diego, CA: 1998. pp. 247–273. [Google Scholar]
- Beach FA. Recent Progress in Hormone Research. Academic Press; New York, NY: 1947. Hormones and mating behaviora in vertebrates. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Beatty PA. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol. 1970;73:446–55. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- Beatty WW, editor. Gonadal hormones and sex differences in nonreproductive behaviors. Plenum Press; New York: 1992. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–25. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker K. Principles and Practice of Endocrinology and Metabolism. J. B. Lippincott Co; Philadelphia: 1995. [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–20. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benice TS, Raber J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learning and Memory. 2009;16:479–485. doi: 10.1101/lm.1428209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav Neurosci. 1997;111:267–74. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's. J Steroid Biochem Mol Biol. 2003;85:473–82. doi: 10.1016/s0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm ACE. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behavioral Neuroscience. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004a;15:2659–63. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm ACE. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behavioral Neuroscience. 2004b;118:707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–42. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15:6050–105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–73. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Fitch RH, Denenberg VH. Neonatal estrogen blockade prevents normal callosal responsiveness to estradiol in adulthood. Brain Res Dev Brain Res. 2000a;122:149–55. doi: 10.1016/s0165-3806(00)00067-5. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Holly Fitch R, Denenberg VH. Adult ovary transfer counteracts the callosal enlargement resulting from prepubertal ovariectomy. Brain Res. 2000b;872:254–7. doi: 10.1016/s0006-8993(00)02505-1. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Mack CM, Stavnezer AJ, Denenberg VH. Ovarian hormones can organize the rat corpus callosum in adulthood. Brain Res Dev Brain Res. 2000c;121:169–77. doi: 10.1016/s0165-3806(00)00043-2. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Turkmen S, Lindblad C, Zhu D, Johansson IM, Backstrom T, Wahlstrom G. GABA(A) receptor changes in acute allopregnanolone tolerance. Eur J Pharmacol. 2006;535:125–34. doi: 10.1016/j.ejphar.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the size of several components of the medial preoptic area in the male rat. J Comp Neurol. 1988;275:613–22. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–7. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol. 2009;21:640–7. doi: 10.1111/j.1365-2826.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279:20539–45. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93:444–53. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Garcia AN, Mennenga SE, Prokai L, Villa SR, Acosta JI, Lefort N, Simard AR, Bimonte-Nelson HA. Cognitive-impairing effects of medroxyprogesterone acetate in the rat: independent and interactive effects across time. Psychopharmacology (Berl) 2011;218:405–18. doi: 10.1007/s00213-011-2322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Baxendale S. Motherhood and memory: a review. Psychoneuroendocrinology. 2001;26:339–62. doi: 10.1016/s0306-4530(01)00003-8. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Watson RI. An evaluation of psychologic effects of sex hormone administration in aged women. I. Results of therapy after six months. J Gerontol. 1952;7:228–44. doi: 10.1093/geronj/7.2.228. [DOI] [PubMed] [Google Scholar]
- Camp BW, Gerson JE, Tsang CW, Villa SR, Acosta JI, Blair Braden B, Hoffman AN, Conrad CD, Bimonte-Nelson HA. High serum androstenedione levels correlate with impaired memory in the surgically menopausal rat: a replication and new findings. Eur J Neurosci. 2012;36:3086–95. doi: 10.1111/j.1460-9568.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Whitehead M. Oestrogen therapy and the menopausal syndrome. Clin Obstet Gynaecol. 1977;4:31–47. [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006;1762:447–52. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269:107–11. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–31. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Horm Behav. 2000;38:234–42. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Chisholm NC, Juraska JM. Long-term replacement of estrogen in combination with medroxyprogesterone acetate improves acquisition of an alternation task in middle-aged female rats. Behav Neurosci. 2012;126:128–36. doi: 10.1037/a0026461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS) The Journal of steroid biochemistry and molecular biology. 2010;118:304–10. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog Brain Res. 2010;182:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer's disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22:67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–25. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–56. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Sulzer JK, Hulst JL. Estrogen increases the sensitivity of ovariectomized rats to the disruptive effects produced by antagonism of D2 but not D1 dopamine receptors during performance of a response learning task. Horm Behav. 2006;49:38–44. doi: 10.1016/j.yhbeh.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci. 1994;108:988–92. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–8. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J Cell Mol Med. 2004;8:537–44. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32:680–97. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–65. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–40. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–7. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–56. [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Estradiol potentiates acetylcholine and glutamate-mediated post-trial memory processing in the hippocampus. Brain Res. 2000;864:263–9. doi: 10.1016/s0006-8993(00)02184-3. [DOI] [PubMed] [Google Scholar]
- Farrag AK, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord. 2002;13:193–8. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang JT. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–7. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Denenberg VH. A role for ovarian hormones in sexual differentiation of the brain. Behav Brain Sci. 1998;21:311–27. doi: 10.1017/s0140525x98001216. discussion 327–52. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–52. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16:1587–93. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51:530–5. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C. A placebo–controlled study of effects of oral progesterone on performance and mood. Br J Clin Pharmacol. 1992;33:293–8. doi: 10.1111/j.1365-2125.1992.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–58. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Posttraining androgens' enhancement of cognitive performance is temporally distinct from androgens' increases in affective behavior. Cogn Affect Behav Neurosci. 2001;1:172–82. doi: 10.3758/cabn.1.2.172. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–64. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gabriel A, Fahim G. Do Depot Medroxyprogesterone Acetate Contraceptive Injections Cause Mood Changes and Memory Impairment? Primary Psychiatry. 2005;12:59–60. [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–26. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. J Neurosci. 1996;16:1049–55. doi: 10.1523/JNEUROSCI.16-03-01049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–33. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000a;101:931–8. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000b;21:107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–57. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–9. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–83. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Hammond R, Nelson D. Galanthamine plus estradiol treatment enhances cognitive performance in aged ovariectomized rats. Horm Behav. 2011a;60:607–16. doi: 10.1016/j.yhbeh.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Nelson D. Donepezil plus estradiol treatment enhances learning and delay-dependent memory performance by young ovariectomized rats with partial loss of septal cholinergic neurons. Horm Behav. 2011b;59:503–11. doi: 10.1016/j.yhbeh.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Handbook of Physiology. 1. Vol. 5. American Physiological Society; Bethesda, MD: 1987. Circuitry of primate prefrontal cortex and the regulation of behavior by representational memory. [Google Scholar]
- Gomez-Gil E, Canizares S, Torres A, de la Torre F, Halperin I, Salamero M. Androgen treatment effects on memory in female-to-male transsexuals. Psychoneuroendocrinology. 2009;34:110–117. doi: 10.1016/j.psyneuen.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Wight RG, Huang MH, Avis N, Gold EB, Joffe H, Seeman T, Vuge M, Karlamangla AS. Menopause-associated symptoms and cognitive performance: Results from the study of Women's Health Across the Nation. Am J Epidemiol. 2010;171:1214–24. doi: 10.1093/aje/kwq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. Possible acceleration of age effects on cognition following menopause. J Psychiatr Res. 1995;29:153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–14. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–10. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol. 2008;51:618–26. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–34. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103:1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28:1670–81. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–98. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–90. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Kantor HI, Michael CM, Shore H. Estrogen for older women. Am J Obstet Gynecol. 1973;116:115–8. doi: 10.1016/0002-9378(73)90894-6. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–79. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23:5354–60. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo SK, O'Neill S, Byrne G, King R, Travers C, Tripcony L. Postmenopausal hormone therapy and cognition: effects of timing and treatment type. Climacteric. 2010;13:259–64. doi: 10.3109/13697130903370316. [DOI] [PubMed] [Google Scholar]
- Kimura D, Hampson E. Cognitive pattern in men and women is influenced by fluctuations in sex hormones. Psychological Science. 1994;3:57–61. [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E. Hysterectomy, oophorectomy, and cognitive function in older women. J Am Geriatr Soc. 2002;50:55–61. doi: 10.1046/j.1532-5415.2002.50008.x. [DOI] [PubMed] [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. No effect of different estrogen receptor ligands on cognition in adult female monkeys. Physiol Behav. 2009;96:448–56. doi: 10.1016/j.physbeh.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun C, Durkin TP, Marighetto A, Jaffard R. A comparison of the working memory performances of young and aged mice combined with parallel measures of testing and drug-induced activations of septo-hippocampal and nbm-cortical cholingergic neurones. Neurobio Aging. 1990;11:515–521. doi: 10.1016/0197-4580(90)90112-d. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–70. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–21. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–43. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lowry NC, Pardon LP, Yates MA, Juraska JM. Effects of long-term treatment with 17 beta-estradiol and medroxyprogesterone acetate on water maze performance in middle aged female rats. Horm Behav. 2010;58:200–7. doi: 10.1016/j.yhbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Luetters C, Huang MH, Seeman T, Buckwalter G, Meyer PM, Avis NE, Sternfeld B, Johnston JM, Greendale GA. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women's health across the nation (SWAN) Journal of Women's Health. 2007;16:331–344. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- Luine VN, McEwen BS. Sex differences in cholinergic enzymes of diagonal band nuclei in the rat preoptic area. Neuroendocrinology. 1983;36:475–82. doi: 10.1159/000123501. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–90. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–44. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav. 1995;29:42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women's Health Initiative 10 years on. Climacteric. 2012;15:256–62. doi: 10.3109/13697137.2012.660613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of Morris water maze. Hormones and Behavior. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–95. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–22. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–81. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71:130–8. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- McClure RE, Barha CK, Galea LA. 17beta-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson HA, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Hormones & Behavior. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan PJ, Singer CA, Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J Neurosci. 1996;16:1860–5. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaul MJ, Young M. Complexities of androgen action. J Am Acad Dermatol. 2001;45:S87–94. doi: 10.1067/mjd.2001.117429. [DOI] [PubMed] [Google Scholar]
- Meites J, Lu JKH. Reproductive aging and neuroendocrine function. In: Charlton HM, editor. Oxford review of reproductive biology. Vol. 16. Oxford Press; New York: 1994. [Google Scholar]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–8. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47:29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- Nauton P, Giry N, Bruhat MA, Alliot J. Effect of administration of an analog of LHRH on appetitive learning in young and middle-aged female rats. Pharmacol Biochem Behav. 1992;43:1005–13. doi: 10.1016/0091-3057(92)90474-t. [DOI] [PubMed] [Google Scholar]
- Neave N, Menaged M, Weightman DR. Sex differences in cognition: the role of testosterone and sexual orientation. Brain Cogn. 1999;41:245–262. doi: 10.1006/brcg.1999.1125. [DOI] [PubMed] [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Horm Behav. 2010;58:878–90. doi: 10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- O'Malley CA, Hautamaki RD, Kelley M, Meyer EM. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Res. 1987;403:389–92. doi: 10.1016/0006-8993(87)90082-5. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocrine Journal. 1994;41:361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia. 1995;6:99–107. doi: 10.1159/000106929. [DOI] [PubMed] [Google Scholar]
- Olsen L, Rasmussen HB, Hansen T, Bagger YZ, Tanko LB, Qin G, Christiansen C, Werge T. Estrogen receptor alpha and risk for cognitive impairment in postmenopausal women. Psychiatr Genet. 2006;16:85–8. doi: 10.1097/01.ypg.0000194445.27555.71. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Hippocampus, space and memory. 1979;2:313–365. [Google Scholar]
- Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progesterone infusions enhance object recognition in young female mice. Pharmacol Biochem Behav. 2009;93:177–82. doi: 10.1016/j.pbb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr PT, Rubin AJ, Fan L, Kent BA, Frick KM. The progesterone-induced enhancement of object recognition memory consolidation involves activation of the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways in the dorsal hippocampus. Horm Behav. 2012;61:487–95. doi: 10.1016/j.yhbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000;85:3840–6. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997a;8:3009–13. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997b;68:172–88. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Pallares M, Darnaudery M, Day J, Le Moal M, Mayo W. The neurosteroid pregnenolone sulfate infused into the nucleus basalis increases both acetylcholine release in the frontal cortex or amygdala and spatial memory. Neuroscience. 1998;87:551–8. doi: 10.1016/s0306-4522(98)00174-2. [DOI] [PubMed] [Google Scholar]
- Pappas CT, Diamond MC, Johnson RE. Morphological changes in the cerebral cortex of rats with altered levels of ovarian hormones. Behav Neural Biol. 1979;26:298–310. doi: 10.1016/s0163-1047(79)91289-5. [DOI] [PubMed] [Google Scholar]
- Parkes AS, Bellerby CW. Studies on the internal secretions of the ovary: I. The distribution in the ovary of the oestrus-producing hormone. J Physiol. 1926;61:562–75. doi: 10.1113/jphysiol.1926.sp002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes AS. The Internal Secretions of the Ovary. Proc R Soc Med. 1927;20:1663–7. doi: 10.1177/003591572702001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau CY, Pau KY, Spies HG. Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol. 1998;146:59–68. doi: 10.1016/s0303-7207(98)00197-x. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–57. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pazol K, Northcutt KV, Patisaul HB, Wallen K, Wilson ME. Progesterone and medroxyprogesterone acetate differentially regulate alpha4 subunit expression of GABA(A) receptors in the CA1 hippocampus of female rats. Physiol Behav. 2009;97:58–61. doi: 10.1016/j.physbeh.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134:81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci. 2008;29:116–23. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Quirion R, Wilson A, Rowe W, Aubert I, Richard J, Doods H, Parent A, White N, Meaney MJ. Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired. The Journal of Neuroscience. 1995;15:1455–1462. doi: 10.1523/JNEUROSCI.15-02-01455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register TC, Shively CA, Lewis CE. Expression of estrogen receptor alpha and beta transcripts in female monkey hippocampus and hypothalamus. Brain Res. 1998;788:320–2. doi: 10.1016/s0006-8993(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72:135–42. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sierra JF. Extended organizational effects of estrogen at puberty. Ann N Y Acad Sci. 1986;474:293–307. doi: 10.1111/j.1749-6632.1986.tb28020.x. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–43. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo VEA, Martienssen RA, Riggs AD. Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory Press; Plainview, N.Y: 1996. [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–93. [PubMed] [Google Scholar]
- Savaskan E, Olivieri G, Meier F, Ravid R, Muller-Spahn F. Hippocampal estrogen beta-receptor immunoreactivity is increased in Alzheimer's disease. Brain Res. 2001;908:113–9. doi: 10.1016/s0006-8993(01)02610-5. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–30. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–51. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005;47:371–5. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–6. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Short RA, Bowen RL, O'Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76:906–9. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, Bowen D, Terrell T, Jones BN. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–21. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Silverman I, Kastuk D, Choi J, Phillips K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology. 1999;24:813–822. doi: 10.1016/s0306-4530(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Singer CA, McMillan PJ, Dobie DJ, Dorsa DM. Effects of estrogen replacement on choline acetyltransferase and trkA mRNA expression in the basal forebrain of aged rats. Brain Res. 1998;789:343–6. doi: 10.1016/s0006-8993(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Singh GK, Kochanek KD, MacDorman MF. Advance report of final mortality statistics, 1994. Monthly Vital Statistics Report. 1996;45:1–80. [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–12. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. Hormonal replacement therapy. Rev Endocr Metab Disord. 2002;3:243–56. doi: 10.1023/a:1020028510797. [DOI] [PubMed] [Google Scholar]
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–7. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiology of Learning and Memory. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Stewart J, Kolb B. The effects of neonatal gonadectomy and prenatal stress on cortical thickness and asymmetry in rats. Behav Neural Biol. 1988;49:344–60. doi: 10.1016/s0163-1047(88)90354-8. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–63. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Engler-Chiurazzi EB, Whiteaker P, Simard AR, Lukas R, Acosta JI, Prokai L, Bimonte-Nelson HA. A component of Premarin((R)) enhances multiple cognitive functions and influences nicotinic receptor expression. Horm Behav. 2010;58:917–28. doi: 10.1016/j.yhbeh.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–65. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Thilers PP, Macdonald SW, Nilsson LG, Herlitz A. Accelerated postmenopausal cognitive decline is restricted to women with normal BMI: longitudinal evidence from the Betula project. Psychoneuroendocrinology. 2010;35:516–24. doi: 10.1016/j.psyneuen.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Timaras P, Quay W, Vernadakis A. Hormones and Aging. CRC Press; Boca Raton, NY: 1995. [Google Scholar]
- Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966;55:1574–81. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res. 1995;700:245–53. doi: 10.1016/0006-8993(95)00971-r. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–65. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–43. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Conjugated equine estrogen enhances rats' cognitive, anxiety, and social behavior. Neuroreport. 2008;19:789–92. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–82. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wallis CJ, Luttge WG. Influence of estrogen and progesterone on glutamic acid decarboxylase activity in discrete regions of rat brain. J Neurochem. 1980;34:609–13. doi: 10.1111/j.1471-4159.1980.tb11187.x. [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–18. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–66. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiology of Learning and Memory. 2000;74:229–240. doi: 10.1006/nlme.1999.3948. [DOI] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber KM, Casadesus G, Bowen RL, Perry G, Smith MA. Evidence for the role of luteinizing hormone in Alzheimer disease. Endocrine and Metabolic Immune Disorders and Drug Targets. 2007;7:300–303. doi: 10.2174/187153007782794326. [DOI] [PubMed] [Google Scholar]
- Weber M, Mapstone M. Memory complaints and memory performance in the menopausal transition. Menopause. 2009;16:694–700. doi: 10.1097/gme.0b013e318196a0c9. [DOI] [PubMed] [Google Scholar]
- Williams CL. Estradiol benzoate facilitates lordosis and ear wiggling of 4- to 6-day-old rats. Behav Neurosci. 1987;101:718–23. doi: 10.1037//0735-7044.101.5.718. [DOI] [PubMed] [Google Scholar]
- Wise PM, Ratner A. Effect of ovariectomy on plasma LH, FSH, estradiol, and progesterone and medial basal hypothalamic LHRH concentrations old and young rats. Neuroendocrinology. 1980;30:15–9. doi: 10.1159/000122968. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–9. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–80. doi: 10.1002/0470870818.ch13. discussion 181–7. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol Psychiatry. 2002;51:677–82. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30:607–14. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lei ZM, Rao CV. Immortalized hippocampal cells contain functional luteinizing hormone/human chorionic gonadotropin receptors. Life Sci. 1999;65:2083–98. doi: 10.1016/s0024-3205(99)00474-9. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci U S A. 2010;107:5605–10. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–51. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Gallagher M. Spatial memory in middle-aged female rats: Assessment of estrogen replacement after ovariectomy. Brain Research. 2005;1052:163–173. doi: 10.1016/j.brainres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–70. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]