Abstract
In response to the ligand-mediated activation of cytokine receptors, cells decide whether to proliferate or to undergo differentiation. D-type Cyclins (Cyclin D1, D2, or D3) and their associated Cyclin-dependent Kinases (CDK4, CDK6) connect signals from cytokines to the cell cycle machinery, and they propel cells through the G1 restriction point and into the S phase, after which growth factor stimulation is no longer essential to complete cell division. D-type Cyclins are upregulated in many human malignancies including breast cancer to promote an uncontrolled proliferation of cancer cells. After summarizing important aspects of the cytokine-mediated transcriptional regulation and the posttranslational modification of D-type Cyclins, this review will highlight the physiological significance of these cell cycle regulators during normal mammary gland development as well as the initiation and promotion of breast cancer. Although the vast majority of published reports focus almost exclusively on the role of Cyclin D1 in breast cancer, we summarize here previous and recent findings that demonstrate an important contribution of the remaining two members of this Cyclin family, in particular Cyclin D3, for the growth of ErbB2-associated breast cancer cells in humans and in mouse models. New data from genetically engineered models as well as the pharmacological inhibition of CDK4/6 suggest that targeting the combined functions of D-type Cyclins could be a suitable strategy for the treatment of ErbB2-positive and potentially other types of breast cancer.
Keywords: Cyclin D, Gene Targeting, Tetracycline Transactivator, ErbB2, Mammary Gland Development, Breast Cancer
D-type Cyclins connect cytokine signaling with the cell cycle machinery
It has been 30 years since Cyclins were discovered by Timothy Hunt and his colleagues as oscillating proteins that drive the entry into mitosis (Evans et al., 1983). Cyclins are being synthesized and destroyed in a tightly controlled manner during distinct phases of the cell cycle (i.e. G1, S, G2, and M phase). One of the major functions of these proteins is to regulate the activity of their catalytic binding partners, the Cyclin-dependent kinases (CDKs). Thus far, twenty nine Cyclins and more than twenty CDKs have been identified in mammalian cells (Malumbres and Barbacid, 2009). All predicted Cyclins share a conserved domain of 150 amino acid residues called the “Cyclin box”, which mediates the binding to CDKs. Ten of these Cyclins belong to four different classes (D, E, A, and B -type Cyclins) and are known to directly regulate the cell cycle. Some of the newly discovered Cyclins lack currently known CDKs or other catalytic partners.
The human Cyclin D1 gene (CCND1) was initially identified as PRAD1, a locus that is rearranged in human parathyroid adenomas. It was also found in a more common t(11; 14) chromosomal translocation, which plays a role in B-cell lymphomas (BCL1) (Arnold et al., 1991; Motokura et al., 1991; Rosenberg et al., 1991; Rosenberg et al., 1993). Subsequently, Cyclin D2 and D3 were cloned based on their homology to Cyclin D1. Cyclin D2 was also independently identified as Vin-1, which is the integration site of a murine leukemia provirus in a mouse T cell leukemia (Tremblay et al., 1992). Cyclin D2 is encoded by the CCND2 gene on human chromosome 12p13, and a t(12; 14)(p13; q32) translocation has been observed in a subset of human mantle cell lymphomas (MCL) (Delmer et al., 1995; Hanna et al., 1993). The gene encoding Cyclin D3 (CCND3) is located on human chromosome 6p21, and several histopathological subtypes of mature B-cell malignancies carry a rare but recurrent t(6;14)(p21.1;q32.3) translocation, which leads to overexpression of Cyclin D3 (Inaba et al., 1992; Motokura et al., 1992).
All three mammalian D-type Cyclins encode 33-36 kDa proteins that share an average of 57% identity over the entire coding region and 78% identity in the N-terminal “Cyclin box” (Won et al., 1992). In addition to this evolutionary conserved “Cyclin box”, D-type Cyclins contain a pRb binding motif and a C-terminal PEST domain [sequence rich in proline, glutamic acid, serine, and threonine], which is required for proteolysis at the end of the G1 phase (Diehl et al., 1997; Diehl et al., 1998). Extensive studies on the Cyclin D1 protein led to the identification of an LxxLL motif and the repressor domain which are suggested to play a role in the transcriptional functions of Cyclin D1 (Knudsen et al., 2006). The three D-type Cyclins are redundant for the activation of CDK4 and CDK6, the phosphorylation of pRb, and the promotion of G1 phase progression. The expression of Cyclins D1, D2, and D3, however, varies considerably among different cell types, and therefore each D-type Cyclin has essential functions in particular tissues (Wianny et al., 1998). Besides directly regulating the cell cycle through phosphorylation of pRb, D-type Cyclins are involved in other, CDK-independent activities such as the transcriptional regulation of genes (Bienvenu et al., 2010).
During the G1 phase of the mammalian cell cycle, various mitogenic and metabolic signals from the extracellular environment integrate to influence the cell cycle clock. Depending on these inputs, the cell decides whether to enter or exit the cell cycle, i.e. whether to proliferate or differentiate. As the rate-limiting and necessary regulators for G1 phase progression, D-type Cyclins (Cyclin D1, D2, or D3) are induced by mitogenic signals and play a significant role in cell cycle entry. Besides promoting gene transcription and protein translation, mitogen-induced signaling pathways also facilitate the assembly of D-type Cyclins with their catalytic subunits, CDK4 and CDK6, and mediate the nuclear translocation of the holoenzymes (Sherr and Roberts, 1999). Progression toward S phase and the initiation of a new round of DNA synthesis is largely dependent upon the phosphorylation and functional inactivation of retinoblastoma protein (pRb) family members (pRb, p107, and p130) by Cyclin D-CDK4/6 complexes (Matsushime et al., 1992; Sherr, 1994). This process controls an E2F-responsive transcriptional program that activates the production of factors required for DNA replication (Trimarchi and Lees, 2002). Early E2F responsive genes include E-type and A-type Cyclins. In the late G1 phase, CDK2 is activated by Cyclin E and completes the phosphorylation of pRb (Sherr and Roberts, 1999; Sherr and Roberts, 2004). This leads to further activation of E2F-mediated transcription, which, in turn, facilitates the passage of the cell cycle through the restriction point at the boundary of the G1/S phase and into S phase initiation. In summary, the cytokine-mediated expression and activation of D-type Cyclins and CDK4/6 propel the cells through the restriction point, after which signaling through these growth factors is no longer essential to complete cell division.
Transcriptional regulation and posttranslational modifications of Cyclin D
Mitogenic signaling pathways induced by WNTs, ErbBs as well as JAKs and STATs are among the best characterized inducers of Cyclin D1 transcription. It has been shown that the canonical MAP kinase pathway can stimulate the expression of Cyclin D1 through activator protein-1 (AP-1) transcription factors (Shaulian and Karin, 2001). Similarly, cytokine receptor-activated Janus tyrosine kinases (JAKs) phosphorylate signal transducers and activators of transcription (STATs) that, in turn, translocate to the nucleus and enhance the transcriptional activation of the Cyclin D1 promoter (Leslie et al., 2006; Matsumura et al., 1999; Mishra and Das, 2005; Sakamoto et al., 2007). It is also well established that Cyclin D1 is a crucial downstream regulator of WNT signaling during mammary gland development and carcinogenesis (Pal and Khanna, 2006). Once cells undergo differentiation, Cyclin D1 is transcriptionally downregulated to maintain a quiescent state during lactation. In mammary epithelial cells, Oct-1 may function as a transcriptional repressor, and it has been proposed that prolactin signaling induces expression of Cyclin D1 by removing Oct-1 from its promoter (Brockman and Schuler, 2005).
Cyclin D levels start to rise in early G1 and continue to accumulate until the G1/S-phase boundary. These proteins have a short half-life of about 30 minutes before they are subjected to regulated proteolysis (Sherr, 1994). The degradation of Cyclin D1 is triggered by phosphorylation of its Threonine 286 (T286) residue through glycogen synthase kinase 3β (GSK3β) (Diehl et al., 1997; Diehl et al., 1998). The cytokine-mediated activation of AKT leads to an inactivation of GSK3β and thereby promotes a prolonged stabilization of Cyclin D1 in the nucleus. Under normal conditions, phosphorylation of Cyclin D1 facilitates nuclear export mediated by CRM1 and subsequent rapid ubiquitin-dependent proteolysis. The ubiquitination of phosphorylated Cyclin D1 is catalyzed by the SCFFbx4/αB-crystallin ligase (Lin et al., 2006). Recent work demonstrated that FBX4 is inactivated through somatic mutations in primary cancers, and overexpression of Cyclin D1 in human cancers can occur as a consequence of deregulated proteolysis (Barbash et al., 2008). Compared to Cyclin D1, significantly fewer studies have assessed the mechanisms by which Cyclin D2 and Cyclin D3 undergo proteolysis. It has recently been shown that threonine 280 plays a role in the stability of Cyclin D2, and the degradation of this protein is inhibited by a mutation of threonine 280 to alanine in NIH-3T3 cells (He et al., 2009). In a similar manner, Russell and colleagues have shown earlier that Cyclin D3 is ubiquitinated by the SCF complex and degraded by the proteosome in breast cancer cell lines (Russell et al., 1999).
Besides transcriptional activation and posttranslational modifications, the functionality of Cyclin D1 can also be altered by other posttranscriptional processes such as mRNA splicing. Specifically, a G870A polymorphism that occurs at the intron 4/exon 5 boundary of Cyclin D1 results in alternative splicing and formation of a distinct transcript (Howe and Lynas, 2001). Individuals harboring the A870 allele have a higher propensity to produce an alternatively spliced Cyclin D1 mRNA, which is called Cyclin D1b. Translation of this transcript produces a variant of the Cyclin D1 protein, which lacks the C-terminal PEST domain and at the T286 residue (Betticher et al., 1995). Similar to the phosphorylation-deficient T286A mutant of Cyclin D1, the D1b isoform is constitutively nuclear but does accumulate to levels above those of wildtype D1 (Alao, 2007; Knudsen et al., 2006; Lu et al., 2003). The absence of a C-terminal PEST sequence or residue T286 suggests that the regulation of Cyclin D1b might be different from wildtype D1 on the functional level (Alao, 2007; Germain et al., 2000). It has been suggested that Cyclin D1b is a poor activator of CDK4 and pRb phosphorylation in vitro (Solomon et al., 2003), but a recent study has reported that the G/A polymorphism is associated with increased cancer risk for a wide variety of malignancies including breast cancer (Knudsen et al., 2006). Then again, unlike K5-Cyclin D1 transgenics that exhibit epidermal hyperproliferation and severe thymic hyperplasia (Robles et al., 1996), expression of the human Cyclin D1b under the control of the bovine keratin 5 (K5) promoter did not cause any significant phenotype or induction of spontaneous tumors in seven founder lines without administration of a chemical carcinogen (Rojas et al., 2009). Reports from breast cancer models are still missing to provide experimental evidence that expression of Cyclin D1b is sufficient to initiate mammary tumorigenesis in an in vivo setting.
Phenotypic consequences of the knockouts of individual D-type Cyclins and their associated kinases
Mice lacking individual D-type Cyclins are viable and develop quite normally. However, these single knockout mice exhibit several cell type-specific abnormalities that are summarized in Table 1. Specifically, Cyclin D1 knockout mice have a reduced body size, neurological abnormalities, and display hypoplastic retinas. It has also been reported that the mammary glands of Cyclin D1 deficient females fail to undergo normal lobuloalveolar development during pregnancy (Fantl et al., 1995; Sicinski et al., 1995). Our own studies show that phenotypic abnormalities associated with lack of Cyclin D1 vary greatly depending on the genetic background (Zhang et al., 2011). Strain-dependent differences in normal mammary gland development will be discussed in the next section of this review, but we also observed that Cyclin D1 deficiency in the FVB background led to a substantial reduction in spermatogenesis and male infertility. This phenotypic abnormality was not observed in a 129 × C57/Bl6 mixed background, and it was therefore feasible to utilize homozygous Cyclin D1 knockout males for crosses with transgenic lines that carry mammary-specific oncogenes (Yu et al., 2001). In this context, it is important to note that the genetic background acts as a modifier for mammary tumorigenesis in particular transgenic lines, and the phenotypic consequences of these strain variations in association with a deletion of Cyclin D1 will be highlighted in the last part in this review.
Table 1. Phenotypes of mice lacking D-type Cyclins.
Adapted from Sherr and Roberts (2004)
| Disrupted gene(s) |
Survival | Pathology | References |
|---|---|---|---|
| Cyclin D1 | viable | Small body size, hypoplastic retinopathy, defective mammary development during pregnancy, and uncharacterized neuropathy with altered clasping reflexes |
(Fantl et al., 1995; Sicinski et al., 1995) |
| Reduction in spermatogenesis and male infertility in FVB background |
(Zhang et al., 2011) | ||
| More extensive lobular development in the mammary gland during pregnancy in Balb/C and FVB |
(Aupperlee et al., 2009; Zhang et al., 2011) |
||
| Cyclin D2 | viable | Defective ovarian granulosa cell development and female sterility. Males have hypoplastic testes but are fertile. Abnormal postnatal cerebellar development due to a reduced number of granule neurons and loss of stellate interneurons. Impaired proliferation of peripheral B-lymphocytes |
(Huard et al., 1999; Kowalczyk et al., 2004; Lam et al., 2000; Sicinski et al., 1996; Solvason et al., 2000) |
| Cyclin D3 | viable | Hypoplastic thymus with loss of T-cell maturation from double-negative (CD4−, CD8−) to double-positive (CD4+, CD8+) cells due to cytokine-independent defects in pre- TCR signaling |
(Sicinska et al., 2003) |
| Cyclin D2 and D3 |
prior to E18.5 | Death likely is due to severe megaloblastic anemia; other hematopoietic lineages were not evaluated. |
(Ciemerych et al., 2002) |
| Cyclin D1 and D3 |
death at P1, some animals survive up to 2 months |
Neuropathy leading to meconium aspiration is cause of early death. Survivors fail to thrive and exhibit hypoplastic retinas. |
(Ciemerych et al., 2002) |
| Cyclin D1 and D2 |
until three weeks postnatally |
Retarded growth and impaired coordination. Inhibited postnatal cerebellar development, and hypoplastic retinas |
(Ciemerych et al., 2002) |
| Cyclins D1, D2, and D3 |
until E16.5 | Severe hematopoietic deficits affecting number and proliferative capacity of stem cells and multipotential progenitors. Fetal liver lacks progenitors and cannot reconstitute lymphoid or myeloid function after transplantation. Death due to anemia and defects in heart development. MEFs can be propagated in culture but exhibit greatly reduced susceptibility to transformation by oncogenic Ras + Myc, E1A, or DN-p53 |
(Kozar et al., 2004) |
Defects in B-lymphocyte and pancreatic β-cell proliferation as well as cerebellar development and adult neurogenesis are phenotypic abnormalities associated with Cyclin D2 deficiency (Huard et al., 1999; Kowalczyk et al., 2004; Sicinski et al., 1996; Solvason et al., 2000). In contrast to the deletion of Cyclin D2, deficiency in Cyclin D3 leads only to a defect in T-lymphocyte development (Sicinska et al., 2003). The diverse range of mild phenotypes in mice lacking individual D-type Cyclins suggests that, despite functional redundancy, each Cyclin can also play an essential role in specific cell types. These cell type-specific defects in single knockouts are likely due to a lack of expression or a difference in the timing of expression of the other D-type Cyclins. In support of this notion, it has been reported that a knockin of the coding regions of Cyclin D2 or Cyclin E into the endogenous Ccnd1 locus rescues phenotypic abnormalities associated with Cyclin D1 deficiency (Carthon et al., 2005; Geng et al., 1999).
Double knockout mice lacking Cyclin D1 and D2 survive up to 3 weeks after birth. They exhibit the combined defects observed in the single knockouts such as reduced body size and hypoplastic cerebella (Ciemerych et al., 2002). In contrast, most of the Cyclin D1/D3 double mutant mice die immediately after birth due to neurological defects, and embryos lacking Cyclins D2 and D3 die at E17.5 and exhibit severe megaloblastic anemia. Cyclin D3 single knockout mice have relatively fewer abnormalities compared to either D1 or D2 deficient mice, but the severity of the phenotypes associated with Cyclin D3 deficiency in combination with a knockout of one of the remaining D-type Cyclins suggests that Cyclin D3 can partially take over cell type-specific functions of D1 and D2. The importance of this phenomenon will be emphasized later in connection with the role of individual D-type Cyclins in mammary cancer initiation and progression.
Similar to embryos lacking Cyclins D2 and D3, deficiency in all three D-type Cyclins causes severe megaloblastic anemia around E16.5 (Kozar et al., 2004). Despite normal proliferation of most cell types, major defects are largely limited to hematopoietic cells and myocardial cells. Transplantation experiments and in vitro assays of hematopoietic progenitors demonstrated that the loss of all D-type Cyclins severely compromised the proliferation of hematopoietic stem cells. These phenotypic abnormalities are also observed in knockout mice that lack the Cyclin D-associated CDKs (Table 2). While mice deficient in either CDK4 or CDK6 are viable, a double knockout of both kinases causes defects in hematopoiesis and embryonic lethality after E14.5 (Malumbres et al., 2004). Like in the Cyclin D deficient mice, possible phenotypic variations in CDK4/6 knockouts in diverse genetic backgrounds have not received much attention in the past.
Table 2.
Developmental abnormalities associated with CDK4 and CDK6 deficiency
| Disrupted gene(s) |
Survival | Pathology | References |
|---|---|---|---|
| CDK4 | viable | Small body size. Most males are sterile due to hypoplastic testes and low sperm counts. Female sterility is due to defects in the hypothalmic–pituitary axis, abnormal estrus, and failure of corpus luteum. Abnormal development of pancreatic β-islet cells leads to insulin-dependent diabetes within the first 2 months of life. MEFs can be propagated in culture with decreased ability to enter the cell cycle from quiescence; they express aberrantly high levels of p21Cip1 and resist transformation by oncogenic Ras + DN-p53. |
(Moons et al., 2002a; Moons et al., 2002b; Rane et al., 1999; Tsutsui et al., 1999; Zou et al., 2002) |
| CDK6 | viable | Thymic and splenic hypoplasia, and mild defects in hematopoiesis. T-lymphocytes exhibit delayed S-phase entry. |
(Malumbres et al., 2004) |
| CDK4 and CDK6 |
progressive embryonic lethality from E14.5 onward; a few pups die after birth. |
Small embryos. Partial failure of hematopoiesis results from reduced multi-potential progenitors and multi-lineage deficits, including severe megaloblastic anemia. MEFs proliferate with increased generation time and reduced Sphase fraction. Some D-type Cyclins associate with and activate CDK2. MEFs resist transformation. |
(Malumbres et al., 2004) |
In summary, the studies on single, double, and triple Cyclin D knockout mice and their associated CDKs revealed that D-type Cyclin complexes have redundant functions in most cell types. Although deletion of two or all three D-type Cyclins as well as CDK4 or CDK6 does not affect the proliferation of many cell types, at least two of the three D-type Cyclins and both associated CDKs are necessary for normal embryogenesis and postpartum development. The significance of this functional redundancy in cancer will be highlighted in the last section of this review.
The role of D-type Cyclins in normal mammary gland development
Similar to genetically engineered mice that are deficient in prolactin signaling, females that lack Cyclin D1 in a 129 × C57/Bl6 mixed background exhibit defective alveologenesis during pregnancy and impaired lactation (Fantl et al., 1995; Sicinski et al., 1995). This supports the notion that Cyclin D1 mediates the proliferative burst of alveolar progenitors in response to pregnancy hormones, in particular prolactin (PRL). While the elongation of mammary ducts following puberty is not dependent on PRL signaling, this hormone and its receptor are required for the numeric expansion and functional differentiation of the milk-producing alveolar cells during pregnancy (Horseman et al., 1997; Ormandy et al., 1997). Using conditional knockout mice or mammary epithelial transplantation, we and others have demonstrated that Jak2 is essential for the activation of Stat5 in response to PRL signaling in vivo. These studies also show that the proliferation of alveolar progenitors and the survival of their differentiated descendants are strictly dependent on these particular signal transducers in diverse genetic backgrounds (Cui et al., 2004; Shillingford et al., 2002; Wagner et al., 2004; Yamaji et al., 2009). Previous observations also suggest that PRL signaling through the Jak2/Stat5 pathway controls the expression and functionality of Cyclin D1 in the mammary epithelium. PRL may regulate the expression of Cyclin D1 indirectly through upregulation of IGF-2 (Brisken et al., 2002). On the other hand, Brockman and colleagues (2002) reported that Stat5 directly mediates the transcriptional activation of the Cyclin D1 promoter. Using Jak2 conditional knockout cells, we demonstrated that signaling through Jak2 and Stat5 not only controls the expression of the Cyclin D1 mRNA but, more importantly, these signal transducers regulate the accumulation of the Cyclin D1 protein in the nucleus through modification of the AKT/GSK3β pathway (Sakamoto et al., 2007). Specifically, active Stat5 binds to p85, the regulatory subunit of the PI3 kinase, in mammary epithelial cells (Sakamoto et al., 2007). Additionally, Stat5 elevates the mRNA expression of Akt1 through transcriptional activation of this gene from a distinct promoter (Creamer et al., 2010). In turn, the PRL-mediated increase in the levels and activation of Akt1 promotes the stabilization of the Cyclin D1 protein through inhibition of GSK3β, which, as discussed earlier, mediates the phosphorylation, nuclear export, and degradation of this cell cycle regulator.
Mice lacking Cyclins D2 or D3 do not show any obvious defects in mammary gland development (Ciemerych et al., 2002). The fact that phenotypic abnormalities as a result of Cyclin D1 deficiency can be rescued through expression of Cyclin D2 or Cyclin E under control of the endogenous Ccnd1 locus (47, 48) might indicate that the level of expression of Cyclin D1 in the mammary epithelium is more important than the specific functions of this particular cell cycle regulator. Geng and colleagues (2001) reported that the functional ablation of p27Kip1 also restored normal alveologenesis in Cyclin D1-deficient females, and they suggested that both kinase-dependent and kinase-independent functions of Cyclin D1 are required for mammary epithelial cell proliferation. As an alternative interpretation of these observations, they proposed that the kinase-dependent role of Cyclin D1 is fully dispensable. Indeed, Landis et al. (2006) showed that knock-in mice expressing the K112E mutant of Cyclin D1, which lacks normal activation of CDK4/6, did not exhibit any defects during mammary gland growth and differentiation. Interestingly, genetically engineered mutants that lack the pRb interaction domain (LxCxE) also did not show any abnormalities in body growth, mammary gland or retinal development (Landis et al., 2007). Hence, Cyclin D1 does not seem to exert its biologically relevant functions by binding to pRb in a manner analogous to the adenoviral protein E1A, the E7 human papilloma virus protein, or the SV40 large T antigen.
At first glance, the biological significance of Cyclin D1 during mammary gland development appears to be well defined, and it is generally assumed that the phenotype associated with Cyclin D1 deficiency in the mammary gland is epithelial cell autonomous (Fantl et al., 1999). It should be noted, however, that the vast majority of studies on Cyclin D1 knockout females were performed only in the 129 or C57/Bl6 genetic backgrounds. We mentioned earlier that phenotypic abnormalities in Cyclin D1 knockout mice can vary depending on the genetic strain, and this phenomenon is particularly obvious with regard to mammary gland development. We recently reported that, unlike in a 129 × C57/Bl6 mixed background, Cyclin D1 is largely dispensable for the proliferation and differentiation of secretory alveoli in pregnant and postpartum FVB females (Zhang et al., 2011). The parity-induced terminal differentiation of the secretory epithelium was not impaired, which was evident by the abundant expression of the late milk protein WAP (whey acidic protein) in Cyclin D1 deficient mammary glands. Similar to our findings, Aupperlee et al. (2009) reported previously that the Cyclin D1 knockout mice show more extensive alveolar development in Balb/C compared to the C57/Bl6 background. In part, this might be due to elevated levels of Cyclin D2 or D3 as shown recently in FVB mice that express exogenous PRL in the mammary epithelium of Cyclin D1 knockout animals (Asher et al., 2012). Using quantitative RT-PCR, we have also observed an approximate 30% increase in Cyclin D3 mRNA in the mid-pregnant mammary gland of FVB Cyclin D1−/− females (Zhang and Wagner, unpublished). All these observations suggest that, depending on the genetic strain, a compensatory upregulation of other D-type Cyclins can occur in response to D1 deficiency. The significance of this phenomenon with regard to the initiation and progression of ErbB2 and PRL-induced mammary cancer will be discussed later this review.
Regardless of the extent of alveologenesis and occasionally some milk in the stomach of pups, Cyclin D1 knockout females in the FVB strain failed to rear their offspring. We propose that the lactation defect in Cyclin D1 knockout mice is not only a consequence of the cell intrinsic functions of Cyclin D1 but appears to be more complex than previously appreciated. This assumption is based on our observation that re-expression of exogenous Cyclin D1 in the Cyclin D1−/− mammary epithelium was insufficient to restore lactation despite the presence of abundant milk within grossly extended alveoli at parturition (Zhang et al., 2011). Almost certainly, the lactation defect in Cyclin D1 knockout females includes a behavioral component. In addition to small size, Cyclin D1 deficient mice possess neurological abnormalities that are evident by impaired movement and postpartum dams failing to care for their waning offspring. To discriminate systemic effects of Cyclin D1 deficiency from intrinsic functions of this cell cycle regulator in the mammary epithelium, it will be necessary in the future to carefully examine a Cyclin D1 conditional knockout mouse, preferably in diverse genetic backgrounds such as FVB, Balb/C, and C57/Bl6.
All three D-type Cyclins are deregulated in human breast cancers
Cyclin D1 is frequently upregulated in a variety of malignancies including breast cancer. Previous studies have shown that the gene encoding this particular D-type Cyclin is amplified in 15% and overexpressed in 30-50% of primary human breast cancers. High levels of the Cyclin D1 protein are more commonly found in ER-positive cases, and the overexpression of this cell cycle regulator is associated with poor outcome. Interestingly, the activity of CDK4 seemed not to strictly follow the Cyclin D1 expression profile in breast cancer cell lines, and it was therefore suggested that CDK4-independent functions of Cyclin D1 may contribute to its biological effect as an oncogene in breast cancer (Arnold and Papanikolaou, 2005; Sutherland and Musgrove, 2004).
Expression of Cyclin D1 under regulation of the mouse mammary tumor virus (MMTV) LTR in transgenic females resulted in precocious alveolar development and the formation of adenocarcinomas with papillary and cribriform elements as well as squamous differentiation (Wang et al., 1994). Tumors appeared in about 75% of mice after a relatively long latency period of 18 months, suggesting that Cyclin D1 is a rather weak oncogene and additional genetic events are required for Cyclin D1 to exert its transforming potential. A mechanism that contributes to a weaker oncogenicity of Cyclin D1 is its GSK3β-mediated phosphorylation and accelerated turnover. Female mice expressing a T286A mutant form of Cyclin D1, which is more resistant to nuclear export and proteasomal degradation, develop mammary adenocarcinomas at an increased rate relative to mice that overexpress wildtype Cyclin D1 (Lin et al., 2008). Collectively, these findings indicate that, in addition to the overexpression of this cell cycle regulator, the temporal control of the subcellular localization and proteolysis of Cyclin D1 is equally important for mammary cancer initiation. It should be noted that the constitutively nuclear T286A mutant of Cyclin D1 effectively binds CDK4 in mammary epithelial cells of transgenic mice (Lin et al., 2008; Zhang et al., 2011), and it is therefore likely that the catalytic activity of CDK4 or CDK6 is involved in neoplastic transformation. Despite the recurring theme in the literature that CDK-independent functions of Cyclin D1 play a significant role in breast cancer, experiments using transgenic mice are still missing to substantiate this claim. For example, a direct comparison of the expression of the D1-K112E mutant with wildtype Cyclin D1, both expressed under a mammary gland-directed promoter (e.g., MMTV-LTR), might provide insight as to whether elevated levels of a Cyclin D1 isoform, which lacks activation of CDK4/6, is sufficient to initiate mammary tumorigenesis.
Among the ~12,000 articles on Cyclin D1 that are currently listed in PubMed, approximately 1,600 show some association to breast cancer. In comparison to the overwhelming interest in this particular D-type Cyclin, it is astonishing to note that the combined number of publications on Cyclin D2 and D3 and breast cancer are roughly only 10% of those on Cyclin D1. In part, this might be due to the suggested inverse relation between Cyclin D2 expression and breast cancer progression. It has been reported that up to 50% of human breast cancers exhibit low expression of Cyclin D2, which, to some extent, is a consequence of promoter silencing through hypermethylation (Evron et al., 2001; Fischer et al., 2002). The notion that this particular D-type Cyclin appears to have a predominantly negative role on the proliferation of mammary epithelial cells might also be supported by the phenotypic examination of MMTV-Cyclin D2 transgenic mice (Kong et al., 2002). Females overexpressing Cyclin D2 showed impaired alveologenesis during pregnancy, and the proliferative defect was associated with an increase in p27 and a decrease in Cyclin D1 levels. Nonetheless, 19% of MMTV-Cyclin D2 transgenics mice developed tumors after a latency of 12.5 months. Unfortunately, a histopathological and molecular characterization of these mammary tumors had not been performed.
Our own analysis of Cyclin D2 protein expression on 2 untransformed and 15 human breast cancer cell lines show that this cell cycle regulator is present at low levels in 14 out of the 17 cell lines (Zhang et al., 2011). In this study, which was primarily focused on ErbB2-positive cancers, we observed that 8 of the 15 breast cancer cell lines exhibited a somewhat lower expression of Cyclin D2 compared to untransformed MCF-10A and MCF-12A cells. However, we also noticed that three cell lines showed elevated levels of Cyclin D2, and this protein was the only D-type Cyclin expressed in poorly differentiated, ErbB2 positive HCC1569 cells. Recently, Escamilla-Hernandez et al. (2010) reported that the Ets transcription factor Elf5 can transcriptionally repress Ccnd2 by directly binding to its proximal promoter region. Elf5 has been demonstrated to be important for alveolar cell specification and differentiation (Oakes et al., 2008). Since loss of this transcription factor may promote epithelial-to-mesenchymal transition (EMT) and cancer progression through release of the transcriptional suppression of Snail2 (Chakrabarti et al., 2012), it might be reasonable to hypothesize that Cyclin D2 is specifically elevated in a subset of poorly differentiated breast cancer cells that exhibit features of EMT and a higher potential for metastatic dissemination.
Although Cyclin D3 is widely expressed in many tissues, it is the least studied member of the D-type Cyclin family in human malignancies. Bartkova et al. (1996) performed an initial immunohistochemical analysis of Cyclin D3 expression in a panel of human cancers, and they were the first to report that this particular cell cycle regulator was overexpressed in breast cancer specimens. Similarly, Russell and colleagues (1999) observed that Cyclin D1 and D3 are synchronously upregulated as a consequence of defective proteolysis in a significant subset of human breast cancer cases. To assess biologically relevant functions of Cyclin D3 in vivo, they generated transgenic mice that express this D-type Cyclin under regulation of the MMTV-LTR. While overexpression of Cyclin D3 had no noticeable effect on normal mammary gland development, 73% of MMTV-Cyclin D3 females developed mammary cancer after multiple pregnancies. The histopathological examination of these mammary tumors revealed that the gain-of-function of Cyclin D3 resulted predominantly in the development of squamous cell carcinoma (Pirkmaier et al., 2003) Since MMTV-Cyclin D1-induced neoplasms are mostly ERα-positive and ERα-negative papillary or glandular adenocarcinomas (Wang et al., 1994), Pirkmaier et al. (2003) suggested that different D-type Cyclins may have the ability to activate alternative oncogenic pathways.
Using western blot analysis and quantitative immunofluorescence (i.e., two independent assays with different antibodies), we recently verified that Cyclin D3 is highly upregulated in primary human breast cancers and in cell lines (Zhang et al., 2011). In particular in ErbB2-positive cases, Cyclin D3 often exceeded the expression of Cyclin D1. Our observations are in line with findings by Wong and colleagues (2001), who reported that Cyclin D3 was more abundant than Cyclin D1 in high-grade breast cancers. In addition, we demonstrated that a knockdown of Cyclin D3 in breast cancer cells resulted in a compensatory upregulation of Cyclin D1 (Zhang et al., 2011), suggesting that the combined pool of D-type Cyclins is critical for breast cancer cell proliferation.
Collectively, the comprehensive analysis of the expression of D-type Cyclins in breast cancer shows that all these cell cycle regulators are, at various degrees, significantly upregulated. Depending on the cancer subtype, the contribution of all three individual Cyclins fuel the numeric expansion of breast cancer cells, and therefore, the singular focus on only Cyclin D1 in 90% of the published studies to date will unlikely provide a definitive insight into the significance of this family of Cyclins in mammary tumor initiation and progression.
Role of D-type Cyclins in breast cancer initiation versus progression
The generation and analysis of transgenic mice provided experimental evidence that overexpression of wildtype Cyclin D1 is sufficient to induce neoplastic transformation and mammary tumorigenesis. In addition, it has been observed that murine mammary cancers induced through overexpression of ErbB2 or oncogenic Ras show an increased expression of endogenous Cyclin D1 (Fu et al., 2004; Sutherland and Musgrove, 2004). Moreover, Yu et al. (2001) reported that the deletion of the Cyclin D1 gene prevented mammary carcinogenesis in MMTV-neu and MMTV-Ha-ras transgenics, but Cyclin D1 deficiency had no effect on tumor onset induced by c-Myc or Wnt1. A null mutation of Cyclin D2 or D3 did not delay neoplastic transformation, and expression of Cyclin E under control of the endogenous Ccnd1 gene promoter was sufficient to restore ErbB2-associated mammary tumorigenesis in the absence of Cyclin D1. As mentioned earlier, knockin mice expressing mutant Cyclin D1, which is deficient in activating CDK4/6, did not exhibit abnormal mammary gland development, but it was reported by Landis and colleagues (2006) that these animals were resistant to ErbB2-indcued neoplastic transformation. The notion that the Cyclin D1-dependent kinase activity is necessary for neoplastic growth was confirmed in mice that express ErbB2 in a CDK4-deficient background (Reddy et al., 2005; Yu et al., 2006). In contrast, the LxCxE domain of Cyclin D1 and binding to pRb appears not to be required for mammary carcinogenesis in MMTV-neu transgenics (Landis et al., 2007).
Collectively, all these studies in genetically engineered mice seem to suggest that targeting specifically Cyclin D1 and its ability to activate CDK4 is sufficient to treat ErbB2 (Her2/neu)-positive breast cancers in humans. However, this general interpretation of these observations in conventional knockout mouse models should be viewed with some skepticism. Overexpressing a particular oncogene in a Cyclin D1 or CDK4 null background is clearly an experimental model for cancer prevention, and the results should not be misperceived as cancer therapy (Matulka and Wagner, 2005). These knockout females do not express any of these cell cycle regulators throughout development, and consequently, these animals did not develop any mammary tumors. This is a very different scenario from a situation where a woman is diagnosed with this dreadful disease and decisions have to be made to treat advanced breast cancer based on the histopathology and biomarker expression. From a standpoint of modeling cancer in animals, it should never be assumed that a cancer-initiating mutation is always required during the entire evolutionary process of a metastatic cancer (Matulka and Wagner, 2005). In contrast to the widely used term “oncogene addition”, it was the first ligand-regulated transgenic cancer model generated in 1996 in the laboratories of Lothar Hennighausen and Priscilla Furth that should have taught us that cancer cells can lose their dependence on transforming oncogenes for the maintenance of the transformed state (Ewald et al., 1996). In this first reversible cancer model, adenocarcinomas were induced through temporally and spatially controlled expression of the SV40 large T antigen, a vial oncoprotein that is known to inhibit pRb downstream of active Cyclin D/CDK4/6 complexes. In subsequent studies, our team has employed conditional knockout mice to assess the importance of signaling networks prior to and after the onset of mammary tumorigenesis (Sakamoto et al., 2009; Sakamoto et al., 2010). Specifically, we have demonstrated that deficiency in Jak2, which acts upstream of Cyclin D1, was sufficient to completely prevent ErbB2- and PRL-induced mammary tumorigenesis. However, the inhibition of this Janus kinase in established tumors had no noticeable effect on cancer cell survival and growth in vivo. This suggests that signaling networks that drive cell proliferation and survival can be fundamentally different between normal and neoplastic cells.
Another major concern of the phenotypic analysis of females that express ErbB2 in the absence of Cyclin D1 or CDK4 is that all previous studies were performed in mixed genetic backgrounds. We mentioned earlier that the extent of the phenotypic abnormalities in the mammary gland and reproductive organs varies considerably among Cyclin D1 deficient mice in different genetic strains; but more importantly, the presence of C57/Bl6 alleles is known to confer resistance towards MMTV-neu-induced mammary tumorigenesis (Rowse et al., 1998). We have shown recently that a knockout of Cyclin D1 can extend the tumor-free survival in MMTV-neu transgenic females (Zhang et al., 2011), but un like previously reported (Yu et al., 2001), deficiency in Cyclin D1 did not prevent the onset of ErbB2-indcued mammary tumorigenesis in the FVB strain. Therefore, targeting Cyclin D1 might be, to some degree, an acceptable strategy for chemoprevention to delay cancer initiation, but cancer cells do eventually evolve over time though selective mechanisms that employ alternative pathways to optimize their growth and survival without Cyclin D1. The fact that ErbB2-indcued cancer cells can form and proliferate in complete absence of Cyclin D1 indicates that targeting of this particular D-type Cyclin is far from optimal for cancer therapy.
Targeting the function of all D-type Cyclins can effectively prevent the proliferation of ErbB2-positive mammary cancer cells
A suitable experimental design to assess the importance of a protein during cancer progression is to downregulate its expression within the cancer cells of an established neoplasm in vivo. Based on this idea, we generated a tetracycline-controlled expression model that allows the targeted ablation of Cyclin D1 in progressing ErbB2-positive mammary cancers (Zhang et al., 2011). Using this experimental design, we found that Cyclin D1 is not essential for the proliferation of cancer cells. This result is in line with our observation that Cyclin D1 knockout mice develop MMTV-neu-induced mammary tumors, and we there proposed that targeting only this particular D-type Cyclin is not a reliable strategy to treat ErbB2-associated breast cancer. The analysis of primary mammary tumors that arose in Cyclin D1 knockout mice revealed that these neoplasms exhibited a compensatory upregulation of Cyclin D3 and, to a much lesser extent, an elevation of Cyclin E. In part, the higher expression of Cyclin D3 was a consequence of an increase in the transcriptional activation of the Ccnd3 gene as determined by quantitative RT-PCR (Zhang and Wagner, unpublished). Unlike a previous report (Bowe et al., 2002), we did not observe a gain-of-function of Cyclin E that is able bypass the importance of all D-type Cyclins. More importantly, we demonstrated that the targeted knockdown of Cyclin D3 in tumor cells that lack Cyclin D1 was sufficient to slow or halt cancer cell proliferation in culture and in vivo (Zhang et al., 2011). The phenomenon that upregulation of Cyclin D3 compensates for the loss of Cyclin D1 during mammary tumorigenesis seems not to be restricted to ErbB2-associated neoplastic transformation. Asher and colleagues (2012) recently reported that mammary tumor formation in NRL-PRL mice, which develop ERα-positive as well as ERα-negative lesions, does not depend on Cyclin D1, and these mammary cancers showed an increased nuclear expression of Cyclin D3. Collectively, these studies in mice parallel our observation that human breast cancer cell lines and primary human breast cancers express high levels of Cyclin D3. It should be noted, however, that unlike in ErbB2-induced mammary tumors in mice that typically express higher levels of Cyclin D1, Cyclin D3 often exceeded the levels of Cyclin D1 in comparable ErbB2-positive primary human breast cancers (Zhang et al., 2011). This was also the case for many ERα-positive cancer cell lines such as T47-D and MCF-7 cells that are being frequently used in experiments. In summary, based on the analysis of tumor formation in Cyclin D1 knockout mice using various oncogenes as well as the expression profile of D-type Cyclins in human cancer specimens, it should be evident that targeting only Cyclin D1 is not sufficient to prevent or treat human breast cancer.
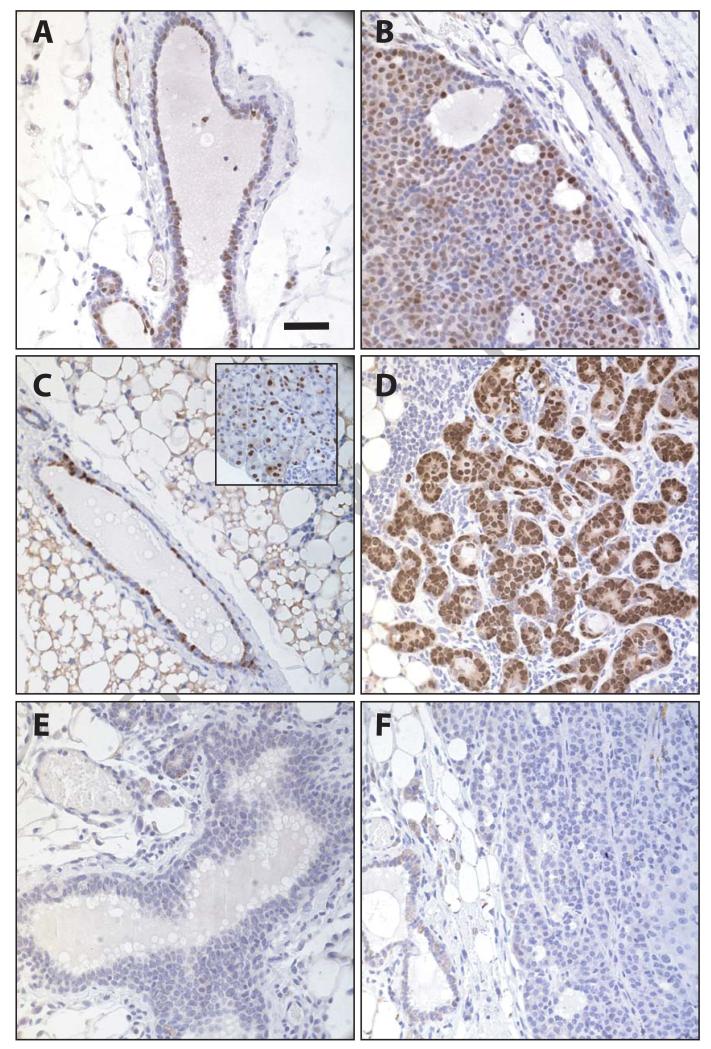
Regardless of the previous reports discussed above, a new article by Choi et al (2012) reiterates the importance of Cyclin D1 in ErbB2-associated mammary cancer progression. These investigators developed a Cyclin D1 conditional knockout mouse; but instead of ablating this gene in the mammary gland or specifically in developing mammary tumors, they used a tamoxifen-inducible Cre strain to delete Cyclin D1 systemically in adult mice that developed MMTV-neu-induced mammary tumors. Based on the findings presented in this new report, the authors concluded that Cyclin D1 is essential for mammary cancer cell proliferation and suppression of cellular senescence. Despite the ubiquitous expression of Cre recombinase, the extent of the deletion of Cyclin D1 in tumors was performed only by semi-quantitative PCR, which does not distinguish specific cell types or discriminate tumor cells from the supporting stroma. Although it is generally perceived that Cyclin D1 acts in a cell autonomous manner (Fantl et al., 1999), it might still be feasible that a systemic deletion of Cyclin D1 can cause indirect effects on cancer cell proliferation that mask the intrinsic functions of this cell cycle regulator within tumor cells. In support of this argument, we observed that overexpression of exogenous Cyclin D1 exclusively in mammary epithelial cells of aging females did not accelerate ErbB2-induced transformation and tumor growth (Fig. 1A, 1B). Both MMTV-neu single transgenics (N=10) and MMTV-neu MMTV-tTA TetO-Cyclin D1 triple transgenic females (N=10) showed a similar tumor latency of about 230 days (P=0.7; Sakamoto and Wagner, unpublished). Also, Cyclin D1 deficient females expressing transgenic Cyclin D1 specifically in the mammary epithelium (FVB, MMTV-neu MMTV-tTA TetO-Cyclin D1 Cyclin D1−/−) showed a delayed tumor onset that was comparable to MMTV-neu single transgenic mice in a Cyclin D1 deficient background (FVB, MMTV-neu Cyclin D1−/−). Although high levels of exogenous Cyclin D1 in the mammary epithelium that lacks both endogenous Cyclin D1 alleles were clearly visible (Fig. 1C), some females did not develop any invasive mammary cancers, and only small lesions of alveolar hyperplasia were detectable in histological sections by 21 months of age (Fig. 1D). More interestingly, MMTV-neu MMTV-tTA TetO-Cyclin D1 Cyclin D1−/− females that developed palpable mammary tumors and invasive lesions exhibited no expression of transgenic Cyclin D1 (Fig. 1E), suggesting that this cell cycle regulator is not required for tumor cell proliferation and survival. In terms of the expression of D-type Cyclins, these cancers are therefore identical to MMTV-neu-induced mammary tumors that arose in complete absence of endogenous and exogenous Cyclin D1 (Fig. 1F). In summary, a mammary-specific overexpression of Cyclin D1 did not rescue the delay in ErbB2-induced mammary carcinogenesis in mice that are systemically deficient in endogenous Cyclin D1. We therefore propose that Cyclin D1 deficiency may cause indirect effects that result in a prolonged latency in mammary tumor formation in MMTV-neu transgenics.
Fig. 1. Expression of exogenous Cyclin D1 in the mammary epithelium of Cyclin D1 knockout mice does not accelerate ErbB2-induced mammary tumorigenesis.
Immunostaining of Cyclin D1 in histologic sections of normal and hyperplastic mammary ducts as well as mammary tumors of MMTV-neu MMTV-tTA TetO-Cyclin D1 triple transgenic females (A, B), MMTV-neu MMTV-tTA TetO-Cyclin D1 Cyclin D1−/− mice (C-E), and MMTV-neu Cyclin D1−/− littermate controls (F). Slides were counterstained with hematoxylin; bar represent 50 μm. Inset in panel C shows a section of the salivary gland, which is serves as a positive control for expression of MMTV-driven transgenes (here MMTV-tTA mediated transactivation of TetO-Cyclin D1).
With regard to the obvious differences between their findings and our previous report, Choi et al. (2012) disregarded the collective observations of our team as a cell culture artifact, which may have resulted in an abnormal expression of Cyclin D3 due do repeated passaging of cells ex vivo. This is certainly an incorrect and misleading recollection of the results of our study since the upregulation of Cyclin D3 was observed in primary tumors of Cyclin D1 deficient mice. In addition to their genetic mouse model, Choi and co-workers used a dual CDK4/6 inhibitor (i.e., PD-0332991) to halt the proliferation of ErbB2-expressing mammary tumor cells, and they concluded that “Cyclin D1 and the Cyclin D1-associated kinase” are essential for the maintenance of ErbB2-driven breast cancers. This conclusion should be viewed with skepticism since a) this inhibitor targets CDKs that associate with all three D-type Cyclins, b) mouse MMTV-neu tumors express Cyclin D3 in addition to Cyclin D1, and, most importantly, c) ErbB2-positive breast cancers in humans express higher levels of Cyclin D3 compared to murine tumors, and the expression of D3 can even exceed the levels of D1 in individual breast cancer specimens.
Collectively, we can agree that targeting the kinase function of all D-type Cyclins is effective to prevent the proliferation of ErbB2-positive cancer cells. As mentioned earlier, a knockdown of Cyclin D3 in Cyclin D1-deficient cancer cells, that typically also expressed very low levels of Cyclin D2, resulted in growth arrest in vitro and in vivo (Zhang et al., 2011). In a study prior to the recent report by Choi et al. (2012), Dean and colleagues (2010) have demonstrated that inhibition of CDK4/6 using the well-tolerated drug PD-0332991 can effectively block the numeric expansion of selected ERα-positive and ERα-negative breast cancer cells that have normal pRb function. Therefore, as long as the proliferation of breast cancer cells can be controlled by the pRb tumor suppressor, targeting the combined functions of D-type Cyclins could be a suitable strategy for the treatment of breast cancer.
Highlights.
D-type Cyclins connect signals from growth factors to the cell cycle machinery
D-type Cyclins, in particular Cyclin D1 and D3 are upregulated in primary human breast cancers and breast cancer cell lines.
Deficiency in Cyclin D1 leads to a compensatory upregulation of D3 and vice versa in mammary tumors of transgenic mice and in human breast cancer cells
Targeting the combined functions of D-type Cyclins though pharmacological inhibition of CDK4/6 could be a suitable strategy for the treatment of ErbB2-positive and potentially other types of breast cancer
Acknowledgements
This work was supported, in part, by the Public Health Service grants CA117930 and CA93797 (K.-U.W.). Additional financial support provided to K.-U.W. by the Nebraska Cancer and Smoking Disease Research Program (NE DHHS LB506 2009-45) was imperative to finance the generation, maintenance, and analysis of MMTV-tTA and TetO-Cyclin D1 transgenic mice described in this article. Q.Z. was supported through a research assistantship from the UNMC Graduate Studies Office. K.S. received a postdoctoral fellowship from the Susan G Komen Breast Cancer Foundation (PDF0600835). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support: PHS grants CA117930 (K.-U.W.) from the National Cancer Institute; the Nebraska Cancer and Smoking Disease Research Program NE DHHS LB506 2009-45 (K.-U.W.); and the Susan G Komen Breast Cancer Foundation fellowship PDF0600835 (K.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have nothing to disclose.
References
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol. Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A, Motokura T, Bloom T, Kronenberg H, Ruderman J, Juppner H, Kim HG. The putative oncogene PRAD1 encodes a novel cyclin. Cold Spring Harb. Symp. Quant. Biol. 1991;56:93–97. doi: 10.1101/sqb.1991.056.01.013. [DOI] [PubMed] [Google Scholar]
- Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J. Clin. Oncol. 2005;23:4215–4224. doi: 10.1200/JCO.2005.05.064. [DOI] [PubMed] [Google Scholar]
- Asher JM, O’Leary KA, Rugowski DE, Arendt LM, Schuler LA. Prolactin promotes mammary pathogenesis independently from cyclin D1. Am. J. Pathol. 2012;181:294–302. doi: 10.1016/j.ajpath.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperlee MD, Drolet AA, Durairaj S, Wang W, Schwartz RC, Haslam SZ. Strain-specific differences in the mechanisms of progesterone regulation of murine mammary gland development. Endocrinology. 2009;150:1485–1494. doi: 10.1210/en.2008-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbash O, Zamfirova P, Lin DI, Chen X, Yang K, Nakagawa H, Lu F, Rustgi AK, Diehl JA. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer Cell. 2008;14:68–78. doi: 10.1016/j.ccr.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J, Zemanova M, Bartek J. Abundance and subcellular localisation of cyclin D3 in human tumours. Int. J. Cancer. 1996;65:323–327. doi: 10.1002/(SICI)1097-0215(19960126)65:3<323::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Betticher DC, Thatcher N, Altermatt HJ, Hoban P, Ryder WD, Heighway J. Alternate splicing produces a novel cyclin D1 transcript. Oncogene. 1995;11:1005–1011. [PubMed] [Google Scholar]
- Bienvenu F, Jirawatnotai S, Elias JE, Meyer CA, Mizeracka K, Marson A, Frampton GM, Cole MF, Odom DT, Odajima J, Geng Y, Zagozdzon A, Jecrois M, Young RA, Liu XS, Cepko CL, Gygi SP, Sicinski P. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature. 2010;463:374–378. doi: 10.1038/nature08684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe DB, Kenney NJ, Adereth Y, Maroulakou IG. Suppression of Neu-induced mammary tumor growth in cyclin D1 deficient mice is compensated for by cyclin E. Oncogene. 2002;21:291–298. doi: 10.1038/sj.onc.1205025. [DOI] [PubMed] [Google Scholar]
- Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA, Jan T. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev. Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Brockman JL, Schroeder MD, Schuler LA. PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol. Endocrinol. 2002;16:774–784. doi: 10.1210/mend.16.4.0817. [DOI] [PubMed] [Google Scholar]
- Brockman JL, Schuler LA. Prolactin signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Mol. Cell Endocrinol. 2005;239:45–53. doi: 10.1016/j.mce.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthon BC, Neumann CA, Das M, Pawlyk B, Li T, Geng Y, Sicinski P. Genetic replacement of cyclin D1 function in mouse development by cyclin D2. Mol. Cell Biol. 2005;25:1081–1088. doi: 10.1128/MCB.25.3.1081-1088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Hwang J, Andres BM, Wei Y, Lukacisin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T, Mercatali L, Amadori D, Haffty BG, Sinha S, Kang Y. Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat. Cell Biol. 2012;14:1212–1222. doi: 10.1038/ncb2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von BH, Sicinski P. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciemerych MA, Kenney AM, Sicinska E, Kalaszczynska I, Bronson RT, Rowitch DH, Gardner H, Sicinski P. Development of mice expressing a single D-type cyclin. Genes Dev. 2002;16:3277–3289. doi: 10.1101/gad.1023602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer BA, Sakamoto K, Schmidt JW, Triplett AA, Moriggl R, Wagner KU. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Mol. Cell Biol. 2010;30:2957–2970. doi: 10.1128/MCB.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JL, McClendon AK, Stengel KR, Knudsen ES. Modeling the effect of the RB tumor suppressor on disease progression: dependence on oncogene network and cellular context. Oncogene. 2010;29:68–80. doi: 10.1038/onc.2009.313. [DOI] [PubMed] [Google Scholar]
- Delmer A, Ajchenbaum-Cymbalista F, Tang R, Ramond S, Faussat AM, Marie JP, Zittoun R. Overexpression of cyclin D2 in chronic B-cell malignancies. Blood. 1995;85:2870–2876. [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- Escamilla-Hernandez R, Chakrabarti R, Romano RA, Smalley K, Zhu Q, Lai W, Halfon MS, Buck MJ, Sinha S. Genome-wide search identifies Ccnd2 as a direct transcriptional target of Elf5 in mouse mammary gland. BMC. Mol. Biol. 2010;11:68. doi: 10.1186/1471-2199-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Evron E, Umbricht CB, Korz D, Raman V, Loeb DM, Niranjan B, Buluwela L, Weitzman SA, Marks J, Sukumar S. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res. 2001;61:2782–2787. [PubMed] [Google Scholar]
- Ewald D, Li M, Efrat S, Auer G, Wall RJ, Furth PA, Hennighausen L. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science. 1996;273:1384–1386. doi: 10.1126/science.273.5280.1384. [DOI] [PubMed] [Google Scholar]
- Fantl V, Edwards PA, Steel JH, Vonderhaar BK, Dickson C. Impaired mammary gland development in Cyl-1(−/−) mice during pregnancy and lactation is epithelial cell autonomous. Dev. Biol. 1999;212:1–11. doi: 10.1006/dbio.1999.9329. [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- Fischer H, Chen J, Skoog L, Lindblom A. Cyclin D2 expression in familial and sporadic breast cancer. Oncol. Rep. 2002;9:1157–1161. [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Geng Y, Whoriskey W, Park MY, Bronson RT, Medema RH, Li T, Weinberg RA, Sicinski P. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–777. doi: 10.1016/s0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Bronson RT, Sicinski P. Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:194–199. doi: 10.1073/pnas.011522998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain D, Russell A, Thompson A, Hendley J. Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J. Biol. Chem. 2000;275:12074–12079. doi: 10.1074/jbc.275.16.12074. [DOI] [PubMed] [Google Scholar]
- Hanna Z, Jankowski M, Tremblay P, Jiang X, Milatovich A, Francke U, Jolicoeur P. The Vin-1 gene, identified by provirus insertional mutagenesis, is the cyclin D2. Oncogene. 1993;8:1661–1666. [PubMed] [Google Scholar]
- He LM, Sartori DJ, Teta M, Opare-Addo LM, Rankin MM, Long SY, Diehl JA, Kushner JA. Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol. Endocrinol. 2009;23:1865–1875. doi: 10.1210/me.2009-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe D, Lynas C. The cyclin D1 alternative transcripts [a] and [b] are expressed in normal and malignant lymphocytes and their relative levels are influenced by the polymorphism at codon 241. Haematologica. 2001;86:563–569. [PubMed] [Google Scholar]
- Huard JM, Forster CC, Carter ML, Sicinski P, Ross ME. Cerebellar histogenesis is disturbed in mice lacking cyclin D2. Development. 1999;126:1927–1935. doi: 10.1242/dev.126.9.1927. [DOI] [PubMed] [Google Scholar]
- Inaba T, Matsushime H, Valentine M, Roussel MF, Sherr CJ, Look AT. Genomic organization, chromosomal localization, and independent expression of human cyclin D genes. Genomics. 1992;13:565–574. doi: 10.1016/0888-7543(92)90126-d. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620–1628. doi: 10.1038/sj.onc.1209371. [DOI] [PubMed] [Google Scholar]
- Kong G, Chua SS, Yijun Y, Kittrell F, Moraes RC, Medina D, Said TK. Functional analysis of cyclin D2 and p27(Kip1) in cyclin D2 transgenic mouse mammary gland during development. Oncogene. 2002;21:7214–7225. doi: 10.1038/sj.onc.1205895. [DOI] [PubMed] [Google Scholar]
- Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, Ciemerych MA, Sicinski P, Kaczmarek L. The critical role of cyclin D2 in adult neurogenesis. J. Cell Biol. 2004;167:209–213. doi: 10.1083/jcb.200404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, Akashi K, Sicinski P. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Lam EW, Glassford J, Banerji L, Thomas NS, Sicinski P, Klaus GG. Cyclin D3 compensates for loss of cyclin D2 in mouse B-lymphocytes activated via the antigen receptor and CD40. J. Biol. Chem. 2000;275:3479–3484. doi: 10.1074/jbc.275.5.3479. [DOI] [PubMed] [Google Scholar]
- Landis MW, Brown NE, Baker GL, Shifrin A, Das M, Geng Y, Sicinski P, Hinds PW. The LxCxE pRb interaction domain of cyclin D1 is dispensable for murine development. Cancer Res. 2007;67:7613–7620. doi: 10.1158/0008-5472.CAN-07-1207. [DOI] [PubMed] [Google Scholar]
- Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22. doi: 10.1016/j.ccr.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, Sakamaki T, Pestell R, Bromberg J. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006;66:2544–2552. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, Rustgi A, Fuchs SY, Diehl JA. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol. Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DI, Lessie MD, Gladden AB, Bassing CH, Wagner KU, Diehl JA. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene. 2008;27:1231–1242. doi: 10.1038/sj.onc.1210738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Gladden AB, Diehl JA. An alternatively spliced cyclin D1 isoform, cyclin D1b, is a nuclear oncogene. Cancer Res. 2003;63:7056–7061. [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell RG, Kanakura Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, Sherr CJ. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Matulka LA, Wagner KU. Models of breast cancer. Drug Discovery Today: Disease Models. 2005;2:1–6. [Google Scholar]
- Mishra R, Das BR. Activation of STAT 5-cyclin D1 pathway in chewing tobacco mediated oral squamous cell carcinoma. Mol. Biol. Rep. 2005;32:159–166. doi: 10.1007/s11033-005-0754-9. [DOI] [PubMed] [Google Scholar]
- Moons DS, Jirawatnotai S, Parlow AF, Gibori G, Kineman RD, Kiyokawa H. Pituitary hypoplasia and lactotroph dysfunction in mice deficient for cyclin-dependent kinase-4. Endocrinology. 2002a;143:3001–3008. doi: 10.1210/endo.143.8.8956. [DOI] [PubMed] [Google Scholar]
- Moons DS, Jirawatnotai S, Tsutsui T, Franks R, Parlow AF, Hales DB, Gibori G, Fazleabas AT, Kiyokawa H. Intact follicular maturation and defective luteal function in mice deficient for cyclin-dependent kinase-4. Endocrinology. 2002b;143:647–654. doi: 10.1210/endo.143.2.8611. [DOI] [PubMed] [Google Scholar]
- Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350:512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Motokura T, Yi HF, Kronenberg HM, McBride OW, Arnold A. Assignment of the human cyclin D3 gene (CCND3) to chromosome 6p----q13. Cytogenet. Cell Genet. 1992;61:5–7. doi: 10.1159/000133359. [DOI] [PubMed] [Google Scholar]
- Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, Kazlauskas M, Pritchard MA, Chodosh LA, Pfeffer PL, Lindeman GJ, Visvader JE, Ormandy CJ. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev. 2008;22:581–586. doi: 10.1101/gad.1614608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Pal R, Khanna A. Role of smad- and wnt-dependent pathways in embryonic cardiac development. Stem Cells Dev. 2006;15:29–39. doi: 10.1089/scd.2006.15.29. [DOI] [PubMed] [Google Scholar]
- Pirkmaier A, Dow R, Ganiatsas S, Waring P, Warren K, Thompson A, Hendley J, Germain D. Alternative mammary oncogenic pathways are induced by D-type cyclins; MMTV-cyclin D3 transgenic mice develop squamous cell carcinoma. Oncogene. 2003;22:4425–4433. doi: 10.1038/sj.onc.1206488. [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- Reddy HK, Mettus RV, Rane SG, Grana X, Litvin J, Reddy EP. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 2005;65:10174–10178. doi: 10.1158/0008-5472.CAN-05-2639. [DOI] [PubMed] [Google Scholar]
- Robles AI, Larcher F, Whalin RB, Murillas R, Richie E, Gimenez-Conti IB, Jorcano JL, Conti CJ. Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7634–7638. doi: 10.1073/pnas.93.15.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas P, Benavides F, Blando J, Perez C, Cardenas K, Richie E, Knudsen ES, Johnson DG, Senderowicz AM, Rodriguez-Puebla ML, Conti CJ. Enhanced skin carcinogenesis and lack of thymus hyperplasia in transgenic mice expressing human cyclin D1b (CCND1b) Mol. Carcinog. 2009;48:508–516. doi: 10.1002/mc.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg CL, Motokura T, Kronenberg HM, Arnold A. Coding sequence of the overexpressed transcript of the putative oncogene PRAD1/cyclin D1 in two primary human tumors. Oncogene. 1993;8:519–521. [PubMed] [Google Scholar]
- Rosenberg CL, Wong E, Petty EM, Bale AE, Tsujimoto Y, Harris NL, Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc. Natl. Acad. Sci. U. S. A. 1991;88:9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowse GJ, Ritland SR, Gendler SJ. Genetic modulation of neu proto-oncogene-induced mammary tumorigenesis. Cancer Res. 1998;58:2675–2679. [PubMed] [Google Scholar]
- Russell A, Thompson MA, Hendley J, Trute L, Armes J, Germain D. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene. 1999;18:1983–1991. doi: 10.1038/sj.onc.1202511. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Creamer BA, Triplett AA, Wagner KU. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol. Endocrinol. 2007;21:1877–1892. doi: 10.1210/me.2006-0316. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Lin WC, Triplett AA, Wagner KU. Targeting janus kinase 2 in Her2/neu-expressing mammary cancer: Implications for cancer prevention and therapy. Cancer Res. 2009;69:6642–6650. doi: 10.1158/0008-5472.CAN-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Triplett AA, Schuler LA, Wagner KU. Janus kinase 2 is required for the initiation but not maintenance of prolactin-induced mammary cancer. Oncogene. 2010;29:5359–5369. doi: 10.1038/onc.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Robinson GW, Grimm SL, Rosen JM, Neubauer H, Pfeffer K, Hennighausen L. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 2002;16:563–570. doi: 10.1210/mend.16.3.0805. [DOI] [PubMed] [Google Scholar]
- Sicinska E, Aifantis I, Le CL, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von BH, Sicinski P. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Wang Y, Fox SR, Lambeck TC, Giesting S, Lan Z, Senderowicz AM, Conti CJ, Knudsen ES. Cyclin D1 splice variants. Differential effects on localization, RB phosphorylation, and cellular transformation. J. Biol. Chem. 2003;278:30339–30347. doi: 10.1074/jbc.M303969200. [DOI] [PubMed] [Google Scholar]
- Solvason N, Wu WW, Parry D, Mahony D, Lam EW, Glassford J, Klaus GG, Sicinski P, Weinberg R, Liu YJ, Howard M, Lees E. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B cell development. Int. Immunol. 2000;12:631–638. doi: 10.1093/intimm/12.5.631. [DOI] [PubMed] [Google Scholar]
- Sutherland RL, Musgrove EA. Cyclins and breast cancer. J. Mammary. Gland. Biol. Neoplasia. 2004;9:95–104. doi: 10.1023/B:JOMG.0000023591.45568.77. [DOI] [PubMed] [Google Scholar]
- Tremblay PJ, Kozak CA, Jolicoeur P. Identification of a novel gene, Vin-1, in murine leukemia virus-induced T-cell leukemias by provirus insertional mutagenesis. J. Virol. 1992;66:1344–1353. doi: 10.1128/jvi.66.3.1344-1353.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling riva lry in the E2F family. Nat. Rev. Mol. Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Hesabi B, Moons DS, Pandolfi PP, Hansel KS, Koff A, Kiyokawa H. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol. Cell Biol. 1999;19:7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J, Rui H. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol. Cell Biol. 2004;24:5510–5520. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Wianny F, Real FX, Mummery CL, Van RM, Lahti J, Samarut J, Savatier P. G1-phase regulators, cyclin D1, cyclin D2, and cyclin D3: up-regulation at gastrulation and dynamic expression during neurulation. Dev. Dyn. 1998;212:49–62. doi: 10.1002/(SICI)1097-0177(199805)212:1<49::AID-AJA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Won KA, Xiong Y, Beach D, Gilman MZ. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1992;89:9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Chan JK, Lee KC, Hsiao WL. Differential expression of p16/p21/p27 and cyclin D1/D3, and their relationships to cell proliferation, apoptosis, and tumour progression in invasive ductal carcinoma of the breast. J. Pathol. 2001;194:35–42. doi: 10.1002/path.838. [DOI] [PubMed] [Google Scholar]
- Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, Hennighausen L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009;23:2382–2387. doi: 10.1101/gad.1840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- Yu Q, Sicinska E, Geng Y, Ahnstrom M, Zagozdzon A, Kong Y, Gardner H, Kiyokawa H, Harris LN, Stal O, Sicinski P. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sakamoto K, Liu C, Triplett AA, Lin WC, Rui H, Wagner KU. Cyclin D3 compensates for the loss of cyclin D1 during ErbB2-induced mammary tumor initiation and progression. Cancer Res. 2011;71:7513–7524. doi: 10.1158/0008-5472.CAN-11-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Ray D, Aziyu A, Christov K, Boiko AD, Gudkov AV, Kiyokawa H. Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence. Genes Dev. 2002;16:2923–2934. doi: 10.1101/gad.1033002. [DOI] [PMC free article] [PubMed] [Google Scholar]