Recent advances in several imaging modalities have improved noninvasive assessment of the pancreas in preclinical animal models and have enabled sequential studies in the same animal, permitting dynamic studies of pancreatic disease. This review outlines progress in the application of small animal imaging to monitor the pancreatic islet, insulitis, and pancreatitis. Preclinical advances in magnetic resonance imaging (MRI) and positron emission tomography (PET) have benefited from higher magnetic field strengths and dedicated small animal scanners, respectively. Novel imaging approaches developed using preclinical MRI and PET scanners may be adaptable to clinical usage. Preclinical imaging of mouse models has also benefited from the recent advent of optical imaging modalities, in which light emission can be detected noninvasively. These optical imaging techniques enable sensitive, high-throughput, and cost-effective studies in preclinical, small animal models.
Pancreatic Islet Imaging
The ability to noninvasively image the pancreatic islet, especially the insulin-producing β-cells of the islet, would provide insight into the pathogenesis of diabetes, enable monitoring of therapeutic interventions, and could provide a predictive biomarker for the development of diabetes. However, the pancreatic islet is difficult to image, owing to its small size, distribution throughout the pancreas, and lack of inherent contrast from the surrounding exocrine pancreas. These imaging challenges have so far prevented successful application of MRI- or CT-based imaging approaches to detect pancreatic islets, but there has been considerable progress in optical- and PET-based imaging. Islet-specific imaging contrast has been achieved with the creation of transgenic animals expressing genetic reporters whose expression is controlled by β-cell–specific DNA regulatory fragments such as the insulin promoter. One such transgenic animal expresses the optical reporter green fluorescence protein (GFP) under control of the mouse insulin promoter.1 A fluorescent optical signal is emitted from the β-cells of the islet, aiding isolation of pancreatic islets and studies of β-cell development, physiology, and blood flow.2 The fluorescent signal emitted from these islets is not detectable noninvasively in the whole mouse; the pancreas must be exposed to image these GFP-expressing islets. A complementary imaging approach uses the firefly luciferase optical reporter, which allows noninvasive bioluminescence imaging (BLI) in the whole mouse (Figure 1). Transgenic mice expressing luciferase under control of the rat insulin promoter or the mouse insulin promoter have allowed investigators to use BLI to image β-cells in conditions of increased β-cell mass, decreased β-cell mass, and after islet transplantation.3–5 Although optical imaging provides a sensitive and quantitative assay of the pancreatic islet, light attenuation by biological tissue precludes clinical application and light scattering limits the spatial resolution, so that individual islets cannot be localized in vivo.
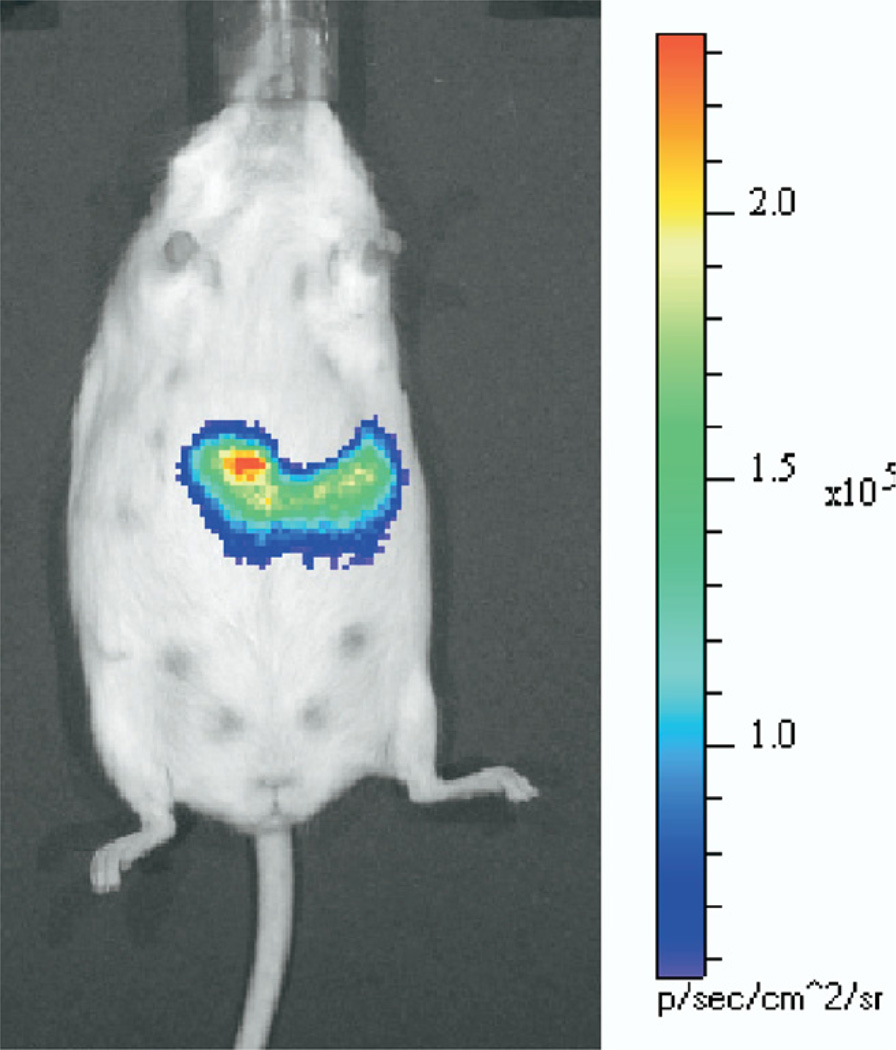
Figure 1.
BLI of the pancreas of a transgenic mouse expressing luciferase under control of the mouse insulin promoter. After administration of the luciferin substrate to the anesthetized mouse (within 30 minutes of injection), light is emitted from the β-cells of the pancreas, which can be imaged noninvasively. Light intensity is pseudocolored according to the adjacent scale. Figure is an unpublished image from Virostko and Powers.
The search for a technique capable of imaging the pancreatic islet in humans has focused largely on targeted contrast agents that are preferentially bound or are retained by the islet. Radiolabeled probes for PET and single-photon emission computed tomography are promising because of their high sensitivity. Owing to the limited volume of islet cell mass, a successful imaging probe must have high affinity and specificity for the islet or β-cell. A number of candidate radiotracers targeting the sulfonylurea receptor, secretory granules, and the glucose metabolism of the β-cell have proven incapable of achieving the requisite specific binding to image the islet.6 A probe using a β-cell–specific monoclonal antibody was capable of detecting altered β-cell mass in excised murine pancreata, but binding to plasma proteins prevented in vivo application.7 Currently, the most promising approach is a PET compound (dihydrotetrabenazine), which targets the type 2 vesicular monoamine transporter (VMAT2) and reflected β-cell mass in a rat model of diabetes.8 Further validation is currently underway on VMAT2-targeted PET compounds to determine their clinical utility for determining β-cell mass.
Insulitis
Imaging of pancreatic islet inflammation may yield insight into the pathogenesis of type 1 diabetes and may serve as an early biomarker of the development of disease. T-lymphocyte infiltration in the pancreas can be imaged by MRI after labeling T cells with an imaging probe such as iron oxide nanoparticles. Moore et al9 demonstrated T-cell migration into the pancreas in a mouse model of type 1 diabetes with this approach. This iron oxide labeling is sensitive but relies on negative contrast mechanisms, confounding detection and quantification of the number of cells that have migrated to the pancreas. An alternate approach labels T cells with 19F for MRI and yielded more specific and quantifiable images of T-cell migration to the pancreas of a diabetic mouse.10
The microvasculature alterations that accompany insulitis also offer a potential contrast mechanism for imaging. Modifications in the pancreatic vasculature, such as increased blood flow, edema, and vascular leakage, could be detected using MRI. Iron oxide particles injected into the blood stream accumulate in the pancreas of mouse models of type 1 diabetes (Figure 2).11 Accumulation of the iron particles likely reflects vascular leakage, blood volume, and probe uptake by phagocytic cells in the islets during the autoimmune process leading to type 1 diabetes. Visualization of the pancreatic islet vasculature may be achievable with MRI after injection of a paramagnetic blood pool contrast agent.12
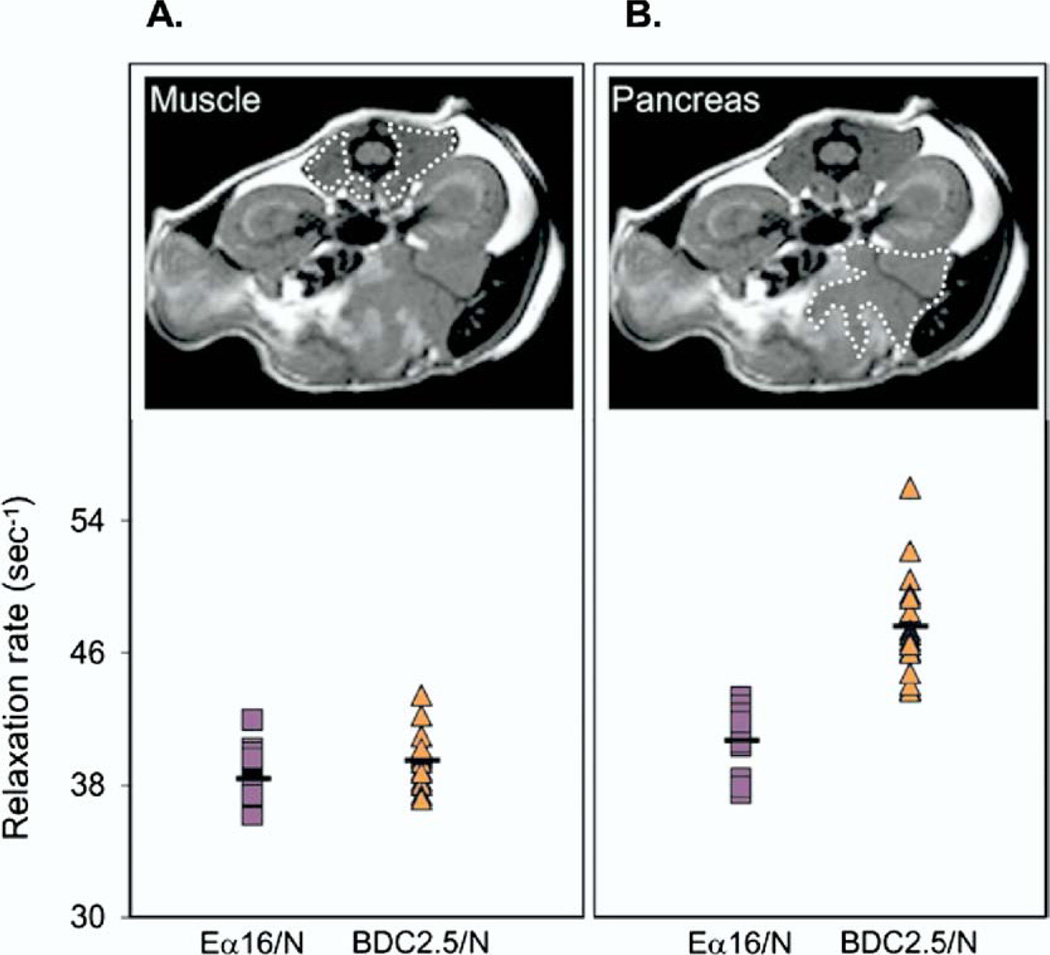
Figure 2.
Magnetofluorescent nanoparticles detected by MRI reveals microvascular alterations in a mouse model of insulitis. Twenty-four hours after injection of magnetofluorescent nanoparticles, MRI was performed in a BDC2.5/NOD mouse with insulitis and in an Eα16/NOD mouse without insulitis. A region of interest was defined on the MRI image over muscle tissue (A, upper) and over the pancreas (B, upper). The accumulation of magnetofluorescent nanoparticles was quantified in muscle tissue (A, lower) and in the pancreas (B, lower) in the 2 strains of mice. (Reproduced with permission from Moore et al,9 copyright ©2004 National Academy of Sciences, USA).
Pancreatitis
Small animal studies of acute pancreatitis have also been aided by molecular imaging modalities. Two different aspects of acute pancreatitis have been studied in mice using optical imaging. The accumulation of bioluminescent macrophages in the pancreas of a mouse model of pancreatitis was tracked using BLI.13 Imaging in this animal model was used to evaluate a new intervention to prevent acute pancreatitis. The inflammation associated with acute pancreatitis has also been studied using a mouse model in which the optical reporter luciferase is under the control of the regulatory region of the inflammatory transcription factor nuclear factor-κB.14 BLI provided insight into the dynamics of inflammatory process of pancreatitis.
Continued advances in molecular imaging technology (optical imaging, MRI, and nuclear imaging) should provide insight into normal pancreatic exocrine and endocrine function and facilitate studies of pathophysiologic processes in the pancreatic islet (lymphocyte infiltration, microvasculature alterations in insulitis, and assessment of islet mass) and in the pancreatic exocrine tissue (pancreatitis).
Acknowledgments
Work by the authors was supported by the Juvenile Diabetes Research Foundation International, a Merit Review Award from the VA Research Service, the National Institutes of Health (DK68764, DK66636, DK69603, DK63439, DK62641, DK68751, T32EB001628), the Vanderbilt Mouse Metabolic Phenotyping Center (DK59637), the Vanderbilt Diabetes Research and Training Center (DK20593), and SAIRP U24 CA126588.
Footnotes
The authors disclose no conflicts.
References
- 1.Hara M, Wang X, Kawamura T, et al. Transgenic mice with green fluorescent protein-labeled pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 2.Nyman LR, Wells KS, Head WS, et al. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest. 2008;118:3790–3797. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aramandla R, Poffenberger G, Virostko J, et al. Non-invasive monitoring of islets within the pancreas and following transplantation using in vivo bioluminescence imaging (BLI) Diabetes. 2006;55:A79. [Google Scholar]
- 4.Park SY, Wang X, Chen Z, et al. Optical imaging of pancreatic beta cells in living mice expressing a mouse insulin I promoter-firefly luciferase transgene. Genesis. 2005;43:80–86. doi: 10.1002/gene.20157. [DOI] [PubMed] [Google Scholar]
- 5.Smith SJ, Zhang H, Clermont AO, et al. In vivo monitoring of pancreatic beta-cells in a transgenic mouse model. Mol Imaging. 2006;5:65–75. [PubMed] [Google Scholar]
- 6.Sweet IR, Cook DL, Lernmark A, et al. Systematic screening of potential beta-cell imaging agents. Biochem Biophys Res Commun. 2004;314:976–983. doi: 10.1016/j.bbrc.2003.12.182. [DOI] [PubMed] [Google Scholar]
- 7.Moore A, Bonner-Weir S, Weissleder R. Noninvasive in vivo measurement of beta-cell mass in mouse model of diabetes. Diabetes. 2001;50:2231–2236. doi: 10.2337/diabetes.50.10.2231. [DOI] [PubMed] [Google Scholar]
- 8.Freeby M, Goland R, Ichise M, et al. VMAT2 quantitation by PET as a biomarker for beta-cell mass in health and disease. Diabetes Obes Metab. 2008;10(Suppl 4):98–108. doi: 10.1111/j.1463-1326.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 9.Moore A, Grimm J, Han B, et al. Tracking the recruitment of diabetogenic CD8+ T-cells to the pancreas in real time. Diabetes. 2004;53:1459–1466. doi: 10.2337/diabetes.53.6.1459. [DOI] [PubMed] [Google Scholar]
- 10.Srinivas M, Morel PA, Ernst LA, et al. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med. 2007;58:725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- 11.Denis MC, Mahmood U, Benoist C, et al. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc Natl Acad Sci U S A. 2004;101:12634–12639. doi: 10.1073/pnas.0404307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medarova Z, Castillo G, Dai G, et al. Noninvasive magnetic resonance imaging of microvascular changes in type 1 diabetes. Diabetes. 2007;56:2677–2682. doi: 10.2337/db07-0822. [DOI] [PubMed] [Google Scholar]
- 13.Nakamichi I, Habtezion A, Zhong B, et al. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray KD, Simovic MO, Chapman WC, et al. Systemic NF-kappaB activation in a transgenic mouse model of acute pancreatitis. J Surg Res. 2003;110:310–314. doi: 10.1016/s0022-4804(03)00024-6. [DOI] [PubMed] [Google Scholar]