SUMMARY
The transfer of molecules between cells during cognate immune cell interactions has been reported, and recently a novel mechanism of transfer of proteins and genetic material such as small RNA between T cells and APCs has been described, involving exchange of extracellular vesicles (EVs) during the formation of the immunological synapse (IS). EVs – a term that encompasses exosomes and microvesicles – have been implicated in cell-cell communication during immune responses associated with tumors, pathogens, allergies and autoimmune diseases. This review focuses on EV transfer as a mechanism for the exchange of molecules during immune cell-cell interactions.
Keywords: Extracellular Vesicles, microRNA, Immune Synapse, T cell, antigen presenting cell
INTRODUCTION
The cells of a multicellular organism communicate through a wide variety of mechanisms that include not only direct cell-cell contact but also the secretion of molecules and vesicles that travel to distant cells (1). Mammalian cells secrete a variety of extracellular vesicles (EVs). EVs were first isolated from cultured cells but have more recently been detected in diverse body fluids such as blood, urine, milk, synovial fluid, bronchoalveolar lavage, amniotic fluid, and malignant ascites. Extensive research showed that EVs contain proteins and RNA molecules that can be transferred from the parent cell to other cells. Contact-independent release of EVs thus potentially allows the exchange of molecular messages over a distance (2).
During cell-cell contact, molecules can be exchanged through different structures, such as nanotubes and gap junctions, and processes such as trogocytosis, trans-endocytosis and, as shown more recently, the transfer of EVs (3). The immune synapse is a highly-adapted device for communication between immune cells that consists of a tightly-structured contact induced during antigen presentation between a lymphocyte and a cognate antigen-presenting cell (APC) (4, 5). This IS concentrates the structures and mechanisms for molecular information exchange at a highly-organized contact area, and the sharing of proteins through the IS has been described (3). Recently the IS was also shown to support the polarized transfer of genetic material (in the form of microRNA) by the shuttling of exosomes from the T cell to the APC (6).
This review summarizes the evidence of EV secretion by immune cells and the mechanisms of protein and RNA exchange between these cells. The review focuses on EV transfer at the IS but also examines other structures for molecular exchange.
IMMUNE CELL EXTRACELLULAR VESICLES
Types and biogenesis of extracellular vesicles
There is as yet no consensus on the nomenclature of EVs, although the term exosome is generally restricted to vesicles derived from endosomal compartments. EVs can thus be classified according to their subcellular origin (2):
ECTOSOMES: EVs that bud directly from the plasma membrane have been termed microparticles, shedding vesicles, ectosomes, or arrestin domain-containing protein 1-mediated microvesicles (ARMMs) (7). They are heterogeneous in size and can be bigger than exosomes (up to 1 micron). Direct vesicle budding from the plasma membrane was recently shown to require recruitment of TSG101 (ESCRT-I) to the plasma membrane by arrestin domain-containing protein 1 (ARRDC1) (8, 9). Additionally, the plasma membrane of certain cells, such as T cells, possess discrete microdomains that support outward vesicle budding, acting as preformed sites for vesicle release (10).
-
EXOSOMES: Cells can also store intracellular vesicles in the endocytic compartment (late endosomes or multivesicular bodies (MVB)). These vesicles are formed by inward budding of the limiting membrane of late endosomes and accumulate inside them as intraluminal vesicles (ILV). Fusion of the MVB limiting membrane with the plasma membrane triggers the release of these 50-100 nm diameter vesicles, which are then called exosomes. Formation and maturation of MVBs is extensively reviewed in (11, 12). Lipids play an important role in the formation of ILVs, and blockade of ceramide production by suppressing neutral sphingomyelinase activity inhibits exosome production (13). Exosome secretion is also promoted by DGKα kinase and phospholipase D (PLD2) activities (14-16).
The endosomal sorting complex required for transport (ESCRT complex) is involved in the sorting of ubiquitinylated proteins to intraluminal vesicles (17). In addition, depletion of components of the ESCRT machinery results in fewer ILVs. Of the ESCRT complexes, TSG101 plays a major role in the biogenesis of MVBs. Alix, a protein that interacts with several ESCRT proteins, regulates the biogenesis of exosomes through its interaction with syndecan and syntenin (18). In addition, small Rab GTPases such as Rab4, Rab11, Rab27A, Rab27 and Rab35 regulate various steps of exosome release, being implicated in cargo sequestration, ILV budding, and the transport and docking of MVBs at the plasma membrane (19-22).
APOPTOTIC BODIES are larger than ectosomes or exosomes (>1μm in diameter). These vesicles originate from apoptotic cells, and contain genomic DNA.
EV composition
The available information on EV composition reflects the mixed populations that have been studied in most of the proteomic and genomic analyses. Since EVs can have overlapping composition, density and size, the different types are very difficult to distinguish, and these difficulties are compounded by the lack of specific markers. Thus a major challenge is to develop methods to distinguish and purify different EV subpopulations according to their composition or origin. Moreover, cells can simultaneously secrete EVs of the same type (e.g. exosomes), but with different composition. This might reflect the presence of different MVB subpopulations, or the presence of different types of ILV inside MVBs. An example of this diversity is provided by epithelial cells, which can secrete exosomes with different protein composition from the apical and basolateral surfaces (23). In fact, cells do not release the entire MVB content in one go, potentially allowing localized release at defined regions of the cell surface. Recent advances have succeeded in blocking production of certain types of EVs. For example, inhibition of Rab27a decreases secretion of a subset of exosomes bearing CD63, Hsp 70, Tsg101 and alix, but does not affect the secretion of vesicles carrying CD9 and Mfge8 (24). Furthermore, silencing of syndecan or syntenin blocks the release of exosomes bearing CD63 and Hsp70 without altering the release of flotillin-positive vesicles (18). Further analysis will be necessary to characterize the molecular composition and distribution of markers in different exosome populations.
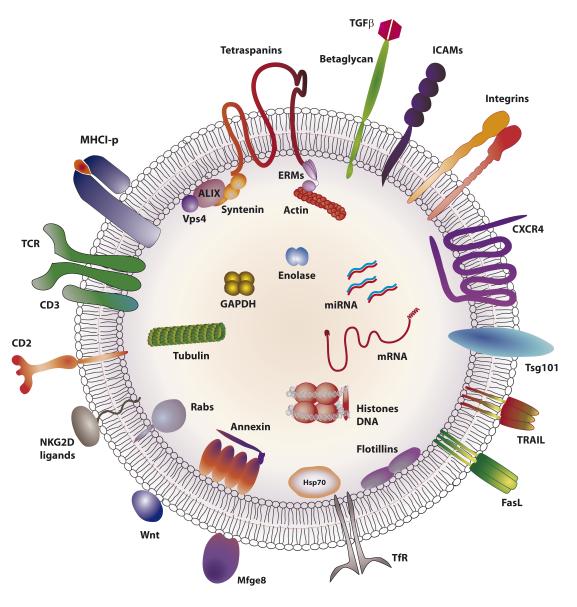
EVs are commonly enriched in a set of proteins (alix/syntenin ,Tsg101, hsc70, Mfge8, acetylcholinesterase (AchE), annexin, flotillins, transferrin receptor (TfR), and tetraspanins) and specific lipids such as phosphatidylserine and ceramide. However, exosomes also contain specific proteins depending on the nature of the producer cell (cell-type fingerprint). For example, exosomes from T cells contain T cell receptor (TCR) and costimulatory molecules (Figure 1), while those from B cells contain B cell receptor (BCR). Similarly, exosomes from tumor cells contain tumor antigens, platelet-derived exosomes contain coagulation factor, and exosomes from dendritic cells (DC) express Major histocompatibility complex II-peptide (MHCII-p) complexes.
Figure 1. Typical molecular composition of T-cell exosomes.
Membrane and luminal distribution of molecules predicted to be found in a typical exosome produced by a T lymphocyte. TCR: T-cell receptor; TGFβ: transforming growth factor beta; ICAMs: intercellular adhesion molecule family; CXCR4: C-X-C chemokine receptor type 4 or CD184; Tsg101: tumor susceptibility gene 101; TRAIL: TNF-related apoptosis-inducing ligand; FasL: Fas ligand or CD95L; TfR: transferrin receptor; Mfge8: milk-fat globule-EGF factor 8; ALIX: ALG-2-interacting protein X; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; ERMs: ezrin, radixin and moesin proteins. For more information about exosome composition see http://www.exocarta.org.
The exosome membrane
The exosome membrane is a lipid bilayer in which integral membrane proteins maintain the same orientation as in the donor-cell plasma membrane. The typical composition of a T cell exosome is shown in Figure 1. Exosomes are enriched in sphingomyelin, phosphatidilserine, glycolipid GM3 and phosphatidethanolamines (25). The exosome membrane is characterized by a symmetric distribution of phosphatidylethanolamine and a rapid flipping of lipids between the two leaflets. Exosomes also contain the MVB-specific lyso-lipid LBPA, and the exosomal membrane is also enriched in the GPI-anchored proteins AchE, DAF, MIRL and LFA-3/CD58 and the complement regulators CD55 and CD59 (APC-derived exosome)(26, 27). The cellular prion protein (PRP(C)), another GPI-anchored protein, is also released on exosomes (28). The GPI-anchored NKG2D ligand ULBP3 has been found in exosomes produced by tumor cells (29) (Figure 1).
The exosomal membrane is highly-enriched in tetraspanins. Tetraspanins are small proteins with four transmembrane domains that organize tetraspanin-enriched microdomains through association with themselves and with other membrane receptors or membrane-proximal signaling proteins (30). Exosomes also contain a number of tetraspanin-associated proteins (31), such as integrins, Ig superfamily receptors, growth-factor receptors, MHC, and costimulation molecules. Tetraspanins are also implicated in the recruitment of transmembrane proteins to exosomes (32). Although tetraspanins are usually palmitoylated, palmitoylation is not required for their exosomal localization (33).
EVs contain receptors involved in antigen (Ag) presentation, including class I and II MHC molecules, the co-stimulatory molecules CD83 and CD40, BCR (B-cell-derived exosomes), TCR (T cells), and FceRI (Mast cells). EVs can also contain cytokines and their receptors, including Tumor necrosis factor-alpha (TNFα), TNFαR, Transforming growth factor-beta (TGF-β), IL-1β, IL-18, IL-32 (34-36), and death receptors ligands such as FASL or TRAIL (37, 38). The TfR is exported from cells through the exosome pathway (39). Other immunoregulatory molecules present in EVs include galectins (40-42). Exosomes also contain components involved in signaling via Wnt (Evi, beta-catenin, Wnt3a, Wnt5a) and Ras (CRK, GRB2) (32, 43-46).
Inside exosomes
In their interior exosomes carry cytoskeletal components (tubulin, actin, cofilin, profilin), proteins involved in intracellular vesicle trafficking (annexins I, II, IV, V, and VII, and the small GTPase family members rab7 and rab11), and cytosolic proteins involved in signal transduction (Gi2α, syntenin, 14-3-3, and beta-catenin) and protein translation (elongation factor-1α and elongation initiation factor-4A). Importantly, exosomes also contain several proteins recently-identified as related to apoptosis, either as markers released by apoptotic cells (histones H2A–H4), or pro- or anti-apoptosis factors (AIP1/alix, thioredoxin peroxidase II (TPxII), 14-3-3, and galectins) (47) (Figure 1).
The endosomal origin of exosomes is reflected in the presence of components of the ESCRT complex, such as Alix and Tsg101. Heat-shock proteins (hsp) and chaperones are also very abundant inside exosomes (48). Many proteins in the exosomal interior are polyubiquitinated (49). Metabolic enzymes contained in exosomes include pyruvate kinase, enolase-α, fatty-acid synthase, phospholipase D2 (pLD2) and glyceraldehyde 3 phosphate dehydrogenase (44, 50).
An unexpected finding was that EVs contain DNA and RNA. EVs derived from embryonic stem or tumor cells contain mRNA (51, 52), and exosomal mRNA content changes in response to cell stress (53, 54). EVs also contain microRNAs (55). The mRNAs and miRNAs contained in EVs differ from the miRNA and mRNA repertoire of the parent cell, indicating the existence an as-yet undefined mechanism for sorting specific RNAs into EVs. In addition to sorting proteins, the ESCRTI complex might also mediate the sorting of RNAs into EVs, since it can directly bind RNA (56) and control RNA silencing mechanisms (57, 58). Some autoantigenic ribonucleoproteins (RNP) have been detected in exosomes, such as the nuclear phosphoproteins DEK (59) and BAT3 (60). An updated repetoire of miRNAs and mRNAs found in exosomes is available at Exocarta (http://exocarta.org/index.html).
Function of immune EVs
B-cell and T-cell EVs
EVs derived from T cells have been shown to have a role in immune response regulation. Like other immune cells, T cells release EVs constitutively, but secretion is enhanced by stimuli such as TCR triggering or T-cell activation (61, 62). In fact, EV secretion by T cells is stimulated by signals that increase intracellular calcium (63). CD4 T cells produce three distinct vesicle subsets, of which one increases more than the others upon T-cell activation depending on the level of co-stimulation, suggesting that the T-cell activation status differentially regulates the release of distinct vesicle subpopulations (62). Activated human T cells release bioactive Fas ligand and APO2 ligand in EVs (37), thereby promoting activation-induced cell death (38). Moreover, EVs from OVA-specific CD8+ T cells are taken up by DCs, leading to downregulation of peptide-MHC expression, induction of apoptosis in OVA –loaded DCs, and inhibition of DC(OVA)-stimulated CD8+ cytotoxic T-lymphocyte (CTL) responses in mouse models of cancer and diabetes (64). In addition, EVs from Jurkat T- and Raji B-cell lines constitutively express NKG2D ligands, which are upregulated during thermal and oxidative stress and inhibit NK cytotoxicity (65) (Figure 2). EVs of activated CD8+ T cells activate ERK and NF-κB in melanoma cells, leading to increased MMP9 expression and thus promoting cancer cell invasion in vitro, suggesting a role for T-cell EVs in tumor progression; however they do not affect tumor-cell apoptosis or proliferation (66). EVs formed from the MVBs of CD8+ CTLs also contain granzymes and perforin (67). These MVBs fuse with the CTL plasma membrane during IS formation with targets cells, thus promoting the release of EVs that may have a role in the killing of the target cell (68).
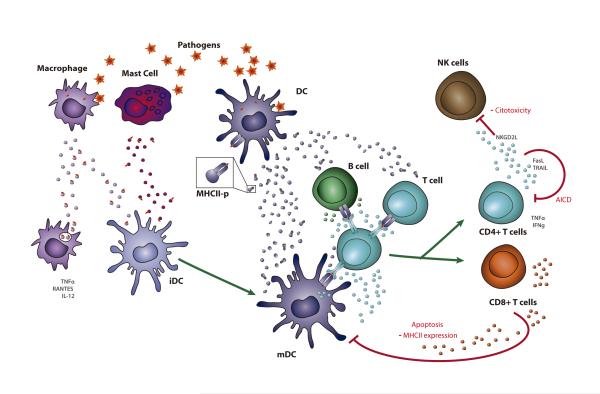
Figure 2. Role of immune-cell-derived EVs during infection.
During infection dendritic cells (DC) produce EVs that carry co-stimulatory molecules, antigens and Ag-MHC-II complexes. These EVs transfer Ag-presentation ability to other DCs and also to B cells and T cells (79, 82, 83), and might directly activate T cells (81, 83). Mast cells and macrophages can also transfer Ag-containing EVs to DCs and induce maturation and presentation of the acquired Ags (102, 152). EVs from macrophages also activate innate immune responses in uninfected macrophages (217). During Ag presentation, TCR and BCR triggering stimulate EV secretion (61, 70), and the formation of a functional immune synapse promotes the functional transfer of EVs (6). Activated T cells produce immune-regulatory EVs that inhibit NK cytotoxicity (65), promote apoptosis in T cells (38) and Ag-carrying DCs (64), and decrease DC antigen-presentation ability (64), thus contributing to homeostasis recovery.
The release of EVs by B cells is stimulated by interaction with Ag-specific CD4 T cells (69), BCR crosslinking (70), and CD40/IL4 signaling(71). B-cell EVs have been reported to induce adaptive immune responses. For example, engagement of antigen-loaded B cells with specific CD4 T cells stimulates release of EVs bearing peptide-MHC-II complexes that directly stimulate primed, but not naïve, CD4+ T cells (69) (Figure 2). Moreover, B-cell EVs loaded with birch allergen Bet v 1 induce proliferation of Bet v 1-specific T cell lines and secretion of IL-5 and IL-13 (72); and EVs from B cells fix and release C3 fragments on EVs, which stimulate T-cell proliferative responses in the presence of suboptimal doses of Ag and recipient APCs (73). Finally, EVs from B cells seem to be important for cell-cell interactions during EBV infection through the transfer of viral proteins (74) and RNA (75) to non-infected B cells and DCs.
Dendritic-cell EVs
DCs also secrete EVs constitutively, but release is reported to be further increased by TLR stimulation (76) and the activation of the purinergic receptor rP2X7 (77). In contrast, other reports show decreased EV release by LPS-matured DCs than by immature DCs (78, 79). EVs secreted by DCs are implicated in the amplification of immune responses in vivo, with EVs from Ag-loaded DCs inducing Ag-specific CD4+ T cell and CD8+ CTL activation (80-82) and Ag-specific humoral responses (81). Whether Ag-loaded EVs can activate T-cell responses directly or only through their capture by recipient DCs is unclear. Some reports show that T-cell activation in vitro by EVs derived from Ag-loaded DCs occurs only in the presence of CD8α-DCs, which can be MHCII-negative but must express CD80 and CD86 (82); in contrast, other reports show that EVs derived from Ag-loaded DC can directly stimulate T-cell proliferation (81, 83) and cytokine secretion (84). EVs from OVA-pulsed DCs can also be taken up by ConA-stimulated bystander CD4+ T cells, which express the acquired exosomal MHCI-OVA complexes and act as APCs able to stimulate OVA-specific CD8+ T-cell proliferation and the generation of memory CD8+ T cells (83). DC-derived EVs are also implicated in alloantigen spreading between host DCs after transplantation (85). These findings suggest that EVs exchange peptides or peptide-MHCII complexes between cells, thus increasing the number of cells presenting an antigen (Figure 2).
A role of DC-derived EVs in the generation and amplification of immune responses has been shown in several contexts (Figure 2). Host immature DCs (iDC) can internalize and process blood-borne allogeneic EVs, load the EV-derived allo-peptide into MHCII for presentation, and activate CD4+T cells, demonstrating a role for EVs in promoting T-cell responses to allogeneic antigens (86). DC-derived EVs can also carry antigens or cross-reactive antigens from different pathogens and induce immune responses against them, thus protecting hosts from infection (78, 87, 88) (Figure 2). These findings suggest that DC-derived EVs have potential in the design of vaccines against different pathogens. The maturation state of DCs influences the ability of DC-derived EVs to stimulate immune responses, with EVs derived from mature DCs (mDC) carrying more MHCII, ICAM-1 and co-stimulatory molecules than iDC-derived EVs and being more potent T-cell stimulators (78, 79). Moreover, EVs from mDCs but not iDCs can transfer the ability to activate naïve T cells to B cells (79). DC maturation state also conditions the cell miRNA profile of DC-derived EVs (89).
DC-derived EVs are implicated in the induction of immune responses to tumors. For example, mDCs pulsed with OVA-EVs stimulate proliferation and differentiation of CD8+ T cells into CTLs, consequently reducing metastatic colonies and protecting mice from growth of established OVA-expressing tumors (90). DC-derived EVs have also been linked to anti-metastatic effects through the promotion of the proliferation and activation of NK cells (91), suggesting their possible use for tumor vaccination. Indeed, EVs from OVA-pulsed DCs are more effective inducers of antitumor immunity than EVs from OVA-expressing tumor cells (92). Also, when combined with CpG adjuvants, DC-derived EVs alone can trigger anti-tumor CD8+ T-cell responses (93). DC-derived EVs can also activate the immune response at other levels. For example, EVs from LPS-treated DCs are internalized by epithelial cells and induce secretion of pro-inflammatory cytokines, revealing a role for DC-derived EVs also in innate immunity (36). DC-derived EVs have also been shown to induce NF-KB activation in microglia cells (94).
Depending on the context or the activation state of the donor DC, it has also been proposed that EVs from DC can also induce tolerance rather than immunogenicity. For example, EVs from iDCs inhibit alloreactive T-cell responses, thus prolonging allograft survival (95, 96), and prevent cytokine production by NK cells (60). The tolerogenic properties of iDCs have led to their proposed use as immunosuppressor agents. Indeed, when combined with LF 15-0195 – an immunosuppressive agent that blocks DC maturation – DC-derived EVs induced a donor-specific allograft tolerance characterized by strong inhibition of the antidonor proliferative response (97). In addition, when modified by transfection with IDO (98) or FasL (99) or by treatment with IL-10 (100), DC-derived EVs suppress immune responses in models of collagen-induced arthritis and delayed-type hypersensitivity.
EVs from other cells of the innate immune system
Communication by the release of EVs has also been described for other immune cell types. Mast cells produce EVs that contain RNA, which can be transferred to other mast cells and translated into proteins in recipient cells (55). EV transfer between mast cells also confers protection against oxidative stress (53), and mast-cell-derived EVs can participate in adaptive immune responses by activating splenocyte proliferation and cytokine secretion (101). Moreover, mast-cell EVs contain endocytosed antigens (Ag) and hsps, and these EVs can induce iDC maturation and acquisition of the capacity to present antigen to Ag-specific T cells (102) (Figure 2). EVs from neutrophils can alter the antigen presentation capacity of DCs by reducing their phagocytic activity, increasing their release of TGF-β1 and inhibiting LPS-induced maturation and reducing their capacity to induce T-cell proliferation (103).
Macrophage-derived EVs might play a role in atherosclerosis, since oxLDL-immune complexes induce the formation and release of EVs containing hsp70 and IL-1β by an A-SMase dependent mechanism (104). EVs derived from monocytes and the plasma of atherosclerosis patients contain and deliver miR-150 to endothelial cells, thus downregulating c-myb and enhancing endothelial-cell migration, a central process in atherosclerosis disease (105).
Endothelial EVs
Endothelial cells play a major role in immune responses and are central to the development of inflammatory diseases (106). EVs derived from endothelial cells have been shown to play a role in atherosclerosis. For example, endothelial-derived apoptotic bodies, which are generated during atherosclerosis, trigger the production of the chemokine CXCL12 in recipient vascular smooth muscle cells (VSMC). The CXCL12 production is mediated by transfer of miR-126, which represses the function of regulator of G-protein signaling 16 (RGS16). Administration of miR-126-containing apoptotic bodies limits atherosclerosis, promotes the incorporation of Sca-1+ progenitor cells, and induces plaque stability features in mouse models of atherosclerosis (107). Also, EVs derived from KLF2-transduced or high-shear-stress-stimulated HUVECs (protected from atherosclerosis formation) contain miR-143/145, which controls target-gene expression in co-cultured SMCs and reduces atherosclerotic lesion formation in the aortas of ApoE(−/−) mice. These results suggest that atheroprotective stimuli induce communication between endothelial cells and VSMCs through a miRNA- and EV-mediated mechanism (108).
EVs derived from endothelial cells have also been implicated in angiogenesis and thrombosis (Recently reviewed in (109)). Endothelial EVs transfer the Dll4 protein to other endothelial cells, resulting in loss of the receptor Notch, inhibition of Notch signaling and enhanced vessel formation (110). EVs derived from endothelial progenitor cells have also been shown to be incorporated into non-descendent endothelial cells and promote endothelial-cell survival, proliferation, and organization into capillary-like structures (111), presumably by the transfer of RNA.
Tumor EVs and the regulation of the immune response
Tumor cells constitutively produce EVs without any need for stimulus, although EV release is increased by stress conditions like heat shock or anticancer drugs (112, 113). Tumor-derived EVs suppress anti-tumor immune responses at many levels, thus promoting tumor progression. Several reports indicate that tumor-derived EVs can inhibit T-cell activation and proliferation and promote their apoptosis (114-117). Activated CD8+ and CD4+ T lymphocytes are suggested to be differentially modulated by tumor-derived EVs based on the observation that tumor EVs inhibit signaling, proliferation and induced apoptosis by activated CD8+ T cells but not CD4+ T cells (118). The effects of tumor-derived EVs on T cells seem to be dependent on EV expression of FasL, TRAIL, membrane-bound TGFβ, CD73, or galectins (34, 41, 116, 117, 119). Furthermore, the suppressor activity of tumor EVs seems to involve induction of the generation, expansion and suppressive function of human regulatory T cells (118, 120). Apoptotic bodies derived from tumor cells are suggested to be the EVs involved in the induction of T-cell regulatory responses (121) (Figure 3).
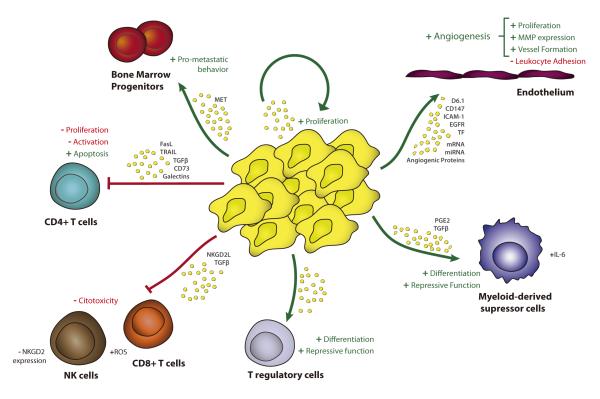
Figure 3. Tumor-derived EVs promote tumor growth via multiple routes.
Tumor-derived EVs suppress anti-tumor immune responses by inhibiting T-cell activation and proliferation and stimulating their apoptosis (34, 41, 114-117, 119). EVs produced by tumor cells also induce regulatory T cells and MDSCs (34, 118, 120, 121, 130) and inhibit the cytotoxicity of NK and CD8+ T cells (34, 122-125). Tumor-derived EVs are taken up by endothelial cells promoting angiogenesis and tumor invasion; the expression of CD147, D6.1A, tissue factor and EGFR in EVs, the transfer of pro-angiogenic components and the induction of MMPs play a role in the pro-angiogenic effects of tumor EVs (52, 132, 134-137). Tumor EVs also contribute to tumor growth by stimulating tumor proliferation and inducing metastatic behavior in bone-marrow progenitors (52, 138, 139).
Tumor-derived EVs can also suppress immune-cell responses by inhibiting the cytotoxic actions of CD8+ T cells and NK cells (34, 122-125). This inhibition is mediated mainly by NKG2D downregulation through a mechanism partly dependent on the expression of NKGD2 ligands (122-124) and TGFβ (123, 125) on the tumor EV surface. The inhibition of cytotoxic T-cell function has also been suggested to be induced in part by the increased T-cell ROS content mediated by melanoma-derived EVs (126) (Figure 3).
Tumor EVs can also suppress immune responses by modifying myeloid precursors toward a more tolerogenic phenotype (Figure 3). For example, EVs derived from mammary tumors inhibit the differentiation of bone marrow precursors into DCs through a mechanism dependent on IL-6 secretion (127). Similarly, CD14+ monocytes incubated with tumor-derived EVs turn into HLA-DR(−/low) cells, which fail to up-regulate co-stimulatory molecules and show TGFβ-dependent suppression of activated T-cell proliferation and cytolytic functions (128). Moreover, tumor EVs induce the differentiation of myeloid cells into myeloid-derived suppressor cells (MDSCs), which promote tumor growth in vivo. EV-mediated induction of MDSCs appears to depend on the tumor environment, since EVs derived from cultured tumor cells express less PGE2 and TGFβ, and are therefore less potent MDSC inducers (129). Tumor EVs are also proposed to activate the suppressor function of MDSCs by inducing autocrine IL-6 production and activating stat-3 (130). Tumor-derived EVs are also suggested to suppress immune-cell responses by releasing EVs bearing membrane-associated ICAM-1, which inhibit leukocyte adhesion to TNFα-activated endothelial cells (131).
Tumor EVs also contribute to tumor progression by inducing angiogenesis, tumor invasion and metastasis (52, 132-137). Tumor-derived EVs contain pro-angiogenic miRNAs, mRNAs and proteins, which can be taken up by endothelial cells and stimulate angiogenesis and the formation of pre-metastatic niches in vivo (52, 135). Highly-metastatic tumor-derived EVs induce a pro-metastatic behavior in bone-marrow progenitors, thereby increasing primary-tumor metastasis, in a process partially dependent on tyrosine-kinase receptor MET expression (138). Several reports suggest that tumor-derived EVs contribute to tumor growth through a direct effect on tumor-cell proliferation (52, 139) (Figure 3). Another way in which tumor-derived EVs can contribute to tumor progression is by sequestering tumor-reactive antibodies and decreasing their binding to tumor cells, thus inhibiting antibody-dependent anti-tumor cytotoxicity (140) and reducing the effectiveness of antibody-based anti-cancer drugs (141).
Despite their capacity to promote tumor progression by inhibiting T-cell- and NK-mediated immune responses, tumor EVs also activate Ag-specific allogeneic and syngeneic anti-tumor immune responses by transferring tumor antigens to APCs (142, 143). The immunogenicity of tumor-derived EVs seems to be related to the state of the producer tumor cell. Thus EVs from heat-stressed tumor cells contain more hsps and induce stronger anti-tumor immunity than EVs from control tumor cells (112, 144-146).
In summary, tumor-derived EVs promote tumor growth by inhibiting anti-tumor immune responses and stimulating tumor proliferation and metastasis. However, under stress conditions tumor cells produce EVs that, in addition to tumor antigens, contain more hsps and stimulate anti-tumor immune responses, making them good candidates for anti-tumor therapies. Moreover, tumor-derived EVs seem to be excellent candidate tumor biomarkers, since they contain tumor antigens as well as cell-origin-specific markers, and are ubiquitous in easily-accessible biofluids.
EVs and pathogens
During infection, EVs from several cell types modify anti-pathogen immune responses, thus favoring immune evasion. For example, Trypanosome cruzi infection promotes secretion of EVs by monocytes, which inhibit complement-mediated lysis of T.cruzi and promote parasite invasion and survival (147). EVs derived from Mycobacterium-infected tumor cells induce IL-10 production by splenic B cells and inhibit T-cell proliferation (148). EVs released by virus-infected cells can also modify immune responses. For example, EVs from rotavirus-infected intestinal epithelial cells inhibit CD4+ T-cell proliferation and promote cell-death through a TGFβ-dependent mechanism (149); and HIV-infected lymphocytes secrete Nef-containing EVs that trigger apoptosis in T cells (150). However, in some cases the release of EVs by infected cells induces effective immune responses against the specific pathogen and contributes to the eradication of the infection. For example, EVs from Mycobacterium-infected macrophages contain PAMPS and stimulate TLR/MyD88 dependent pro-inflammatory responses in uninfected macrophages (151) and also induce DC maturation and Ag-specific T-cell responses (152) (Figure 2). In addition, EVs from reticulocytes infected with Plasmodium yoelii increase parasite clearance (153). Sometimes microorganisms themselves secrete EVS that enhance immune responses against the pathogens (154, 155).
Other immune-related-scenarios
Other non-immune cells secrete EVs that affect immune responses against diverse antigens, thus promoting immunogenic or tolerogenic effects. EVs from intestinal epithelial cells carrying OVA and αvβ6 induce the secretion of active TGFβ by DCs, the generation of FOXP3+ regulatory T cells, and the inhibition of Ag-specific Th2 responses against food Ag, revealing a role for intestinal epithelial EVs in oral tolerance (156). EVs from serum of OVA-fed mice protect against allergic sensitization with OVA in a mouse model of allergic asthma by decreasing the number of eosinophils and the level of OVA-specific IgE and inducing regulatory T cells (157). Also, EVs from bronchoalveolar fluid of ole-e1-tolerized mice inhibit allergen-specific responses in ole-challenged mice (158). The tolerogenic properties of EVs have also been shown in the context of pregnancy. Placental EVs carry NKG2D ligands on their surface and induce downregulation of the NKG2D receptor on NK, CD8+ and γδ T cells, reducing their cytotoxicity (159). In addition, EVs from serum of pregnant women delivering at term express higher levels of FasL than EVs from pregnant women delivering preterm, and inhibit IL-2 production by CD4+ T cells (160), revealing a possible role for FasL+ EVs in the induction of tolerance to the fetus.
EVs from intestinal epithelial cells may have an immunogenic role by activating Ag-specific T-cell responses through the transfer of Ag to DCs (161). Similarly, oral adjuvants are proposed to be transported across the intestinal epithelium through the secretion of EVs by epithelial cells, resulting in the activation of naïve macrophages (162). EVs might also aggravate immune responses to autoantigens in autoimmune diseases. For example, EVs from an insulinoma cell line express candidate diabetes autoantigens and induce APC expression of co-stimulatory molecules, splenocyte proliferation, and pro-inflammatory cytokine secretion; moreover, injection of these EVs into diabetes-resistant mice accelerates insulinitis development (163). Also, EVs from oligodendrocytes express multiple-sclerosis-related autoantigens that are preferentially taken up and subsequently degraded by MHCII-negative microglia cells; however, these cells do not appear to be activated or to provoke an immune response (164).
TRANSFER OF EVs DURING IMMUNE SYNAPSIS
As mentioned, T lymphocytes and DCs can acquire exosomes from the extracellular milieu (79, 85, 165, 166). Uptake of isolated exosomes seems to be influenced by activation of donor T cells (167), the maturation state of recipient DCs (165), and the presence of specific proteins like LFA-1 or ICAM-1 on exosomal or cellular membranes (64, 79, 167). TCR specificity was suggested not to be required for exosomal uptake (167); however, others found that TCR specificity and the presence of antigen enhance their acquisition (6, 64).
Despite these discrepancies about the role of the TCR and antigen specificity in exosome uptake from the extracellular milieu, it is clear that antigen specificity, through the formation of the IS, provides ample opportunity for cell-cell communication. Intercellular contacts like the IS and other immune-cell interactions are so intimate that they often involve membrane fusion and the formation of several membrane structures. These processes facilitate the exchange of molecules not only from cell surfaces but also from the cytoplasm of the cells involved. Moreover, the exchange of exosomes through the IS has been described to promote the uptake of membrane and intraluminal cargo by recipient cells (6). IS-dependent transfer is supported by the finding that exosomal proteins are not transferred when the IS is disrupted or in the absence of antigen. However, the exact mechanism of IS-driven exosome transfer is still unresolved. At least two possibilities can be considered: i) transfer may occur through the IS itself; ii) exosomes could be released to the surrounding medium and acquired by the recipient cell, the IS thus making uptake more efficient than in the absence of cell-cell contact. Both possible routes have been described for IS-dependent transfer of cytokines and lytic factors (168). While cytokines such as TNFα and the chemokine CCL3 are released multidirectionally, IL2 and IFNγ are secreted within the IS between CD4+ T cell and APC (169). Directional secretion also occurs with lytic granules and factors released by CD8+ T cells during cytotoxic synapse with a target cell. In this case, directional and confined communication is especially important to safeguard surrounding cells from the cytotoxic action (170).
Polarized secretion is guided by the reorientation of the microtubule-organizing center (MTOC) toward the IS, which is accompanied either by the Golgi apparatus in cytokine-releasing helper T cells or by lytic granules in cytotoxic T cells (171). Preliminary evidence suggests that the mechanism of MTOC reorientation and MVB traffic for polarized secretion at the IS involves local production of second messenger lipids such as diacylglcyerol and its negative regulator, diacylgycerol kinase α (172). Further work is needed to better define the mechanism of directed exosomal release and uptake. Nevertheless, plasma-membrane fusion of MVBs at the IS zone is supported by the observed specific translocation of T-cell MVBs toward a functional IS and the selective uptake of exosomes only by APCs expressing MHCII and therefore able to form this functional IS (6). The MVBs of APCs, whether DCs or B cells, do not translocate to the IS, indicating that although APCs might release exosomes during cognate interaction, polarized release as occurs in T cells is not feasible (6). Intraflagelar transport proteins (IFT), specifically IFT20, have been described to be recruited to the IS on the T-cell side, and might regulate the polarized delivery of endosomal recycling vesicles containing the TCR (173).
In the IS between a CD4+ T cell and an APC, which occurs in the context of a cell-dense scenario such as a lymph node, directionality may ensure exclusive delivery of the exosomal signal to the specific target cell, without affecting surrounding bystander cells. Safeguarding bystander cells is even more of an imperative in the case of contacts between CTLs and target cells. Similarities between lytic-granule release, exosome release at the IS, and neurotransmitter secretion at neuronal synapses are becoming increasingly evident (168). However, the release of EVs other than exosomes is not influenced by the polarization of MVBs, and their release would therefore be undirected, not polarized, and may occur bidirectionally between the two participant cells.
In order for the exosomal cargo (both protein and genetic material) to be functional after delivery, it must avoid degradation in the recipient cell. Using fluorescently labeled exosomes, several studies have demonstrated internalization of exosomes by DCs and other phagocytes (85, 89, 165, 174). Although internalized exosomes colocalize with late endosomes/lysosomes (86, 174), allopeptides of exosomal origin are processed and later presented on the DC surface, demonstrating that exosomal cargo is not completely degraded (86). Moreover, tumor-derived exosomes have been shown to fuse directly with melanoma cells at low pH (175), although in other cell lines internalization occurs via the endocytic pathway, with proteins directed to lysosomes while lipids appear to be recycled (176). In a recent study, luciferin-loaded exosomes induced luciferase activity in luciferase-transfected DCs, indicating release of exosomal content into the cytosol (89). Further clarification of this issue awaits conclusive direct live-cell imaging of exosome fusion.
It has been reported that only phagocytic cells can internalize exosomes. (174). Given that exosomal content depends on the cell of origin, it is feasible that exosome-cell interaction and the function of this communication are determined by the cellular context of both the recipient and the donor cell.
Exosomes have a similar protein and lipid content to retroviruses, and both use the same biogenesis pathway and cell-to-cell transmission mechanisms (165, 177). The finding that HIV can be delivered through the virological synapse between infected and uninfected T cells and successfully infect the recipient cell (178) thus strengthens the case for exosomal delivery of functional cargo via the IS. In this regard, the use of 3′UTR-luciferase vectors to sense miRNA activity demonstrates that exosomally-transferred miRNA is functional only in IS-mediated cell-cell contacts: isolated exosomes added directly to recipient cells do not transfer functional miRNA (6). However, other reports have detected the transfer of functional mRNA and viral miRNA by isolated exosomes (55, 75). We propose that, whatever the precise delivery route, the IS acts as a highly-efficient platform that enhances functional, antigen-specific delivery of exosomal cargo to the cytosol of the recipient APC. It is therefore conceivable that in other systems (for example with a more phagocytic target) free exosomes might be able to deliver functional cargo. The elucidation of the mechanism by which exosomes are taken up by the recipient cell will help to explain these differences.
Evidence for exosome-mediated miRNA transfer is provided by the finding that miRNA transfer is inhibited when exosome release is impaired (6). Transfer of synthetic miRNA and viral miRNAs between T and B lymphocytes has been reported, although it is not clear if this exchange is mediated by exosomes or other mechanisms (179). There are thus numerous mechanisms for molecular exchange during immune cell-cell contacts. The following sections describe other possible mechanisms for protein exchange during IS formation: trogocytosis, trans-endocytosis, nanotubes and gap junctions (Figure 4).
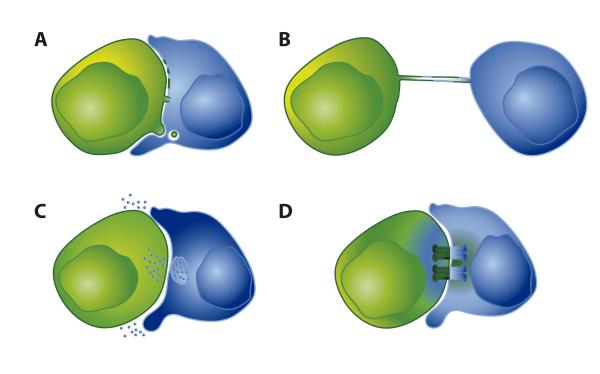
Figure 4. Mechanisms of protein and genetic-material exchange during immune-cell contacts.
A: Trogocytosis consists of the rapid transfer of membrane patches, allowing exchange of intact membrane proteins (180). Transfer of intact membrane proteins between cells is also mediated by trans-endocytosis of membranous material (192). Membrane bridges can be formed between cells, connecting their cytoplasms (170). B: Nanotubes are tubular membranous connections between cells that are either limited to the exchange of membranes between cells or also allow communication between the two cytoplasms (218). C: EVs are exchanged during IS formation. It is possible that MVB-derived exosomes are delivered directionally at the IS, while other EVs are delivered adirectionally into the extracellular space near the recipient cell. D: Gap junctions are composed of connexin proteins that form a channel directly connecting the cytoplasms of two cells, allowing the transfer of material. It is also possible that gap junctions formed at the IS discharge material into the intercellular space between the two cells.
OTHER MECHANISMS FOR TRANSFER OF MOLECULES DURING IS
Trogocytosis
The term trogocytosis, from the Greek trogo, meaning gnaw or nibble, refers to the process of intercellular exchange of intact membrane patches that occurs during IS between lymphocytes and APCs (180). Trogocytosis is very rapid compared with other membrane-transfer mechanisms and allows the transfer of specific and intact proteins (3). One of the best-known examples of trogocytosis is transfer of peptide-MHC complexes from APCs to T cells (181-183), and other studies have demonstrated a similar ‘nibbling’ of other cell-surface molecules by B and NK cells (3). Trogocytosis of MHC-peptide proteins by the T cell is antigen dependent. More recently, bidirectional trogocytosis has been described, in which mouse APCs acquire the TCR and the counterpart T cell acquires peptide-bound MHC (184). Although the mechanism of membrane patch transfer is unclear (3), advances in the study of the structure of the CD4 T-cell–APC IS are helping to shed light on it. Using electron microscopy and 3D tomography, T cells were recently shown to form invasive pseudopodia that penetrate the APC (185). This could provide the tension that, together with the high avidity of receptor interactions, would allow the tearing of membrane patches. Moreover, another recent study identified the small Rho GTPases TC21 and RhoG as mediators of trogocytosis at the IS and of TCR internalization (186). Exchange of membrane proteins has also been described during IS formation between CTLs and target cells, and membrane fusion between these cells leads to the formation of a membrane bridge (170). B-cell trogocytosis differs slightly from other systems, in that it does not depend on signaling or actin and is not inhibited at 4°C (187, 188). Moreover, trogocytosis is a constitutive process in most cell types, whereas uptake of MHC-peptide complexes by T cells requires Ag recognition (189)
It should also be noted that although some studies suggest that trogocytosis is much more rapid than other protein-transfer mechanisms (190), given the scarcity of the molecular details about this phenomenon it remains possible that the protein transfer in some of these studies is mediated by exosomes.
Trans-endocytosis
Trans-endocytosis is a protein-transfer mechanism described in several contexts, including immune-system cells (191-193). Trans-endocytosis refers to the internalization by one cell of specifically-bound receptor proteins on the surface of another cell. This occurs with the CTLA-4 ligands CD80 and CD86, which can be ‘captured’ from the surface of one cell by a separate cell expressing CTLA-4 (192). A possible effect of this ‘molecular kidnap’ might be to deprive the interacting cell of the capacity to activate other cells, since the negative regulator molecule CTLA-4 shares these ligands with the costimulatory molecule CD28. Bidirectional trans-endocytosis has been described for the molecule pair CD47-SHPS-1 (191). This mechanism seems to act as a mechanism to curtail signaling by a specific molecule exposed on the membrane of the interacting cell.
Membrane nanotubes and tunneling nanotubes
Tubular structures containing membrane and filamentous (F) actin have been reported to readily form connections between a variety of cell types. Since their first description as connectors between neurons (194), a wide variety of nanotubular structures have been described in non-immune and immune contexts. Some studies suggest that nanotubes are formed de novo by membrane protrusions that extend from one cell to another, in a process driven by the actin cytoskeleton (194) Other reports, however, claim that in the IS membrane nanotubes might be created upon disassembly of the contact, as the cells move apart (195, 196). Exchange of membrane proteins and lipids has been demonstrated between NK cells and their target cells (195-197). Glycosylphosphatidylinositol-(GPI)-anchored and palmitoylated fluorescent proteins have both been observed to move along nanotubes, indicating that proteins anchored to the inner or outer plasma-membrane leaflet can be transferred (194, 196, 198). Bulges along the intercellular nanotubes observed in different studies (194, 196, 199) suggest the traffic of cargo, not only membrane proteins, between immune cells. Vesicles can be transferred through nanotubes in different contexts such as between neurons (194) or between macrophages (199). Bacteria travel between macrophages through ‘thin’ nanotubes containing F-actin, while mitochondria travel through ‘thick’ nanotubes containing both F-actin and microtubules (199). HIV-1 can also be transported between T cells through nanotubes (198); however, the T-cell nanotubes examined in this study were not open-ended. Evidence for communication through open-ended nanotubes (called tunneling nanotubes or TNTs) was found between macrophages and DCs, where the propagation of calcium flux was detected through nanotubes, presumably inducing functional responses in recipient cells (200). Nanotubes have also been detected in vivo between DCs (201). A link between nanotubes and exosomes has recently been described in cultured endothelial cells, suggesting a possible association of these mechanisms of intercellular communication (202). Transfer of genetic material through TNTs has not been described, although the detection of calcium flux along them suggests that such transfer is feasible.
The literature contains descriptions of several kinds of intercellular tubular structures. Although the structural diversity of nanotubes suggests diverse functionality, transfer of proteins and vesicles along these tubes has been described between immune cells, confirming that membrane nanotubes provide a possible mechanism for intercellular communication when they are formed after the IS.
Gap junctions
Gap-junction-mediated intercellular communication (GJIC) is a widespread homeostatic mechanism that takes place not only in compact tissues and organs but also in the immune system, in lymphoid organs and between peripheral lymphocytes (203). Gap junctions (GJ) are built by the oligomerization of connexin (Cx) proteins in hexameric hemichannels which, upon docking with a partner on a neighbouring cell, form the gap junction channel. Transport via gap junctions was historically thought to be restricted to molecules and ions below ~1.2 kDa, but recently the passage of siRNAs and synthetic morpholino oligonucleotides of approximately 2-4 kDa was reported (204).
The possible role of GJIC in intercellular communication in the immune system was first pointed out by Oviedo-Orta and colleages. They described how cocultures of B and T lymphocytes treated with Cx inhibitors are unable to secrete antibodies and show impaired intercellular signaling (205). More recent work has described the recruitment of Cx43 to the IS between T cells and DCs and showed the importance of functional gap junctions for T-cell activation (206, 207). GJs and stand-alone hemichannels composed of Cx43, the main GJ protein in the immune system, accumulate at the IS in an antigen-dependent manner. Moreover, redistribution of Cx43 to the IS is dependent on the actin cytoskeleton but not on microtubules, suggesting that Cx43 is relocated to the IS exclusively from the pre-existing pool at the plasma membrane (207). Bidirectional transfer of GJ-permeable dye was described not only during IS formation (207) but also during other immune cell contacts such as homotypic DC-DC interactions (208, 209) or contacts between follicular DCs and B-cells (210), as well as unidirectionally from DCs to T cells (206). GJs transfer ions and small signaling molecules (211), but GJIC of small peptides and antigens has also been reported between the cytoplasm of Cx43-transfected carcinoma cell lines and monocytes (212) and between DCs (209), contributing to the subsequent phenomenon of cross-presentation. However, these studies do not rule out the possible involvement of other mechanisms of protein transfer.
Transfer of genetic material through GJs is physically possible. The transfer of small fluorescent oligonucleotides between Hela cells is suggested to occur through GJs formed by Cx43, but not through GJs composed of other connexins expressed in other cell types (204). GJIC-mediated transfer of miRNA has been reported between cardiac cells (213, 214), glioma cells (215), and bone-marrow cells and breast-cancer cells (216). Although miRNA transfer via GJs has not yet been reported between immune cells or during IS formation, its occurance is feasible given previous observations of GJIC at the IS and the fact that Cx43, the main T-cell connexin, is the connexin reported to be permeable to small nucleotides (204). Moreover, connexin hemichannels that relocate to the IS not only form a GJ by docking with a second hemichannel on the APC, but can also form channels (207) that potentially enable release of material to the intercellular space formed between the two cells at the IS.
CONCLUDING REMARKS AND PERSPECTIVES
EVs have been extensively described as devices for intercellular communication in diverse physiological and pathological contexts. The number of molecules reported to be shuttled in EVs has grown in recent years, and now ranges from membrane proteins to small RNAs. Due to the presence of specific biomolecules in EVs that can reflect a particular pathological situation, they have been proposed as potential biomarkers.
How EVs and their content are incorporated into a recipient cell remains unclear, but the mechanism and the subsequent fate of the cargo seem to depend on the cell type and context. We propose that the immune synapse and other types of connecting structures between immune cells enhance the specific transfer of EVs to ensure functional delivery of their cargo, specifically genetic material in the form of microRNA. However, there several issues require further investigation. The packaging of specific RNA repertoires into EVs that differ from that of the parent cell indicates that specific mechanisms must exist for sorting RNA molecules to EVs. Moreover, different activation states alter the repertoire of RNAs packaged into the EVs, so it is possible that different types of immune cells, for example differentiated T-helper lymphocyte subsets (Th1, Th2, Th17 and Treg), might secrete different EV-shuttled RNAs.
The physiological relevance of protein exchange during IS formation is still unclear, although its occurrence has been widely demonstrated. Some authors claim that trogocytosis-mediated uptake of MHC-peptide complexes by T cells might aid the selection of high-affinity T cells, or in contrast induce ‘fratricidal’ killing by CTLs. A broader function of this kind of exchange, involving transfer of other molecules, might enable cells to acquire molecules (proteins, lipids and RNAs) that they do not synthesize themselves. It is very likely that these processes will affect signal transduction, allowing intercellular communication to modify the physiology of the recipient cell. The mechanism of the transfer of microRNA and other genetic material at the IS still needs to be addressed, as does the impact of this transfer on the function of the recipient cell.
This knowledge will give us a better understanding of how the IS works and of what are the consequences of this mode of communication. Detailed understanding of how EVs are exchanged between immune cells would permit therapeutic exploitation of these vesicles, which are promising vehicles for gene therapy in diseases of the immune system and in other scenarios.
AKNOWLEDGEMENTS
We thank M. Yañez-Mo, M. Izquierdo, M. Valés-Gómez, M. Martínez-Picado, M. Vicente-Manzanares and L. Fernandez-Messina for critical reading of the mansucript. We are grateful to D. Mateos San Martín for help with artwork. C.G.-V.is supported by the Comunidad de Madrid, C.V.-B. by the Spanish Ministry of Education, M.M. by the Instituto de Salud Carlos III and F.S-.M. by grants SAF2011-25834 and ERC-2011-AdG 294340-GENTRIS. S. Bartlett provided English editorial support. The Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Spanish Ministry of the Economy and the Pro-CNIC Foundation.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 3.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 4.Dustin ML. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 5.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 6.Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Kuo L, Freed EO. ARRDC1 as a mediator of microvesicle budding. Proc Natl Acad Sci U S A. 2012;109:4025–4026. doi: 10.1073/pnas.1201441109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109:4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huotari J, Helenius A. Endosome maturation. Embo J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 13.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 14.Alonso R, et al. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011;18:1161–1173. doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso R, Rodriguez MC, Pindado J, Merino E, Merida I, Izquierdo M. Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J Biol Chem. 2005;280:28439–28450. doi: 10.1074/jbc.M501112200. [DOI] [PubMed] [Google Scholar]
- 16.Laulagnier K, et al. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572:11–14. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 17.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 18.Baietti MF, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012 doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 19.Hsu C, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11-13. [DOI] [PubMed] [Google Scholar]
- 21.Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 22.Vidal MJ, Stahl PD. The small GTP-binding proteins Rab4 and ARF are associated with released exosomes during reticulocyte maturation. Eur J Cell Biol. 1993;60:261–267. [PubMed] [Google Scholar]
- 23.van Niel G, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 24.Bobrie A, Colombo M, Krumerich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisims are present in exosome preparations obtained by differential ultracentrifugation. Journal of Extracellular Vesicles. 2012;1:18397. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laulagnier K, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33:522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 27.Rabesandratana H, Toutant JP, Reggio H, Vidal M. Decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59) are released within exosomes during In vitro maturation of reticulocytes. Blood. 1998;91:2573–2580. [PubMed] [Google Scholar]
- 28.Fevrier B, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Messina L, et al. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J Biol Chem. 2010;285:8543–8551. doi: 10.1074/jbc.M109.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 32.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abache T, Le Naour F, Planchon S, Harper F, Boucheix C, Rubinstein E. The transferrin receptor and the tetraspanin web molecules CD9, CD81, and CD9P-1 are differentially sorted into exosomes after TPA treatment of K562 cells. J Cell Biochem. 2007;102:650–664. doi: 10.1002/jcb.21318. [DOI] [PubMed] [Google Scholar]
- 34.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa H, Thomas HJ, Schooley K, Born TL. Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein. Cytokine. 2011;53:74–83. doi: 10.1016/j.cyto.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Pathol. 2009;175:696–705. doi: 10.2353/ajpath.2009.080716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Lorenzo MJ, et al. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163:1274–1281. [PubMed] [Google Scholar]
- 38.Monleon I, et al. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–6744. doi: 10.4049/jimmunol.167.12.6736. [DOI] [PubMed] [Google Scholar]
- 39.Mathew A, Bell A, Johnstone RM. Hsp-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem J. 1995;308(Pt 3):823–830. doi: 10.1042/bj3080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barres C, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 41.Klibi J, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 42.Thery C, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 43.Koles K, et al. Mechanism of evenness interrupted (evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subra C, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51:2105–2120. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren Y, et al. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol. 2011;51:280–287. doi: 10.1016/j.yjmcc.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tauro BJ, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 48.Thery C, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35:398–403. doi: 10.1016/j.bcmd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Soo CY, et al. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136:192–197. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baj-Krzyworzeka M, et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eldh M, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PloS one. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Jong OG, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. Journal of Extracellular Vesicles. 2012;1:18396. doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 56.Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–558. doi: 10.1038/nature05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 58.Lee YS, et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009;11:1150–1156. doi: 10.1038/ncb1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mor-Vaknin N, et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol. 2006;26:9484–9496. doi: 10.1128/MCB.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simhadri VR, et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PloS one. 2008;3:e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanchard N, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 62.van der Vlist EJ, Arkesteijn GJA, van der Lest CHA, Stoorvogel W, Nolte-’t Hoen EN, Wauben MH. CD4+ T cell activation promotes the differential release of distinct populations of nanosized vesicles. Journal of Extracellular Vesicles. 2012;1:18364. doi: 10.3402/jev.v1i0.18364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 64.Xie Y, et al. Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to inhibition of CD8+ CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity. J Immunol. 2010;185:5268–5278. doi: 10.4049/jimmunol.1000386. [DOI] [PubMed] [Google Scholar]
- 65.Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PloS one. 2011;6:e16899. doi: 10.1371/journal.pone.0016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai Z, et al. Activated T Cell Exosomes Promote Tumor Invasion via Fas Signaling Pathway. J Immunol. 2012 doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 67.Peters PJ, et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peters PJ, et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol. 1989;19:1469–1475. doi: 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- 69.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. Embo J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rialland P, Lankar D, Raposo G, Bonnerot C, Hubert P. BCR-bound antigen is targeted to exosomes in human follicular lymphoma B-cells. Biol Cell. 2006;98:491–501. doi: 10.1042/BC20060027. [DOI] [PubMed] [Google Scholar]
- 71.McLellan AD. Exosome release by primary B cells. Crit Rev Immunol. 2009;29:203–217. doi: 10.1615/critrevimmunol.v29.i3.20. [DOI] [PubMed] [Google Scholar]
- 72.Admyre C, et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 73.Papp K, et al. B lymphocytes and macrophages release cell membrane deposited C3-fragments on exosomes with T cell response-enhancing capacity. Mol Immunol. 2008;45:2343–2351. doi: 10.1016/j.molimm.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 74.Vallhov H, et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J Immunol. 2011;186:73–82. doi: 10.4049/jimmunol.1001145. [DOI] [PubMed] [Google Scholar]
- 75.Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Obregon C, Rothen-Rutishauser B, Gitahi SK, Gehr P, Nicod LP. Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006;169:2127–2136. doi: 10.2353/ajpath.2006.060453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qu Y, Dubyak GR. P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 2009;5:163–173. doi: 10.1007/s11302-009-9132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J Immunol. 2006;177:3757–3762. doi: 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- 79.Segura E, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 80.Andre F, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 81.Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113:2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 82.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 83.Hao S, Yuan J, Xiang J. Nonspecific CD4(+) T cells with uptake of antigen-specific dendritic cell-released exosomes stimulate antigen-specific CD8(+) CTL responses and long-term T cell memory. J Leukoc Biol. 2007;82:829–838. doi: 10.1189/jlb.0407249. [DOI] [PubMed] [Google Scholar]
- 84.Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 85.Montecalvo A, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180:3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 86.Morelli AE, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 87.Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004;72:4127–4137. doi: 10.1128/IAI.72.7.4127-4137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colino J, Snapper CM. Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect Immun. 2007;75:220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montecalvo A, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120:90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viaud S, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PloS one. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3:205–211. [PubMed] [Google Scholar]
- 93.Chaput N, et al. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 94.Teo BH, Wong SH. MHC class II-associated invariant chain (Ii) modulates dendritic cells-derived microvesicles (DCMV)-mediated activation of microglia. Biochem Biophys Res Commun. 2010;400:673–678. doi: 10.1016/j.bbrc.2010.08.126. [DOI] [PubMed] [Google Scholar]
- 95.Yang X, Meng S, Jiang H, Zhu C, Wu W. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J Surg Res. 2011;171:826–832. doi: 10.1016/j.jss.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 96.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503–1510. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 97.Peche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant. 2006;6:1541–1550. doi: 10.1111/j.1600-6143.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 98.Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009;60:380–389. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim SH, et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 100.Ruffner MA, Kim SH, Bianco NR, Francisco LM, Sharpe AH, Robbins PD. B7-1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. Eur J Immunol. 2009;39:3084–3090. doi: 10.1002/eji.200939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skokos D, et al. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 102.Skokos D, et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 103.Eken C, Gasser O, Zenhaeusern G, Oehri I, Hess C, Schifferli JA. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J Immunol. 2008;180:817–824. doi: 10.4049/jimmunol.180.2.817. [DOI] [PubMed] [Google Scholar]
- 104.Truman JP, et al. Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release. Immunology. 2012;136:30–45. doi: 10.1111/j.1365-2567.2012.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 106.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zernecke A, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 108.Hergenreider E, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 109.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 110.Sheldon H, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 111.Deregibus MC, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 112.Lv LH, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lespagnol A, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 114.Andreola G, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abusamra AJ, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 117.Huber V, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 118.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 120.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PloS one. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xie Y, et al. Tumor apoptotic bodies inhibit CTL responses and antitumor immunity via membrane-bound transforming growth factor-beta1 inducing CD8+ T-cell anergy and CD4+ Tr1 cell responses. Cancer Res. 2009;69:7756–7766. doi: 10.1158/0008-5472.CAN-09-0496. [DOI] [PubMed] [Google Scholar]
- 122.Ashiru O, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70:481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 124.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34:206–213. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 125.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96:1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soderberg A, Barral AM, Soderstrom M, Sander B, Rosen A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med. 2007;43:90–99. doi: 10.1016/j.freeradbiomed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 127.Yu S, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 128.Valenti R, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 129.Xiang X, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee HM, et al. A membranous form of ICAM-1 on exosomes efficiently blocks leukocyte adhesion to activated endothelial cells. Biochem Biophys Res Commun. 2010;397:251–256. doi: 10.1016/j.bbrc.2010.05.094. [DOI] [PubMed] [Google Scholar]
- 132.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deng Z, et al. Tumor cell cross talk with tumor-associated leukocytes leads to induction of tumor exosomal fibronectin and promotes tumor progression. Am J Pathol. 2012;180:390–398. doi: 10.1016/j.ajpath.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 134.Gesierich S, Berezovskiy I, Ryschich E, Zoller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- 135.Grange C, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 136.Millimaggi D, et al. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia. 2007;9:349–357. doi: 10.1593/neo.07133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Svensson KJ, et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci U S A. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012 doi: 10.1038/nm.2753. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qu JL, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis. 2009;41:875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 140.Battke C, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60:639–648. doi: 10.1007/s00262-011-0979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aung T, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108:15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cho JA, et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 143.Wolfers J, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 144.Cho JA, Lee YS, Kim SH, Ko JK, Kim CW. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275:256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 145.Dai S, et al. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005;11:7554–7563. doi: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- 146.Xie Y, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med. 2010;14:2655–2666. doi: 10.1111/j.1582-4934.2009.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI. Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol. 2012;188:1942–1952. doi: 10.4049/jimmunol.1102053. [DOI] [PubMed] [Google Scholar]
- 148.Yang C, Chalasani G, Ng YH, Robbins PD. Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PloS one. 2012;7:e36138. doi: 10.1371/journal.pone.0036138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barreto A, et al. Membrane vesicles released by intestinal epithelial cells infected with rotavirus inhibit T-cell function. Viral Immunol. 2010;23:595–608. doi: 10.1089/vim.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lenassi M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PloS one. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PloS one. 2011;6:e26588. doi: 10.1371/journal.pone.0026588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Roy K, Hamilton DJ, Munson GP, Fleckenstein JM. Outer membrane vesicles induce immune responses to virulence proteins and protect against colonization by enterotoxigenic Escherichia coli. Clin Vaccine Immunol. 2011;18:1803–1808. doi: 10.1128/CVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bishop AL, Tarique AA, Patimalla B, Calderwood SB, Qadri F, Camilli A. Immunization of mice with vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission. J Infect Dis. 2012;205:412–421. doi: 10.1093/infdis/jir756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen X, et al. Intestinal epithelial cell-derived integrin alphabeta6 plays an important role in the induction of regulatory T cells and inhibits an antigen-specific Th2 response. J Leukoc Biol. 2011;90:751–759. doi: 10.1189/jlb.1210696. [DOI] [PubMed] [Google Scholar]
- 157.Almqvist N, Lonnqvist A, Hultkrantz S, Rask C, Telemo E. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008;125:21–27. doi: 10.1111/j.1365-2567.2008.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prado N, et al. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol. 2008;181:1519–1525. doi: 10.4049/jimmunol.181.2.1519. [DOI] [PubMed] [Google Scholar]
- 159.Hedlund M, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183:340–351. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 160.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 161.Mallegol J, et al. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007;132:1866–1876. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 162.Bu HF, Wang X, Tang Y, Koti V, Tan XD. Toll-like receptor 2-mediated peptidoglycan uptake by immature intestinal epithelial cells from apical side and exosome-associated transcellular transcytosis. J Cell Physiol. 2010;222:658–668. doi: 10.1002/jcp.21985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sheng H, et al. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J Immunol. 2011;187:1591–1600. doi: 10.4049/jimmunol.1100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fitzner D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 165.Izquierdo-Useros N, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 167.Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 168.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 170.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 171.Martin-Cofreces NB, Alarcon B, Sanchez-Madrid F. Tubulin and actin interplay at the T cell and antigen-presenting cell interface. Front Immunol. 2011;2:24. doi: 10.3389/fimmu.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 173.Finetti F, Paccani SR, Rosenbaum J, Baldari CT. Intraflagellar transport: a new player at the immune synapse. Trends Immunol. 2011;32:139–145. doi: 10.1016/j.it.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Feng D, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 175.Parolini I, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 177.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hubner W, et al. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rechavi O, et al. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 2009;23:1971–1979. doi: 10.1101/gad.1789609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 181.Huang JF, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 182.Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol. 1999;163:5201–5210. [PubMed] [Google Scholar]
- 183.Wetzel SA, McKeithan TW, Parker DC. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174:80–89. doi: 10.4049/jimmunol.174.1.80. [DOI] [PubMed] [Google Scholar]
- 184.He T, et al. Bidirectional membrane molecule transfer between dendritic and T cells. Biochem Biophys Res Commun. 2007;359:202–208. doi: 10.1016/j.bbrc.2007.05.099. [DOI] [PubMed] [Google Scholar]
- 185.Ueda H, Morphew MK, McIntosh JR, Davis MM. CD4+ T-cell synapses involve multiple distinct stages. Proc Natl Acad Sci U S A. 2011;108:17099–17104. doi: 10.1073/pnas.1113703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martinez-Martin N, et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Quah BJ, Barlow VP, McPhun V, Matthaei KI, Hulett MD, Parish CR. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer. Proc Natl Acad Sci U S A. 2008;105:4259–4264. doi: 10.1073/pnas.0800259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111:5621–5628. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patel DM, Mannie MD. Intercellular exchange of class II major histocompatibility complex/peptide complexes is a conserved process that requires activation of T cells but is constitutive in other types of antigen presenting cell. Cell Immunol. 2001;214:165–172. doi: 10.1006/cimm.2001.1897. [DOI] [PubMed] [Google Scholar]
- 190.Espinosa E, Tabiasco J, Hudrisier D, Fournie JJ. Synaptic transfer by human gamma delta T cells stimulated with soluble or cellular antigens. J Immunol. 2002;168:6336–6343. doi: 10.4049/jimmunol.168.12.6336. [DOI] [PubMed] [Google Scholar]
- 191.Kusakari S, et al. Trans-endocytosis of CD47 and SHPS-1 and its role in regulation of the CD47-SHPS-1 system. J Cell Sci. 2008;121:1213–1223. doi: 10.1242/jcs.025015. [DOI] [PubMed] [Google Scholar]
- 192.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sotelo-Silveira JR, Calliari A, Kun A, Koenig E, Sotelo JR. RNA trafficking in axons. Traffic. 2006;7:508–515. doi: 10.1111/j.1600-0854.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 194.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 195.Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A. 2010;107:5545–5550. doi: 10.1073/pnas.0910074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 197.Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc Natl Acad Sci U S A. 2006;103:11258–11263. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sowinski S, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 199.Onfelt B, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 200.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 201.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oviedo-Orta E, Howard Evans W. Gap junctions and connexin-mediated communication in the immune system. Biochim Biophys Acta. 2004;1662:102–112. doi: 10.1016/j.bbamem.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 204.Valiunas V, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Oviedo-Orta E, Gasque P, Evans WH. Immunoglobulin and cytokine expression in mixed lymphocyte cultures is reduced by disruption of gap junction intercellular communication. FASEB J. 2001;15:768–774. doi: 10.1096/fj.00-0288com. [DOI] [PubMed] [Google Scholar]
- 206.Elgueta R, et al. Gap junctions at the dendritic cell-T cell interface are key elements for antigen-dependent T cell activation. J Immunol. 2009;183:277–284. doi: 10.4049/jimmunol.0801854. [DOI] [PubMed] [Google Scholar]
- 207.Mendoza-Naranjo A, et al. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol. 2011;187:3121–3132. doi: 10.4049/jimmunol.1100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Matsue H, et al. Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J Immunol. 2006;176:181–190. doi: 10.4049/jimmunol.176.1.181. [DOI] [PubMed] [Google Scholar]
- 209.Mendoza-Naranjo A, et al. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J Immunol. 2007;178:6949–6957. doi: 10.4049/jimmunol.178.11.6949. [DOI] [PubMed] [Google Scholar]
- 210.Krenacs T, van Dartel M, Lindhout E, Rosendaal M. Direct cell/cell communication in the lymphoid germinal center: connexin43 gap junctions functionally couple follicular dendritic cells to each other and to B lymphocytes. Eur J Immunol. 1997;27:1489–1497. doi: 10.1002/eji.1830270627. [DOI] [PubMed] [Google Scholar]
- 211.Bopp T, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 213.Hosoda T, et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 214.Kizana E, Cingolani E, Marban E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 2009;16:1163–1168. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- 215.Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lim PK, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 217.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]