Abstract
Hierarchical organization is widespread in the societies of humans and other animals, both in social structure and in decision-making contexts. In the case of collective motion, the majority of case studies report that dominant individuals lead group movements, in agreement with the common conflation of the terms “dominance” and “leadership.” From a theoretical perspective, if social relationships influence interactions during collective motion, then social structure could also affect leadership in large, swarm-like groups, such as fish shoals and bird flocks. Here we use computer-vision–based methods and miniature GPS tracking to study, respectively, social dominance and in-flight leader–follower relations in pigeons. In both types of behavior we find hierarchically structured networks of directed interactions. However, instead of being conflated, dominance and leadership hierarchies are completely independent of each other. Although dominance is an important aspect of variation among pigeons, correlated with aggression and access to food, our results imply that the stable leadership hierarchies in the air must be based on a different set of individual competences. In addition to confirming the existence of independent and context-specific hierarchies in pigeons, we succeed in setting out a robust, scalable method for the automated analysis of dominance relationships, and thus of social structure, applicable to many species. Our results, as well as our methods, will help to incorporate the broader context of animal social organization into the study of collective behavior.
Keywords: collective animal behavior, hierarchy, high-throughput ethology, leadership, dominance network
For a group of humans or nonhuman animals, networks can be constructed from a number of different types of interaction and across a range of contexts, including association, aggression, courtship, and leadership (1–4). This aspect of interaction networks raises the question as to whether network structure is maintained across contexts because of stable relationships or underlying individual differences, or whether network structure reorganizes in every new situation, where the same individuals may have different competences. For example, does social dominance routinely endow individuals with leadership roles within the group? By leadership, we refer to an individual’s degree of influence over a group’s decision: in the case of collective travel, this largely concerns the timing or direction of the group’s movements (5). Dominance signifies the consistent winning of agonistic interactions (6). Most studies of the relationship between leadership and social structure have focused on mammals, especially primates. In species with highly asymmetrical dominance relationships, such as gray wolves (7), mountain gorillas (8), and chacma baboons (9), there is a consistent trend for dominants to lead. This effect may be mediated by factors other than dominance, such as the central position of dominants in the association network (10) or their greater metabolic needs as a result of body size. Decision-making is more egalitarian in species where dominance relationships are weak or absent, such as in Tonkean macaques (11).
However, the relationship between social structure and leadership is still unknown in some of the most rapid, large-scale, and impressive examples of collective motion: bird flocks and fish shoals. Initial simulations of these groups posed anonymous, homogeneous interaction rules (12, 13), but individual differences are now the focus of both theoretical and empirical investigations (3, 14–19). Elucidating how social structure and individual differences affect group decision-making across a range of taxonomic groups will put the study of collective behavior into a broader biological context. Addressing these questions requires improved tracking technology and computational methods for quantifying interactions in large groups of identified individuals. These high-throughput tools can thus open up new areas of research in social behavior (20, 21).
In this study, we develop automated methods for assessing social dominance, and use them to compare dominance and leadership in domestic pigeons (Columba livia). Agonistic encounters in pigeons are characterized by frequent reversals of aggression (22), requiring prolonged observation to determine which bird dominates within a dyad. We build on advances in high-throughput video tracking by using computer-vision and custom-made algorithms to find interactions between marked individuals as they feed indoors in groups of 10 or 30. We measure leadership hierarchies in the same groups of birds by tracking free flights around the home lofts using custom-made high-resolution GPS loggers. Applying two tracking technologies to the same animal groups allows us to test: (i) the degree of hierarchical structure in both dominance and leader-follower interactions and (ii) whether dominance and leadership correlate.
Results
Social Dominance.
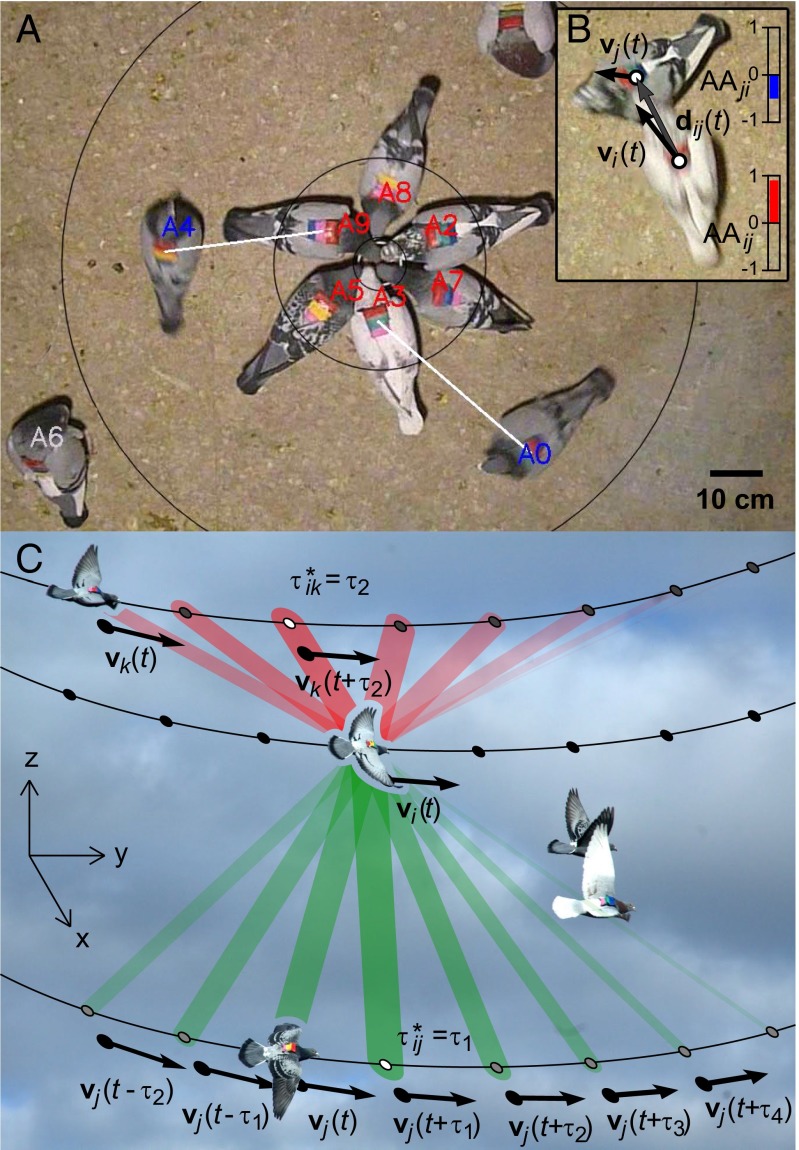
We analyzed videos of groups of 10 and 30 pigeons as they fed from a cup in the center of the camera’s field of view (Fig. 1 A and B, and Movies S1 and S2). Each pigeon carried a unique three-color barcode on its back, which enabled computer-vision–based reconstruction of individual trajectories. The accuracy of the automated method was confirmed through detailed manual identification (SI Materials and Methods). We quantified dominance relationships from the video tracking data using two metrics: feeding-queuing (FQ) and approach-avoidance (AA). FQ is the pairwise asymmetry in access to food, taking into account the spatial positions of the birds relative to the food cup (Fig. 1A and Movie S1). AA, on the other hand, uses velocity and relative position to determine the degree to which pairs of birds tend to approach and avoid each other (Fig. 1B and Movie S2).
Fig. 1.
Automated analysis of dominance and leader–follower relationships. (A) Frame from feeding experiment. Color of identified IDs indicates automated behavior categorization based on the concentric zones: red, feeding (5–20 cm); blue, queuing (20–60 cm); gray, outside zone of interest. White lines indicate FQ interactions between queuing birds and their respective closest feeding neighbors. (B) Illustration of AA calculation. For birds i and j AA (shown by bars on the right) is the dot product of i’s velocity (vi) and the direction from i to j (dij). AAij ∼1: bird i is approaching; AAji < 0: bird j is avoiding. (C) Schematic illustration of leader-follower analysis, superimposed on a photo of subjects carrying GPS devices. For three birds (i, j, and k) a segment of trajectory is shown, with arrows indicating direction of motion, vi(t) in the horizontal plane. For each pair (i ≠ j), vi(t) · vj (t+τ) is the dot product of the normalized velocity of bird i at time t and that of bird j at time t+τ, indicated by the width of the colored edge. τ* is the time delay with the maximal correlation (marked with white dot) with which birds j and k are following bird i.
Having constructed interaction matrices for each group, we computed their transitivity and symmetry. The full interaction matrix describes a weighted directed network with two edges reciprocally connecting every pair of vertices (i.e., a complete directed graph). We decompose it into: (i) a weighted undirected network, representing the amount of interference/aggression that is reciprocated by the other bird, which we call the “common part,” and (ii) a weighted directed network, now with only one edge at most connecting each pair of vertices, representing the asymmetry in those birds’ interactions, which we call the “dominant part” (SI Materials and Methods and Fig. S1). Transitivity (T) is calculated from the dominant part; it ranges from 0.5 to 1, with a score of 1 for fully transitive hierarchies (i.e., networks containing no directed loops). Symmetry (S) is a measure of hierarchy flatness, ranging from 0 to 1, with a score of 1 if the two-way interaction is symmetrical within every pair. We calculate S as the total weight of the common part relative to the total weight of all interactions.
We found highly transitive dominance hierarchies in all three groups of 10 (designated groups A, B, and C), based on both the FQ (TA = 0.960, TB = 0.998, TC = 0.959; P < 0.001 for all groups) and AA [TA= 0.892 (P = 0.021), TB = 0.896 (P = 0.018), TC = 0.951 (P < 0.001)] metrics (FQ shown in Fig. 2 A–C). These transitivity values are all significantly higher than would be expected from randomly directed interactions (Fig. S2; see SI Materials and Methods for details of the randomization). In addition to being highly transitive, the degree of symmetry in FQ matrices demonstrates that a normally subordinate bird frequently blocked the dominant from accessing food (SA = 0.660, SB = 0.402, SC = 0.614), in line with previous reports of frequent reversal events in pigeon social dominance (22). Despite these reversal events, we amassed enough data through automated video tracking to construct a hierarchy based on which bird in a pair dominated a greater proportion of the time. Because of the high level of transitivity, we could summarize dominance by calculating a score for each individual (23). We chose the normalized David’s score (NormDS) (24) because its assumptions best matched our dataset; however, all calculated scores showed close agreement with each other (SI Materials and Methods).
Fig. 2.
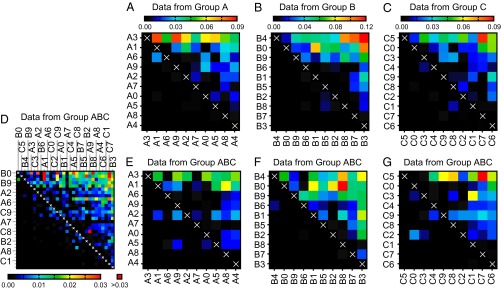
Dominant part of the FQ interactions, comparing the groups of 10 and the combined group of 30. The color-coding illustrates the strength of the dominant part of the FQ interaction matrix. In all panels, the more values above the diagonal, the higher the transitivity of the matrix. (A–C) Interactions measured in the groups of 10. Individuals are ordered according to their NormDS. (D) Interactions measured in the combined group of all 30 individuals. Individuals are ordered according to NormDS from the group of 30. (E–G) To visualize the stability of dominance (and of our dominance metric) across independent measurements involving 10 and 30 individuals, we plotted on E–G the same data as on D but with individuals in the same order as in A–C (i.e., using NormDS calculated within groups of 10). The matrices for groups A (in A and E), B (in B and F), and C (in C and G) are highly similar, meaning that the dominance relationships in the groups of 10 were also detected in the group of 30. The corresponding interaction matrices for leadership are shown in Fig. S3.
To test whether the automated dominance metrics above correspond with more traditional measures of dominance, we manually identified pecking, fighting, and chasing events on approximately half of the videos (10.4 h of 22.2 h; see SI Materials and Methods for details of scoring method). We use the term “pecking order” (PO) for dominance estimates based on these manually scored interactions, to distinguish them from those derived from the automated methods. For each method, we tested whether the NormDS values correlate with those from the other methods (Table 1), as well as testing for correlations between the pairwise interaction matrix values from different methods (Table S1). Both types of comparison produce very similar results. PO dominance correlates positively with FQ dominance, and has a weaker positive correlation with AA dominance. FQ dominance is also a good predictor of a pigeon’s total time-at-feeder (TAF), a measure that is often used as a proxy for dominance or competitive ability in the absence of interaction data (6, 25). Finally, FQ dominance correlated positively with body mass but not with age (Table 1).
Table 1.
Correlation between dominance, leadership, and other parameters
| Dominance | Other parameters | Leadership | 10 vs. 30 | |||||
| AA | FQ | PO | TAF | Size | Age | GFL | ||
| Dominance | ||||||||
| AA | X | <0.001 | <0.001 | 0.044 | 0.768 | 0.730‾ | 0.183 | 0.025 |
| FQ | <0.001 | x | <0.001 | <0.001 | 0.020 | 0.649‾ | 0.104 | <0.001 |
| PO | <0.001 | <0.001 | x | 0.007 | 0.421 | 0.866‾ | 0.411 | <0.001 |
| Other parameters | ||||||||
| TAF | 0.044 | <0.001 | 0.007 | x | 0.002 | 0.787‾ | 0.126 | <0.001 |
| Size | 0.768 | 0.020 | 0.421 | 0.002 | x | 0.213‾ | 0.522‾ | x |
| Age | 0.730‾ | 0.649‾ | 0.866‾ | 0.787‾ | 0.213‾ | x | 0.298 | x |
| Leadership | ||||||||
| GFL | 0.183 | 0.104 | 0.411 | 0.126 | 0.522‾ | 0.298 | x | 0.010 |
The table shows P values of the meta-analysis of the Pearson correlations for the three groups of 10 (A, B, C), using Fisher’s combined probability test (29). For each group, we calculated two-tailed Pearson correlations for the NormDS from the antisymmetrized interaction matrices. The final column contains P values for Pearson correlations between individual scores of the same 30 birds when measured in their respective groups of 10 and their scores in the group of 30 (n = 30), using NormDS to score dominance and leadership. Cells that contain significant correlations (P < 0.05) are in bold and strong significant correlations (P < 0.01) are highlighted in gray. Superscript “–” shows a correlation where the correlation coefficient is negative. “x” indicates cells where correlations are not applicable. See main text and Table S3 for descriptions of variables. See Table S1 for correlation coefficients and P values of all groups, calculated using pairwise interaction values as well as NormDS.
As an additional test of the robustness of our dominance measurements and of the pigeons’ social structure, we compared the three groups of 10 to the combined group of all 30 birds (group ABC). All of these groups were subsamples from a freely interacting population of ca. 100. For the FQ and manual measurements of dominance, the scores in the groups of 10 correlate positively with the scores in the group of 30 (Fig. 2 and Table 1, final column). This finding suggests that the dominance relationships measured via the FQ method are robust to third-party interference and are indicative of inherent behavioral variance in the population, with individual qualities that serve as predictors of dominance maintained across different group configurations. Compared with FQ, the AA score has a weaker positive correlation between groups of 10 and 30 (Table 1, final column). This score considers any neighbor within 50 cm as a potential interactor, and is perhaps less scalable to high densities than the FQ score, which considers only the nearest feeding neighbor.
Leadership.
We analyzed leader–follower relationships from high-resolution GPS tracks of flock flights (Movie S3). For each dyad in a flock, we quantified leadership as the mean time delay between the two birds’ directional choices (τ) (Fig. 1C) (3). These pairwise time delays constitute a weighted (i.e., nonbinary) network based on data from multiple flights. In the rest of the article we focus on the directed edges (nonzero time delays) as a proxy for leadership. It is also possible to form an undirected network from those pairs where the measurable time delays are close to zero (26). This alternative analysis, containing both mutual and directed connections, produced very similar results to the weighted directed case (for details, see SI Materials and Methods).
The directed networks from flock flight trajectories revealed transitive leadership hierarchies in all three groups of 10 and in the combined group of 30 (TA = 1, TB = 0.989, TC = 1, TABC = 0.986, P < 0.001 in all cases) (Fig. S3). We thus confirm Nagy et al.’s (3) earlier finding of hierarchical leadership, and extend both the methods and results to larger flocks of 30. Individual leadership scores were consistent between the groups of 10 and 30 (Pearson’s r = 0.56, n = 30, P = 0.002), indicating that a pigeon’s propensity for leadership does not depend on group composition, but more likely arises from some individual attribute that changes little from flight to flight. Leadership correlated neither with body mass nor with age (Table 1). In agreement with Nagy et al. (3), we found that leaders tended to be positioned toward the front compared with the average direction of motion of the whole flock [groups of 10: r = 0.87, P < 0.001, n = 30 (3 × 10); group ABC: r = 0.92, P < 0.001, n = 30].
Comparison of Hierarchies.
We performed a thorough correlation analysis between the stable hierarchies emerging in the contexts of social dominance and leadership, both at the level of individual ranks and using the more detailed pairwise interaction matrices (Table 1, and Tables S1 and S2). We used Fisher’s combined probability test to summarize the correlations for the three independent groups of 10 (Fig. 3, Table 1, and Figs. S4 and S5). For all our measures of social dominance, correlations with flight leadership remained nonsignificant (P > 0.05). Given the reliability of our measurements of individual scores in both contexts (see above for tests of robustness of each of our metrics), this lack of correlation is unlikely to be because of a failure to detect an existing relationship. Thus, crucially, we found that leadership was independent of social dominance, despite the robustness and transitivity of both the dominance and leadership hierarchies.
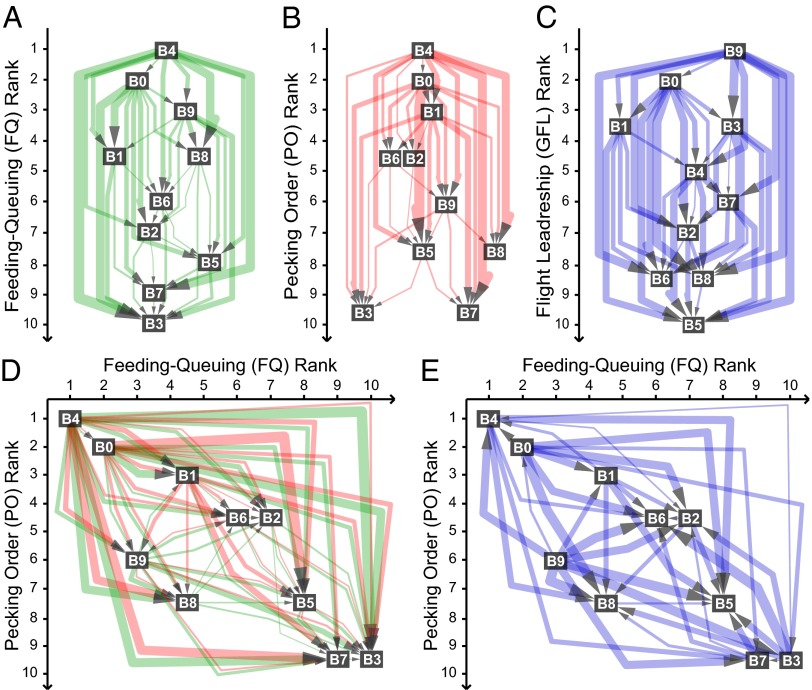
Fig. 3.
Dominance and leadership networks. (A) FQ, (B) PO, (C) group flight leadership (GFL) networks for group B. Directed edges point from the dominant or leader to the subordinate or follower, with edge widths corresponding to interaction strength. Nodes are ordered vertically according to rank, with dominants/leaders at the top. (D) Comparison of FQ (green) and PO (red) networks (same edges as in A and B). Horizontal position of nodes corresponds to FQ rank, vertical to PO rank. Nodes close to the diagonal and the similarity of green/red edges indicate high correlation between FQ and PO. (E) Flight leadership network superimposed on the layout created from dominance ranks FQ and PO (i.e., same node positions as in D, same edges as in C). The absence of correlation between the GFL and FQ/PO rankings is apparent from the random direction of the arrows. See Fig. S4 for the corresponding networks for groups A and C.
Discussion
Our results clearly demonstrate that multiple, context-dependent hierarchies can coexist simultaneously in the same group of animals. The computer-vision–based analysis of interactions among feeding pigeons revealed transitive social dominance hierarchies, significantly different from randomly directed networks. When we compared social dominance to leader–follower relationships in the air, we found that the stable, hierarchical pattern of in-flight leadership does not build upon the stable, hierarchical social dominance structure evident in the same birds. Instead, in the case of pigeon flocks, the emergence of leadership and dominance hierarchies are each affected by different factors. By ignoring social dominance when in flight, flocks of pigeons potentially make better navigational decisions because leadership can emerge from relevant attributes, such as local experience and route fidelity (27, 28). In despotically organized societies of mosquitofish and meerkats, it has also been observed that the dominant individual is not necessarily the leader (18, 29); however, in neither of these cases were dominance relationships quantified as multilevel networks.
The dissociation between dominance and leadership in pigeons suggests that pigeons have a different mechanism, either of dominance or of leadership, compared with species where dominants lead (e.g., gray wolves, chacma baboons) (7, 9, 30). In common with those species, dominance in pigeons is associated with aggression and large body size. Therefore, it is likely that what makes pigeons different from, for example, baboons, is the way leadership emerges. An airborne flock deciding on a direction cannot interact aggressively in the same way as it can on the ground, which may isolate leadership from dominance to a greater extent than in purely terrestrial animals.
Dominance is not the only aspect of individual variability that may be relevant to compare with leadership. We expect that our study will motivate more research into how leadership relates to other individual differences, either measured from other contexts of interaction network (association, courtship, mate choice, and so forth) or from biophysical parameters. We did not find a correlation between leadership and age, but all of our subjects were experienced adults, so this does not rule out a difference between adults and juveniles. Age-related leadership has been reported in African elephants (31) and in some migratory birds, such as broad-winged hawks, where adults tend to fly in front of juveniles (32). In these wild migratory species, experience accumulates linearly with age, which is not necessarily the case in domestic pigeons.
In addition to our findings concerning dominance and leadership, our automated dominance analysis methods could be applied to other forms of tracking data (e.g., radio-frequency identification tags, GPS loggers), even in species with prolonged, noisy contests (Table S3). The results of our automated methods were in close agreement with the more traditional method of manually scoring aggressive encounters, with the added benefit that the automated methods can monitor multiple pairwise interactions occurring in parallel within large groups. Our study demonstrates the benefit of applying different types of tracking technology to the same group of animals, to investigate the wider biological context of patterns in collective motion. We provide previously unused tools for studying social complexity within the emerging field of high-throughput ethology (20, 21), applicable across a wide range of group-living species from insects to mammals, including humans. A broader taxonomic perspective, including our current results on pigeons, will aid in understanding how the structure of interaction networks changes across different behavioral contexts.
Materials and Methods
Subjects.
We used 30 homing pigeons (Columba livia), aged 2.8 ± 1.6 y (mean ±SD), from two neighboring lofts at the Oxford University Field Station. Birds were allocated to three groups of 10 (A, B, and C) and also tested in a combined group of 30 (group ABC). Groups A and B contained individuals only from lofts 1 and 2, respectively. Group C contained birds from both lofts. Feeding trials took place in the home loft (groups A and B) or alternated between the two lofts (groups C and ABC). Both feeding and flock flight experiments were interspersed over a period of 2 mo. The procedures outlined in this article were approved by the Ethical Review Committee of Oxford University’s Department of Zoology.
Feeding Experiments.
Groups of 10 or 30 pigeons were given access to a single food source (a small ceramic cup containing grain mix) (Fig. 1A) inside the pigeon loft. Food was replenished every 12 min, and trials were terminated when 10 min passed with only one pigeon feeding. Each pigeon participated in no more than one trial per day. All trials were video recorded by a camera fixed to the ceiling (Panasonic DMC-FS10, 2.1 × 1.2 m2 field of view, 1,280 × 720 resolution, 30 fps). Eight, 6, 8, and 10 trials were conducted for groups A, B, C, and ABC, respectively, producing 22.2 h of video. Each pigeon carried a unique three-color barcode on its back, enabling computer-vision–based reconstruction of individual trajectories resulting in 10 million identified pigeon positions on the 2.4 million frames of video. The recorded video sequences were analyzed off-line and verified against frame-by-frame manual identification (see SI Materials and Methods for details).
Flock Flights.
GPS data were collected from free flights of flocks around the loft (7 flights each for groups A, B, C; 10 for group ABC). A maximum of two flights were conducted per day. The GPS logger weighed 13 g, was based on a commercially available embedded device (Gmsu1LP), logged time-stamped longitude, latitude, and altitude data at 10 Hz, and was affixed to a pigeon’s back with an elastic harness. Loggers were randomly allocated to pigeons before every flight. In other respects, the flight experimental procedure and data handling were identical to those in Nagy et al. (3). Of the 360 individual trajectories flown, 25 were partly or entirely missing due to device error. In total, GPS devices logged 90.5 h of flight time, representing >3,250,000 datapoints.
Automated Dominance Analysis.
For automated dominance analysis, we used two methods: feeding-queuing and approach-avoidance, both of which are pairwise measures calculated for each pair of birds. FQ provided an estimate of the relative frequency with which bird i was in a position that excluded bird j from accessing food. We classified a bird as “feeding” if it was located within a radius of <20 cm from the cup, with the head pointing toward the cup’s center (±30°). We classified a bird as “queuing” if it was in the vicinity of the food cup (<60 cm from the cup) but was not feeding according to our criteria (Fig. 1A and Movie S1). When bird j was queuing, we calculated the total time that bird i was its closest feeding neighbor, and divided this by the total time that i or j was classified as feeding or queuing. FQ relations were detected in a total of 4 million position pairs.
AA was defined for each pair of birds (i ≠ j) as the time-averaged dot product of i's velocity (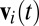 ) and the direction from i to j (
) and the direction from i to j (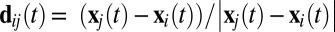 ):
): 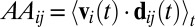 . We averaged AAij across all frames when i and j were within 50 cm of each other (
. We averaged AAij across all frames when i and j were within 50 cm of each other (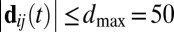 cm) and i was moving at least 0.05 ms−1 (
cm) and i was moving at least 0.05 ms−1 (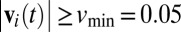 ms−1). AAij is positive if i tends to approach j and negative if i tends to avoid j (Fig. 1B and Movie S2).
ms−1). AAij is positive if i tends to approach j and negative if i tends to avoid j (Fig. 1B and Movie S2).
To check both types of automated dominance analysis against human observation, we manually scored agonistic interactions in approximately half of the videos. We recorded pecking, wing slapping, chasing, and whether the attacked bird retreated or fought back (SI Materials and Methods and Movie S2). From these events, we compiled a third type of interaction matrix for each group, which we termed pecking order, and then compared the three dominance analysis methods PO, FQ, and AA.
Supplementary Material
Acknowledgments
We thank Zsuzsa Ákos and Max Gray for their technical help throughout the flight experiments. This work was partly supported by the European Research Council COLLMOT project (Grant 227878); a Royal Society Newton International Fellowship, Somerville College, Oxford, and European Social Fund Grant TÁMOP-4.2.1/B-09/1/KMR (to M.N.); European Social Fund Grant TÁMOP-4.2.4.A/1-11-1-2012-0001 (to G.V.); a Royal Society University Research Fellowship (to D.B.); and a Biotechnology and Biological Sciences Research Council Doctoral Training grant (to B.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305552110/-/DCSupplemental.
References
- 1.Krause J, Lusseau D, James R. Animal social networks: An introduction. Behav Ecol Sociobiol. 2009;63(7):967–973. [Google Scholar]
- 2.Barrett L, Henzi SP, Lusseau D. Taking sociality seriously: The structure of multi-dimensional social networks as a source of information for individuals. Philos Trans R Soc Lond B Biol Sci. 2012;367(1599):2108–2118. doi: 10.1098/rstb.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy M, Akos Z, Biro D, Vicsek T. Hierarchical group dynamics in pigeon flocks. Nature. 2010;464(7290):890–893. doi: 10.1038/nature08891. [DOI] [PubMed] [Google Scholar]
- 4.Vicsek T, Zafeiris A. Collective motion. Phys Rep. 2012;517(3):71–140. [Google Scholar]
- 5.Conradt L, Roper TJ. Consensus decision making in animals. Trends Ecol Evol. 2005;20(8):449–456. doi: 10.1016/j.tree.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Drews C. The concept and definition of dominance in animal behaviour. Behaviour. 1993;125(3-4):283–313. [Google Scholar]
- 7.Peterson RO, Jacobs AK, Drummer TD, Mech LD, Smith DW. Leadership behavior in relation to dominance and reproductive status in gray wolves, Canis lupus. Can J Zool. 2002;80(8):1405–1412. [Google Scholar]
- 8.Schaller GB. The Mountain Gorilla: Ecology and Behavior. Chicago: Univ of Chicago Press; 1963. [Google Scholar]
- 9.King AJ, Douglas CM, Huchard E, Isaac NJ, Cowlishaw G. Dominance and affiliation mediate despotism in a social primate. Curr Biol. 2008;18(23):1833–1838. doi: 10.1016/j.cub.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 10.Sueur C, Jacobs A, Amblard F, Petit O, King AJ. How can social network analysis improve the study of primate behavior? Am J Primatol. 2011;73(8):703–719. doi: 10.1002/ajp.20915. [DOI] [PubMed] [Google Scholar]
- 11.Sueur C, Petit O. Shared or unshared consensus decision in macaques? Behav Processes. 2008;78(1):84–92. doi: 10.1016/j.beproc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Vicsek T, Czirók A, Ben-Jacob E, Cohen I, Shochet O. Novel type of phase transition in a system of self-driven particles. Phys Rev Lett. 1995;75(6):1226–1229. doi: 10.1103/PhysRevLett.75.1226. [DOI] [PubMed] [Google Scholar]
- 13.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. Collective memory and spatial sorting in animal groups. J Theor Biol. 2002;218(1):1–11. doi: 10.1006/jtbi.2002.3065. [DOI] [PubMed] [Google Scholar]
- 14.Bode NWF, Wood AJ, Franks DW. The impact of social networks on animal collective motion. Anim Behav. 2011;82(1):29–38. [Google Scholar]
- 15.Bode N, Franks D, Wood A. Leading from the front? Social networks in navigating groups. Behav Ecol Sociobiol. 2012;66(6):835–843. [Google Scholar]
- 16.Sueur C, Deneubourg J-L, Petit O. From social network (centralized vs. decentralized) to collective decision-making (unshared vs. shared consensus) PLoS ONE. 2012;7(2):e32566. doi: 10.1371/journal.pone.0032566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conradt L, Krause J, Couzin ID, Roper TJ. “Leading according to need” in self-organizing groups. Am Nat. 2009;173(3):304–312. doi: 10.1086/596532. [DOI] [PubMed] [Google Scholar]
- 18.Burns ALJ, Herbert-Read JE, Morrell LJ, Ward AJW. Consistency of leadership in shoals of mosquitofish (Gambusia holbrooki) in novel and in familiar environments. PLoS ONE. 2012;7(5):e36567. doi: 10.1371/journal.pone.0036567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama S, Harcourt JL, Johnstone RA, Manica A. Initiative, personality and leadership in pairs of foraging fish. PLoS ONE. 2012;7(5):e36606. doi: 10.1371/journal.pone.0036606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6(6):451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mersch DP, Crespi A, Keller L. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science. 2013;340(6136):1090–1093. doi: 10.1126/science.1234316. [DOI] [PubMed] [Google Scholar]
- 22.Masure RH, Allee WC. The social order in flocks of the common chicken and the pigeon. Auk. 1934;51(3):306–327. [Google Scholar]
- 23.Bayly KL, Evans CS, Taylor A. Measuring social structure: A comparison of eight dominance indices. Behav Processes. 2006;73(1):1–12. doi: 10.1016/j.beproc.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.De Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Anim Behav. 2006;71(3):585–592. [Google Scholar]
- 25.Cole EF, Quinn JL. Personality and problem-solving performance explain competitive ability in the wild. Proc Biol Sci. 2012;279(1731):1168–1175. doi: 10.1098/rspb.2011.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X-K, Kattas GD, Small M. Reciprocal relationships in collective flights of homing pigeons. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;85(2 Pt 2):026120. doi: 10.1103/PhysRevE.85.026120. [DOI] [PubMed] [Google Scholar]
- 27.Flack A, Pettit B, Freeman R, Guilford T, Biro D. What are leaders made of? The role of individual experience in determining leader–follower relations in homing pigeons. Anim Behav. 2012;83(3):703–709. [Google Scholar]
- 28.Freeman R, Mann R, Guilford T, Biro D. Group decisions and individual differences: Route fidelity predicts flight leadership in homing pigeons (Columba livia) Biol Lett. 2011;7(1):63–66. doi: 10.1098/rsbl.2010.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousquet CAH, Manser MB. Resolution of experimentally induced symmetrical conflicts of interest in meerkats. Anim Behav. 2011;81(6):1101–1107. [Google Scholar]
- 30.King AJ, Sueur C. Where next? Group coordination and collective decision making by primates. Int J Primatol. 2011;32(6):1245–1267. doi: 10.1007/s10764-011-9524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McComb K, Moss C, Durant SM, Baker L, Sayialel S. Matriarchs as repositories of social knowledge in African elephants. Science. 2001;292(5516):491–494. doi: 10.1126/science.1057895. [DOI] [PubMed] [Google Scholar]
- 32.Maransky BP, Bildstein KL. Follow your elders: Age-related differences in the migration behavior of Broad-winged Hawks at Hawk Mountain Sanctuary, Pennsylvania. Wilson Bull. 2001;113(3):350–353. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.