Abstract
Purpose:
To report the results of imaging using high-resolution, Fourier domain anterior segment optical coherence tomography (AS-OCT) to evaluate patients with a type 1 Boston Keratoprosthesis (KPro).
Methods:
We performed a retrospective comparative study of patients in whom we implanted the Boston KPro. A total of 26 eyes of 23 patients from the Cornea Service at the University of California Davis Eye Center were included. Subjects were evaluated with the Spectralis AS-OCT (Heidelberg Engineering GmbH).
Results:
Preoperative diagnoses for KPro surgery included failed corneal transplant (69%), chemical burn (23%), and aniridia (8%). The average age of patients was 63.2 years (range, 17–88 years). Fifty-four percent of the patients were female. The mean duration between the KPro surgery and the acquisition of high-resolution AS-OCT imaging was 35.8 months (range, 2–90 months). The most commonly observed finding was retroprosthetic membrane formation, which we found in 77% of KPro eyes. In 65% of KPro eyes, we identified epithelium behind the front plate, and in 54%, we identified an epithelial lip over the anterior surface of the KPro front plate. In 31% of KPro eyes, we identified periprosthetic cysts, gaps or spaces, and thinning in the corneal carrier graft.
Conclusions:
Fourier domain AS-OCT is a useful noninvasive imaging technique in patients with a KPro and provides the ability to identify changes that are sometimes difficult to appreciate by clinical evaluation. The higher resolution Fourier domain systems may aid in the clinical diagnosis and management of pathology that might not be imaged with instruments of lower resolution. AS-OCT has the potential for monitoring the anatomic stability of an implanted KPro and may also help to monitor for complications. Moreover, high-resolution imaging may enhance our understanding of periprosthetic anatomy.
Keywords: Boston type 1 Keratoprosthesis, anterior segment optical coherence tomography, Spectralis, spectral domain, Fourier domain, KPro, OCT
The restoration of vision in patients with corneal blindness has become increasingly successful because of advances in standard penetrating keratoplasty (PK) and ocular surface reconstruction achieved over recent decades. The type 1 Boston Keratoprosthesis (KPro) has been a useful tool in treating corneal blindness in eyes that are poor candidates for PK or that have failed primary corneal transplant surgery in the past.1-9
Situations in which PK carries a poor prognosis include patients with Stevens-Johnson syndrome, ocular cicatricial pemphigoid, end-stage dry eye, chemical burns, and other stem cell deficiency states such as aniridia.1-4 In addition, patients with repeated graft failures may have a poor prognosis for subsequent keratoplasty because of immune reaction.
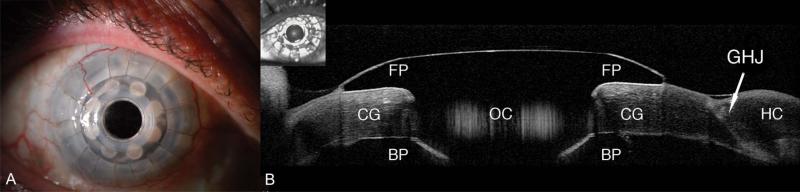
The functional corneal anatomy and certain pathological processes that accompany the Boston KPro are not always easily appreciated in vivo at the slit lamp because of opacification of both the host tissue and the carrier graft (Fig. 1A). Anterior segment optical coherence tomography (AS-OCT) allows the measurement and visualization of microscopic structures of the anterior segment, including detailed visualization of the implanted KPro and its relationship to surrounding tissues.10-12 Commercially available Fourier domain (also called spectral domain) AS-OCT is a noncontact examination that permits imaging with higher resolution and higher speed than traditional time domain AS-OCT.
FIGURE 1.
A, In vivo slit-lamp photograph of the Boston KPro. B, AS-OCT of the Boston KPro. The front plate (FP) and optical cylinder (OC) are clearly visible over the corneal graft (CG). The back plate (BP), like the rest of the KPro, appears dark on OCT. It is also possible to identify the graft–host junction (GHJ).
In this study, we report in vivo corneal findings seen in patients with a KPro, using the Spectralis (Heidelberg Engineering GmbH, Germany) high-resolution Fourier domain ASOCT. This instrument requires only an additional lens adapter to the traditional posterior segment Spectralis OCT instrument used in everyday practice. The Spectralis AS-OCT received clearance from the Food and Drug Administration (FDA) in March 2012. A limited number of cases using spectral domain AS-OCT in patients with a KPro have been reported recently.13,14
MATERIALS AND METHODS
This retrospective comparative study was approved by the Institutional Review Board of the University of California Davis and conforms to the tenets of the Helsinki Declaration and the Health Insurance Portability and Accountability Act of 1996.
We included patients who underwent surgery with implantation of a KPro and were examined at the University of California Davis Eye Center between September 28, 2011, and March 14, 2012. A total of 26 eyes of 23 patients were included. Subjects were examined using the Spectralis Anterior Segment Module Optical Coherence Tomography (Heidelberg Engineering GmbH, Germany) using raster and single-scan with high-resolution acquisition.
All KPro devices examined were manufactured by the Massachusetts Eye and Ear Infirmary. Details of the KPro and the surgical technique for implantation have been described previously in detail.15,16 The surgical technique and postoperative management at the University of California Davis did not differ significantly from that described in the initial Boston Keratoprosthesis Study Group report.
RESULTS
Patient Demographics
Preoperative diagnoses and the indications for KPro surgery in the study cohort included failed corneal transplant in 69% (n = 18/26), chemical burn in 23% (n = 6/26), and aniridia in 8% (n = 2/26) of eyes. Three eyes (12%) were aphakic, and 23 eyes (88%) were pseudophakic. The mean age was 63 years (range, 17–86 years).
Fifty-four percent of patients were female, and the mean duration between the KPro surgery and AS-OCT imaging was 35 months (range, 2–90 months).
Imaging
Using AS-OCT, we obtained high-resolution images of the KPro, visible as a T-shaped cylinder with corrugated sides through the center of the cornea. The bandage contact lens, front plate, optical cylinder, back plate, the carrier corneal graft, and the graft–host junction were distinctly visualized in the images obtained (Fig. 1B).
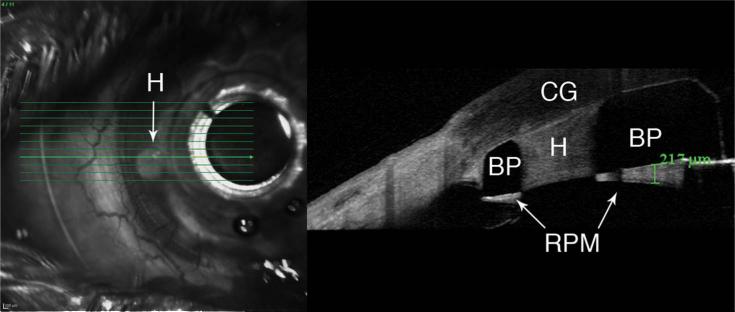
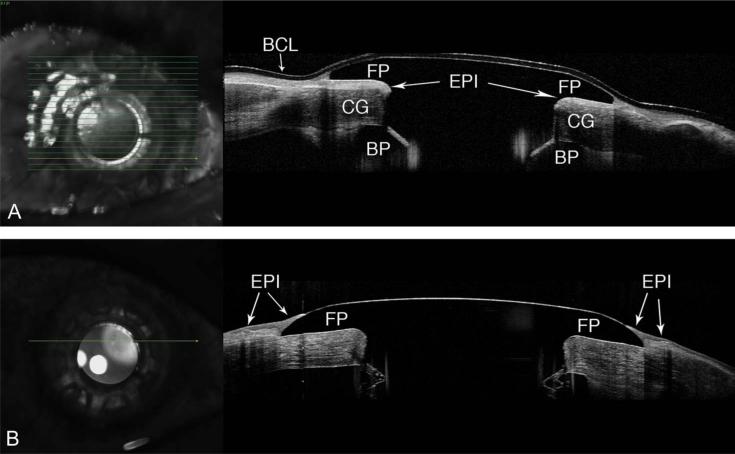
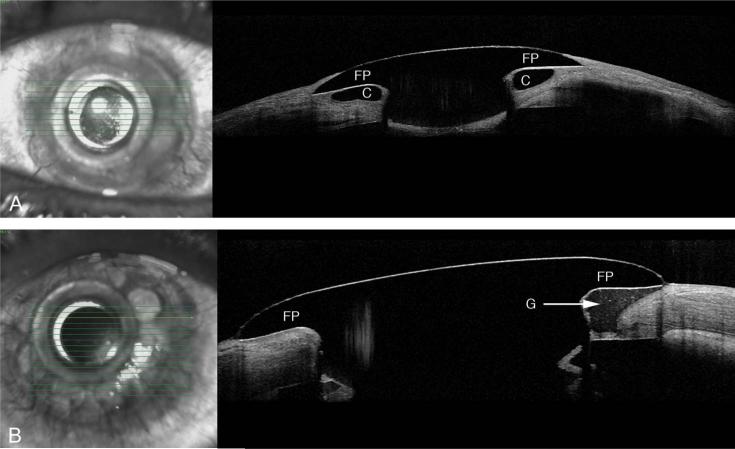
The presence of retroprosthetic membrane was observed in 77% (n = 20/26) of KPro eyes (Fig. 2). In 65% (n = 17/26) of eyes, we were able to identify epithelium behind the front plate (Fig. 3A), and in 54% (n = 14/26), we identified an epithelial lip over the periphery of the anterior surface of the KPro front plate (Fig. 3B). In 38% (10/26), we identified epithelium both over and under the front plate. In 31% (n = 8/26) of KPro eyes, we identified periprosthetic cysts (Fig. 4A), gaps or spaces (Fig. 4B), which were not appreciated at the slit lamp, and areas of corneal thinning in the carrier graft (Fig. 5). The holes in the back plate were visible in all KPro eyes (Fig. 2).
FIGURE 2.
Boston KPro with a retroprosthetic membrane (RPM). Corneal graft (CG), the KPro back plate (BP), and one of the holes (H) manufactured into the KPro back plate are visible. The hole (H) has been filled with fibrous tissue. Posterior to the back plate, we can see an RPM.
FIGURE 3.
A, In this image, the epithelium (EPI) is clearly visible posterior to the front plate (FP) and anterior to the corneal graft (CG). The bandage contact lens (BCL) and back plate (BP) are also identified. B, Epithelium (EPI) growing over the peripheral edge of the FP of Boston KPro eyes.
FIGURE 4.
A, Raster scan showing a periprosthetic cyst (C) in the Corneal graft behind to the front plate (FP). B, AS-OCT showing a KPro patient with a gap (G) in the corneal graft posterior to the FP.
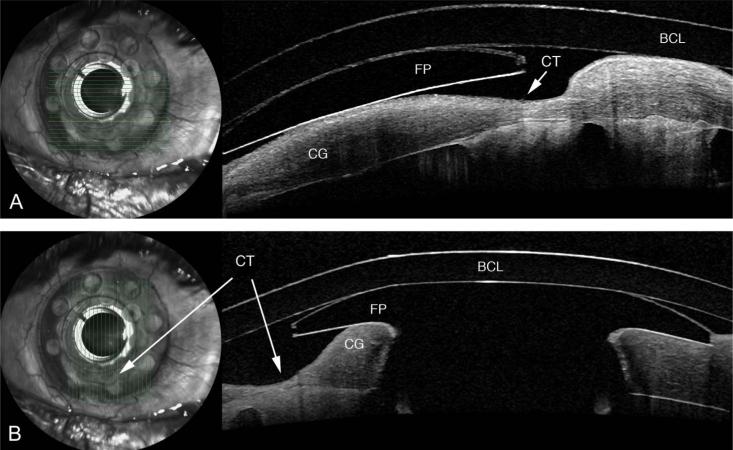
FIGURE 5.
Horizontal (A) and vertical (B) scan showing a KPro patient with corneal thinning (CT) in the graft behind to the front plate (FP). The corneal thinning is unmistakable on AS-OCT but was difficult to detect at the slit lamp. A bandage contact lens (BCL) is also identified.
DISCUSSION
Fourier domain OCT technology provides more rapid and higher resolution scans and, therefore, better visualization of anterior segment detail and delineation of fine structures than can be obtained with traditional time domain OCT systems.17 The Spectralis AS-OCT has an acquisition speed of 40,000 A-scans per second, with an axial resolution of 3.9 to 7 μm and a transverse resolution of 14 μm. This enables the Spectralis system to provide higher resolution and definition, as seen in the images presented here, compared with commercially available time domain OCT systems, such as the Visante (Carl Zeiss AG, Germany), which has a lower axial resolution of 18 μm and a transverse resolution of 60 μm. There are, nonetheless, limitations associated with the Spectralis images. These include a shallow scan depth (only 1.9 mm compared with 6 mm in the Visante) and the poor ability to penetrate tissues that produce high light scatter (e.g., sclera, limbus). These limitations prevent the Spectralis AS-OCT from capturing a cross-section of the entire anterior segment (or often even the entire cornea or KPro) in one image, as can be done with the Visante OCT instrument.
Reported complications in the literature in patients with the KPro include retroprosthetic membrane, infectious and sterile endophthalmitis, periprosthetic corneal melting, extrusion of the KPro, epithelial downgrowth, retinal detachment, and glaucoma.18-20 In our cohort imaged with Fourier domain AS-OCT, we were able to visualize periprosthetic anatomy that is potentially germane to the recognized complications. We were able to distinguish precise components of the KPro and carrier graft and to visualize exquisitely the relationship of the epithelium to both the anterior and the posterior surfaces of the KPro front plate. This configuration of the epithelium may be important. The presence of an intact epithelial layer covering the junction between the carrier donor corneal tissue and the edge of the KPro front plate in its entire circumference would presumably decrease the infection risk by constituting a barrier for entry of microorganisms between the front plate and the carrier graft.12,14
An interesting finding in our study was the visualization of periprosthetic corneal gaps, spaces, or cysts that were difficult to visualize in clinical examination with the slit lamp. The importance of these periprosthetic cysts or gaps is not yet clear, but their observation adds to increased elucidation of the anatomic relationships that develop after implantation of the device.
AS-OCT can be used to evaluate the components of the assembled KPro in vivo, with visualization of the donor cornea interface and to assess the presence of a potential space between the KPro front plate, the optical cylinder, and the corneal graft (Fig. 1B). Furthermore, AS-OCT can be used to monitor, in greater detail, complications such as retroprosthetic membrane formation (Fig. 2), epithelial downgrowth, stromal necrosis, and corneal thinning (Fig. 5).
In conclusion, high-resolution Fourier domain AS-OCT provides novel and highly detailed visual information not previously available for patients with a KPro. AS-OCT is a noninvasive technique that is capable of monitoring patients postoperatively and provides detailed visualization of subtle changes that may be difficult or impossible to appreciate at the slit lamp or with devices of lesser resolution. Although the Spectralis AS-OCT has a narrow window depth, the high resolution offers an advantage that may augment both our ability to detect pathological changes and our understanding of the healing process associated with these devices.
Acknowledgments
Supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY) and the Heed Ophthalmic Foundation (Cleveland, OH).
REFERENCES
- 1.Dohlman CH, Doane MG. Some factors influencing outcome after keratoprosthesis surgery. Cornea. 1994;13:214–218. doi: 10.1097/00003226-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Doane MG, Dohlman CH, Bearse G. Fabrication of a keratoprosthesis. Cornea. 1996;15:179–184. doi: 10.1097/00003226-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Tugal-Tutkun I, Akova YA, Foster CS. Penetrating keratoplasty in cicatrizing conjunctival diseases. Ophthalmology. 1995;102:576–585. doi: 10.1016/s0161-6420(95)30980-3. [DOI] [PubMed] [Google Scholar]
- 4.Soong HK. Penetrating keratoplasty in ocular surface disease. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea: Surgery of the Cornea and Conjunctiva. Mosby; St Louis, MO: 1997. pp. 1781–1788. [Google Scholar]
- 5.Yaghouti F, Dohlman CH. Innovations in keratoprosthesis: proved and unproved. Int Ophthalmol Clin. 1999;39:27–36. doi: 10.1097/00004397-199903910-00005. [DOI] [PubMed] [Google Scholar]
- 6.Dohlman CH, Waller SG, Netland PA. Keratoprosthesis surgery. In: Lindquist TD, Lindstrom RL, editors. Ophthalmic Surgery Update. 4th Mosby; Chicago, IL: 1997. pp. 1–31. [Google Scholar]
- 7.Yaghouti F, Nouri M, Abad JC, et al. Keratoprosthesis: preoperative prognostic categories. Cornea. 2001;20:19–23. doi: 10.1097/00003226-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Khan B, Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: an update. Curr Opin Ophthalmol. 2001;12:282–287. doi: 10.1097/00055735-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dohlman CH, Dudenhoefer EJ, Khan BF, et al. Protection of the ocular surface after keratoprosthesis surgery: the role of soft contact lenses. CLAO J. 2002;28:72–74. [PubMed] [Google Scholar]
- 10.Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112:1584–1589. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JP, Jr, de la Cruz J, Rosen RB, et al. Imaging implanted keratoprostheses with anterior-segment optical coherence tomography and ultrasound biomicroscopy. Cornea. 2008;27:180–188. doi: 10.1097/ICO.0b013e318159bc7d. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JP, Jr, Ritterband DC, Buxton DF, et al. Evaluation of the stability of Boston type I keratoprosthesis-donor cornea interface using anterior segment optical coherence tomography. Cornea. 2010;29:1031–1035. doi: 10.1097/ICO.0b013e3181ca2ea5. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez AG, Radcliffe NM, Sippel KC, et al. Boston type I keratoprosthesis-donor cornea interface evaluated by high-definition spectral-domain anterior segment optical coherence tomography. Clin Ophthalmol. 2012;6:1355–1359. doi: 10.2147/OPTH.S34787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiang L, Rosenblatt MI, Sartaj R, et al. Surface epithelialization of the type I Boston keratoprosthesis front plate: immunohistochemical and high-definition optical coherence tomography characterization. Graefes Arch Clin Exp Ophthalmol. 2012;250:1195–1199. doi: 10.1007/s00417-012-1960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zerbe BL, Belin MW, Ciolino JB, et al. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113:1779. doi: 10.1016/j.ophtha.2006.05.015. e1-7. [DOI] [PubMed] [Google Scholar]
- 16.Bradley JC, Hernandez EG, Schwab IR, et al. Boston type 1 keratoprosthesis: the University of California Davis experience. Cornea. 2009;28: 321–327. doi: 10.1097/ICO.0b013e31818b8bfa. [DOI] [PubMed] [Google Scholar]
- 17.Chin EK, Sedeek RW, Li Y, et al. Reproducibility of macular thickness measurement among five OCT instruments: effects of image resolution, image registration, and eye tracking. Ophthalmic Surg Lasers Imaging. 2012;43:97–108. doi: 10.3928/15428877-20111222-02. [DOI] [PubMed] [Google Scholar]
- 18.Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118:1543–1550. doi: 10.1016/j.ophtha.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Li JY, Greiner MA, Brandt JD, et al. Long-term complications associated with glaucoma drainage devices and Boston keratoprosthesis. Am J Ophthalmol. 2011;152:209–218. doi: 10.1016/j.ajo.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Dudenhoefer EJ, Nouri M, Gipson IK, et al. Histopathology of explanted collar button keratoprostheses: a clinicopathologic correlation. Cornea. 2003;22:424–428. doi: 10.1097/00003226-200307000-00007. [DOI] [PubMed] [Google Scholar]