Abstract
BACKGROUND
In low-renin hypertension (LRH), serum aldosterone levels are higher in those subjects with primary aldosteronism and may be lower in those with non-aldosterone mineralocorticoid excess or primary renal sodium retention. We investigated the hypothesis that the frequency distribution of aldosterone in LRH is bimodal.
METHODS
Of the 3,532 attendees at the sixth examination cycle of the Framingham Offspring Study, 1,831 were included in this cross-sectional analysis after we excluded those with conditions or taking medications such as antihypertensive drugs that might affect renin or aldosterone.
RESULTS
Three hundred three subjects (17%) had untreated hypertension (SBP ≥140mm Hg or DBP ≥90mm Hg). LRH, defined as plasma renin ≤5 mU/L, was present in 93 of those 303 hypertensive subjects (31%). Aldosterone values were adjusted statistically for age, sex, and the urinary sodium/creatinine ratio. In the subjects with LRH, the adjusted aldosterone distribution was bimodal (dip test for unimodality, P = 0.008). The adjusted aldosterone distribution was unimodal in the normal subjects (P = 0.98) and in the hypertensive subjects with normal plasma renin (P = 0.94).
CONCLUSIONS
In this community-based sample of white subjects, those with low-renin hypertension had a bimodal adjusted aldosterone distribution. Subjects with normal-renin hypertension and subjects with normal blood pressure had unimodal adjusted aldosterone distributions. These findings suggest 2 pathophysiological variants of LRH, one that is aldosterone-dependent and one that is non-aldosterone-dependent.
Keywords: aldosterone, blood pressure, hypertension, low-renin hypertension, renin.
Plasma renin is lower than normal in about one-quarter of persons with hypertension.1–5 Primary aldosteronism (Conn’s syndrome) is characterized by low renin as well as by elevated aldosterone levels, and morphologic adrenal abnormalities are common incidental autopsy findings in hypertensive patients. These observations led Conn to suggest that primary aldosteronism might be a common cause of hypertension, responsible for as much as 20% of cases rather than the previously estimated 1% or less.6 But studies of patients with low-renin hypertension (LRH) consistently failed to find elevated aldosterone levels in more than 1% or 2%, and the cause of the renin suppression in most cases remained unexplained.1,3–5
Proposed causes of LRH, in the absence of hyperaldosteronism, include mineralocorticoid excess due to steroids other than aldosterone7 and extracellular fluid volume expansion caused by primary renal retention of sodium.8,9 Aldosterone production, largely dependent on renin, would be expected to be low. Indeed low circulating aldosterone levels have been found in many of these disorders.8,10,11
Recently a new concept of primary aldosteronism, based on an elevated aldosterone/renin ratio coupled with evidence of nonsuppressible serum aldosterone, even in persons with normal serum potassium and aldosterone levels, has greatly increased the estimates of the prevalence of primary aldosteronism.12 A recent review of 18 studies found the median prevalence of primary aldosteronism in persons with hypertension to be 8.9%.13 Common causes of LRH, then, may be primary aldosteronism or other disorders that cause volume expansion with resulting suppression of renin and aldosterone.
These considerations lead us to propose the following hypothesis: LRH consists of aldosterone-dependent disorders (various forms of primary aldosteronism), and non-aldosterone-dependent disorders; the expected elevation of serum aldosterone levels in the first group of disorders and lowered circulating aldosterone levels in the second group suggest the possibility of a bimodal distribution of serum aldosterone levels in LRH. This study was designed to test that hypothesis.
METHODS
Participants
The Framingham Heart Study began in 1948 as a prospective, community-based observational cohort study of 5,209 men and women. The Framingham Offspring Study, started in 1971, consisted of a cohort of 5,135 individuals who were the children, and their spouses, of the original members of the Framingham Heart Study.14 At the sixth examination cycle of the Framingham Offspring Study, performed from 1995 to 1998, blood was drawn that was later assayed for serum aldosterone and plasma renin concentrations. Blood pressure was measured, and other data based on medical history, physical examination, and laboratory measurements were collected. Participants in our study are the 3,345 persons, out of a total of 3,532 attendees at examination cycle 6, for whom complete aldosterone, renin, and blood pressure data were available. All patients studied in the Framingham Heart Study and Framingham Offspring Study were considered by the investigators to be white.
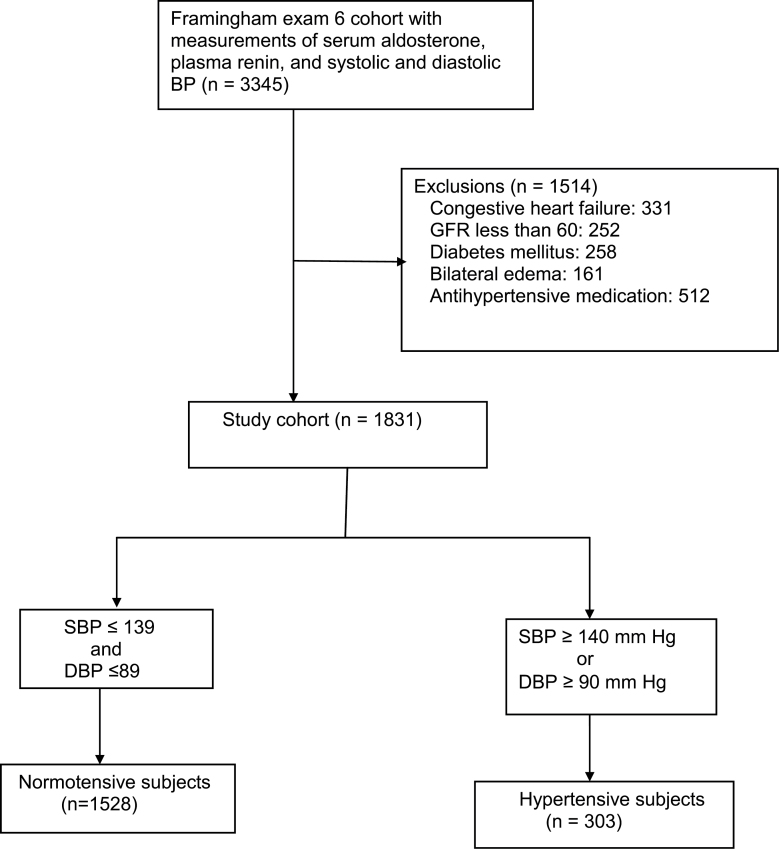
We sought to exclude those subjects who had disorders or were taking medications that would be likely to substantially affect either aldosterone or renin levels. Subjects with congestive heart failure, estimated glomerular filtration rate of <60ml/minute, diabetes mellitus, or bilateral edema and those using any antihypertensive medication were excluded. Subjects taking diuretics, beta-blockers, angiotensin converting-enzyme inhibitors, calcium-channel blockers, and alpha-1 adrenergic receptor blockers were excluded. After exclusions, our study sample consisted of 1,831 individuals aged 29–86 years (Figure 1).
Figure 1.
Flow chart showing the derivation of the 1,831 subjects of this study. Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; GFR, glomerular filtration rate; SBP, systolic blood pressure.
Clinical and laboratory assessment
Blood pressure was measured in the left arm by a Heart Study physician using a mercury column sphygmomanometer and a standardized protocol. Participants rested in the seated position for 5 minutes before blood pressure was measured. Blood pressure was measured twice, and the average of the 2 readings was taken as the blood pressure for the study.
Blood for aldosterone and renin measurement was drawn between 8 am and 10 am. Subjects were fasting and ambulatory but remained in a recumbent position for 5–10 minutes before blood was drawn. Specimens were promptly stored at −80 °C. Serum aldosterone was measured by a sensitive radioimmunoassay (Quest Diagnostics, Cambridge, MA).15 The sensitivity of the assay is <1ng/dl, with an intra-assay coefficient of variation ranging from 3.8% at high levels to 6.0% at low levels. Corresponding interassay coefficient of variation ranged 4.0%–9.8%.
Plasma renin concentration (in milliunits per liter) was measured between June and September 2004 using an immunochemiluminometric assay (Nichols Advantage Direct Renin Assay, Quest Diagnostics Nichols Institute, San Juan Capistrano, CA).16 Intra-assay coefficient of variation ranged 1.7%–5.3%; interassay coefficient of variation ranged 2.7%–8.2%.
Statistical analysis
To compare levels of continuous variables in 2 groups, we used independent samples t tests, Mann–Whitney–Wilcoxon tests, or permutation tests as appropriate. Fisher exact tests were used to compare proportions. Because the data suggest differences in sex distribution and the urine sodium/creatinine ratio among the groups studied (Table 1), and because age-related differences in aldosterone levels have been reported,17 we adjusted for these variables. We regressed natural-log-transformed values of aldosterone on age, sex, and the urinary sodium/creatinine ratio and graphed the exponentiated predicted values of natural logarithm of aldosterone (ln(aldo)). Those back-transformed values hereafter will be called adjusted aldosterone. Regression diagnostics were performed for the analysis of hypertensives with low plasma renin (≤5 mU/L). These included a normal QQ residual plot and tests for normality of residuals, linearity of ln(aldo), heterogeneity of variance, omitted variables, and multicollinearity. These all showed that the model specification was adequate.
Table 1.
Demographic, physical examination, and laboratory characteristics of the study subjects
| Normotensive (n = 1,528) | Hypertensive (n = 303) | Hypertensive, renin >5 mU/L (n = 210) | Hypertensive, renin ≤5 mU/L (n = 93) | |
|---|---|---|---|---|
| Age in years, mean (SD) | 55.1 (9.0) | 60.3 (8.9) | 60.1 (9.3) | 60.6 (7.9) |
| Male, No. (%) | 707 (46.3) | 161 (53.1) | 127 (60.5) | 34 (36.6) |
| Body mass index, kg/m2, mean (SD) | 26.7 (4.4) (n = 1,524) | 28.0 (4.9) | 28.0 (4.9) | 28.0 (4.9) |
| Systolic blood pressure, mm Hg, mean (SD) | 118 (11.5) | 152 (13.9) | 150.6 (12.5) | 154.5 (15.8) |
| Diastolic blood pressure, mm Hg, mean (SD) | 73 (7.7) | 85 (9.6) | 84.6 (9.6) | 84.5 (9.8) |
| Current smoking, No. (%) | 268 (17.5) | 50 (16.5) | 32 (15.2) | 18 (19.4) |
| Urine sodium/creatinine, mmol/g, mean (SD) | 109.8 (71.0) (n = 1,326) | 118.0 (73.9) (n = 262) | 109.9 (68.8) | 136.2 (81.7) |
| Family history of hypertension, No. (%) | 736/1,159 (63.5) | 152/221 (68.8) | 106/159 (66.7) | 46/62 (74.2) |
To determine whether the distribution of the aldosterone values (both unadjusted and adjusted) in LRH was bimodal, we used dotplots and the dip test for unimodality.18 In the dip test, if P < 0.05, we rejected the null hypothesis of unimodality and concluded that the distribution has >1 mode. Statistical tests were two-sided. All analyses were performed using Stata 11 (StataCorp, College Station, TX).
RESULTS
Figure 1 shows the exclusion process that resulted in the final sample of 1,831 subjects. One thousand five hundred twenty-eight persons had normal blood pressure, and 303 had untreated hypertension, as defined in Figure 1.
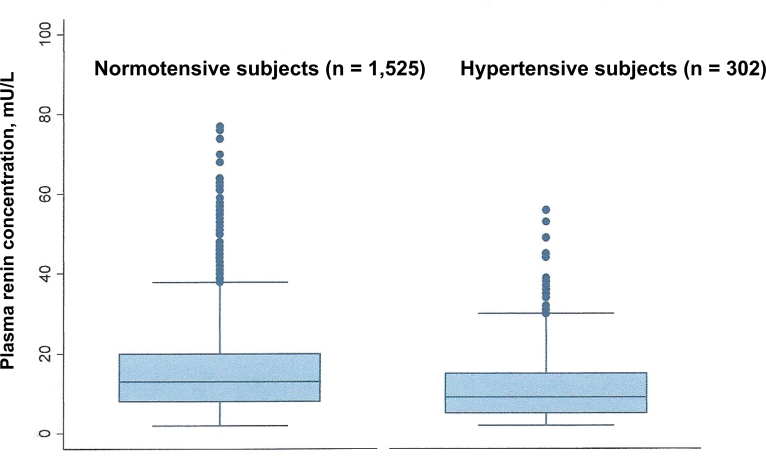
Figure 2 shows the distribution of renin levels in the normotensive and hypertensive groups. An increased frequency of renin levels in the lower ranges was evident in hypertensive subjects. The median renin concentration was 13 mU/L in the normotensive subjects, compared with 9 mU/L in the hypertensive subjects. There was no evident excess of high renin levels in the hypertensive group, presumably because we excluded many individuals who may have had secondary aldosteronism. Only 1.7% of hypertensive subjects had renin levels above the 97.5 percentile of the normotensive group.
Figure 2.
Box plots showing the plasma renin concentrations in the normotensive and hypertensive subjects. The horizontal lines above and below the box (whiskers) show the lower and upper values that are the furthest observations within 1.5 times the interquartile range of the lower and upper ends of the box. The mean renin concentration in normotensive subjects was 15.9 mU/L; the mean renin concentration in hypertensive subjects was 12.7 mU/L. The mean difference between normotensive and hypertensive subjects was 3.2 mU/L (95% confidence interval = 1.2–5.0; P = 0.001). Four normotensive and 1 hypertensive subject with renin >100 mU/L are not shown.
There was no clear criterion for defining LRH. Previous studies1,4 have shown that consideration only of baseline renin levels fails to identify many persons with renin that is unresponsive to dietary or diuretic-induced salt depletion, a more sensitive indicator of renin suppression. Also, renin values were expressed only as whole numbers, so it was not possible to calculate percentiles (e.g., the 22 hypertensive subjects with a renin value of 2 mU/L could not be ranked individually). For this reason, in examining the distribution of aldosterone levels, we looked at each of the subsets of hypertensive subjects created by using renin values ≤3, 4, 5, 6, 7, and 8 mU/L as cutoff points to define low renin. These corresponded to low-renin subsets that constituted 14.9%, 21.5%, 30.7%, 36.6%, 44.2%, and 49.5% of the hypertensive subjects, respectively. We selected the cutpoint of renin ≤5 mU/L for the comparison of demographic and other characteristics of the low-renin and normal-renin hypertensive subjects (Table 1) and for the distribution of aldosterone values in normal-renin hypertensive subjects.
Table 1 shows demographic and other characteristics of the study subjects in 4 groups: normotensive, all hypertensive, low-renin hypertensive, and normal-renin hypertensive.
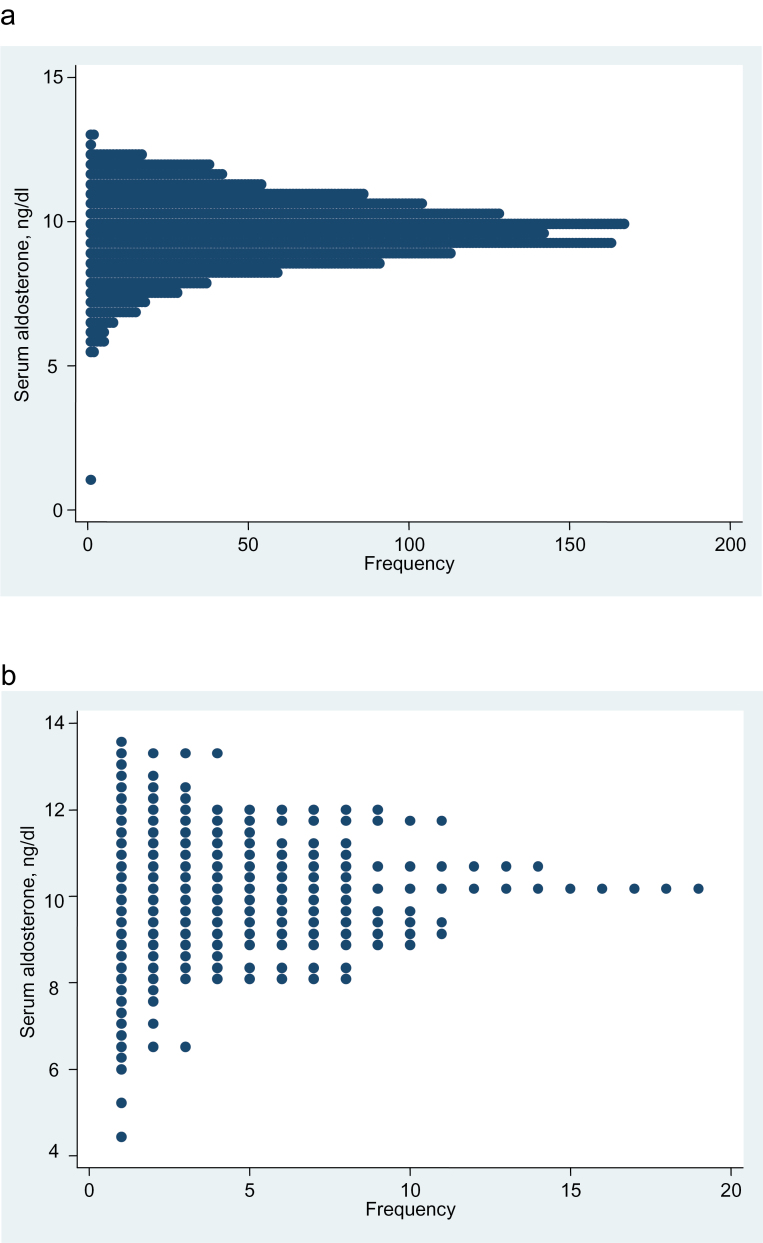
The distribution of adjusted aldosterone values was unimodal in the normotensive subjects (dip test for unimodality, P = 0.98) and in the hypertensive subjects with normal renin, which was defined as plasma renin of ≥6 mU/L (P = 0.94) (Figure 3).
Figure 3.
Dotplot showing the aldosterone frequency distribution, etc. (a) Aldosterone frequency distribution, adjusted for age, sex, and urine sodium/creatinine ratio, in the 1,326 of the 1,528 normotensive subjects for whom the urine sodium/creatinine ratio was available. Dip test for unimodality, P = 0.98. (b) Aldosterone frequency distribution, adjusted for age, sex, and urine sodium/creatinine ratio, in the 181 of the 210 subjects with hypertension and plasma renin concentration ≥6 mU/L, for whom the urine sodium/creatinine ratio was available. Dip test for unimodality, P = 0.94.
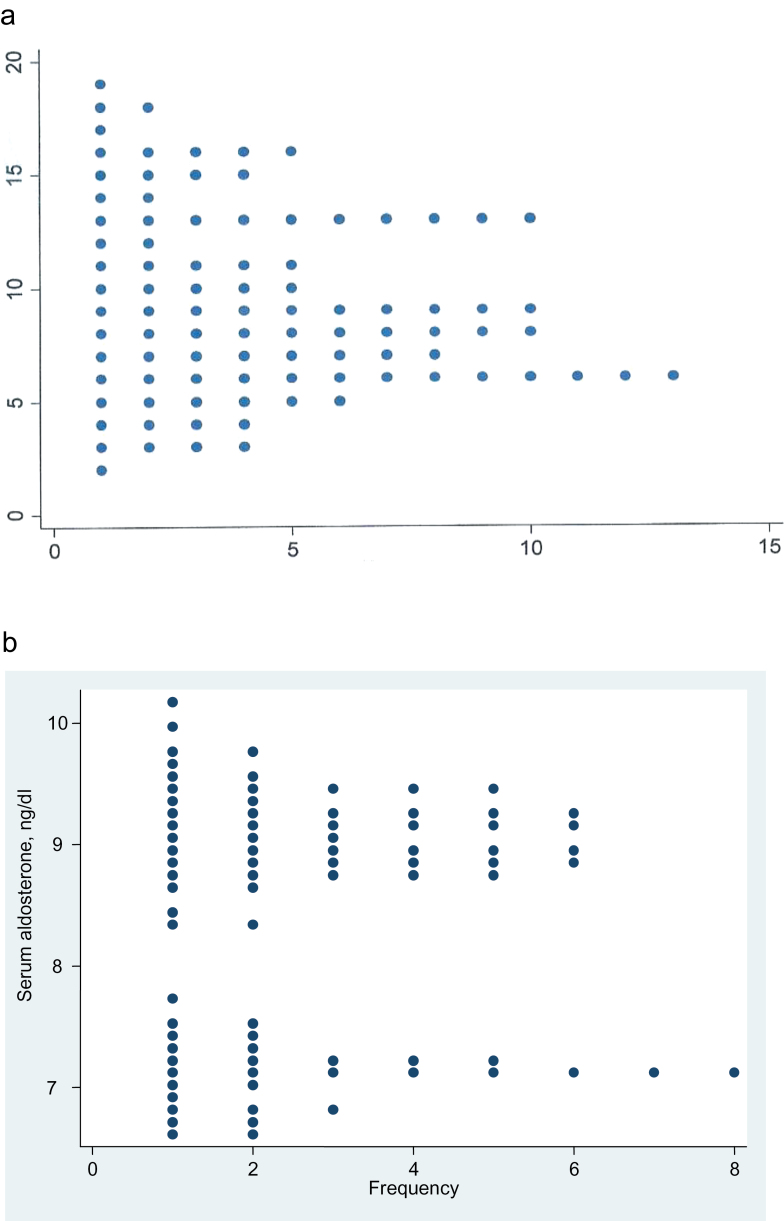
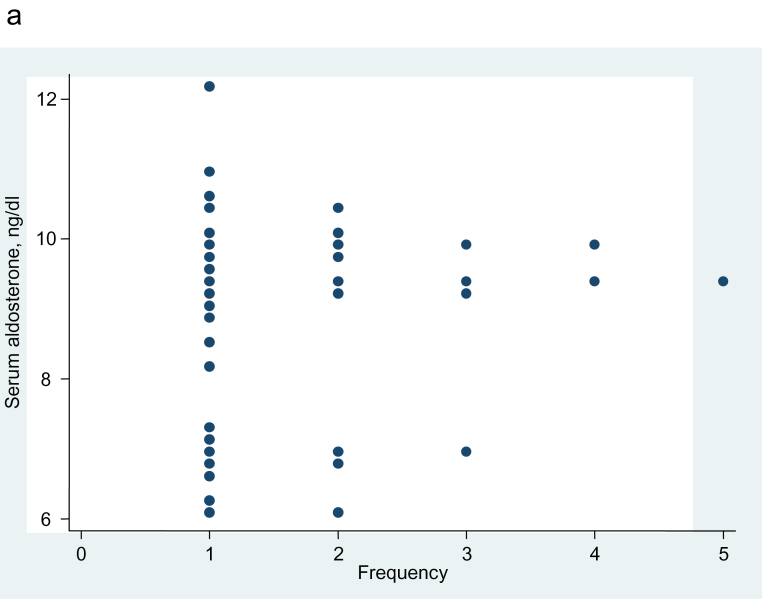
In the subjects with LRH, which was defined as renin ≤5 mU/L, the plot of unadjusted aldosterone values appeared as if it might be bimodal, but this possibility was not confirmed by the dip test for unimodality (P = 0.11) (Figure 4a). But after adjustment for age, sex, and urinary sodium/creatinine ratio, the aldosterone distribution is clearly bimodal in the dotplot and by the dip test (P = 0.008) (Figure 4b).
Figure 4.
Dotplot showing the aldosterone frequency distribution, etc. (a) Aldosterone frequency distribution, unadjusted, in 93 subjects with untreated hypertension and plasma renin concentration ≤5 mU/L. Dip test for unimodality, P = 0.11. (b) Aldosterone frequency distribution, adjusted for age, sex, and urine sodium/creatinine ratio, in the 81 of the 93 subjects with hypertension and plasma renin concentration ≤5 mU/L, for whom the urine sodium/creatinine ratio was available. Dip test for unimodality, P = 0.008.
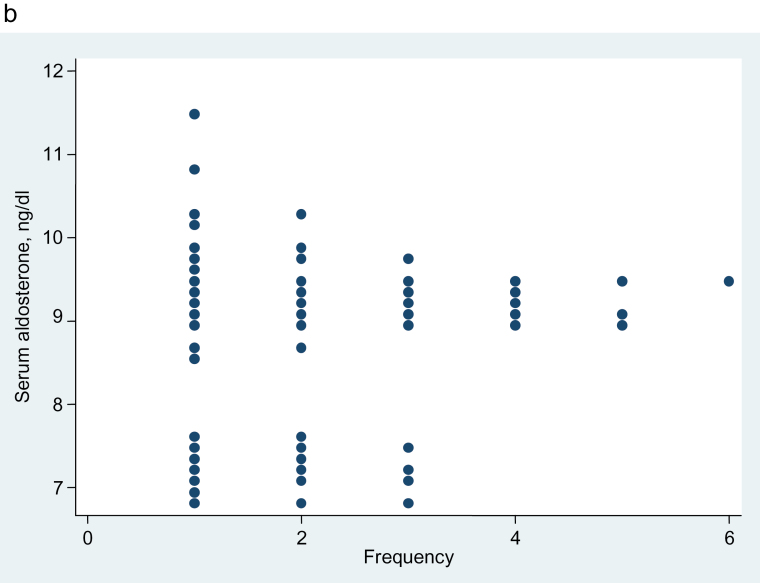
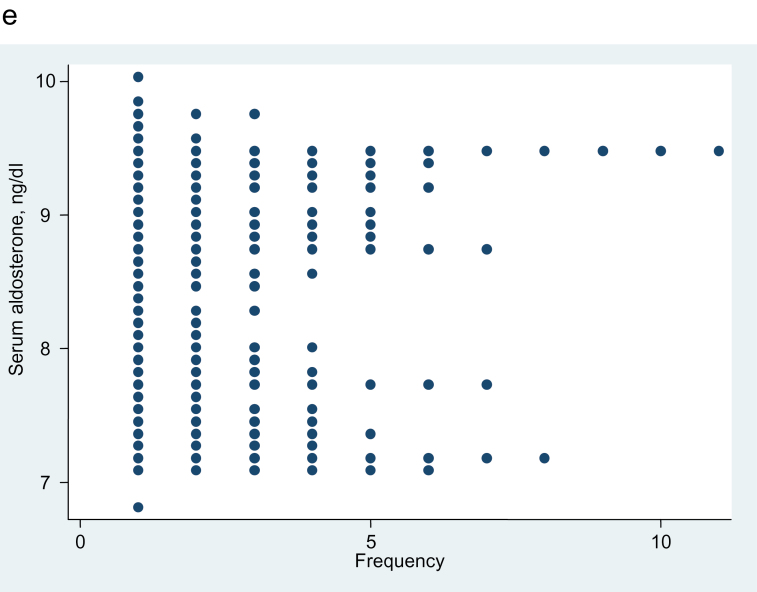
Figure 5 shows the distribution of adjusted aldosterone values in LRH subsets defined by increasing cutoff points for plasma renin from 3 through 8 mU/L (except for 5 mU/L, which is shown in Figure 4). For the plasma renin with cutoff points of 3, 4, and 5, the separation of adjusted aldosterone values in the low-renin groups is complete (Figure 5). Although there is less separation between the 2 parts of the frequency distributions of adjusted aldosterone as subjects with higher renin levels are added (cutoff points of 6, 7, and 8), those dotplots still suggest the possibility of bimodality.
Figure 5.
Dotplot showing the aldosterone frequency distribution, etc. Aldosterone frequency distribution, adjusted for age, sex, and urine sodium/creatinine ratio. (a) Thirty-seven hypertensive subjects with plasma renin concentration ≤3 mU/L. Dip test for unimodality, P = 0.25. (b) Fifty-five hypertensive subjects with plasma renin concentration ≤4 mU/L. Dip test for unimodality, P = 0.06. (c) Ninety-six hypertensive subjects with plasma renin concentration ≤6 mU/L. Dip test for unimodality, P = 0.43. (d) One hundred fifteen hypertensive subjects with plasma renin concentration ≤7 mU/L. Dip test for unimodality, P = 0.06. (e) One hundred twenty-nine hypertensive subjects with plasma renin concentration ≤8 mU/L. Dip test for unimodality, P = 0.11.
DISCUSSION
We have analyzed the distribution of serum aldosterone in a community-based sample of 1,831 persons, after excluding those who were taking antihypertensive medications or were believed to have comorbidities likely to affect aldosterone or renin levels. In normotensive subjects and in hypertensive subjects with normal plasma renin concentration, we observed a unimodal distribution of serum aldosterone levels. In the hypertensive subjects with low plasma renin concentration the adjusted aldosterone distribution was bimodal.
The finding of a bimodal aldosterone distribution in LRH may offer clues to the pathophysiology of LRH. High serum aldosterone levels in one group of patients with LRH suggests an aldosterone-dependent form of the disorder, caused by 1 of the several forms of primary aldosteronism; the combination of low renin and high aldosterone is considered pathognomonic of primary aldosteronism.19
The finding of a distinct group of LRH patients with lower aldosterone levels suggests a non-aldosterone-dependent form of the disorder. LRH without elevated aldosterone is often referred to as low-renin essential hypertension and is widely believed to result from sodium retention and subsequent expansion of extracellular fluid volume, in most cases of undetermined cause. Mineralocorticoid excess due to steroids other than aldosterone has been suggested by a number of findings, including a low salivary sodium/potassium ratio (an indicator of mineralocorticoid excess)20,21 and by selective response of the hypertension to a steroid antagonist.22 A number of mineralocorticoids and mineralocorticoid-related syndromes have been found to cause LRH, but none has been implicated in more than a small number of cases.5,7 Interest continues in the syndrome of apparent mineralocorticoid excess, in which deficiency of 11β-hydroxysteroid dehydrogenase type 2 causes cortisol to act as a potent mineralocorticoid.23,24 The activity of this enzyme has recently been found to be decreased in LRH25 and to be decreased with aging26 and in salt-sensitivity,27 both of which are associated with LRH.
Causes other than mineralocorticoid excess that may cause LRH with decreased serum aldosterone levels include primary renal sodium retention, as in Liddle’s syndrome,8 and perhaps suppression of renin by the effects of arterial hypertension itself.28 Excess dietary sodium ingestion has been proposed, but there is no evidence that this is an important factor.29
It is often stated that serum aldosterone levels are normal in LRH but that the normal levels are inappropriately elevated in the face of low renin, suggesting a possible role for altered aldosterone responsiveness to angiotensin in the pathogenesis of the hypertension.30–33 Also, the “normal” serum aldosterone levels in LRH have been cited as evidence that another mineralocorticoid could not be the cause of the hypertension because a lowered aldosterone level would then be expected.34,35 Our finding that serum aldosterone is bimodally distributed challenges these notions and is consistent with the idea that a mineralocorticoid other than aldosterone is associated with the lowered aldosterone seen in some cases of LRH.
Studies of the pathophysiology and therapy of LRH frequently consider it to be a single entity, with normal aldosterone levels. Our data suggest that an increase in serum aldosterone levels in an aldosterone-dependent subset of LRH may be masked by a decrease in aldosterone levels in a non-aldosterone-dependent subset if the aldosterone level is averaged in all individuals with LRH. Future studies of LRH should consider this possibility.
This study has several strengths. The Framingham data are derived from a large, community-based sample that has been carefully studied under uniform conditions. The numbers were large enough to allow exclusion of many subjects with disorders or taking medications that are known to affect the renin-aldosterone system.
But there are also many limitations. The 2 blood pressure measurements in each participant were obtained on a single day; the diagnosis of hypertension is therefore much less certain than if the measurements had been obtained on multiple occasions over a period of time. The subjects of the Framingham Heart Study were exclusively white; our findings cannot be extended to nonwhite individuals, especially in view of the many studies that have showed differences in the prevalence of LRH in black populations. Some disorders that may affect the renin-aldosterone axis, such as Cushing syndrome, hepatic cirrhosis, and nephrotic syndrome could not be excluded because the Framingham data did not identify them. Data on serum and urine potassium levels, which may affect aldosterone, also were not available. LRH is most reliably identified by examining plasma renin levels that have been stimulated by salt restriction or by rapidly acting diuretics, as well as several hours of upright posture. The renin levels in our study were stimulated only by the upright posture assumed between rising in the morning and the blood drawing between 8 am and 10 am; this may have diminished the accuracy of our identification of the LRH cohort. It is likely, however, that these limitations would have biased our findings toward the null hypothesis–that is, would have decreased the likelihood of our finding a bimodal serum aldosterone distribution.
These observations, although consistent with the existence of 2 pathophysiologically different variants of LRH, shed no light on the underlying causes of aldosterone-dependent and non-aldosterone-dependent LRH. A prospective study of hormonal and renal factors in an untreated, multiethnic hypertensive population may be needed to further advance our understanding of this common disorder.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
R.S.V. is supported in part by contract NO1HL25195 from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1. Channick BJ, Adlin EV, Marks AD. Suppressed plasma renin activity in hypertension. Arch Intern Med 1969; 123:131–140 [PubMed] [Google Scholar]
- 2. Brunner HR, Laragh JH, Baer L, Newton NA, Goodwin FT, Krakoff LR, Bard RH, Buhler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med 1972; 286:441–449 [DOI] [PubMed] [Google Scholar]
- 3. Dunn MJ, Tannen RL. Low-renin hypertension. Kidney Int 1974; 5:317–3–25 [DOI] [PubMed] [Google Scholar]
- 4. Gunnells JC, Jr, McGuffin WL., Jr Low renin hypertension. Annu Rev Med 1975; 26:259–275 [DOI] [PubMed] [Google Scholar]
- 5. Ganguly A, Weinberger MH. Low renin hypertension: A current review of definitions and controversies. Amer Heart J 1979; 98:642–652 [DOI] [PubMed] [Google Scholar]
- 6. Conn JW. Plasma renin activity in primary aldosteronism: Importance in differential diagnosis and in research of essential hypertension. JAMA 1964; 190:222–225 [PubMed] [Google Scholar]
- 7. Gomez-Sanchez CE. The role of steroids in human essential hypertension. Biochem Pharmacol 1982; 31:893–897 [DOI] [PubMed] [Google Scholar]
- 8. Liddle GW, Bledsoe T, Coppage WS., Jr A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians 1963; 76:199–213 [Google Scholar]
- 9. Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 2002; 346:913–923 [DOI] [PubMed] [Google Scholar]
- 10. White PC, New MI, Dupont B. Congenital adrenal hyperplasia. N Engl J Med 1987; 316:1580–1586 [DOI] [PubMed] [Google Scholar]
- 11. Epstein MT, Espiner EA, Donald RA, Hughes H. Effect of eating liquorice on the renin-angiotensin aldosterone axis in normal subjects. Brit Med J 1977; 1:488–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulatero P, Stowasser M, Loh K-C, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez C.E, Veglio F, Young WF., Jr Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004; 89:1045–1050 [DOI] [PubMed] [Google Scholar]
- 13. Rossi GP. Primary aldosteronism: needle in a haystack or yellow cab on Fifth Avenue? Curr Hypertens Rep 2004; 6:1–4 [DOI] [PubMed] [Google Scholar]
- 14. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol 1979; 110:281–290 [DOI] [PubMed] [Google Scholar]
- 15. Ito T, Woo J, Haning R, Horton R. A radioimmunoassay for aldosterone in human peripheral plasma including a comparison of alternate techniques. J Clin Endocrinol Metab 1972; 34:106–112 [DOI] [PubMed] [Google Scholar]
- 16. De Bruin RA, Bouhuizen A, Diederich S, Perschel FH, Boomsma F, Deinum J. Validation of a new automated renin assay. Clin Chem 2004; 50:2111–2116 [DOI] [PubMed] [Google Scholar]
- 17. Weidmann P, De Myttenaere-Bursztein S, Maxwell MH, De Lima J. Effect of aging on plasma renin and aldosterone in normal man. Kidney Int 1975; 8:325–333 [DOI] [PubMed] [Google Scholar]
- 18. Hartigan JA, Hartigan PM. The dip test of unimodality. Ann Statistics 1985; 13:70–84 [Google Scholar]
- 19. Conn JW, Cohen EL, Rovner DR. Suppression of plasma renin activity in primary aldosteronism: distinguishing primary from secondary aldosteronism in hypertensive disease. JAMA 1964; 190:213–221 [DOI] [PubMed] [Google Scholar]
- 20. Adlin EV, Channick BJ, Marks AD. Salivary sodium-potassium ratio and plasma renin activity in hypertension. Circulation 1969; 39:685–692 [DOI] [PubMed] [Google Scholar]
- 21. Crane MG, Harris JJ, Johns VJ., Jr Hyporeninemic hypertension. Am J Med 1972; 52:457–466 [DOI] [PubMed] [Google Scholar]
- 22. Woods JW, Liddle GW, Stant EG, Jr, Michelakis AM, Brill AB. Effect of an adrenal inhibitor in hypertensive patients with suppressed renin. Arch Intern Med 1969; 123:366–370 [PubMed] [Google Scholar]
- 23. White PC, Mune T, Agarwal AK. 11β-hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocrinol Rev 1997; 18:135–156 [DOI] [PubMed] [Google Scholar]
- 24. Wilson RC, Dave-Sharma S, Wei J-Q, Obeyesekere VR, Li K, Ferrari P, Krozowski ZS, Shackleton CHL, Bradlow L, Wiens T, New MI. A genetic defect resulting in mild low-renin hypertension. Proc Natl Acad Sci 1998; 95:10200–10205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carvajal CA, Romero DG, Mosso LM, Gonzalez AA, Campino C, Montero J, Fardella CE. Biochemical and genetic characterization of 11 β-hydroxysteroid dehydrogenase type 2 in low-renin essential hypertensives. J Hypertens 2005; 23:71–77 [DOI] [PubMed] [Google Scholar]
- 26. Henschkowski J, Stuck AE, Frey BM, Gillmann G, Dick B, Frey FJ, Mohaupt MG. Age-dependent decrease in 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) activity in hypertensive patients. Am J Hypertens 2008; 21:644–649 [DOI] [PubMed] [Google Scholar]
- 27. Lovati E, Ferrari P, Dick B, Jostarndt K, Frey BM, Frey FJ, Schorr U, Sharma AM. Molecular basis of human salt sensitivity: the role of the 11β-hydroxysteroid dehydrogenase type 2. J Clin Endocrinol Metab 1999; 84:3745–3749 [DOI] [PubMed] [Google Scholar]
- 28. Meade TW, Imeson JD, Gordon D, Peart WS. The epidemiology of plasma renin. Clin Sci 1983; 64:273–280 [DOI] [PubMed] [Google Scholar]
- 29. Adlin EV, Biddle CM, Channick BJ. Dietary salt intake in hypertensive patients with normal and low plasma renin activity. Am J Med Sci 1971; 261:67–71 [DOI] [PubMed] [Google Scholar]
- 30. Buhler FR, Laragh JH, Sealey JE, Brunner HR. Plasma aldosterone-renin relationships in various forms of essential hypertension: studies using a rapid assay of plasma aldosterone. Am J Cardiol 1973; 32:554–561 [DOI] [PubMed] [Google Scholar]
- 31. Grim CE. Low renin “essential” hypertension: a variant of classic primary aldosteronism? Arch Intern Med 1975; 135:347–350 [PubMed] [Google Scholar]
- 32. Mitchell JR, Taylor AA, Pool JL, Lake CR, Rollins DE, Bartter FC. NIH conference: renin-aldosterone profiling in hypertension. Ann Intern Med 1977; 87:596–612 [DOI] [PubMed] [Google Scholar]
- 33. Griffing GT, Wilson TE, Melby JC. Alterations in aldosterone secretion and metabolism in low renin hypertension. J Clin Endocrinol Metab 1990; 71:1454–1460 [DOI] [PubMed] [Google Scholar]
- 34. Laragh JH, Sealey J, Brunner HR. The control of aldosterone secretion in normal and hypertensive man: abnormal renin:aldosterone patterns in low renin hypertension. Am J Med 1972; 53:649–663 [DOI] [PubMed] [Google Scholar]
- 35. Taylor AA, Mitchell JR, Bartter FC, Snodgrass WR, McMurtry RJ, Gill JR., Jr Effect of aminoglutethimide on blood pressure and steroid secretion in patients with low renin essential hypertension. J Clin Invest 1978; 62:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]