Abstract
Many arboviral proteins are phosphorylated in infected mammalian cells, but it is unknown if the same phosphorylation events occur when insects are similarly infected. One of the mammalian kinases responsible for phosphorylation, protein kinase G (PKG), has been implicated in the behavior of multiple nonvector insects, but is unstudied in mosquitoes. PKG from Aedes aegypti was cloned, and phosphorylation of specific viral sites was monitored by mass spectrometry from biochemical and cell culture experiments. PKG from Aedes mosquitoes is able to phosphorylate dengue nonstructural protein 5 (NS5) at specific sites in cell culture and cell-free systems and autophosphorylates its own regulatory domain in a cell-free system. Injecting Aedes aegypti and Anopheles gambiae mosquitoes with a pharmacological PKG activator resulted in increased Aedes wing activity during periods of their natural diurnal/crepuscular activity and increased Anopheles nocturnal locomotor/flight activity. Thus, perturbation of the PKG signaling pathway in mosquitoes alters flight behavior. The demonstrated effect of PKG alterations is consistent with a viral PKG substrate triggering increased PKG activity. This increased PKG activity could be the mechanism by which dengue virus increases flight behavior and possibly facilitates transmission. Whether or not PKG is part of the mechanism by which dengue increases flight behavior, this report is the first to show PKG can modulate behavior in hematophagous disease vectors.
Key Words: Protein kinase G, Phosphorylation, Nonstructural protein 5, Wing activity, Dengue virus
Introduction
The flavivirus genus (family Flaviviridae) includes a number of medically relevant viruses, often transmitted by an arthropod vector (such as mosquito or tick) to a mammalian or avian host. For example, dengue virus (DENV) is transmitted primarily by Aedes mosquitoes, particularly Aedes aegypti (Black et al. 2002, Vasilakis et al. 2008), and West Nile virus (WNV) is transmitted primarily by Culex mosquitoes (Hayes et al. 2005). Both mosquito-borne and tick-borne flaviviral nonstructural 5 (NS5) proteins, which contain a methyltransferase (MTase) domain and an RNA-dependent RNA polymerase domain, are phosphorylated in flavivirus-infected mammalian cells (Morozova et al. 1997, Reed et al. 1998, Bhattacharya et al. 2008, Bhattacharya et al. 2009, Keating et al. 2010). Potentially, multiple mammalian kinases phosphorylate NS5. The best-characterized phosphorylation is mediated by mammalian protein kinase G (PKG), a cyclic guanosine monophosphate (cGMP)-dependent serine/threonine kinase, that phosphorylates mosquito-borne flaviviruses DENV and WNV NS5 protein at multiple sites, but not tick-borne flaviviral NS5 (Langat virus) (Bhattacharya et al. 2009, Keating et al. 2010).
The addition of a negatively charged phosphate group to a protein by a kinase can affect that protein's enzymatic activity, stability, and/or its interactions with other viral and cellular host proteins during an infection (Jakubiec et al. 2007). Viruses may be phosphorylated by viral or host kinases, and roles for many phosphorylations continue to be determined (Keating et al. 2011). As an example of the potential importance of phosphorylations during flaviviral infections in mammalian cells, the yellow fever virus NS5 protein is phosphorylated at Ser56, and evidence suggests this phosphorylation inhibits the MTase activity of NS5 (Bhattacharya et al. 2008). Despite these recent advances in understanding the role of phosphorylation in flaviviral infections, the presence and identity of phosphorylation sites, as well as the potential impact of any phosphorylation events, during a vector flaviviral infection is unknown.
Interestingly, PKG activity is also related to various behavioral modulations in a number of insects, including Drosophila (Osborne et al. 1997, Kaun et al. 2007), honeybees (Apis mellifera) (Ben-Shahar et al. 2002), and ants (Pheidole pallidula) (Lucas et al. 2009). For example, increased levels of PKG activity [encoded by the foraging or forager (for) gene] is associated with increased foraging behavior in naturally occurring Drosophila variants (Osborne et al. 1997), and an increase in PKG expression over the individual honeybee's life span corresponds with a transition from hive work to foraging behaviors (Ben-Shahar et al. 2002). A potential for gene in mosquitoes was identified on the basis of its sequence homology to Drosophila and human PKGI (Nene et al. 2007). We hypothesized that the protein encoded by this gene in mosquitoes phosphorylates flaviviral proteins, and that alteration of the PKG signaling pathway would modulate mosquito behavior, possibly facilitating viral transmission. Prior to this work, PKG's effects on insect behavior have not been demonstrated in any vector of human disease.
In the present study, we demonstrate that flaviviral NS5 is phosphorylated during an infection in mosquito cells. A. aegypti PKG protein (AePKG) expressed in a cell-free system has catalytic activity and is able to phosphorylate mosquito-borne flaviviral NS5 substrates. AePKG also autophosphorylates, and specific autophosphorylation sites were mapped, which has not previously been performed for any for insect homolog. Additionally, we demonstrate that treatment of mosquitoes (both flaviviral vectors and nonflaviral vectors) with a PKG-activating compound is associated with increased behavioral activity. These findings begin to shed light on the molecular and organismal effects of PKG and flaviviral vector infections.
Materials and Methods
Cell culture and viral infection
Aedes albopictus [c6/36, Americam Type Culture Collection (ATCC) # CRL-1660] cells were maintained at 28°C in L-15 medium (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. Stocks of DENV-2 strain 16681 (GenBank accession number U87411, Centers for Disease Control and Prevention, Fort Collins, CO) were prepared in c6/36 cells as previously described (Lim et al. 2010).
c6/36 cells were infected with DENV-2 (16681) at a multiplicity of infection (MOI)=10 in serum-free L-15 medium. Infected cells were incubated for 1 h at 28°C; FBS was added to the medium at a final concentration of 2%. The infected cells were incubated for 48 h at 28°C, and cells were lysed by homogenization. NS5 was immunoprecipitated from the infected cell lysate using a custom-made rabbit polyclonal antibody against WNV NS5 (Covance, Princeton, NJ).
Mosquito maintenance and flight monitoring
A. aegypti
A. aegypti (Liverpool strain) mosquitoes were maintained in a 27°C, humidity-controlled insectary at the University of Wisconsin–Madison and fed 0.3 M sucrose solution ad libitum (Christensen et al. 1984). Twenty-four hours after hatching, female mosquitoes were injected with a pharmacological PKG activator, 8-(4-chlorophenylthio) (guanosine-3′-5′-cyclic monophosphate (8-pCPT-cGMP; EMD Biosciences, Gibbstown, NJ) diluted in Aedes saline (150 mM NaCl, 1 mM CaCl2, 2 mM KCl, 1 mM NaHCO3) or Aedes saline alone (vehicle control). Injection procedures have been published previously (Dasgupta et al. 2007). Twenty-four hours after injection, flying mosquitoes were selected for flight monitoring in a custom-made flight chamber (Berry et al. 1987, Rowley et al. 1987). Within the large (70 inches×24 inches) flight chamber, multiple small soundproof chambers (5 inches×4.5 inches) allowed for the monitoring of individual mosquitoes. For each mosquito, the flight monitor measured the number of seconds in which the mosquito was beating its wings within 30-min intervals. Activity of each mosquito was measured for approximately 7 days in a controlled 16 h light/8 h dark environment. Mosquitoes fed on sucrose ad libitum. The wing activity during each 30-min interval was averaged among all PKG activator-treated versus all control mosquitoes. This wing activity experiment was repeated three times. The numbers of mosquitoes used in each experimental run were based on variability data from a pilot experiment to increase power in statistical calculations. Mosquitoes that died during the course of monitoring wing activity (within the flight chamber) were removed from final data analysis. For run 1 (see Fig. 6, below), 16 control mosquitoes and 10 PKG activator-treated mosquitoes (2.5 mM) were used; there was a 100% survival rate for both groups during the course of wing activity monitoring. Run 2 (Fig. S1)(Supplementary Data are available at www.liebertonline/vbz/) used 14 control mosquitoes (64% survival) and 6 PKG activator-treated mosquitoes (2.5 mM) (83% survival). Run 3 (Fig. S2) used 5 control mosquitoes (80% survival) and 10 PKG activator-treated mosquitoes (5 mM) (100% survival).
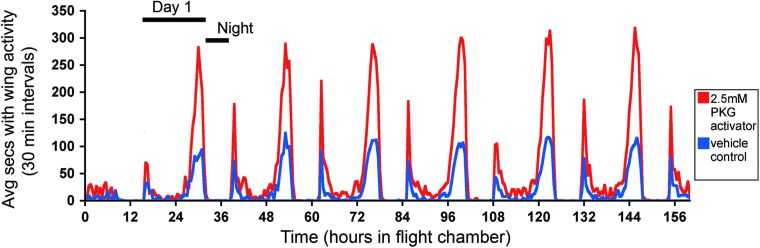
FIG. 6.
Aedes mosquitoes treated with protein kinase G (PKG) activator shows increased peaks of flight activity in morning and evening. Female A. aegypti mosquitoes were injected with a PKG activator drug [8-(4-chlorophenylthio) (guanosine-3′-5′-cyclic monophosphate (8-pCPT-cGMP)] or vehicle control. Twenty-four hours after injection, healthy individual mosquitoes were placed in soundproof glass chambers with an attached microphone to measure the number of seconds in 30-min intervals that each mosquito was beating its wings. Mosquitoes were measured over the course of 160 h, with night (8 dark hours) and each “day” (16 light hours) notated on the figure. The average amount of time in flight (30-min intervals) for mosquitoes treated with PKG activator is plotted in red and for control mosquitoes is in blue.
Anopheles gambiae
Never-blood-fed, mated female Anopheles gambiae Pimperena S form mosquitoes [MRA-861, Malaria Research and Reference Reagent Resource Center (MR4), ATCC] were maintained at the University of Notre Dame in a humidity-controlled environment at 27±1°C. Injections began when mosquitoes were between 4–5 days postemergence.
Mosquitoes were injected with 0.2 μL of 10 mM 8-pCPT-cGMP, 20% dimethyl sulfoxide (DMSO), 80% saline (150 mM NaCl, 1 mM CaCl2, 2 mM KCl, 1 mM NaHCO3) solution or a DMSO/saline vehicle control (0 mM). The day after injection, flying mosquitoes were selected for 5 full days of individual locomotor/flight monitoring in a Locomotor Activity Monitor 25 (L.A.M. 25) system (TriKinetics, Waltham, MA). This system has previously been used to monitor A. gambiae locomotor/flight activity (Rund et al. 2011, Rund et al 2012). Individual mosquitoes were placed in 25-×150-mm clear glass tubes with access to fructose in the tubes provided ad libitum. Flight activity for each individual animal was detected as the animals broke infrared beams passing through the glass tubes. Each L.A.M. unit allows simultaneous monitoring of 32 mosquitoes in a 4×8 vertical by horizontal matrix. All recordings occurred in a lightproof box with its own lighting system: 11 h full light (∼94 lux), 11 h darkness, and 1 h dawn and 1 h dusk transitions environment. The number of minutes of detectable locomotor/flight activity within 60-min intervals was determined for both PKG activator-treated and control mosquitoes.
This locomotor/flight activity experiment was repeated three times. There was a 47%±16% [mean±standard error of the mean (SEM)] survival rate of control-treated mosquitoes and 45%±21% (mean±SEM) for PKG activator-treated mosquitoes during the 5-day course of monitoring flight activity (within the flight chamber). Mosquitoes that died within the flight chamber during the 5 days of recording were removed from final data analysis. Data from a total of 20 control and 19 PKG activator treated mosquitoes was subsequently analyzed.
For both Aedes and Anopheles flight monitoring experiments, time elapsed in the chambers is listed as hours in Figs. 6 and 7.
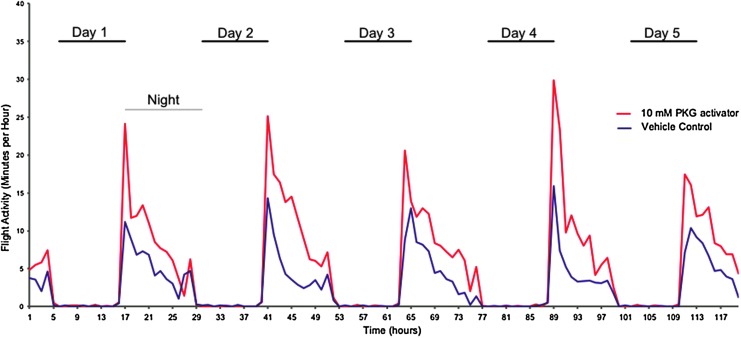
FIG. 7.
A. gambiae mosquitoes treated with protein kinase G (PKG) activator show increased nocturnal locomotor/flight activity. Female A. gambiae mosquitoes were injected with PKG activator drug [8-(4-chlorophenylthio) (guanosine-3′-5′-cyclic monophosphate (8-pCPT-cGMP)] or vehicle control. At 24 h after injection, mosquitoes were placed in the Locomotor Activity Monitor 25 (L.A.M. 25) system, and locomotor/flight activity was monitored over the course of 120 h. Data shown are mean data for 20 control and 19 PKG activator-treated mosquitoes from three separate recording trials. Mosquitoes were subjected to 11 h of dark (“night”) and 11 h of 100% light (“day”) notated on the figure; there were also 1 h of “dawn” and “dusk” transitional light periods between “night” and “day.” The number of minutes with detectable locomotor/flight activity within 60-min intervals was plotted for both PKG activator-treated mosquitoes (red) and control mosquitoes (blue).
Extraction of Aedes aegypti RNA
Five female A. aegypti whole bodies (including heads) were collected in an Eppendorf tube. RNA extraction was performed with RNAzol solution [4 M guanidine isothiocyanate, 0.0225 M sodium citrate (pH=7.0), 0.5% Sarkosyl; Fisher Scientific, Pittsburgh, PA]. Then 750 μL of RNAzol solution was added to the tube for grinding with a pestle. RNA was purified by chloroform extraction and ethanol precipitation, treated with DNase (Ambion, Grand Island, NY), and resuspended in diethylpyrocarbonate-treated water.
Cloning and expression of Aedes aegypti and human PKG
The total RNA extract from A. aegypti bodies was used as a template for reverse transcription PCR with the SuperScript III One-Step RT-PCR system with Platinum Taq (Invitrogen, Grand Island, NY). Primers to amplify A. aegypti PKG were based on National Center for Bioinformatics Information (NCBI) accession number XM_001652896 (forward primer, 5′-ATGTCCTCTCCAAAGCCAGCGATGGCTGGTGG-3′, and reverse primer, 5′-GAAATCTTCATCCCATCCGGAAAGATCATCTGG-3′, total length 2482 base pairs). The reverse transcription step was performed at 55°C for 30 min, then 40 cycles of PCR (59°C annealing temperature and a 3-min extension time) amplified the gene. A. aegypti PKG cDNA produced from the RT-PCR reaction was cloned into the pCI-Neo vector (Promega, Madison, WI) with an amino-terminal FLAG tag, using the restriction enzymes XhoI and NotI (New England Biolabs, Ipswich, MA). Human PKGIβ was obtained from Dr. Kuan-Teh Jeang (Lee et al. 2007) and cloned into the pCI-Neo vector (Promega, Madison, WI) with a carboxy-terminal Flag tag, using XhoI and NotI. FLAG-tagged A. aegypti and human PKG protein were produced by a transcription and translation (TNT) reaction (TNT T7 Quick Coupled Transcription/Translation System, Promega). PKG activity was measured with the CycLex Cyclic GMP dependent protein kinase assay kit (MBL International, Woburn, MA) according to the manufacturer's protocol.
Protein purification from bacteria
His-tagged viral DENV MTase was expressed in M15/pREP4 Escherichia coli (Qiagen). Protein expression was induced by incubating E. coli with 1 mM isopropyl-β-D-thiogalactopyranoside (Invitrogen) for 7 h. Bacteria cells were collected by centrifugation and lysed by sonication in buffer containing 50 mM Tris (pH 8.0), 300 mM NaCl, and 10 mM imidazole. Cleared lysate was incubated with Ni-NTA Beads (Qiagen) for His-tag binding, and bound protein was eluted in buffer containing 50 mM Tris (pH 8.0), 300 mM NaCl, and 250 mM imidazole.
In vitro kinase assay
FLAG-tagged AePKG was purified from TNT reactions by immunoprecipitation using monoclonal α-Flag antibody (Sigma, St. Louis, MO) and rProtein G Agarose beads (Invitrogen). An immunoprecipitation using α-FLAG was also done on pCI-Neo empty vector DNA (negative control) TNT reactions. Beads with attached AePKG or negative control were resuspended in 30 μL of PKG storage buffer [20 mM HEPES, 1 mg/mL bovine serum albumin, 1 mM EDTA, 6 mM dithiothreitol (DTT), 50% glycerol, 1×protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL), 1×phosphatase inhibitor cocktail (Thermo Fisher Scientific)]. A 10-μL amount of the bead suspension was used as the kinase in each in vitro kinase reaction, with 1 μg of His-tagged DENV MTase [amino acids (aa) Met1-Gln296, expressed and purified from bacteria] as substrate. The reactions included 200 μM adenosine triphosphate (ATP), 10 μM cGMP (Calbiochem, Gibbstown, NJ), and PKG reaction buffer, as described previously (Bhattacharya et al. 2009), with a final sample volume of 32.4 μL. The samples were incubated at 30°C for 60 min, and reactions were terminated by adding 8 μL of (4×) Laemmli buffer and incubating the samples at 95°C for 3 min.
Mass spectrometry sample preparation, loading, and analysis
Samples containing proteins of interest (NS5, MTase, and/or AePKG from an infection or in vitro kinase assay) were run on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and stained with GelCode Blue (Thermo Fisher Scientific). Proteins were identified by size (∼100 kD full-length DENV NS5, ∼33 kD NS5 MTase domain, and ∼91 kD AePKG), and the appropriate gel bands were excised for in-gel trypsin digestion (Trypsin Gold Mass Spectrometry Grade, Promega) in the presence of proteaseMAX surfactant (Promega). For comparison, half of each sample was dephosphorylated with 20 units of calf intestinal alkaline phosphatase for 2 h at 37°C. Mass spectrometry was performed on the matrix-assisted laser desorption/ionization time of flight/time of flight (MALDI-TOF/TOF; Applied Biosystems, Carlsbad, CA) and Finnigan LTQ Linear Ion Trap (Thermo Fisher) mass spectrometers, and sample preparation and analysis were performed as previously described (Bhattacharya et al. 2009).
Statistical analysis
Two-tailed unpaired Student's t-tests (GraphPad, San Diego, CA) were used to test the differences between PKG activities (empty vector vs. human PKG, empty vector vs. A. aegypti PKG, and human PKG vs. A. aegypti PKG). For Aedes wing activity experiments, the SAS v.9.1.3 PROCPOWER program was used to estimate the number of mosquitoes needed for each experimental group (treated vs. control) to achieve a 90% statistical power. The wing activity pattern shape and amplitude between experimental and control populations was tabulated. Daily morning and evening peak hours were identified in each group, as well as total wing activity time for the day. The area under the curve was computed for wing activity and flight activity at the times of interest (4- to 6-h time intervals spanning peak flight times). For Anopheles data, the locomotor/flight activity of each individual animal was summed into 4-h intervals over the entire 5 days. For both Aedes and Anopheles, Wilcoxon rank-sum tests were used to test the differences between the treated and control animals. For all tests, a p value<0.05 was considered statistically significant.
Results
DENV NS5 is phosphorylated during an infection in c6/36 cells
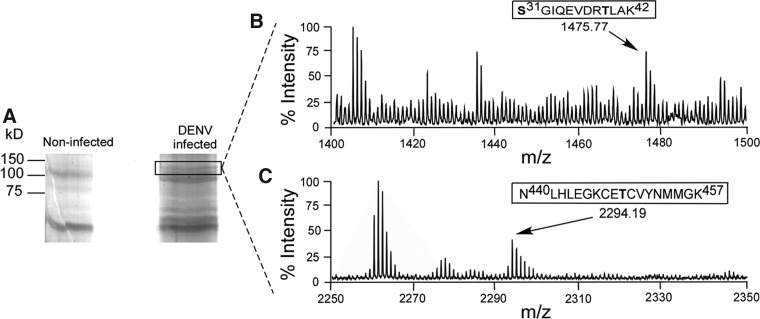
Although flaviviral NS5 phosphorylation has been documented in mammalian cells, NS5 phosphorylation has not previously been studied in insect cells. To determine the phosphorylation state of NS5 during an infection in mosquito cells, A. albopictus c6/36 cells were infected with DENV and NS5 was purified via immunoprecipitation and SDS-PAGE gel purification from the infected cells. While other experiments described in this paper were performed with A. aegypti PKG, c6/36 cells were the only mosquito cell line available to us. Additionally, a putative PKG gene was isolated from c6/36 cells for sequencing. The translated sequence showed ∼99.5% amino acid sequence conservation with A. aegypti PKG (data not shown), so c6/36 cells should serve as a suitable substitute for A. aegypti cells. The phosphorylation status of DENV NS5 in vector cells during an infection was determined by MALDI-TOF mass spectrometry. Several sites in NS5 are phosphorylated, including Ser31, Thr39, and Thr449 (Fig. 1). The identification of these sites suggests that NS5 is also a phosphoprotein during infection of mosquito vectors. Additionally, these three specific sites have been previously identified as phosphorylated in an infection in mammalian cells and in vitro by mammalian PKG (Bhattacharya et al. 2009, Keating et al. 2010) retrospective analysis of our previously published mass spectrometry data. Although these mosquito and mammalian experiments were done using the DENV-2 serotype, serine/threonine phosphoacceptors at these sites are conserved in all four serotypes of DENV. So the phosphorylation of Ser31, Thr39, and Thr449 in DENV NS5 appears consistent for DENV infections in both mosquito vector and mammalian host cells.
FIG. 1.
Dengue virus (DENV) nonstructural 5 protein (NS5) is phosphorylated during an infection in c6/36 cells. A. albopictus c6/36 cells were noninfected or infected with DENV [multiplicity of infection (MOI)=10]. The cells were harvested and lysed at 48 h postinfection, and NS5 was immunoprecipitated with α-WNV NS5. (A) The immunoprecipitated samples were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the NS5 band from DENV-infected cells (boxed) was excised from the gel for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) analysis. (B) The arrow highlights the amino acid 31–42 phosphopeptide on the spectrum. This phosphopeptide's mass, 1475.77 Da, is approximately 160 Da higher than its expected mass, indicating the presence of two phosphorylations (Ser31 and Thr39). (C) The arrow on the spectrum indicates the amino acid 440–457 phosphopeptide. The peptide's mass of 2294.19 Da includes a 79.9-Da phosphate group, corresponding to one phosphorylation (Thr449).
Mosquito PKG is able to phosphorylate a canonical PKG substrate
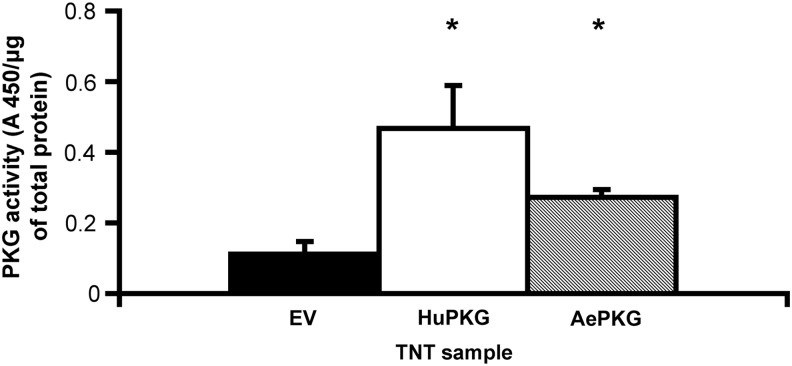
Because the gene product of the sequence corresponding to the putative PKG in A. aegypti had not previously been biochemically characterized, we tested to determine if AePKG in fact had kinase activity and an ability to phosphorylate a canonical PKG substrate. Full-length AePKG, full-length human PKGIβ (HuPKG), and empty vector (EV, negative control) were produced by TNT reaction. In all, 100 μg of total protein from the TNT reaction samples was used in a CycLex PKG activity assay, a colorimetric assay that measures relative levels of phosphorylation of a recombinant PKG substrate peptide sequence. Fig. 2 shows the relative levels of PKG activity of HuPKG and AePKG; both showed significantly more phosphorylation of the substrate than the EV-negative control. There was no statistically significant difference between HuPKG and AePKG activity (p=0.15). So, AePKG is a kinase and capable of phosphorylating a canonical PKG substrate.
FIG. 2.
Mosquito protein kinase G (PKG) phosphorylates a canonical PKG substrate. Full-length human PKGIβ (HuPKG), A. aegypti PKG (AePKG), and empty vector (EV) were expressed by a transcription and translation (TNT) reaction. A 100-μg amount of total protein from the TNT reactions was used in an in vitro CycLex PKG activity assay. Phosphorylation of a canonical PKG substrate was measured to determine the relative levels of PKG activity in each sample. Significant p values between EV and PKG samples are indicated as follows: (*) p<0.05. Samples were measured in duplicate; the figure is representative of two experimental replications.
Mosquito PKG phosphorylates flaviviral MTase substrates in vitro
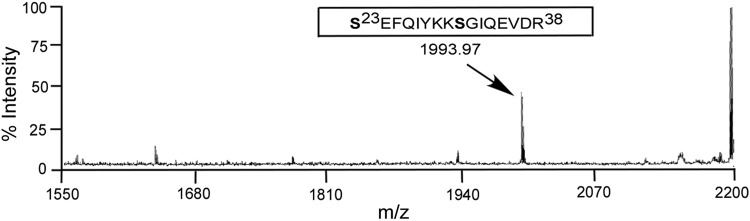
DENV MTase, phosphorylated in vitro by AePKG, was subjected to MALDI-TOF mass spectrometry to identify sites phosphorylated by AePKG. Mass spectrometric data in Fig. 3 shows the phosphopeptide Ser23-Arg38, with an 80-Da mass change corresponding to a single phosphorylation. While there are two potential serine phosphosites in this peptide, serine 31 has previously been identified as being phosphorylated during an infection in c6/36 cells (Fig. 1) and phosphorylated by mammalian PKG (unpublished data). Therefore, Ser31 of AePKG is likely phosphorylated specifically by PKG in vitro.
FIG. 3.
Mass spectrometry analysis of dengue virus (DENV) methyltransferase (MTase) phosphorylated by A. aegypti protein kinase G (AePKG) in vitro. Bacterially purified DENV MTase (amino acids Met1-Gln 296) was incubated with purified AePKG, cyclic guanosine monophosphate (cGMP), and non-radiolabeled adenosine triphosphate (ATP) in vitro. The reaction was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the DENV MTase band was extracted from the gel and subjected to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) analysis. The spectrum indicates the monoisotopic peak phosphorylated Ser23-Arg38 peptide. The mass of the peptide was calculated by one potential phosphosite with two deamidation of glutamines and a water loss during time of flying.
Multiple unique sites in Aedes PKG may be autophosphorylated
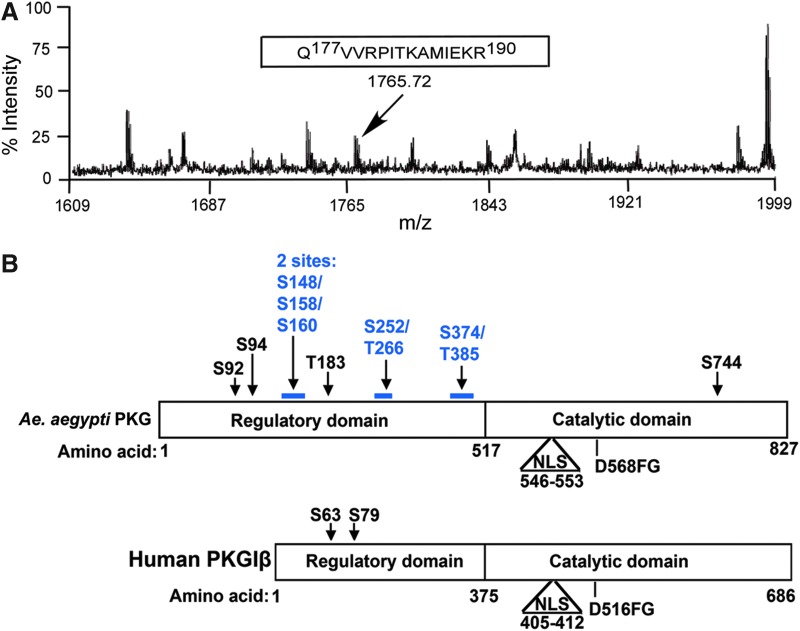
Mammalian PKG is autophosphorylated as part of its enzymatic activation process (Francis et al. 1996, Smith et al. 1996, Chu et al. 1998). As autophosphorylation is an important prerequisite for the activity of many kinases, we examined whether mosquito PKG was also autophosphorylated. To identify specific phosphosites in AePKG, AePKG was used as the enzyme in an in vitro kinase assay with DENV MTase as a substrate. After the kinase assay, AePKG was subjected to MALDI-TOF mass spectrometry to determine which, if any, sites might be autophosphorylated. Fig. 4A shows mass spectrometry data corresponding to the phosphopeptide Gln177-Arg190, which has a mass change corresponding to a single phosphorylation. Thr183 is the only serine, threonine, or tyrosine residue in this phosphopeptide, suggesting this specific site is autophosphorylated. Similar mass spectrometry data identified several other potential autophosphorylation sites, indicated in Figure 4B. A schematic comparing AePKG and human PKGIβ (Fig. 4B) lists the autophosphorylated phosphopeptides identified in AePKG and shows sequence similarities between AePKG and HuPKGIβ. The nuclear localization signal (NLS) and DFG motif (involved in PKGIβ's catalytic activity) are characterized for human PKGIβ; while not yet biochemically characterized, similar features are also found in the AePKG amino acid sequence. The schematic also shows potential autophosphorylation sites in AePKG, identified by mass spectrometry. The majority of potentially autophosphorylated sites are in the amino-terminal region, which is consistent with the localization of autophosphorylated sites in mammalian PKG (shown here for PKGIβ, one of two mammalian PKGI isoforms that differ due to alternative splicing at the extreme amino-terminus (Smith et al. 1996). While the carboxy-terminal catalytic region sequence is well conserved between insect and mammalian PKGs, the amino-terminal region is variable between and among insect and mammalian PKGs. The variable amino-terminal PKG amino acid sequences for A. aegypti (XP_001652946), A. gambiae (AGAP008863), and Drosophila melanogaster (foraging isoform A, NP_477487) are aligned in Fig. 5. The potential autophosphorylation sites in AePKG are marked with an asterisk. A BLAST analysis of AePKG amino acid sequence shows considerable amino acid sequence conservation between AePKG and the above proteins (maximum identities of 85% with Drosophila PKG and 97% with Anopheles PKG). There is no consistent conservation of serine or threonine between these insect PKG sequences at the identified sites, so PKG autophosphorylation patterns may vary among insect species.
FIG. 4.
A. aegypti protein kinase G (AePKG) is autophosphorylated in vitro. In vitro phosphorylated AePKG was subjected to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry to identify potential autophosphorylation sites. (A) The spectrum indicates a monoisotopic peak of Gln177-Arg190 peptide. The peptide's mass was 80 Da greater than expected, suggesting the presence of a single phosphorylation. (B) A schematic represents the phosphopeptides in AePKG found by our mass spectrometry analysis, as well as a comparison between AePKG and human PKGIβ sequences. Putative domains (regulatory and catalytic) and residues of interest are indicated for both AePKG and human PKG. The amino acid numbering for AePKG is based on sequence homology to known human PKG domains, nuclear localization signal (NLS), and DFG motif. Known human PKGIβ autophosphorylation sites (Smith et al. 1996) and AePKG autophosphorylation sites we have identified are denoted with arrows. For AePKG, serine or threonine resides present in phosphopeptides with only one potential serine/threonine phosphorylation site are shown in black. Phosphopeptides with multiple potential phosphosites are shown in blue.
FIG. 5.
Specific autophosphorylation sites in A. aegypti protein kinase G (AePKG) are not conserved with other insect protein kinases G (PKGs). An amino acid sequence comparison between A. aegypti, A. gambiae, and D. melanogaster PKG amino-terminal regulatory domains is shown. Potential AePKG autophosphorylation sites identified by mass spectrometry are in bold and marked with an asterisk.
A. aegypti mosquitoes treated with PKG activator have increased wing activity
Previous studies have connected increased PKG activity with an increase in food searching (foraging) behavior in insects (Osborne et al. 1997, Ben-Shahar et al. 2002, Ben-Shahar 2005), but this effect had not been shown in insect vectors that transmit pathogens for human disease (human disease vectors). Because we lacked the facilities for full DENV infections of mosquitoes, we performed an experiment similar to those done in other insects in which PKG activation was used to test if enhancement of PKG activity resulted in increased foraging behavior (Osborne et al. 1997, Ben-Shahar et al. 2002). Adult female A. aegypti mosquitoes were injected with 2.5 mM PKG activator (a cGMP analog) or vehicle control for monitoring of wing activity. In Fig. 6, both treated and control groups of mosquitoes show a clear diurnal and crepuscular pattern of wing activity, with two discrete peaks of activity—one upon lights turning on (“dawn”) and the other during the late day toward lights turning off (“dusk”)—and with lower levels of activity during the mid-day phase. There is minimal activity during the “night” period (8 h dark), which is consistent with previously reported A. aegypti locomotor/flight patterns and host-seeking behavior in the wild (Corbet et al. 1974, Gentile et al. 2009). However, PKG activator-treated mosquitoes (in red) showed up to an approximately three-fold increase in the average wing activity over control mosquitoes (in blue). The increase was most pronounced during the peak activity times (“dawn” and “dusk”), but a slight increase was also observed during the lower-activity daytime periods for treated mosquitoes. Table 1 includes the statistical analyses of the wing activity between control and PKG activator-treated mosquitoes. p values are displayed for various time intervals that encompass morning and evening peak activity times, with experimental hours corresponding to the hours in Fig. 6. Significance is defined as p<0.05. Additional experimental repeats showed the same trend, with PKG activator-treated mosquitoes consistently showing a higher level of wing activity than control-treated mosquitoes (Fig. S1), and this effect was also seen with a higher drug concentration (Fig. S2). Thus, treating the flaviviral vector A. aegypti with a PKG activator alters mosquito wing activity.
Table 1.
A Formal Comparison of 2.5 mM and Control Groups in Terms of the Area under the Aedes aegypti Wing Activity Intensity Curve Calculated at Peak Times
| |
|
Average sum of seconds with wing activity in time intervals |
Exact Wilcoxon rank-sum test |
||
|---|---|---|---|---|---|
| Day | Time interval (experimental hours) (AM/PM peak) | Vehicle control | 2.5 mM PKG activator | W | p value |
| 1 | 16–20 (AM) | 147.3 | 218.4 | 52 | 0.144 |
| 1 | 27–33 (PM) | 573.2 | 1455.1 | 43 | 0.053 |
| 2 | 40–44 (AM) | 81.4 | 248.9 | 59 | 0.273 |
| 2 | 49–55 (PM) | 673.2 | 1730.9 | 38 | 0.027 |
| 3 | 62–66 (AM) | 166.8 | 516 | 41 | 0.041 |
| 3 | 72–78 (PM) | 755.1 | 1847.5 | 36 | 0.020 |
| 4 | 85–89 (AM) | 153.6 | 417.9 | 42 | 0.047 |
| 4 | 95–101 (PM) | 717.9 | 2020.2 | 30 | 0.007 |
| 5 | 108–112 (AM) | 151.6 | 399.7 | 30 | 0.007 |
| 5 | 119–125 (PM) | 774 | 1949.6 | 29 | 0.006 |
| 6 | 132–136 (AM) | 170.7 | 503.8 | 40 | 0.036 |
| 6 | 141–147 (PM) | 780.9 | 1955.2 | 28 | 0.005 |
A. gambiae mosquitoes treated with PKG activator have increased locomotor/flight activity
While A. gambiae is not a flaviviral vector, we were interested in whether PKG would alter behavior in Anopheles as well due to its importance as a malarial vector and its nocturnal rather than daytime activity. In this experiment, locomotor/flight activity was directly detected, as compared to the acoustical detection of wing beats (wing activity) measured for A. aegypti. The number of minutes with locomotor activity detected for both PKG activator-treated and vehicle control-treated mosquitoes was compiled into 60-min intervals. Fig. 7 shows the nocturnal flight pattern of Anopheles mosquitoes, with minimal flight activity during the 12-h light phase and an increased level of flight activity during the 12-h dark phase. Anopheles mosquitoes that were treated with PKG activator again showed an enhancement in locomotor/flight activity as compared to the control mosquitoes and almost exclusively during night phase of the light:dark cycle. A statistical analysis of these data is shown in Table 2, with significance defined as p<0.05. The “experimental hours” correspond to the hours shown in Fig. 7, whereas ZT hours correspond to the experimental light:dark cycle (ZT0–11 is full light, ZT12–23 is dark, with dusk/dawn transitions of decreasing/increasing light at ZT11–12 and ZT23–0, respectively). While the light cycles for the Aedes and Anopheles experiments differed, and the natural flight patterns differ between Aedes and Anopheles mosquitoes, both mosquito species exhibited increased activity specifically during natural flight times upon treatment with the PKG activator, but not during natural rest periods.
Table 2.
A Formal Comparison of 10 mM and Control Anopheles Locomotor Activity
| |
Time interval |
Average sum of minutes with flight activity in time intervals |
Exact Wilcoxon rank-sum test |
|||
|---|---|---|---|---|---|---|
| Day | Elapsed time | ZT hours | Vehicle control | 10 mM protein kinase G activator | W | p value |
| 1 | 12–16 | 7.5–11.5 | 0.50 | 0.68 | 180 | 0.563 |
| 1 | 16–20 | 11.5–15.5 | 34.20 | 61.16 | 110 | 0.025 |
| 1 | 20–24 | 15.5–19.5 | 19.25 | 34.42 | 113.5 | 0.031 |
| 1 | 24–28 | 19.5–23.5 | 13.00 | 17.47 | 124 | 0.062 |
| 1–2 | 28–32 | 23.5–3.5 | 0.70 | 0.32 | 189 | 0.979 |
| 2 | 32–36 | 3.5–7.5 | 0.55 | 0.16 | 172.5 | 0.446 |
| 2 | 36–40 | 7.5–11.5 | 0.65 | 0.68 | 180 | 0.646 |
| 2 | 40–44 | 11.5–15.5 | 34.45 | 72.68 | 88.5 | 0.005 |
| 2 | 44–48 | 15.5–19.5 | 11.70 | 41.42 | 56 | <0.001 |
| 2 | 48–52 | 19.5–23.5 | 8.8 | 19.89 | 95.5 | 0.008 |
| 2–3 | 52–56 | 23.5–3.5 | 0.05 | 0.26 | 149.5 | 0.072 |
| 3 | 56–60 | 3.5–7.5 | 0.35 | 0.16 | 182 | 0.717 |
| 3 | 60–64 | 7.5–11.5 | 0.85 | 0.42 | 190 | 1 |
| 3 | 64–68 | 11.5–15.5 | 39.7 | 64.16 | 121 | 0.054 |
| 3 | 68–72 | 15.5–19.5 | 15.9 | 40.95 | 88 | 0.004 |
| 3 | 72–76 | 19.5–23.5 | 5.00 | 20.89 | 82.5 | 0.002 |
| 3–4 | 76–80 | 23.5–3.5 | 0.20 | 0.26 | 188.5 | 0.957 |
| 4 | 80–84 | 3.5–7.5 | 0.20 | 0.26 | 171 | 0.324 |
| 4 | 84–88 | 7.5–11.5 | 0.80 | 0.95 | 180 | 0.646 |
| 4 | 88–92 | 11.5–15.5 | 32.35 | 75.00 | 80.5 | 0.002 |
| 4 | 92–96 | 15.5–19.5 | 13.20 | 31.21 | 95 | 0.008 |
| 4 | 96–100 | 19.5–23.5 | 6.8 | 27.95 | 104 | 0.014 |
| 4–5 | 100–104 | 23.5–3.5 | 0.3 | 0.32 | 180.5 | 0.663 |
| 5 | 104–108 | 3.5–7.5 | 0.3 | 0.37 | 188.5 | 0.957 |
| 5 | 108–112 | 7.5–11.5 | 1.0 | 0.95 | 184 | 0.834 |
| 5 | 112–116 | 11.5–15.5 | 32.55 | 74.37 | 77.5 | 0.002 |
| 5 | 116–120 | 15.5–19.5 | 15.1 | 33.11 | 91 | 0.006 |
Discussion
Our data here provide the first studies of the biochemistry and molecular biology of mosquito (A. aegypti) PKG. In many insects, the for gene locus is not associated with an increase in general locomotory activity, but specifically with foraging behavior (Osborne et al. 1997, Ben-Shahar 2005). In multiple insect species, either the expression levels of the product of the for gene or manipulation of cGMP signaling alters insect behavior, but how PKG activity is modulated physiologically in insects is unknown. In mammalian PKG systems, control of PKG activity is modulated in part by autophosphorylation of the regulatory domain, which changes the binding of cGMP to the regulatory domain of PKG (Hofmann et al. 1985, Kotera et al. 2003). The catalytic domains of mammalian PKG and insect PKG are well conserved, but the regulatory domains are not, and prior to this work there was no evidence for autophosphorylation of the for gene product in insects. Autophosphorylation has been shown for the homolog of PKGII (expressed from a separate gene than PKGI in mammals) in Drosophila (Foster et al. 1996); however, these sites have not been mapped and are not homologous to the for gene product, so these data provide only limited information on the regulation of the PKGI-homolog enzyme. We have shown that the mosquito protein that is homologous to human PKGI does, in fact, have kinase activity against a canonical PKG substrate and is able to autophosphorylate and phosphorylate a flaviviral substrate.
In our previous work, we found a strict separation between mosquito-borne and tick-borne flaviviruses. Mosquito-transmitted flaviviruses have conserved PKG phosphorylation sites, while flaviviruses transmitted by ticks, or flaviviruses without an arthropod vector, have no conserved serine/threonine phosphoacceptor for PKG (Bhattacharya et al. 2008, Bhattacharya et al. 2009, Keating et al. 2010). This information led to the hypothesis that PKG is somehow important in the mosquito-borne flaviviral life cycle; however, it is not clear that PKG is required for flaviviral replication. The findings that AePKG phosphorylates a mosquito-borne flaviviral substrate and that treatment with a PKG-activating drug alters mosquito behavior raise the possibility that flaviviruses could use PKG to modulate vector behavior. In fact, DENV-infected mosquitoes show an increase in flight as compared to uninfected female A. aegypti mosquitoes (Lima-Camara et al. 2011), in a pattern similar to the increase seen here in PKG activator-treated A. aegypti females. Because A. aegypti is the first hematophagous insect whose behavior has been linked to PKG, it could be that all food-seeking behavior, and not only blood-seeking behavior by females, is increased by PKG. We also showed that female A. gambiae mosquitoes treated with PKG activator had an increase in locomotor/flight activity over control-treated mosquitoes. Because the two mosquito species here (A. aegypti and A. gambiae) were tested in different flight-monitoring systems (for Aedes, wing activity was measured using sound, and for Anopheles, movement was measured by infrared beam breaks; these experiments were also completed under different light cycles), their results cannot be directly compared. However, flight alterations seem to be correlated with the addition of a PKG-activating drug, even in mosquitoes that are not DENV or flaviviral vectors (Anopheles). Additionally, for gene expression, encoding PKG, is found to be rhythmic under both circadian (constant darkness) and/or light:dark cycle conditions in female A. gambiae mosquitoes (AGAP008863, JTK_CYCLE algorithm, q<0.05, expression in heads and bodies, peak phase in the late day) (Rund et al. 2011), and rhythmic under light:dark cycle conditions in D. melanogaster (FBgn0000721, COSOPT algorithm, p<0.1, expression in bodies peaks at the dawn phase) (Ceriani et al. 2002) and A. aegypti (AAEL007826, autocorrelation <0.05, expression in heads peaks in the middle of the night phase) (Ptitsyn et al. 2011). Work in Drosophila did not distinguish any effects of PKG between males and females (Osborne et al. 1997). Overall, additional flight studies into PKG's effects on different mosquito species (and other vector insects) may provide more information about the role PKG clearly plays in behavior and possibly in disease transmission.
How either DENV infection or PKG activation results in increased flight remains to be determined, but at least PKG activation likely acts on the neuronal level. When Aedes is parenterally infected, the neuronal tissue is an early site of high viral amplification, possibly the primary site of viral replication (Linthicum et al. 1996). While fewer studies have examined mosquito tissue after oral infection, Salazar, et al. found that the head and salivary glands were the only sites of early and prolonged antigenemia (Salazar et al. 2007). PKG expression and activity is present in Drosophila (and other insect) heads (Foster et al. 1996, MacPherson et al. 2004) and the pharmacological PKG activator used in our studies has been shown to affect neuronal activity (Levitan et al. 1988). Therefore, flaviviral infection does not need to turn on PKG transcription to modulate behavior. PKG expression in mosquito brain and neuronal tissue could facilitate flaviviral replication, as we have demonstrated for mammalian PKG in cell culture (Bhattacharya et al. 2009). High neuronal levels of viral antigens that are substrates for mosquito PKG, as shown here, would likely drive autophosphorylation and enhance PKG activity in a positive feedback loop. While this work demonstrates that like other insects, mosquito PKG can regulate behavior, the link with flaviviral infection is indirect at the present time. Further studies are necessary to directly correlate PKG's activity to the DENV-induced increase in flight, but the current data are supportive of the hypothesis that viral infection may increase flight via PKG.
Conclusions
The A. aegypti gene product that is homologous to human PKGI has kinase activity against a canonical PKG substrate. PKG phosphorylates DENV NS5 in both mammalian and Aedes mosquito cells, suggesting that PKG may play a role in both the vertebrate host and insect vector phases of the flaviviral transmission cycle. PKG activation is associated with an increase in the daytime flight of A. aegypti mosquitoes, the primary vector for DENV transmission, and nighttime flight of A. gambiae, the primary vector for malaria transmission.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Jeremy Fuchs, George Mayhew, and Bruce Christensen for their assistance with the mosquito flight experiments; Dave Hilgendorf for writing a Perlscript program to transform flight data; and Adin-Cristian Andrei for his assistance with statistical analyses. J.A.K. is funded by the National Institutes of Health (NIH)-funded Cellular and Molecular Parasitology Training Program (2T32AI007414), and G.E.D. by a grant from the Eck Institute for Global Health, University of Notre Dame.
Author Disclosure Statement
No competing financial interests exist.
References
- Ben-Shahar Y. Robichon A. Sokolowski MB. Robinson GE. Influence of gene action across different time scales on behavior. Science. 2002;296:741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y. The foraging gene, behavioral plasticity, and honeybee division of labor. J Comp Physiol A. 2005;191:987–994. doi: 10.1007/s00359-005-0025-1. [DOI] [PubMed] [Google Scholar]
- Berry WJ. Rowley WA. Christensen BM. Influence of developing Dirofilaria immitis on the spontaneous flight activity of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1987;24:699–701. doi: 10.1093/jmedent/24.6.699. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D. Hoover S. Falk SP. Weisblum B, et al. Phosphorylation of yellow fever virus NS5 alters methyltransferase activity. Virology. 2008;380:276–284. doi: 10.1016/j.virol.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D. Mayuri Best SM. Perera R, et al. Protein kinase G phosphorylates mosquito-borne flavivirus NS5. J Virol. 2009;83:9195–9205. doi: 10.1128/JVI.00271-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WC. Bennett KE. Gorrchotegui-Escalante Barillas-Mury , et al. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- Ceriani MF. Hogenesch JB. Yanovsky M. Panda S, et al. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BM. Sutherland DR. Brugia pahangi: Exsheathment and midgut penetration in Aedes aegypti. Trans Am Microscop Soc. 1984;103:423–433. [Google Scholar]
- Chu DM. Francis SH. Thomas JW. Maksymovitch EA, et al. Activation by autophosphorylation or cGMP binding produces a similar apparent conformational change in cGMP-dependent protein kinase. J Biol Chem. 1998;273:14649–14656. doi: 10.1074/jbc.273.23.14649. [DOI] [PubMed] [Google Scholar]
- Corbet PM. Smith SM. Diel periodicities of nulliparous and parous Aedes aegypti (L.) at Dar es Salaam, Tanzania (Diptera:Culicidae) Bull Entomol Res. 1974;64:111–121. [Google Scholar]
- Dasgupta R. Free HM. Zietlow SL. Paskewitz SM, et al. Replication of Flock House Virus in three genera of medically important insects. J Med Entomol. 2007;44:102–110. doi: 10.1603/0022-2585(2007)44[102:rofhvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Foster JL. Higgins GC. Jackson FR. Biochemical properties and cellular localization of the Drosophila DG1 cGMP-dependent protein kinase. J Biol Chem. 1996;271:23322–23328. doi: 10.1074/jbc.271.38.23322. [DOI] [PubMed] [Google Scholar]
- Francis SH. Smith JA. Colbran JL. Grimes K, et al. Arginine 75 in the pseudosubstrate sequence of type Ib cGMP-dependent protein kinase is critical for autoinhibition, although autophosphorylates serine 63 is outside of this sequence. J Biol Chem. 1996;271:20748–20755. [PubMed] [Google Scholar]
- Gentile C. Rivas GB. Meireles-Filho AC. Lima JB, et al. Circadian expression of clock genes in two mosquito disease vectors: cry2 is different. J Biol Rhythms. 2009;24:444–451. doi: 10.1177/0748730409349169. [DOI] [PubMed] [Google Scholar]
- Hayes EB. Komar N. Nasci RS. Montgomery SP, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F. Gensheimer HP. Gobel C. cGMP-dependent protein kinase. Autophosphorylation changes the characteristics of binding site 1. Eur J Biochem. 1985;147:361–365. doi: 10.1111/j.1432-1033.1985.tb08758.x. [DOI] [PubMed] [Google Scholar]
- Jakubiec A. Jupin I. Regulation of positive-strand RNA virus replication: The emerging role of phosphorylation. Virus Res. 2007;129:73–79. doi: 10.1016/j.virusres.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR. Riedl CAL. Chakaborty-Chatterjee M. Belay AT, et al. Natural variation in food acquisitin mediated via a Drosophila cGMP-dependent protein kinase. J Exp Biol. 2007;210:3547–3558. doi: 10.1242/jeb.006924. [DOI] [PubMed] [Google Scholar]
- Keating JA. Bhattacharya D. Striker R. Coevolutionary implications of mosquito cGMP-dependent kinase and mosquito-borne flaviviruses. Am Soc Trop Med Hyg. 2010 59th Meeting. [Google Scholar]
- Keating JA. Striker R. Phosphorylation events in viral infections provide potential therapeutic targets. Rev Med Virol. 2011;22:166–181. doi: 10.1002/rmv.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera J. Grimes KA. Corbin JD. Francis SH. cGMP-dependent protein kinase protects cGMP from hydrolysis by phosphodiesterase-5. Biochem J. 2003;372:419–426. doi: 10.1042/BJ20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH. Yedavalli VR. Jeang KT. Activation of HIV-1 expression and replication by cGMP dependent protein kinase type 1-beta (PKG1beta) Retrovirology. 2007;4:91. doi: 10.1186/1742-4690-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan ES. Levitan IB. A cyclic GMP analog decreases the currents underlying bursting activity in the Aplysia neuron R15. J Neurosci. 1988;8:1162–1171. doi: 10.1523/JNEUROSCI.08-04-01162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PY. Louie KL. Styer LM. Shi PY, et al. Viral pathogenesis in mice is similar for West Nile virus derived from mosquito and mammalian cells. Virology. 2010;400:93–103. doi: 10.1016/j.virol.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Camara TN. Bruno RV. Luz PM. Castro MG, et al. Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS One. 2011;6:e17690. doi: 10.1371/journal.pone.0017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum KJ. Platt K. Myint KS. Lerdthusnee K, et al. Dengue 3 virus distribution in the mosquito Aedes aegypti: An immunocytochemical study. Med Vet Entomol. 1996;10:87–92. doi: 10.1111/j.1365-2915.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Lucas C. Sokolowski MB. Molecular basis for changes in behavioral state in ant social behaviors. Proc Natl Acad Sci USA. 2009;106:6351–6356. doi: 10.1073/pnas.0809463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson MR. Lohmann SM. Davies SA. Analysis of Drosophila cGMP-dependent protein kinases and assessment of their in vivo roles by targeted expression in a renal transporting epithelium. J Biol Chem. 2004;279:40026–40034. doi: 10.1074/jbc.M405619200. [DOI] [PubMed] [Google Scholar]
- Morozova OV. Tsekhanovskaya NA. Maksimova TG. Bachvalova VN, et al. Phosphorylation of tick-borne encephalitis virus NS5 protein. Virus Res. 1997;49:9–15. doi: 10.1016/s0168-1702(96)01433-5. [DOI] [PubMed] [Google Scholar]
- Nene V. Wortman JR. Lawson D. Haas B, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne KA. Robichon A. Burgess E. Butland S, et al. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Ptitsyn AA. Reyes-Solis G. Saavedra-Rodriguez K. Betz J, et al. Rhythms and synchronization patterns in gene expression in the Aedes aegypti mosquito. BMC Genomics. 2011;12:153. doi: 10.1186/1471-2164-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KE. Gorbalenya AE. Rice CM. The NS5A/NS5 proteins of viruses from three genera of the family Flaviviridae are phosphorylated by associated serine/threonine kinases. J Virol. 1998;72:6199–6206. doi: 10.1128/jvi.72.7.6199-6206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley WA. Jones MDR. Jacobson DW. Clarke JL. A microcomputer-monitored mosquito flight activity system. Ann Entomol Soc Am. 1987;80:534–538. [Google Scholar]
- Rund SSC. Hou TY. Ward SM. Collins FH, et al. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2011;108:E421–E430. doi: 10.1073/pnas.1100584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund SSC. Lee SJ. Bush BR. Duffield GE. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J Insect Physiol. 2012;58:1609–1619. doi: 10.1016/j.jinsphys.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Salazar MI. Richardson JH. Sanchez-Vargas I. Olson KE, et al. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA. Francis SH. Walsh KA. Kumar S, et al. Autophosphorylation of type Ib cGMP-dependent protein kinase increases basal catalytic activity and enhances allosteric activation by cGMP or cAMP. J Biol Chem. 1996;271:10756–10762. doi: 10.1074/jbc.271.34.20756. [DOI] [PubMed] [Google Scholar]
- Vasilakis N. Weaver SC. The history and evolution of human dengue emergence. Adv Virus Res. 2008;72:1–76. doi: 10.1016/S0065-3527(08)00401-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.