Abstract
The production of conidia of entomopathogenic Beauveria bassiana by solid-state fermentation was studied for the development of a biocontrol agent against aphid Myzus persicae. The optimal conditions for conidia production on polished white rice were 40% moisture content, 25℃ culture temperature, 2-day-old seeding culture grown in 3% corn meal, 2% rice bran, 2% corn steep powder medium, initial conidia concentration of 107 conidia/g in the wet rice, 10% inoculum size, and use of a polyethylene bag as a container. The polyethylene bag containing inoculated rice was hand-shaken every 12 hr during fermentation. Using optimal conditions, the maximum conidia production obtained was 4.05 g conidia/100 g dry rice after 14 days of cultivation, a rate 2.83 times higher than conidia yield of pre-optimization.
Keywords: Beauveria bassiana, Conidia production, Optimal culture conditions, Solid-state fermentation
Entomopathogenic fungi are recognized as important natural enemies of insect pests. Such species include Verticillium lecanii (Zimmerman) Viegas, Metarhizium anisopliae (Metsch.), M. flavoviride (Metsch.), Nomuraea rileyi (Farlow) Samson, Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Clavicipitaceae), and Paecilomyces farinosus (Holm ex S. F. Gray) Brown & Smith [1]. Mycoinsecticides are garnering increased attention as environmentally-friendly insect control agents. Although thousands of fungal species infect insects, few have received serious consideration as potential commercial candidates. B. bassiana appears to have the broadest potential as a viable insect control agent. Adhesion of fungal spores to the host cuticle along with germination are prerequisites for the efficacy of fungal pathogens [2-4].
Aphids are distributed worldwide but are most common in temperate climates. Aphids transmit various pathogenic plant viruses and are very difficult to control using only organic pesticides due to increased insecticide resistance and rapid increases in population size. Among the various biocontrol agents using entomopathogenic fungi to control aphids, B. bassiana (Balsamo) is the most promising [5-7].
Production of B. bassiana spores can be achievied using different methodologies, which can be classified as either low input or industrial technologies [2]. Both solid and liquid fermentation systems are used for the mass production of biocontrol agents [8, 9]. Solid state fermentation (SSF) allows several types of fungi to produce hardly and healthy conidia [10]. Though blastospores are produced by submerged liquid fermentation, they are hydrophilic and lose viability relatively quickly during storage [11, 12]. Therefore, SSF is considered the proper system for the mass production of conidia to be used in oil formulation [13]. SSF has additional advantages compared to submerged fermentation; SSF is more simple and productive, requires lower capital and energy, uses simpler fermentation media, does not require rigorous control of fermentation parameters, uses less water, produces less wastewater, easily controls bacterial contamination, and has cheaper downstream processing [14, 15].
Previously, we isolated the entomopathogenic fungus B. bassiana KK5, which has high infectivity and virulence to aphids (Myzus persicae). Despite its high infectivity, the large scale application of this fungus for aphid control is only possible if high titers of spores are grown and produced on widely available substrates.
Therefore, the aim of this study was the optimization of B. bassiana KK5 conidia production on polished rice using SSF.
Materials and Methods
Microorganism
The original strain of B. bassiana KK5 was preserved at -80℃ in sterile cryovials containing 10% glycerol (in sterilie 0.02% of Tween 80 solution). The fungal strain was cultured on potato dextrose agar (PDA) at 25℃ for 10 days and stored at 4℃ until use.
Preparation of the seeding inoculums
Aerial conidia taken from a stock culture growing on a PDA agar plate were suspended in sterile water containing 0.02% Tween 80. The number of conidia in the suspension was counted using a haemocytometer (Superior Marienfeeld, Lauda-Königshofen, Germany), followed by dilution to 1.0 × 108 conidia/mL with 0.02% Tween 80. One milliliter of the conidium suspension was inoculated into a 250 mL Erlenmeyer flask containing 100 mL of liquid media, followed by culture at 25 ± 0.1℃ using a rotary shaking incubator operated at 200 rpm. The resulting suspension was used as the seeding inoculum for SSF.
Solid-state fermentation for aerial conidia production
The moistened solid medium was transferred into a container (see "Effect of solid medium container on aerial conidia production" in Materials and Methods) and autoclaved at 121℃ for 25 min. The container with moistened solid media was then cooled, after which the seeding culture was added at 10% inoculum size (107 conidia/g wet medium) to each container, followed by thorough mixing using a sterilized spoon. The specimens were then incubated at various temperatures and relative humidities (RH) under still culture conditions for a desired time period, depending on experimental protocols. The contents of the container were gently hand-shaken every 12 hr. Unless otherwise mentioned, these conditions were maintained throughout all experiments. Conidia were counted using a haemocytometer after growth for 10 days. For all experiments, three replicates of each treatment were used.
Counting conidia
Two grams of conidiated rice was mixed with 18 mL of 0.02% Tween 80. The 100 mL flask containing the mixture of conidiated rice was agitated in a rotary shaking incubator operated at 200 rpm. After 60 min of agitation, the mixture of conidiated rice was filtered through three layers of cheese cloth. The number of conidia was determined using a haemocytometer (Neubauer improved; Superior Marienfeld, Germany).
Harvesting conidia
Conidiated rice cultivated for various desired times were dried at 35℃ for 24 hr and then harvested through three sieves with mesh sizes of 25 (710 µm), 80 (180 µm), and 200 (75 µm) using a sieving vibrator (Model 3PRO; Fritsch Co. Ltd., Idar-Oberstein, Germany). The conidia were then collected and dried using a vacuum dryer at room temperature in order to lower the moisture content to less than 5%.
Effect of different substrates on aerial conidia production
Several kinds of solid substrates were tested, including polished rice, brown rice, and rice husk. The substrates were mixed with water to achieve a moisture level of 35%, then put into a plastic bottle (62 × 92 × 160 mm3). The solid medium in the plastic bottle was inoculated with 2-day-old liquid culture grown in medium composed of 3% corn meal, 2% rice bran, and 2% corn steep powder, followed by incubation at 25℃, 75% relative humidities (RH). After 14 days of cultivation, the fermented medium was dried at 35℃ for 24 hr, after which the conidia yield was determined.
Effect of temperature on aerial conidia production
Inoculated, steamed polished rice at a moisture level of 35% was incubated in a plastic bottle at 25, 27, and 30℃ at 75% RH. The number of conidia per g wet conidiated rice was determined after 12 days of cultivation.
Effect of liquid seeding culture on aerial conidia production
Sterilized steamed rice at a moisture level of 35% in plastic bottles (62 × 92 × 160 mm3) were inoculated with two-day-old cultures grown in different liquid media, such as potato dextrose broth (PDB), wheat bran (WB; 3% corn meal, 2% wheat bran, 3.5% corn steep liquor), corn step liquor (CSL; 3% corn meal, 2% rice bran, 3.5% corn steep liquor), and rice bran (RB; 3% corn meal, 2% rice bran, 2% corn steep powder), at an inoculum size of 10% (v/w). Conidia production on rice was conducted at 25℃ and 75% RH for 14 days, after which the conidia were dried at 35℃ for 24 hr and then harvested using a sieve vibrator.
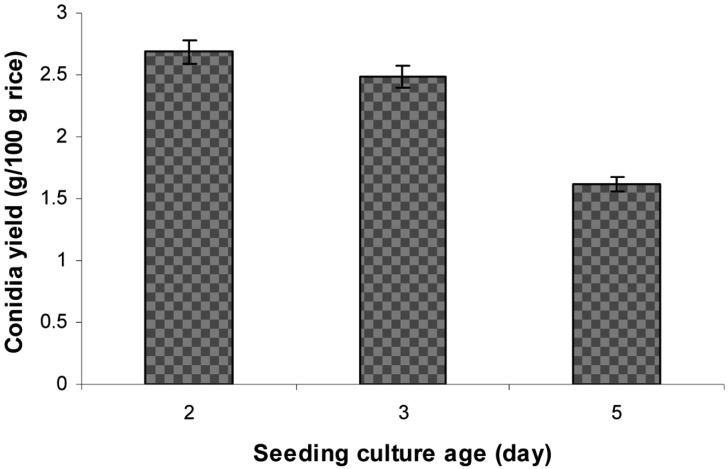
Effect of seeding culture time on aerial conidia production
Seeding cultures grown in RB medium for different time periods (2, 3, or 5 days) were inoculated onto rice. The initial inoculum sizes were 10% (v/w). Conidia production on rice was conducted at 25℃ and 75% RH for 14 days, after which the conidia were dried at 35℃ for 24 hr and then harvested using a sieve vibrator.
Effect of inoculum size on aerial conidia production
Sterilized polished rice in plastic bottles were inoculated with different inoculum sizes (10, 15, and 20% v/w). The inoculated rice were incubated at 25℃ and 75% RH for 14 days, after which the conidia were dried at 35℃ for 24 hr and then harvested using a sieve vibrator.
Effect of inoculum concentration on aerial conidia production
The steamed rice in plastic bottles at a 35% moisture level were inoculated with different concentrations of 10% inoculum in order to achieve an initial concentration of 106, 107, and 108 conidia/g wet rice. And then conidia were produced on the rice as described above.
Effect of solid medium container on aerial conidia production
The rice were submerged and drained in order to achieve a moisture level of 40%. The moistened rice were then placed into either plastic bottles (62 × 92 × 160 mm3 and 70 × 92 × 160 mm3) or polyethylene bags [325 × 435 mm2 with two small aeration filters (diameter, 36 mm) or two large aeration filters (diameter, 79 mm)]. The plastic bottle was plugged with a ventilated cap in order to minimize contamination and allow passive aeration during growth and conidiogenesis. The moistened rice were next sterilized, cooled, and inoculated with 2-day-old RB culture, followed by additional incubation as shown above. After 14 days of cultivation, the conidiated rice were dried at 35℃ for 24 hr, after which conidia were harvested using a sieve vibrator and dried using a vacuum dryer at room temperature to a moisture level lower than 5%. Lastly, the conidia yield was determined.
Effect of moisture content of rice on aerial conidia production
The polished rice were submerged in tap water for 2~3 hr, followed by draining for different time periods to achieve moisture levels of 30, 35, 40, and 50%. One hundred grams of moistened rice was dried at 105℃ for 24 hr, followed by weighing to determine the water content. Polyethylene bags with small aeration filters containing the inoculated rice were then incubated at 25℃ for 12~17 days, after which the number of conidia/g wet conidiated rice was determined.
Effect of different ambient RH on aerial conidia production
Sterilized moistened rice in the polyethylene bag with two small aeration filters were inoculated with 2-day-old liquid culture as shown above, followed by incubation at 25℃ at different ambient RHs of 45, 60, and 75%. The conidia yield was determined after 15 days after inoculation.
Effect of incubation period on aerial conidia production
Conidia production on sterilized rice at a moisture level of 40% was conducted at 25℃ and 75% RH in a polyethylene bag with two small aeration filters. After 10 days of cultivation and a 1 day interval, the number of conidia per g rice was determined.
Results and Discussion
Effect of different substrates on aerial conidia production
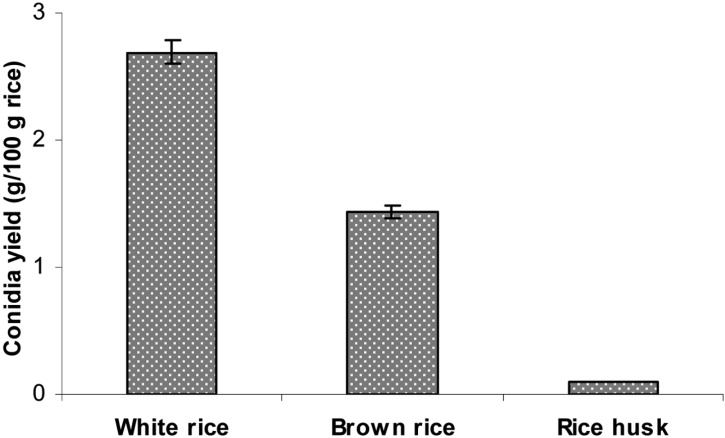
After 14 days of cultivation, the highest amount of aerial conidia was produced using steamed polished white rice (2.69 g conidia/100 g substrate), followed by brown rice (1.43 g conidia/100 g substrate) (Fig. 1). Conversely, the results show that rice husk was not suitable for producing aerial conidia of B. bassiana KK5. According to Feng et al. [16], the conidia yields of V. lecanii on cooked rice and rice bran are 1.5 × 109 and 1.4 × 109 conidia/g solid culture, respectively, which are significantly higher than those of rice husk and a mixture of rice and rice bran under identical culture conditions. The maximum conidia yield for B. bassiana is 4.38 × 109 conidia/g wet rice [17]. The conidia yield of B. brongniartii cultured on media consisting of 70% cotton seed-shell powder, 25% wheat bran, and 5% corn flour (plus water) is 2.9 × 109 conidia/g dry powder [18]. When B. brongniartii was cultured for 42 days on a mixture of shelled barley, sunflower oil, and water, the conidia yield was found to be 2 × 109 [19]. Therefore, the optimal substrate for the high yield production of conidia varies depending on the fungal species.
Fig. 1.
Aerial conidia production on different solid culture media. Polished rice, brown rice, and rice husk at a 35% moisture level in plastic bottles were inoculated with 2-day-old liquid culture and then incubated at 25℃ and 75% relative humidities for 14 days.
The most commonly selected substrate for the production of fungal conidia is by far white rice [2, 3, 20-23], which was also selected as the best solid substrate in this study. This is probably due to a combination of factors including nutritional balance, cost, worldwide availability, physical characteristics such as grain size and shape, hydration properties, and structural integrity even after colonization by fungi [13].
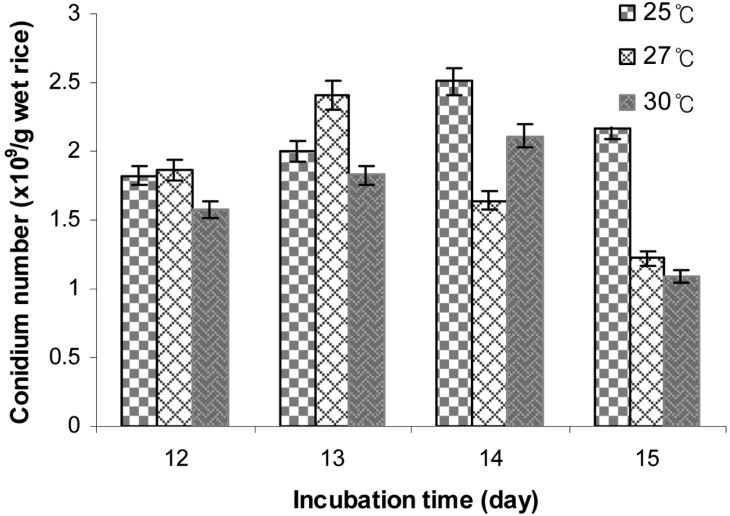
Effect of temperature on aerial conidia production
The result depicted in Fig. 2 indicates that incubation temperature markedly affected conidia yield. The optimal temperature for conidia production was 27℃ for 12 and 13 day incubations, whereas 25℃ was optimal for 14 and 15 day incubations. The optimal temperature for conidia production was 25℃ for the 14 day incubation. In this case, the number of conidia produced was 2.51 × 109/g wet conidiated rice. According to Vu et al. [9], the optimal temperature for conidia production of V. lecanii 41185 is 25℃, with the number of conidia produced 6.13 × 109 conidia/g. In this study, the optimal temperature of 25℃ matched that for liquid culture of B. bassiana KK5 [8]. Furthermore, Thomas and Jenkins [24] showed that the incubation temperatures of the liquid and solid production stages matched those of the germination and mycelial growth of M. flavoviride.
Fig. 2.
Effect of temperature on aerial conidia production. The inoculated steamed polished rice at a 35% moisture level in a plastic bottle was incubated at 25, 27, and 30℃ and 75% relative humidities for 12~15 days.
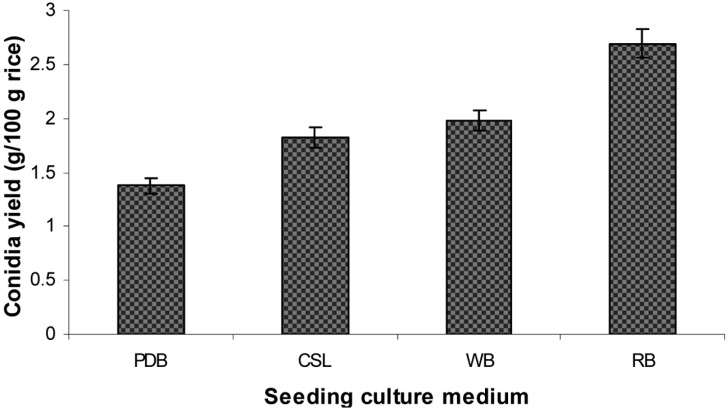
Effect of liquid seeding culture medium on aerial conidia production
Different seeding culture media including PDB, WB, CSL, and RB were tested. The results in Fig. 3 show that conidia production significantly depended on liquid culture medium. Rice inoculated with the culture grown in RB medium produced a significantly higher number of conidia (2.69 g conidia/100 g rice) compared to those grown on other seeding culture media. Rice inoculated with the PDB culture produced the lowest number of aerial conidia.
Fig. 3.
Effect of seeding culture medium on aerial conidia production. PDB, potato dextrose broth; wheat bran (WB), 3% corn meal, 2% wheat bran, 3.5% corn steep liquor; corn step liquor (CSL), 3% corn meal, 2% rice bran, 3.5% corn steep liquor; and RB, 3% corn meal, 2% rice bran, 2% corn steep powder. Various seeding cultures grown in different liquid media were inoculated onto sterilized steamed rice at a 35% moisture level in plastic bottles at an inoculum size of 10% (v/w). Determination of conidia production was conducted at 25℃ and 75% relative humidities for 14 days.
Effect of seeding culture time on aerial conidia production
The RB seeding culture was inoculated onto rice for 2, 3, or 5 days at 10% inoculum size, after which conidia yield after 14 days of cultivation was determined. The results in Fig. 4 show that conidial production on rice inoculated with 2-day-old liquid culture was the highest, whereas rice inoculated with 5-day-old liquid culture produced the lowest yield of aerial conidia.
Fig. 4.
Effect of seeding culture time on aerial conidia production. Ten percent (v/w) seeding cultures grown in 3% corn meal, 2% rice bran, 2% corn steep powder medium for different times were inoculated onto steamed rice, after which the inoculated rice were incubated at 25℃ and 75% relative humidities for 14 days.
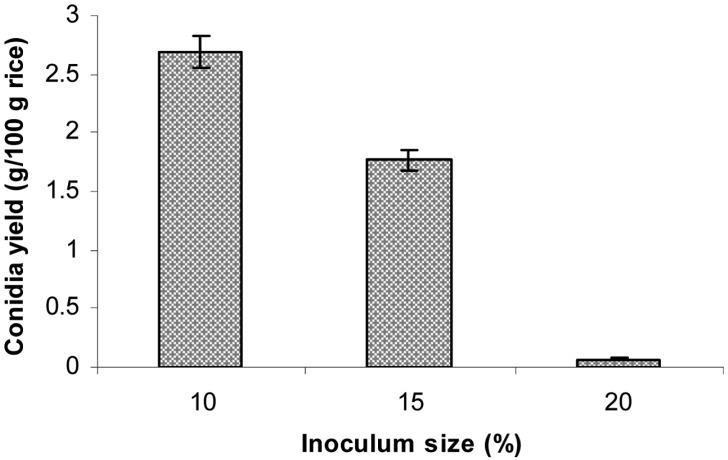
Effect of inoculum size on aerial conidia production
Rice were inoculated with different sizes of inoculum and then incubated for 14 days, after which the conidiated rice was dried at 35℃ for 24 hr. The dried conidia powder was then harvested to determine conidia yield. The results shown in Fig. 5 indicate that the conidia yield of rice inoculated with increasing sizes of inoculum was decreased, due possibly to increased moisture content and, in turn, decreased aeration in rice. The highest conidial yield was achieved after 14 days of cultivation using 10% inoculum size of 2-day-old seeding culture.
Fig. 5.
Effect of inoculum size on aerial conidia production. The sterilized polished rice in plastic bottles were inoculated with different inoculum sizes, such as 10, 15, and 20%, of seeding culture. The inoculated rice were incubated at 25℃ and 75% relative humidities for 14 days.
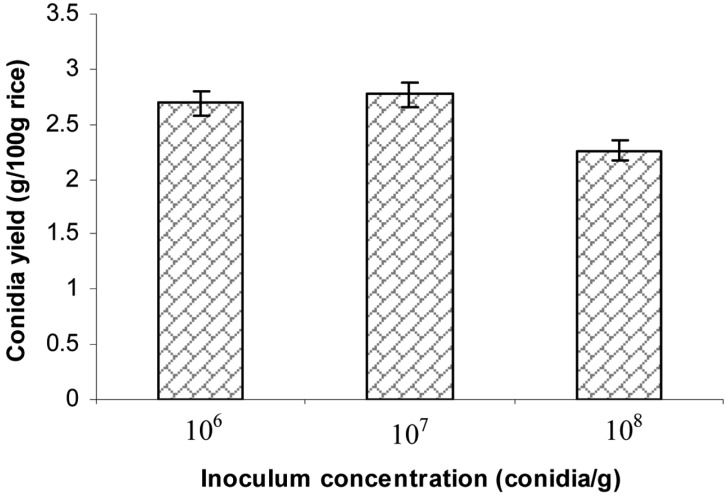
Effect of inoculum concentration on aerial conidia production
Rice were incubated with 106, 107, or 108 conidia/g wet rice for 14 days after which conidial yield were examined. The results shown in Fig. 6 indicate that the highest conidial yield (2.77 g conidia/100 g rice) was achieved using an initial concentration of 107 conidia/g wet rice.
Fig. 6.
Effect of initial conidia concentration on aerial conidia production. The steamed rice at a 35% moisture level in plastic bottles were inoculated with different conidium concentrations of 10% inoculum in order to achieve initial substrate concentrations of 106, 107 and 108 conidia/g wet rice, followed by incubation of inoculated rice at 25℃ and 75% relative humidities for 14 days.
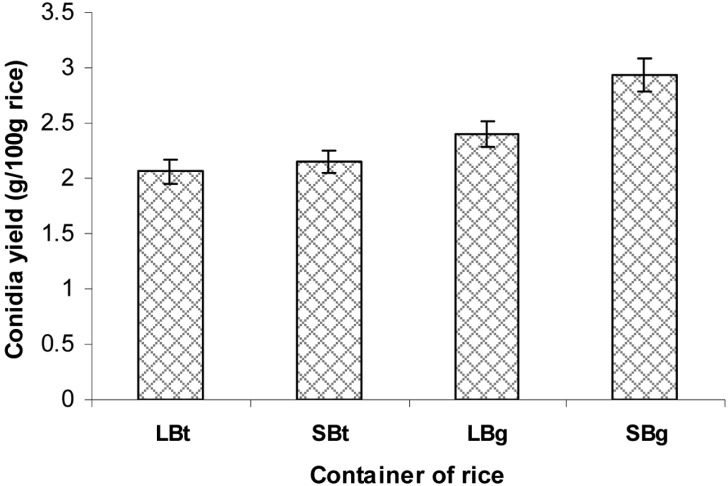
Effect of rice container on aerial conidia production
Four different types of rice containers were compared regarding their conidia production (Fig. 7). The two polyethylene bags produced more conidia than the two platic bottles. The conidia yield in the container with two small aeration filters was higher than that containing a larger aeration filter. In general, aeration during cultivation was important for conidia production. However, the production of conidia can be affected by the fact that aeration through a large aeration filter alters the water content of rice via absorption of outside moisture from the humid culture environment. Rice in the polyethylene bag with two small aeration filters produced the most number of conidia (2.91 g conidia/100 g rice) compared to other containers.
Fig. 7.
Effect of rice container on aerial conidia production. LBt, large plastic bottle; SBt, small plastic bottle; SBg, small aerated area polyethylene bag; and LBg, large aerated area polyethylene bag. The polished rice at a 40% moisture level were placed into different containers such as plastic bottles (62 × 92 × 160 mm3 and 70 × 92 × 160 mm3) with a ventilated cap and polyethylene bags (325 × 435 mm2) with small aeration filters (diameter, 36 mm) or large aeration filters (diameter, 79 mm). The moistened rice in the container were sterilized, cooled, and inoculated with 2-day-old 3% corn meal, 2% rice bran, 2% corn steep powder culture, followed by incubation at 25℃ and 75% relative humidities for 14 days.
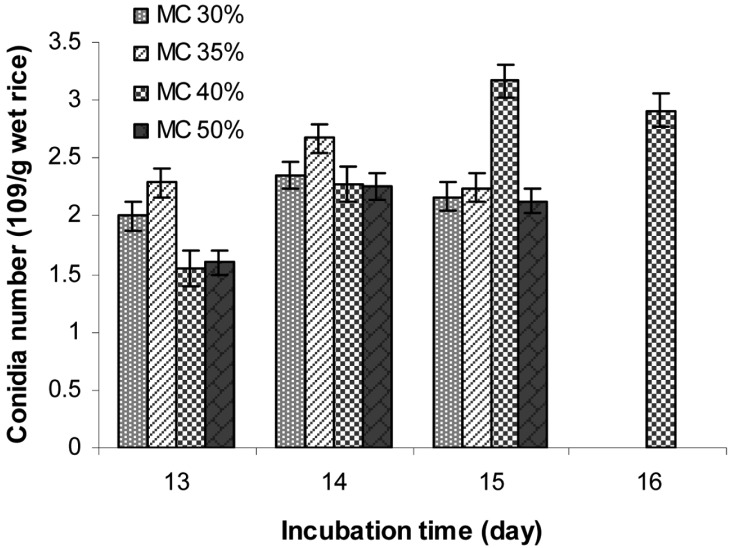
Effect of moisture content of rice on aerial conidia production
Conidia production on rice with different moisture contents of 30, 35, 40, and 50% were examined and the results are shown in Fig. 8. The results show that rice with a moisture level of 40% had the highest conidia yield (3.17 × 109 conidia/g wet conidiated rice) after 15 days of incubation. Moisture content of the substrate plays a significant role in the final yield of conidia [13]. Most mitosporic fungi prefer humid environments. However, different substrates vary in their moisture sorption curves, reaching maximum adsorption at different moisture levels. The conidia yield in this study was increased significantly with increasing incubation time. The results demonstrate that conidia yield strongly depended on the moisture content of rice. In another report [25], initial moisture levels from 35 to 80% were utilized in solid fermentation, depending on the fungal strain and substrate.
Fig. 8.
Effect of moisture content of rice on aerial conidia production. The polished rice with desired moisture contents (MC) of 30, 35, 40, and 50% in polyethylene bags with small aeration filters were inoculated with 10% inoculum in order to achieve an initial concentration of 107 conidia/g wet rice. The inoculated rice were then incubated at 25℃ for 12~17 days in order to determine the conidia number/g wet conidiated rice.
The moisture content of the medium changed during fermentation as a result of evaporation and metabolic activities. Therefore, the optimum moisture level of the substrate is very important [26]. There was a close relationship between moisture content and oxygen availability; increases in the moisture content of the substrate tends to decrease oxygen availability as the inter-particular spaces become filled with water and air is forced out [13, 27].
Effect of different ambient RH on aerial conidia production
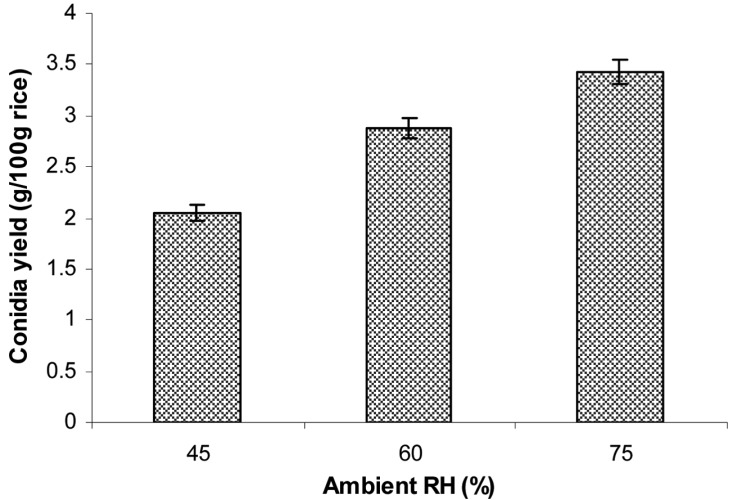
The conidia were produced on rice at a moisture level of 40% inoculated with 10% inoculum size of 107 conidia/g wet rice, followed by incubation at 25℃ at different ambient RHs of 45, 60, and 75%. The conidial yield was determined after 15 days of inoculation. The results in Fig. 9 indicate that the amount of aerial conidia significantly depended on ambient RH. For example, the amount of conidia produced at 75% RH (3.43 g conidia/100 g rice) was significantly higher than that at 45 or 60% RH.
Fig. 9.
Effect of ambient relative humidities (RH) on aerial conidia production. The sterilized moistened rice in a polyethylene bag with small aeration filter were inoculated with 2-day-old 3% corn meal, 2% rice bran, 2% corn steep powder liquid culture at 10% inoculum size, followed by incubation at 25℃ and different ambient RHs (45, 60, and 75%) for 15 days.
Effect of incubation period on aerial conidia production
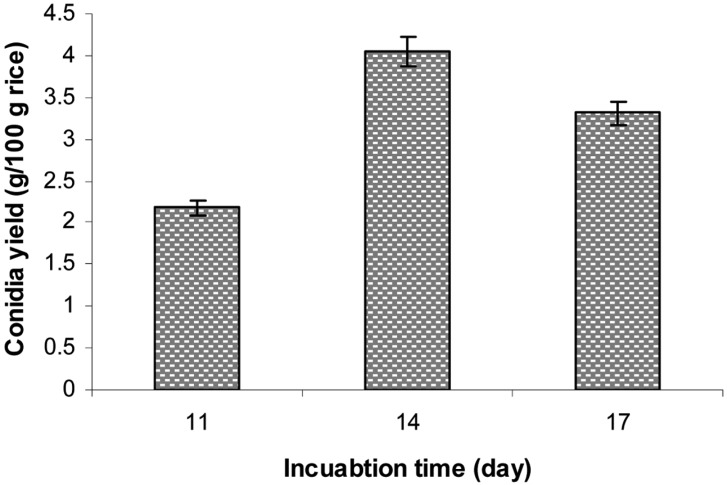
The conidia production of B. bassiana KK5 was conducted at 25℃ and 75% ambient RH using rice at a moisture level of 40% in a polyethylene bag with two small aeration filters and 2-day-old seeding culture grown in RB medium. Under these optimal conditions, the effect of incubation period (11, 14, or 17 days) on conidia production was examined, the results of which are shown in Fig. 10. After 14 days of incubation, the conidia yield was 4.05 g conidia/100 g rice (1.82 × 109 conidia/g rice), which is more than 2.83 times higher than that before optimization (1.43 g conidia/100 g rice). This value was also higher than the conidia yields of 3 g conidia/100 g rice and 2.28 g conidia/100 g as reported by Alves and Pereira [20] and Posada-Flórez [28], respectively, for B. bassiana. Using a mixture of 70% cotton seed-shell powder, 25% wheat bran, and 5% corn flour (plus water), the conidia yield of B. brongniartii was 2.9 × 109 conidia/g dry powder [17]. However, when B. brongniartii was cultured for 42 days on a mixture of shelled barley, sunflower oil, and water, the conidia yield was 2 × 109 [18].
Fig. 10.
Effect of incubation period on aerial conidia production. Conidia production was conducted at 25℃ and 75% relative humidities using polished rice at a 40% moisture level in a polyethylene bag with small aeration filter for 10~17 days.
In conclusion, we evaluated the suitability of various substrates, moisture content, temperature, seeding culture time, etc. on the aerial conidia production of B. bassiana KK5. The results show that the optimal conditions of conidia production on rice were 40% moisture content of whole polished rice, 25℃ culture temperature, 2-day-old seeding culture in 3% corn meal, 2% rice bran, and 2% corn steep powder, inoculum concentration of 107 conidia/g wet rice, 10% inoculum size, a fermentation container comprising a polyethylene bag with two small aeration filters. Under optimal conditions, maximum conidia production (4.05 g conidia/100 g dry white rice) was obtained after 14 days of cultivation and was 2.83 times higher than that pre-optimization.
Acknowledgements
We acknowledge the financial support provided by the Korean Rural Development Administration (PJ006640).
References
- 1.Hajek AE, StLeger RJ. Interactions between fungal pathogens and insect hosts. Annu Rev Entomol. 1994;39:293–322. [Google Scholar]
- 2.Feng MG, Poprawski TJ, Khachatourians GG. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol. 1994;4:3–34. [Google Scholar]
- 3.Feng MG, Chen B, Ying SH. Trials of Beauveria bassiana, Paecilomyces fumosoroseus and imidacloprid for management of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci Technol. 2004;14:531–544. [Google Scholar]
- 4.Sharma HC. Pest Management and the Environment. In: Sharma HC, editor. Biotechnological approaches for pest management and ecological sustainability. Boca Raton: Taylor & Francis Group/CRC Press; 2009. pp. 1–18. [Google Scholar]
- 5.Chen B, Li ZY, Feng MG. Occurrence of entomopathogenic fungi in migratory alate aphids in Yunnan Province of China. Biocontrol. 2008;53:317–326. [Google Scholar]
- 6.Hagen KS, van den Bosch R. Impact of pathogens, parasites, and predators on aphids. Annu Rev Entomol. 1968;13:325–384. [Google Scholar]
- 7.Rashki M, Kharazi-pakdel A, Allahyari H, van Alphen JJ. Interactions among the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales), the parasitoid, Aphidius matricariae (Hymenoptera: Braconidae), and its host, Myzus persicae (Homoptera: Aphididae) Biol Control. 2009;50:324–328. [Google Scholar]
- 8.Pham TA, Kim JJ, Kim S, Kim K. Production of blastospore of entomopathogenic Beauveria bassiana in a submerged batch culture. Mycobiology. 2009;37:218–224. doi: 10.4489/MYCO.2009.37.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vu VH, Hong SI, Kim K. Production of aerial conidia of Lecanicillium lecanii 41185 by solid state fermentation for use as mycoinsecticides. Mycobiology. 2008;36:183–189. doi: 10.4489/MYCO.2008.36.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett MC, Jaronski ST. Mass production of entomogenous fungi for biological control of insects. In: Burge MN, editor. Fungi in biological control systems. Manchester/NY: Manchester University Press; 1988. pp. 65–85. [Google Scholar]
- 11.Rombach MC. Production of Beauveria bassiana [Deuteromycotina: Hyphomycetes] sympoduloconidia in submerged culture. Entomophaga. 1989;34:45–52. [Google Scholar]
- 12.Kleespies RG, Zimmermann G. Production of blastospores by three strains of Metarhizium anisopliae (Metch.) Sorok in submerged culture. Biocontrol Sci Technol. 1992;2:127–135. [Google Scholar]
- 13.Jenkins NE, Heviefo G, Langewald J, Cherry AJ, Lomer CJ. Development of mass production technology for aerial conidia for use as mycopesticides. Biocontrol News Inf. 1998;19:21–31. [Google Scholar]
- 14.Pandey A. Solid-state fermentation. New Delhi: Wiley Eastern Limited; 1994. pp. 12–17. [Google Scholar]
- 15.Babu KR, Satyanarayana T. α-amylase production by thermophilic Bacillus coagulans in solid-state fermentation. Process Biochem. 1995;30:305–309. [Google Scholar]
- 16.Feng KC, Liu BL, Tzeng YM. Verticillium lecanii spore production in solid-state and liquid-state fermentations. Bioprocess Eng. 2000;23:25–29. [Google Scholar]
- 17.Nelson TL, Low A, Glare TR. Large scale production of New Zealand strains of Beauveria and Metarhizium; Procceeding of the 49th New Zealand Plant Protection Conference; 1996 Aug 13-15; Nelson, New Zealand. Paihia: New Zealand Plant Protection Society; 1996. pp. 257–261. [Google Scholar]
- 18.Lin LX, Yu YC, Hu JW. Li YW, Li ZZ, Liang ZQ, Wu JW, Wu ZK, Xu QF. Study and application of entomogenous fungi in China. Vol.1. Beijing: Academic Periodical Press; 1988. Study on white grub control by Beauveria brongiartii in soybean field, in study and application of Entomogenous Fungi in China; pp. 135–139. [Google Scholar]
- 19.Aregger E. Conidia production of the fungus Beauveria brongniartii on barley and quality evaluation during storage at 2℃. J Invertebr Pathol. 1992;5:2–10. [Google Scholar]
- 20.Alves SB, Pereira RM. Production of Metarhizium anisopliae (Metsch.) Sorok and Beauveria bassiana (Bals.) Vuill in plastic trays. Ecossistema. 1989;14:188–192. [Google Scholar]
- 21.Mendonca AF. Mass production, application and formulation of Metarhizium flavoviride for control of sugarcane grasshopper in Brazil. In: Lomer CJ, Prior C, editors. Biological control of locusts and grasshoppers. Wallingford: CAB International; 1992. pp. 239–254. [Google Scholar]
- 22.Ibrahim YB, Low W. Potential of mass-production and field efficacy of isolates of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus against Plutella xylostella. Int J Pest Manag. 1993;39:288–292. [Google Scholar]
- 23.Milner RJ, Rogers DJ, McRae CM, Huppatz RJ, Brier H. Corey SA, Dall DJ, Milner WM. Pest control in sustainable agriculture. Melbourne: CSIRO Press; 1993. Preliminary evaluation of the use of Metarhizium anisopliae as a mycopesticide for control of peanut scarabs; pp. 253–255. [Google Scholar]
- 24.Thomas MB, Jenkins NE. Effects of temperature on growth of Metarhizium flavoviride and virulence to the variegated grasshopper Zonocerus variegatus. Mycol Res. 1997;101:469–474. [Google Scholar]
- 25.Raimbault M. General and microbiological aspects of solid substrate fermentation. EJB Electron J Biotechnol. 1998;1:1–20. [Google Scholar]
- 26.Baysal Z, Uyar F, Aytekin C. Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem. 2003;38:1665–1668. [Google Scholar]
- 27.M MY, Moreira AR, Tengerdy RP. Principles of solid substrate fermentation. In: Smith JE, Berry DR, Kristiansen B, editors. Fungal technology. London: Edward Arnold; 1983. pp. 117–144. [Google Scholar]
- 28.Posada-Flórez FJ. Production of Beauveria bassiana fungal spores on rice to control the coffee berry borer, Hypothenemus hampei, in Colombia. J Insect Sci. 2008;8:1–13. [Google Scholar]