Abstract
Responses to drought, heat, and combined stress were compared in tobacco (Nicotiana tabacum L.) plants ectopically expressing the cytokinin oxidase/dehydrogenase CKX1 gene of Arabidopsis thaliana L. under the control of either the predominantly root-expressed WRKY6 promoter or the constitutive 35S promoter, and in the wild type. WRKY6:CKX1 plants exhibited high CKX activity in the roots under control conditions. Under stress, the activity of the WRKY6 promoter was down-regulated and the concomitantly reduced cytokinin degradation coincided with raised bioactive cytokinin levels during the early phase of the stress response, which might contribute to enhanced stress tolerance of this genotype. Constitutive expression of CKX1 resulted in an enlarged root system, a stunted, dwarf shoot phenotype, and a low basal level of expression of the dehydration marker gene ERD10B. The high drought tolerance of this genotype was associated with a relatively moderate drop in leaf water potential and a significant decrease in leaf osmotic potential. Basal expression of the proline biosynthetic gene P5CSA was raised. Both wild-type and WRKY6:CKX1 plants responded to heat stress by transient elevation of stomatal conductance, which correlated with an enhanced abscisic acid catabolism. 35S:CKX1 transgenic plants exhibited a small and delayed stomatal response. Nevertheless, they maintained a lower leaf temperature than the other genotypes. Heat shock applied to drought-stressed plants exaggerated the negative stress effects, probably due to the additional water loss caused by a transient stimulation of transpiration. The results indicate that modulation of cytokinin levels may positively affect plant responses to abiotic stress through a variety of physiological mechanisms.
Key words: Abscisic acid, cytokinin, cytokinin oxidase/dehydrogenase, drought stress, heat stress, tobacco
Introduction
In agriculture, drought stress is one of the most important constraints on crop yield. As a result of global climate change, the negative impact of drought on yield is expected to increase in future, especially in combination with a rise in temperature. Consequently, an understanding of the mechanisms involved in plant tolerance to drought stress is of vital importance.
Plants have evolved various means to cope with drought stress. One strategy is through a change in plant morphology, including changes in root system architecture. The size of the root system has been found to correlate positively with resistance to water deficit (Tuberosa et al., 2002), with larger root systems improving water and nutrient use efficiency.
One of the key regulators of root system architecture is the hormone cytokinin (CK). Reduction in CK levels retards differentiation of cells in the root meristem (Werner et al., 2001, 2003). CKs also regulate the distance between adjacent lateral root primordia (Werner et al., 2003; Shkolnik-Inbar and Bar-Zvi, 2010). Thus, a decrease in the CK level or a reduction in CK signalling can lead to an enlarged root system (Werner et al., 2001, 2003; Miyawaki et al., 2006; Riefler et al., 2006; Heyl et al., 2008). CK down-regulation may be achieved either by stimulation of CK degradation by overexpression of the main CK-deactivating enzyme, cytokinin oxidase/dehydrogenase (CKX; EC 1.4.3.18/1.5.99.12) (Werner et al., 2003), or by down-regulation of CK biosynthesis (Miyawaki et al., 2006). Overexpression of CKX has been studied extensively and CKX-overexpressing plants have been shown to exhibit increased drought and salinity tolerance (Werner et al., 2010; Nishiyama et al., 2011).
Constitutively enhanced CKX activity has a positive effect on the growth of roots, but a strong negative effect on the growth and development of shoot systems, resulting in a dwarf phenotype with delayed organogenesis. In order to counteract this drawback, enhanced CKX expression was limited mainly to the roots by using root-specific promoters such as the WRKY6 gene (Werner et al., 2010). Tobacco plants expressing a chimeric WRKY6:CKX1 gene exhibited a greatly increased size of the root system (by 27–39%), while their development was very similar to that of the wild type (WT). Similarly, root-specific CKX gene expression in Arabidopsis also resulted in an enlarged root system (Werner et al., 2010).
Here, the responses to drought and heat stress (HS) of tobacco plants exhibiting root-specifically enhanced CKX gene expression, constitutive CKX overexpression, and the corresponding WT are compared in more detail. Taking into account the frequent simultaneous occurrence of drought and HS under natural conditions, the responses of the three genotypes to combined drought and HS have also been compared. The results demonstrate the importance of CKs in plant responses to abiotic stresses.
Materials and methods
Plant material and stress application
WT tobacco (Nicotiana tabacum L. cv. Samsun NN), WRKY6:CKX1 (line W6:CKX1-29) (Werner et al., 2010), and 35S:CKX1 (line 35S:CKX1-50) plants (Werner et al., 2001) were grown in soil (pot volume 300ml) in a growth chamber (SANYO MLR 350H, Osaka, Japan) for 6 weeks in a 16h photoperiod at 130 μmol m–2 s–1, 25/23 °C, and a relative humidity (RH) of ~80%. Drought stress was imposed by cessation of watering for 10 d at reduced RH (~35%). Combined drought and HS was achieved by transferring the plants to 40 °C for 2h at the very end of the drought period. Recovery of drought-stressed plants was determined 24h after re-watering. Heat treatment (40 °C for 2h or 6h) was also applied to well-watered plants. Upper leaf (the youngest fully developed leaf), lower leaf (the oldest still green true leaf), and root (apical part, 1.5cm) samples were frozen in liquid nitrogen and stored at –80 °C. Three independent biological experiments were performed.
RNA extraction and reverse transcription
Total RNA from leaves or roots was extracted using an RNeasy Plant Kit (Qiagen, Hilden, Germany) and treated with DNAse I (DNA-free; Ambion, Huntingdon, UK). RNA was transcribed with Transcriptor Reverse Transcriptase (Roche Applied Science, Mannheim, Germany), using oligo(dT) primers and Protector RNase Inhibitor (Roche Applied Science, Mannheim, Germany).
Quantitative real-time reverse transcription–polymerase chain reaction (qRT–PCR)
qRT–PCR was performed using a FastStart DNA MasterPLUS SYBR Green I Kit (Roche Applied Science) by LightCycler 1.2 (Roche Applied Science). The primer sequences and annealing temperatures are shown in Supplementary Table S1 available at JXB online. CKX1 primers specific for the Arabidopsis gene were used. cDNA derived from calibrator RNA was included in each LighCycler run to correct run-to-run differences. The transcript levels were normalized using the Act9 gene as a reference.
Water and osmotic potential
Leaf water potential was determined using a pressure chamber (Labio, Czech Republic), following the procedure described by Boyer (1995). The balancing pressure was measured in individual leaves. Osmotic potential was determined psychrometrically from leaf sap using a Wescor HR-33 microvoltmeter (USA) with C52 sample chambers. Mean values of two independent experiments, each with three replications, are presented.
Gas exchange measurement
Gas exchange parameters [net photosynthetic rate (A) and stomatal conductance for water vapour (g s)] were determined using the LI-6400 portable photosynthesis system (Li-Cor, Lincoln, NE, USA) with a leaf chamber of 2×3cm and an integrated light source (LI-6400-02B). Fully expanded leaves were measured at 130 μmol m–2 s–1 (if not specified otherwise), 40 °C (closed cabinet incubated to 40 °C), and RH ~60–70%. The external CO2 concentration in air C a[CO2] was maintained at 380 μmol mol–1 in the reference cuvette. Measurements were recorded every 30 s over a 1.5h period for three plants of each genotype.
Thermal imaging
All thermal images were obtained with an infrared camera (FLIR P660; FLIR, Sweden), which operates in the wavebands (spectral resolution) 7.5–13 μm, with a thermal resolution 0.06 °C and a spatial resolution of 640×480 pixels. A 45 ° objective was used. Images were analysed using ThermaCAM Reporter 8 Professional software. Emissivity for leaf measurements was set at 0.95 throughout.
Photosynthetic pigment analysis
Xanthophyll cycle pigments were determined in acetone extracts by HPLC (ECOM, Prague, Czech Republic) according to Haisel et al. (2008). Data were analysed using the Clarity software package (DataApex, Prague, Czech Republic). DEPS (de-epoxidation state of xanthophyll cycle pigments) was calculated as the ratio of de-epoxidated zeaxanthin+0.5 antheraxanthin to the total content of xanthophyll cycle pigments (i.e. violaxanthin, antheraxanthin, and zeaxanthin).
Quantification of cytokinin and abscisic acid metabolites
Sample [~0.5g fresh weight (FW)] extraction and purification for endogenous CK analysis was performed according to Novák et al. (2003). The CK levels were quantified by ultraperformance liquid chromatography–electrospray tandem mass spectrometry (UPLC-MS/MS) (Novák et al., 2008). Abscisic acid (ABA) metabolites were determined using solid-phase extraction (Oasis HLB cartridges, 60mg, 3ml; Waters, Milford, MA, USA), followed by a derivatization step, ABA-specific immunoaffinity extraction, and quantification by the UPLC-MS/MS system according to Turečková et al. (2009).
Determination of cytokinin oxidase/dehydrogenase activity
CKX enzyme was extracted and partially purified according to Motyka et al. (2003). CKX activity was determined by in vitro assays based on the conversion of [2-3H]N 6-(2-isopentenyl)adenine (iP; prepared by the Isotope Laboratory, IEB AS CR, Prague, Czech Republic) to [3H]adenine. CKX activity was determined in three independent experiments, which showed the same tendencies (with different absolute values). The results of one representative experiment (mean value of three parallel assays for each of three replicate protein preparations) are presented.
Statistical analysis
All data presented are from at least three independent experiments. Mean values and standard errors of the means were calculated, and the significance of differences was evaluated by Student’s t-test. Analysis of variance performed by the least mean square (LMS) method was used to evaluate the significance of differences among treatments, tissues, as well as genotypes.
Results
Transgene expression and CKX activity were determined in both leaves and roots in order to characterize the genotypes used in this study. The upper and lower leaves were analysed separately, as they differ substantially in their physiological behaviour, especially under drought stress (Supplementary Fig. S1 at JXB online), when the sink strength of the upper leaves is enhanced and the lower leaves gradually become a source of nutrients (Havlova et al., 2008). Subsequently, different parameters relevant to drought stress tolerance were investigated, namely expression of the drought stress marker genes ERD10 and P5CSA, water and osmotic potentials, and levels of chlorophyll and xanthophyll cycle pigments. The estimated stress tolerance was correlated with the endogenous CK content as well as with the metabolite profile of ABA, which is the key hormone in abiotic stress defence.
Ectopic expression of the CKX1 gene and cytokinin oxidase/dehydrogenase activity
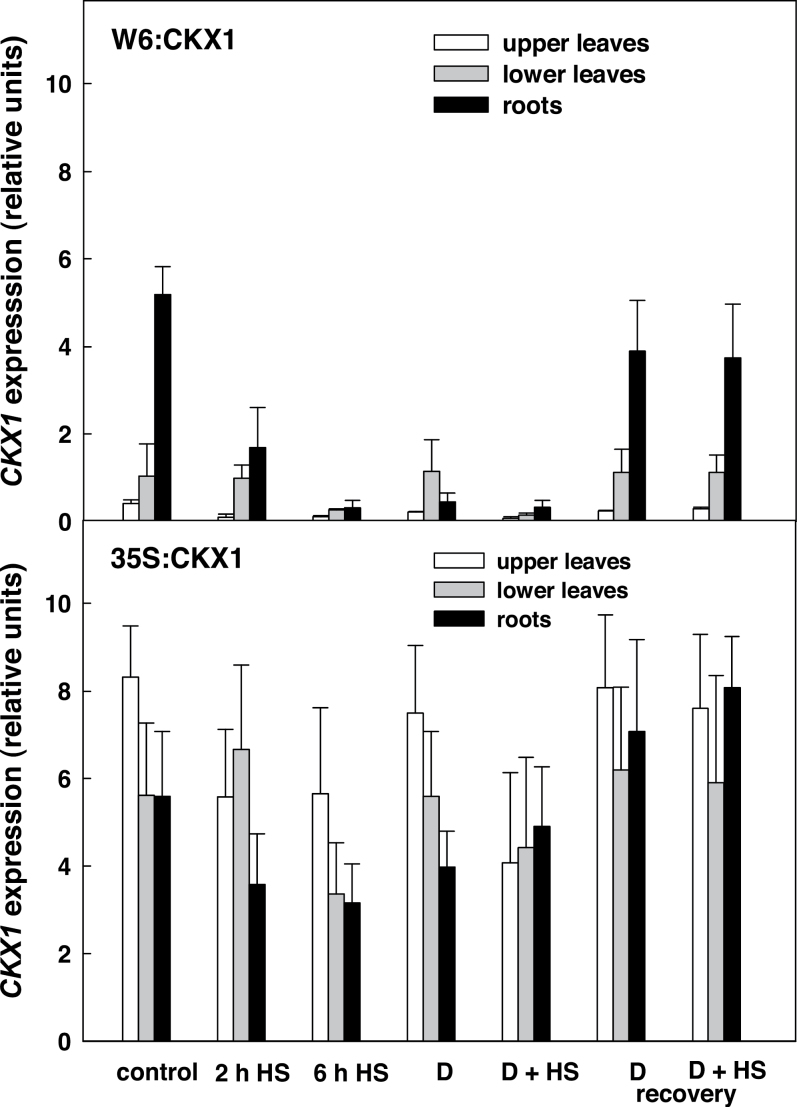
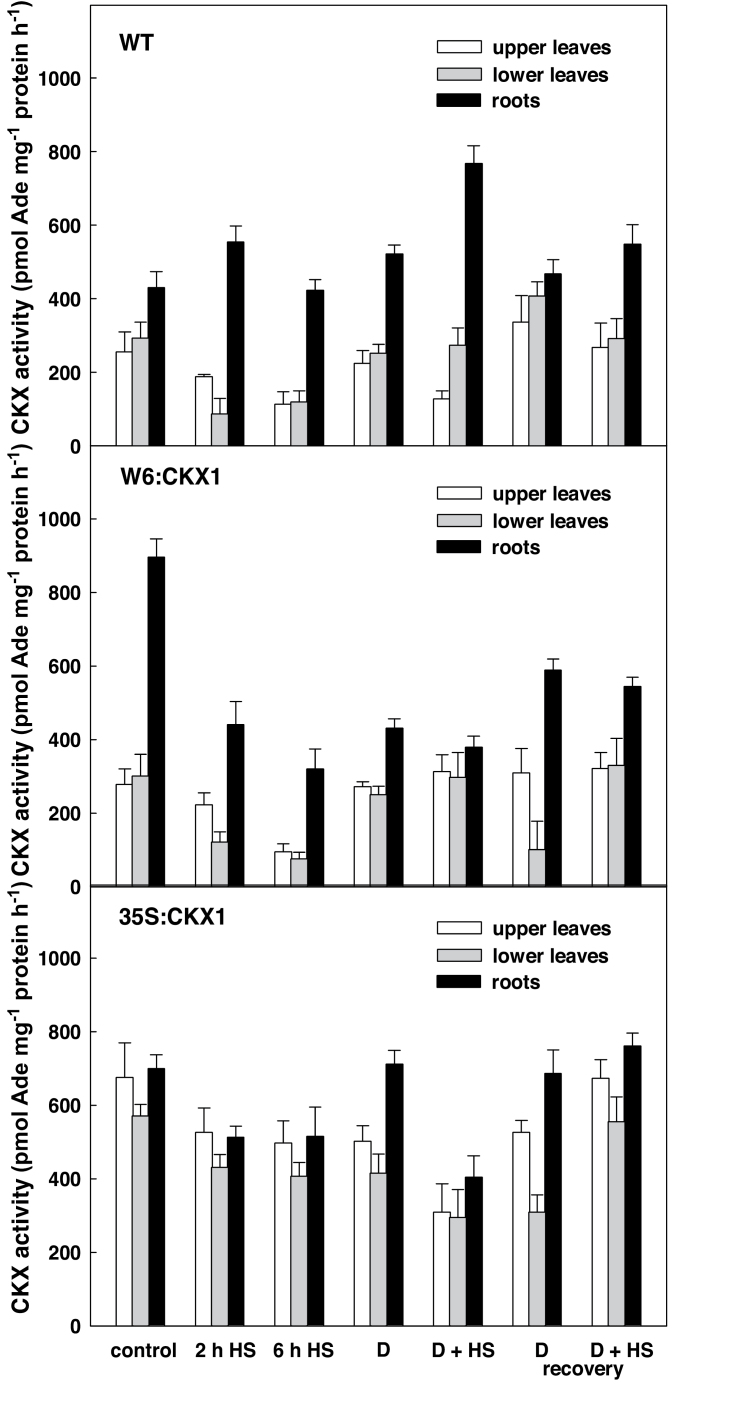
The expression of the CKX1 gene was studied in upper and lower leaves and roots under control conditions, during the stress responses, and following recovery. Under control conditions, the level of CKX1 expression in roots, driven by the WRKY6 promoter (W6:CKX1 plants), was comparable with that in 35S:CKX1 plants (Fig. 1). This corresponded well to the high CKX activity in W6:CKX1 roots (Fig. 2). WRKY6:CKX1 expression was much lower (5–18% of the root values) in leaves, which was in accordance with a substantially lower CKX activity in this tissue, comparable with that in the WT. Both drought and HS were associated with strong down-regulation of WRKY6 promoter activity in the roots, declining to the leaf level after 6h at 40 °C (Fig. 1). The drop in WRKY6:CKX1 expression was reflected by a very low CKX activity. Upon recovery, WRKY6 promoter activity started increasing again in the roots.
Fig. 1.
Transcript level of the CKX1 gene in leaves and roots of CKX1 transgenic tobacco plants. Control, control (hydrated) conditions; D, drought stress (10 d dehydration); D+HS, combined drought and heat stress (10 d dehydration+40 °C for 2h); D recovery, 24h recovery following rehydration; D+HS recovery, 24h recovery after combined stress; 2h HS; heat stress (40 °C for 2h); 6h HS, heat stress (40 °C for 6h).
Fig. 2.
Total cytokinin oxidase/dehydrogenase activity in leaves and roots of wild-type (WT) and CKX1 transgenic tobacco plants. Details for designation of individual variants are as described in Fig. 1.
35S:CKX1 plants displayed high levels of CKX1 expression in both leaves and roots and under all experimental conditions (Fig. 1). CKX activity in the leaves of 35S:CKX1 plants was about three times higher than in WT leaves under control conditions, but only twice higher than in roots (Fig. 2), in spite of the high CKX1 gene expression in this organ.
The expression data revealed considerable differences between both CKX1-expressing transgenic lines, especially under stress. Switching off the activity of the WRKY6 promoter in stressed roots resulted in a significant suppression of CKX activity, which affected the endogenous CK pool.
Changes in the cytokinin content after stress treatment
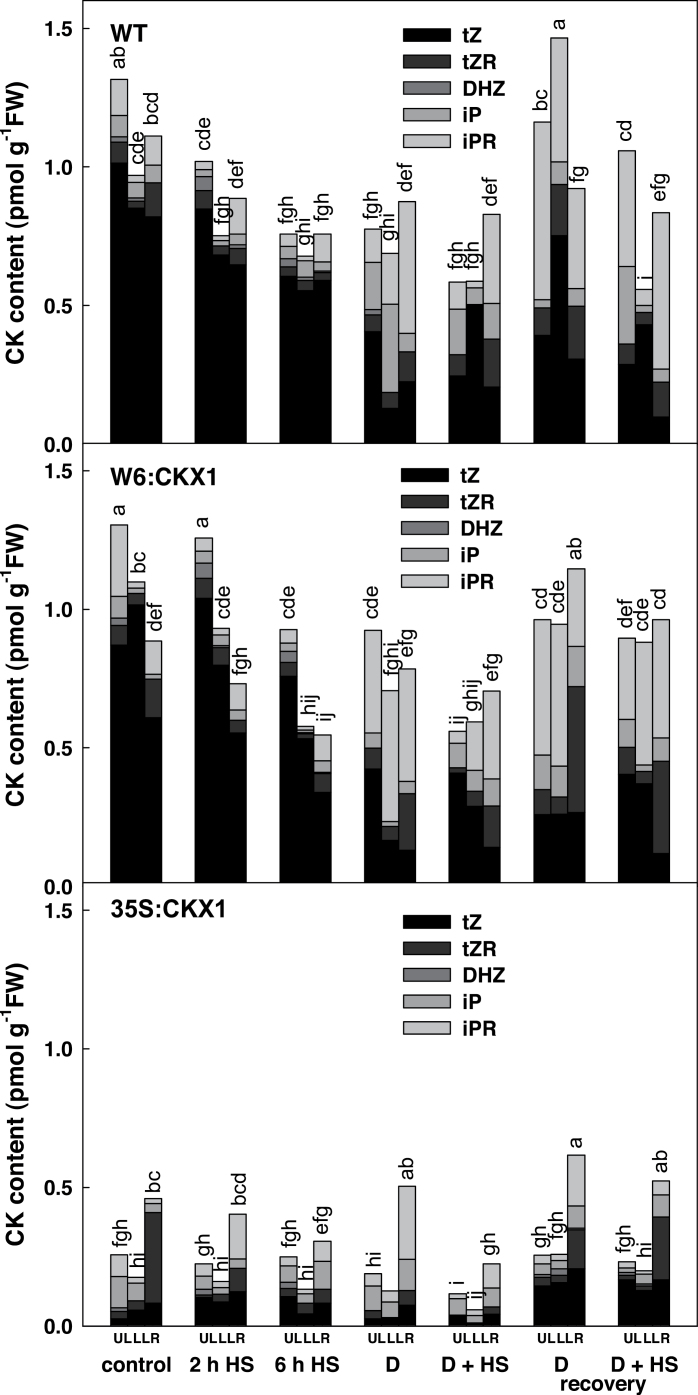
The impact of CKX1 expression and the resulting changes in CKX activity on the CK pool was determined before and during the stress responses, and during recovery (Fig. 3). Under control conditions, leaves of W6:CKX1 plants exhibited levels of bioactive CKs similar to leaves of the WT (Fig. 3). This finding agrees with their shoot phenotype, which did not differ from that of the WT. The difference in bioactive CKs in the roots of W6:CKX1 and WT plants was statistically significant (decrease by 30%, LMS variance analysis). This relatively small reduction might be due to the fact that only the apical parts of roots (~1.5cm) were used for CK analysis. 35S:CKX1 plants exhibited diminished levels of bioactive CKs in leaves (27% of WT) and roots (42% of WT) (Fig. 3).
Fig. 3.
Content of endogenous bioactive cytokinins (trans-zeatin, isopentenyladenine, and their corresponding ribosides and dihydrozeatin) in leaves and roots of wild-type (WT) and CKX1 transgenic tobacco plants. Details for designation of individual variants are as described in Fig. 1. Different letters indicate statistically different values.
Drought stress caused a substantial decrease in the level of the most physiologically active CK trans-zeatin (tZ) and its riboside in all genotypes. The levels of bioactive CKs strongly decreased in WT leaves (P=0.0006); in WT roots, a substantial tZ decrease coincided with moderate elevation of isopentenyladenosine (iPR) (Fig. 3). The decrease in bioactive CKs was smaller in the leaves of the more drought-tolerant W6:CKX1 plants (P=0.001). Down-regulation of tZ was accompanied in the whole plant by elevation of iPR. Drought stress resulted in only a moderate CK decrease in leaves of the highly drought-tolerant 35S:CKX1 plants, the decrease in tZ riboside in the roots being compensated by iPR elevation (Fig. 3). Application of HS to drought-stressed plants resulted in a further decrease of bioactive CKs, especially in the leaves of WT and W6:CKX1 plants (Fig. 3). Re-watering resulted in an increase of bioactive CKs in all genotypes. This increase was relatively lower in the leaves of W6:CKX1 plants, probably due to reactivation of the WRKY6 promoter in the roots and thus a diminished CK export via the xylem.
The WT exhibited a small decrease of bioactive CKs in leaves after a 2h HS without prior drought stress, while only minor changes were found in W6:CKX1 plants. Prolonged HS (6h) was associated with a decrease in bioactive CKs in both genotypes. HS had negligible effects on the CK levels in 35S:CKX1 plants.
The contents of immediate precursors of bioactive CKs (CK phosphates) correlated with the levels of bioactive CKs in control conditions in all genotypes, with the exception of W6:CKX1 roots, where their level was relatively higher (Supplementary Fig. S2 at JXB online). A substantial decrease of CK phosphates was found during drought stress, in particular in lower leaves. Re-watering was associated with a profound elevation of CK phosphate levels in all genotypes. The contents of CK O-glucosides were relatively low, especially in 35S:CKX1 plants, which reflects a diminished CK deactivation by glucosylation in CKX1-expressing genotypes. Levels of CK deactivation products, CK N7- and N9-glucosides, were substantially increased during prolonged drought stress—with the exception of 35S:CKX1 plants. They were not affected by a relatively short-term HS.
In contrast to the decrease in tZ, its riboside, and phosphate under drought stress in WT and W6:CKX1 plants, the content of corresponding cis-zeatin metabolites increased after drought and even more after combined stress (Supplementary Fig. S2 at JXB online). After a 2h HS in the absence of drought stress, these cis-zeatin metabolites were slightly elevated in the upper leaves of the WT and more so in those of W6:CKX1 plants. Their increase was delayed in 35S:CKX1 plants, being observed only after 6h of HS. These data are in agreement with the proposed function of cis-zeatin under stress conditions (Gajdosova et al., 2011).
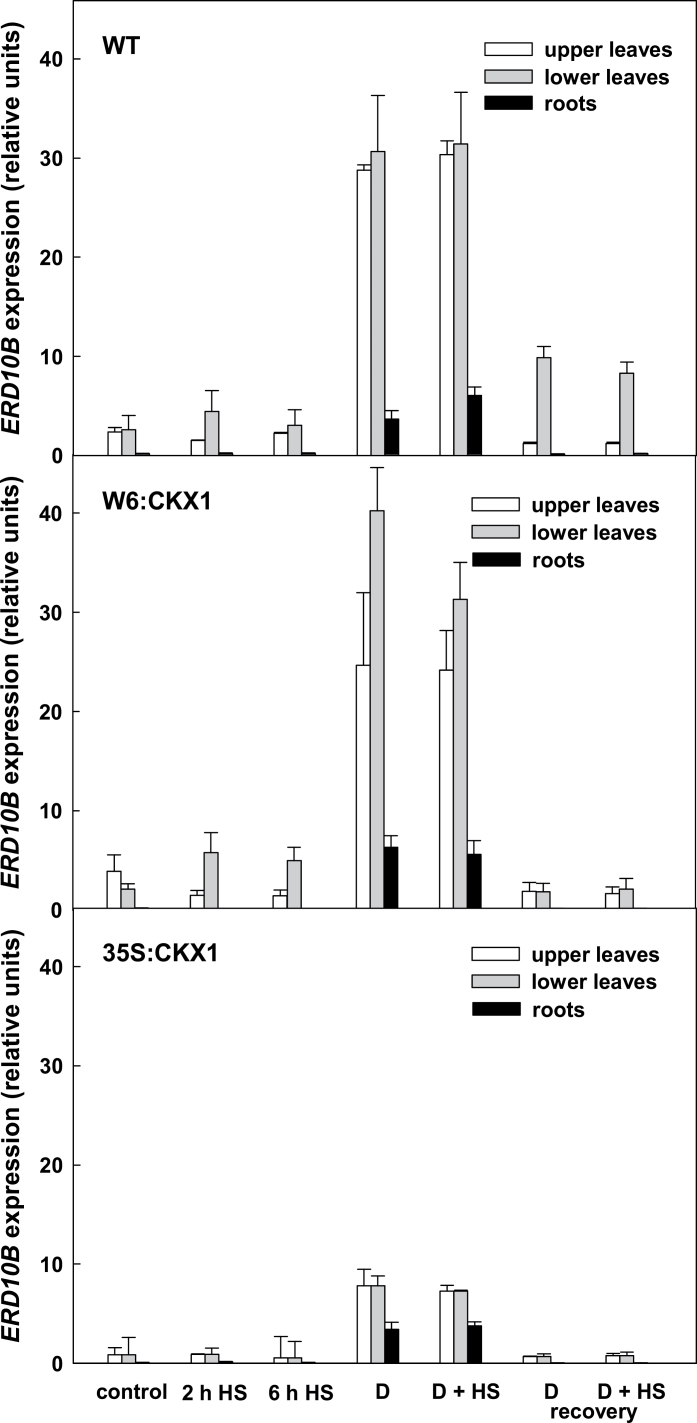
Expression of the dehydrin gene ERD10B and P5CSA
Next, the impact of drought on the individual genotypes was analysed and the dehydrin-encoding gene, ERD10 (Dobra et al., 2011), was used as a marker of dehydration stress (Fig. 4). WT plants exhibited a low level of dehydrin expression in leaves and only a marginal expression in roots under control conditions. Drought stress resulted in a ~10-fold up-regulation of ERD10B expression in WT leaves (P=0.005), which was further enhanced in combined stress. In contrast, only a weak elevation of ERD10B expression was observed in leaves of well-watered WT plants exposed to HS (Fig. 4).
Fig. 4.
Transcript levels of the ERD10B gene in leaves and roots of wild-type (WT) and CKX1 transgenic tobacco plants. Details for designation of individual variants are as described in Fig. 1.
W6:CKX1 plants exhibited a slightly higher ERD10B expression level than the WT under control conditions. ERD10B expression was greater in W6:CKX1 than in the WT under drought stress, especially in the middle and lower leaves (P=0.004). 35S:CKX1 plants exhibited only about one-third of the ERD10B expression level shown by the other two genotypes. ERD10B expression under drought conditions increased throughout the plant (P=0.03). The expression was stimulated ~8-fold in the leaves, reaching ~25% of the WT levels.
Another drought-inducible gene, P5CSA, which codes for the rate-limiting proline biosynthetic enzyme Δ1-pyrroline-5-carboxylate synthetase (Dobrá et al., 2011), exhibited a similar expression profile to that of ERD10B in WT and W6:CKX1 plants (Supplementary Fig. S3 at JXB online). In 35S:CKX1 plants, P5CSA showed an enhanced basal level, especially in lower leaves. Enhanced expression of P5CSA in lower leaves was also maintained in this genotype during drought and combined stress (Supplementary Fig. S3).
Water and osmotic potential
The effect of drought stress on water relations in WT, W6:CKX1, and 35S:CKX1 plants was compared. Under control conditions, all genotypes tested exhibited a similar water potential in both upper and lower leaves (Table 1). Under drought stress, WT and W6:CKX1 plants had a similarly decreased water potential in the upper leaves, which coincided with their comparably mild wilting. The lower leaves of the WT exhibited strong wilting after 10 d of drought, which was associated with a decrease in their water potential. In contrast, W6:CKX1 plants retained a higher water potential in the lower leaves (P=0.03), consistent with the reduced wilting shown by these plants. Under drought stress, 35S:CKX1 plants maintained a higher water potential in all leaves (P=0.01). No leaf wilting was observed in these plants during the whole drought period (10 d).
Table 1.
Water and osmotic potential after 10 d of drought.
| Water potential (MPa) | Osmotic potential (MPa) | |||||
|---|---|---|---|---|---|---|
| WT | W6:CKX | 35S:CKX | WT | W6:CKX | 35S:CKX | |
| UL, C | –0.23±0.01 | –0.21±0.06 | –0.19±0.02 | –0.66±0.02 | –0.87±0.04 | –0.82±0.05 |
| LL, C | –0.22±0.03 | –0.24±0.04 | –0.18±0.02 | –0.68±0.17 | –0.71±0.11 | –0.75±0.02 |
| UL, stress | –0.72±0.13 | –0.73±0.01 | –0.65±0.14 | –1.22±0.01 | –1.47±0.08 | –1.75±0.09 |
| LL, stress | –1.42±0.14 | –1.2±0.01 | –1.02±0.11 | –1.41±0.14 | –1.55±0.14 | –1.71±0.01 |
Water and osmotic potential were determined in upper (UL) and lower leaves (LL) under control conditions (C) and after 10 d of drought (stress) in CKX1 transgenic tobacco plants. WT, wild-type.
The osmotic potential of the lower leaves was comparable in all genotypes under control conditions (Supplementary Table S2 at JXB online). In the upper leaves, both CKX1 lines had a more negative potential than the WT. The response to drought in all genotypes was correlated with an increase in osmolarity. W6:CKX1 plants showed a more negative osmotic potential than the WT, but did not reach the value observed in the 35S:CKX1 plants. Thus, drought stress-induced changes in water potential showed that 35S:CKX1 plants represent the most tolerant genotype, followed by W6:CKX1 plants. Osmolyte accumulation correlated positively with drought resistance, being one of the important defence mechanisms (Wahid et al., 2007).
Chlorophyll and xanthophyll cycle pigments
The activation of defence pathways in order to cope with stress is a highly energy-demanding process. As the main source of energy for plants is photosynthesis, preservation of the photosynthetic machinery and its protection contribute significantly to the plant’s ability to withstand the stress. The relative levels of chlorophyll, the main light-harvesting pigment, were measured in a non-invasive way by a SPAD meter in individual leaves during drought stress progression. Drought resulted in elevation of the chlorophyll content in the upper leaves of both WT and W6:CKX1 plants (Supplementary Fig. S4 at JXB online). In the lower leaves, stress accelerated senescence in WT plants but it had only a minor effect in W6:CKX1 plants. In contrast, drought stress did not impose any stimulatory effect on the upper leaves of 35S:CKX1 plants and its effect on lower leaves was not significant (after 6 d).
The content of the light-harvesting carotenoid neoxanthin increased early during drought stress in both WT and W6:CKX1 plants, but it fell quickly upon rehydration (data not shown). 35S:CKX1 plants exhibited lower neoxanthin contents than WT and W6:CKX1 plants under control conditions. Drought stress-induced elevation of this pigment was also lower, but proportionally similar to both the other genotypes. In addition, the increased level of this pigment was maintained at least 24h after re-watering in 35S:CKX1 plants (data not shown).
Under control conditions, the pigments of the xanthophyll cycle, which dissipate excess light energy in the form of heat to avoid damage to the photosynthetic apparatus, were most abundant in 35S:CKX1 plants (Supplementary Table S2 at JXB online). The ratio of de-epoxidated xanthophyll cycle pigments to their total sum (DEPS) increased during drought stress in the leaves of all genotypes, increasing most rapidly in WT and W6:CKX1 plants.
Taken together, the results indicate that formation of a chlorophyll gradient with the highest concentrations in the younger, upper leaves occurs in both WT and W6:CKX1 plants. This drought stress-induced polarization preferentially protects the upper, younger, and more efficient leaves. Such polarization was not observed in the more stress-tolerant 35S:CKX1 plants. These plants had a higher basal level of protective xanthophyll cycle pigments, which might contribute to their higher stress tolerance.
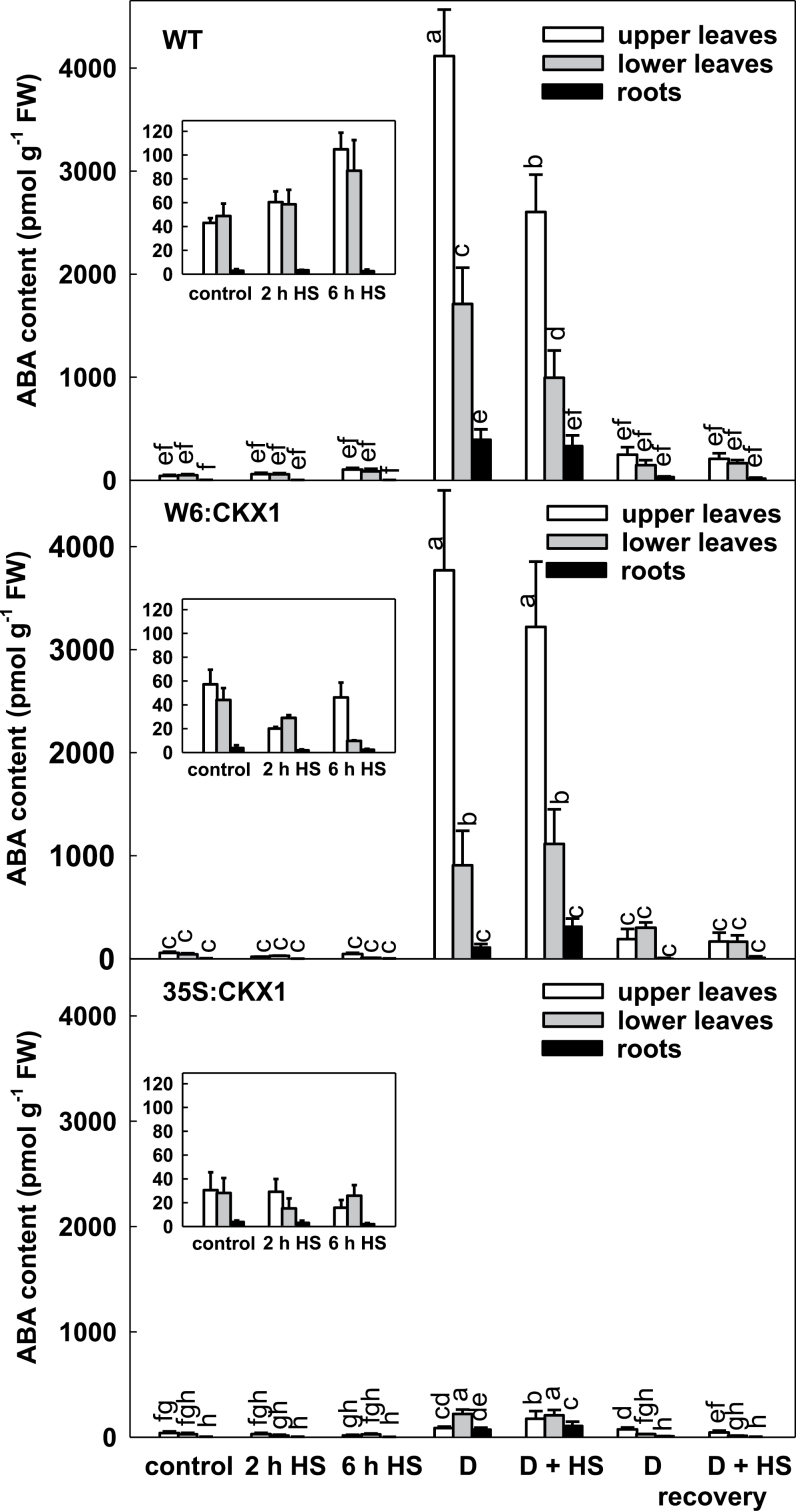
The content of abscisic acid
Determination of the levels of ABA and its metabolites, a key hormone in the response to abiotic stresses, was performed. The ABA content in all genotypes was higher in the leaves than in the roots (Fig. 5). Under control conditions, ABA levels were similar in WT and W6:CKX1 plants and lower in 35S:CKX1 plants. Drought caused a very strong increase in ABA content in the upper leaves of WT and W6:CKX1 plants of two orders of magnitude and a lower ~7-fold increase in 35S:CKX1 plants. A 24h rehydration coincided with a substantial ABA decrease in both leaves and roots, although its level still remained well above the basal level. After 2h HS, the ABA content increased in WT leaves but dropped below the basal level in both CKX1 transgenic lines. ABA in W6:CKX1 plants started increasing after 2h HS, while 35S:CKX1 plants maintained their ABA content below the control level during the whole period tested.
Fig. 5.
Abscisic acid content in leaves and roots of wild-type (WT) and CKX1 transgenic tobacco plants. Details for designation of individual variants are as described in Fig. 1. Different letters indicate statistically different values.
Determination of the content of ABA catabolites—phaseic acid (PA), dihydrophaseic acid, neophaseic acid, and its storage form (ABA glucosylester; ABA-GE)—allowed an insight into the dynamics of ABA metabolism during the stress responses. The most abundant ABA catabolite was PA (Supplementary Fig. S5 at JXB online). Drought caused PA elevation in all genotypes. Furthermore, significant PA up-regulation was observed after the application of HS at the end of drought stress. Recovery was associated with high PA levels in all genotypes. Surprisingly, PA was strongly elevated as early as after 2h HS in WT and W6:CKX1 plants, but only slightly in 35S:CKX1 plants.
Under control conditions ABA-GE levels were highest in 35S:CKX1 plants, which exhibited the lowest content of free ABA. Drought was associated with a remarkable ABA-GE increase, especially in WT and W6:CKX1 plants, but smaller in 35S:CKX1. ABA-GE accumulation in all genotypes was high upon rehydration, its stimulation being most remarkable in 35S:CKX1. Thus, a lower content of bioactive CKs in 35S:CKX1 plants coincided with a lower ABA content. Drought stress was associated with a very significant ABA increase in all genotypes. Elevation of PA content after 2h HS indicated a transient down-regulation of the ABA content at an early phase in the HS response.
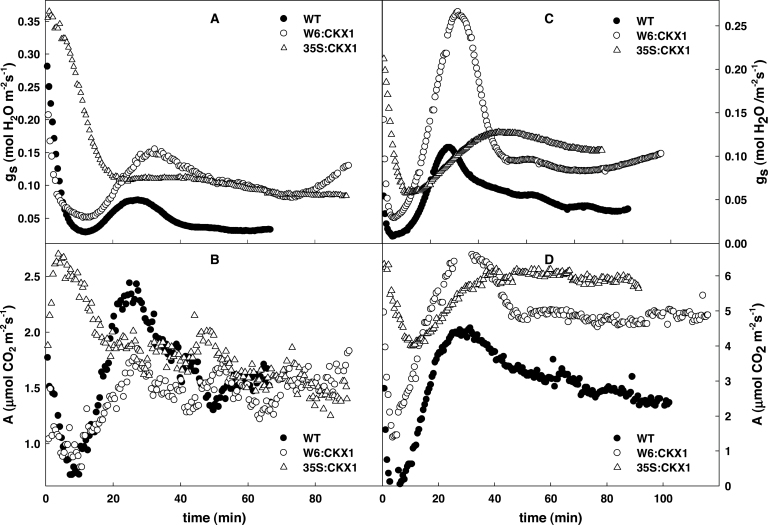
Stomatal conductance, photosynthesis rate, and leaf temperature during the heat stress response
The accumulation of ABA catabolites observed after 2h HS indicated that the early phase of the HS response was associated with a substantial deactivation of ABA, the key regulator of stomatal conductance (g s). Therefore changes in stomatal conductance were measured during heat stress. Stomatal conductance was followed at 130 μmol m–2 s–1 at 30 s intervals upon exposure of plants to high temperature (Fig. 6A). After an initial drop in conductance caused by the technical parameters of the set-up, both WT and W6:CKX1 plants exhibited a transient elevation of g s, which indicated enhanced transpiration. 35S:CKX1 plants exhibited a much milder and delayed response. When g s was followed under high light conditions (300 μmol m–2 s–1), the plant response was enhanced, particularly in the case of W6:CKX1 (Fig. 6C). The photosynthetic rate under both light intensities followed a similar profile to g s (Fig. 6B, D).
Fig. 6.
Gas exchange parameters in wild-type (WT) and CKX1 transgenic tobacco plants during drought stress. Stomatal conductance for water vapour, g s, and net photosynthetic rate, A, were measured in fully expanded leaves of tobacco WT, W6:CKX1, and 35S:CKX1 plants in a closed cabinet at 40 °C. (A and B) PAR 130 μmol m–2 s–1, (C and D) PAR 300 μmol m–2 s–1. Measurements were recorded every 30 s over a 1.5h period for three plants of each genotype.
Thermocamera data showed that 35S:CKX1 plants were able to maintain a lower leaf temperature than either of the other genotypes at the beginning of HS (Supplementary Fig. S6 at JXB online). Later on, the leaf temperature was consistently maintained at least 3 °C lower in 35S:CKX1 plants.
Discussion
Drought stress
Drought stress is among the most frequent challenges to which plants are exposed, often in combination with HS. It is generally believed and supported by correlative evidence that a larger root system might be advantageous to cope with drought stress conditions (Tuberosa et al., 2002). This view has been underpinned recently by a comparison of near-isogenic lines that differed essentially in the size of their root system. Genetically engineered transgenic tobacco plantlets with a larger root system due to a lowered CK content survived severe drought stress conditions better than the corresponding WT (Werner et al., 2010). Here, the physiological parameters which are affected by drought stress in plants over-expressing CKX1 under two different promoters have been investigated in more detail. The results indicate that the strength of promoter activity as well as its organ and time specificity significantly affects the physiological performance of plants. Therefore, the two transgenic lines analysed in this study are discussed separately.
Multiple parameters are altered in drought-stressed 35S:CKX1 plants
The 35S:CKX1 plants under control conditions exhibited a higher content of the xanthophyll cycle pigments (Supplementary Table S3 at JXB online), important for the protection of the photosynthetic apparatus under stress conditions (Haisel et al., 2008), as well as a higher basal expression level of the P5CSA gene, coding for an enzyme involved in the production of proline, the main osmolyte in tobacco. In response to drought stress, 35S:CKX1 plants showed a more negative osmotic potential than the WT (Table 1). The absence of leaf wilting following drought stress (Supplementary Fig. S1) and the analysis of water potential (Table 1) showed unequivocally that 35S:CKX1 plants are highly drought resistant. Furthermore, 35S:CKX1 plants were characterized by a lower ABA content, which was only moderately increased during drought stress, contrasting with the strong drought-induced increase of the ABA level in the WT (Fig. 5). The lower expression of the drought stress marker gene ERD10B (Fig. 3) indicated that the leaves of 35S:CKX1 plants suffered less from water deficit in comparison with the other genotypes tested (Table 1). However, although the transcript level of this gene was lower in 35S:CKX1 plants, its relative drought stimulation was comparable with that of the WT (i.e. 8-fold compared with 10-fold induction), indicating that the stress response was efficiently activated in the transgenic lines. A fast and efficient activation of defence by 35S:CKX1 transgenic lines is also indicated by the fast increase in the content of the xanthophyll cycle pigments (Supplementary Table S3).
The reduced CK content in 35S:CKX1 plants could influence the parameters listed above in a direct fashion; alternatively, they could be indirectly affected by the obvious morphological changes apparent in 35S:CKX1 plants. One important factor could be their increased root to shoot ratio, which will be discussed further below for W6:CKX1 plants. Another possibly relevant feature is formation of smaller and thicker leaves composed of larger cells (Werner et al., 2001). As plants lose water mainly through their guard cells, one possible explanation for the better preservation of water potential might be faster stomatal closure or a decreased stomatal density, as has been demonstrated in strawberry plants (Orsini et al., 2012). Indeed, an increased distance between individual stomata was found in 35S:CKX1 leaves (Werner et al., 2001). Furthermore, 35S:CKX1 plants grow more slowly compared with the WT, which is associated with a lower energy demand (Nishiyama et al., 2011). These plants can, therefore, probably allocate more resources to stress defence.
An enhanced root system and stress-induced modulation of CK degradation are major causes of improved drought resistance of W6:CKX1 plants
W6:CKX1 plants have been shown to have an enlarged root system but to lack the detrimental morphological effects of CK deficiency on shoot growth (Werner et al., 2010). In fact, several of the parameters analysed in W6:CKX1 plants, including changes in ABA (Fig. 5) and pigment content (Supplementary Table S3 at JXB online), resembled those in WT plants. However, W6:CKX1 plants retained a higher water potential in the lower leaves and showed reduced wilting compared with the WT (Supplementary Fig. S1). W6:CKX1 plants also showed a more negative osmotic potential than the WT (Table 1).
It is concluded that one of the parameters contributing to the enhanced drought resistance of W6:CKX1 plants is their larger root system and their higher root to shoot ratio. Apart from better access to deeper soil levels with an additional water supply (especially in field conditions), an enlarged root system may also improve accumulation of ions, including Mg2+, which seems to play a positive role in chlorophyll retention under drought stress (Werner et al., 2010). W6:CKX1 consistently showed a weaker decrease in the chlorophyll content in lower leaves in comparison with the WT.
In addition, the expression analysis revealed that the activity of the WRKY6 promoter under drought or HS was quickly down-regulated (Fig. 1), resulting in a decreased CKX activity in roots (Fig. 2). This coincided with a lower reduction of bioactive CK content in the leaves compared with the WT (Fig. 3). The higher CK content in the leaves of W6:CKX1 plants might contribute, in part, to alleviate the consequences of drought stress, operating in a way that might resemble a stress-dependent increase in CK synthesis driven by stress- or senescence-inducible promoters. An example of this are transgenic SARK:IPT tobacco or rice plants which showed enhanced drought stress resistance (Rivero et al., 2007; Peleg et al., 2011). One of the reasons reported was a diminished suppression of photosynthetic activity (Rivero et al., 2009), which is in accordance with the present finding of a higher chlorophyll content in the leaves of stressed W6:CKX1 plants.
Heat shock: acute stress associated with fast, transient stimulation of stomatal conductance
Detailed measurement of stomatal conductance at the very beginning of HS revealed that both W6:CKX1 and WT plants responded with a transient elevation of stomatal conductance (Fig. 6). This is a necessary prerequisite for an increase in transpiration, which is the main cooling mechanism of leaves in response to a sudden elevation of temperature (Wahid et al., 2007). Enhanced transpiration, however, resulted in water deficit, as reflected by mild elevation of the expression of ERD10B (Fig. 3), which stimulates an elevation in ABA levels and, thus, stomatal closure. The transient character of the elevation of stomatal aperture observed (Fig. 6A, C) agrees with the results of Vysotskaya et al. (2010), who found down-regulation of transpiration in tobacco during recovery after HS.
The stronger stomatal response of W6:CKX1 plants (in comparison with the WT) may be explained by the delivery of a higher amount of CKs by the transpiration stream, as a consequence of diminished CK degradation in the root system after HS. This assumption is in accordance with a positive correlation between the amount of CKs transported via xylem and the transpiration rate (Aloni et al., 2005; Boonman et al., 2007). The promoting effect on stomatal opening in W6:CKX1 plants was even stronger in high light intensity (Fig. 6C), which corresponds well to the difference in transpiration reported between shaded and light-exposed leaves (Boonman et al., 2007).
Drought and HS: much more than an additive effect of combined stresses
The decrease in ABA levels in WT drought-stressed leaves following transfer of plants to 40 °C indicates that a transient stimulation of transpiration occurred even under conditions of limited water supply. As a consequence, the leaf water potential of the preferentially protected upper leaves decreased to the level of the lower leaves, paralleling the changes in the levels of bioactive CKs. This additional water loss was probably the main reason for the greater effect of the combined stress in comparison with either of the individual stress treatments. The drop in bioactive CKs was significant in the more drought-tolerant W6:CKX1 and 35S:CKX1 plants, but the CK gradient between the upper and lower leaves was maintained.
Conclusions
The results showed that constitutive overexpression of CKX1, which is associated with an enlarged root system and dwarf, stunted shoots, confers enhanced tolerance to both drought and heat stresses. Furthermore, targeting of CKX1 overexpression to the roots using the WRKY6 promoter, which avoided the negative impacts on shoot growth, also resulted in enhanced drought and heat tolerance. Together, the results demonstrate the importance of tissue- and time-dependent gene activation in responses to drought and heat stresses. Suitable promoter–gene combinations offer significant potential for targeted manipulation of the plant hormone pool and, thereby, modulation of stress responses.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Photographs of the tested genotypes under control and drought conditions.
Figure S2. Cytokinin metabolites.
Figure S3. Expression profile of Δ 1 -pyrroline-5-carboxylate synthetase A.
Figure S4. Chlorophyll levels in the individual leaves.
Figure S5. ABA metabolites.
Figure S6. Infrared photography of heat-stressed plants.
Table S1. Primers used for qRT–PCR.
Table S2. Xanthophyll cycle pigments and DEPS.
Acknowledgements
This work was supported by the Czech Science Foundation, project no. 206/09/2062.
References
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. 2005. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany 56, 1535–1544. [DOI] [PubMed] [Google Scholar]
- Boonman A, Prinsen E, Gilmer F, Schurr U, Peeters AJM, Voesenek LACJ, Pons TL. 2007. Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiology 143, 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. 1995. Measuring the water status of plants and soils. New York: Academic Press. [Google Scholar]
- Dobra J, Vankova R, Havlova M, Burman AJ, Libus J, Storchova H. 2011. Tobacco leaves and roots differ in the expression of proline metabolism-related genes in the course of drought stress and subsequent recovery. Journal of Plant Physiology 168, 1588–1597. [DOI] [PubMed] [Google Scholar]
- Gajdosova S, Spichal L, Kaminek M, et al. 2011. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. Journal of Experimental Botany 62, 2827–2840. [DOI] [PubMed] [Google Scholar]
- Haisel D, Vankova R, Synkova H, Pospisilova J. 2008. The impact of trans-zeatin O-glucosyltransferase gene over-expression in tobacco on pigment content and gas exchange. Biologia Plantarum 52, 49–58. [Google Scholar]
- Havlova M, Dobrev PI, Motyka V, Storchova H, Libus J, Dobra J, Malbeck J, Gaudinova A, Vankova R. 2008. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant, Cell and Environment 31, 341–353. [DOI] [PubMed] [Google Scholar]
- Heyl A, Ramireddy E, Brenner WG, Riefler M, Allemeersch J, Schmülling T. 2008. The transcriptional silencer ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis. Plant Physiology 147, 1380–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences, USA 103, 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyka V, Vankova R, Capkova V, Petrasek J, Kaminek M, Schmülling T. 2003. Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion. Physiologia Plantarum 117, 11–21. [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, et al. 2011. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. The Plant Cell 23, 2169–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak O, Hauserova E, Amakorova P, Dolezal K, Strnad M. 2008. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry 69, 2214–2224. [DOI] [PubMed] [Google Scholar]
- Novak O, Tarkowski P, Tarkowska D, Dolezal K, Lenobel R, Strnad M. 2003. Quantitative analysis of cytokinins in plants by liquid chromatography/single-quadrupole mass spectrometry. Analytica Chimica Acta 480, 207–218. [Google Scholar]
- Orsini F, Alnayef M, Bona S, Maggio A, Gianquinto G. 2012. Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environmental and Experimental Botany 81, 1–10. [Google Scholar]
- Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E. 2011. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnology Journal 9, 747– –758. [DOI] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T. 2006. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell 18, 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. 2007. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proceedings of the National Academy of Sciences, USA 104, 19631–19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Shulaev V, Blumwald E. 2009. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiology 150, 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnik-Inbar D, Bar-Zvi D. 2010. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. The Plant Cell 22, 3560–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuberosa R, Sanguineti MC, Landi P, Giuliani M, Salvi S, Conti S. 2002. Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Molecular Biology 48, 697–712. [DOI] [PubMed] [Google Scholar]
- Tureckova V, Novak O, Strnad M. 2009. Profiling ABA metabolites in Nicotiana tabacum L. leaves by ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Talanta 80, 390–399. [DOI] [PubMed] [Google Scholar]
- Vysotskaya LB, Veselov SYu, Kudoyarova GR. 2010. Effect on shoot water relations, and cytokinin and abscisic acid levels of inducing expression of a gene coding for isopentenyltransferase in roots of transgenic tobacco plants. Journal of Exerimental Botany 61, 3709–3717. [DOI] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. 2007. Heat tolerance in plants: an overview. Enviromental and Experimental Botany 61, 199–223. [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15, 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. 2001. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA 98, 10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmülling T. 2010. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. The Plant Cell 22, 3905–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.