Abstract
Animal models of hemophilia and related diseases are important for development of novel treatments and to understand the pathophysiology of bleeding disorders in humans. Testing in animals with the equivalent human disorder provides informed estimates of doses and measures of efficacy, which aids in design of human trials. Many models of hemophilia A, hemophilia B, and von Willebrand disease have been developed from animals with spontaneous mutations (hemophilia A dogs, rats, sheep; hemophilia B dogs; and von Willebrand disease pigs and dogs), or by targeted gene disruption in mice to create hemophilia A, B, or VWD models. Animal models have been used to generate new insights into the pathophysiology of each bleeding disorder and also to perform pre-clinical assessments of standard protein replacement therapies as well as novel gene transfer technology. Both the differences between species and differences in underlying causative mutations must be considered in choosing the best animal for a specific scientific study
Importance of Animal Models for Study of Human Bleeding Disorders
Animal models are important for FDA-required pre-clinical assessment of safety prior to testing of novel therapeutics in humans. If testing can be done in animals with the equivalent of the human disease under study, better informed estimates of dose finding can be obtained (pharmacokinetics), and measures of efficacy can be tested (pharmacodynamics), which aid in pre-clinical assessment of the drug and design of human trials. Apart from drug development, animal models of human disease are also important for understanding the pathophysiology of those diseases.
There are many potential disadvantages and limitations to animal models of human diseases, including immune reactions to human proteins, different metabolism or clearance of human proteins by different species, and different tissue tropism of viral vectors in animals compared to humans. Despite these limitations, animal models of hemophilia and related bleeding disorders provide insights into the pathophysiology of human disease.
Several animal models identified after spontaneous occurrences or engineered by targeted gene disruption recapitulate human hemophilia and other bleeding diseases. Models arising from spontaneous mutations usually have clear hemorrhagic phenotypes, since they are only identified by overtly abnormal bleeding. “Knock-out” animals (usually mice) often do not have spontaneous bleeding despite equally low levels of coagulant proteins. For hemophilia and related bleeding disorders, animal models (spontaneous or engineered) have not only informed our understanding of the natural history and pathophysiology of the disease but also guided development of therapeutics in humans. Each model can provide complementary information on the pathophysiology of bleeding disorders and novel therapeutics for their treatment--especially important for development of gene therapy, in which unique toxicities such as insertional mutagenesis, germ line gene transfer, and viral toxicities, must be studied.
Hemophilia A Animals
Hemophilia A Dogs
The first hemophilia animal model arose when a dog breeder noticing prolonged bleeding after clipping of the nails of a particular Irish setter dog (Figure 1). This was brought to the attention of pathologist Kenneth M. Brinkhous at the University of North Carolina at Chapel Hill who showed complete deficiency of coagulation factor VIII.1, 2 From that animal he established a colony of hemophilia A dogs that has been studied since 1947. The bleeding phenotype replicates that of severe hemophilia A in humans, with spontaneous soft tissue hemorrhage, hemarthroses, and occasional mucosal bleeding. Thrombin generation in vitro is defective. From the observation of prolonged bleeding with nail clipping came a standardized cuticle bleeding time method that predicts hemostasis.3 Bleeding can be treated or prevented by administration of canine plasma, canine cryoprecipitate, or recombinant canine factor VIII,4 as is true for humans with hemophilia A. The basis of the factor VIII deficiency is a gene inversion in which recombination occurs between an actively transcribed gene that is inside intron 22 of the factor VIII gene on the X chromosome and a copy ~0.5 Mb upstream of the factor VIII gene.5 This results in the first 22 exons of the factor VIII gene being transcribed normally, but exons 23-26 are replaced with a non-factor VIII sequence.
Figure 1.
Irish Setter hemophilia A index case, University of North Carolina at Chapel Hill (photo by William Brinkhous).
At Queens University in Toronto, Canada, a separate hemophilia A dog colony was established in 1980 from an affected miniature schnauzer (Figure 2).6 The bleeding phenotype was severe, as in the Chapel Hill colony. As in the Chapel Hill colony, there is no circulating factor VIII, and interestingly, the Canadian colony has the same aberrant factor VIII transcript is seen in the Chapel Hill dogs.7 An identical factor VIII transcript arising in different breeds, different places and different decades suggests a common mutation mechanism. This involves repeated DNA elements like those that undergo spontaneous homologous recombination in humans.8-10 Dogs from the Queens University hemophilia A colony notably have a greater rate of forming inhibitor antibodies (“inhibitors”) to dog factor VIII when transfused to treat or prevent bleeding.11 Notably, the hemophilia A dogs in Canada have two amino acid polymorphisms at position 909 (Gly/Ser) and 1184 (Leu/Pro) that differ from factor VIII from normal dogs used for replacement therapy.12 This finding raises the possibility that factor VIII peptides differing at these sites may be recognized as foreign, and thereby provoke antibody formation. A similar explanation has been proposed for the increased risk of inhibitor development in humans of African ancestry with hemophilia A, who have a greater prevalence of rare factor VIII polymorphisms.13 The Chapel Hill and Queens University hemophilia A dog colonies, with a common gene inversion mutation could be used to study the contribution to inhibitor risk of minor mismatches in protein coding sequence.
Figure 2.
Queen's University Hemophilia A dogs, early in existence of the colony. Pictures courtesy of Dr. David Lillicrap, Queen's University, Kingston, Ontario, CA.
Other spontaneous mutations in the factor VIII gene have been observed in hemophilia A dogs.14 For the most part, these are pets that are not used in biomedical research. However their existence makes it clear that various mutations in the factor VIII gene do occur spontaneously. One mutation causes a premature stop codon in exon 12 that is analogous to a human mutation associated with a severe hemophilia (unpublished data, J Lozier and TC Nichols).
The hemophilia A dogs in Chapel Hill and Queens University have been used for pre-clinical trials of human factor VIII concentrates,15-18 novel hemophilia A therapeutics,19-21 and recombinant activated factor VII, a direct activator of factor X that circumvents factor VIII inhibitors.22-24 More recently, gene therapy for hemophilia A is progressing to clinical applications in man, and hemophilia A dogs have been used to test various adenovirus and adeno-associated virus (AAV) vectors both for safety and efficacy.25-33
Hemophilia A Sheep
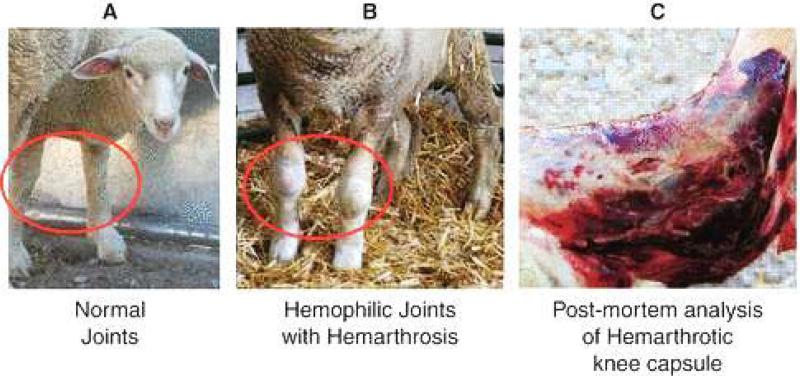
In 1979, Swiss researchers documented occurrence of severe hemophilia A in male offspring of an alpine white ewe, manifesting initially as umbilical cord bleeding after birth. Laboratory testing indicated < 1% factor VIII activity, consistent with severe hemophilia A.34,35 It was also demonstrated that the factor VIII gene in sheep maps to the X chromosome, like humans and dogs.36 Further breeding of the same ewe gave rise to additional affected progeny, and though the line was not continuously maintained, semen from affected males was cryopreserved. In 2009, Porada et al reported re-establishment of the hemophilia A sheep by multiple ovulation embryo transfer, and in vitro fertilization using intracytoplasmic sperm injection.37 They also were able to amplify the factor VIII mRNA from the spleen of an affected animal, and identified a premature stop codon in exon 14 of the factor VIII gene.37 Soft tissue hemorrhage, hemarthroses, and hematuria occurred spontaneously, and were responsive to treatment with human factor VIII (Figure 3). Like dogs with hemophilia A, there was prolonged bleeding with cuticle clipping. The hemarthropathy of the hemophilia A sheep is more pronounced than in the hemophilia A dogs, but replacement therapy is more aggressive in the dogs. Although the sheep can be kept from bleeding to death with human factor VIII, they are prone to make anti-human factor VIII antibodies, due to the differences between each species’ factor VIII. If sheep factor VIII were more readily available for prophylaxis and treatment of bleeding, this species would be a more practical model.
Figure 3.
Sheep with hemophilia A, depicting swollen and hemorrhagic knees from acute and chronic hemarthoses. Figure modified from Porada, CD, et al.37
Recently, correction of the bleeding phenotype in hemophilia A sheep by implantation of genetically modified mesenchymal stromal cells (MSCs) obtained from bone marrow was reported. Two sheep with hemophilia A treated with human factor VIII developed antibodies to the human protein, and became refractory to treatment. MSCs derived from a parent of the sheep were transduced with a lentivirus vector containing the porcine factor VIII gene, and transduced MSCs were repeatedly implanted into the peritoneum of each hemophilia A sheep. The bleeding, especially the hemarthroses, diminished dramatically in each animal.38 Eventually inhibitor titers rose dramatically in each animal, due to antibodies that cross-reacted with porcine and human factor VIII. On necropsy, lentiviral transduced MSCs were distributed widely, including the synovium of affected joints. Thus, local delivery of factor VIII to tissues in amounts that are not detectable in the systemic circulation may ameliorate bleeding.
Hemophilia A Mice
Mice are less expensive to house and maintain in large numbers, so researchers sought examples of mice with hemophilia A but were unsuccessful, even though hemophilia A dogs and sheep were readily identified. In theory, complete deficiency of factor VIII could be an embryonic lethal event. Conversely, the bleeding phenotype of factor VIII deficiency in animals such as mice may be mild and therefore undetectable. Eventually, through embryonic stem cell manipulation, two factor VIII deficient knockout mouse strains were engineered by Bi et al in 1995.39 In one strain exon 16 of the mouse factor VIII gene was interrupted by cre-lox mediated recombination strategies, and in another, exon 17 was disrupted. The mice had no detectable circulating factor VIII, but somewhat surprisingly, little or no apparent spontaneous bleeding, in contrast to hemophilia A dogs and sheep.40 Incised wounds and tail clipping reliably resulted in fatal bleeding, but blood collection from the retro-orbital venous plexus with glass capillaries was surprisingly uneventful (unpublished observations, JNL). This has practical importance for investigators, since collection of blood by retro-orbital puncture is quick and requires no precautions to prevent exsanguination, whereas tail transection to collect blood requires cauterization to prevent exsanguination. In hemophilic mice, methods to assess hemostasis by transection of the tail at a site of defined size/caliber, and quantifying blood loss can assess the amount of factor VIII in circulation.41 The factor VIII knockout mice have no circulating factor VIII protein and therefore are a fairly “clean” system in which to measure factor VIII after administration of the protein or factor VIII gene transfer. Mice typically make antibodies to human factor VIII, whether exposed to factor VIII protein or subjected to factor VIII gene transfer in vivo. In normal mice the predilection towards making antibodies is strain-dependent, and likely related to MHC genes. Hemophilia A mice can be bred into immunodeficient strains of mice to create hemophilic mice that do not make antibodies to human factor VIII protein (e.g., NOD-SCID hemophilia A mice).42 In such animals, experiments in factor VIII gene transfer can be undertaken with no risk of developing neutralizing antibodies to human factor VIII, for instance.
Although the mouse has the equivalent of the F8A gene seen in humans and dogs, there is only one copy of that gene, residing outside the nearby mouse factor VIII gene on the X chromosome.43 This molecular biology explains why there can be no homologous recombination to mediate a factor VIII gene inversion. Although in mice spontaneous factor VIII gene mutations could occur by other mechanisms, the factor VIII knockout mice suggest spontaneous bleeding probably would not occur. The body mass of a mouse is negligible compared to that of larger mammals, and the weight is carried much closer to the ground. Finally, mice carry body weight on four joints (rather than two). The spontaneous hemarthroses routinely seen in humans, dogs, and sheep are most common in the weight-bearing surfaces of the knee, ankle, or hip that bear much more weight than for the mouse.
The hemophilia A knockout mice remain a critical “entry level” animal model for testing of therapeutics of all kinds (protein replacement, gene therapy, etc.) since small amounts of precious research reagents can serve to test many small animals prior to scale up in larger animals. One key limitation of mice is that functional assays of factor VIII coagulant activity require large volumes of blood, a significant practical problem.
Hemophilia A Rats
A group at Yale identified inbred animals from the WAG/RijY rat strain in which there is bruising, soft-tissue hemorrhage, and hemarthroses of the tarsal joints typical of human hemophilia A (Figure 4).44 The affected animals, designated WAG/RijYcb, came to attention after a subcutaneous injection in a male rat caused a hematoma. Some male and female littermates of the index case were likewise prone to bleed and all had prolonged activated partial thromboplastin times. A missense mutation of the factor VIII gene was found in which proline is substituted for leucine at amino acid 176 of the factor VIII protein.45 In contrast to humans, dogs, sheep, and mice, the factor VIII gene in rats is located on chromosome 18, rather than the X chromosome, which explains the autosomal recessive pattern of inheritance. The presence of an autosomal hemophilia animal model permits future study of carriers with a mild/minimal bleeding phenotype, as well as the severely affected homozygotes.
Figure 4.
Hemophilia A Rat WAG/RijYcb strain, with tarsal joint bleed. From Booth CJ, Brooks MB, Rockwell S.44
Hemophilia B Animals
Hemophilia B Dogs
Severe bleeding with trauma or surgery, and arthritic signs/symptoms of recurrent hemarthroses were noted in Cairn terriers in Toronto, Canada that were shown to be deficient in coagulation factor IX (Figure 5).46 The inheritance pattern was sex-linked, like human hemophilia B. Complementation studies with serum from humans deficient in factor VIII, IX, XI, or XII confirmed factor IX deficiency. The gene defect was found to be a point mutation in factor IX that results in substitution of glutamic acid for glycine at amino acid 379 in the catalytic domain.47 The mutation is remarkable in that there is no detectable circulating factor IX protein in the plasma, and apparently the protein is so unstable that it is not secreted into the circulation.
Figure 5.
Hemophilia B dog, a Keagle (mix of Cairn terrier and beagle) from University of North Carolina at Chapel Hill.
A different hemophilia B dog colony was established at Auburn in Alabama after severe bleeding was observed in Lhasa Apso dogs that were deficient in factor IX. The factor IX gene sequence showed a 5 bp deletion at nucleotides 772-776 and a C to T transition at nucleotide 777 resulting in a frame-shift and a premature stop codon at amino acid 146 in the factor IX protein.48 The mutation eliminates the catalytic domain of the factor IX protein, and there is almost no detectable mRNA or translated protein. In contrast to the Chapel Hill hemophilia B dogs, the Auburn dogs are prone to develop factor IX inhibitors when exposed to canine factor IX.49 It is not clear if this is due to the different underlying mutations, or whether the particular dogs into which these mutations have been bred have different histocompatibility locus genes, for instance. Inhibitor antibodies to canine factor IX have also been demonstrated in a Labrador retriever dog with hemophilia B in which there is a complete deletion of the canine factor IX gene.50
Hemophilia B Mice
There have been no mice identified with hemophilia B from spontaneous factor IX mutations, perhaps for the same reason that no mice were identified with hemophilia A from spontaneous factor VIII mutations. Therefore a series of factor IX deficient mice were engineered by targeted disruption of the mouse factor IX gene.51-53 These mice have a bleeding phenotype like that of the hemophilia A knockout mice: little spontaneous bleeding and prolonged (even fatal) bleeding after tail clipping or most other invasive procedures. Also, like the hemophilia A mice, blood collection by retro-orbital puncture does not cause serious bleeding (JNL, unpublished observations).
After generation of factor IX knock-out mice, it became possible to engineer “knock-in” mice that express human factor IX.54 This permitted in vivo testing of structure/function relationships using human factor IX constructs. A knock-in mouse has been described in which glutamine replaces arginine at amino acid 333 in the catalytic domain of human factor IX (R333Q-hFIX), a point mutation seen in some humans with hemophilia B.54 Another knock-in mouse has been created in which lysine at amino acid 5 is mutated to alanine, which impairs binding of the factor IX protein to type IV collagen.55 Interestingly, in vitro factor IX activity of this variant is normal, but in vivo hemostasis is impaired, though not as much as in the factor IX knockout mouse.55 These animals are useful in studies on the effect of factor IX mutations in residual circulating protein on immunogenicity of factor IX. For instance, human factor IX expression in knockout mice by adeno-associated virus vectors typically results in antibody formation, whereas in the R333Q-hFIX knock-in, there is no inhibitor antibody formation after human factor IX gene expression.56 Factor IX protein (even if non-functional), is protective against antibody formation. Most humans with hemophilia B have (defective) circulating factor IX protein and are not prone to make inhibitors when treated with factor IX concentrates.
Immunologic insights can also be derived from normal (non-hemophilic) miceA survey of various mouse strains indicated that A/J mice were high responders to human factor IX and C57BL/6J mice were non-responders.57 By measuring antibodies to human factor IX in recombinant inbred and congenic mice derived from A/J and C57BL/6J strains it was possible to show that the MHC complex on chromosome 17, IL10 and interferon-γ genes were linked to the immune response to human factor IX.57
Gene therapy in humans has advanced more rapidly for hemophilia B than for hemophilia A or VWD since the shorter factor IX coding sequence makes gene transfer vector construction easier, and factor IX gets into the circulation readily after expression in most tissues types. Hemophilia B dogs and mice have been essential for pre-clinical testing of factor IX gene transfer vectors that are being developed. Hemophilia B models have predicted the dose of vector required to achieve clinically significant factor IX levels, the immunogenicity of expressed factor IX, toxicity of vectors, and the effect of novel factor IX variants on gene expression in vivo. 58-65 Information derived in mice and dogs is usually comparable, but each animal provides complementary information, as described for hemophilia A models. The most important recent work in hemophilia B animal models has been in support of clinical trials of AAV-factor IX gene transfer into skeletal muscle66 or liver67, 68 for hemophilia B in humans.
Von Willebrand Disease Animals
VWF is a large plasma protein that exists as a globular protein under low shear conditions, but under high shear conditions it unfolds into a linear protein.69 It binds subendothelial collagen and platelets through the various platelet receptors and plasma proteins (e.g., GpIb/IX/V, GpVI, GpIV, thrombospondin, fibrinogen, etc.),70 thereby mediating platelet adhesion to injured endothelium. Under high-shear conditions, binding to GpIb is most critical.71 VWF can form multimers with enhanced ability to bind platelets to each other or subendothelial collagen. VWF is synthesized in megakaryocytes and in the endothelial cell, and is stored in the alpha granule of the platelet and Weibel-Palade body of the endothelial cell. Virtually all plasma VWF is derived from endothelial cells in humans, and platelet VWF is secreted only with platelet activation. A second critical role of VWF is to stabilize factor VIII. The absence of VWF leads to accelerated clearance of factor VIII and in mild (type 1) VWD where the VWF level is ~30-50% of normal, the factor VIII may be low enough to slightly prolong the activated partial thromboplastin time. However, in type 1 VWD, the bleeding manifestations are primarily those of impaired platelet function, namely mucosal bleeding.72 In severe (type 3) von Willebrand disease, VWF levels are less than 10%, factor VIII levels are also typically less than 10%, and there can be soft tissue hemorrhage and hemarthroses typical of hemophilia in addition to the mucosal bleeding from VWF deficiency.72
Many insights into VWF biochemistry and physiology are derived from the animal models of VWD in mice pigs, and dogs. Dogs do not have VWF in their platelets,73, 74 whereas pigs have both megakaryocyte75 and endothelial cell-derived VWF76 like humans, which is a critical issue for modeling human VWD. Inheritance of VWD is autosomal in humans, dogs, pigs and mice, in contrast to sex-linked hemophilias A and B. Pigs and dogs share 84-86% VWF amino acid sequence identity (with many additional highly conserved amino acids), and have conservation of the major domains for binding of platelets, factor VIII, collagen, and heparin.77,78 In pigs and dogs with VWD, human VWF mediates platelet adhesion in vitro and in vivo, likely through the conserved RGD integrin motif found in all three species that interacts with the platelet αIIb/βIII surface receptor. Human factor VIII is stabilized by porcine or canine VWF and human VWF stabilizes porcine or canine factor VIII. Thus, these two species are well-suited for study of VWD.
von Willebrand Disease Dogs
Von Willebrand disease is fairly common in dogs, and a model of VWD was developed from a Scottish Terrier show dog, first identified in 1978, and studied thereafter at the University of North Carolina at Chapel Hill (Figure 6).79 The causative mutation is a single nucleotide deletion in exon 4 of the VWF gene that causes premature termination of the protein; there is no detectable VWF mRNA or protein.73 The dogs can be bred either to produce severe (type 3) VWD animals with two defective alleles, or the milder (type 1) VWD animals in which one copy of the gene is defective. The former are especially useful for pre-clinical study of human VWF concentrates.80, 81 In the mild (type 1) VWD dogs, desmopressin can be used to discharge VWF from Weibel-Palade bodies in the endothelial cells. It has been shown in VWD dogs that IL-11 results in increased VWF message transcription and increased plasma levels of VWF.82 Weibel-Palade body stores of VWF releasable with desmopressin are also increased with IL-11. Unlike type 1 VWD where desmopressin therapy results in tachyphylaxis after a few doses, the responsiveness to desmopressin persists when given after/during administration of IL-11. As expected, in severe VWD where both gene copies are defective, there is no effect of desmopressin or IL-11.82 The studies in VWF dogs were the basis for successful phase II clinical trials in humans reported by Ragni et al.83-85
Figure 6.
Scottish terrier dog with von Willebrand disease, from University of North Carolina at Chapel Hill.
Von Willebrand Disease Pigs
Pigs with VWD have been studied in detail over the years (Figure 7), and have provided many insights into the pathophysiology of VWD in humans. Like dogs VWD in pigs is transmitted as an autosomal trait. In contrast to the VWD dogs the VWF gene mutation in the pig is not known. However, linkage to the VWF gene has been demonstrated, and there are no gross rearrangements of the VWF gene, suggesting a point mutation or small deletion may be responsible.86 The VWF message is present but at reduced levels, and low amounts of VWF antigen can be detected in VWD pigs’ platelets and endothelial cells.76
Figure 7.
von Willbrand disease pig from University of North Carolina at Chapel Hill.
Von Willebrand Disease Mice
The level of von Willebrand factor in mice varies widely (not unlike humans) and in 1990 the inbred mouse strain RIIIs/J was noted to have ~50% of the normal mouse VWF level and a prolonged bleeding time after tail clipping; accordingly, it was proposed to be a model of human type 1 VWD.87 By taking advantage of the marked variation of VWF levels between various mouse strains, it was possible to identify the locus Mvwf (for “modifier of VWF”) on chromosome 11 (distinct from the VWF gene on mouse chromosome 12) associated with the low VWF levels88, 89 As it turns out, Mvwf is a polymorphism in Galgt2, a glycosyltransferase gene.90 The mutated Galgt2 gene is expressed abnormally in endothelial cells, and altered glycosylation of VWF in the RIIIS/J strain causes accelerated turnover and decreased levels of VWF.
In addition to spontaneous VWD in certain mouse strains of due to modifier genes, a knockout mouse with no detectable VWF in the circulation was generated by targeted VWF gene disruption.91 Spontaneous bleeding is not typical for the knockout mouse, but there is prolonged bleeding with tail clipping. Like dogs and pigs with VWD, mice with two defective alleles have severe (type 3) VWD and mice with one defective allele have mild (type 1) VWD. As typical of all VWD animal models, factor VIII levels are decreased to 15-20% in the severe homozygous mice with type 3 VWD, and factor VIII levels of 50-60% are seen in heterozygotes.91 Since VWD mice can be bred into other strains with well-defined genetic make-up, and mice are less expensive to maintain than large animals, the VWD mice are being used in more laboratories than the large animals with VWD.
Von Willebrand disease animal models have been used to show that the Weibel-Palade body of the endothelial cell is comprised largely of condensed multimers of VWF; the Weibel-Palade body is missing in type 3 VWD pigs, dogs, or knockout mouse endothelial cells and expression of canine VWF in VWD dog endothelial cells restored them.73, 92, 93
Summary
Animal models of hemophilia and related bleeding disorders have provided us with fascinating insights into pathophysiology of bleeding, common mechanisms for gene mutation, normal and abnormal biochemistry/metabolism of coagulation factors, and strategies for treatment of human disease. Their use will continue to be critical for ongoing development of novel therapies, especially gene therapy.
Acknowledgments
Supported in part by a Career Development Award from the National Hemophilia Foundation and the NIH Intramural Research Program (JNL) and R24 HL63098 (TCN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jay N. Lozier, Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, MD 20892.
Timothy C. Nichols, Departments of Medicine and Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, Department of Laboratory Medicine of the National Institutes of Health Clinical Center, Bethesda, MD 20892.
References
- 1.Graham JB, Buckwalter JA, Hartley LJ, Brinkhous KM. Canine hemophilia: Observations on the course, the clotting anomaly, and the effects of blood transfusion. J Exp Med. 1949;90:97–111. doi: 10.1084/jem.90.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkhous KM, Graham JB. Hemophilia in the female dog. Science. 1950;111:723–4. doi: 10.1126/science.111.2896.723. [DOI] [PubMed] [Google Scholar]
- 3.Pijnappels MI, Briet E, Van Der Zwet GT, Huisden R, Van Tilburg NH, Eulderink F. Evaluation of the cuticle bleeding time in canine haemophilia A. Thromb Haemost. 1986;55:70–3. [PubMed] [Google Scholar]
- 4.Sabatino DE, Freguia CF, Toso R, et al. Recombinant canine B-domain deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model. Blood. 2009;114:4562–5. doi: 10.1182/blood-2009-05-220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozier JN, Dutra A, Pak E, et al. The Chapel Hill hemophilia: A dog colony exhibits an inversion of the factor VIII gene. Proc Natl Acad Sci USA. 2002;99:12991–6. doi: 10.1073/pnas.192219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles AR, Tinlin S, Greenwood R. A canine model of hemophilic (factor VIII:C deficiency) bleeding. Blood. 1982;60:727–30. [PubMed] [Google Scholar]
- 7.Hough C, Kamisue S, Cameron C, et al. Aberrant splicing and premature termination of transcription of the FVIII gene as a cause of severe canine hemophilia A: Similarities with the intron 22 inversion mutation in human hemophilia. Thromb Haemost. 2002;87:659–65. [PubMed] [Google Scholar]
- 8.Levinson B, Kenwrick S, Lakich D, Hammonds G, Jr, Gitschier J. A transcribed gene in an intron of the human factor VIII gene. Genomics. 1990;7:1–11. doi: 10.1016/0888-7543(90)90512-s. [DOI] [PubMed] [Google Scholar]
- 9.Lakich D, Kazazian HH, Jr, Antonarakis SE, Gitschier J. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat Genet. 1993;5:236–41. doi: 10.1038/ng1193-236. [DOI] [PubMed] [Google Scholar]
- 10.Naylor JA, Nicholson P, Goodeve A, et al. A novel DNA inversion causing severe hemophilia A. Blood. 1996;87:3255–61. [PubMed] [Google Scholar]
- 11.Tinlin S, Webster S, Giles AR. The development of homologous (canine/anti-canine) antibodies in dogs with haemophilia A (factor VIII deficiency): A ten-year longitudinal study. Thromb Haemost. 1993;69:21–24. [PubMed] [Google Scholar]
- 12.Cameron C, Notley C, Hoyle S, et al. The canine factor VIII cDNA and 5' flanking sequence. Thromb Haemost. 1998;79:317–22. [PubMed] [Google Scholar]
- 13.Viel KR, Ameri A, Abshire TC, et al. Inhibitors of factor VIII in black patients with hemophilia. N Engl J Med. 2009;360:1618–27. doi: 10.1056/NEJMoa075760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph SA, Brooks MB, Coccari PJ, Riback SC. Hemophilia A in a German shorthaired pointer: clinical presentations and diagnosis. J Am Anim Hosp Assoc. 1996;32:25–8. doi: 10.5326/15473317-32-1-25. [DOI] [PubMed] [Google Scholar]
- 15.Brinkhous KM, Sandberg H, Garris JB, et al. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci U S A. 1985;82:8752–6. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles AR, Tinlin S, Hoogendoorn H, Fournel MA, Ng P, Pancham N. In vivo characterization of recombinant factor VIII in a canine model of hemophilia A (factor VIII deficiency). Blood. 1988;72:335–9. [PubMed] [Google Scholar]
- 17.Nichols TC, Dillow AM, Franck HW, et al. Protein replacement therapy and gene transfer in canine models of hemophilia A, hemophilia B, von Willebrand disease, and factor VII deficiency. ILAR J. 2009;50:144–67. doi: 10.1093/ilar.50.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karpf DM, Kjalke M, Thim L, et al. Pharmacokinetics and ex vivo whole blood clot formation of a new recombinant FVIII (N8) in haemophilia A dogs. Haemophilia. 2011;17:963–8. doi: 10.1111/j.1365-2516.2011.02580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad S, Lillicrap D, Labelle A, et al. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood. 2008;111:672–9. doi: 10.1182/blood-2007-07-098913. [DOI] [PubMed] [Google Scholar]
- 20.Agersø H, Stennicke HR, Pelzer H, et al. Pharmacokinetics and pharmacodynamics of turoctocog alfa and N8-GP in haemophilia A dogs. Haemophilia. 2012;18:941–7. doi: 10.1111/j.1365-2516.2012.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumont JA, Liu T, Low SC, et al. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119:3024–30. doi: 10.1182/blood-2011-08-367813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkhous KM, Hedner U, Garris JB, Diness V, Read MS. Effect of recombinant factor VIIa on the hemostatic defect in dogs with hemophilia A, hemophilia B, and von Willebrand disease. I. Proc Natl Acad Sci U S A. 1989;86:1382–6. doi: 10.1073/pnas.86.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudsen T, Kristensen AT, Nichols TC, et al. Pharmacokinetics, pharmacodynamics and safety of recombinant canine FVIIa in a study dosing one haemophilia A and one haemostatically normal dog. Haemophilia. 2011;17:962–70. doi: 10.1111/j.1365-2516.2011.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen LC, Karpf DM, Agersø H, et al. Intravascular inhibition of factor VIIa and the analogue NN1731 by antithrombin. Br J Haematol. 2011;152:99–107. doi: 10.1111/j.1365-2141.2010.08432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, Lillicrap D. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–10. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Nichols TC, Sarkar R, McCorquodale S, Bellinger DA, Ponder KP. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci U S A. 2005;102:6080–5. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Lillicrap D, Patarroyo-White S, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–15. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 28.McCormack WM, Jr, Seiler MP, Bertin TK, et al. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J Thromb Haemost. 2006;4:1218–25. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar R, Mucci M, Addya S, et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. 2006;17:427–39. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- 30.Arruda VR. Toward gene therapy for hemophilia A with novel adenoviral vectors: successes and limitations in canine models. J Thromb Haemost. 2006;4:1215–7. doi: 10.1111/j.1538-7836.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- 31.Margaritis P, Roy E, Aljamali MN, et al. Successful treatment of canine hemophilia by continuous expression of canine FVIIa. Blood. 2009;113:3682–9. doi: 10.1182/blood-2008-07-168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milbauer LC, Enenstein JA, Roney M, et al. Blood outgrowth endothelial cell migration and trapping in vivo: a window into gene therapy. Transl Res. 2009;153:179–89. doi: 10.1016/j.trsl.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finn JD, Ozelo MC, Sabatino DE, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116:5842–8. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuenschwander S, Kissling-Albrecht L, Heiniger J, Backfisch W, Stranzinger G, Pliska V. Inherited defect of blood clotting factor VIII (haemophilia A) in sheep. Thromb Haemost. 1992;68:618–20. [PubMed] [Google Scholar]
- 35.Neuenschwander S, Pliska V. Factor VIII in blood plasma of haemophilic sheep: analysis of clotting time-plasma dilution curves. Haemostasis. 1994;24:27–35. doi: 10.1159/000217077. [DOI] [PubMed] [Google Scholar]
- 36.Backfisch W, Neuenschwander S, Giger U, Stranzinger G, Pliska V. Carrier detection of ovine hemophilia A using an RFLP marker, and mapping of the factor VIII gene on the ovine X-chromosome. J Hered. 1994;85:474–8. doi: 10.1093/oxfordjournals.jhered.a111503. [DOI] [PubMed] [Google Scholar]
- 37.Porada CD, Sanada C, Long CR, et al. Clinical and molecular characterization of a re-established line of sheep exhibiting hemophilia A. J Thromb Haemost. 2010;8:276–85. doi: 10.1111/j.1538-7836.2009.03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porada CD, Sanada C, Kuo CJ, et al. Phenotypic correction of hemophilia A in sheep by postnatal intraperitoneal transplantation of FVIII-expressing MSC. Exp Hematol. 2011;39:1124–35. doi: 10.1016/j.exphem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bi L, Lawler AM, Antonarakis SE, et al. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–21. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 40.Bi L, Sarkar R, Naas T, et al. Further characterization of factor VIII-deficient mice created by gene targeting: RNA and protein studies. Blood. 1996;88:3446–50. [PubMed] [Google Scholar]
- 41.Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM. Towards a standardization of the murine tail bleeding model. J Thromb Haemost. 2010;8:2820–2. doi: 10.1111/j.1538-7836.2010.04084.x. [DOI] [PubMed] [Google Scholar]
- 42.Follenzi A, Raut S, Merlin S, Sarkar R, Gupta S. Role of bone marrow transplantation for correcting hemophilia A in mice. Blood. 2012;119:5532–42. doi: 10.1182/blood-2011-07-367680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levinson B, Bermingham JR, Jr., Metzenberg A, Kenwrick S, Chapman V, Gitschier J. Sequence of the human factor VIII-associated gene is conserved in mouse. Genomics. 1992;13:862–5. doi: 10.1016/0888-7543(92)90170-w. [DOI] [PubMed] [Google Scholar]
- 44.Booth CJ, Brooks MB, Rockwell S. Spontaneous coagulopathy in inbred WAG/RijYcb rats. Comp Med. 2010;60:25–30. [PMC free article] [PubMed] [Google Scholar]
- 45.Booth CJ, Brooks MB, Rockwell S, et al. WAG-F8m1Ycb Rats harboring a factor VIII gene mutation provide a new animal model for hemophilia A. J Thromb Haemost. 2010;8:2472–7. doi: 10.1111/j.1538-7836.2010.03978.x. [DOI] [PubMed] [Google Scholar]
- 46.Mustard JF, Rowsell HC, Robinson GA, et al. Canine haemophilia B (Christmas disease). Br J Haematol. 1960;6:259–266. doi: 10.1111/j.1365-2141.1960.tb06241.x. [DOI] [PubMed] [Google Scholar]
- 47.Evans JP, Brinkhous KM, Brayer G, et al. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A. 1989;86:10095–9. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mauser AE, Whitlark J, Whitney KM, Lothrop CD. A deletion mutation causes hemophilia B in Lhasa Apso dogs. Blood. 1996;88:3451–5. [PubMed] [Google Scholar]
- 49.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD. Muscle-directed gene transfer and transient immune suppression result in sustained and partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 50.Brooks MB, Gu W, Ray K. Complete deletion of factor IX gene and inhibition of factor IX activity in a Labrador retriever with hemophilia B. J Am Vet Med Assoc. 1997;211:1418–21. [PubMed] [Google Scholar]
- 51.Lin H-F, Maeda N, Smithies O, et al. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90:3962–6. [PubMed] [Google Scholar]
- 52.Wang L, Zoppe M, Hackeng TM, et al. A factor IX-deficient mouse model for hemophilia B gene therapy. Proc Natl Acad Sci USA. 1997;94:11563–6. doi: 10.1073/pnas.94.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kundu RK, Sangiorgi F, Wu LY, et al. Targeted inactivation of the coagulation factor IX gene causes hemophilia B in mice. Blood. 1998;92:168–74. [PubMed] [Google Scholar]
- 54.Jin DY, Zhang TP, Gui T, Stafford DW, Monahan PE. Creation of a mouse expressing defective human factor IX. Blood. 2004;104:1733–9. doi: 10.1182/blood-2004-01-0138. [DOI] [PubMed] [Google Scholar]
- 55.Gui T, Reheman A, Ni H, Gross PL, Yin F, Monroe D, Monahan PE, Stafford DW. Abnormal hemostasis in a knock-in mouse carrying a variant of factor IX with impaired binding to collagen type IV. J Thromb Haemost. 2009;7:1843–51. doi: 10.1111/j.1538-7836.2009.03545.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang TP, Jin DY, Wardrop RM, 3rd, et al. Transgene expression levels and kinetics determine risk of humoral immune response modeled in factor IX knockout and missense mutant mice. Gene Ther. 2007 Mar;14:429–40. doi: 10.1038/sj.gt.3302881. [DOI] [PubMed] [Google Scholar]
- 57.Lozier JN, Tayebi N, Zhang P. Mapping of genes that control the antibody response to human factor IX in mice. Blood. 2005;105:1029–35. doi: 10.1182/blood-2004-03-1126. [DOI] [PubMed] [Google Scholar]
- 58.Herzog R, Hagstrom N, Kung S, et al. Stable gene transfer and expression of human FIX following intramuscular injection of recombinant AAV. Proc Natl Acad Sci U S A. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 60.Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–6. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 61.Snyder RO, Miao C, Meuse L, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Nichols TC, Read MS, Bellinger DA, Verma IM. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther. 2000;1:154–8. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- 63.Nathwani AC, Gray JT, Ng CY, et al. Self complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–61. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finn JD, Nichols TC, Svoronos N, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene. Blood. 2012;120:4521–3. doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cantore E, Nair N, Della Valle P, et al. Hyper-functional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012;120:4517–20. doi: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 66.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2002;101:2963–72. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 67.Manno CS, Pierce GF, Arruda V, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–7. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 68.Nathwani AC, Tuddenham EGD, Rangaragan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siedlecki CA, Lestinie BJ, Koggke-Marchant K, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent change in the three dimensional structur of human von Willebrand factor. Blood. 1996;88:2939–50. [PubMed] [Google Scholar]
- 70.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circulation Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 71.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 72.Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, The National Heart, Lung, and Blood Institute (NHLBI) expert panel report (USA). Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 73.Haberichter SL, Merricks EP, Fahs SA, Christopherson PA, Nichols TC, Montgomery RR. Re-establishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells. Blood. 2005;105:145–52. doi: 10.1182/blood-2004-02-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nichols TC, Bellinger DA, Reddick RL, et al. The roles of von Willebrand factor and factor VIII in arterial thrombosis: studies in canine von Willebrand disease and hemophilia A. Blood. 1993;81:2644–51. [PubMed] [Google Scholar]
- 75.Bowie EJW, Solberg LA, Jr, Fass DN. Transplantation of normal bone marrow into a pig with severe von Willebrand disease. J Clin Invest. 1986;78:26–30. doi: 10.1172/JCI112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu QY, Drouet L, Carrier JL. Differential distribution of von Willebrand factor in endothelial cells. Comparison between normal pigs and pigs with von Willebrand disease. Arteriosclerosis. 1987;7:47–54. doi: 10.1161/01.atv.7.1.47. [DOI] [PubMed] [Google Scholar]
- 77.Bahnak BR, Lavergne, Ferreira V, Kerbiriou-Nabias D, Meyer D. Comparison of the primary structure of the functional domains of human and porcine von Willebrand factor that mediate platelet adhesion. Biochem Biophys Res Comm. 1992;182:561–8. doi: 10.1016/0006-291x(92)91769-m. [DOI] [PubMed] [Google Scholar]
- 78.Haberichter SL, Fahs SA, Montgomery RR. Von Willebrand factor storage and multimerization: 2 independent processes. Blood. 2000;96:1808–15. [PubMed] [Google Scholar]
- 79.Read MS, Shermer RW, Brinkhous KM. Venom coagglutinin: an activator of platelet aggregation dependent on von Willebrand factor. Proc Natl Acad Sci U S A. 1978;75:4514–8. doi: 10.1073/pnas.75.9.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwarz HP, Dorner F, Mitterer A, et al. Evaluation of recombinant human von Willebrand factor in a canine model of von Willebrand disease. Haemophilia. 1998;4:53–62. doi: 10.1046/j.1365-2516.1998.0040s3053.x. [DOI] [PubMed] [Google Scholar]
- 81.Turecek PL, Gritsch H, Pichler L, et al. In vivo characterization of recombinant von Willebrand factor in dogs with von Willebrand disease. Blood. 1997;90:3555–67. [PubMed] [Google Scholar]
- 82.Olsen EH, McCain AS, Merricks EP, et al. Comparative response of plasma VWF in dogs to upregulation of VWF mRNA by interleukin-11 versus Wiebel-Palade body release by desmopressin (DDAVP). Blood. 2003;102:436–41. doi: 10.1182/blood-2003-01-0290. [DOI] [PubMed] [Google Scholar]
- 83.Ragni MV, Jankowitz RC, Chapman HL, et al. A phase II prospective open-label escalating dose trial of recombinant interleukin-11 in mild von Willebrand disease. Haemophilia. 2008;14:968–77. doi: 10.1111/j.1365-2516.2008.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ragni MV, Jankowitz RC, Jaworski K, Merricks EP, Kloos MT, Nichols TC. Phase II prospective open-label trial of recombinant interleukin-11 in women with mild von Willebrand disease and refractory menorrhagia. Thromb Haemost. 2011;106:641–645. doi: 10.1160/TH11-04-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ragni MV, Novelli EM, Murshed A, Merricks EP, Kloos MT, Nichols TC. Phase II prospective open-label trial of recombinant interleukin-11 in desmopressin-unresponsive von Willebrand disease and mild or moderate haemophilia A. Thromb Haemost. 2012:109. doi: 10.1160/TH12-06-0447. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bahou WF, Bowie EJW, Fass DN, Ginsburg D. Molecular genetic analysis of porcine von Willebrand disease: tight linkage to the fon Willebrand factor locus. Blood. 1988;72:308–13. [PubMed] [Google Scholar]
- 87.Sweeney JD, Novak EK, Reddington M, Takeuchi KH, Swank RT. The RIIIS/J inbred mouse strain as a model for von Willebrand disease. Blood. 1990;76:2258–65. [PubMed] [Google Scholar]
- 88.Nichols WC, Cooney KA, Mohlke KL, et al. von Willebrand disease in the RIIIS/J mouse is caused by a defect outside of the von Willebrand factor gene. Blood. 1994;83:3225–31. [PubMed] [Google Scholar]
- 89.Mohlke KL, Nichols WC, Westrick RJ, et al. A novel modifier gene for plasma von Willebrand factor level maps to distal mouse chromosome 11. Proc Natl Acad Sci USA. 1996;93:15352–7. doi: 10.1073/pnas.93.26.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohlke KL, Purkayastha AA, Westrick RJ, et al. Mvwf, a dominant modifier of murine von Willebrand factor, results from altered lineage-specific expression of a glycosyltransferase. Cell. 1999;96:111–20. doi: 10.1016/s0092-8674(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 91.Denis C, Methia N, Frenette PS, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95:9524–9. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Denis CV, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:4072–7. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gebrane-Younes J, Drouet L, Caen JP, Orcel L. Heterogeneous distribution of Weibel-Palade bodies and von Willebrand factor along the porcine vascular tree. Am J Pathol. 1991;139:1471–84. [PMC free article] [PubMed] [Google Scholar]