Abstract
Rationale
Cardiac hypertrophy results from the complex interplay of differentially regulated cascades based upon the phosphorylation status of involved signaling molecules. While numerous critical regulatory kinases and phosphatases have been identified in the myocardium, the intracellular mechanism for temporal regulation of signaling duration and intensity remains obscure. In the non-myocyte context, control of folding, activity, and stability of proteins is mediated by the prolyl isomerase Pin1, but the role of Pin1 in the heart is unknown.
Objective
To establish the role of Pin1 in the heart.
Methods and Results
Here we show that either genetic deletion or cardiac over-expression of Pin1 blunts hypertrophic responses induced by transaortic constriction and consequent cardiac failure in vivo. Mechanistically, we find that Pin1 directly binds to Akt, MEK and Raf-1 in cultured cardiomyocytes following hypertrophic stimulation. Furthermore, loss of Pin1 leads to diminished hypertrophic signaling of Akt and MEK, while over-expression of Pin1 increases Raf-1 phosphorylation on the auto-inhibitory site Ser259 leading to reduced MEK activation.
Conclusions
Collectively, these data support a role for Pin1 as a central modulator of the intensity and duration of two major hypertrophic signaling pathways, thereby providing a novel target for regulation and control of cardiac hypertrophy.
Keywords: Pin1, cardiomyocyte, Akt, Raf-MEK-ERK, hypertrophy, signal transduction, molecular biology, heart failure
Introduction
Molecular signaling cascades are characteristically based upon interwoven networks held in regulated balance. Tuning the network by selectively altering signal duration and intensity provides another layer of regulation above and beyond binary “on/off” switching mechanisms. This capacity to influence triggered signaling cascades characterizes the unique property of Pin-1. Almost 15 years ago the loss of the Pin1 in both yeast and human cells was shown to cause defects in cytokinesis.1 From this seminal observation, hundreds of studies contributed to deciphering the mechanism, function, and biological consequences of Pin1 activity 2, 3 By way of analogy, Pin1 acts as a “molecular orchestrator” that does not choose the music, but rather sets the amplitude and duration. Pin1 accomplishes this feat by post-translational modification of protein structure based upon specific recognition of a major regulatory phosphorylation motif (Ser/Thr–Pro) belonging to the family of Proline (Pro)-directed protein kinases that includes Akt, Pim-1, cyclin-dependent protein kinases (CDKs), and many more. Presence of a proline residue means the molecule can adopt either cis or trans conformations, often with widely divergent biological activities depending upon configuration. Pin1 is a highly conserved peptidyl-prolyl isomerase (PPI) that lowers the activation energy necessary for isomerization around the Pro-directed neighboring phosphorylation site. Isomerization modification leads to changes in stabilization of proteins in active configurations, enhanced degradation, or even accessibility for further modifications by other enzymes. Clearly, Pin1 is a critical element of survival and proliferative signaling in the field of cancer, but the role of this critical regulatory molecule in the myocardium has not been previously examined.
The consequences of Pin1 activation are multifaceted, including transcriptional reprogramming4-7 as well as altered signaling cascades involved in proliferation, cell survival, lineage commitment, and cellular aging. Elevation of Pin1 expression in cancer suggests a role in cellular proliferation,8 and Pin1 has been touted as a potential therapeutic target for slowing proliferation 9, 10 since inhibition of Pin1 leads to mitotic arrest and apoptosis.11, 12 These postulates are consistent with Pin1 induction by growth factors as well as the family of Pin1 target substrates responsible for regulation of mitosis.1 Presumably increased Pin1 expression correlates with mitosis to preserve the delicate rhythm of molecular signals leading to cell division, but Pin1 can be a double edged sword for cell survival. Numerous mediators of apoptosis are regulated by Pin1 activity. However, since Pin1 does not “choose the music, but instead propagates the rhythm” the outcome can be either to enhance survival or to accentuate death signaling. Several targets of Pin1 regulate survival including Akt, which is stabilized by Pin1 resulting in prolonged and persistent activation.13, 14 Pin1 suppresses apoptosis mediated by cell death-associated proteins.15 Pin1 also may promote survival via increasing VEGF expression,16 enhanced NFκB signaling,17 or tuning autophagy.18 Clues are emerging to implicate Pin1 in the regulation of cell commitment to the differentiated state through two canonical signaling pathways mediated by Nanog and Notch.7, 19 One of the most intriguing aspects of Pin1 biology is the link to aging, particularly in the context of neurobiology. Genetic deletion of Pin1 in mice leads to an early onset neurodegeneration syndrome resembling Alzheimers disease. Conversely, Pin1 overexpression decreases β-amyloid production, restores tau function, and promotes cell cycle reentry in neuronal cells,20, 21 whereas inhibition of Pin1 halts proliferation.10, 22 These observations have led to speculation that Pin1 links neurodegenerative disease, cancer, and aging, with Pin1 playing a protective role to antagonize the aging phenotype in neurons.20, 23, 24 In fact, the phenotype of Pin1 knockout mice recapitulates the premature aging observed in telomerase-deficient mice.25
Extrapolating two essential points from the assembled Pin1 literature: 1) Pin1 is a facilitator of timing and intensity for multiple distinct signaling cascades, and 2) Pin1 potentiates the biological consequences of signal transduction without serving to initiate them. Targets of Pin1 action are well known meditators of myocardial signaling including the Akt and MEK/ERK cascades.26, 27 The impact of Pin1 in areas of proliferation, survival, aging, and cell fate determination place this molecule central to many of the most significant areas of current myocardial signal transduction under investigation.2 Turning to the myocardium where the functions of Pin1 are yet to be determined, the present study documents differential regulated expression of Pin1 in the heart during development and following pressure overload. Furthermore, we elucidate the significance of Pin1 in cardiac hypertrophy.
Methods
Detailed methods can be found in the supplementary methods online.
Mice
All experimental procedures were performed according to the guidelines established by San Diego State University for experiments in animals and all protocols were approved by Institutional Animal Care and Use Committee. Animal model surgery, echocardiographic and hemodynamic analyses were performed as previous described. 28, 29
Histology and staining
Hearts fixed in 10% formalin were embedded in paraffin, sectioned at 4μm thickness, and were used for immunohistochemistry.30
Immunoblot analysis
Whole cell lysates were resolved by SDS-polyacrylamide gel electrophoresis. Immunoblot analyses were performed with Pin1, phosphorylated Akt, phosphorylated MEK, phosphorylated Raf-1, phosphorylated ERK, ERK (CST), GAPDH (Chemicon), Akt (Santa Cruz), and MEK (Transduction Lab). There are two closely migrating immunoreactive bands for Pin1 evident by immunoblot analysis. However, the relationship of these two immunoreactive bands relative to Pin1 biological function remains unclear. Intensity of the lower band remained consistently detectable but with varying intensity throughout our immunolabeling studies. Staining with a phosphospecific antibody towards Ser71 did not correlate conclusively with either band in the immunoblot analysis (Online Figure IA). Alternative to phosphorylation status, we speculated that mobility may correlate with subcellular localization, but subcellular fractionation findings were inconclusive. Further possible explanations include alternative splicing or posttranslational modification other than Ser71 phosphorylation. Delineation of the functional differences, if any, for these two related Pin1 bands remains the subject of future studies. Intensities of immunoblot bands were measured using ImageJ software (NIH).
Cardiomyocyte culture
Neonatal rat cardiomyocytes (NRCMs) were prepared from ventricles of one-day-old Wistar rats and cultured by standard protocol.30 NRCMs were treated with serum or phenylephrine (PE) for indicated times.
Adenovirus and siRNA
Recombinant adenovirus strains harboring EGFP or human Pin1 with EGFP were generated as previous described.31 NRCMs were transduced with the adenovirus at a multiplicity of infection of 25. NRCMs were transfected with siRNA to Pin1 (Invitrogen) by using HiPerfect (Qiagen). Functional comparability for EGFP, Pin1 or EGFP-Pin1 was assessed in direct comparison studies in HeLa cells, because HeLa cells have previously been used for demonstration of Pin1-mediated effects.9, 32 Impact of Pin1 upon ERK activation was observed by phosphorylation status of the kinase. ERK activation is extended by Pin1 overexpression in HeLa cells treated with EGF. Similar to previously reported findings,33 ERK phosphorylation was increased by EGF stimulation at the 1 minute time point. However, activation returned to basal levels in MOCK or EGFP-expressing cells by the 30 minute time point whereas, in comparison, overexpression of Pin1 or Pin1-EGFP delayed ERK de-phosphorylation (Online Figure II). Therefore, with respect to potentiation of ERK phosphorylation, the function of Pin1-GFP fusion protein is comparable to Pin1 alone.
RNA isolation and quantitative RT-PCR
Total RNA was isolated from NRCMs with Quick-RNA MiniPrep kit (Zymo Research) according to the manufacturer's instructions. cDNA synthesis of RNA was carried out by using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and quantitative real time (RT)-PCR was performed by using Quanti-Tect SYBR Green PCR kit (Qiagen). Relative levels of gene expressions were normalized to HRPT expression using the Delta Delta Ct method.
Adeno-associated Virus Serotype 9 (AAV9)
AAV9 harboring human Pin1 was generated. The virus (1×1011 total virus particles) was injected 6 weeks old mice via tail vein and mice were subjected to pressure overload at 6 weeks post-AAV injection.
Proximity ligation assay (PLA)
PLA was performed to detect protein-protein interaction according to manufacturer's instruction.
Statistical analysis
Data are shown as mean ± SEM. Statistical analysis was performed using GraphPad Prism (Graphpad Software Inc.). Multiple group comparison was performed by one-way or two-way analysis of variance followed by the Bonferroni procedure for comparison of means. Comparisons between two groups were performed using Student's t-test. Values of P< 0.05 were considered statistically significant.
Results
Pin1 expression decreases with age and is upregulated after pressure overload in the heart
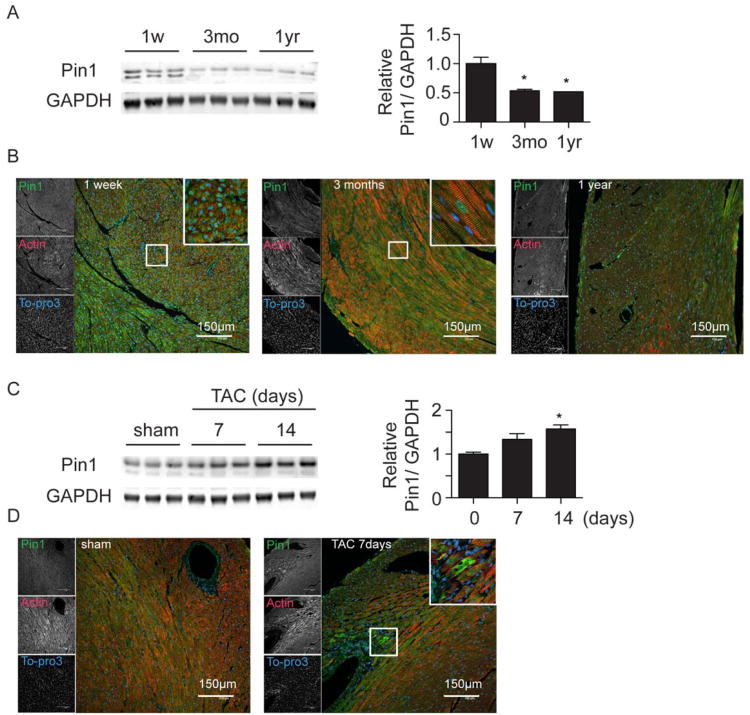
Pin1 was highly expressed in postnatal hearts at 1 week, decreasing by 46% at 3 months and 48% by one year of age (Figure 1A). Pin1 cellular localization shifted during development from predominantly nuclear in neonatal mouse hearts to increasingly cytosolic in adult mouse hearts (Figure 1B). In the pathologically challenged heart exposed to pressure overload through transaortic constriction (TAC), Pin1 was evident in perivascular areas (Figure 1D) and expression was increased (157% induction at 14 days, Figure 1C). Phosphorylation of Pin1 on Ser71, which inhibits its catalytic activity,34 increased with age (Online Figure IA) but decreased after TAC (Online Figure IB). These results suggest that Pin1 is important for cardiac development and plays a role in regulation of cardiac signal transduction following hypertrophic and/or mechanical stress since perivascular regions are hot spots for strain, remodeling, and increased cardioactive signaling in the heart.
Figure 1. Pin1 decreases with aging and is induced after pressure overload in the murine heart.
A, Immunoblot showing decline of Pin1 expression in the heart during development at 1 week (1w), 3 months (3mo), and 1 year (1yr) of age (left) with densitometric quantification on the right. * p<0.05 versus 1 week. n=3. B, Paraffin embedded sections from mouse hearts at the same time points as in A were stained for Pin1 (green), Actin (red), and To-pro3 (blue). Boxed regions are shown at higher magnification in the upper right corner. C, Immunoblot showing induction of Pin1 expression in the heart 7 and 14 days after transaortic constriction (TAC, left) with densitometric quantification on the right. * p<0.05 versus day0. n=3. D, Paraffin embedded sections from mouse hearts 7 days after TAC were stained with Pin1 (green), Actin (red), and To-pro3 (blue). Boxed regions are shown at higher magnification in the upper right corner.
Loss of Pin1 attenuates hypertrophy and preserves cardiac function upon pressure overload
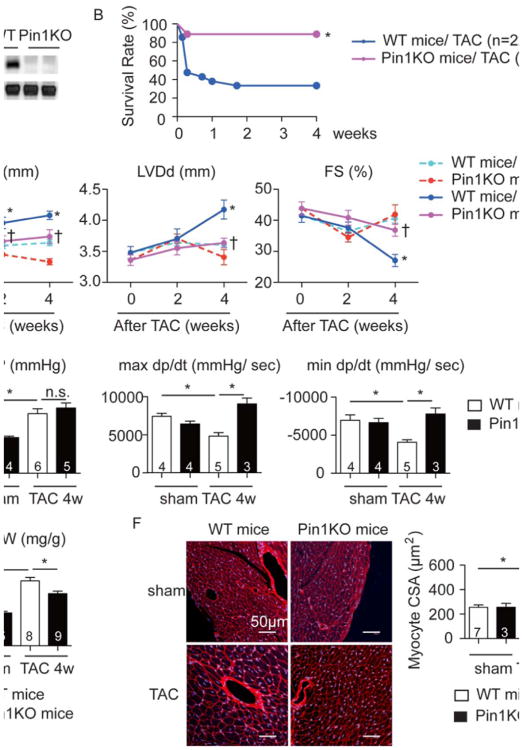
Global Pin1 knockout (KO) mice35 were shown to exhibit a multitude of age-associated diseases,20, 21, 36 but cardiac function was not assessed. Echocardiographic analysis of hearts in Pin1 KO mice up to 6 months of age (Figure 2A) showed a normal cardiac phenotype under basal conditions (Online Table). Pin1 KO mice subjected to pathologic challenge by TAC showed significantly lower mortality rate during four weeks following TAC (11%) relative to wild type (WT) littermate controls (63%) as depicted by Kaplan-Meier analysis (Figure 2B). Pressure overload induced cardiac hypertrophy (127% increase) without affecting cardiac function in WT mice at 2 weeks post TAC, whereas increased wall thickness was attenuated in Pin1 KO mice (Figure 2C). Posterior wall thickness (Pwd) and left ventricular diameter at diastole (LVDd) increased by 132% and 144% respectively, in conjunction with a loss of fractional shortening (FS) by 33% in WT mice (Figure 2C) at 4 weeks after TAC. Increased LVDd was diminished and cardiac function was preserved in Pin1 KO mice (Figure 2C). Equivalent systolic arterial blood pressure between WT and TAC hearts confirmed uniform constriction of the aorta in both groups (Figure 2D). Echocardiographic data were corroborated by terminal invasive hemodynamic assessment at 4 weeks after TAC, confirming that maximal and minimal developed pressure over time (max and min dp/dt) were higher in Pin1KO mice compared to WT controls (Figure 2D). The heart weight to body weight ratios (HW/BW) were also decreased in Pin1 KO mice (Figure 2E). Cross-sectional area (CSA) of myocytes increased in WT mice by 177% after TAC, but Pin1 KO mice showed blunted cellular enlargement (Figure 2F). Collectively, these data indicate that deletion of Pin1 attenuates the hypertrophic response to pressure overload and preserves cardiac function.
Figure 2. Loss of Pin1 attenuates hypertrophy and preserves cardiac function upon pressure overload.
A, Immunoblot showing Pin1 expression in the heart of wild type (WT) and Pin1 knockout (Pin1 KO) mice. B, Kaplan-Meier curve after TAC procesure. * p<0.05 versus WT mice exposed with TAC. C, Echocardiographic analysis at the indicated time points after TAC procedure showing posterior wall thickness at diastole (Pwd), left ventricular dimension at diastole (LVDd), and fractional shortening (FS). * p<0.05 vs WT sham, † P<0.05 versus WT TAC at the same time point. D, Invasive hemodynamic assessment 4 weeks after TAC showing arterial systolic blood pressure (BP), maximal (max dp/dt) and minimal (min dp/dt) changes of developed pressure over time. * p<0.05. E, Heart weight to body weight ratio (HW/ BW) at 4 weeks after TAC. *p<0.05. F, Sections 4 weeks after TAC stained with Wheat Germ Agglutinin (WGA, red) and To-pro3 (blue) (left), quantification of cross sectional area of cardiomyocytes (CSA, right). *p<0.05.
Loss of Pin1 dampens cultured cardiomyocyte hypertrophy via inhibition of Akt and MEK activation
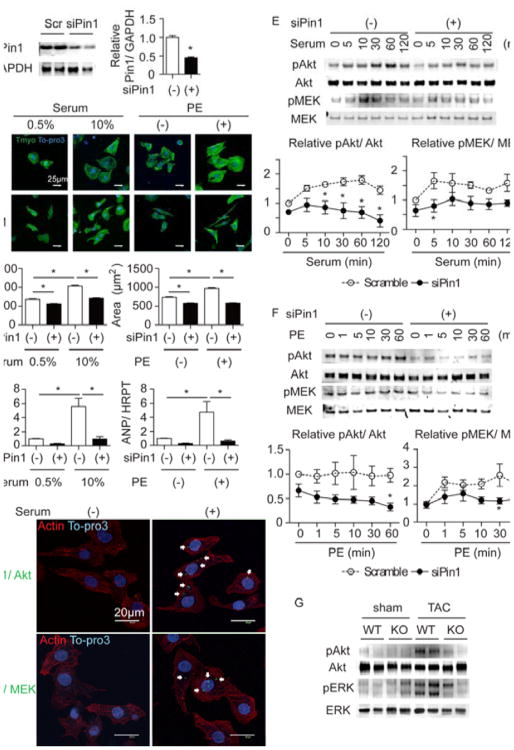
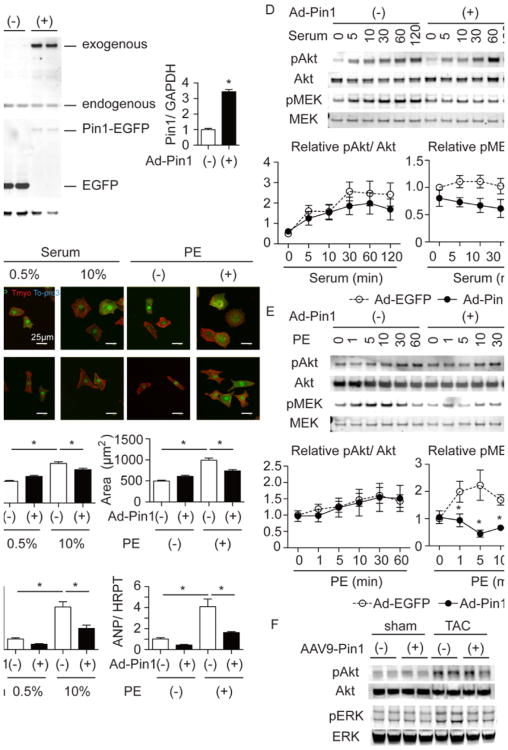
The molecular mechanism underlying attenuation of cardiac hypertophy by Pin1 deletion was determined using siRNA to deplete Pin1 in neonatal rat cardiomyocytes (NRCMs) followed by hypertrophic stimulation with serum or phenylephrine (PE). Successful knockdown of Pin1 protein was confirmed by immunoblot (Figure 3A). Cell size increased by 153% and ANP levels rose by 556% upon growth induction by serum stimulation in NRCM treated with scrambled RNA sequence as control (Figure 3B and 3C). Additionally, PE stimulation increased cell area by 133% and ANP by 470% (Figure 3B and 3C). In contrast, increases in cell size and ANP expression upon hypertrophic stimulation were mitigated by siRNA knockdown of Pin1 (siPin1; Figure 3B and 3C). Known binding partners of Pin1 in non-cardiac cell types include Akt and MEK,13, 33 both of which are established mediators of hypertrophic signaling.26, 27 As such, participation of Akt and MEK in Pin1-mediated regulation of hypertrophic remodeling was determined following Pin1 depletion. Direct interaction between Pin1 and Akt or MEK was demonstrated by proximity ligation assay (PLA, Figure 3D) as well as immunoprecipitation (Online Figure III) in NRCMs. Interestingly, the interaction of Pin1 with Akt and MEK was increased under hypertrophic growth conditions (Figure 3D). Pin1 binding modifies target protein conformation and activity after phosphorylation, therefore phosphorylation of Akt and MEK following serum or PE treatment was measured in siPin1-treated NRCMs. Sustained Akt phosphorylation up to 120 min following serum stimulation or MEK activation within 1 min after PE exposure were attenuated by siPin1 (Figure 3E and 3F). Therefore, attenuation of hypertrophy after siPin1 treatment is likely due in part to combined interference with well-accepted Akt- and MEK-dependent pro-hypertrophic signaling pathways. Confirmation of MEK pathway activation is corroborated by phosphorylation of ERK, the direct downstream target of MEK (Online Figure IVA and IVB). Extrapolation to the in vivo context shows Akt and ERK phosphorylation were increased in hearts of WT mice, however activation of both enzymes was blunted in Pin1 KO hearts measured after 4 days of TAC (Figure 3G). Collectively, these data show that loss of Pin1 compromises Akt and MEK activation, thereby attenuating cardiomyocyte hypertrophy in vitro and in vivo.
Figure 3. Loss Pin1 attenuates cardiomyocyte hypertrophy in vitro via regulating Akt and MEK pathway.
A, Immunoblot showing Pin1 expression after scramble (scr) or Pin1 specific siRNA treatment (siPin1) in neonatal rat cardiomyocytes (NRCMs, left) and densitometric quantification (right). *p<0.05 versus Scr. n=8. B, Immuncytochemistry of NRCMs treated with 10% serum or phenylephrine (PE) and stained for tropomyosin (Tmyo, green) and To-pro3 (blue) after scramble or siRNA treatment (upper panel). Quantification of cardiomyocyte area on the right. *p<0.05. n=3. C, Quantitative RT-PCR showing ANP expression in NRCMs under the same conditions as in B. *p<0.05. n=3. D, Proximity ligation assay (PLA) showing direct interaction of Pin1 with Akt (upper panel) or MEK (lower panel) in cardiomyocytes (green dots, white arrows) with (right) or without stimulation (left). Cardiomyocytes were also stained with Tmyo (red) and To-pro3 (blue). E, Immunoblots showing time course analysis for Akt and MEK phosphorylation after serum stimulation in scramble and siPin1 treated NRCMs (upper panel). Densitometric quantification is shown in the lower panel for Akt (left) and MEK (right). n=5. F, Immunoblot showing time course analysis for Akt and MEK phosphorylation after PE stimulation in scramble and siPin1 treated NRCMs (upper panel). Densitometric quantification is shown in the lower panel for Akt (left) and MEK (right). n=6. G, Immunoblot showing Akt and ERK phosphorylation in the heart of WT and Pin1 KO (KO) mice at 4 days after TAC.
Pin1 over-expression preserves cardiac function after pressure overload
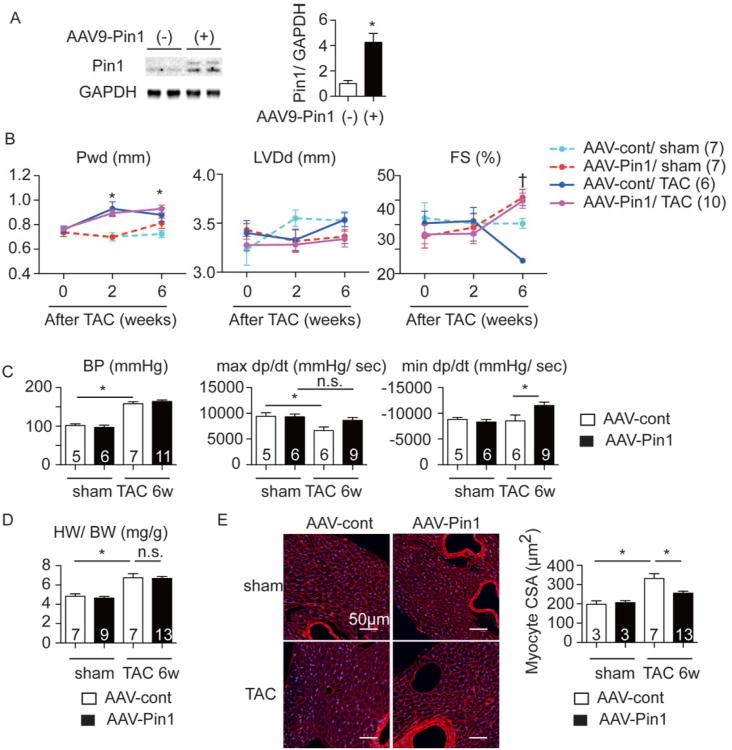
Cardiac-specific Pin1 over-expression was accomplished using an adeno-associated virus serotype 9 (AAV9) harboring Pin1 downstream of CMV-enhanced cardiac specific myosin light chain (MLC) promoter (AAV-Pin1).37 Adult mice were infected with AAV-Pin1 and allowed to accumulate Pin1 protein in the heart for 6 weeks following viral exposure (Figure 4A). Basal cardiac function between mice receiving control virus (AAV-cont) and AAV-Pin1 was comparable at six weeks after viral exposure (data not shown). Hearts of normal versus AAV-Pin1 treated mice were then challenged by TAC surgery at 12 weeks of age. Systolic blood pressure increased to the same extent in both AAV-control and AAV-Pin1 groups (Figure 4C). Posterior wall thickness (Pwd) increased by 121% with a loss of fractional shortening (FS) by 19% in AAV-cont mice (Figure 4B) 6 weeks after TAC. In comparison, cardiac function was preserved in AAV-Pin1 mice (Figure 4B) and subsequently confirmed by invasive hemodynamic assessments (Figure 4C). Comparable results were observed in AAV-cont and AAV-Pin1-treated mice when either wall thickness (Figure 4B) or HW/ BW ratios were examined (Figure 4D). However, at the cellular level, cardiomyocyte size was increased by 167% in AAV-cont mice after TAC, whereas AAV-Pin1 treatment blunted cellular hypertrophy (Figure 4E). Survival was comparable between all groups (6/9 for the AAV control versus 11/12 for the AAV-Pin1 group). In summary, cardiac-specific Pin1 over-expression preserves cardiac function and blunts cardiac hypertrophy at the cellular level in vivo upon pressure overload.
Figure 4. AAV9-Pin1 attenuates hypertrophy and preserves cardiac function upon pressure overload.
A, Immunoblot showing Pin1 expression in the heart of mice injected with empty AAV9 vector (AAV-cont) and with AAV9 harboring Pin1 (AAV-Pin1). The right panel shows densitometric quantification of Pin1 expression. n=3-4. B, Echocardiographic analysis at the indicated time points after TAC procedure showing posterior wall thickness at diastole (Pwd), left ventricular dimension at diastole (LVDd), and fractional shortening (FS). * p<0.05 vs WT sham, † P<0.05 versus WT TAC at the same time point. C, Invasive hemodynamic assessment 6 weeks after TAC showing arterial systolic blood pressure (BP), maximal (max dp/dt) and minimal (min dp/dt) changes of developed pressure over time. * p<0.05. n.s., not significant. D, Heart weight to body weight ratio (HW/ BW) at 6 weeks after TAC. *p<0.05. n.s., not significant. E, Sections 6 weeks after TAC stained with Wheat Germ Agglutinin (WGA, red) and To-pro3 (blue) (left), quantification of cross sectional area of cardiomyocytes (CSA, right). *p<0.05.
Over-expression of Pin1 attenuates cardiomyocyte hypertrophy in vitro through inhibition of MEK but not Akt
The molecular basis for Pin1-mediated attenuation of cardiac hypertrophy was delineated in further analyses involving NRCM subjected to hypertrophic stimuli in vitro. Pin1 over-expression in NRCM (Figure 5A) attenuated hypertrophic responses relative to the eGFP-expressing control cells where cell size increased by 183% and ANP expression by 396% after serum treatment (Figure 5B and 5C). Furthermore, in control NRCMs cell area increased by 200% and ANP increased by 397% after PE stimulation, both of which are inhibited by Pin1 over-expression. Exogenously expressed Pin1 localized to both cytosol and nuclear compartments (Figure 5B), which was confirmed using cell fractionation samples (data not shown). The kinetics of Akt phosphorylation, which are indicative of activation in response to serum or PE stimulation, was similar in control and Pin1 over-expressing NRCMs, suggesting that Akt is not responsible for Pin1 over-expression-mediated decreases in the hypertrophic response (Figure 5D and 5E). In contrast, in NRCMs treated with serum or PE, MEK and ERK activation were significantly decreased by Pin1 over-expression (Figure 5D and 5E and Online Figure IV). Involvement of the MEK/ERK cascade in Pin1 over-expression signaling was corroborated, in vivo, by measuring phosphorylated Akt and ERK in the hearts of mice treated with AAV-cont or AAV-Pin1 and subjected to TAC. ERK, but not Akt activation, was blunted in AAV-Pin1 TAC hearts compared to controls (Figure 5F). Collectively, these data indicate Pin1 over-expression blunts TAC-induced hypertrophy via inhibition of MEK but not Akt signaling.
Figure 5. Over-expression of Pin1 attenuates cardiomyocyte hypertrophy in vitro via regulating MEK pathway.
A, Immunoblot showing Pin1 and GFP expressions in NRCM transduced with adenovirus expressing EGFP (Ad-EGFP) or Pin1-EGFP (Ad-Pin1). The right panel shows densitometric quantification of Pin1 expression. * p<0.05. n=6. B, Immuncytochemistry of NRCMs treated with 10% serum or phenylephrine (PE) and stained for GFP (green), tropomyosin (Tmyo, red) and To-pro3 (blue) after Ad-EGFP or Ad-Pin1 induction (upper panel). Lower panel showed cell size quantification only of cardiomyocytes expressing EGFP. *p<0.05. n=3. C, Quantitative RT-PCR showing ANP expression in NRCMs under the same conditions as in B. * p<0.05. n=3. D, Immunoblot showing time course analysis for Akt and MEK phosphorylation after serum stimulation in Ad-EGFP and Ad-Pin1 transduced NRCMs (upper panel). Densitometric quantification is shown in the lower panel for Akt (left) and MEK (right). n=7. * p<0.05 versus control at the same time point. E, Immunoblot showing time course analysis for Akt and MEK phosphorylation after PE stimulation in Ad-EGFP and Ad-Pin1 transduced NRCMs (upper panel). Densitometric quantification is shown in the lower panel for Akt (left) and MEK (right). n=6. * p<0.05 versus control at the same time point. F, Immunoblot showing Akt and ERK phosphorylation in the heart of AAV-cont and AAV-Pin1 mice at 4 days after TAC.
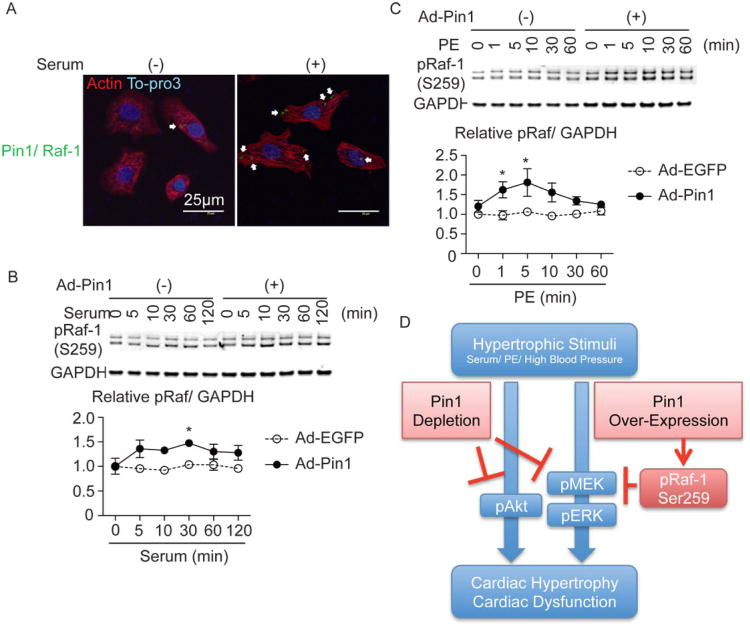
The seemingly paradoxical finding that Pin1 inhibition and over-expression blunted MEK activation can be reconciled by the involvement of additional inhibitory signaling upstream of MEK, ultimately leading to reduced MEK activation. Specifically, Raf-1 is a direct activator of MEK and a target of Pin1, but phosphorylation of Raf-1 on Ser259 inhibits kinase activity leading to reduced MEK phosphorylation.38 Indeed, Pin1 binding to Raf-1 was confirmed by PLA (Figure 6A) and, compared to GFP-expressing controls, phosphorylation of the RafSer259 inhibitory site was induced by serum, as well as PE in NRCMs over-expressing Pin1 (Figure 6B and 6C). Thus, Pin-1 over-expression blunts MEK activation through phosphorylation of the RafSer259 inhibitory site.
Figure 6. Raf-1 phosphorylation on an autoinhibitory site is increased in the cardiomyocyte transduced with Ad-Pin1.
A, Proximity ligation assay (PLA) showing direct interaction of Pin1 with Raf-1 in cardiomyocytes (green dots, white arrows) with (right) or without stimulation (left). Cardiomyocytes were also stained with tropomyosin (Tmyo, red) and To-pro3 (blue). B, Immunoblots showing time course analysis for Raf-1 phosphorylation on Ser259 (pRaf) after serum stimulation in EGFP and Pin1 treated NRCMs (upper panel). Densitometric quantification is shown in the lower panel for pRaf. n=5. * p<0.05 versus control at the same time point. pRaf protein levels were normalized with GAPDH as a loading control. C, Immunoblots showing time course analysis for pRaf after phenylephrine (PE) stimulation (upper panel). Densitometric quantification is shown in the lower panel for pRaf. n=5. * p<0.05 versus control at the same time point. pRaf protein levels were normalized with GAPDH as a loading control. D, Schematic showing the mechanistic impact of Pin1 depletion as well as over-expression upon cardiac hypertrophy.
Discussion
Elevation of Pin1 in cancer cells suggests a role in regulation of cellular growth 8 consistent with Pin1 induction by growth factors including IGF1.39 In comparison, unlike transformed proliferative cells, the post-mitotic nature of mature cardiomyocytes underscores a distinct context-dependent nature for Pin1 effects. Extrapolating from Pin1 literature, neurons (like cardiomyocytes) are also notoriously resistant to growth stimuli prompting comparisons between post-mitotic neuronal cells versus transformed cells. Comparing these two divergent cell types, Pin1 expression acts as molecular timer for signaling to maintain healthy aging in postmitotic cells such as neurons,20, 25 whereas dysregulation of Pin1 in proliferating cells alters regulators of cell cycle and commitment leading to oncogenic transformation.7, 12, 19, 32 Mature cardiomyocytes respond to growth stimuli by remodeling40, 41 and our findings support a role for Pin1 as a regulator of myocardial hypertrophy on both cellular and organ levels. Significance for Pin1 in the myocardial context is the novel finding that canonical hypertrophic signaling pathways are influenced by Pin1 activity, ultimately determining the adaptive cellular response and overall outcome of the heart when challenged by hypertrophic stimulation.
Pin1 increases proliferation and survival in tumor cells through maintenance of oncogenic proteins such as Akt.13 In the cardiac context, Akt is a well-accepted mediator of cardiac hypertrophy.27 Constitutive over-expression of Akt induces cardiomyocyte hypertrophy in vitro and in vivo ultimately leading to heart failure,42, 43 whereas transient Akt activation also induces cardiac hypertrophy, however, cardiac function is preserved.43 Therefore, the intensity and duration of Akt activation are critical for physiologic versus pathologic remodeling. Akt activation in cardiomyocytes treated with serum peaks at 60min, whereas Akt activation coupled with Pin1 silencing is weaker and returns to basal levels within 10min after stimulation (Figure 3E). Conversely, Akt activation in the cardiomyocyte context is unaffected by Pin1 overexpression (Figure 5D and 5E). Although increased Pin1 expression can correspond to enhanced Akt activation this correlation does not hold true for all cancer entities13 and obviously does not apply for cardiomyocytes. Therefore, we posit that facilitation of Akt activation by Pin1 is likely to be cell-type dependent and, as the heart possesses rather high Pin1 expression, endogenous Pin1 may be sufficient to saturate Akt regulation with additional Pin1 overexpression of no further consequence. However, loss-of-function studies confirm that Pin1 is a powerful modulator of Akt signal duration and intensity to implicate Pin1 in regulation of cardiomyocyte hypertrophy.
The MEK-ERK cascade regulates fundamental cellular processes that govern cell transformation, differentiation, proliferation, and survival.44 Like Akt in the cardiac context, the MEK-ERK cascade is another major inducer of hypertrophy.26, 45 In tumor cells, Pin1 modulates MEK activity through direct binding and maintenance of activation.33 Similarly, Pin1 binds directly to MEK in cardiomyocytes after hypertrophic stimuli (Figure 3D) and Pin1 silencing attenuated MEK/ERK induction after stimulation independent of Akt (Figure 3E and 3F, Online Figure IVA and IVB). These data clearly show that Pin1 regulates the MEK-ERK pathway in cardiomyocytes and is needed for full hypertrophic response in vivo and in vitro. The overtly paradoxical finding that Pin1 over-expression reduced MEK activation prompted assessment of Raf-1 autoinhibition, revealing MEK activation was blocked due to increased RafSer259 inhibitory site phosphorylation (Figure 5D and 5E, 6B, and 6C). The precise mechanism of how over-expression Pin1 increases RafSer259 inhibitory site phosphorylation remains unclear, and Pin1 has an opposite effect upon Raf-1 activation by regulating dephosphorylation at alternate phosphorylation sites in NIH3T3 cells,46 requiring further studies to clarify the role of Pin1 upon Raf-1 activation in NRCMs. Mechanistically, the influence of Pin1 can be attributed, at least in part, to alterations in the intensity and duration of Akt and MEK-dependent signaling cascades influenced by Raf-1-dependent feedback regulation of Pin1 activity.
Pin1 expression is likely to be tightly regulated within a narrow window to facilitate appropriate hypertrophic responses, as attenuation of cardiac growth was observed in response to both loss as well as over-expression of Pin1 after TAC (Figures 2 and 4) and as demonstrated by the ability to blunt MEK/ERK signaling through direct mechanisms in silencing studies or indirect feedback though the Raf-1 feedback cascade.
Groups receiving AAV treatment underwent surgery at postnatal 12 week that includes a requisite 6 week incubation period for sufficient Pin1-AAV expression when delivered at 6 weeks of age. The 6 week post-delivery AAV incubation necessitated use of a 26 gauge needle for to allow for survival of the 12 week old animals at time of TAC banding. Therefore, the appropriate control group for Pin1-AAV injections is the control-AAV cohort treated and operated upon at identical age. In comparison, the surgical protocol for mice not receiving AAV treatment consisted of TAC banding at 8 weeks of age since AAV incubation time for expression was not necessary. Since these young mice cope better with pressure overload a 27 gauge needle was used for constriction surgery resulting in greater constriction relative to the virus-treated groups at 12 weeks, leading to increased wild-type mouse mortality, although measured developed systolic pressure was comparable at time of sacrifice as quantified by invasive hemodynamic assessment.
In the present study, we focused on two major well-established pro-hypertrophic pathways namely Akt- and the Raf-MEK-ERK cascade. Pin1 regulates activity or stability of phospho-proteins only, therefore activation per se of Akt and ERK are still possible in NRCMs treated with siRNA or hearts from Pin1KO mice. Signal duration and intensity are altered by modulation of Pin1, whereas in the Akt/ERK KO mice these respective pathways are deleted completely. 47-49 Other signaling pathways might be involved and contributing to the observed phenotype and is noted as a limit for interpretations in our study.
Collectively, our results indicate a pivotal role for Pin1 as a regulator of the intensity and duration of the pro-hypertrophic signaling network in the heart. Previously documented effects of Pin1 in the non-cardiac context will be the focus of future studies involving the multifaceted nature of Pin-1 mediated consequences for many of significant areas of current myocardial signal transduction under investigation including proliferation, survival, aging, and cell fate determination.
Supplementary Material
Novelty and Significance.
What Is Known?
Cardiac hypertrophy is regulated not only by activation of specific signal transduction pathways, but also by the intensity and duration of the respective enzymatic activities.
Pin1 regulates the duration and intensity of activity by enzymes responsible for cell growth and survival in cancer cells.
What New Information Does This Article Contribute?
Pin1 binds directly to key mediators of cardiac remodeling following hypertrophic stimulation in cardiomyocytes in vitro.
Loss of Pin1 attenuates hypertrophic signaling of Akt and MEK.
Over-expression of Pin1 increases Raf-1 phosphorylation at the auto-inhibitory site, leading to impaired MEK activation.
The role of Pin1 as a regulator of cell growth and survival has been described in cancer cells. This is the first report elucidating the role of Pin1 as a modulator of Akt and MEK signaling during pressure overload in the heart. Our data show that Pin1 operates within a restricted range, as both over-expression and down-regulation of Pin1 attenuate cardiac hypertrophy. Our findings suggest that Pin1 represents a novel target for therapeutic intervention during pathological cardiac hypertrophy.
Acknowledgments
We thank the Sussman lab members for their critical review of the manuscript. We thank Dr. Roger J. Hajjar, Mt Sinai School of Medicine, for the generous gift of pTRUF and Dr. Giannino Del Sal, Laboratorio Nazionale CIB, for the generous gift of human Pin1 cDNA.
Sources of Funding: This study was supported by the National Institute of Health to M.A.S. (R21HL102714, R01HL067245, R37HL091102, P01HL085577, RC1HL100891, R21HL102613, R21HL104544, and R01HL105759) and to C.C.G. (RO1 HL75573, RO1 HL104535, RO3 EB011698), American Heart Association (11POST7610164), The Uehara Memorial Foundation and Mochida Memorial Foundation for Medical and Pharmaceutical Research to H.T., Deutsche Forschungsgemeinschaft DFG to M.V. (1659/1-1) and to M.H.K. (3900/1-1), DZHK (German Centre for Cardiovascular Research) and BMBF (German Ministry of Education and Research) to O.J.M., and The Rees-Stealy Research Foundation, the San Diego Chapter of the Achievement Rewards for College Scientists (ARCS) Foundation, the American Heart Association (Predoctoral Fellowship 10PRE3410005) and the Inamori Foundation to S.D..
Nonstandard Abbreviations
- AAV9
Adeno-associated Virus Serotype 9
- AAV-Pin1
AAV9 harboring Pin1 downstream of the MLC2v promoter
- CDKs
cyclin-dependent protein kinases
- CSA
cross sectional area
- FS
fractional shortening
- KO
knockout
- LVDd
left ventricular diameter at diastole
- max dp/dt
maximal developed pressure over time
- min dp/dt
minimal developed pressure over time
- MLC
myosin light chain
- NRCMs
neonatal rat cardiomyocytes
- PE
phenylephrine
- PLA
proximity ligation assay
- PPI
peptidyl-prolyl isomerase
- Pro
proline
- Pwd
posterior wall thickness
- siPin1
siRNA knockdown of Pin1
- TAC
transaortic constriction
- WT
wild type
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- 2.Lu KP, Zhou XZ. The prolyl isomerase pin1: A pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 3.Lu KP, Finn G, Lee TH, Nicholson LK. Prolyl cis-trans isomerization as a molecular timer. Nat Chem Biol. 2007;3:619–629. doi: 10.1038/nchembio.2007.35. [DOI] [PubMed] [Google Scholar]
- 4.Chao SH, Greenleaf AL, Price DH. Juglone, an inhibitor of the peptidyl-prolyl isomerase pin1, also directly blocks transcription. Nucleic Acids Res. 2001;29:767–773. doi: 10.1093/nar/29.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lufei C, Koh TH, Uchida T, Cao X. Pin1 is required for the ser727 phosphorylation-dependent stat3 activity. Oncogene. 2007;26:7656–7664. doi: 10.1038/sj.onc.1210567. [DOI] [PubMed] [Google Scholar]
- 6.Monje P, Hernandez-Losa J, Lyons RJ, Castellone MD, Gutkind JS. Regulation of the transcriptional activity of c-fos by erk. A novel role for the prolyl isomerase pin1. J Biol Chem. 2005;280:35081–35084. doi: 10.1074/jbc.C500353200. [DOI] [PubMed] [Google Scholar]
- 7.Moretto-Zita M, Jin H, Shen Z, Zhao T, Briggs SP, Xu Y. Phosphorylation stabilizes nanog by promoting its interaction with pin1. Proc Natl Acad Sci U S A. 2010;107:13312–13317. doi: 10.1073/pnas.1005847107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG. Prevalent overexpression of prolyl isomerase pin1 in human cancers. Am J Pathol. 2004;164:1727–1737. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu KP, Suizu F, Zhou XZ, Finn G, Lam P, Wulf G. Targeting carcinogenesis: A role for the prolyl isomerase pin1? Mol Carcinog. 2006;45:397–402. doi: 10.1002/mc.20216. [DOI] [PubMed] [Google Scholar]
- 10.Xu GG, Etzkorn FA. Pin1 as an anticancer drug target. Drug News Perspect. 2009;22:399–407. doi: 10.1358/dnp.2009.22.7.1381751. [DOI] [PubMed] [Google Scholar]
- 11.Lu KP. Phosphorylation-dependent prolyl isomerization: A novel cell cycle regulatory mechanism. Prog Cell Cycle Res. 2000;4:83–96. doi: 10.1007/978-1-4615-4253-7_8. [DOI] [PubMed] [Google Scholar]
- 12.Lv L, Zhou Z, Huang X, Zhao Y, Zhang L, Shi Y, Sun M, Zhang J. Inhibition of peptidyl-prolyl cis/trans isomerase pin1 induces cell cycle arrest and apoptosis in vascular smooth muscle cells. Apoptosis. 2010;15:41–54. doi: 10.1007/s10495-009-0409-8. [DOI] [PubMed] [Google Scholar]
- 13.Liao Y, Wei Y, Zhou X, Yang JY, Dai C, Chen YJ, Agarwal NK, Sarbassov D, Shi D, Yu D, Hung MC. Peptidyl-prolyl cis/trans isomerase pin1 is critical for the regulation of pkb/akt stability and activation phosphorylation. Oncogene. 2009;28:2436–2445. doi: 10.1038/onc.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Hung MC. Physiological regulation of akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Shen ZJ, Esnault S, Schinzel A, Borner C, Malter JS. The peptidyl-prolyl isomerase pin1 facilitates cytokine-induced survival of eosinophils by suppressing bax activation. Nat Immunol. 2009;10:257–265. doi: 10.1038/ni.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MR, Choi HS, Heo TH, Hwang SW, Kang KW. Induction of vascular endothelial growth factor by peptidyl-prolyl isomerase pin1 in breast cancer cells. Biochem Biophys Res Commun. 2008;369:547–553. doi: 10.1016/j.bbrc.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 17.Atchison FW, Capel B, Means AR. Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development. 2003;130:3579–3586. doi: 10.1242/dev.00584. [DOI] [PubMed] [Google Scholar]
- 18.Namgoong GM, Khanal P, Cho HG, Lim SC, Oh YK, Kang BS, Shim JH, Yoo JC, Choi HS. The prolyl isomerase pin1 induces lc-3 expression and mediates tamoxifen resistance in breast cancer. J Biol Chem. 2010;285:23829–23841. doi: 10.1074/jbc.M109.092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rustighi A, Tiberi L, Soldano A, Napoli M, Nuciforo P, Rosato A, Kaplan F, Capobianco A, Pece S, Di Fiore PP, Del Sal G. The prolyl-isomerase pin1 is a notch1 target that enhances notch1 activation in cancer. Nat Cell Biol. 2009;11:133–142. doi: 10.1038/ncb1822. [DOI] [PubMed] [Google Scholar]
- 20.Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, Uchida T, Bronson R, Bing G, Li X, Hunter T, Lu KP. Role of the prolyl isomerase pin1 in protecting against age-dependent neurodegeneration. Nature. 2003;424:556–561. doi: 10.1038/nature01832. [DOI] [PubMed] [Google Scholar]
- 21.Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, Xia W, Nicholson LK, Lu KP. The prolyl isomerase pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature. 2006;440:528–534. doi: 10.1038/nature04543. [DOI] [PubMed] [Google Scholar]
- 22.Yeh ES, Means AR. Pin1, the cell cycle and cancer. Nat Rev Cancer. 2007;7:381–388. doi: 10.1038/nrc2107. [DOI] [PubMed] [Google Scholar]
- 23.Behrens MI, Lendon C, Roe CM. A common biological mechanism in cancer and alzheimer's disease? Curr Alzheimer Res. 2009;6:196–204. doi: 10.2174/156720509788486608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashemzadeh-Bonehi L, Phillips RG, Cairns NJ, Mosaheb S, Thorpe JR. Pin1 protein associates with neuronal lipofuscin: Potential consequences in age-related neurodegeneration. Exp Neurol. 2006;199:328–338. doi: 10.1016/j.expneurol.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Lee TH, Tun-Kyi A, Shi R, Lim J, Soohoo C, Finn G, Balastik M, Pastorino L, Wulf G, Zhou XZ, Lu KP. Essential role of pin1 in the regulation of trf1 stability and telomere maintenance. Nat Cell Biol. 2009;11:97–105. doi: 10.1038/ncb1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: Targeting raf/mek/erk1/2-signaling. Int J Biochem Cell Biol. 2009;41:2351–2355. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, Quijada P, Mason M, Konstandin MH, Malhowski A, Cheng Z, Khan M, McGregor M. Myocardial akt: The omnipresent nexus. Physiol Rev. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quijada P, Toko H, Fischer KM, Bailey B, Reilly P, Hunt KD, Gude NA, Avitabile D, Sussman MA. Preservation of myocardial structure is enhanced by pim-1 engineering of bone marrow cells. Circ Res. 2012;111:77–86. doi: 10.1161/CIRCRESAHA.112.265207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 30.Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, Sussman MA. Nuclear targeting of akt antagonizes aspects of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avitabile D, Bailey B, Cottage CT, Sundararaman B, Joyo A, McGregor M, Gude N, Truffa S, Zarrabi A, Konstandin M, Khan M, Mohsin S, Volkers M, Toko H, Mason M, Cheng Z, Din S, Alvarez R, Jr, Fischer K, Sussman MA. Nucleolar stress is an early response to myocardial damage involving nucleolar proteins nucleostemin and nucleophosmin. Proc Natl Acad Sci U S A. 2011;108:6145–6150. doi: 10.1073/pnas.1017935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Wang S, Zhu T, Zhou J, Xu Q, Lu Y, Ma D. Pin1 contributes to cervical tumorigenesis by regulating cyclin d1 expression. Oncol Rep. 2006;16:491–496. [PubMed] [Google Scholar]
- 33.Khanal P, Namgoong GM, Kang BS, Woo ER, Choi HS. The prolyl isomerase pin1 enhances her-2 expression and cellular transformation via its interaction with mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1. Mol Cancer Ther. 2010;9:606–616. doi: 10.1158/1535-7163.MCT-09-0560. [DOI] [PubMed] [Google Scholar]
- 34.Lee TH, Chen CH, Suizu F, Huang P, Schiene-Fischer C, Daum S, Zhang YJ, Goate A, Chen RH, Zhou XZ, Lu KP. Death-associated protein kinase 1 phosphorylates pin1 and inhibits its prolyl isomerase activity and cellular function. Mol Cell. 2011;42:147–159. doi: 10.1016/j.molcel.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimori F, Takahashi K, Uchida C, Uchida T. Mice lacking pin1 develop normally, but are defective in entering cell cycle from g(0) arrest. Biochem Biophys Res Commun. 1999;265:658–663. doi: 10.1006/bbrc.1999.1736. [DOI] [PubMed] [Google Scholar]
- 36.Liou YC, Ryo A, Huang HK, Lu PJ, Bronson R, Fujimori F, Uchida T, Hunter T, Lu KP. Loss of pin1 function in the mouse causes phenotypes resembling cyclin d1-null phenotypes. Proc Natl Acad Sci U S A. 2002;99:1335–1340. doi: 10.1073/pnas.032404099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller OJ, Leuchs B, Pleger ST, Grimm D, Franz WM, Katus HA, Kleinschmidt JA. Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors. Cardiovasc Res. 2006;70:70–78. doi: 10.1016/j.cardiores.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Dhillon AS, von Kriegsheim A, Grindlay J, Kolch W. Phosphatase and feedback regulation of raf-1 signaling. Cell Cycle. 2007;6:3–7. doi: 10.4161/cc.6.1.3593. [DOI] [PubMed] [Google Scholar]
- 39.You H, Zheng H, Murray SA, Yu Q, Uchida T, Fan D, Xiao ZX. Igf-1 induces pin1 expression in promoting cell cycle s-phase entry. J Cell Biochem. 2002;84:211–216. doi: 10.1002/jcb.10037. [DOI] [PubMed] [Google Scholar]
- 40.McMullen JR, Izumo S. Role of the insulin-like growth factor 1 (igf1)/phosphoinositide-3-kinase (pi3k) pathway mediating physiological cardiac hypertrophy. Novartis Found Symp. 2006;274:90–111. discussion 111-117, 152-115, 272-116. [PubMed] [Google Scholar]
- 41.Schluter KD, Wenzel S. Angiotensin ii: A hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol Ther. 2008;119:311–325. doi: 10.1016/j.pharmthera.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 43.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, Tafuri A, Bonati A, Basecke J, Cocco L, Evangelisti C, Martelli AM, Montalto G, Cervello M, McCubrey JA. Roles of the raf/mek/erk and pi3k/pten/akt/mtor pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 46.Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrads TP, Veenstra TD, Lu KP, Morrison DK. Regulation of raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 47.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac erk1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.