Abstract
The effector function of CD8 T cells is mediated via cell-mediated cytotoxicity and production of cytokines like gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). While the roles of perforin-dependent cytotoxicity, IFN-γ, and TNF-α in controlling acute viral infections are well studied, their relative importance in defense against chronic viral infections is not well understood. Using mice deficient for TNF receptor (TNFR) I and/or II, we show that TNF-TNFR interactions have a dual role in mediating viral clearance and downregulating CD8 and CD4 T-cell responses during a chronic lymphocytic choriomeningitis virus (LCMV) infection. While wild-type (+/+) and TNFR II-deficient (p75−/−) mice cleared LCMV from the liver and lung, mice deficient in TNFR I (p55−/−) or both TNFR I and TNFR II (double knockout [DKO]) exhibited impaired viral clearance. The inability of p55−/− and DKO mice to clear LCMV was not a sequel to either suboptimal activation of virus-specific CD8 or CD4 T cells or impairment in trafficking of LCMV-specific CD8 T cells to the liver and lung. In fact, the expansion of LCMV-specific CD8 and CD4 T cells was significantly higher in DKO mice compared to that in +/+, p55−/−, and p75−/− mice. TNFR deficiency did not preclude the physical deletion of CD8 T cells specific for nucleoprotein 396 to 404 but delayed the contraction of CD8 T-cell responses to the epitopes GP33-41 and GP276-285 in the viral glycoprotein. The antibody response to LCMV was not significantly altered by TNFR deficiency. Taken together, these findings have implications in development of immunotherapy in chronic viral infections of humans.
It is well established that CD8 T cells play an important role in defense against viral infections, including human immunodeficiency virus, hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus, Epstein-Barr virus, lymphocytic choriomeningitis virus (LCMV), and influenza virus (4, 6, 8, 9, 20, 26, 29, 44, 54, 56). CD8 T cells recognize and respond to foreign peptides presented by self major histocompatibility complex class I (MHC-I) molecules. The antiviral effects of CD8 T cells are mediated by MHC-I-restricted lysis of infected cells and/or by production of cytokines like gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (25). The lysis of virus-infected cells by CD8 T cells occurs by perforin- or Fas-dependent pathways. Studies in perforin-deficient mice have demonstrated that perforin-dependent cytotoxicity plays a role in resolving infections with LCMV, influenza virus, Theiler's virus, and herpes simplex virus (27, 29, 51, 54, 56). However, noncytocidal effector mechanisms of CD8 T cells mediated by cytokines like IFN-γ and/or TNF-α can contribute to clearance of viruses, including LCMV, adenovirus, mouse hepatitis virus, and coxsackievirus (7, 14, 19, 35, 45, 48, 60). Further, elegant studies using HBV transgenic mice have clearly ascribed a role for cytokine-mediated noncytolytic effector mechanisms in viral control (15, 18, 21-23). Infection of mice with LCMV has been extensively used as a model system to decipher the mechanisms underlying the CD8 T-cell-mediated clearance of a noncytopathic virus. LCMV can cause an acute or a chronic infection in mice, depending upon the viral strain used; while an acute LCMV infection is cleared in 8 to 10 days, a chronic infection lasts up to several months (1, 2, 59). Clearance of an acute LCMV infection is dependent upon CD8 T-cell-mediated perforin-dependent cytotoxicity and does not require TNF activity (29, 32, 56). IFN-γ or IFN-γ receptor deficiency causes a slight delay in the control of an acute LCMV infection (28, 35, 37, 39, 45). Unlike in an acute infection, the effector mechanisms of LCMV clearance in a chronic infection are not well understood. It has been previously shown that local induction of TNF-α can lead to clearance of LCMV from hepatocytes in persistently infected mice (19). However, it is still unclear if TNF-TNF receptor (TNFR) interactions are required to resolve a chronic LCMV infection. Since TNF-α exerts its cellular effects via two receptors, TNFR I (p55R) and TNFR II (p75R), the role of TNFR I versus TNFR II in mediating the antiviral effects of TNF-α needs investigation.
In recent years, it has become increasingly clear that effector molecules of CD8 T cells, namely, perforin, Fas ligand, IFN-γ, and TNF-α, have important immunoregulatory functions (24). These molecules possess the dual capacity of mediating T-cell effector function and dampening T-cell responses in vivo (24). Establishment of a chronic LCMV infection is associated with deletion (exhaustion) and/or functional impairment of CD8 T cells, and impaired clearance of HBV and HCV in humans has been linked to loss of virus-specific CD8 T cells (30, 33, 34, 40, 59). Therefore, augmentation of CD8 T-cell responses is being considered as a therapeutic strategy to treat chronic viral infections in humans. To this end, there is considerable interest towards understanding mechanisms that downregulate the CD8 T-cell response during a chronic viral infection. Previous studies have indicated that perforin, Fas, and IFN-γ may suppress CD8 T-cell responses by affecting expansion or deletion of virus-specific CD8 T cells in a persistent LCMV infection (43, 62). Since TNF-α can induce apoptosis of activated CD8 T cells in vitro, efforts have been made to understand the role of TNF-α in downregulating CD8 T-cell responses in a chronic LCMV infection. During a persistent LCMV infection, the exhaustion of virus-specific CD8 T cells is delayed in TNFR I-deficient mice (62). Notably, it is not TNFR I but TNFR II, the second receptor for TNF-α, which has been shown to cause apoptosis of activated CD8 T cells in vitro (3, 61). However, the role of TNFR II in downregulation of the CD8 T-cell response during a chronic LCMV infection has not been examined. Moreover, the existence of functional redundancies between TNFR I and TNFR II in regulating CD8 T-cell responses has not been examined using TNFR I and II double-deficient mice. To dissect the antiviral and immunoregulatory effects of TNF-α, in this study we have compared the kinetics of viral clearance and virus-specific CD8 and CD4 T-cell responses between wild type (+/+), TNFR I-deficient (p55−/−), TNFR II-deficient (p75−/−), and TNFR I and II double-deficient (double knockout [DKO]) mice during a chronic LCMV infection. These studies showed that TNFR I and TNFR II might have redundant roles in mediating LCMV clearance and downregulating CD8 and CD4 T-cell responses during a chronic infection.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine). p55−/−, p75−/−, and DKO mice on the C57BL/6 background (49) were kindly provided by Jacques Peschon (Immunex Corporation, Seattle, Wash.). Mice were used when they were 6 to 8 weeks of age. All experiments were conducted in strict accordance with the guidelines of the institutional animal care committee.
Virus.
The Armstrong (LCMV-Arm) and clone 13 (LCMV-clone 13) strains of LCMV were used in this study (1, 2). Mice were infected with 2 × 106 PFU of LCMV-clone 13 by intravenous injection or 2 × 105 PFU of LCMV-Arm by intraperitoneal injection. Infectious LCMV in the tissues was quantitated by a plaque assay using Vero cell monolayers (1, 2).
Cytotoxicity assay.
The MHC-I-restricted cytotoxic activity in the spleen was measured directly ex vivo using a 51Cr-release assay as described elsewhere (2).
Identification of LCMV-specific CD8 T cells by MHC class I tetramers.
The preparation and use of MHC-I tetramers specific to the Db-restricted immunodominant epitopes nucleoprotein 396-404 (NP396) and glycoprotein 33-41 (GP33) have been described previously (46). Single-cell suspensions of splenocytes were prepared by standard procedures. Mononuclear cells were isolated from the liver and lung as described elsewhere (41). Single-cell suspensions of splenocytes or mononuclear cells from the liver or lung were stained with phycoerythrin-labeled anti-CD8, fluorescein isothiocyanate-labeled anti-CD44, and allophycocyanin-labeled MHC-I tetramers in fluorescence-activated cell sorting buffer (phosphate-buffered saline containing 2% bovine serum albumin and 0.02% NaN3) for 1 h at 4°C. Following staining, cells were fixed in 2% paraformaldehyde and acquired on a FACSCalibur flow cytometer (BD Biosciences, San Jose, Calif.). Flow cytometry data were analyzed using CellQuest software (BD Biosciences). All antibodies were purchased from BD-Pharmingen (San Diego, Calif.).
Intracellular cytokine staining.
CD8 T cells specific to LCMV cytotoxic T-lymphocyte (CTL) epitopes NP396, GP33, and GP276-286 (GP276) were quantitated by intracellular staining for IFN-γ as described previously (46). Briefly, splenocytes were stimulated with various LCMV CTL epitope peptides in vitro in the presence of brefeldin A and human recombinant interleukin-2 for 5 to 6 h at 37°C. To measure CD4 T-cell responses, splenocytes were stimulated with an MHC-II-restricted epitope peptide, GP61-80. Following culture, cells were stained for cell surface CD8 or CD4 and intracellular IFN-γ using the Cytofix/Cytoperm kit (Pharmingen). The number of IFN-γ-producing CD8 and CD4 T cells was determined by flow cytometry as described above.
ELISA.
LCMV-specific antibody in the serum was quantitated by a solid-phase enzyme-linked immunosorbent assay (ELISA) as described previously (2).
Statistical analysis.
Data were analyzed using commercially available statistical software (SYSTAT version 8.0; Chicago, Ill.). Groups were compared by Student's t test, and significance was defined at a P level of ≤0.05.
RESULTS
Effect of TNFR deficiency on clearance of a chronic LCMV infection.
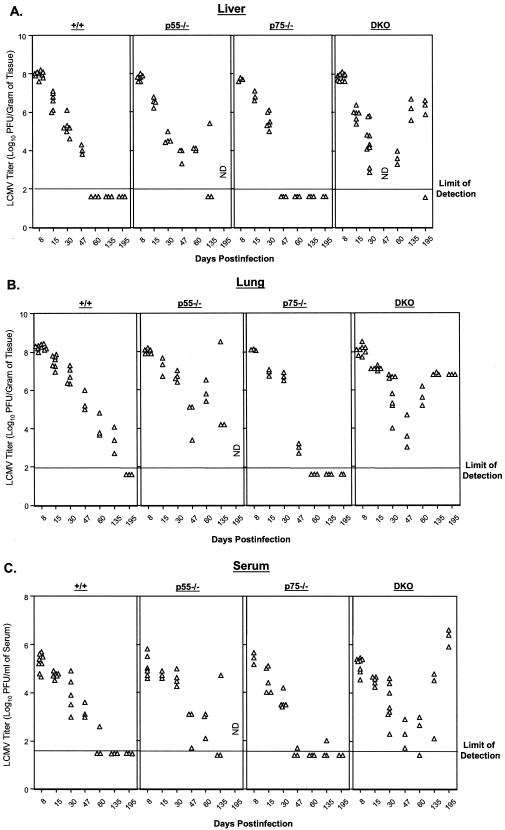
Previous studies have shown that exposure of adult immunocompetent mice to LCMV-clone 13 results in a disseminated infection lasting between 3 and 6 months (2, 59). Although it has been documented that CD8 and CD4 T cells are essential to resolve an LCMV-clone 13 infection, the underlying effector mechanisms of viral clearance are unclear (42). To examine the requirement for TNF-TNFR interactions in resolving a chronic LCMV infection, groups of +/+, p55−/−, p75−/−, and DKO mice were infected with LCMV-clone 13, and the kinetics of viral clearance was monitored in the liver, lung, and serum (Fig. 1). As shown in Fig. 1A, in the livers of +/+ mice, peak viral load was attained on day 8 postinfection (p.i.), but viral titers gradually declined in the ensuing 40 days. By day 60 p.i., +/+ mice had successfully resolved the viral infection in the liver and the levels of infectious LCMV-clone 13 were below the level of detection. The livers of p75−/− mice had viral titers comparable to those of +/+ mice on day 8 p.i. but seemed to clear LCMV-clone 13 a little earlier than in +/+ mice. Compared to +/+ and p75−/− mice, the hepatic clearance of LCMV-clone 13 was delayed in p55−/− mice. However, 66% of the p55−/− mice cleared LCMV-clone 13 in the liver by 135 days p.i. Strikingly, DKO mice initially exhibited some control of viral load, but the livers of six of seven DKO mice continued to harbor a high viral burden until at least 195 days p.i. It is likely that these DKO mice might fail to clear LCMV-clone 13 and become carriers for life. Taken together, these data suggested that TNFRs p55 and p75 might play redundant roles in mediating LCMV-clone 13 clearance from the liver. However, TNFR I (p55R) appeared to be more important than TNFR II (p75R) in mediating hepatic clearance of LCMV-clone 13.
FIG. 1.
Role of TNFRs in viral clearance during a chronic viral infection. Groups of +/+, p55−/−, p75−/−, and DKO mice were infected with LCMV-clone 13, and the levels of infectious LCMV in the liver (A), lung (B), and serum (C) were determined by a plaque assay using Vero cell monolayers. Each data point represents the viral titer of an individual mouse. ND, not available.
Data in Fig. 1B show the kinetics of LCMV-clone 13 clearance from the lung. The viral titer in the lung of +/+ mice was highest on day 8 p.i. but exhibited a progressive decline thereafter. By 195 days p.i., infectious LCMV-clone 13 was undetectable in the lungs of +/+ mice. Upon comparing Fig. 1A and B, it is clear that clearance of LCMV-clone 13 from the liver was faster than clearance from the lung in the +/+ mice. Loss of p75R had a minimal impact on the kinetics of LCMV clearance from the lung. Akin to the liver, the clearance of LCMV-clone 13 from the lungs of p75−/− mice was slightly accelerated compared to that in +/+ mice. The resolution of LCMV-clone 13 from the lung occurred less efficiently in p55−/− mice; on day 60 p.i., the viral load in the lung of p55−/− mice (5.9 ± 0.6 log10 PFU) was ∼100-fold higher than that in +/+ mice (4.0 ± 0.6 log10 PFU). In striking contrast to +/+ mice, DKO mice showed no viral control and harbored high levels of infectious LCMV at least until day 195 p.i. On day 135 p.i., the viral titers in the lungs of DKO mice (6.8 ± 0.05 log10 PFU) were >1,000-fold higher than those in +/+ mice (3.4 ± 0.6 log10 PFU). In summary, these data provide strong evidence supporting a role for TNFRs in clearance of LCMV-clone 13 from the lung.
As an overall indicator of virus control, we also quantitated viral load in the serum of LCMV-clone 13-infected +/+ and TNFR-deficient mice (Fig. 1C). Consistent with viral clearance from the liver and lung (Fig. 1A and B), both +/+ and p75−/− mice exhibited efficient control of viremia by day 60 p.i. The kinetics of LCMV-clone 13 clearance in the serum of p55−/− mice was delayed, compared to that in +/+ mice. DKO mice were able to achieve transient control of viremia until day 60 p.i., but circulating virus levels rebounded to high levels thereafter (Fig. 1C).
Effect of TNFR deficiency on primary virus-specific T-cell responses.
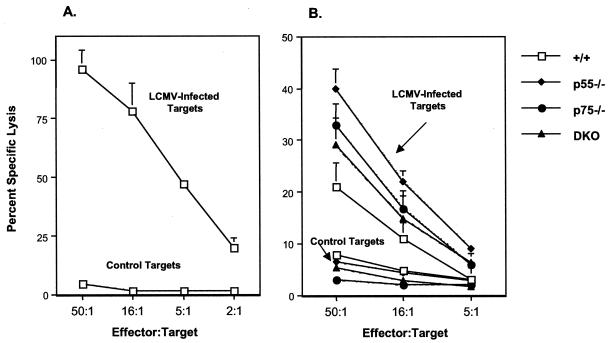
It is well established that clearance of LCMV is dependent upon CD8 T-cell responses (29, 42, 56). Data in Fig. 1 showed that DKO mice which are deficient in both p55 and p75 TNFRs are unable to control LCMV-clone 13 infection in the liver and lung. The inability of DKO mice to clear LCMV-clone 13 might be due to the elicitation of a suboptimal antiviral T-cell response. To address this issue, we examined primary virus-specific CD8 T-cell responses in +/+, p55−/−, p75−/−, and DKO mice infected with LCMV-clone 13. For comparison, we also infected a group of +/+ mice with LCMV-Arm (cleared by day 8 p.i.), which elicits a strong CTL response. On day 8 p.i., we quantitated LCMV-specific MHC- I-restricted cytotoxic activity in the spleen directly ex vivo. As shown in Fig. 2A, as expected, spleens of +/+ mice infected with LCMV-Arm contained high levels of CTL activity. In comparison to LCMV-Arm-infected mice, splenocytes from LCMV-clone 13-infected +/+, p55−/−, p75−/−, and DKO mice showed poor CTL activity (Fig. 2B). However, it is worth noting that splenocytes from p55−/−, p75−/−, and DKO mice exhibited slightly higher CTL activities than those in LCMV-clone 13-infected +/+ mice. These data show that TNFR deficiency had no significant effect on the CTL activity in LCMV-clone 13-infected mice.
FIG. 2.
MHC-I-restricted cytotoxicity in the spleens of LCMV-clone 13-infected mice. (A) Eight days after infection with LCMV-Arm, the LCMV-specific MHC-I-restricted cytotoxic activity in the spleens of +/+ mice was quantitated by a 51Cr-release assay using syngeneic LCMV-infected and uninfected MC57G cells as target cells. (B) Groups of +/+ and TNFR-deficient mice were infected with LCMV-clone 13, and cytotoxic activity in the spleen was quantitated 8 days later as described for panel A. Data are the mean of three to six mice per group, ± the standard deviation.
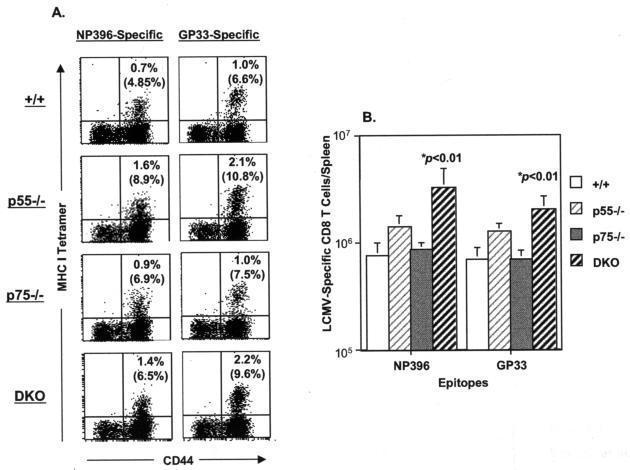
We next investigated the effect of TNFR deficiency on the activation and expansion of LCMV-specific CD8 T cells in the spleen. Fluorochrome-labeled peptide-MHC-I tetramers were used to visualize CD8 T cells specific to the two immunodominant epitopes of LCMV, namely NP396 and GP33. As illustrated in Fig. 3A, on day 8 p.i., virus-specific CD8 T cells were readily detected in the spleens of +/+ mice. The percentages of LCMV-specific CD8 T cells in the spleens of p75−/− mice were comparable to those in +/+ mice. However, spleens of p55−/− and DKO mice contained higher percentages of LCMV-specific CD8 T cells than those of +/+ mice. The absolute numbers of LCMV-specific CD8 T cells in the spleen on day 8 p.i. are depicted in Fig. 3B. Deficiency of p75R had no detectable effect on the expansion of LCMV-specific CD8 T cells in vivo. In the p55−/− mice, although percentages of LCMV-specific CD8 T cells in the spleens were substantially higher than in +/+ mice (Fig. 3A), the absolute numbers (Fig. 3B) were determined to be not statistically significant (P > 0.05). Strikingly, the spleens of DKO mice contained substantially higher numbers of NP396- and GP33-specific CD8 T cells, compared to levels in +/+ and p75−/− mice (P < 0.01). The total number of NP396- and GP33-specific CD8 T cells in the spleens of DKO mice was approximately five- and threefold more, respectively, than in +/+ and p75−/− mice (Fig. 3B). Taken together, these data suggested the following: (i) TNFRs might play a role in downregulating the expansion of virus-specific CD8 T cells during a chronic LCMV infection; and (ii) the inability of DKO mice to effectively clear LCMV-clone 13 was not due to a defect in the activation and expansion of virus-specific CD8 T cells during the primary response.
FIG. 3.
Effect of TNFR deficiency on the expansion of virus-specific CD8 T cells during a chronic LCMV infection. Groups of +/+, p55−/−, p75−/−, and DKO mice were infected with LCMV-clone 13. On the eighth day after infection, splenocytes were stained with anti-CD8, anti-CD44, and MHC-I tetramers (Db) loaded with the CTL epitope peptides NP396 or GP33 and analyzed by flow cytometry. (A) Dot plots gated on total CD8 T cells; the numbers represent percentages of epitope-specific CD8 T cells among splenocytes, and numbers in parentheses are percentages of epitope-specific CD8 T cells per total CD8 T cells in the spleen. (B) Total number of LCMV-specific CD8 T cells in the spleen; the data are the means of three to nine mice per group ± the standard deviation.
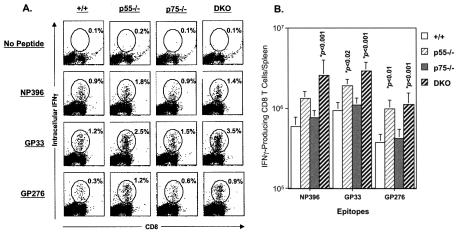
Production of IFN-γ is one of the important effector mechanisms of CD8 T cells (25, 58). Lack of IFN-γ-mediated effector function could delay the clearance of an acute LCMV infection (28). As a measure of functional competence, we examined the ability of LCMV-specific CD8 T cells to produce IFN-γ in response to stimulation with various LCMV CTL epitope peptides. On day 8 p.i., CD8 T cells in the spleens of +/+ mice were fully functional and produced readily detectable levels of IFN-γ upon in vitro stimulation (Fig. 4A). CD8 T cells from p55−/−, p75−/−, and DKO mice also produced comparable levels of IFN-γ, but the percentages of cytokine-producing CD8 T cells were consistently higher in the spleens of p55−/− and DKO mice (Fig. 4A). These data suggested that TNFR deficiency did not affect the development of effector functions (cytotoxic activity and IFN-γ production) in LCMV-specific CD8 T cells during a chronic LCMV infection. The total number of epitope-specific IFN-γ-producing CD8 T cells in the spleens of +/+ and TNFR-deficient mice is shown in Fig. 4B. The total number of LCMV-specific CD8 T cells in the spleens of p75−/− mice was comparable to that in +/+ mice. There was a general trend that spleens of p55−/− mice contained more LCMV-specific CD8 T cells than spleens of +/+ and p75−/− mice; specifically, the total number of GP33- and GP276-specific CD8 T cells was significantly higher in p55−/− mice than in +/+ and p75−/− mice. Remarkably, however, irrespective of epitope specificity, the spleens of DKO mice contained substantially higher numbers of LCMV-specific CD8 T cells than in +/+, p55−/−, and p75−/− mice. Consistent with tetramer data (Fig. 3), these data clearly show that TNFRs might downregulate the expansion of CD8 T cells during a chronic LCMV infection.
FIG. 4.
Effect of TNFR deficiency on the expansion of virus-specific IFN-γ-producing CD8 cells during a chronic LCMV infection. On the eighth day after infection with LCMV-clone 13, splenocytes from +/+, p55−/−, p75−/−, and DKO mice were stimulated in vitro with the indicated LCMV CTL epitope peptides. Following in vitro stimulation, cells were stained for cell surface CD8 and intracellular IFN-γ. The number of IFN-γ-producing CD8 T cells was determined by flow cytometry. (A) Dot plots gated on total splenocytes; the numbers are the percentages of IFN-γ-producing CD8 T cells per total splenocytes. (B) Total number of epitope-specific IFN-γ-producing CD8 T cells; the data are the means of three to nine mice per group ± the standard deviation.
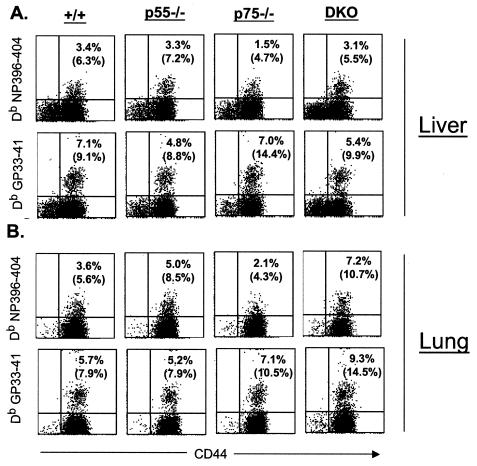
Activation and expansion of virus-specific CD8 T cells in the secondary lymphoid organs are essential but not sufficient to clear the infection from the tissues. Virus-specific effector CD8 T cells need to extravasate into the peripheral tissues to achieve viral clearance. We investigated the effect of TNFR deficiency on the trafficking of virus-specific CD8 T cells into the peripheral tissues following LCMV-clone 13 infection. On day 8 p.i., we quantitated nonlymphoid LCMV-specific CD8 T cells in the lungs and livers of +/+, p55−/−, p75−/−, and DKO mice. As illustrated in Fig. 5, LCMV-specific CD8 T cells were detected in significant numbers in the livers and lungs of +/+ mice. The percentages of LCMV-specific CD8 T cells in the lungs and livers of p55−/−, p75−/−, and DKO mice were comparable to those in +/+ mice (Fig. 5). These data suggested that TNFRs are not essential for trafficking of effector CD8 T cells into the peripheral tissues during a chronic LCMV infection.
FIG. 5.
Virus-specific CD8 T cells in the nonlymphoid organs during a chronic LCMV infection. Eight days after infection with LCMV-clone 13, mononuclear cells were isolated from the liver and lung and stained with anti-CD8, anti-CD44, and MHC-I tetramers (Db) (loaded with CTL epitope peptides NP396 or GP33). The number of tetramer-binding CD8 T cells was quantitated by flow cytometry. The dot plots are gated on total CD8 T cells, and the numbers are the percentages of tetramer-binding CD8 T cells per total splenocytes. The numbers in parentheses are percentages of tetramer-binding CD8 T cells per total CD8 T cells.
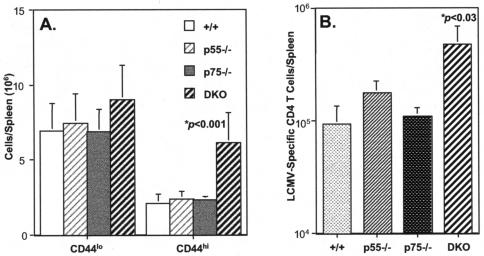
As mentioned before, clearance of a chronic LCMV infection is dependent upon the functions of both CD8 and CD4 T cells (42). We examined whether impaired clearance of LCMV-clone 13 in DKO mice was related to ineffective induction of CD4 T-cell responses. Activation of T cells is associated with the upregulation of the cell surface expression of CD44; while naive T cells are CD44lo, activated or memory T cells are CD44hi. First, we assessed activation of CD4 T cells by comparing the number of naive and activated or memory CD4 T cells between +/+, p55−/−, p75−/−, and DKO mice on day 8 following LCMV-clone 13 infection. As shown in Fig. 6A, the number of naive (CD44lo) CD4 T cells was comparable between +/+, p55−/−, p75−/−, and DKO mice. However, strikingly, the number of activated or memory (CD44hi) CD4 T cells was significantly higher in the spleens of DKO mice, compared to that in +/+, p55−/−, and p75−/− mice. We also quantitated the number of LCMV-specific CD4 T cells in +/+ and TNFR-deficient mice by intracellular staining for IFN-γ. Figure 6B shows the total number of IFN-γ-producing LCMV-specific CD4 T cells in the spleens of LCMV-clone 13-infected +/+, p55−/−, p75−/−, and DKO mice (day 8 p.i.). Notably, LCMV-clone 13-infected DKO mice exhibited significantly higher expansion of LCMV-specific CD4 T cells than in +/+, p55−/−, and p75−/− mice. Loss of either p55 or p75 alone had a minimal impact on the activation and expansion of CD4 T cells during a chronic LCMV infection. These data suggested that TNFRs p55 and p75 might play redundant roles in downregulating activation of CD4 T cells in LCMV-clone 13-infected mice. In summary, data presented in Fig. 3 to 6 strongly suggest that TNFRs might play an important role in downregulating virus-specific CD8 and CD4 T-cell responses during a chronic LCMV infection.
FIG. 6.
Effect of TNFR deficiency on the expansion of CD4 T cells during a chronic LCMV infection. Groups of +/+, p55−/−, p75−/−, and DKO mice were infected with LCMV-clone 13, and CD4 T-cell responses were examined 8 days later. (A) On the eighth day after infection, splenocytes were stained with anti-CD4 and anti-CD44 antibodies and the numbers of naive (CD44lo) and activated (CD44hi) CD4 T cells were determined by flow cytometry. (B) Eight days after infection, splenocytes were stimulated in vitro with MHC-II-restricted epitope peptide GP61-80, and the number of IFN-γ-producing CD4 T cells was determined by intracellular staining and flow cytometry. Data are the means of three to nine mice per group ± the standard deviation.
Contraction and maintenance of T-cell responses in TNFR-deficient mice.
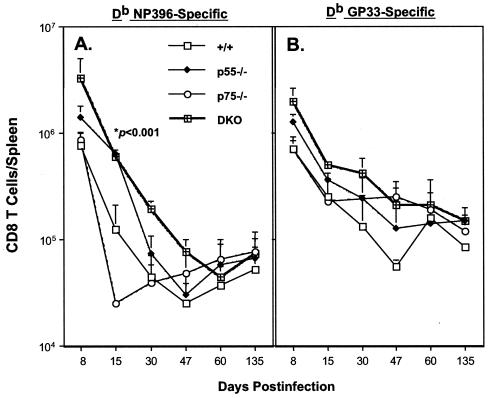
Next, we performed a series of experiments to determine the kinetics of LCMV-specific CD8 T-cell responses in +/+ and TNFR-deficient mice. Following infection with LCMV-clone 13, virus-specific CD8 T cells in the spleen were enumerated by flow cytometry using MHC-I tetramers. As shown in Fig. 7, ensuing day 8 p.i., the CD8 T-cell response underwent a contraction phase in all groups of mice. The kinetics of the NP396-specific CD8 T-cell response is shown in Fig. 7A. In the +/+ mice, between days 8 and 15 p.i., there was a sixfold reduction in the number of NP396-specific CD8 T cells (Fig. 7A). In striking contrast, p55−/− mice showed only a twofold drop in the number of NP396-specific CD8 T cells between days 8 and 15 p.i. Interestingly, in the p75−/− mice the contraction phase of the NP396-specific CD8 T-cell response seemed to be slightly accelerated, compared to the response in +/+ mice. There was a fivefold drop in NP396-specific CD8 T cells in DKO mice between days 8 and 15 p.i. By day 30 p.i., >95% of NP396-specific CD8 T cells were deleted in all groups of mice, and by day 47 p.i. NP396-specific CD8 T cells were barely detectable in a large proportion of LCMV-clone 13-infected mice. However, it is worth noting that (i) on day 15 p.i., spleens of p55−/− contained significantly higher numbers of NP396-specific CD8 T cells than those in +/+ and p75−/− mice; (ii) on both days 15 and 30 p.i., the total number of NP396-specific CD8 T cells was substantially higher in DKO mice than in +/+ and p75−/− mice (Fig. 7A). Data in Fig. 7B illustrate the kinetics of CD8 T-cell responses to the epitope GP33 in +/+ and TNFR-deficient mice. Compared to +/+ mice, DKO mice exhibited a slight delay in the contraction of GP33-specific CD8 T cells following LCMV-clone 13 infection. While in the +/+ mice there was a ∼10-fold drop in the number of GP33-specific CD8 T cells between days 8 and 30 p.i., in the DKO mice there was only a 5-fold reduction. However, by day 60 p.i., the total number of GP33-specific CD8 T cells in p55−/−, p75−/−, DKO, and +/+ mice had reached comparable levels and were maintained for at least until day 135 p.i.
FIG. 7.
MHC-I tetramers were used to study the kinetics of LCMV-specific CD8 T-cell responses in chronically infected TNFR-deficient mice. Groups of +/+, p55−/−, p75−/−, and DKO mice were infected with LCMV-clone 13, and the numbers of CD8 T cells specific to the two immunodominant epitopes NP396 (A) and GP33 (B) in the spleen were determined by staining with MHC-I tetramers. The data are the means of at least three mice per group at each time point ± the standard deviation.
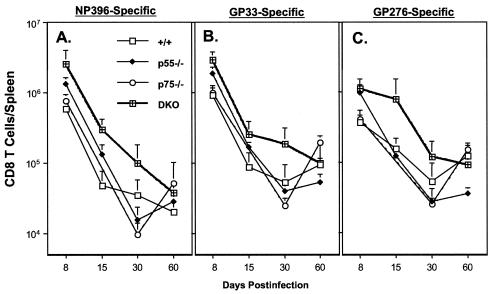
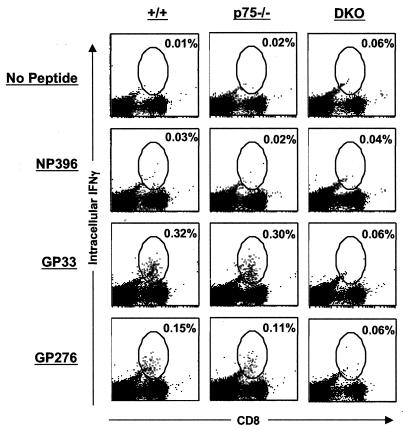
We also compared the kinetics of “functional” LCMV-specific CD8 T cells between +/+ and TNFR-deficient mice by intracellular staining for IFN-γ (Fig. 8). The effect of TNFR deficiency on the kinetics of the anti-LCMV CD8 T-cell response seemed to be epitope dependent (Fig. 8). As shown in Fig. 8A, the kinetics of the NP396-specific CD8 T-cell response were largely comparable between +/+ and TNFR-deficient mice. However, the spleens of DKO mice contained ∼6- and 3-fold more IFN-γ-producing NP396-specific CD8 T cells on days 15 and 30 p.i., respectively, than those of +/+ mice. Data in Fig. 8B show the progressive drop in the number of IFN-γ-producing GP33-specific CD8 T cells between days 8 and 30 p.i. in all groups of mice. The kinetics of the GP33-specific response were comparable between +/+, p55−/−, and p75−/− mice. However, on day 30 p.i., there were significantly more IFN-γ-producing GP33-specific CD8 T cells in DKO mice than in +/+ mice. Figure 8C illustrates the kinetics of the CD8 T-cell response to the GP276 epitope in +/+ and TNFR-deficient mice. The kinetics of the GP276-specific CD8 T-cell response were comparable between +/+, p55−/−, and p75−/− mice. Quite remarkably, DKO mice exhibited a delay in the contraction in the number of IFN-γ-producing GP276-specific CD8 T cells, compared to that in +/+ mice; between days 8 and 15 p.i., DKO mice showed minimal reduction in the number of GP276-specific IFN-γ-producing CD8 T cells, compared to responses in +/+, p55−/−, and p75−/− mice. Notably, between days 30 and 60 p.i., there was an increase in the number of IFN-γ-producing GP33- and GP276-specific CD8 T cells only in +/+ and p75−/− mice (Fig. 8B and C). Several studies have documented that high LCMV load in the infected host is associated with functional unresponsiveness of virus-specific CD8 T cells (inability to produce cytokines upon ex vivo stimulation) (10, 13, 16, 17, 36, 38, 55, 57). However, with decreasing viral burden, these dysfunctional CD8 T cells gradually regain their ability to produce cytokines. The observed increase in the numbers of GP33- and GP276-specific CD8 T cells between days 30 and 60 p.i. likely reflect reacquisition of a cytokine-producing ability associated with decreasing viral load in spleens and other organs of +/+ and p75−/− mice (Table 1 and Fig. 1). In contrast to +/+ and p75−/− mice, p55−/− and DKO mice continued to harbor high levels of LCMV in their tissues after 60 days p.i. (Fig. 1). On day 195 p.i., we examined the long-term effect of virus persistence on the functional responsiveness of LCMV-specific CD8 T cells in DKO mice by intracellular staining for IFN-γ. As shown in Fig. 9, on day 195 p.i., consistent with viral clearance (Fig. 1), IFN-γ-producing GP33- and GP276-specific CD8 T cells were readily detected in +/+ and p75−/− mice. In striking contrast, CD8 T cells from DKO mice were functionally unresponsive and failed to produce detectable levels of IFN-γ upon antigenic stimulation in vitro. Our inability to detect IFN-γ-producing cells in response to stimulation with GP33 peptide in DKO mice was not due to clonal deletion of GP33-specific CD8 T cells. This is because GP33-specific CD8 T cells were present in these DKO mice, as detected by MHC-I tetramers (Fig. 7). However, in the case of NP396-specific CD8 T cells, lack of IFN-γ production in all groups of mice was due to physical deletion and not due to functional unresponsiveness. In summary, at least until day 60 p.i., a time point by which +/+ mice had cleared LCMV to a large extent, DKO mice had comparable or higher numbers of IFN-γ-producing (functional) CD8 T cells. Taken together these data suggested that impaired LCMV clearance in DKO mice might not be due to defective CD8 T-cell activation but are likely due to lack of TNF-dependent antiviral effects.
FIG. 8.
Kinetics of IFN-γ-producing CD8 T cells in TNFR-deficient mice during a chronic LCMV infection. On the indicated days after infection with LCMV-clone 13, splenocytes were stimulated with the indicated CTL epitope peptide (NP396, GP33, and GP276) and the number of IFN-γ-producing CD8 T cells was determined by intracellular staining followed by flow cytometry. The data are the means of at least three mice per group at each time point ± the standard deviation.
TABLE 1.
Viral titers in the spleens of LCMV-clone 13-infected mice
| Group | LCMV titer (log10 PFU/spleen)a
|
|||
|---|---|---|---|---|
| Day 8 | Day 15 | Day 30 | Day 60 | |
| +/+ | 7.3 | 6.4 | 5.3 | 3.7 |
| 7.2 | 6.3 | 5.0 | <2.0 | |
| 7.8 | 6.3 | 5.6 | <2.0 | |
| p55−/− | 7.3 | 5.9 | 5.3 | 3.0 |
| 7.3 | 5.8 | 5.4 | 4.0 | |
| 7.7 | ND | ND | 3.9 | |
| p75−/− | 7.4 | 6.0 | 5.0 | <2.0 |
| 7.5 | 6.0 | 5.1 | <2.0 | |
| 7.6 | ND | 4.7 | <2.0 | |
| DKO | 7.4 | 6.1 | 5.3 | 2.0 |
| 7.6 | 5.9 | 5.0 | 5.0 | |
| 7.7 | 6.1 | 5.0 | 3.7 | |
Groups of +/+, p55−/−, p75−/−, and DKO mice were infected with LCMV-clone 13, and viral titers in the spleens were determined by a plaque assay on the indicated day after infection. The data are the viral titers of individual mice. ND, not done.
FIG. 9.
Functional unresponsiveness of CD8 T cells in persistently infected TNFR-deficient mice. At 195 days after infection with LCMV-clone 13, splenocytes were stimulated with the indicated LCMV CTL epitope peptide and the number of IFN-γ-producing CD8 T cells was determined by intracellular staining. The dot plots are gated on total splenocytes, and the numbers represent percentages of IFN-γ-producing CD8 T cells per total splenocytes.
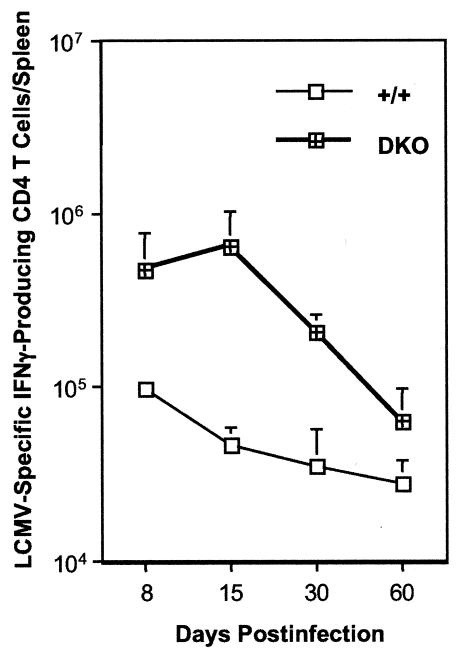
Maintenance of CD8 T-cell responses during a chronic LCMV infection is dependent upon CD4 T cells (42). We examined the effect of TNFR deficiency on the kinetics of LCMV-specific CD4 T-cell response by intracellular staining for IFN-γ (Fig. 10). As shown in Fig. 10, on days 8, 15, and 30 p.i., the spleens of DKO mice contained considerably more LCMV-specific CD4 T cells than in +/+ mice; there were 14-fold and 6-fold more LCMV-specific CD4 T cells in DKO mice on days 15 and 30 p.i., respectively, compared to levels in +/+ mice. However, by day 60 p.i., the total number of IFN-γ-producing CD4 T cells in DKO mice were reduced to levels comparable to those in +/+ mice. After 60 days p.i., CD4 T cells from both +/+ and DKO mice did not produce detectable levels of IFN-γ upon in vitro stimulation. Nonetheless, these data implied that impairment of LCMV clearance in DKO mice is not likely due to ineffective activation and maintenance of CD4 T cells.
FIG. 10.
Kinetics of CD4 T-cell responses in TNFR-deficient mice during a chronic LCMV infection. Groups of mice were infected with LCMV-clone 13, and on the indicated days postinfection virus-specific CD4 T-cell responses were quantitated by intracellular staining for IFN-γ following in vitro stimulation with the MHC-II-restricted epitope peptide GP61-80. The data are the means of at least three mice per group at each time point ± the standard deviation.
Effect of TNFR deficiency on antibody responses to LCMV-clone 13.
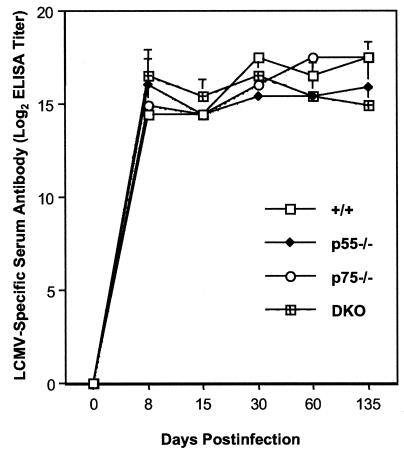
While the importance of T cells in the resolution of LCMV-clone 13 infection is well documented, the role of antibodies is unclear. Long-term control of a persistent LCMV infection, especially under conditions of CTL deficiency, seems to be dependent upon antibodies (50). We investigated the effect of TNFR deficiency on the elicitation of antibody responses during a chronic LCMV infection. Data in Fig. 11 show the kinetics of antibody responses to LCMV-clone 13 in +/+ and TNFR-deficient mice. In all groups of mice, high titers of anti-LCMV antibodies were detected by day 8 p.i. and were maintained thereafter at least until day 135 p.i. On day 135 p.i., the titers of anti-LCMV antibodies in sera of p55−/− and DKO mice were slightly lower than those in +/+ and p75−/− mice. Overall, loss of TNFRs did not significantly affect the kinetics and magnitude of antibody responses to LCMV-clone 13.
FIG. 11.
Kinetics of antibody responses in TNFR-deficient mice during a chronic LCMV infection. Groups of +/+, p55−/−, p75−/−, and DKO mice were infected with LCMV-clone 13. On the indicated days postinfection, LCMV-specific antibody titers in the serum were determined by an ELISA. The data are the means of three mice per group ± the standard deviation at each time point.
DISCUSSION
TNF is a prototypic proinflammatory cytokine produced predominantly by activated macrophages and T cells. In addition to its importance in orchestrating the inflammatory cascade, TNF exerts potent antimicrobial and immune regulatory effects (24). In this study, we have documented the role of TNF-TNFR interactions in mediating viral clearance and regulating the kinetics of the T-cell response during a chronic infection with LCMV in mice. We have shown for the first time that TNF-TNFR interactions might be required for the resolution of a chronic infection with a noncytopathic virus, LCMV. We further showed that TNFRs play distinct roles in downregulating LCMV-specific CD8 and CD4 T-cell responses during a chronic LCMV infection.
Elucidation of effector mechanisms of viral clearance is an area of intense investigation in an effort to identify specific antiviral agents to purge an ongoing infection or to develop effective vaccines that can provide protective immunity in the event of an infection. It is well established that CD8 T cells play an important role in controlling several viral infections in humans and mice (58). As mentioned before, CD8 T cells exert their effector function by lysing virally infected cells via perforin-dependent cell-mediated cytotoxicity or the production of cytokines like IFN-γ and TNF (25). LCMV-Arm replicates primarily in the spleen and induces an acute infection that is cleared in 8 to 10 days (2). Although control of an acute LCMV infection is critically dependent upon perforin-dependent cytotoxicity of CD8 T cells, IFN-γ deficiency leads to a delay in viral clearance (29, 35, 39, 45, 56). TNF activity does not seem to be essential to clear an acute LCMV infection (32). In contrast to LCMV-Arm, LCMV-clone 13 replicates to very high levels in multiple organs, resulting in a disseminated infection lasting 3 to 6 months (1, 2). The molecular mechanisms underlying the resolution of a chronic LCMV infection are not well understood. In this study, we showed that TNF-TNFR interactions might be important in the clearance of LCMV-clone 13 from liver and lung. While deficiency in TNFR II alone did not affect the control of LCMV-clone 13, loss of TNFR I caused a delay in viral clearance from liver. Loss of both TNFR I and TNFR II resulted in uncontrolled viral replication in the liver and LCMV persistence. Similar to the liver, LCMV clearance from the lung did not depend upon TNFR II. Notably, deficiency of TNFR I alone was sufficient to inhibit LCMV clearance from the lung. Taken together, these data strongly suggest an important role for TNFR I in the successful resolution of a chronic LCMV infection from the liver and lung. The control of LCMV-clone 13 is dependent upon both CD8 and CD4 T cells (42). However, the impairment in the clearance of LCMV-clone 13 in TNFR I and DKO mice is not due to a defect in the activation and expansion of LCMV-specific CD8 and CD4 T cells. Further, similar numbers of LCMV-specific CD8 T cells were detected in the livers and lungs of +/+ and TNFR-deficient mice. Therefore, it is unlikely that suboptimal activation or defective trafficking of CD8 T cells into the lung and liver accounts for ineffective LCMV clearance in TNFR I or DKO mice. Under conditions of CTL deficiency, neutralizing antibodies might be important in controlling a persistent LCMV infection (50). Although our studies showed that TNFR deficiency did not significantly impact the titers of anti-LCMV antibodies in the serum of LCMV-clone 13-infected mice, further studies are warranted for qualitative characterization of antibody responses in TNFR-deficient mice.
The CD8 T-cell response in acute and chronic LCMV infections has been studied extensively (reviewed in reference 58). The CD8 T-cell response to an acute LCMV infection comprises three distinct phases: (i) an expansion phase, when naive CD8 T cells are activated to undergo extensive proliferation and differentiation into effector cells; (ii) a contraction phase, when ∼90% of the expanded CD8 T cells are eliminated by apoptosis; and (iii) a memory phase, when a stable number of memory CD8 T cells are maintained for an extended period of time. In striking contrast to an acute LCMV infection, viral persistence during a chronic LCMV infection is associated with suppressed CD8 T-cell responses. The suppression of the CD8 T-cell response during a chronic LCMV infection includes greatly reduced CD8 T-cell expansion and complete loss of CD8 T cells specific to the immunodominant epitope, NP396 (57, 59). The present study suggests that TNF-TNFR interactions might contribute to the suppression of T-cell expansion during a chronic LCMV infection; spleens of DKO mice contained significantly higher numbers of LCMV-specific CD8 and CD4 T cells on day 8 p.i. than those of +/+ mice. Markedly increased expansion of LCMV-specific T cells seen in DKO mice and not in either p55−/− or p75−/− mice suggests some degree of redundancy between TNFRs I and II to suppress T-cell responses in vivo. A previous study has shown that interleukin-12 treatment-induced suppression of CD8 T-cell responses to LCMV is TNF dependent (47).
Similar to an acute LCMV infection, ensuing the expansion phase, CD8 T-cell responses undergo a programmed contraction phase in a chronic infection (5, 46, 57). However, it is worth pointing out that the contraction of the CD8 T cells seen in an acute infection coincides with viral clearance, and contraction seen in a chronic infection occurs in the presence of high viral load. Therefore, the mechanisms of CD8 T-cell contraction are likely different between acute and chronic infections. The magnitude of contraction of CD8 T cells in a chronic LCMV infection varies in an epitope-dependent fashion; while CD8 T cells specific to all epitopes are lost in significant number, CD8 T cells specific to the epitope NP396 are completely deleted (59). It was recently shown that the selective deletion (exhaustion) of NP396-specific CD8 T cells occurs because NP396 peptide is presented at a higher level on infected cells, compared to other epitopes (57). Repeated encounter with antigen might lead to deletion of NP396-specific CD8 T cells by a process called activation-induced cell death (AICD). Several mechanisms, including perforin, Fas, and TNF, can cause AICD of activated T cells in vitro (52, 53, 61). The deletion of NP396-specific CD8 T cells is highly attenuated in persistently infected perforin-deficient mice (62). However, during a persistent LCMV infection, loss of Fas or TNFR I interactions only slightly delayed but did not prevent the deletion of NP396-specific CD8 T cells (62). Consistent with this report, our studies showed that deletion of NP396-specific CD8 T cells was delayed in TNFR I-deficient mice (62). Although TNFR II (p75) has been shown to mediate TNF-induced AICD of T cells in vitro (61), surprisingly, the exhaustion of NP396-specific CD8 T cells in chronically infected p75−/− or DKO mice was unperturbed. Even though CD8 T cells specific to GP33 and GP276 were spared from deletion, a substantial number of these cells were lost between days 8 and 30 after infection with LCMV-clone 13 in the +/+ mice. In this study, the contraction of GP33- and GP276-specific CD8 T cells was delayed only in DKO mice and not in p55−/− or p75−/− mice. These studies indicated that TNFRs might play an ancillary role in regulating clonal deletion of LCMV-specific CD8 T cells during a chronic LCMV infection. Perhaps perforin is the major player in mediating the programmed contraction of CD8 T-cell responses, with significant contributions from both Fas and TNFRs.
What are the implications of the findings documented in this report? The outcome of a viral infection with a noncytopathic virus like LCMV is dependent upon complex interactions between the virus and the immune system. LCMV-clone 13 establishes a disseminated infection in mice, which includes rampant replication in vital organs like the liver, lung, and heart (1, 2). Notably, LCMV-clone 13 is highly hepatotropic, and titers in the liver can be as high as 8.0 log10 PFU/gram of tissue. An uncontrolled CD8 CTL response in an LCMV-clone 13 infection can result in massive tissue damage and hepatic failure. To avoid such a catastrophe, there is a need to dampen the CD8 T-cell response and/or achieve viral clearance by noncytopathic means via cytokines that can purge viral replication with minimum cellular damage. Our study shows that TNF-TNFR interactions are critical in both downregulating CD8 T-cell responses and mediating LCMV clearance from the tissues. These findings might have implications in therapy of HBV- or HCV-infected people, because poor CD8 T-cell response in HBV infection is associated with viral persistence and a protracted course of the disease (30, 34, 40). Perhaps, short-term immunotherapy of HBV- or HCV-infected patients to modulate TNF activity might enhance CD8 T-cell responses without compromising viral clearance. Alternatively, TNF administration might be used as an adjunct with other treatment modalities to accelerate HBV clearance. Prolonged anti-TNF therapy is commonly used to treat immune-mediated diseases like rheumatoid arthritis (11, 12). Anti-TNF therapy arguably has tremendous beneficial effects clinically due to suppression of joint inflammation. However, the present study and earlier reports (31, 37) suggest that anti-TNF therapy might neutralize the T-cell-suppressive function of TNF, which in turn might lead to enhanced activity of joint-reactive T cells. Furthermore, our data suggest that prolonged neutralization of TNF in vivo might predispose patients to infections, which is consistent with increased upper respiratory tract infections in arthritic patients undergoing treatment with TNF inhibitors (11). In summary, the findings presented in this paper should enhance our understanding about the pathobiology of TNF in vivo in the context of a chronic viral infection.
Acknowledgments
This work was supported by Public Health Service grant AI48785 from the National Institutes of Health and a research grant (RG3092A1/T) from the National Multiple Sclerosis Society to M. Suresh.
REFERENCES
- 1.Ahmed, R., and M. B. A. Oldstone. 1988. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 167:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander-Miller, M. A., M. A. Derby, A. Sarin, P. A. Henkart, and J. A. Berzofsky. 1998. Supraoptimal peptide-major histocompatibility complex causes a decrease in bc1-2 levels and allows tumor necrosis factor alpha receptor II-mediated apoptosis of cytotoxic T lymphocytes. J. Exp. Med. 188:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3:619-626. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., and N. L. Letvin. 2001. CD8+ cytotoxic T lymphocyte responses to lentiviruses and herpesviruses. Curr. Opin. Immunol. 13:479-482. [DOI] [PubMed] [Google Scholar]
- 7.Benihoud, K., I. Saggio, P. Opolon, B. Salone, F. Amiot, E. Connault, C. Chianale, F. Dautry P. Yeh, and M. Perricaudet. 1998. Efficient, repeated adenovirus-mediated gene transfer in mice lacking both tumor necrosis factor alpha and lymphotoxin alpha. J. Virol. 72:9514-9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr. Opin. Immunol. 11:451. [DOI] [PubMed] [Google Scholar]
- 9.Callan, M. F., C. Fazou, H. Yang, T. Rostron, K. Poon, C. Hatton, and A. J. McMichael. 2000. CD8+ T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Investig. 106:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmann, M., and R. N. Maini. 2001. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol. 19:163-196. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann, M. 2001. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2:364-371. [DOI] [PubMed] [Google Scholar]
- 13.Gallimore, A., A. Glithero, A. Godkin, A. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhard, J. R., C. M. Perry, S. Harkins, T. Lane, I. Mena, V. C. Asensio, I. L. Campbell, and J. L. Whitton. 1998. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am. J. Pathol. 153:417-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilles, P. N., G. Fey, and F. V. Chisari. 1992. Tumor necrosis factor-alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J. Virol. 66:3955-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidotti, L. G., K. Ando, M. V. Hobbs, T. Ishikawa, R. D. Runkel, R. D. Schreiber, and F. V. Chisari. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. USA 91:3764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti, L. G., P. Borrow, A. Brown, H. McClary, R. Koch, and F. V. Chisari. 1999. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J. Exp. Med. 189:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti, L. G., S. Guilhot, and F. V. Chisari. 1994. Interleukin 2 and interferon alpha/beta downregulate hepatitis B virus gene expression in vivo by tumor necrosis factor dependent and independent pathways. J. Virol. 68:1265-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 23.Guidotti, L. G., R. Rochford, L. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 24.Harty, J. T., and V. P. Badovinac. 2002. Influence of effector molecules on the CD8+ T cell response to infection. Curr. Opin. Immunol. 14:360-365. [DOI] [PubMed] [Google Scholar]
- 25.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 26.Hislop, A. D., N. E. Annels, N. H. Gudgeon, A. M. Leese, and A. B. Rickinson. 2002. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein-Barr virus infection. J. Exp. Med. 195:893-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holterman, A. X., K. Rogers, K. Edelmann, D. M. Koelle, L. Corey, and C. B. Wilson. 1999. An important role for major histocompatibility complex class I-restricted T cells, and a limited role for gamma interferon, in protection of mice against lethal herpes simplex virus infection. J. Virol. 73:2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 29.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, J. Olsen, E. R. Podack, R. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 30.Kantzanou, M., M. Lucas, E. Barnes, H. Komatsu, G. Dusheiko, S. Ward, G. Harcourt, and P. Klenerman. 2003. Viral escape and T cell exhaustion in hepatitis C virus infection analysed using class I peptide tetramers. Immunol. Lett. 85:165-171. [DOI] [PubMed] [Google Scholar]
- 31.Kassiotis, G., and G. Kollias. 2001. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193:427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klavinskis, L. S., R. Geckeler, and M. B. Oldstone. 1989. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumour necrosis factor alpha, displays antiviral activity in vivo. J. Gen. Virol. 70:3317-3325. [DOI] [PubMed] [Google Scholar]
- 33.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 34.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leist, T. P., M. Eppler, and R. M. Zinkernagel. 1989. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J. Virol. 63:2813-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98:1667-1677. [DOI] [PubMed] [Google Scholar]
- 37.Liu, J., M. W. Marino, G. Wong, D. Grail, A. Dunn, J. Bettadapura, A. J. Slavin, L. Old, and C. C. Bernard. 1998. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat. Med. 4:78-83. [DOI] [PubMed] [Google Scholar]
- 38.Liu, H., S. Andreansky, G. Diaz, T. Hogg, and P. C. Doherty. 2002. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J. Immunol. 168:3477-3483. [DOI] [PubMed] [Google Scholar]
- 39.Lohman, B. L., and R. M. Welsh. 1998. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon knockout mice. J. Virol. 72:7815-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maini, M. K., C. Boni, C. K. Lee, J. R. Larrubia, S. Reignat, G. S. Ogg, A. S. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N. V. Naoumov, C. Ferrari, and A. Bertoletti. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 42.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matloubian, M., M. Suresh, A. Glass, M. Galvan, K. Chow, J. K. Whitmire, C. M. Walsh, W. R. Clark, and R. Ahmed. 1999. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 73:2527-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 45.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 46.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 47.Orange, J. S., T. P. Salazar-Mather, S. M. Opal, R. L. Spencer, A. H. Miller, B. S. McEwen, and C. A. Biron. 1995. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J. Exp. Med. 181:901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 49.Peschon, J. J., D. S. Torrance, K. L. Stocking, M. B. Glaccum, C. Otten, C. R. Willis, K. Charrier, P. J. Morrissey, C. B. Ware, and K. M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160:943-952. [PubMed] [Google Scholar]
- 50.Planz, O., S. Ehl, E. Furrer, E. Horvath, M. A. Brundler, H. Hengartner, and R. M. Zinkernagel. 1997. A critical role for neutralizing-antibody-producing B cells, CD4+ T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc. Natl. Acad. Sci. USA 94:6874-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi, C. P., A. McAllister, M. Tanguy, D. Kagi, and M. Brahic. 1998. Theiler's virus infection of perforin-deficient mice. J. Virol. 72:4515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell, J. H. 1995. Activation-induced death of mature T cells in the regulation of immune responses. Curr. Opin. Immunol. 7:382-388. [DOI] [PubMed] [Google Scholar]
- 53.Spaner, D., K. Raju, B. Rabinovich, and R. G. Miller. 1999. A role for perforin in activation-induced T cell death in vivo: increased expansion of allogeneic perforin-deficient T cells in SCID mice. J. Immunol. 162:1192-1199. [PubMed] [Google Scholar]
- 54.Topham, D. J., R. A. Tripp, and P. C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197-5200. [PubMed] [Google Scholar]
- 55.Vogel, T. U., T. M. Allen, J. D. Altman, and D. I. Watkins. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 75:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh, C. M., M. Matloubian, C.-C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. Huang, J. D.-E. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong, P., and E. G. Pamer. 2003. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21:29-70. [DOI] [PubMed] [Google Scholar]
- 59.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, H. G., T. Zhou, P. Yang, C. K. Edwards, D. T. Curiel, and J. D. Mountz. 1998. Inhibition of tumor necrosis factor alpha decreases inflammation and prolongs adenovirus gene expression in lung and liver. Hum. Gene Ther. 9:1875-1884. [DOI] [PubMed] [Google Scholar]
- 61.Zheng, L., G. Fisher, R. E. Miller, J. Peschon, D. H. Lynch, and M. J. Lenardo. 1995. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 377:348-351. [DOI] [PubMed] [Google Scholar]
- 62.Zhou, S., R. Ou, L. Huang, and D. Moskophidis. 2002. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 76:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]