Abstract
Adipose tissue influences tumor development in two major ways. First, obese individuals have a higher risk of developing certain cancers (endometrial, esophageal, and renal cell cancer). However, the risk of developing other cancers (melanoma, rectal, and ovarian) is not altered by body mass. In obesity, hypertrophied adipose tissue depots are characterized by a state of low grade inflammation. In this activated state, adipocytes and inflammatory cells secrete adipokines and cytokines which are known to promote tumor development. In addition, the adipocyte mediated conversion of androgens to estrogen specifically contributes to the development of endometrial cancer, which shows the greatest relative risk (6.3-fold) increase between lean and obese individuals. Second, many tumor types (gastric, breast, colon, renal, and ovarian) grow in the anatomical vicinity of adipose tissue. During their interaction with cancer cells, adipocytes dedifferentiate into pre-adipocytes or are reprogrammed into cancer-associated adipocytes (CAA). CAA secrete adipokines which stimulate the adhesion, migration, and invasion of tumor cells. Cancer cells and CAA also undergo a dynamic exchange of metabolites. Specifically, CAA release fatty acids through lipolysis which are then transferred to cancer cells and used for energy production through β-oxidation. The abundant availability of lipids from adipocytes in the tumor microenvironment supports tumor progression and uncontrolled growth. Given that adipocytes are a major source of adipokines and energy for the cancer cell, understanding the mechanisms of metabolic symbiosis between cancer cells and adipocytes should reveal new therapeutic possibilities.
Keywords: obesity, adipocytes, visceral adipose tissue, cancer, metastasis, metabolic symbiosis
1 Introduction
The role of adipose tissue, and more specifically adipocytes, in tumor initiation, growth, and metastasis, is a relatively new area of investigation. Intially, adipose tissue was regarded as an insulating and mechanically supportive site of energy storage and mobilization for peripheral organs during times of increased energy demand. However, after the discovery of leptin in 1994, the traditional role of adipose tissue has evolved to a fully functioning endocrine organ, capable of regulating systemic energy and metabolic homeostasis. A role for adipose tissue in cancer is emerging based on two key observations: (i) epidemiologic studies have demonstrated an association between obesity and some cancers (e.g. esophageal and endometrial), and (ii) adipocytes constitute a major component of the tumor microenvironment for breast and abdominally metastasizing cancers (e.g. gastric, colon, and ovarian) promoting tumor growth.
Several similarities can be drawn between an adipose tissue-dominated tumor microenvironment and the well-described function of adipose tissue in type 2 diabetes and obesity (Figure 1). The two microenvironments share similar histologic features, including the presence of inflammatory cells, especially macrophages which secrete inflammatory cytokines. Adipocytes in both patients with obesity-associated type 2 diabetes and cancer, secrete adipokines, sustaining the activated state of the tissues, and promoting the progression of both diseases [1]. In patients with diabetes, adipokines sustain the activated state of the adipose tissue environment and in patients with cancer adipokines support tumor cell growth. Expanding the analogy between the activated inflammatory adipose tissue in obesity and the cancer microenvironment, adipose tissue in obese patients is often characterized by chronic inflammation, while the tumor microenvironment is characterized by intratumoral inflammation which promotes progressive tumor growth and angiogenesis as described by Virchow (1863, “lymporeticular infiltrate in cancer”) and Dvorak (1986, “Tumors are wounds that do not heal”).
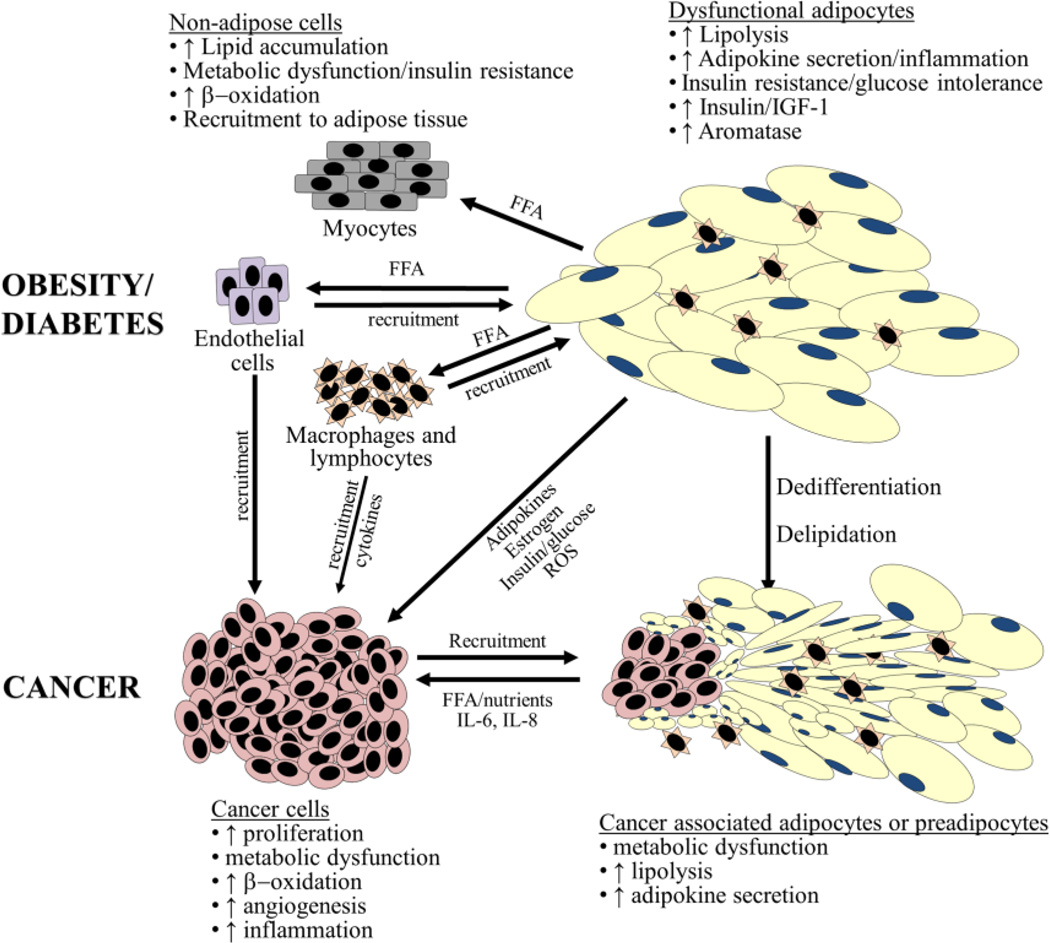
Figure 1. Adipocytes in metabolic disease and cancer.
Striking similarities exist between the involvement of adipocytes in both tumorigenesis and obesity/type 2 diabetes. These disease states result in the recruitment of immune cells including macrophages and lymphocytes. Cytokine secretion from adipocytes (e.g., leptin, IL-6, IL-8, and TNF-α) are also involved in both disease states. Activated adipocytes in obesity/diabetes and/or cancer are delipidated and potentially dedifferentiate to fibroblast-like cells. Expelled nutrients (e.g. FFA) can be taken up by both cancer cells as well as other non-adipose cells (e.g., myocytes, macrophages, and vascular endothelial cells) resulting in metabolic dysfunction.
Abbreviations: CAA, cancer-associated adipocytes; FFA, free-fatty acid(s); IGF-1, insulin-like growth factor protein-1; IL; interleukin; MCP-1, monocyte chemoattractant protein-1; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α.
The expansion in cancer research from a cancer cell-centric approach to include adjacent “normal” cells that make significant contributions to cancer initiation and progression, has brought forth an exciting wave of research [2–5]. This review will summarize what is known about how adipose tissue and adipocytes promote tumor development and progression. The first part of the review will discuss adipose tissue physiology, pathology, and biochemistry as they support the role of adipocytes in the tumor microenvironment. The latter part will review data from cancer-specific epidemiologic and experimental studies on obesity and adipocytes in carcinogenesis. Our goal is to provide a concise synopsis of the complex reciprocal relationship between tumor cells and adipocytes, while highlighting interactions that may serve as unique clinical targets for cancer treatment and prevention.
2 Adipose tissue and adipocyte physiology and pathology
Adipose tissue is largely composed of adipocytes, but also contains a stromal vascular fraction made up of pericytes, endothelial cells, monocytes, macrophages, and pluripotent stem cells [6;7]. Interestingly, adipocytes from different adipose tissue sites have unique metabolic functions, replicative and developmental potentials, cytokine production, and responses to external stimuli [8;9]. For example, a proteomic analysis comparing human omental (i.e. visceral) and subcutaneous adipose depots found omental adipose tissue expressed higher levels of proteins involved in lipid and glucose metabolism and consistently overexpressed epithelial cytokeratins (CK) such as CK7, CK-8/18, and CK-19 [10]. Differences in gene expression and lipolytic activity have also been reported in subcutaneous tissue from different anatomic regions of the body (abdomen versus hip) [11].
White adipocytes are considered the dominant adipocyte subtype in adult humans. The main depots of white adipose tissue in humans are found in subcutaneous and visceral (omental and bowel mesentery) sites. Alternative sites of white adipocytes include the perirenal fat pad, postmenopausal breast, and perivascular fat pads. Differentiated white adipocytes specialize in the storage of lipids, primarily in the form of triacylglycerol, which are released as free fatty acids (FFA). Brown adipocytes produce heat following cold exposure by expressing high levels of uncoupling protein 1 (UCP1), although very few are present in adults. A third type of adipocyte, the beige adipocyte, has recently been identified which has characteristics of both white and brown adipocytes. Like white adipocytes, basal UCP1 expression is low in beige adipocytes. However like brown adipocytes, beige adipocytes respond to cyclic AMP (cAMP) stimulation with increased UCP1 expression [12]. Brown and white adipocytes arise from a similar mesenchymal stem cell which is driven by brown or white adipocyte-specific transcription factors to differentiate towards white and brown adipogenesis in early development [13]. In contrast, beige adipocytes further differentiate along the white adipocyte lineage [13;14]. A critical step in white adipocyte physiology is the terminal differentiation of preadipocytes in to adipocytes which allows increased storage of fatty acids, in the form of triacylglycerol (adipogenesis). The nuclear transcription factor, peroxisome proliferator-activated receptor (PPAR-γ), is a central regulator of this differentiation process. PPAR-γ activates transcription through binding to the PPAR response element after heterodimerizing with the retinoid X receptor (RXR). The PPAR-γ activated transcriptional program regulates expression of hormone sensitive lipase (HSL), adiponectin, and fatty acid binding protein (FABP)-4, all of which participate in the interaction between tumor cells and adipocytes.
Once terminally differentiated, the white adipocytes maintain energy homeostasis by storing and mobilizing lipids (Figure 2). In acute positive energy balance (such as after a meal) adipocytes increase the storage of triacylglycerol by inducing the expression of enzymes (e.g. acetyl CoA carboxylase, ACC) involved in triglyceride synthesis and adipogenesis [15;16]. To further reduce peripheral lipid levels, adipocytes promote β-oxidation in non-adipose tissue (e.g. liver) [17] and secrete the adipokine, leptin, to reduce caloric intake.
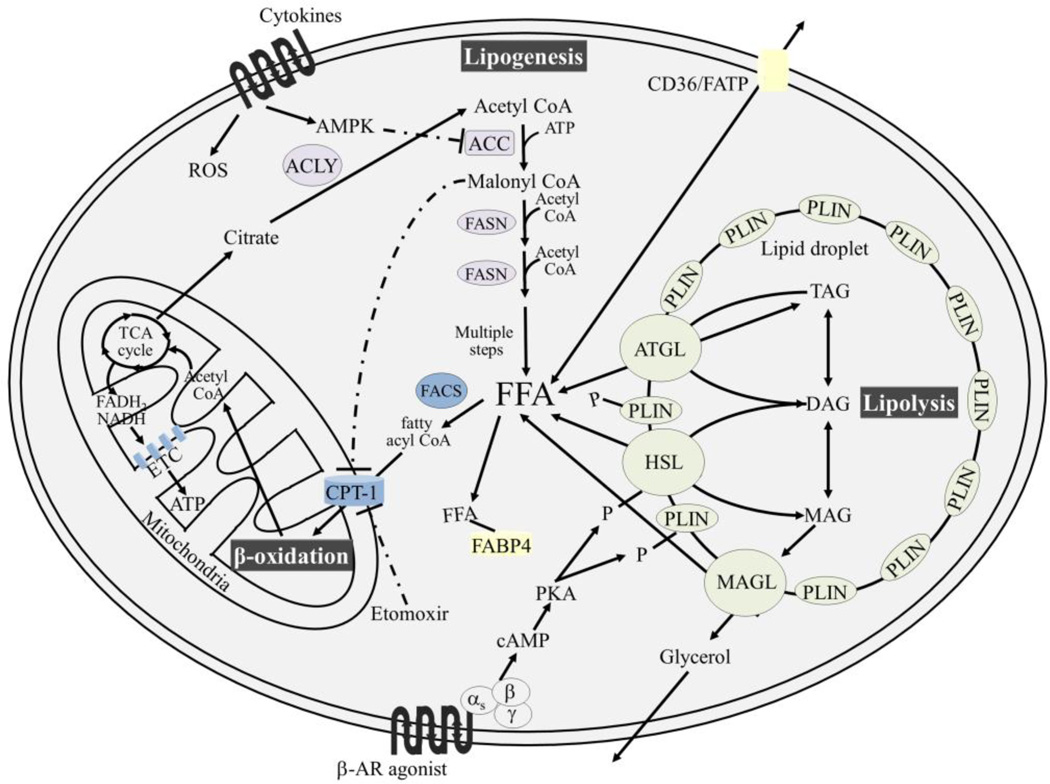
Figure 2. An overview of adipocyte lipid metabolism.
Lipogenesis occurs in the cytoplasm of the cell and requires acetyl CoA which can be generated from citrate, through the activity of ACLY. The irreversible formation of malonyl CoA from acetyl CoA initiates lipogenesis and requires the activity of ACC. AMPK phosphorylates and inactivates ACC. Cytokines (e.g., leptin, IL-6, IL-8, MCP1, and TNF-α) can trigger both ROS production and AMPK activation. Lipogenesis proceeds in a repeating reaction sequence which involves the addition of two carbon units to a growing fatty acid chain, through the activity FASN. Accumulation of malonyl CoA during energy surplus results in allosteric inhibition of CPT-1, the rate-limiting enzyme involved in shuttling acylated free fatty acids (via FACS) into the mitochondria for β-oxidation. Etomoxir prevents β-oxidation by inhibiting CPT-1. In contrast, under the condition of energy deficit, citrate becomes limited and malonyl CoA concentrations decrease within the cell, lifting the allosteric regulation of CPT-1 and allowing ATP generation through β-oxidation. To prevent lipotoxicity, fatty acids bound to glycerol are stored in lipid droplets, primarily as TAG. TAG can be mobilized by β-AR agonists (e.g., epinephrine and norepinephrine) which initiate a G-protein coupled cascade leading to the phosphoryation of HSL and PLIN. Phosphorylation results in a conformational change in PLIN, allowing HSL access to the lipid droplet. Triacylglycerols are completely hydrolyzed by the activity of (i) ATGL which removes one fatty acid forming DAG, (ii) HSL which remove another fatty acid from DAG resulting in MAG, and (iii) MAGL which removes the final fatty acid from the glycerol backbone. FFA can be exported and imported through fatty acid receptors such as CD36 and FATPs. FABP4 chaperones free fatty acid within the cell.
Abbreviations: ACC, acetyl CoA carboxylase; ACSL, acyl CoA synthetase lyase; ACLY, ATP citrate lyase; AMPK, AMP kinase; AR, adrenergic; ATGL, adipose triglyceride lipase; CPT-1, carnitine-palmitoyltransferase 1; DAG, diacylglycerol(s); ETC, electron transport chain; FABP4, fatty acid binding protein 4; FACS, fatty acyl CoA synthetase; FASN, fatty acid synthase; FATP, fatty acid transport proteins; FFA, free-fatty acids; HSL, hormone-sensitive lipase; MAG, monacylglycerol(s); MAGL, monacylglycerol lipase; MCP-1, monocyte chemoattractant protein-1; P, phosphorylation; PKA, protein kinase A; PLIN, perilipin; TAG, triacylglycerol(s); TCA; tricarboxylic acid; TNF-α, tumor necrosis factor-α.
Due to the robust endocrine function of adipose tissue, numerous pathologies result when adipocytes become dysfunctional. For example, the excess fatty acids produced by dysfunctional adipocytes, disrupt the cell membranes of adjacent cells, resulting in endoplasmic reticulum stress and mitochondrial damage [17]. To attenuate the toxicity, free fatty acids are esterified to glycerol, yielding inert triacylglycerol. With increasing energy imbalance in the direction of surplus, excess triglyceride accumulation results in adipocyte hypertrophy and ultimately obesity. Obesity, particularly visceral obesity, is considered a major risk factor for metabolic disease including type 2 diabetes, cardiovascular disease, and cancer [18]. The combination of type 2 diabetes/hyperglycemia, insulin insensitivity, hypertension, central obesity, and dyslipidemia is termed “the metabolic syndrome”.
When the state of excess energy is chronic (obesity, metabolic syndrome), the ability of adipose tissue to function as an endocrine organ deteriorates. In particular, hypertrophied adipocytes secrete increasing amounts of pro-inflammatory adipokines, including monocyte chemoattractant protein (MCP) −1, tumor necrosis factor (TNF)-α, IL-6, IL-8, PAI-1, and leptin [9]. Increased secretion of inflammatory peptides results in the infiltration of lymphocytes, macrophages, and stromal cells; significantly altering the adipose tissue microenvironment. In fact, macrophages and inflammatory cells may comprise up to 50% of the adipose tissue cellular content in obese subjects, compared to 5–10% in lean subjects [19]. The massive infiltration of macrophages into adipose tissue leads to chronic inflammation that not only modifies local metabolism, but also influences systemic energy homeostasis [20–22]. Activated macrophages in adipose tissue are an essential contributor of pro-inflammatory cytokines. Consistent with the central role of inflammation in cytokine secretion, it has been shown that deletion or inhibition of the pro-inflammatory adipokine MCP-1, reduces macrophage accumulation and insulin resistance in mice [21]. These cytokine mediators, synergistically secreted by both dysfunctional adipocytes and newly recruited macrophages [23], led to increased lipolysis and reduced triglyceride synthesis in the adipocytes which elevated circulating free fatty acids [24] and negatively affected metabolic homeostasis. The liberated free fatty acids accumulate in non-adipose tissue, predominantly in the liver, skeletal muscle, β-cells of the pancreas, and the endothelium, resulting in organ steatosis, insulin resistance, and ultimately type 2 diabetes [17].
3 Adipocyte Biochemistry
Adipocytes tightly control energy storage and utilization. Excess energy is stored in the form of triacylglycerol and the hydrolysis of triacylglycerol occurs during times of increased energy demands. The cycle of fatty acid storage and release is regulated, at least in part, by insulin. Insulin induces fatty acid uptake into adipocytes, inhibits lipolysis, and stimulates lipogenesis of triacylglycerol.
Triglyceride synthesis and storage occurs in lipid droplets through the glycerol-phosphate pathway (reviewed in [25]). In this process two molecules of fatty acyl CoA are formed from free fatty acids by acyl CoA synthetase. The fatty acyl CoA molecules are acylated to glycerol 3-phosphate yielding phosphatidate, which is dephosphorylated to form diacylglycerol. The final step of triglyceride synthesis occurs through diacylgycerol acyltransferase, which joins a third fatty acyl CoA to the glycerol backbone [26]. The major rate limiting enzyme for fatty acid synthesis is ACC.
Lipolysis is the process by which energy is mobilized from adipocytes and is required for the release of free fatty acids. In this process triacylglycerol are hydrolyzed to free fatty acids and glycerol. This requires the activity of three critical enzymes, each removing one fatty acid from the glycerol moiety (Fig 2). The first enzyme, adipose triglyceride lipase (ATGL), converts the triglyceride to a diacyglycerol. The second step involves HSL, resulting in monacylglycerol, and the final hydrolysis reaction occurs through the activity of monacylglycerol lipase (MAGL, Figure 2) [27;28]. Although, it has been suggested that more than 95% of triglyceride hydrolysis in murine adipocytes occurs through ATGL and HSL [29]. Aggressive epithelial tumor cell lines and primary tumors reportedly overexpress MAGL which contributes to the production of pro-tumorigenic fatty acid products [30]. When MAGL is overexpressed, it induces invasion and migration of ovarian and prostate cancer cells. MAGL expressing cancer cell lines are more aggressive, undergo epithelial-mesenchymal transition, and express stem cell markers [31]. These findings suggest that MAGL may be an attractive treatment target.
The control of adipocyte energy stores is tightly regulated by both hormonal and sympathetic signals. Of these, insulin and catecholamine signaling are the most thoroughly investigated. During fasting, catecholamines (e.g., epinephrine and norepinephrine) bind to β-adrenergic receptors on the surface of the adipocyte. Receptor-binding activates a G-protein coupled cascade, including the second messenger cAMP and protein kinase A (PKA), and ultimately results in the activation/phosphorylation of HSL [32]. In addition, glucocorticoids are released during fasting which encourages lipolysis by increasing transcription of ATGL. In contrast, postprandial insulin release stimulates the esterification of free fatty acids to glycerol (triacylglycerol synthesis) by preventing the accumulation of cAMP and activation of PKA. In addition to these systemic forms of energy regulation, there are equally important forms of local regulation of lipolysis both by secreted factors from adipocytes themselves (autocrine) and adjacent resident cells of the adipose tissue (paracrine). These secreted factors include cytokines and prostaglandins.
4 The role of obesity in tumorigenesis and cancer progression
Several epidemiological studies suggest an association between obesity and both cancer incidence and mortality [33;34]. Not all cancers are associated with obesity and the relative risk (RR) seems to vary among cancer sites. In epidemiologic studies cancer risk is often quantified as the proportional change in risk per 5 kg/m2 increase in body mass index (BMI), a measure of adiposity. Using this formula, the cancers most strongly associated with obesity are endometrial cancer (RR 1.52), esophageal cancer in men (RR 1.59) and renal cancer (RR 1.24 in men and RR 1.34 in women). Some studies have reported a RR for endometrial cancer as high as 6.3 in obese individuals [35]. In addition, both metabolic syndrome and insulin resistance are associated with higher risk of endometrial cancer [36]. For most other cancers (e.g. melanoma, rectum, gallbladder, leukemia) the RR per 5 kg/m2 is less than 1.17 or insignificant (pancreas, prostate, gastric, and ovarian cancer) [33]. Therefore, compared to smoking, which increases an individual’s risk for lung cancer by 10 to 20-fold [37], the increase in cancer risk associated with obesity is modest. The only exception is endometrial cancer.
Obesity may not only affect the risk of developing cancer but also impact cancer survival. Overall, obesity is associated with a 52% and 88% increase in cancer mortality rate among men and women, respectively [35]. This increase is not solely obesity related. Patients who are obese often have co-morbidities, including stroke, cardiovascular disease, renal disease, and/or metabolic syndrome, which may contribute to higher mortality in this cohort. Given the low overall risk of all cancers with obesity, it is unlikely that obesity causes cancer, but should instead be considered as a tumor promoter. There are currently three wide spread hypotheses that explain how obesity might contribute to cancer development and growth. All involve the endocrine and metabolic functions of adipose tissue [38] and are outlined below:
The unopposed estrogen cancer hypothesis
Adipose tissue is the predominant source of aromatase, an enzyme which converts the androgen, androstendione, to estrone, leading to excess estrogen production. This phenomenon is highly relevant in endometrial cancer because given that epithelial endometrial cells express estrogen receptors they are very hormone responsive. In obesity, continuous stimulation of the endometrial lining by adipocyte-generated estrone is an important risk factor for the development of well-differentiated endometrial cancer. The unopposed estrogen hypothesis largely explains the increased risk of endometrial cancer in obese women [39].
The adipokine cancer hypothesis
Adipose tissue in obesity is in a state of low grade chronic inflammation, as evidenced by the presence of inflammatory cells (lymphocytes, macrophages) which generate reactive oxygen species (ROS). These ROS have mitogenic properties at low concentrations and could thus be considered tumor promoters [40;41]. Furthermore these inflammatory cells, together with adipocytes, secrete significant amounts of adipokines and other cytokines, which have been implicated in the promotion of tumor growth through various mechanisms [38]. Adipokines function not only as local paracrine signaling cytokines, but also have a systemic effect through secretion in the serum and communication with distant sites. In fact, several adipokines secreted by adipose tissue including TNF-α, IL-6, IL-8, MCP-1 (CCL2) have been implicated in tumor progression [42–44]. In a genetic mouse model of pancreatic cancer driven by pancreatic specific activation of K-ras a high fat diet induced obesity and secretion of TNF-α and IL-6. Compared to lean, the obese mice showed a higher rate of β-oxidation and early tumor growth which could be blocked by crossing the K-ras mice with TNF receptor 1 knock-out mice thereby inhibiting TNF signaling [45].
The Framingham Heart Study also provides a possible explanation for the higher risk of cancer development and mortality in obese patients. In this study, the volume of visceral and subcutaneous adipose tissue was quantified and correlated with serum levels of pro-inflammatory cytokines. Greater adipose tissue mass/volume correlated with C-reactive protein, MCP-1 and, IL-6 serum levels, while subcutaneous fat volume correlated with fibrinogen levels [46]. This chronically increased systemic secretion of pro-inflammatory cytokines and ROS in obesity likely promotes tumorigenesis. In contrast, adiponectin, an adipokine which increases the sensitivity of cells to insulin and is elevated in lean individuals, may protect against cancer. In endometrial cancer, an inverse association was found between circulating adiponectin levels and the occurrence of endometrial cancer in young patients (<65 years). This effect was independent of the patient’s BMI [47].
The insulin cancer hypothesis
There is accumulating evidence that the metabolic effects of obesity through insulin resistance are risk factors for cancer development. This evidence is derived from epidemiologic studies indicating patients with metabolic syndrome have a higher incidence of cancer [38]. In patients with insulin resistance, the reduced sensitivity of tissues to insulin results in elevated blood glucose and insulin levels. Chronic hyperinsulinaemia promotes secretion of IGF-1 and reduces production of IGF binding proteins, which in turn further increases circulating levels of IGF. Through the IGF receptor, IGF activates downstream signaling pathways that promote mitogenic and proangiogenic pathways and inhibit apoptosis [48]. Insulin itself is mitogenic and antiapoptotic. However, most of insulin’s proliferative effects are mediated through IGF.
The insulin and adipokine cancer hypotheses overlap, since the insulin-resistant state is mediated, at least in part, by cytokine-mediated inflammation. Simply, cytokines inhibit insulin signaling promoting insulin resistance and activate adipose tissue environment, creating a microenvironment of low grade inflammation which is tumor promoting (Figure 1).
Considering the uncertain association between obesity and most cancers, it is possible that the effect of obesity in cancer is mediated by confounding factors such as type 2 diabetes, a high fat diet (“western diet”), or factors that are currently unknown [49]. Alternatively, other hypotheses may explain the tumor promoting role of obesity. Activated adipose tissue releases reactive species (H2O2, NO) that are known to be tumor promoting. Moreover, adipose tissue in obese individuals is characterized by chronic hypoxia, an environment that is tumor-promoting through activation of hypoxia-inducible factor (HIF) pathways. Activated adipose tissue is also a rich source of stromal cells. Stromal cells from adipose tissue have been shown to travel hematogenously to distant tumor sites where they differentiate in to vascular pericytes or intratumoral adipocytes [50]. Experimental evidence suggests adipose-derived stromal cells and endothelial cells which originate from remote fat depots, may promote cancer progression through the secretion of proangiogenic factors [50;51]. These data indicate obesity-associated shedding of adipocytes to tumors sites contributes to tumor cell survival, angiogenesis, and ultimately tumor growth [51].
5 Adipocytes: Active contributors to the tumor microenvironment
Tumorigenesis involves constant communication between tumor cells and neighboring normal cells. The tumor microenvironment, i.e. the normal cells that interact with premalignant and malignant cells, has recently been credited with supporting the acquisition of the necessary hallmark traits for tumorigenesis [49;52–54]. In fact, an understanding of cancer is impossible without a comprehensive description of the tumor microenvironment. Most studies of the tumor microenvironment focus on the tumor-promoting role of cancer-associated fibroblasts (CAF) [2] and the transition of normal fibroblasts to CAFs [55]. The biologic functions of CAFs may inform our understanding of adipocyte function as well, since pre-adipocytes resemble fibroblasts and are derived from the same mesenchymal stem cell lineage [13]. These insights led the Dirat group (University of Toulouse, France) to coin the term “cancer-associated adipocytes” in 2010 [1]. As described in more detail below several studies have shown that the putative cancer-associated adipocytes in the vicinity of cancer cells promote tumor growth either through adipokines or direct contact.
Adipocytes are the predominant cell type in benign adipose tissue, but other cell types are also present, i.e. vascular cells (including the endothelial cells lining vessels), macrophages, fibroblasts, and adipose precursor cells. Given our understanding of the transition from a benign fibroblast to a CAF, it is reasonable to speculate that all the elements of adipose tissue may be recruited by cancer cells and used to promote tumor growth. Several reports suggest that in the presence of cancer cells, adipocytes revert from mature, differentiated adipocytes to pre-adipocytes by delipidation, a process during which adipocytes secrete their lipids [56;57]. Moreover, adipocytes not only function in the local microenvironment, but systemically as endocrine cells (Figure 1).
A model in which cancer cells induce metabolic changes in adipocytes resulting in enhanced lipolytic activity is also supported by findings in patients with cancer-associated cachexia. Cachexia is common in cancer patients with advanced disease and typically results in adipose tissue atrophy and both increased lipolysis and an inability to properly store triacylglycerol in adipocytes [58]. High lipolytic enzyme activity as well as free fatty acid and glycerol release is evident in adipose tissue of cachectic cancer patients [59]. One of the mechanisms identified is increased HSL activity in adipocytes. Accordingly, mice lacking the ATGL or HSL genes were protected from cancer-associated cachexia [58].
6 Adipocytes and cancer
A number of tumors grow in the vicinity of adipocytes (e.g. breast cancer) or metastasize to the predominantly adipocyte-dominated host environment in the abdominal cavity (e.g. gastric and ovarian cancers). Studies linking adipocytes to tumorigenesis have increased in number over the last decade, most focused on breast, prostate, and colon cancer. A possible role for adipocytes in tumor development was first suggested in the mid-1960’s with pioneering work by Spector (NIH, Bethesda). Dr. Spector showed that while 40–50% of the 14C-palmitate injected directly into the peritoneal fluid was incorporated into Ehrlich ascites tumor cells, only 1 % of 14C-glucose was incorporated into cellular lipids [60]. A later study examined mice harboring Ehrlich ascites tumors injected either intraperitoneally or intravenously with tritiated palmitate. Intraperitoneally injected palmitate was rapidly incorporated into tumor cell lipids. However, only minimal incorporation of external lipids in the tumor resulted from intravenous injection of palmitate. The authors hypothesized that rather than using external lipids from the plasma the tumors utilized fatty acids directly transferred from the host intraperitoneal fluid [61].
In tumors growing in an adipose tissue-dominated microenvironment, adipocytes dissapear, fibroblast-like cells accumulate, and a desmoplastic stroma ensues. As an example, in both ovarian cancer and renal cell cancer, tumor cells replace and invade the adipocytes microenvironment and the adipocytes vanish (Figure 3). Histological studies of these and other abdominally metastasizing cancers (colon, gastric, pancreatic) confirm that adipocytes at the tumor invasive front become smaller and the number of fibroblast-like cells increases, raising the interesting possibility that the fibroblast-like cells might be preadipocytes derived from dedifferentiated mature adipocytes.
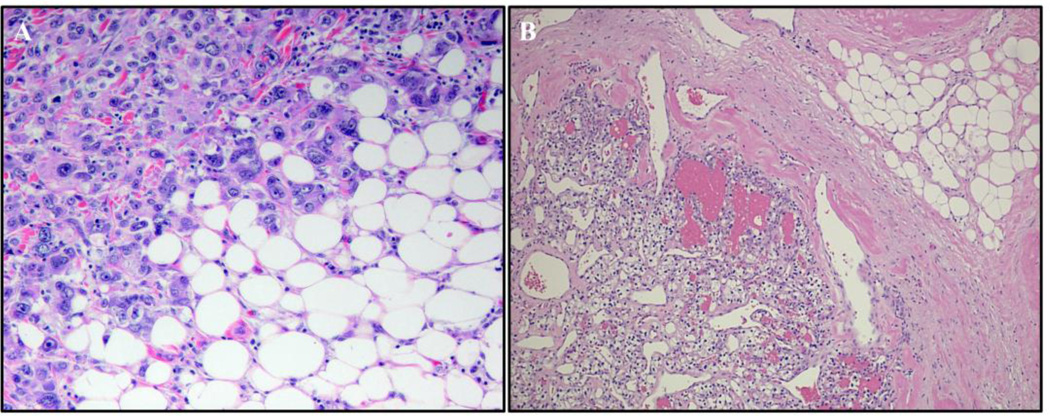
Figure 3. Breast and renal cell cancer invading adipose tissue.
Similar to ovarian cancer, breast and renal cancers both invade and devour neighboring adipose tissue. (A) Ductal breast cancer in a postmenopausal patient infiltrating the adjacent breast adipose tissue. The adipocytes farther away from the tumor are of normal size, while those in close proximity are much smaller. This observation suggests dedifferentiation and delipidation of mature adipocytes to pre-adipocytes (400x). (B) Renal cell cancer invading the perirenal fat pad (100x).
6.1 Adipocytes and breast cancer
Studies in cell culture using the differentiated murine preadipocyte cell line, 3T3-L1, suggest adipocytes promote the growth, proliferation, and survival of human breast cancer cell lines in serum-restricted conditions. Conditioned-medium from differentiated 3T3-L1 cells, initiated a transcriptional program that supported invasion of breast cancer cells through AP-1 transcription factors and proliferation through NFκB and cyclin D1 [62]. Notably, both adipocytes and fibroblasts provided survival benefits for the breast cancer cell lines. However, while the survival benefits were limited to a few days with fibroblasts, they were sustained with adipocytes. Similarly, conditioned-medium from both adipocytes and fibroblasts supported a survival transcriptional program, but the number of genes affected and the magnitude of change induced by fibroblasts was far less than the changes induced by adipocytes [62]. Conditioned-medium from primary human breast preadipocytes differentiated in culture was also reported to increase motility of both normal breast and invasive breast cancer cell lines [63]. However, it is unclear which factors in the conditioned media mediate this effect (e.g., growth factors, fatty acids, and ROS).
Additional evidence establishing cross-talk between adipocytes and breast cancer cells was reported in a very important study using differentiated murine 3T3-F442A preadipocytes. The coculture of the adipocytes with both human and mouse breast cancer cell lines, increased invasiveness of the cancer cells in vitro [57]. It is important to note that the cells were cultured in a transwell system preventing direct contact between cancer cells and adipocytes, indicating that adipocyte-secreted factors were likely responsible for the increased invasion of breast cancer cells. In another study, mouse breast cancer cellscultured with mature adipocytes and injected into the tail vein of mice, resulted in enhanced metastatic tumor burden as compared to cancer cells that were not cultured with adipocytes. The investigators in this study also indicated that phenotypic changes occured in the adipocytes exposed to breast cancer cells, including delipidation, loss of terminal differentiation markers (APN, resistin, and FABP4), and increased expression of proinflammatory cytokines, including IL-6 and PAI-1 [57]. An additional example of cross-talk between adipocytes and cancer cells is data indicating cancer cells induce the reversion of adipocytes to a more fibroblast/preadipocyte phenotype. This is compatible with the theory that adipocytes and fibroblasts originate from the same mesenchymal stem cell [64]. At least in breast cancer models, adipose-tissue mediated mitogenesis might be dependent on estrogen and inhibited by progesterone [65].
The cross-talk between adipocytes and cancer cells may represent a novel target for cancer prevention. Adipocyte-secreted factors, TGF-β and TNF-α, and the upregulation of matrix metalloproteinase (MMP)-11 in the adipocytes, are likely partially responsible for the tumor-promoting effect of preadipocytes. Specifically, MMP-11 is induced in adipocytes in proximity to invading cancer cells by those cancer cells. Activated adipocytes dedifferentiate in the presence of MMP-11, into preadipocyte/fibroblast like cells, which in turn sustain cancer cell invasion [42;56]. If this dedifferentiation of normal adipocytes could be blocked, the early steps of carcinogenesis might be prevented.
Adipocyte-secreted extracellular matrix (ECM) proteins participate in the adaptive response to obesity and cancer which was shown in several elegant studies from the Scherer laboratory (University of Texas Southwestern Medical Center, Dallas) [63;66–68]. In activated adipose tissue collagen VI, an adipocyte-secreted ECM protein, was enriched and contributed to fibrosis. The deletion of collagen VI prolonged adipocyte survival and improved metabolism. Indeed, collagen VI, was responsible for the promotion of early growth and survival of breast cancer cells [63;66;67]. Using a collagen VI-deficient mouse on the background of a mammary cancer model, tumor growth was reduced as compared to wild-type littermates. Similarly, the tumor size in nude mice was reduced when breast cancer cells were subcutaneously injected with adipocytes isolated from collagen VI-deficient mice as compared to adipocytes from wild-type mice [63;66]. The collagen VI α-chain cleavage product, known as endotrophin, reportedly mediated the mammary tumor growth, which is a critical mediator of angiogenesis and inflammation through macrophage recruitment [68]. Taken together, these studies outline a role for adipocytes and adipocyte-secreted factors in both breast cancer tumorigenesis and metastasis.
6.2 Adipocytes and prostate cancer
Adipocytes also play an important role in prostate cancer progression. Accordingly, the extracapsular extension of prostate cancer cells into periprostatic adipose tissue is an adverse prognostic factor. Primary murine adipocytes support colony formation in several prostate cancer cell lines, although this effect varies depending on androgen-dependence. Adipocytes did not stimulate proliferation or the PI3-kinase pathway in androgen-independent cell lines [69]. These data suggest that the tumor-promoting effect of adipocytes is most significant in highly differentiated, hormone-dependent cancer cells. Poorly differentiated cancer cells are more autonomous and less dependent on the adipocyte microenvironment. Periprostatic and visceral adipose tissue has also been implicated in the support of prostate cancer cell growth and motility through the secretion of the MMP-9 [70]. Periprostatic adipose tissue was collected from patients undergoing prostatectomies and used to prepare conditioned-medium. Cytokines secreted into the conditioned-medium were compared to corresponding patient serum. IL-6, a multifunctional cytokine involved in cell proliferation, angiogenesis and tumor progression, was excreted at high levels from adipose tissue and correlated with pathological grade of the tumor, compared to patient-matched serum [70;71]. Adipocytes may also provide lipid mediators to support prostate tumorigenesis, as evidenced by translocation of lipids from adipocytes to prostate cancer cells visualized by FTIR spectroscopy [72]. It is interesting to point out that in prostate cancer cells β-oxidation is a major source of energy [73] and glucose utilization is low, limiting the utility of F18-2DG PET imaging in well-differentiated tumors [74].
6.3 Adipocytes in colon, ovarian, and other cancer types
Two additional studies in colon and ovarian cancer warrant review, since they provide mechanistic insight in the relationship between cancer and adipocytes. In the first study, several colon cancer cell lines were cultured with adipose tissue, adipocytes, or preadipocytes from leptin-deficient (ob/ob) or wild-type mice in a three dimensional culture system containing collagen. All conditions supported the proliferation of colon cancer cells. However, the proliferative effect provided by the adipocytes was found to be leptin-dependent. The mature adipocytes from leptin-deficient mice did not induce cancer cell proliferation, but could be rescued by exogenous leptin administration [75].
The biology of ovarian cancer is different from breast, colon, and prostate cancer, since distant metastasis is rare and often confined to the peritoneal cavity [76]. The most common site of metastasis is the human omentum, a large fat pad (20x20x10 cm), which extends to the pelvis and is positioned in front of the small bowel. In an effort to understand why ovarian cancer metastasizes to the omentum we showed that primary human omental adipocytes induce breast, colon, and ovarian cancer cell proliferation and invasion in vitro and ovarian cancer growth in vivo [77]. It became apparent that adipocyte-secreted cytokines (IL-8 and IL-6) attract ovarian cancer cells to the omentum when the cytokine receptors responsible for binding IL-6 and IL-8 were blocked and fewer cancer cells homed to the omentum. These data showed that cytokines secreted by adipocytes attract cancer cells. Once the ovarian cancer cells interact with the adipocytes they initiate HSL-mediated lipolysis in the adipocyte, releasing fatty acids which are then taken up by the ovarian cancer cells for energy production. This was observed as an increase in β-oxidation which was inhibited by the carnitine-palmitoyltransferase 1 (CPT-1) inhibitor, etomoxir (Figure 2). These data are direct evidence for high-energy lipids provided by the adipocytes stimulating mitochondrial metabolism in the cancer cells, supporting fast tumor growth. Lipid accumulation was also evident in breast and colon cancer cells cultured with adipocytes and metastatic human ovarian cancer tissue sections [77]. Furthermore, there is evidence that an abundant adipocyte protein, fatty acid binding protein 4 (FABP4, aP2, or A-FABP), may support tumor growth. Metastatic tumor burden was drastically reduced in a FABP4-deficient mouse model of ovarian cancer, compared to wild-type controls [77].
When adipocytes are predominant in the tumor microenvironment, as in ovarian or gastric cancers, the tumor promoting role of adipocytes is a local effect mediated by direct contact and paracrine factors; likely independent of obesity or diabetes. Consequently, only a weak, [35] if any, [33] association has been reported between obesity and ovarian cancer, which almost always metastasizes to the omentum. In fact, ovarian cancer patients with a BMI below 25, still present with omental metastases (EL, unpublished observations).
The studies reviewed above suggest that tumorigenesis is supported by the metabolic reprogramming of adipocytes in the microenvironment. In addition, a 2010 study suggests adipocytes may contribute to chemotherapy resistance. Behan et al., [78] reported adipocytes impaired the antitumor effect of vincristine, daunorubicin, and dexamethasone, contributing to the leukemia cell survival. The mechanisms elucidated included a decrease in leukemia cell apoptosis in the presence of adipocytes and an induction of the cell cycle in the presence of chemotherapy drugs.
7 Metabolic symbiosis in cancer
Cancer cells do not reside in plastic tissue culture dishes as solitary cell types where they are typically studied, or as Greenstein put it in 1954, “the tumor does not develop in a vacuum”. Tumor cells are part of a complex tissue environment which defines their metabolism. As the tumor grows, regions evolve specific metabolic functions due to changes in their microenvironement. Dewhirst and colleagues (Duke University, Durham) first demonstrated that hypoxic regions of the tumor generate large amounts of lactate, which is converted to pyruvate and utilized in the mitochondria by the better oxygenated tumor cells. They named this process, metabolic symbiosis and further showed that blocking the monocarboxylate transporter causes death of the hypoxic region of the tumor [79]. In addition, the tissue microenvironment can greatly influence the growth of tumor cells. This is exemplified by the, ground-breaking work of the Lisanti group (Thomas Jefferson University, Philadelphia) which showed that metabolites from CAF are transferred and utilized by cancer cells, promoting tumor growth and metastasis [53;80]. They coined this phenomenon, the “Reverse Warburg effect”, in which CAFs perform aerobic glycolysis, providing lactate to cancer cells which then convert lactate to pyruvate for use in the mitochondria to generate energy. These studies suggest that tumor cells have a high degree of metabolic flexibility and given ample oxygen supply, utilize mitochondria to generate energy and important metabolic intermediates [3]. Cancer cells that grow in the presence of stromal cells including fibroblasts or adipocytes will adapt their metabolism (oxidative phosphorylation and β-oxidation) to take full advantage of the metabolites (lactate, ketones, glutamine, fatty acids) provided by the local host cells [54]. The role of mitochondrial oxidative phosphorylation in metastatic melanoma has been recently been examined. Surprisingly, cells freshly isolated from metastatic melanoma showed high levels of oxidative phosphorylation and tissue microarrays showed high levels of complex V in metastatic melanoma [81]. These data suggest mitochondrial function is important in metastasis and consistent with the idea that fatty acids could help fuel this bioenergetic demand. The metabolic interactions between adipocytes and cancer cells ([77] discussed in [54]), are consistent with the “metabolic symbiosis” [3] between cancer cells and host stromal cells. These studies also strongly suggest that tumor cells growing in an adipocyte-dominated microenvironment are highly reliant on mitochondrial β-oxidation for ATP production.
8 Conclusions and future areas of research
In summary, the current evidence clearly supports a model in which cancer cells reprogram adipocytes to CAA. Reprogrammed adipocytes produce growth promoting cytokines and provide lipids and other metabolites to cancer cells, promoting uncontrolled tumor growth.
The tumor promoting functions of adipocytes are a result of both the systemic actions of hormones involved in lipid hemostasis and local effects of adipocytes in the tumor microenvironment. Alterations in lipid metabolism during carcinogenesis may represent unique targets for cancer prevention and treatment. In fact, a commonly used diabetic medication, metformin, targets several of the pathways discussed. Prospective clinical trials are underway to test the efficacy of metformin as an adjuvant treatment in breast cancer [82]. Inhibition of enzymes regulating lipolysis, such as HSL or MAGL, may prove to be additional targets [27–29].
While our understanding of the role of adipocytes in cancer biology has increased dramatically over the last several years; many important questions remain. Future investigations should focus on determining whether adipocytes play a role in tumor initiation or progression. The literature supports a role in metastasis and tumor growth but do adipocytes play a role in tumor initiation and promotion during the early stages of tumorigenesis? The answer to this question will be critically important in understanding whether obesity and adipocytes are viable targets for the primary prevention or adjuvant treatment of cancer. A second novel area of investigation is understanding how cancer cells reprogram adipose and stromal cells in the tumor microenvironment. What are the paracrine signals sent by cancer cells which reprogram adipocytes into CAA? What is the relative contribution of mitochondrial β-oxidation, as compared to glycolysis of tumor and stromal cells? A third area of study is to understand if there is a difference in the interaction of tumor cells with adipocytes in lean as compared to obese individuals? In other words, does activated adipose tissue in obesity, with its high content of inflammatory cells and increased cytokine secretion, affect the interaction of adipocytes with tumor cells?
Understanding the biology of adipocytes in the microenvironment will identify metabolic targets and allow us to introduce a new class of compounds for the treatment of cancer. To this end, weight loss and exercise could be considered as a strategy for cancer prevention and improving survival, particularly for breast and colorectal cancer [83;84]. Further, restoring metabolic and endocrine function in cancer-associated adipocytes may prove to be a worthy direction of study for cancer prevention and treatment.
Highlights.
We discuss the role of adipocytes and adipose tissue in tumor progression.
Cancer cells recruit adipocytes and use their lipids for rapid tumor growth.
Understanding the interactions between cancer cells and adipocytes should reveal new targets
Acknowledgments
Dr. Nieman is supported by a National Research Service Award from the National Cancer Institute. Dr. Romero is supported by grants from the Reproductive Scientist Development Program and the Prevent Cancer Foundation. Dr. Van Houten is supported by UPCI start-up and the Pennsylvania Department of Health, PA CURE. Dr. Lengyel is supported by a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund, the Ovarian Cancer Research Fund, the National Cancer Institute, and Bears Care, the charitable beneficiary of the Chicago Bears Football Club.
Footnotes
This article is part of a Special Issue entitled Lipid Biology in Cancer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- 1.Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: A role for cancer-associated adipocytes. Endocr Dev. 2010;19:45–52. doi: 10.1159/000316896. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: Functions of cell recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima EC, Van Houten B. Metabolic symbiosis in cancer: Refocusing the Warburg lens. Mol Carcinog. 2012:1–9. doi: 10.1002/mc.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Outschoorn UE, Whitaker-Menezes D, Pavlides S, Chiavarina B, Bonuccelli G, Trimmer C, Tsirigos A, Migneco G, Witkiewicz AK, Balliet R, Mercier I, Wang C, Flomenberg N, Howell A, Lin Z, Caro J, Pestell RG, Sotgia F, Lisanti MP. The autophagic tumor stroma model of cancer or "battery-operated tumor growth" a simple solution to the autophagy paradox. Cell Cycle. 2010;9:4297–4306. doi: 10.4161/cc.9.21.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS Journal. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 6.Sethi JK, Vidal-Piug AJ. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 8.Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol Scand. 2005;183:13–30. doi: 10.1111/j.1365-201X.2004.01385.x. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic diseases. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Perez R, Ortega-Delgado FJ, Garcia-Santos E, Lopez JA, Camafeita E, Ricart W, Fernandez-Real JM, Peral B. Differential proteomics of omental and subcutanous adpose tissue reflects their unalike biochemical and metabolic properties. J Proteome Res. 2009;8:1682–1693. doi: 10.1021/pr800942k. [DOI] [PubMed] [Google Scholar]
- 11.Rehrer C, Karimpour-Fard A, Hernandez TL, Law C, Stob N, Hunter L, Eckel RH. Regional differences in subcutaneous adipose tissue gene expression. Obesity. 2012;20:2168–2173. doi: 10.1038/oby.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, Van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristancho AG, Lazar MA. Forming functional fat: A growing understanding of adipocyte differentiation. Nature Rev. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Clinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frayn KN, Shadid S, Hamlani R, Humphreys SM, L Clark M, Fielding BA, Boland O, Coppack SW. Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol. 1994;266:E308–E317. doi: 10.1152/ajpendo.1994.266.3.E308. [DOI] [PubMed] [Google Scholar]
- 16.Christianson JL, Nicoloro S, Straubhaar J, Czech MP. Stearoyo-CoA desaturase 2 is required for peroxisome proliferator-activated receptor y expression and adipogenesis in cultured. J Biol Chem. 2008;283:2906–2916. doi: 10.1074/jbc.M705656200. [DOI] [PubMed] [Google Scholar]
- 17.Unger R, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Guh DP, Zhang W, Bansback N, Amarsi Z, Laird Birmingham C, Anis AH. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. Biomed Central Public Health. 2009;9:1–20. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg SP, McCann D, Desai M, Rosenbaum M, L Leibel R, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E656–E665. doi: 10.1152/ajpendo.00127.2007. [DOI] [PubMed] [Google Scholar]
- 21.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa KI, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 23.Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-κB expression and activity. Am J Physiol Endocrinol Metab. 2004;287:E1187–E1188. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- 24.Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Hormone and Metabolic Research Supplement. 1988;19:26–29. [PubMed] [Google Scholar]
- 25.Hertzel AV, Thompson BR, Wiczer BM, Bernlohr DA. Lipid metabolism in adipose tissue. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and membranes. Amsterdam: Elsevier; 2008. pp. 277–304. [Google Scholar]
- 26.Cases S, Smith SJ, Zhent YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV., Jr Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodgriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 29.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 30.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura DK, Lombardi DP, Chang JW, Niessen S, Ward AM, Long JZ, Hoover HH, Cravatt BF. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1–G4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 34.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the million women study: Cohort study. BMJ. 2012:1–11. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calle EE, Rodriguez C, Walker-Thurmond K, Thun M. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 36.Friedenreich CM, Biel RK, Lau DC, Csizmadi I, Courneya K, Magliocco A, Yasui Y, Cook L. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2384–2395. doi: 10.1158/1055-9965.EPI-11-0715. [DOI] [PubMed] [Google Scholar]
- 37.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 39.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–757. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS) --induced genetic and epigenetic alterations in human carcinogenesis. Mutant Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Kim YJ, Kim EH, Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. Journal of Gastroenterol Hepatology. 2012;27:1004–1010. doi: 10.1111/j.1440-1746.2012.07108.x. [DOI] [PubMed] [Google Scholar]
- 42.Guerrero J, Tobar N, Cáceres M, Espinoza L, Escobar P, Dotor J, Smith PC, Martìnez J. Soluble factors derived from tumor mammary cell lines induce a stromal mammary adipose reversion in human and mice adipose cells. Possible role of TGF- β1 and TNF-x . Breast Cancer Res Treat. 2010;119:497–508. doi: 10.1007/s10549-009-0491-1. [DOI] [PubMed] [Google Scholar]
- 43.Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 44.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khasawneh J, Schulz M, Walch A, Rozman J, Hrabe de Angelis M, Klingenspor M, Buck A, Schwaiger M, Saur D, Schmid RM, Klöppel G, Sipos B, Greten FR, Arkan MC. Inflammation and mitochondrial fatty acid β-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci USA. 2009;106:3354–3359. doi: 10.1073/pnas.0802864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pou KM, Massaro JM, Hoffman U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 47.Dal Maso L, Augustin L, Karalis A, Talamini R, Franceschi S, Trichopoulos D, Mantzoros CS, La Vecchia C. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- 48.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: Overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 49.Taubes G. Epidemiology faces its limits. Science. 1995;269:164–169. doi: 10.1126/science.7618077. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Daquinag A, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- 52.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Maza O, Sotgia F, Lisanti MP. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Power surge: Supporting cells "fuel" cancer cell mitochondria. Cell Metab. 2012;15:4–5. doi: 10.1016/j.cmet.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 55.Mitra AK, Zillhardt M, Hua YJ, Tiwari P, Murmann A, Peter ME, Lengyel E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2:1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andarawewa KL, Montrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, Rio MC. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65:10862–10871. doi: 10.1158/0008-5472.CAN-05-1231. [DOI] [PubMed] [Google Scholar]
- 57.Dirat B, Bochet L, Dabek M, Daviaud D, Dauviller S, Majad B, Wang YY, Meulle A, Sailes B, LeGonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 58.Tisdale M. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–870. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 59.Agustsson T, Rydén M, Hoffstedt J, van Harmelen V, Dicker A, Laurencikiene J, Isaksson B, Permert J, Arner P. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67:5531–5537. doi: 10.1158/0008-5472.CAN-06-4585. [DOI] [PubMed] [Google Scholar]
- 60.Spector A. The importance of free fatty acid in tumor nutrition. Cancer Res. 1967;27:1580–1586. [PubMed] [Google Scholar]
- 61.Mermier P, Baker N. Flux of free fatty acids among host tissues, ascites fluid, and Ehrlich ascites carcinoma cells. J Lipid Res. 1974;15:339–351. [PubMed] [Google Scholar]
- 62.Adib T, Henderson S, Perrett C, Bourmpoulia D, Lederman J, Boshoff C. Predicting biomarkers for ovarian cancer using gene-expression microarrays. Br J Cancer. 2004;90:686–692. doi: 10.1038/sj.bjc.6601603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter JC, Church FC. Mature breast adipocytes promote breast cancer cell motility. Experimental and Molecular Pathology. 2012;92:312–317. doi: 10.1016/j.yexmp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: A vicious tumor progression cycle. Biol Chem. 2008;389:1037–1041. doi: 10.1515/BC.2008.110. [DOI] [PubMed] [Google Scholar]
- 65.Elliott BE, Tam SP, Dexter D, Chen ZQ. Capacity of adipose tissue to promote growth and metastasis of a murine mammary carcinoma: Effect of estrogen and progesterone. Int J Cancer. 1992;51:416–424. doi: 10.1002/ijc.2910510314. [DOI] [PubMed] [Google Scholar]
- 66.Iyengar P, Combs TP, Shah SJ, Gouon V, Pollard J, Albanase C, Flanagan L, Tenniswood MP, Guha C, Lisanti MP, Pestell RG, Scherer PE. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 67.Iyengar P, Espina V, Williams T, Lin Y, Berry D, Jelicks L, Lee H, Temple K, Graves R, Pollard J, Chopra N, Russell R, Sasisekharan R, Trock B, Lippman M, Calvert V, Petricoin E, Liotta L, Dadachova E, Pestell RG, Lisanti MP, Bonaldo P, Scherer PE. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenviroment. J Clin Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko A, Satoh Y, Tokuda Y, Fujiyama C, Udo K, Uozumi J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. International Journal of Urology. 2010;17:369–376. doi: 10.1111/j.1442-2042.2010.02472.x. [DOI] [PubMed] [Google Scholar]
- 70.Ribeiro R, Monteiro C, Cunha V, Oliveira MJ, Freitas M, Fraga A, Principe P, Lobato C, Morais A, Silva V, Sanchez-Magalhaes J, Oliveira J, Pina F, Mota-Pinto A, Lopes C, Medeiros R. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro . Journal of Experimental & Clinical Cancer Research. 2012;31:1–12. doi: 10.1186/1756-9966-31-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin FE, Zaldivar F, Santos R, Tyson DR, Ornstein DK. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. The Journal of Urology. 2009;182:1621–1627. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Gazi E, Gardner P, Lockyer N, Hart C, Brown M, Clarke N. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846. doi: 10.1194/jlr.M700131-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostate Diseases. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology. 2007;72:226–233. doi: 10.1159/000112946. [DOI] [PubMed] [Google Scholar]
- 75.Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, Shiraishi R, Sakata Y, Tsunada S, Iwakiri R, Fujimoto K. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2006;292:G923–G929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- 76.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt M, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Behan JW, Yun JP, Proektor MP, Ehsanipour EA, Arutyunyan A, Moses AS, Avramis VI, Louie SG, Butturini A, Heisterkamp N, Mittleman S. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867–7874. doi: 10.1158/0008-5472.CAN-09-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonveaux P, Schroeder VF, Wergin MC, Verrax J, Rabbani ZN, DeSaedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, Sotgia F, Brody JR, Mitchell EP, Lisanti MP. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–143. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho J, deMoura MB, Lin Y, Vincent G, Thorne S, Duncan LM, Hui-Min L, Kirkwood JM, Becker D, Van Houten B, Moschos SJ. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol Cancer. 2012;11 doi: 10.1186/1476-4598-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dowling R, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: Translational challenges. J Mol Endocrinol. 2012;48:31–43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- 83.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 84.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1548–1561. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]