Abstract
Human T-cell leukemia virus type 1 (HTLV-1) causes adult T-cell leukemia (ATL). Although the viral transactivation factor, Tax, has been known to have apparent transforming ability, the exact function of Tax in ATL development is still not clear. To understand the role of Tax in ATL development, we introduced short-interfering RNAs (siRNAs) against Tax in a rat HTLV-1-infected T-cell line. Our results demonstrated that expression of siRNA targeting Tax successfully downregulated Tax expression. Repression of Tax expression was associated with resistance of the HTLV-1-infected T cells to Tax-specific cytotoxic-T-lymphocyte killing. This may be due to the direct effect of decreased Tax expression, because the Tax siRNA did not alter the expression of MHC-I, CD80, or CD86. Furthermore, T cells with Tax downregulation appeared to lose the ability to develop tumors in T-cell-deficient nude rats, in which the parental HTLV-1-infected cells induce ATL-like lymphoproliferative disease. These results indicated the importance of Tax both for activating host immune response against the virus and for maintaining the growth ability of infected cells in vivo. Our results provide insights into the mechanisms how the host immune system can survey and inhibit the growth of HTLV-1-infected cells during the long latent period before the onset of ATL.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL) (17, 40) and a chronic progressive neurological disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (12, 38). Since examination of the viral nucleotide sequences among different disease groups has not revealed any specific determinants that distinguish a particular HTLV-1-associated disease, it has been speculated that a primary determinant of HTLV-1-associated disease is host related (3, 26, 53).
ATL is an aggressive malignancy of CD4+ T cells, affecting a subgroup of middle-aged HTLV-1 carriers characterized by the presence of mature T-cell phenotype (51). The HTLV-1 has been shown to activate and immortalize human T cells in vitro, resulting in polyclonal proliferation of infected cells and subsequent oligoclonal or monoclonal growth (11, 52). The HTLV-1 genome contains a unique 3′ region, designated pX, that encodes the viral transactivator protein, Tax (42). Tax transactivates not only the viral long terminal repeat (7, 43, 47) but also the promoters of cellular genes such as interleukin-2 (IL-2) (45), IL-2 receptor (18), myc (5), and fos (8). Thus, it is speculated that Tax plays a central role in HTLV-1-associated immortalization and transformation of T cells, which may lead to the development of ATL.
Despite the apparent transforming ability of Tax in HTLV-1 infection under experimental conditions, most HTLV-1 carriers are asymptomatic. One explanation for this is that HTLV-1 is controlled by host immunity in most carriers, as is the case in many other viruses. In addition, Tax is known as a major target protein recognized by cytotoxic T lymphocytes (CTL) of HTLV-1 carriers (19, 21). It has been reported that the levels of HTLV-1-specific CTL are quite diverse among HTLV-1 carriers and that ATL patients have impaired levels of HTLV-1 specific CTL in contrast to the high levels of CTL response in HTLV-1 carriers with HAM/TSP (23, 24, 39). Since HTLV-1 Tax-specific CTL can recognize and lyse ATL cells in vitro (22), it is reasonable to assume that the low CTL activity in ATL patients is disadvantageous since it may allow uncontrolled proliferation and evolution of HTLV-1-infected cells in vivo.
On the other hand, it is also known that Tax expression is rarely detected in fresh peripheral blood mononuclear cells from HTLV-1-infected individuals (22) and that the expression level of Tax mRNA in ATL is lower than that in HAM/TSP or asymptomatic carriers (25). This observation raised the possibility that HTLV-1-infected cells that do not require Tax expression are selected in the course of ATL development and that the appearance of these cells may lead to the reduction of HTLV-1-specific CTL activities. Thus, to understand the pathogenesis of ATL, it is important to study the interplay between host immune responses and HTLV-1-infected T cells in vivo. For this purpose, establishment of a suitable animal model is required. We have previously established a rat model of ATL-like disease, which allows examination of the growth and spread of HTLV-1-infected cells, as well as assessment of the effects of immune T cells on the development of the disease (16, 35). By using this model system, we recently reported the therapeutic effect of Tax-coding DNA or peptide against the disease (15, 36). Since the HTLV-1-infected cells used in this model predominantly express Tax protein, this system is thought to be useful for further analyzing the role of Tax in the course of ATL development.
RNA interference is a mechanism of posttranscriptional gene silencing and has become a powerful and widely used tool for the analysis of gene function in plants, invertebrates (44) and, more recently, in mammalian cells (6). Recent reports demonstrated that genes encoding short hairpin RNAs could be engineered into plasmid vectors for stable expression in target cells (2, 54). In this study, we introduced small interfering RNAs (siRNAs) targeted against Tax in an HTLV-1-infected rat T-cell line and examined the role of Tax in our rat model system. Our results demonstrated that repression of Tax expression in the rat cells by the siRNAs resulted in impaired ability to activate Tax-specific proliferative response and CTL activity. Furthermore, T cells with Tax downregulation appeared to lose the ability to develop tumors in T-cell-deficient nude rats, in which the parental HTLV-1-infected cells can induce ATL-like lymphoproliferative disease. These results indicated the significant roles of Tax both for activating host immune response to the virus and for maintaining the growth ability of infected cells in vivo.
MATERIALS AND METHODS
Animals.
Female F344/N Jcl-rnu/rnu (nu/nu) rats and F344/N Jcl-rnu/+ (nu/+) rats were purchased from Clea Japan, Inc. (Tokyo, Japan). All rats were maintained at the experimental animal facilities of Tokyo Medical and Dental University. The experimental protocol was approved by the Animal Ethics Review Committee of our university.
Cell lines.
Mouse lymphoma cell lines, EL4, EL4/Gax, and EL4/enhanced green fluorescent protein (EGFP), were previously described (10). An HTLV-1-immortalized cell line, FPM1, was established in our laboratory by cocultivating thymocytes of a nu/+ rat with the HTLV-1-producing human cell line, MT-2, which was treated with mitomycin C (50 μg/ml) for 30 min at 37°C (28). FPM1.BP was a subclone of FPM1 and was obtained by treating IL-2-dependent FPM1 cells with 100 μg of chemical carcinogen benzo(a)pyrene diol epoxide (BPDE)/ml (H. Tateno et al., unpublished data). The cells were maintained in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS; Whittaker, Walkersville, Md.), penicillin, and streptomycin. A CD8+ Tax-specific CTL line, 4O1/C8, and an IL-2-dependent HTLV-1-negative cell line, G14, were also established in our laboratory from nu/+ rats and were maintained in RPMI 1640 medium with 10% FCS and 10 to 20 U of IL-2 (Shionogi, Osaka, Japan)/ml as described previously (36, 37).
Expression plasmids.
The mouse U6 promoter was isolated by PCR from mouse genomic DNA as described previously with minor modifications (54). Briefly, for PCR amplification, we used a primer pair of 5′-CCCGAATTCATCCGACGCCGCCATCTCTA-3′ and 5′-GCTCGAGGAAGACCACAAACAAGGCTTTTCTCCAA-3′, which has EcoRI and XhoI sites at each 5′ end, respectively. The PCR products were digested, cloned into EcoRI-XhoI sites of the vector pBluescript SK(+), and designated pBS/mU6. The accuracy of the U6 promoter sequence was confirmed by ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif.). Hairpin siRNA sequences targeted against EGFP or Tax were synthesized as two cDNA oligonucleotides (for sequences, see Fig. 1A), annealed, and ligated between the EcoRI and XhoI sites of the vector pEGFP-1 (Clontech, Palo Alto, Calif.), together with pBS/mU6-derived U6 promoter digested with BbsI and EcoRI. The obtained siGFP- or siTax-expressing vectors contain neomycin-resistant genes for in vitro selection and were designated pEGFP-1/mU6/siGFP or pEGFP-1/mU6/siTax, respectively.
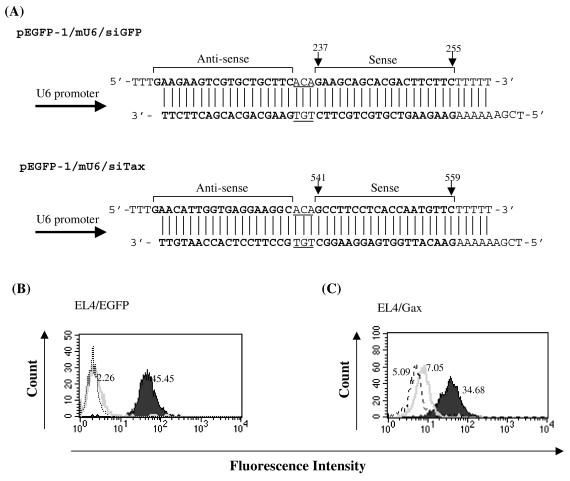
FIG. 1.
Repression of EGFP and Tax expression by mouse U6-driven siRNAs in mouse lymphoma cells. (A) Schematic drawing of the transcribed region of pEGFP-1/mU6/siGFP and pEGFP-1/mU6/siTax vectors. The mouse U6 promoter was cloned in front of the gene-specific targeting sequence. Nineteen-nucleotide sequences (sense) from EGFP (positions 237 to 255) or Tax (positions 541 to 559) are separated by a short spacer (underline) from the reverse complement of the same sequence (antisense). Five thymidines (T) are added as termination signal. (B) EGFP expression in EL4 (dotted histogram), EL4/EGFP (solid histogram), or EL4/EGFP-siGFP (open histogram) was examined by flow cytometric analysis. (C) Expression of EGFP-Tax fusion protein in EL4/Gax (solid histogram), EL4/Gax-siTax (open histogram), or EL4/Gax-siGFP (dotted histogram) was examined by flow cytometric analysis. The values shown indicate the mean fluorescence intensity in each histogram.
Transfection.
Cells were electrically transfected with pEGFP-1/mU6/siGFP or pEGFP-1/mU6/siTax by using GenePulser II systems (Bio-Rad, Hercules, Calif.), and stable transfectants were selected with 400 μg of Geneticin (Sigma, St. Louis, Mo.)/ml. After 4 weeks of Geneticin selection, surviving cells were cloned by limiting dilution method and examined for EGFP or Tax expression by flow cytometry by using a FACScalibur (Becton Dickinson, San Jose, Calif.).
Detection of intracellular Tax protein.
For intracellular analysis, 106 cells were fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS) containing 20 μg of Lysolecitin (Sigma) for 2 min at room temperature, centrifuged, and resuspended in cold methanol. The cells were then stored at 4°C for 15 min, centrifuged, and incubated in 0.1% Triton X-100 in PBS at 4°C for 5 min. After centrifugation, the cells were stained with mouse anti-Tax monoclonal antibody Lt-4 (30) for 30 min at room temperature, washed 1 time with staining buffer (1% FCS, 0.1% NaN3 in PBS), and then stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G plus M antibody (IgG+IgM monoclonal antibody; Immunotech, Marseille, France) for 30 min at room temperature. Finally, the cells were washed twice and fixed with 1% formalin in PBS prior to analysis on a FACScalibur (Becton Dickinson).
Protein analysis.
Cells were resuspended in ice-cold extraction buffer (20 mmol of HEPES [pH 7.9], 10 mmol of KCl, 1 mmol of MgCl2, 150 mmol of NaCl, 1% Triton X-100, 0.5 mmol of dithiothreitol, and 0.5 mmol of phenylmethylsulfonyl fluoride/liter; 1 μg of aprotinin/ml; 1 μg of leupeptin/ml) and gently rocked for 30 min. After centrifugation at 14,000 × g for 20 min at 4°C, the supernatant was collected as a whole-cell extract. The protein concentration of each sample was determined by using a protein assay kit (Bio-Rad). A total of 140 μg of whole-cell extracts was separated by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis and transferred to a nitrocellulose filter. After incubation with blocking buffer (2% bovine serum albumin in 10 mmol of Tris-HCl [pH 7.5] and 100 mmol of NaCl/liter), the filter was incubated with 1:500-diluted anti-Tax monoclonal antibody, Lt-4, and then with an anti-mouse immunoglobulin antibody conjugated to horseradish peroxidase (Amersham, Arlington Heights, Ill.). Antibodies bound to the filter were detected by the enhanced chemiluminescence method (Amersham).
Detection of HTLV-1 P19.
FPM1B-siGFP1, FPM1B-siGFP2, FPM1B-siTax27, or FPM1B-siTax32 cells (104/well) were cultured in each well of 96-well flat-bottom microtiter plates for 4 days. The amount of HTLV-1 p19 protein in the culture supernatant was quantified by using HTLV-1/II p19 antigen enzyme-linked immunosorbent assay (ELISA; ZeptoMetrix Co., Buffalo, N.Y.) in accordance with the manufacturer's instructions.
Analysis of cell surface markers.
Expression of cell surface markers was examined by flow cytometry. Briefly, 106 cells were stained with various mouse monoclonal antibodies for 30 min on ice, washed three times with 1% FCS in PBS, and then stained with FITC-conjugated goat anti-mouse IgG+IgM. After being washed, the cells were fixed with 1% formalin in PBS prior to analysis on a FACScalibur (Becton Dickinson). Anti-rat major histocompatibility complex class I (MHC-I; RT1.A) and MHC-II (RT1.B) were purchased from Cedarlane Laboratories (Hornby, Ontario, Canada), and an anti-rat CD25 antibody was from Chemicon International, Inc. (Temecula, Calif.). Antibodies against rat CD80 and CD86 were generously provided by Ko Okumura and Hideo Yagita (Juntendo University, Tokyo, Japan) (32).
T-cell proliferation assay.
A CD8+ Tax-specific CTL line, 4O1/C8 (1 × 105 or 5 × 104 cells/well) was cocultured with formalin-fixed FPM1B-siGFP1, FPM1B-siGFP2, FPM1B-siTax27, FPM1B-siTax32, or G14 cells (5 × 104 cells/well) in 96-well round-bottom culture plates at 37°C for 72 h. Cultures were pulsed with [3H]thymidine ([3H]TdR; 37 kBq/well) for the last 18 h to assess cell proliferation. Cells were harvested with a Micro 96 Harvester (Skatron, Lier, Norway), and [3H]TdR uptake into cells (reported as the mean ± the standard deviation [SD]) was measured in a Microplate β-Counter (Micro Beta Plus; Wallac, Turku, Finland).
51Cr-release cytotoxicity assay.
CTL activity against HTLV-1-infected cells was measured by 6-h 51Cr-release assay at various effector/target ratios. A Tax-specific rat CTL line, 4O1/C8, was used as effector cells. 51Cr-labeled FPM1.BP, FPM1B-siGFP1, FPM1B-siTax27, or FPM1B-siTax32 cells were used as target cells. The 51Cr-labeled target cells (104 cells/well) were cocultured with various numbers of effector cells in 96-well round-bottom culture plates at 37°C for 6 h, and then the 51Cr activities released in the supernatants were measured. The percent specific cytotoxicity was calculated as follows: (experimental 51Cr release − spontaneous 51Cr release/maximum 51Cr release − spontaneous 51Cr release]) × 100.
Cell growth assay.
FPM1B-siGFP1, FPM1B-siGFP2, FPM1B-siTax27, or FPM1B-siTax32 cells (104/well) were cultured in each well of 96-well flat-bottom microtiter plates for 4 days. The number of growing cells was determined by using a Cell Counting Kit-8 (Dojinndo Laboratories, Kumamoto, Japan) in accordance with the manufacturer's instructions.
Inoculation of HTLV-1-immortalized cells.
A total of 2 × 107 FPM1.BP, FPM1B-siGFP1, FPM1B-siGFP2, FPM1B-siTax27, or FPM1B-siTax32 cells were inoculated subcutaneously into 3-week-old nu/nu rats. The growth of subcutaneous tumor was measured every other day and recorded as the longest surface length (a [in millimeters]) and width (b [in millimeters]). Tumor volume (V [in cubic millimeters]) was calculated according to the following formula: V = a × b2 × 0.5, as described previously (35).
RESULTS
The siRNA targeted against Tax reduced the expression of Tax in both mouse and rat T cells.
Based on the sequence previously reported (54), we constructed a vector, which can express EGFP-targeted siRNA under the control of the mouse U6 promoter, and designated the resulting vector pEGFP-1/mU6/siGFP (Fig. 1A). We introduced the vector into the EGFP-expressing mouse T-cell line, EL4/EGFP, selected the stable transfectants by using 400 μg of neomycin/ml, and cloned the survived cells by the limiting dilution method. We then examined the EGFP expression in each clone by flow cytometry to pick up clones with reduced EGFP expression. Among nine clones tested, three clones exhibited >60% reduction of EGFP expression compared to parental EL4/EGFP cells (Table 1). A representative result of maximum EGFP downregulation observed in the EL4/EGFP-siGFP clone was shown in Fig. 1B. In this clone, the expression of EGFP was almost completely inhibited (95% inhibition), indicating that mouse U6 promoter-driven siRNA targeted against EGFP is effective in EL4/EGFP cells. We next constructed a vector, which replaced the sequence targeted against EGFP to that directed to Tax in pEGFP-1/mU6/siGFP (Fig. 1A) and obtained pEGFP-1/mU6/siTax. It was anticipated that the EGFP expression in EL4/Gax cells should be inhibited by Tax-targeting siRNAs, because both EGFP and Tax transcripts are on the same RNAs. As shown in Fig. 1C, EL4/Gax-siTax cells, which had been selected with 400 μg of neomycin/ml after transfection and cloned, expressed 80% less EGFP than did parental EL4/Gax cells. We obtained a total of four clones that showed >60% reduction of EGFP expression of seven clones tested (Table 1), indicating that pEGFP-1/mU6/siTax efficiently reduced the Tax mRNA in the mouse cells. We also introduced pEGFP-1/mU6/siGFP vector into EL4/Gax cells to directly evaluate the relative effectiveness of the Tax siRNA plasmid compared to the EGFP siRNA plasmid. As shown in Table 1, of 24 clones tested, we were able to obtain 13 clones that showed >60% reduction of EGFP expression compared to parental EL4/Gax cells. Although the cloning efficiency to pick up cells with >60% reduction of EGFP-fused Tax expression were similar, the maximum reduction of EGFP-fused Tax by the EGFP siRNA (86% inhibition) was apparently more than that induced by the Tax siRNA (80% inhibition) as shown in Fig. 1C. These results suggested that the EGFP siRNA could inhibit EGFP-fused Tax expression more effectively than the Tax siRNA.
TABLE 1.
Isolation of clones with the siRNA-induced reduction of target gene expression
| Cell | Vector | No. of clones tested | No. of clones with % downregulationa of: |
% clones with >60% of downregulation | |||
|---|---|---|---|---|---|---|---|
| <40% | 40-50% | 50-60% | >60% | ||||
| EL4/EGFP | pEGFP-1/mU6/siGFP | 9 | 5 | 0 | 1 | 3 | 33 |
| EL4/Gax | pEGFP-1/mU6/siGFP | 24 | 5 | 2 | 4 | 13 | 54 |
| EL4/Gax | pEGFP-1/mU6/siTax | 7 | 1 | 1 | 1 | 4 | 57 |
| FPM1.BP | pEGFP-1/mU6/siTax | 45 | 41 | 3 | 1 | 0 | 0 |
Downregulation of EGFP or Tax was determined by fluorescence-activated cell sorting.
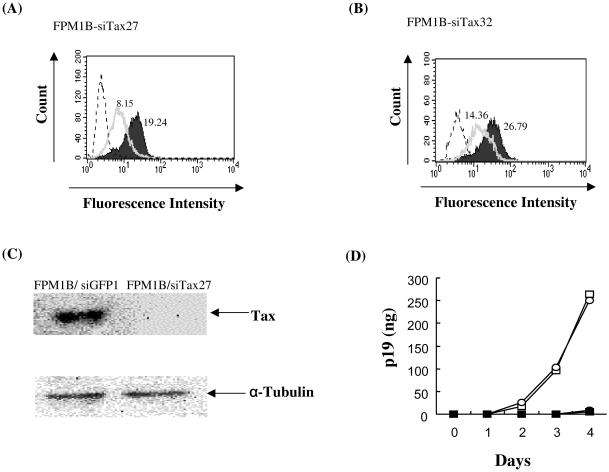
We next introduced pEGFP-1/mU6/siTax plasmids into an HTLV-1-infected rat T-cell line, FPM1.BP, to determine whether the vector can also inhibit the Tax expression in rat T cells. A flow cytometric assay was performed to detect intracellular Tax protein expression. Of 45 clones tested, we obtained only one clone, FPM1B-siTax27, that showed >50% reduction of Tax expression compared to the FPM1B-siGFP1 clone, which was transfected with pEGFP-1/mU6/siGFP plasmids as a control (Table 1). As shown in Fig. 2A, FPM1B-siTax27 cells exhibited the severest downregulation of Tax and expressed 58% less Tax than did FPM1B-siGFP1 cells. Another clone, FPM1B-siTax32, showed the second-severest downregulation of Tax and expressed 46% less Tax than did FPM1B-siGFP1 cells (Fig. 2B). The reduction of Tax protein expression in FPM1B-siTax27 cells was also confirmed by Western blot analysis as shown in Fig. 2C. These results indicated that the pEGFP-1/mU6/siTax plasmids were able to inhibit Tax expression in the HTLV-1-infected rat T-cell line.
FIG. 2.
Repression of Tax expression by the siRNAs targeting Tax in rat HTLV-1-infected T cells. (A and B) FPM1B-siGFP1 (solid histogram), FPM1B-siTax27 (open histogram in panel A) or FPM1B-siTax32 (open histogram in panel B) cells were stained with the anti-Tax monoclonal antibody for 30 min and then labeled with FITC-conjugated anti-mouse IgG and IgM. After being washed, cells were subjected to flow cytometric analysis to assess intracellular Tax expression. Histogram of FPM1B-siGFP1 cells stained only with FITC-conjugated second antibody was represented by the dotted line. (C) Expression of Tax protein in FPM1B-siGFP1 or FPM1B-siTax27 cells was analyzed by Western blotting. Whole-cell extracts were isolated from each cell line, and 140 μg of each protein was separated by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was reacted with Lt-4 and then with horseradish peroxidase-conjugated anti-mouse immunoglobulin antibody. Antibodies bound to the membrane were detected by the enhanced chemiluminescence method. The expression of α-tubulin was also examined as an internal control. (D) Production of p19 protein in culture supernatant was analyzed by ELISA. FPM1B-siGFP1 (○), FPM1B-siGFP2 (□), FPM1B-siTax27 (•), or FPM1B-siTax32 (▪) cells (104/well) were cultured in each well of 96-well flat-bottom microtiter plates for 4 days. The amount of HTLV-1 p19 protein in 200 μl of the culture supernatant was quantified by using HTLV-1/II p19 antigen ELISA (ZeptoMetrix) in accordance with the manufacturer's instructions.
It was anticipated that the pEGFP-1/mU6/siTax plasmids should also downregulate the expression of other HTLV-1 genes in addition to Tax. Thus, we have attempted to examine the alteration of other viral protein expression after the Tax siRNA introduction. Since we have previously reported the very low production of viral structural proteins by parental FPM1 cells (28), we utilized a sensitive ELISA system to detect HTLV-1 p19 protein. As shown in Fig. 2D, FPM1B-siGFP1 and FPM1B-siGFP2, another control clone transfected with pEGFP-1/mU6/siGFP, produced detectable amounts of p19 after 3 days of cultivation. In contrast, p19 production by FPM1B-siTax27 and FPM1B-siTax32 was not detected by 3 days and was kept at a very low level after 4 days of cultivation. These results suggested that the expression of the HTLV-1 structural protein was also inhibited by the siRNA targeted against Tax.
Repression of Tax expression was associated with evasion of Tax-specific CTL lysis.
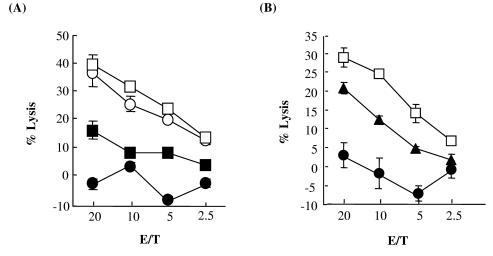
In HTLV-1 infection, it has been suggested that scarcity of viral antigens in vivo is one of the strategies by which the virus can escape host immune surveillance (22, 23). Thus, it was speculated that the insufficiency of Tax protein in FPM1B-siTax27 cells could lead to evasion of CTL lysis by the HTLV-1-infected cells. To assess this possibility, we examined whether FPM1B-siTax27 cells are still susceptible to killing by a CD8+ Tax-specific CTL line, 4O1/C8. As target cells, we used FPM1.BP, FPM1B-siGFP1, and HTLV-1-negative G14 cells together with FPM1B-siTax27 cells. As shown in Fig. 3A, 4O1/C8 cells showed a strong cytotoxic activity against FPM1.BP and FPM1B-siGFP1 cells but not against G14 cells. The susceptibility of FPM1B-siTax27 clone to killing by the CTL was intermediate between that of positive and negative controls. FPM1B-siTax32 clone also showed intermediate susceptibility between FPM1B-siGFP1 and G14 cells (Fig. 3B). These results indicated that downregulation of Tax expression could lead to the evasion of Tax-specific CTLs in the HTLV-1-infected T cells.
FIG. 3.
The repression of Tax expression was associated with evasion of Tax-specific CTL lysis. FPM1.BP (○), FPM1B-siGFP1 (□), FPM1B-siTax27 (▪, panel A), FPM1B-siTax32 (▴, panel B), or G14 (•) cells (104 cells/well) were labeled with 51Cr for 2 h and used as target cells. 4O1/C8 cells were used as effectors at the indicated effector/target ratios (E/T). The results are expressed as the mean percent lysis ± the SD of triplicate wells. Similar results were obtained in three independent experiments.
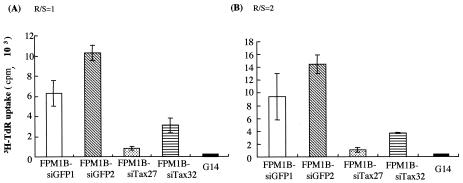
We also examined the proliferative responses of 4O1/C8 against FPM1B-siTax27 and FPM1B-siTax32 cells to confirm the effect of downregulation of Tax on T-cell stimulation. 4O1/C8 cells were cocultured with formalin-fixed stimulator cells for 72 h at two different ratios of responder to stimulator, and TdR incorporation in 4O1/C8 cells was measured. As shown in Fig. 4, 4O1/C8 cells cocultured with FPM1B-siTax27 or FPM1B-siTax32 cells showed a lower level of proliferation than did those cocultured with FPM1B-siGFP1 or FPM1B-siGFP2 cells. These results indicated the possibility that the HTLV-1-specific proliferative response was greatly reduced by downregulation of Tax expression.
FIG. 4.
Impaired proliferative response of 4O1/C8 CTL stimulated with FPM1B-siTax27 or FPM1B-siTax32 cells. Either 5 × 104 (A) or 105 (B) 4O1/C8 cells were cocultured with 5 × 104 cells/well of formalin-fixed FPM1B-siGFP1 (□), FPM1B-siGFP2 (▧), FPM1B-siTax27 (░⃞), FPM1B-siTax32 (▤), or G14 (▪) cells for 54 h at the indicated responder/stimulator ratio (R/S). [3H]TdR incorporation was measured during the last 18 h. The data represent the mean ± the SD of triplicate wells. Similar results were obtained in three independent experiments.
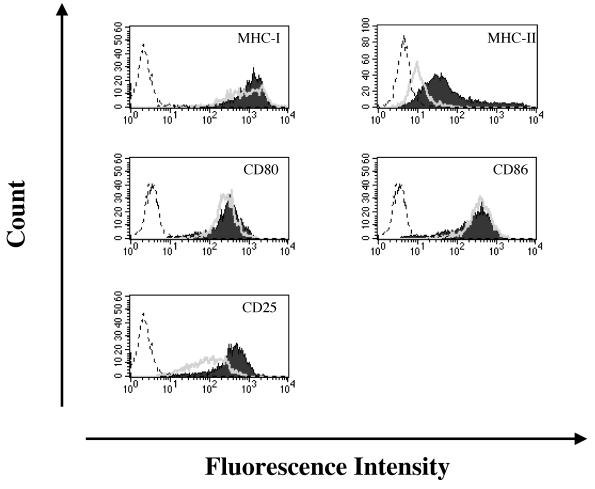
Repression of Tax expression was associated with downregulation of MHC-II and CD25 but not MHC-I, CD80, or CD86.
It has been reported that HTLV-1-infected T cells express cell surface molecules related to T-cell activation (4, 29, 41, 48). Thus, we examined the expression of MHC-I, MHC-II, CD25, CD80, and CD86 on FPM1B-siTax27 cells to determine whether the evasion of Tax-specific CTLs was associated with the expression levels of these molecules. Our results indicated that MHC-II and CD25 expression was significantly downregulated in FPM1B-siTax27 cells, whereas all of the othermolecules were equivalently expressed both in FPM1B-siTax27 and in FPM1B-siGFP1 cells (Fig. 5). Since the activation of 4O1/C8 cells is restricted with MHC-I (37), it is likely that the evasion of Tax-specific CTL was mainly due to the direct effect of the downregulation of Tax expression but not due to the alteration of cell surface molecules related to T-cell activation. However, it is of note that FPM1B-siTax27 cells showed decreased levels of MHC-II and CD25 expression, which may affect the activation of host immune responses against HTLV-1 in vivo. We have detected similar patterns of the surface molecule expression on FPM1B-siTax32 cells (data not shown).
FIG. 5.
The expression of MHC-II and CD25 was downregulated in FPM1B-siTax27 cells. FPM1B-siGFP1 (solid histogram) or FPM1B-siTax27 (open histogram) cells were stained with anti-rat CD80, CD86, MHC-I, MHC-II, or CD25 antibodies for 30 min and labeled with FITC-conjugated anti-mouse IgG+IgM. After being washed, labeled cells were subjected to flow cytometric analysis. Histograms of FPM1B-siGFP1 cells stained only with FITC-conjugated second antibody were represented by the dotted line.
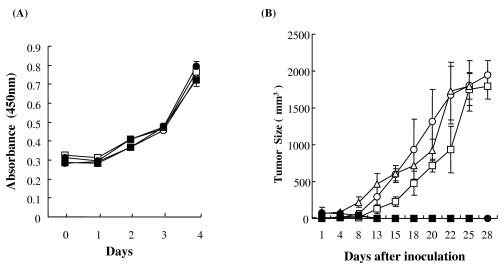
Downregulation of Tax altered the growth ability of HTLV-1-infected T cells in vivo but not in vitro.
It has been demonstrated that Tax plays an important role in the growth of HTLV-1-infected T cells (46, 50). Thus, we investigated whether downregulation of Tax altered the cell growth of HTLV-1-infected cells both in vitro and in vivo. As shown in Fig. 6A, FPM1B-siTax27 and FPM1B-siTax32 cells were able to grow in vitro as well as control FPM1B-siGFP1 and FPM1B-siGFP2 cells did. In contrast, rats subcutaneously inoculated with 2 × 107 FPM1B-siTax27 or FPM1B-siTax32 cells did not show the growth of tumor cells at the inoculation sites. In addition, no apparent distant metastatic tumors were detected in these rats. On the other hand, FPM1B-siGFP1 and FPM1B-siGFP2 cells, as well as parental FPM1.BP cells, formed solid nodules in nu/nu rats, and the tumors continued to grow for 4 weeks (Fig. 6B). All rats inoculated with FPM1.BP, FPM1B-siGFP1, or FPM1B-siGFP2 cells developed metastases, preferably in lymph nodes and lungs (data not shown). These results clearly indicated the correlation of in vivo growth ability of HTLV-1-infected T cells with the expression levels of Tax protein.
FIG. 6.
Repression of Tax expression was associated with impaired tumorigenicity in nu/nu rats. (A) The growth of FPM1B-siTax27 and FPM1B-siTax32 cells in vitro. FPM1B-siGFP1 (□), FPM1B-siGFP2 (○), FPM1B-siTax27 (•), or FPM1B-siTax32 (▪) cells (104/well) were cultured in each well of 96-well flat-bottom microtiter plates for 4 days. The number of growing cells was determined by Cell Counting Kit-8 and indicated as the absorbance at 450 nm. The results were expressed as the mean ± the SD of triplicate wells. (B) The growth of FPM1B-siTax27 and FPM1B-siTax32 cells in vivo. Three-week-old nu/nu rats were subcutaneously inoculated with 2 × 107 FPM1.BP (▵), FPM1B-siGFP1 (□), FPM1B-siGFP2 (○), FPM1B-siTax27 (•), or FPM1B-siTax32 (▪) cells. The tumor size was measured every other day and is expressed in cubic millimeters as determined by the formula described in Materials and Methods. The results were expressed as the mean ± the SD for each group of three (FPM1.BP and FPM1B-siGFP1), four (FPM1B-siGFP2 and FPM1B-siTax32), or six (FPM1B-siTax27) rats.
DISCUSSION
There remains an unsolved question regarding the requirement of Tax in the long latent period before the onset of ATL. To examine this issue, we targeted Tax expression here by using a recently established system of siRNA and investigated the role of Tax in HTLV-1-infected T cells in the rat model of ATL-like disease. Our results indicated that the downregulation of Tax expression was associated with the reduced levels of immune responses against the cells by Tax-specific CTL. This indicates the possibility that these cells with reduced Tax expression have an advantage in surviving in vivo, because they could escape from major immune responses to HTLV-1. However, we also showed that these cells lost the ability to form tumor nodules and to metastasize to other organs in nude rats. This was not due to the alteration of sensitivity to natural killer (NK) cells, because neither FPM1B-siGFP1 nor FPM1B-siTax27 cells were susceptible to killing by NK cells (data not shown). These observations clearly indicated the essential role of Tax for the growth of the infected cells in vivo and further suggested a dynamic balance between immunogenicity and tumorigenicity in HTLV-1-infected cells, involving a tripartite interaction among Tax, the virus-infected T cells, and host immune components. It is likely that HTLV-1-infected cells used in the present study are still in the middle of ATL development and need some additional changes to become ATL cells, many of which contain defective viruses unable to express Tax (49).
In association with the reduction of Tax expression, downregulation of MHC-II, and CD25 was also observed in FPM1B-siTax27 and FPM1B-siTax32 cells. This may be due to the direct effect of the repression of Tax expression because HTLV-1-infected T cells are known to express these molecules (18, 48). Since MHC-II expressed on HTLV-1-infected T cells plays a role in the activation of helper T cells (48), it is possible that this downregulation could also result in the reduction of HTLV-1-specific immune responses. On the other hand, FPM1B-siTax27 and FPM1B.siTax32 cells did not show the downregulation of CD80, CD86, and MHC-I, whose expression is also shown to be induced by Tax (4, 41). It is likely that the expression of these proteins required lower levels of Tax and that the 58% reduction of Tax expression observed here did not affect the expression of these proteins. Nevertheless, we observed the reduction of both the proliferation response and CTL activity of Tax-specific CTL directed against FPM1B-siTax27 and FPM1B.siTax32 cells. It is possible that partial reduction of Tax expression observed in the present study was enough for HTLV-1-infected cells to evade Tax-specific CTL without altering the expression levels of some T-cell stimulatory molecules.
It is well documented that the expression of the viral protein is repressed in most cases of HTLV-1-infected individuals (22, 23). This may be one of the strategies by which the virus can escape from the host immune system. In agreement with this idea, we show here that the downregulation of Tax in HTLV-1-infected T cells leads to the escape from Tax-specific CTL. With regard to Tax expression, controversy has existed as to whether a low level of HTLV-1 expression in vivo is sufficient (i) for immortalizing infected cells, (ii) to cause infection of other cells in order to establish a variable repertoire of infected clones, and (iii) for the activation of host immune responses. We showed here that partial reduction of Tax expression dramatically affected the in vivo growth ability of HTLV-1-infected T cells, strongly suggesting the importance of Tax for tumor formation. This is in consistent with previous reports, which demonstrated the pivotal role of Tax for oncogenic transformation (46, 50). Thus, these results raised the hypothesis that HTLV-1-infected cells usually cannot grow in vivo when they lose Tax expression and that exceptional cells may exist which can proliferate in vivo despite the downregulation of Tax expression. These cells can escape from host immune responses and may be the candidates of ATL cells. To understand the pathogenesis of ATL, it is important to identify these candidates and determine the factors involving Tax-independent growth of infected cells. The HTLV-1-infected rat T cells with Tax downregulation such as FPM1B-siTax27 and FPM1B-siTax32 may be good materials for identifying these factors if we could render the cells capable of growing in vivo. In addition, we should also point out that our present findings are based on the use of two clones derived from a single HTLV-1-infected cell line and a rat model system. Cell lines from other sources would be useful for generalizing the present findings. Future studies utilizing CTL and HTLV-1-infected cells from HTLV-1 carriers will also help us to understand the clinical significance of these observations.
We have previously shown that HTLV-1-infected T cells obtained the ability to evade Tax-specific CTL by downregulating MHC-I expression after a long period of mixed culture with the CD8+ CTL (37). In addition, there are other mechanisms related to evasion of CTL lysis, such as generation of sequence variants in CTL epitopes (9, 33) and resistance to Fas-mediated killing (1, 27). It is possible that HTLV-1-infected cells utilize these mechanisms in combination with the downregulation of Tax expression in the course of ATL development to maintain appropriate levels of Tax expression both for evasion of the immune system and for growth of HTLV-1-infected cells in vivo.
As an assay for the siRNAs targeted against Tax, we used EL4/Gax cells, which express Tax fused with EGFP (10). This cell line is useful to determine an effective sequence to inhibit Tax expression. Indeed, we were able to determine the siRNA sequence, which can induce 80% reduction of Tax expression in EL4/Gax cells. However, the sequence used in the present study may not be ideal to completely inhibit Tax expression, because EGFP expression was much more efficiently inhibited both in EL4/EGFP and EL4/Gax cells by using the EGFP siRNA sequence reported previously (54). Moreover, our present results suggested the possibility that the siRNA targeted against Tax was less effective in rat cells than mouse cells. This may be due to the species difference since we used mouse U6 promoter for the expression of siRNA. Alternatively, it is also possible that the rat cell line used in the present study was not able to survive if the Tax expression decreased below 42%, since a certain level of Tax expression may be required to immortalize primary CD4+ T cells in vitro as reported previously (13). Consistent with this idea, we have never obtained FPM1.BP clones with >60% reduction of Tax expression (Table 1). This is a dramatic contrast to the high frequency of clones with Tax reduction obtained from EL4/Gax cells, which do not depend on Tax for their proliferation. To further support the pivotal role of Tax in the growth of certain HTLV-1-infected cells, our preliminary experiment revealed that an HTLV-1-infected human T-cell line could not survive in vitro when it completely lost Tax protein after infection of lentivirus encoding the Tax siRNA (data not shown). It is of interest to determine the minimum amount of Tax required for the growth of HTLV-1-infected cells in vitro and in vivo. Materials used in the present study, including EL4/Gax cells, a rat ATL-like disease model and siRNAs, will be useful to address such an issue.
Posttranscriptional silencing mediated by siRNA has been shown to be a potentially powerful tool to inhibit replication of a number of viruses, including human immunodeficiency virus type 1 (20, 31, 34). These studies suggested the possibility of using siRNA to prevent virus-related diseases by inhibiting the viral replication. Moreover, it has been recently reported that RNA interference of human papillomavirus oncoproteins E6 and E7 induced senescence in the virus-infected cells (14), suggesting the possibility of applying siRNA to prevent virus-inducing tumor development. Our present findings also supported this possibility because downregulation of Tax dramatically reduced the tumor development in a rat model. Although many ATL cell lines are known to lack Tax expression, Tax-targeting siRNA should still be applicable to prevent the proliferation of HTLV-1-infected cells before Tax expression is lost. In addition, we should also point out that the Tax siRNA may inhibit the expression of other HTLV-1 proteins, as we show in Fig. 2D. Thus, it is possible that other viral proteins could also be responsible for the altered phenotype observed in cells with Tax downregulation. Further studies are required to clarify the involvement of these proteins in the phenotypic alteration induced by Tax-targeting siRNA and to develop effective methods to inhibit the growth of HTLV-1 and the virus-infected cells.
In conclusion, we evaluated the role of Tax in HTLV-1-infected T cells by using Tax-targeting siRNA and the rat model system. Our results demonstrated the importance of Tax both for activating host immune responses and for maintaining in vivo growth ability of the infected cells. The phenomena observed in association with the alteration of Tax expression may reflect some aspects of the events happening in HTLV-1-infected individuals during the long latent period before the onset of ATL. Although the observation may be limited to certain HTLV-1-infected cells, the methods developed in the present study will be useful for further analysis of the responsibility of Tax for the development of ATL. These studies could provide important information for understanding the mechanism of ATL development and further establishing effective therapies against the disease.
Acknowledgments
We thank Ko Okumura and Hideo Yagita (Juntendo University, Tokyo, Japan) for providing anti-rat CD80 and CD86 antibodies. We are also grateful to Mitsuhiko Yanagisawa and Shu Endo for cooperation with the maintenance of animals at the P3 level facilities.
This study was supported in part by grants from the Ministry of Education, Science, Culture, and Sports of Japan.
REFERENCES
- 1.Arai, M., M. Kannagi, M. Matsuoka, T. Sato, N. Yamamoto, and M. Fujii. 1998. Expression of FAP-1 (Fas-associated phosphatase) and resistance to Fas-mediated apoptosis in T-cell lines derived from human T-cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis patients. AIDS Res. Hum. Retrovir. 14:261-267. [DOI] [PubMed] [Google Scholar]
- 2.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 3.Daenke, S., S. Nightingale, J. K. Cruickshank, and C. R. Bangham. 1990. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J. Virol. 64:1278-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dezzutti, C. S., D. L. Rudolph, C. R. Roberts, and R. B. Lal. 1993. Characterization of human T-lymphotropic virus type I- and II-infected T-cell lines: antigenic, phenotypic, and genotypic analysis. Virus Res. 29:59-70. [DOI] [PubMed] [Google Scholar]
- 5.Duyao, M. P., D. J. Kessler, D. B. Spicer, C. Bartholomew, J. L. Cleveland, M. Siekevitz, and G. E. Sonenshein. 1992. Transactivation of the c-myc promoter by human T cell leukemia virus type 1 tax is mediated by NF-κB. J. Biol. Chem. 267:16288-16291. [PubMed] [Google Scholar]
- 6.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 7.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-1 is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 8.Fujii, M., P. Sassone-Corsi, and I. M. Verma. 1988. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 85:8526-8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa, Y., R. Kubota, M. Tara, S. Izumo, and M. Osame. 2001. Existence of escape mutant in HTLV-1 tax during the development of adult T-cell leukemia. Blood 97:987-993. [DOI] [PubMed] [Google Scholar]
- 10.Furuta, R. A., K. Sugiura, S. Kawakita, T. Inada, S. Ikehara, T. Matsuda, and J. Fujisawa. 2002. Mouse model for the equilibration interaction between the host immune system and human T-cell leukemia virus type 1 gene expression. J. Virol. 76:2703-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazzolo, L., and M. Duc Dodon. 1987. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature 326:714-717. [DOI] [PubMed] [Google Scholar]
- 12.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 13.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall, A. H., and K. A. Alexander. 2003. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J. Virol. 77:6066-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, A. Hasegawa, F. Takemura, T. Masuda, and M. Kannagi. 2001. Regression of human T-cell leukemia virus type I (HTLV-1)-associated lymphomas in a rat model: peptide-induced T-cell immunity. J. Natl. Cancer Inst. 93:1775-1783. [DOI] [PubMed] [Google Scholar]
- 16.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, F. Takemura, K. Hirokawa, T. Yoshiki, H. Yagita, K. Okumura, and M. Kannagi. 2000. Development of human T-cell leukemia virus type 1-transformed tumors in rats following suppression of T-cell immunity by CD80 and CD86 blockade. J. Virol. 74:428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue, J., M. Seiki, T. Taniguchi, S. Tsuru, and M. Yoshida. 1986. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 5:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-1 pX in patients with HTLV-1 associated neurological disease. Nature 348:245-248. [DOI] [PubMed] [Google Scholar]
- 20.Jacque, J. M., K. Triques, and M. Stevenson. 2002. Modulation of HIV-1 replication by RNA interference. Nature 418:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannagi, M., S. Harada, I. Maruyama, H. Inoko, H. Igarashi, G. Kuwashima, S. Sato, M. Morita, M. Kidokoro, M. Sugimoto, M. Funahashi, M. Osame, and H. Shida. 1991. Predominant recognition of human T-cell leukemia virus type I (HTLV-1) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int. Immunol. 3:761-767. [DOI] [PubMed] [Google Scholar]
- 22.Kannagi, M., S. Matsushita, and S. Harada. 1993. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int. J. Cancer 54:582-588. [DOI] [PubMed] [Google Scholar]
- 23.Kannagi, M., K. Sugamura, K. Kinoshita, H. Uchino, and Y. Hinuma. 1984. Specific cytolysis of fresh tumor cells by an autologous killer T-cell line derived from an adult T-cell leukemia/lymphoma patient. J. Immunol. 133:1037-1041. [PubMed] [Google Scholar]
- 24.Kannagi, M., K. Sugamura, H. Sato, K. Okochi, H. Uchino, and Y. Hinuma. 1983. Establishment of human cytotoxic T-cell lines specific for human adult T-cell leukemia virus-bearing cells. J. Immunol. 130:2942-2946. [PubMed] [Google Scholar]
- 25.Kinoshita, T., M. Shimoyama, K. Tobinai, M. Ito, S. Ito, S. Ikeda, K. Tajima, K. Shimotohno, and T. Sugimura. 1989. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86:5620-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinoshita, T., A. Tsujimoto, and K. Shimotohno. 1991. Sequence variations in LTR and env regions of HTLV-1 do not discriminate between the virus from patients with HTLV-1-associated myelopathy and adult T-cell leukemia. Int. J. Cancer 47:491-495. [DOI] [PubMed] [Google Scholar]
- 27.Kishi, S., S. Saijyo, M. Arai, S. Karasawa, S. Ueda, M. Kannagi, Y. Iwakura, M. Fujii, and S. Yonehara. 1997. Resistance to fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-1) transgenic mice with autoimmune arthropathy. J. Exp. Med. 186:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koya, Y., T. Ohashi, H. Kato, S. Hanabuchi, T. Tsukahara, F. Takemura, K. Etoh, M. Matsuoka, M. Fujii, and M. Kannagi. 1999. Establishment of a seronegative human T-cell leukemia virus type 1 (HTLV-1) carrier state in rats inoculated with a syngeneic HTLV-1-immortalized T-cell line preferentially expressing Tax. J. Virol. 73:6436-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lal, R. B., D. L. Rudolph, C. S. Dezzutti, P. S. Linsley, and H. E. Prince. 1996. Costimulatory effects of T cell proliferation during infection with human T lymphotropic virus types I and II are mediated through CD80 and CD86 ligands. J. Immunol. 157:1288-1296. [PubMed] [Google Scholar]
- 30.Lee, B., Y. Tanaka, and H. Tozawa. 1989. Monoclonal antibody defining tax protein of human T-cell leukemia virus type-I. Tohoku J. Exp. Med. 157:1-11. [DOI] [PubMed] [Google Scholar]
- 31.Lee, N. S., T. Dohjima, G. Bauer, H. Li, M. J. Li, A. Ehsani, P. Salvaterra, and J. Rossi. 2002. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol. 20:500-505. [DOI] [PubMed] [Google Scholar]
- 32.Maeda, K., T. Sato, M. Azuma, H. Yagita, and K. Okumura. 1997. Characterization of rat CD80 and CD86 by molecular cloning and MAb. Int. Immunol. 9:993-1000. [DOI] [PubMed] [Google Scholar]
- 33.Niewiesk, S., S. Daenke, C. E. Parker, G. Taylor, J. Weber, S. Nightingale, and C. R. Bangham. 1995. Naturally occurring variants of human T-cell leukemia virus type I Tax protein impair its recognition by cytotoxic T lymphocytes and the transactivation function of Tax. J. Virol. 69:2649-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novina, C. D., M. F. Murray, D. M. Dykxhoorn, P. J. Beresford, J. Riess, S. K. Lee, R. G. Collman, J. Lieberman, P. Shankar, and P. A. Sharp. 2002. siRNA-directed inhibition of HIV-1 infection. Nat. Med. 8:681-686. [DOI] [PubMed] [Google Scholar]
- 35.Ohashi, T., S. Hanabuchi, H. Kato, Y. Koya, F. Takemura, K. Hirokawa, T. Yoshiki, Y. Tanaka, M. Fujii, and M. Kannagi. 1999. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J. Virol. 73:6031-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi, T., S. Hanabuchi, H. Kato, H. Tateno, F. Takemura, T. Tsukahara, Y. Koya, A. Hasegawa, T. Masuda, and M. Kannagi. 2000. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J. Virol. 74:9610-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi, T., S. Hanabuchi, R. Suzuki, H. Kato, T. Masuda, and M. Kannagi. 2002. Correlation of major histocompatibility complex class I downregulation with resistance of human T-cell leukemia virus type 1-infected T cells to cytotoxic T-lymphocyte killing in a rat model. J. Virol. 76:7010-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-1 associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 39.Parker, C. E., S. Daenke, S. Nightingale, and C. R. Bangham. 1992. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology 188:628-636. [DOI] [PubMed] [Google Scholar]
- 40.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawada, M., A. Suzumura, M. Yoshida, and T. Marunouchi. 1990. Human T-cell leukemia virus type I trans activator induces class I major histocompatibility complex antigen expression in glial cells. J. Virol. 64:4002-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seiki, M., A. Hikikoshi, T. Taniguchi, and M. Yoshida. 1985. Expression of the pX gene of HTLV-1: general splicing mechanism in the HTLV family. Science 228:1532-1534. [DOI] [PubMed] [Google Scholar]
- 43.Seiki, M., J. Inoue, T. Takeda, and M. Yoshida. 1986. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 5:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp, P. A. 2001. RNA interference-2001. Genes Dev. 15:485-490. [DOI] [PubMed] [Google Scholar]
- 45.Siekevitz, M., M. B. Feinberg, N. Holbrook, F. Wong-Staal, and W. C. Greene. 1987. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc. Natl. Acad. Sci. USA 84:5389-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, M. R., and W. C. Greene. 1991. Type I human T-cell leukemia virus tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J. Clin. Investig. 88:1038-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sodroski, J., C. Rosen, W. C. Goh, and W. Haseltine. 1985. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science 228:1430-1434. [DOI] [PubMed] [Google Scholar]
- 48.Takamoto, T., M. Makino, M. Azuma, T. Kanzaki, M. Baba, and S. Sonoda. 1997. HTLV-1-infected T cells activate autologous CD4+ T cells susceptible to HTLV-1 infection in a costimulatory molecule-dependent fashion. Eur. J. Immunol. 27:1427-1432. [DOI] [PubMed] [Google Scholar]
- 49.Tamiya, S., M. Matsuoka, K. Etoh, T. Watanabe, S. Kamihira, K. Yamaguchi, and K. Takatsuki. 1996. Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood 88:3065-3073. [PubMed] [Google Scholar]
- 50.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 52.Yamamoto, N., M. Okada, Y. Koyanagi, M. Kannagi, and Y. Hinuma. 1982. Transformation of human leukocytes by cocultivation with an adult T-cell leukemia virus producer cell line. Science 217:737-739. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida, M., M. Osame, K. Usuku, M. Matsumoto, and A. Igata. 1987. Viruses detected in HTLV-1-associated myelopathy and adult T-cell leukaemia are identical on DNA blotting. Lancet i:1085-1086. [DOI] [PubMed] [Google Scholar]
- 54.Yu, J. Y., S. L. DeRuiter, and D. L. Turner. 2002. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc. Natl. Acad. Sci. USA 99:6047-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]