Abstract
Adaptive immune responses in which CD8+ T cells recognize pathogen-derived peptides in the context of major histocompatibility complex class I molecules play a major role in the host defense against infection with intracellular pathogens. Cells infected with intracellular bacteria such as Listeria monocytogenes, Salmonella enterica serovar Typhimurium, or Mycobacterium tuberculosis are directly lysed by cytotoxic CD8+ T cells. For this reason, current vaccines for intracellular pathogens, such as subunit vaccines or viable bacterial vaccines, aim to generate robust cytotoxic T-cell responses. In order to investigate the capacity of a herpes simplex virus type 1 (HSV-1) vector to induce strong cytotoxic effector cell responses and protection from infection with intracellular pathogens, we developed a replication-deficient, recombinant HSV-1 (rHSV-1) vaccine. We demonstrate in side-by-side comparison with DNA vaccination that rHSV-1 vaccination induces very strong CD8+ effector T-cell responses. While both vaccines provided protection from infection with L. monocytogenes at low, but lethal doses, only rHSV-1 vaccines could protect from higher infectious doses; HSV-1 induced potent memory cytotoxic T lymphocytes that, upon challenge by pathogens, efficiently protected the animals. Despite the stimulation of relatively low humoral and CD4-T-cell responses, rHSV-1 vectors are strong candidates for future vaccine strategies that confer efficient protection from subsequent infection with intracellular bacteria.
Major histocompatibility complex (MHC) class I-restricted CD8+ T cells recognizing antigenic peptides derived from pathogens play major roles in protection against intracellular bacteria like Mycobacterium tuberculosis, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes (for a review, see references 15 and 16). Vaccines utilizing inactivated or recombinant bacteria have been demonstrated to elicit both CD4- and CD8-T-cell activation (16), but they seem to be inefficient stimulators of effector (25) and memory (34) T cells if compared side by side with live bacteria (25) or recombinant viral vaccines (34). In this respect, inactivated intracellular bacterial vaccines are similar to natural M. tuberculosis (44) and L. monocytogenes (33) infections, which fail to induce sufficient immunological memory to prevent recurrent infections. DNA vaccines have been demonstrated to induce protection against infections with M. tuberculosis (43) and L. monocytogenes (9). However, it was demonstrated that combining DNA vaccines with attenuated bacteria (12), protein antigen (42), or modified vaccinia virus (28) in heterologous prime-boost vaccination protocols could further optimize protection. In other words, DNA vaccines alone are not sufficient to induce maximal protective immunity and, in a clinical setting, might require complex vaccination arrangements. Similarly, recombinant vaccinia virus could elicit only limited protection against intracellular bacteria and delayed, rather than prevented death after infection (2). Thus, as stated previously elsewhere (16), efficacious “single-shot” vaccines to intracellular infection have yet to be developed.
While most intracellular bacteria replicate in maturation-arrested phagosomes (17), L. monocytogenes bacteria egress from the phagosome and gain access to the cytoplasm of infected cells (37). This led initially to the assumption that, because their antigens have access to the cytosolic MHC class I presentation pathway, only the latter would induce CD8-T-cell responses. In contrast, bacteria localized in phagosomes were thought to preferentially trigger CD4-T-cell responses via the phagosomal MHC class II presentation pathway. However, it has been demonstrated in mice (39) as well as in humans (41) that cytotoxic T lymphocytes (CTL) are important players in the control of both M. tuberculosis and L. monocytogenes infections (24, 29), and human MHC class I peptides derived from M. tuberculosis were recently identified (6). In addition to lysis of infected cells, CTL expressing granulysin have been shown to directly kill extracellular bacteria (11, 40). CD8+ T cells seem to be the effector cells of choice for intracellular bacteria and are currently major targets for vaccination studies (for review see (16).
Replication-deficient (10, 30) or disabled infection with single-cycle herpes simplex virus (HSV) (3, 27) has been shown to induce strong immune responses against HSV-derived antigens. Besides vaccination against HSV infections (26), the application of replication-deficient HSV vectors has been largely restricted to suicide (20) or cytokine (22) gene therapy against tumors. Vaccination with replication-defective recombinant HSV (rHSV) encoding simian immunodeficiency virus Env and Nef proteins could also be shown to induce partial protection against simian immunodeficiency virus in rhesus monkeys (32). However, neither in vivo analysis of humoral and cellular responses following recombinant HSV type 1 (rHSV-1) vaccination nor results of rHSV-1-based vaccination against bacterial infection has been reported.
In order to examine the potential of rHSV-1 vaccines for vaccination against intracellular bacteria, we used recombinant replication-defective HSV-1, which was modified to encode OVA as a model antigen. We demonstrate that in contrast to gene gun DNA vaccination, rHSV-1 induce neither strong humoral responses nor CD4-T-cell responses specific for the model antigen OVA. However, when ex vivo CD8-T-cell responses were compared, rHSV-1 was much more potent than DNA vaccination and resulted in immediate as well as long-term memory protection against infection with recombinant L. monocytogenes expressing OVA. Thus, rHSV-1 represents a promising “single-shot” vaccine with the potential to induce long-term protective CTL responses.
MATERIALS AND METHODS
Mice.
All mice were bred and maintained under standard conditions in the animal facilities of the Institute for Immunology, Ludwig-Maximilians-University Munich; the Institute for Microbiology, Immunology and Hygiene, Technical University Munich; or the Department of Pharmacy, University of Ferrara. DO11.10 mice (expressing transgenic T-cell receptors [TCR] specific for OVA323-339/MHC class II I-Ad) were obtained from Jackson Laboratory, Bar Harbor, Maine.
Plasmid construction and preparation of recombinant, replication-deficient HSV-1 vaccines.
A BamHI/XhoI fragment of rabbit βglobin was cloned into a BamHI/XhoI-opened pcDNA3 vector (Invitrogen) to create pcDNA3-βglobin. The pcDNA3-OVA vector encoding the secreted form of chicken ovalbumin (OVA) was constructed by cloning a 1.9-kb EcoRI fragment from the plasmid pAc-Neo-OVA (provided by F. Carbone, Melbourne, Walter and Eliza Hall Institute, Australia), which contained the entire coding sequence of OVA, into the EcoRI site of pcDNA3-βglobin. Plasmids were prepared from Escherichia coli with Qiagen (Hilden, Germany) Mega Kits. A recombination plasmid (pB410H:OVA) was constructed by introduction of HCMV-βglobin OVA cDNA expression cassette into the Ul41 locus of HSV-1. The cDNA under the transcriptional control of the human cytomegalovirus promoter was inserted in a SmaI/XbaI-opened pBBSK-plasmid between the two UL41 fragments (map positions 93,858 to 92,230 and 91,631 to 90,145) 100 bp downstream of the HSV immediate-early ICP0 promoter. This plasmid (pB410H:OVA) was recombined with the genome of T0ZGFP using the previously described Pac-facilitated lacZ substitution method (19). T0ZGFP is a nonreplication HSV viral vector background that has low toxicity due to the deletion in three immediate early genes (ICP4 and ICP27, which are essential for viral replication, and ICP22) with cDNA encoding GFP inserted into the ICP22 locus and an insertion of LacZ in the UL41 locus. The recombination was carried out using standard calcium phosphate transfection of 5 μg of viral DNA and 1 μg of linear recombination plasmid pB410H:OVA. Transfection and isolation of the recombinant virus was performed in 7b Vero cells (African green monkey kidney cells CCL81; American Type Culture Collection, Manassas, Va.) capable of providing the essential ICP4 and ICP27 HSV gene products. The recombinant virus containing the OVA cDNA was identified by isolation of a clear plaque phenotype after X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining. T0H-OVA virus was purified by three rounds of limiting dilution and the presence of the transgene was verified by Southern blot analysis. Viral stocks of the T0H-OVA virus and the control vector T0-GFP (derived from T0ZGFP without lacZ reporter gene in UL41 locus) were prepared and titrated using 7b cells.
Adoptive transfer.
DO11.10 cells were prepared from lymph nodes and spleens of transgenic mice. Briefly, spleen and lymph nodes were taken out, and single-cell suspensions were prepared. Erythrocytes were removed by osmotic lysis, and after determining the percentage of DO11.10 TCR-transgenic T cells by flow cytometry, 2.5 × 106 transgenic T cells were injected intravenously into the recipient mice.
Vaccination.
Naked DNA immunization was performed by gene gun administration (Bio-Rad Laboratories, Hercules, Calif.). Cartridges of DNA-coated gold particles were prepared according to the manufacturer's instructions. For each preparation, gold particles (25 mg; diameter, 1 μm) were coated with 200 μg of DNA. Mice were anesthetized prior to vaccination with a mixture of ketaminhydrochloride and xylazinhydrochloride in phosphate-buffered saline (PBS). A total of 8 μg of plasmid DNA was delivered to the shaved abdominal skin of adult mice with a discharge pressure of 400 lb/in2. For T0H-OVA vaccination, frozen virus stocks were thawed on ice, diluted in PBS to 4 × 106 T0H-OVA/200 μl, and injected intravenously (i.v.).
Enzyme-linked immunosorbent assay.
For the detection of OVA-specific antibodies, 96-well microtiter plates (Nunc Maxisorp, Nunc, Wiesbaden, Germany) were coated with OVA (15 μg/ml; Sigma Chemical Co., St. Louis, Mo.) at room temperature overnight. Plates were blocked (PBS, 0.5% milk powder, 0.05% NaN3), and immune sera (diluted 1:100 in blocking buffer) were incubated for 2 h at room temperature. After washing five times with PBS, horseradish peroxidase-labeled second-step goat sera specific for mouse immunoglobulin M (IgM) or IgG (Serotec Ltd., Oxford, England) or IgG1 or IgG2a (Southern Biotechnology Assoc. Inc., Birmingham, Ala.) in PBS (0.5% milk powder, 0.05% Tween20) was added and incubated for 2 h. After five washing steps, the amount of bound antibody was determined by addition of substrate solution (1 mM 3,3′,5,5′ tetramethylbenzidine, 30% H2O2 [0.3 μl/ml] in 0.2 M potassium acetate). The reaction was stopped by addition of 2 N H2SO4, and the absorbance at 450 nm was determined with a Vmax microplate reader (Molecular Devices Corporation, Sunnyvale, Calif.).
MAbs, tetramers, and flow cytometry.
Lymphocytes were analyzed using the following monoclonal antibodies (MAbs): anti-CD4-PerCP (L3T4), anti-CD8a-PerCP (Ly2), and anti-CD62L (Mel-14) from PharMingen (San Diego, Calif.) and KJ1-26-fluorescein isothiocyanate specific for DO11.10 TCR, anti-CD8a-APC (Ly2), and anti-CD44-PE from Caltag (Burlingame, Calif.). Biotinylated MAbs were detected with streptavidin-APC (Caltag). Analytic flow cytometry was performed on a FACScalibur (Becton Dickinson, Mountain View, Calif.), and the data were analyzed using CellQuest software (Becton Dickinson). Tetrameric H2-Kb/Ova257-264 complexes were generated as previously described (5). In brief, refolded and biotinylated MHC-peptide complexes were multimerized with the addition of phycoerythrin-conjugated streptavidin (Molecular Probes, Eugene, Oreg.). Tetrameric complexes were stored at 2 mg/ml at 4°C in PBS (pH 8.0) containing 0.03% sodium azide, pepstatin (1 μg/ml), leupeptin (1 μg/ml), and 1 mM EDTA. The reagents were frequently tested on antigen-specific T-cell lines to document staining quality.
Listeria.
Mice were infected i.v. with L. monocytogenes expressing the secreted form of OVA (36) (L.m.-OVA, kindly provided by Hao Chen, Philadelphia, Pa.). Viable bacterial counts within spleen and liver were determined by homogenizing the respective tissue in PBS containing 0.05% Triton X-100 and plating on brain heart infusion agar plates (Life Technologies, Gaithersburg, Md.). L. monocytogenes colonies were identified by their characteristic morphology and by Gram staining.
RESULTS
Induction of CTL responses by rHSV-1 vaccines.
The goal of the present study was to investigate the capacities of rHSV-1 vectors to induce protective primary and long-term immune responses against intracellular bacterial infection in vivo. Earlier attempts to use HSV-1-derived vectors in gene therapy approaches were complicated by viral cytotoxicity and transient expression of transgenes. Replication-defective HSV-1 strains, in which nonreverting mutations have been incorporated into mandatory viral genes, retain the immunogenicity of wild-type HSV but are much safer. Deletion of a series of viral immediate-early genes such as ICP4, ICP22, and ICP27 substantially reduces cytotoxicity and enhances long-term transgene expression (21). Further studies have shown that these or similar mutant vectors do not interfere with MHC class I expression in the infected neurons (20) or fibroblasts (46), an important consideration with respect to antigen presentation. For our studies, a replication-incompetent, low-cytotoxicity, ICP4−, ICP22−, and ICP27− triple mutant HSV-1 virus was modified to express the model antigen chicken OVA under control of the human cytomegalovirus (CMV) promoter. This virus (T0H-OVA) was used to vaccinate mice. As positive vaccination control, we employed gene gun DNA vaccination, a method previously proven to confer CD8-T cell-mediated protective immunity against bacterial challenge (9). The plasmid used for this approach was pcDNA3-OVA, containing the same CMV-OVA-expression cassette as T0H-OVA.
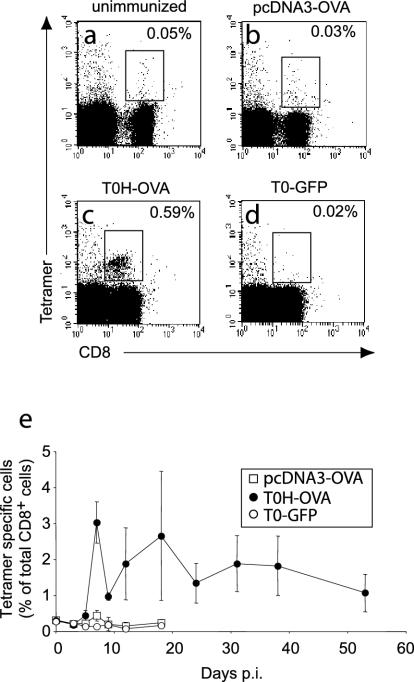
Using OVA as a model antigen, we first investigated the capacity of the different vaccines to induce OVA-specific CD8-T-cell activation and expansion in vivo. With tetrameric H2-Kb/Ova257-264 complexes (Tet), we determined that the frequency of OVA-specific Tet+ CD8+ T cells in normal nonimmune C57BL/6 mice was, on average, 0.05% of all peripheral blood lymphocytes (Fig. 1a). This percentage did not increase upon gene gun (0.03%) (Fig. 1b) or control rHSV-1 (TO-GFP) vaccination (0.02%) (Fig. 1d). In contrast, T0H-OVA induced a 10-fold expansion of OVA-specific CTL compared to the background (0.59%) (Fig. 1c). When the kinetics of CD8+ Tet+ T-cell expansion in peripheral blood was analyzed as a percentage of total CD8+ T cells (Fig. 1e), a weak, but statistically significant (Student's t test, P = 0.0026) expansion was detectable at day 7 after gene gun vaccination (0.43% ± 0.15%) compared to negative-control mice vaccinated with T0-GFP (0.145% ± 0.03%). However, a significant expansion after gene gun vaccination could not be observed in all of our experiments (see Fig. 4). In contrast, T0H-OVA vaccination induced >21-fold expansion of Tet+ CD8+ CTL over background (Fig. 1e) (3.03% ± 0.57%) (except as noted, values are presented as means ± standard deviations). At the peak of the response (day 7), the frequency of Tet+ CD8+ T cells induced by T0H-OVA was sevenfold larger than following gene gun vaccination. While levels of Tet+ CD8+ CTL remained elevated in T0H-OVA-vaccinated mice for more than 50 days, the expansion in gene gun-vaccinated mice was transient and short-lived, declining rapidly to background levels (Fig. 1e). As CD8-T-cell expansion in animals vaccinated with T0H-OVA was variable between different experiments, we could not observe elevated levels of Tet+ CD8+ CTL for such a long period in each experiment performed (see Fig. 4b). When the peripheral blood of the same animals was analyzed for nonspecific (Tet−) augmentation of the CD8+-T-cell compartment, T0H-OVA and T0-GFP induced an approximately twofold increase of Tet− CD8+ T cells (data not shown). This effect was probably due to a proinflammatory response in rHSV-1-vaccinated mice and was not observed in the DNA-vaccinated group. These results show that T0H-OVA induce a severalfold stronger expansion of antigen-specific CD8+ T cells compared to gene gun vaccination.
FIG. 1.
Visualization of antigen-specific CTL expansion after vaccination. PBL were stained with anti-CD8-APC and H-2Kb/OVA257-264 tetramers-phycoerythrin and analyzed by flow cytometry before (a) and 7 days after vaccination with pcDNA3-OVA (b), T0H-OVA (c), or T0-GFP (4 × 106 virus particles i.v.) (d). The percentage of CD8+ Tet+ CTL among PBL is indicated on each plot. (e) The frequency of antigen-specific cells among peripheral blood CD8+ T cells was determined by H-2Kb/OVA257-264 tetramer staining for pcDNA3-OVA-, T0H-OVA-, and T0-GFP-immunized mice as shown (a to d). Data represent average values ± standard deviations (error bars) obtained from five mice per group at each time point. p.i., postimmunization.
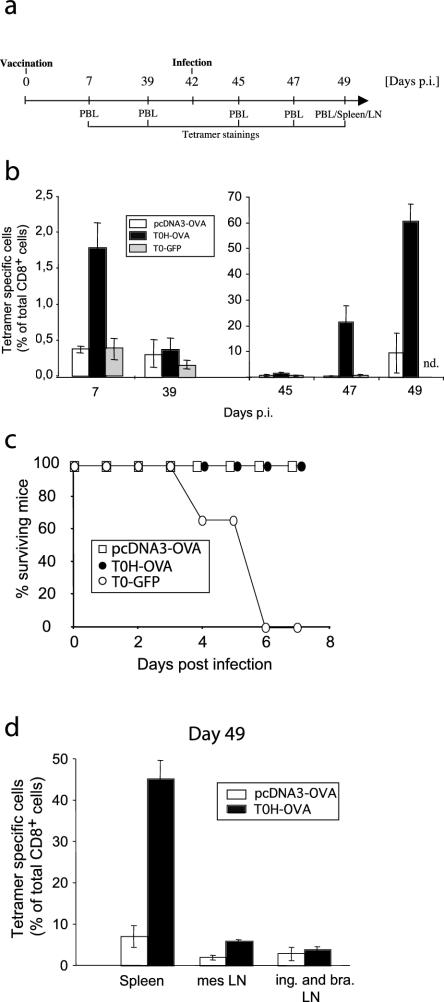
FIG. 4.
Long-term protection against L.m.-OVA infection. (a) Experimental protocol: C57BL/6 mice were vaccinated with pcDNA3-OVA, T0H-OVA, or T0-GFP (4 × 106 virus particles i.v.) and infected i.v. with 5 × 104 (two times the LD50) L.m.-OVA cells 6 weeks later. (b) The percentage of H-2Kb/OVA257-264 tetramer-specific cells among CD8+ T lymphocytes in the blood was determined by flow cytometry at the days indicated. (c) Survival of the mice was monitored daily; no death occurred after day 7 postinfection. (d) On day 7 after infection (day 49 postvaccination), spleen, mesenteric (mes), inguinal, and brachial lymph nodes were isolated and analyzed by flow cytometry; inguinal and brachial lymph nodes (ing. and bra. LN) were pooled for each mouse. The bar graphs depict the percentage (mean ± standard deviation [error bars]) of H-2Kb/OVA257-264 tetramer-positive cells among CD8+ T cells (n = 4 to 5 per group).
Recombinant HSV-1 vaccination protects against L. monocytogenes infection.
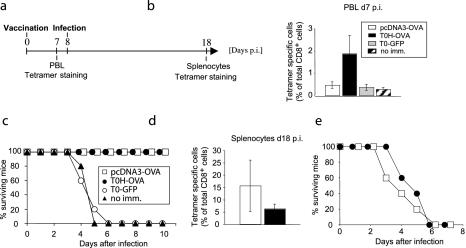
While infection of mice with sublethal doses of L. monocytogenes leads to rapid clearance of the bacterium and long-lived protective immunity, larger doses result in death of the animals within a few days. Despite a relatively weak induction of antigen-specific CTL expansion, as demonstrated in Fig. 1, gene gun DNA vaccination has been reported to provide protective immunity against L. monocytogenes infection in mice (9). We wondered if vaccination with T0H-OVA would have similar protective properties. To directly compare both vaccination strategies, we challenged rHSV-1 or gene gun-vaccinated mice with a lethal dose (5 × 104, two times the 50% lethal dose [LD50]) of L. monocytogenes genetically modified to express the model antigen OVA (L.m.-OVA [36]). In order to determine the expansion of OVA-specific CTL after vaccination and L.m.-OVA challenge, the experimental protocol was designed as shown in Fig. 2a, including Tet analysis at two stages during the experiment. Tet-analysis at day 7 postvaccination confirmed the findings shown in Fig. 1; a strong increase of Tet+ CD8+ CTL could be detected in T0H-OVA immunized mice, while DNA vaccination induced a weak, but significant (P < 0.05) increase compared to nonimmunized mice (Fig. 2b). Despite the relatively weak expansion of Tet+ CTL following gene gun vaccination, all mice from this group were protected from the subsequent L.m.-OVA challenge, whereas all control animals (unimmunized or control-vaccinated with T0-GFP) died between day 3 and day 6 postinfection (Fig. 2c). T0H-OVA-vaccinated mice were also fully protected (Fig. 2c). These results demonstrate that under these conditions of bacterial challenge (low dose) the strength of the CD8+-T-cell response did not correlate with protection. As expected, elevated levels of Tet+ CD8+ CTL were detectable in both gene gun- and T0H-OVA-vaccinated mice 18 days postvaccination (10 days postinfection, Fig. 2d). While the average of Tet+ CD8+ CTL was higher in DNA-vaccinated mice than in the T0H-OVA-vaccinated group, the difference was not statistically significant (Fig. 2d). To control the specificity of protection, we infected gene gun- and T0H-OVA-vaccinated mice with wild-type L. monocytogenes not expressing the model antigen OVA (Fig. 2e). As expected, neither group was protected, and all animals died from infection. Together with the results from control vaccination using T0-GFP (Fig. 2c), where mice were not protected from lethal infection with L.m.-OVA, these data exclude the possibility that protection induced by T0H-OVA is due to unspecific HSV-1-mediated effects. These findings indicate that T0H-OVA induce strong CTL responses specific for recombinant antigen and are sufficient to protect mice from subsequent bacterial infection.
FIG. 2.
Protection against L.m.-OVA infection. (a) C57BL/6 mice were left unimmunized or vaccinated with pcDNA3-OVA, T0H-OVA, or T0-GFP (4 × 106 virus particles i.v.) (day 0) and infected i.v. with 5 × 104 (two times the LD50) L.m.-OVA cells 8 days later. H-2Kb/OVA257-264 tetramer staining for OVA-specific CD8+ T cells in blood was performed at day 7 postvaccination (b), and survival of the mice was monitored for 10 days after infection (c). At day 10 postinfection (day 18 postimmunization), spleens of surviving mice were analyzed for presence of Tet+ CTL (d). The bar graphs (b and d) depict the percentage (mean ± standard deviation [error bars]) of Tet-positive cells among total CD8+ T cells (five mice/group). (e) As a control for the antigen-specificity of protection, vaccinated mice were infected with 5 × 104 wild-type L. monocytogenes cells (not expressing OVA), and survival was monitored. Abbreviations: no imm, no immunization; p.i., postimmunization.
Protection from infection with a high dose of L. monocytogenes is induced by HSV-1 vaccines but not by DNA vaccination.
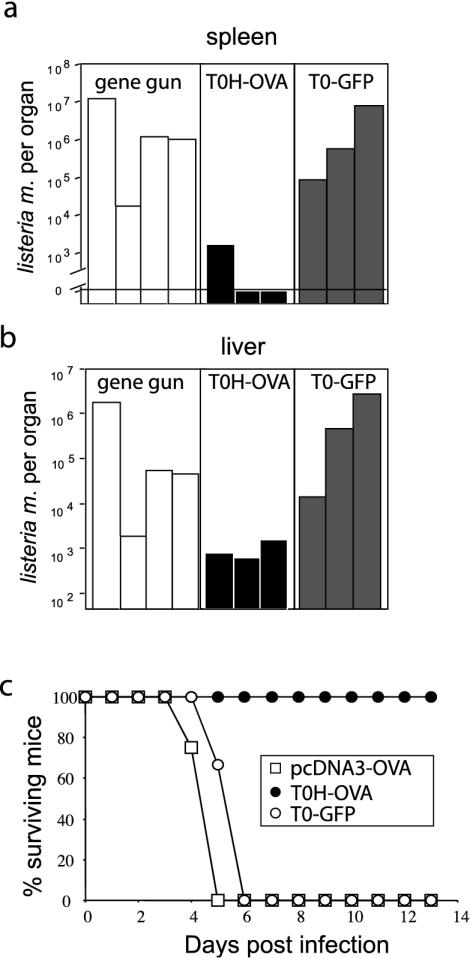
Although DNA vaccination induced much lower numbers of circulating antigen-specific Tet+ CTL compared to T0H-OVA, both groups were protected from a lethal (two times the LD50) L.m.-OVA challenge (Fig. 2). We wondered, however, if DNA vaccination would also provide protection from higher doses of L. monocytogenes. We therefore repeated the experiment shown in Fig. 2a, infecting the mice with a twofold-higher dose of L.m.-OVA (105 cells, four times the LD50). When spleens and livers were analyzed 3 days postinfection for presence of viable Listeria, striking differences between gene gun- and T0H-OVA-vaccinated mice became evident: spleens from two out of three T0H-OVA-vaccinated animals were completely free of bacteria, while bacterial counts in the third were approximately 6,000-fold lower than those from gene gun-vaccinated mice (Fig. 3a). The latter had bacterial numbers similar to the control-vaccinated (T0-GFP) group. Livers of the same mice showed a similar picture, with an average of >430-fold-lower bacterial counts in livers of T0H-OVA-vaccinated mice than in gene gun- or control virus (T0-GFP)-vaccinated mice (Fig. 3b). In order to test if these differences in bacterial numbers correlate with survival, we infected vaccinated mice with 10-fold more L.m.-OVA (5 × 105 cells, 20 times the LD50) than the amounts used for the experiment depicted in Fig. 2, and then we monitored survival (Fig. 3c). With this high dose, a direct relationship between the frequency of antigen-specific Tet+ CTL induced by the vaccine and protection from infection became clear. The T0H-OVA-vaccinated mice survived without signs of disease (data not shown), while all gene gun- and T0-GFP-vaccinated mice died (Fig. 3c). These data show that while the DNA vaccine induces protection against infection with a low, but lethal dose of L.m.-OVA, T0H-OVA induces much stronger responses capable of protecting against a much more potent infectious dose.
FIG. 3.
Protection against infection with high dose of L.m.-OVA. C57BL/6 mice were vaccinated with T0H-OVA, T0-GFP (4 × 106 virus particles i.v.), or pcDNA3-OVA and infected with 105 (four times the LD50) L.m.-OVA cells 7 days postvaccination. Mice were sacrificed 3 days after infection, and bacterial counts in spleen (a) and livers (b) were determined from organ homogenates (n = 3 to 4 mice per group). (c) Differentially vaccinated mice (three or four per group) were infected with 5 × 105 (20 times the LD50) L.m.-OVA cells, and survival was monitored for 13 days.
Long-lived CTL immunity to L. monocytogenes induced by recombinant replication-deficient HSV-1.
The induction of long-term memory responses is a prerequisite for optimal vaccine efficacy. In order to test if T0H-OVA is able to induce antigen-specific long-term protection against intracellular bacterial infection, we vaccinated mice with T0H-OVA or control-vaccines and challenged the mice 6 weeks later with a lethal dose of L.m.-OVA (5 × 104 cells, two times the LD50). Expansion of Tet+ CD8+ CTL was monitored in the blood following vaccination and challenge. As observed previously (Fig. 1 and 2), only T0H-OVA induced strong expansion of Tet+ CD8+ CTL 7 days postvaccination (Fig. 4b). This increase was transient and after the contraction of Tet+ CTL, lower levels of Tet+ CD8+ CTL could be detected in the vaccinated mice 5 weeks after vaccination (day 39) (Fig. 4b). Following challenge with L.m.-OVA, changes were first detectable at day 5 (day 47) (Fig. 4b), with significantly elevated levels of Tet+ CD8+ CTL in T0H-OVA-vaccinated animals compared to the gene gun group or controls (P < 0.001); more than 20% of all CD8+ T cells in the blood of T0H-OVA-vaccinated mice were Tet+. Elevated levels of antigen-specific Tet+ CD8+ CTL in gene gun-vaccinated mice could be detected at day 49, 7 days postinfection, where they represented approximately 10% of all CD8+ CTL, an expansion sixfold lower than that of the T0H-OVA group (Fig. 4b).
Despite these differences in antigen-specific CTL frequency, both T0H-OVA- and gene gun DNA-vaccinated mice were well protected from low-dose (5 × 104 cells) L.m.-OVA challenge, showing no signs of serious illness. In contrast, all control T0-GFP-vaccinated mice died within 6 days postinfection (Fig. 4c).
At day 7 postinfection, we sacrificed the surviving mice and analyzed spleens and livers for presence of L. monocytogenes. The spleens of all animals from both surviving groups were bacteria free (data not shown). However, while the livers of T0H-OVA-vaccinated mice were also free of L. monocytogenes, 50% of the gene gun-vaccinated mice had not yet cleared the bacteria (3 × 104 to 9 × 104 bacteria per liver; data not shown). These data indicate that despite survival of all mice from both groups, the T0H-OVA vaccine results in more complete or, alternatively, more rapid bacterial clearance.
The different frequencies of antigen-specific CTL observed in T0H-OVA- versus gene gun-vaccinated mice (Fig. 4b) is a systemic phenomenon, observed not only in the peripheral blood, but also in spleens of surviving mice (day 49) (Fig. 4d). The mesenteric lymph nodes of T0H-OVA immunized mice also contained significantly more Tet+ CD8+ CTL than detected in gene gun-vaccinated animals (P < 0.005), although the difference was not as great as observed in spleen or blood (Fig. 4d). This analysis excludes the possibility that the differences in frequencies of Tet+ CD8+ CTL detected in peripheral blood lymphocytes (PBL) following T0H-OVA or gene gun vaccination were a result of differential homing properties of the stimulated T cells.
Taken together this analysis shows that T0H-OVA-derived vaccines induce potent long-term protection against infection with the facultative intracellular bacterium L. monocytogenes and generate a large memory CTL pool capable of clearing even large bacterial loads very efficiently. In contrast, gene gun vaccination induces lower frequencies of specific CTL sufficient for the protection of mice from challenge with lower but lethal Listeria-doses but are less efficient at achieving complete bacterial clearance.
Induction of antigen-specific CD4+ T helper responses and antibodies by recombinant replication-deficient HSV-1.
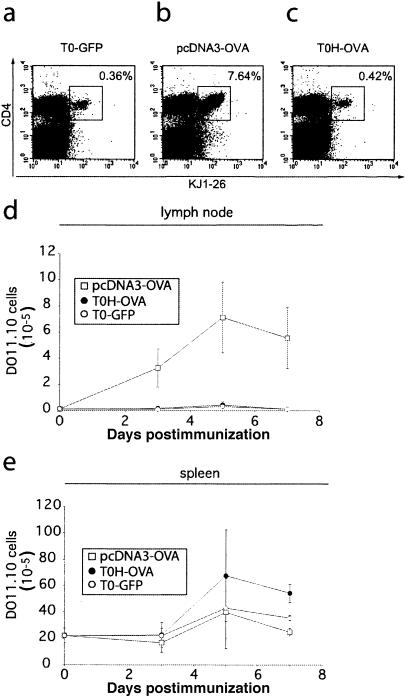
In order to directly monitor the capacity of T0H-OVA to induce CD4+-T-cell responses against their encoded recombinant antigen in vivo, we vaccinated mice that had previously received adoptively transferred TCR-transgenic OVA-specific DO11.10 CD4+ T cells (18). DO11.10 transgenic T cells recognize the OVA323-339 peptide in the context of MHC class II I-Ad and can be detected with the clonotypic TCR-specific MAb KJ1-26 facilitating the monitoring of their activation and expansion following vaccination with the specific antigen. While control vaccinated mice contained very few CD4+ KJ1-26+ T cells (T0-GFP) (Fig. 5a and d), the percentages increased 5- to 10-fold and total numbers increased 10- to 30-fold in the draining lymph nodes in gene gun-vaccinated animals (Fig. 5b and d) 5 days after vaccination. In contrast, vaccination with T0H-OVA did neither augment the frequencies (Fig. 5c) nor cell numbers (Fig. 5d) of antigen-specific DO11.10 T cells. Simultaneously, we monitored the activation status of DO11.10 T cells with a MAb specific for CD44, a cell surface marker modulated from moderate levels on naive T cells to high expression on activated T cells (data not shown). While CD4+ KJ1− 26+ T cells demonstrated a highly activated phenotype following gene gun vaccination, in lymph nodes of control (T0-GFP-), and T0H-OVA-immunized mice they did not gain an activated phenotype (data not shown). A CD4-T-cell response, following i.v. T0H-OVA vaccination would be expected primarily in the spleen. Surprisingly, the kinetics of DO11.10 expansion in the spleen following T0H-OVA vaccination was also not significantly different from the control immunized mice during the first 5 days postvaccination (Fig. 5e). A twofold expansion of CD4+ KJ1-26+ T cells (Fig. 5e) with high levels of CD44 (data not shown) was first detected in the spleens of T0H-OVA-vaccinated mice at day 7 postvaccination compared to gene gun-vaccinated animals. Other vaccination routes for T0H-OVA such as intraperitoneal, subcutaneous, and intradermal routes did not induce greater expansion of antigen-specific CD4 T cells (data not shown). These findings indicate that rHSV-1 vaccines elicit poorer CD4 T helper responses than gene gun vaccination.
FIG. 5.
Monitoring of OVA-specific CD4+-T-cell responses in vivo following rHSV-1 vaccination. BALB/c mice received 2.5 × 106 naive DO11.10 cells at day −1 and were immunized with gene gun (pcDNA3-OVA), T0H-OVA, or T0-GFP (4 × 106 virus particles) i.v. at day 0. The frequency of DO11.10 T cells present in the inguinal lymph nodes and spleen was measured by flow cytometry. (a to c) Cell suspensions were stained with anti-CD4, and KJ1-26-fluorescein isothiocyanate (DO11.10 TCR-specific). The percentages of CD4+/KJ1-26+ cells in lymph nodes of control (T0-GFP)-, pcDNA3-OVA-, and T0H-OVA-immunized mice at day 5 postimmunization are indicated in the dot plots. Kinetics of DO11.10 T-cell expansion is shown for lymph nodes (d) and spleen (e). In panels d and e, the total cell numbers ± standard deviations (error bars) of CD4+ KJ1-26+ cells are indicated (three to four animals per group).
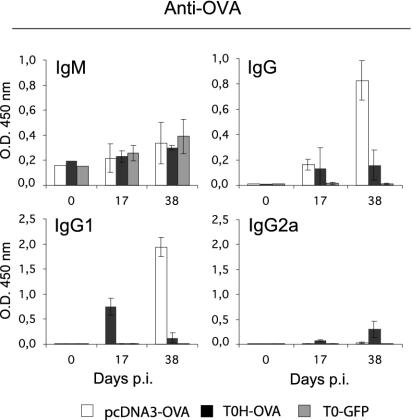
Poor induction of CD4 helper T-cell responses by HSV-1-derived vaccines could result in similarly weak induction of T dependent antibody responses. To address this possibility, we analyzed the sera of vaccinated mice for OVA-specific antibodies. While none of the vaccinations resulted in significant levels of specific IgM (Fig. 6), specific IgG was induced at later time points after vaccination (Fig. 6). The recombinant virus (T0H-OVA) and gene gun immunization induced similar IgG levels 17 days postvaccination, but only gene gun immunization resulted in further increase at later time points (Fig. 6). No specific IgG was detected following control (T0-GFP) immunization (Fig. 6). Further analysis of IgG subclasses revealed that gene gun vaccination induces a Th2-dominated response with OVA-specific IgG1 antibodies (Fig. 6), while a weak Th1-like IgG2a-dominated response is induced by T0H-OVA (Fig. 6). These data show that vaccination with rHSV-1-derived vaccines leads to the induction of a significantly weaker humoral response than gene gun immunization with additional skewing of the response towards Th1.
FIG. 6.
OVA-specific antibody responses in immunized BALB/c mice. Sera were obtained from mice immunized with pcDNA3-OVA, T0H-OVA, or T0-GFP (4 × 106 virus particles i.v.) at the indicated time points postvaccination, and OVA-specific antibody serum levels were determined by enzyme-linked immunosorbent assay. Preimmune serum (day 0) from each group was determined with a pool of sera. Results are expressed as the mean of optical density at 450 nm (O.D. 450 nm) ± standard deviation (error bars) from at least three individual mice per group.
Thus, recombinant HSV-1-derived vaccines are weak inducers of CD4 T helper and antibody responses but activate a large and efficient CD8+-T-cell response.
DISCUSSION
Protective vaccination against bacteria with a facultative intracellular life style is dependent on the induction of cytotoxic T-cell responses (17, 39). Since natural Listeria-derived CTL epitopes have not been identified in H-2b mice, we studied and visualized the vaccine efficacy of rHSV-1 vectors by using the model antigen OVA; mice were vaccinated with rHSV-1 encoding OVA and then challenged with recombinant OVA-expressing L. monocytogenes. As a control we used gene gun DNA vaccination, which has been shown to induce reliable protective CTL-mediated immunity to intracellular L. monocytogenes infection (9). Analysis of vaccinated mice with H-2Kb-OVA peptide tetramers revealed that rHSV-1 vaccines induce strong activation and expansion of OVA-specific CD8+ T cells, resulting in peak frequencies much greater than those observed following gene gun vaccination (Fig. 1). These data are in concordance with recent reports showing that DNA vaccines induce very low numbers of epitope-specific CTL which are poorly detectable ex vivo by flow cytometry after a single DNA vaccination via either gene gun (1) or intramuscular application (45).
At the peak of the response following T0H-OVA vaccination, 2 to 5% of all CD8+ T cells in the blood and spleen were specific for OVA257-264. This frequency is two- to threefold lower than that shown in a recently published report for HSV-1 glycoprotein epitope gB498-505 specific CTL in H-2b mice infected with replication competent HSV-1 (7). The lower frequencies of specific CTL in our system might be explained by our use of a replication-deficient (ICP4−, ICP22−, and ICP27− triple mutant) rHSV-1 vaccine, which reduces the number of infected cells compared to replication competent HSV-1. However, side-by-side comparison of OVA- and gB-specific CTL in the same animal infected with the same HSV-1 variant would be necessary to validate these speculations.
The efficiency of a vaccine is directly proportional to its capacity to activate T cells and to generate a memory T-cell pool. Development of memory T cells has been shown to be directly proportional to the intensity of the primary response (31), and in several viral infection models, the viral dose correlated positively with T-cell memory development (23, 34, 35). Recombinant replication-deficient mutant HSV-1 not only induced strong expansion of CD8+ CTL (Fig. 1), it also proved to be an extremely efficient vaccine against infection with an intracellular bacterium. Gene gun DNA vaccination protected equally well when we mice were challenged with low but lethal doses of bacteria, but following infection with higher doses of Listeria, only T0H-OVA-vaccinated animals showed low bacterial counts in spleen and liver (Fig. 3a) and were completely protected (Fig. 3c). However, further experiments will have to clarify if long-term memory response as shown in Fig. 4 for a low-dose challenge will be sufficient to protect the animals from high doses of infectious material. Thus, as a consequence of the low frequencies of memory precursor CTL generated in gene gun-vaccinated mice, the response to a large infectious challenge was insufficient to offer protection (Fig. 4). These data are in line with findings that gene gun vaccination can be further enhanced by combining it in heterologous prime-boost protocols with other vaccines (12, 28, 42); rHSV-1 vaccine, on the other hand, is by itself a very efficient vaccine against even high doses of infection with intracellular bacteria. Interestingly, T0H-OVA, unlike DNA vaccination, did not induce significant CD4 T helper cell or antibody responses specific for the recombinant antigen. This may be irrelevant, as protection against intracellular bacterial pathogens such as L. monocytogenes is largely CTL mediated (17, 39). Although antibody responses could be important to neutralize L. monocytogenes immediately after bacterial entry into the host and might therefore be considered a prerequisite for the establishment of an efficient long-term vaccination effect against intracellular bacteria (8), a lack of pathogen-specific antibodies did not negatively affect the ability of HSV-1-derived vaccines to provide long-term memory protection in our system.
Many different recombinant viral vector systems, including alpha-, adeno-, pox- and poliovirus systems, have been developed for vaccination. One concern common to all these vaccines is that their efficiency might be negatively affected by preexisting immunity to the viruses, as has proven true in the case of adenovirus (38) and poxvirus vectors (14). It has recently been demonstrated that HSV-1-derived vectors are not affected by preexisting immunity to HSV-1 (4). This aspect is currently being investigated in our laboratory for the specific rHSV-1 vaccine presented here. In the light of 75% of the adult human population have been previously exposed to HSV-1, this is an important prerequisite for the efficient usage of these vaccines (13). In addition, the strength of CTL response we observed upon vaccination with T0H-OVA indicates that, in contrast to other vaccines tested in the infectious Listeria model (for a review, see reference 16), further homologous or heterologous prime-boost protocols may be superfluous in the case of rHSV-1.
We have evaluated the efficacy of rHSV-1 vaccines in mice and demonstrate in the present report that a single vaccination is sufficient to elicit a protective response specific for a vaccine-encoded, nonviral protein. We have directly compared the cellular and humoral responses elicited by rHSV-1 vaccines and gene gun DNA vaccination and demonstrate significant differences in the strength and quality of the elicited responses. To our knowledge this is the first report analyzing the capacity of a recombinant HSV-1 vaccine to directly induce primary and memory CTL, CD4 T helper cells, and humoral responses specific for a nonviral antigen. We demonstrate for the first time that HSV-1-derived vectors induce strong CTL responses, making them a promising candidate for vaccines against intracellular bacterial infection. Further experiments with HSV-1-derived vaccines will be necessary to evaluate if our findings with the model antigen OVA can be extended to more relevant bacterial antigens, which may have different immunogenic properties. Experiments will have to be performed to confirm the usefulness of HSV-1 as a vector for human immunization.
Acknowledgments
This work was supported by a grant from the European Commission (EUROAMP; contract QLK2-CT-1999-00055) to A.L.E., P. Mavromara, R.M., P. Marconi, and T.B; D.H.B. and T.B. were supported by grants SFB576-A8 and SFB456 from the Deutsche Forschungsgemeinschaft. P. Marconi was supported by an Italian grant from the Instituto Superiore di Sanità (ICAV, Italian Concerted Action on HIV-AIDS Vaccine Development).
We thank A. Bol and W. Mertl for expert help in the animal facility of the LMU.
REFERENCES
- 1.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 2.An, L. L., E. Pamer, and J. L. Whitton. 1996. A recombinant minigene vaccine containing a nonameric cytotoxic-T-lymphocyte epitope confers limited protection against Listeria monocytogenes infection. Infect. Immun. 64:1685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boursnell, M. E., C. Entwisle, D. Blakeley, C. Roberts, I. A. Duncan, S. E. Chisholm, G. M. Martin, R. Jennings, D. Ni Challanain, I. Sobek, S. C. Inglis, and C. S. McLean. 1997. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J. Infect. Dis. 175:16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockman, M. A., and D. M. Knipe. 2002. Herpes simplex virus vectors elicit durable immune responses in the presence of preexisting host immunity. J. Virol. 76:3678-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busch, D. H., I. M. Pilip, S. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353-362. [DOI] [PubMed] [Google Scholar]
- 6.Cho, S., V. Mehra, S. Thoma-Uszynski, S. Stenger, N. Serbina, R. J. Mazzaccaro, J. L. Flynn, P. F. Barnes, S. Southwood, E. Celis, B. R. Bloom, R. L. Modlin, and A. Sette. 2000. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. USA 97:12210-12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coles, R. M., S. N. Mueller, W. R. Heath, F. R. Carbone, and A. G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168:834-838. [DOI] [PubMed] [Google Scholar]
- 8.Collins, H. L., and S. H. Kaufmann. 2001. Prospects for better tuberculosis vaccines. Lancet Infect. Dis. 1:21-28. [DOI] [PubMed] [Google Scholar]
- 9.Cornell, K. A., H. G. Bouwer, D. J. Hinrichs, and R. A. Barry. 1999. Genetic immunization of mice against Listeria monocytogenes using plasmid DNA encoding listeriolysin O. J. Immunol. 163:322-329. [PubMed] [Google Scholar]
- 10.Da Costa, X. J., C. A. Jones, and D. M. Knipe. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. USA 96:6994-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst, W. A., S. Thoma-Uszynski, R. Teitelbaum, C. Ko, D. A. Hanson, C. Clayberger, A. M. Krensky, M. Leippe, B. R. Bloom, T. Ganz, and R. L. Modlin. 2000. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J. Immunol. 165:7102-7108. [DOI] [PubMed] [Google Scholar]
- 12.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. S. Malin, and W. J. Britton. 2001. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis bacille Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 14.Flexner, C., B. R. Murphy, J. F. Rooney, C. Wohlenberg, V. Yuferov, A. L. Notkins, and B. Moss. 1988. Successful vaccination with a polyvalent live vector despite existing immunity to an expressed antigen. Nature 335:259-262. [DOI] [PubMed] [Google Scholar]
- 15.Harty, J. T., and M. J. Bevan. 1999. Responses of CD8+ T cells to intracellular bacteria. Curr. Opin. Immunol. 11:89-93. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann, S. H. E. 1998. Immunity to intracellular bacteria, p. 1335-1371. In W. E. Paul (ed.), Fundamental immunology. Raven Press Ltd., New York, N.Y.
- 18.Kearney, E. R., K. A. Pape, D. Y. Loh, and M. K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1:327-339. [DOI] [PubMed] [Google Scholar]
- 19.Krisky, D. M., P. C. Marconi, T. Oligino, R. J. Rouse, D. J. Fink, and J. C. Glorioso. 1997. Rapid method for construction of recombinant HSV gene transfer vectors. Gene Ther. 4:1120-1125. [DOI] [PubMed] [Google Scholar]
- 20.Krisky, D. M., P. C. Marconi, T. J. Oligino, R. J. Rouse, D. J. Fink, J. B. Cohen, S. C. Watkins, and J. C. Glorioso. 1998. Development of herpes simplex virus replication-defective multigene vectors for combination gene therapy applications. Gene Ther. 5:1517-1530. [DOI] [PubMed] [Google Scholar]
- 21.Krisky, D. M., D. Wolfe, W. F. Goins, P. C. Marconi, R. Ramakrishnan, M. Mata, R. J. Rouse, D. J. Fink, and J. C. Glorioso. 1998. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther. 5:1593-1603. [DOI] [PubMed] [Google Scholar]
- 22.Kuklin, N. A., M. Daheshia, P. C. Marconi, D. M. Krisky, R. J. Rouse, J. C. Glorioso, E. Manican, and B. T. Rouse. 1998. Modulation of mucosal and systemic immunity by enteric administration of nonreplicating herpes simplex virus expressing cytokines. Virology 240:245-253. [DOI] [PubMed] [Google Scholar]
- 23.Kundig, T. M., M. F. Bachmann, S. Oehen, U. W. Hoffmann, J. J. Simard, C. P. Kalberer, H. Pircher, P. S. Ohashi, H. Hengartner, and R. M. Zinkernagel. 1996. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl. Acad. Sci. USA 93:9716-9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladel, C. H., I. E. Flesch, J. Arnoldi, and S. H. Kaufmann. 1994. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 153:3116-3122. [PubMed] [Google Scholar]
- 25.Lauvau, G., S. Vijh, P. Kong, T. Horng, K. Kerksiek, N. Serbina, R. A. Tuma, and E. G. Pamer. 2001. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294:1735-1739. [DOI] [PubMed] [Google Scholar]
- 26.McLean, C. S., M. Erturk, R. Jennings, D. N. Challanain, A. C. Minson, I. Duncan, M. E. Boursnell, and S. C. Inglis. 1994. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J. Infect. Dis. 170:1100-1109. [DOI] [PubMed] [Google Scholar]
- 27.McLean, C. S., D. Ni Challanain, I. Duncan, M. E. Boursnell, R. Jennings, and S. C. Inglis. 1996. Induction of a protective immune response by mucosal vaccination with a DISC HSV-1 vaccine. Vaccine 14:987-992. [DOI] [PubMed] [Google Scholar]
- 28.McShane, H., R. Brookes, S. C. Gilbert, and A. V. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mielke, M. E., G. Niedobitek, H. Stein, and H. Hahn. 1989. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J. Exp. Med. 170:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 220:402-413. [DOI] [PubMed] [Google Scholar]
- 31.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.North, R. J. 1973. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production J. Exp. Med. 138:342-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochsenbein, A. F., U. Karrer, P. Klenerman, A. Althage, A. Ciurea, H. Shen, J. F. Miller, J. L. Whitton, H. Hengartner, and R. M. Zinkernagel. 1999. A comparison of T cell memory against the same antigen induced by virus versus intracellular bacteria. Proc. Natl. Acad. Sci. USA 96:9293-9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oehen, S., H. Waldner, T. M. Kundig, H. Hengartner, and R. M. Zinkernagel. 1992. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J. Exp. Med. 176:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pope, C., S. K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402-3409. [DOI] [PubMed] [Google Scholar]
- 37.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulick, A. H., G. Vassalli, P. F. Dunn, G. Dong, J. J. Rade, C. Zamarron, and D. A. Dichek. 1997. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J. Clin. Investig. 99:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sousa, A. O., R. J. Mazzaccaro, R. G. Russell, F. K. Lee, O. C. Turner, S. Hong, L. Van Kaer, and B. R. Bloom. 2000. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 97:4204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 41.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 42.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 69:3041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tascon, R. E., M. J. Colston, S. Ragno, E. Stavropoulos, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. Nat. Med. 2:888-892. [DOI] [PubMed] [Google Scholar]
- 44.van Rie, A., R. Warren, M. Richardson, T. C. Victor, R. P. Gie, D. A. Enarson, N. Beyers, and P. D. van Helden. 1999. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 341:1174-1179. [DOI] [PubMed] [Google Scholar]
- 45.Wolkers, M. C., M. Toebes, M. Okabe, J. B. Haanen, and T. N. Schumacher. 2002. Optimizing the efficacy of epitope-directed DNA vaccination. J. Immunol. 168:4998-5004. [DOI] [PubMed] [Google Scholar]
- 46.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]