Abstract
Expression of the human organic anion transporting polypeptides OATP1B1 and OATP1B3 have been previously believed to be restricted to hepatocytes. Here we demonstrate that the gene encoding OATP1B3, but not OATP1B1, is abundantly expressed in multiple human solid tumors that include hepatocellular, lung, and ovarian carcinomas. Surprisingly, OATP1B3 expression in a panel of 60 human tumor cell lines was linked with sensitivity to multiple cytotoxic agents, including the platinum anticancer drugs cisplatin, carboplatin, and oxaliplatin. In addition, overexpression of OATP1B3 in mammalian cells increased cellular accumulation of platinum agents and decreased cell survival. In mice with a targeted disruption of the ortholog transporter Oatp1b2, the liver-to-plasma ratio of cisplatin was significantly reduced compared with wildtype mice, without concurrent changes in expression profiles of other transporter genes. Our findings indicate an unexpected role for tumoral and host OATP1B-type carriers in the toxicity and disposition of platinum anticancer drugs, and may provide a foundation for understanding the extensive interindividual pharmacodynamic variability seen with these drugs in patients.
Keywords: OATP1B3, Cisplatin, Transporter, Liver
INTRODUCTION
Membrane-localized transport proteins have evolved to catalyze migration or expulsion of an endogenous or exogenous molecule across biological membranes. Such evolution has been necessary because membrane lipid bilayers would otherwise present virtually impenetrable barriers to many molecules or, alternatively, permit excessive intracellular accumulation. Although most of the currently known transporters have unknown physiologic function, several have gained interest within the scientific community due to the recognition that drugs may ‘hitchhike’ on these proteins that normally act on endogenous substrates (1).
While the role of ABC transporters in drug resistance has historically been a major focus of research, recent years have seen a greater emphasis on studies devoted to the so-called solute carriers, which comprise of over 300 proteins organized into 51 distinct families and are involved in the uptake of molecules into cells (http://www.bioparadigms.org/slc/). One reason contributing to the apparent historic lack of interest in solute carrier research is the long-held belief that most xenobiotics are transported across membranes via passive diffusion at a rate related to their hydrophobicity (2). Not only is this paradigm itself poorly supported by experimental data, but evidence accumulated over the last decade now supports the notion that solute carrier-mediated migration of drugs across the highly organized lipid bilayers of biological membranes is a predominant mechanism of cellular uptake (3).
Among the most widely studied solute carrier family are the SLCO cluster of genes that encode organic anion transporting polypeptides (OATP) (4). Two of these proteins, namely OATP1B1 (formerly OATP2, OATP-C, LST-1; gene symbol, SLCO1B1, SLC21A6) and OATP1B3 (formerly OATP8, LST-2; SLCO1B3, SLC21A8), are highly expressed in hepatocytes, and are of particular importance for hepatic drug elimination. Unlike OATP1B1, OATP1B3 is also overexpressed in various human cancer tissues as well as in several cancer cell lines derived from solid tumors (5), and is therefore a potential therapeutic target to enhance drug delivery into cancer cells. Previous studies have identified a number of widely used anticancer drugs as substrates of OATP1B3, including paclitaxel (6), docetaxel (7), imatinib (8), and methotrexate (9). However, a systematic approach to evaluate the ability of anticancer drugs to interact with OATP1B3 and subsequently affect tumor sensitivity and drug disposition is still lacking. In the current study, we exploited the NCI60 database (10) to correlate gene expression data with efficacy data for 118 anticancer drugs, unexpectedly identified several platinum chemotherapeutics as OATP1B3 substrates, and validated these findings in vitro using overexpressing cell models and in vivo with OATP-deficient mice.
MATERIALS AND METHODS
Real-time PCR
Tissue plates containing cDNA from 68 normal human tissues and 312 human cancer tissues were obtained from Origene. RNA and DNA from the NCI anti-cancer screening panel (NCI60) were provided by the National Cancer Institute tumor repository. RNA was reverse transcribed using SuperScript III first strand synthesis supermix for real-time RT-PCR (Invitrogen) according to manufacturer’s recommendations. Gene transcripts were quantified using SYBR Green PCR mastermix (Qiagen) and primers obtained from Origene that were specific to OATP1B1 (HP209396) and OATP1B3 (HP213461). Reactions were carried out in triplicate as previously reported (11). Transcripts of each sample were normalized to the housekeeping gene, GAPDH.
Correlation analyses
Growth inhibition data for the compounds of interest were obtained from the Developmental Therapeutics Program at the National Cancer Institute (http://dtp.cancer.gov/). The database comprises information on 60 different human tumor cell lines (NCI60), representing leukemia, melanoma and cancers of the lung, colon, brain, ovary, breast, prostate, and kidney. The 60 cell line dose response produced by a given compound results in a biological response pattern which can be utilized in a pattern recognition algorithm known as COMPARE (see: http://dtp.nci.nih.gov/docs/compare/compare.html). Using this algorithm, Pearson correlation coefficients were calculated for assessment of SLCO1B3-drug relationships. For in vivo associations, each cell line was classified as a low expresser if SLCO1B3 expression fell at or below the median expression of all cell lines, while cell lines above the median were considered high expressers. The two classifications of SLCO1B3 expressers were then correlated with cisplatin-sensitivity (at a dose of 2.5 mg/kg) in xenograft mouse models (reported as a ratio of treatment versus control) that were available from the In Vivo Screen Summary Data (see: http://dtp.nci.nih.gov/dtpstandard/InvivoSummary/index.jsp).
Cellular transport
Xenopus laevis oocytes injected with OATP1B1 or OATP1B3 cRNA along with water-injected controls were obtained from BD Biosciences. OATP1B1 or OATP1B3 overexpressing human embryonal kidney (HEK293) cells were created by stably transfecting the respective cDNA fragments spliced from TrueClone plasmids (OriGene Technologies), cloned into a pIRES2-EGFP vector (BD Biosciences). HEK293 cells were obtained from Invitrogen (Aug 2006) and no authentication was performed by the authors. These cells were functionally characterized with the OATP1B1/OATP1B3 substrate [6,7-3H(N)]estradiol-17β-D-glucuronide (E2-Glu) [radiochemical purity of 99% and specific activity of 45.8 Ci/mmol; obtained from American Radiolabeled Chemicals Inc. (Lot No. 080709)]. Additional uptake experiments were performed as described previously (12) using 500 μM of cisplatin, carboplatin or oxaliplatin in Xenopus laevis oocytes or HEK293 cells, with results normalized to uptake values in water-injected controls or cells transfected with an empty vector. The concentration of cisplatin used and the 30-min time point were selected on the basis of feasibility and sensitivity of currently available analytical methods for the determination of Pt concentrations (12). Previous experiments indicated that Phenol Red, a pH indicator in trypsin used to re-suspend cultured cells, influenced OATP1B-mediated uptake of substrates (7), and therefore these studies were performed in Phenol Red free conditions.
Animal experiments
Pharmacokinetic studies were performed in adult male Oatp1b2(−/−)mice (13) and age-matched wildtype mice (Taconic), both on a DBA1/lacJ background, that were housed in a temperature-controlled environment with a 12-hour light cycle, and given a standard diet and water ad libitum. Cisplatin was administered by i.p. injection at a dose of 10 mg/kg, after which plasma and liver from each animal were collected at 5, 10, 15, and 30 min. Urine was collected from animals housed in metabolic cages for 72 hours after cisplatin administration. All experiments involving animals were approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital.
Determination of platinum concentrations
Total platinum was determined using an AAnalyst 600 atomic absorption spectrometer with Zeeman background correction (Perkin Elmer). Concentrations in unknown samples were interpolated on linear calibration curves ranging 0.2 to 3 μg/mL that were constructed fresh daily in the relevant matrix. The percentage deviation from nominal values and the within-run and between-run precision of quality control samples spiked with known amounts of cisplatin were always <15%. Pharmacokinetic parameters were calculated using PK Solutions 2.0 (Summit Research Services).
Gene expression analysis of mouse liver and kidney
RNA was extracted using the RNEasy mini kit (Qiagen), and samples were amplified from 3 animals per group and then analyzed using the Mouse 430v2 GeneChip array (Affymetrix). Volcano plots were constructed using all probe sets or using select genes, including those that encode enzymes (N = 27 probe sets), nuclear receptors (N = 27), ABC transporters (N = 56) and solute carriers (N = 293). The data was summarized and normalized by the RMA algorithm. Then t tests were performed using the Partek Genomics Suite 6.4 software. A Bonferroni correction method was applied to the resulting P-values.
Statistical calculations
All graphed data is presented as mean values (symbols or bars) with standard error (error bars). Two-tailed P-values of less than 0.05 were considered as statistically significant. All statistical calculations were performed using the software package NCSS version 2004 (Number Cruncher Statistical System).
RESULTS
SLCO1B1/SLCO1B3 expression in normal and human tumor tissue
To provide insight into the tumor-specific expression profile of the OATP1B1 and OATP1B3 genes, real-time PCR analysis was performed on a panel of normal human tissues and corresponding tumor specimens. SLCO1B1 was only detectable in normal and tumor tissue of the liver (Fig. 1A). In contrast, SLCO1B3 was expressed in various normal and tumor tissues with the exception of the adrenal gland, breast or kidney (Fig. 1B). Interestingly, the expression of SLCO1B3 was higher in colon, endometrium, esophagus, lung, ovary, prostate, stomach, testis, and bladder tumors, when compared to the baseline expression in the corresponding normal tissue (Fig. 1B). These findings are consistent with recent expression data for these transporters that were reported by Pressler et al. (5) in a similar set of human samples.
Figure 1. Tumoral expression of the OATP1B1 and OATP1B3 genes.
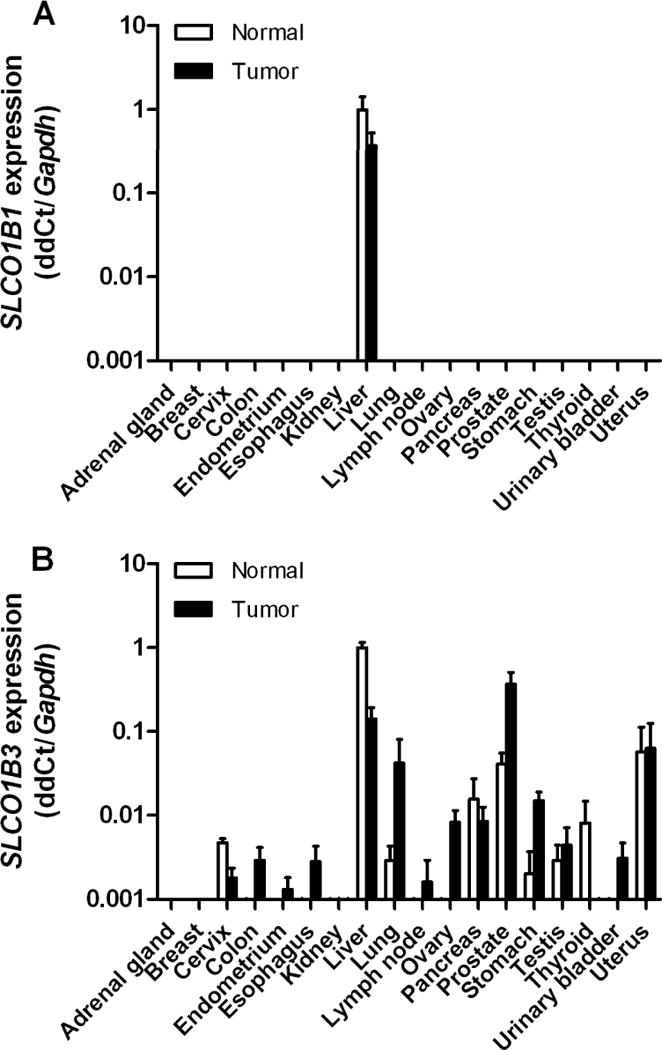
A, Expression of the OATP1B1 gene SLCO1B1 and B expression of the OATP1B3 gene SLCO1B3 in human normal tissues and human tumor samples. Tissue and tumor plates containing cDNA tissues were used for real-time PCR analysis, and normalized to the house keeping gene GAPDH. Data are shown as mean values (bars) with standard errors (error bars) of duplicate plates and expressed relative to values observed in normal human liver, which was set to a value of 1.
Association of SLCO1B3 expression with anti-cancer drug sensitivity
Since expression of the OATP1B3 gene was detectable in all cell lines of the NCI60 panel (Fig. 2A), a COMPARE analysis was performed on the growth-inhibitory potential of 118 well-characterized anti-cancer drugs (14). Using a cut-off correlation coefficient of 0.3, corresponding to a P-value of 0.025, we found that the cytotoxicity of 9 anticancer drugs of different classes was statistically significantly associated with SLCO1B3 expression (Table 1 and Supplementary Table S1). Among the top-ranking compounds were 3 platinum-based chemotherapeutics (Fig. 2B), including cisplatin, carboplatin, and (trans-l-1,2-diaminocyclo-hexane)platinum-II (DACH-Pt), which is structurally related to the anticancer agent oxaliplatin (15). Using the DTP in vivo screen summary data obtained from xenograft studies, we found that OATP1B3 may contribute in vivo to the tumor sensitivity of cisplatin, since cells lines with low SLCO1B3 expression are less sensitive to treatment than cells with high SLCO1B3 expression (Fig. 2C).
Figure 2. Expression of the OATP1B3 gene in the NCI60 cell line panel.
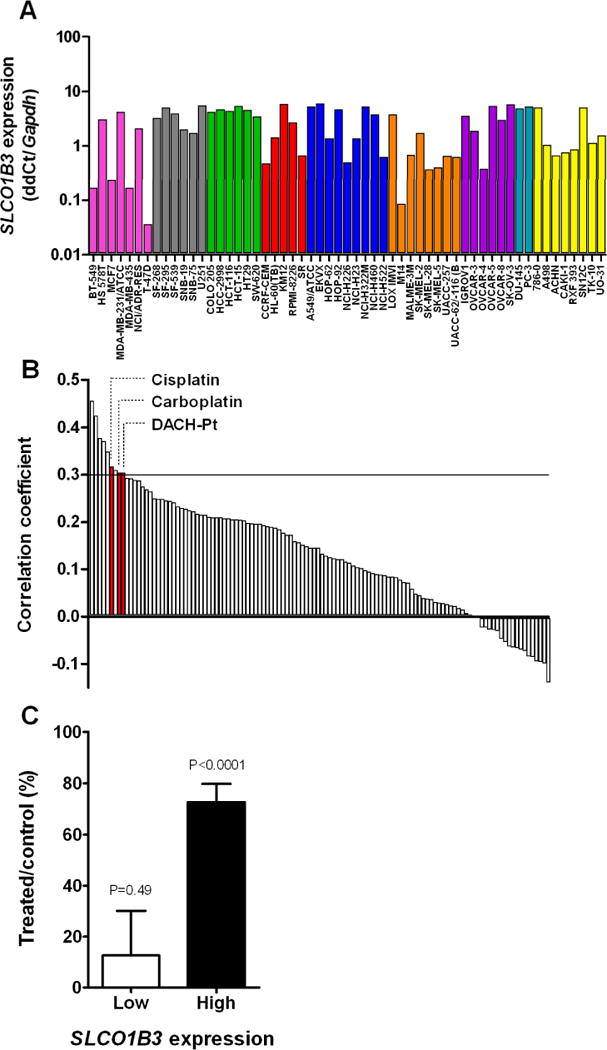
A, Plates containing cDNA samples of each cell line were used for real-time PCR analysis, and normalized to the house keeping gene GAPDH. Data are shown as mean values (bars) of duplicate plates and expressed relative to the median expression value in all lines, which was set to a value of 1. The graph is color-coded by tissue of origin: Red for leukemia, blue for lung cancer, green for colon cancer, grey for CNS cancer, coral for melanoma, purple for ovarian cancer, gold for renal cancer, turquoise for prostate cancer and pink for breast cancer cell lines. B, Sorted correlation coefficients (R) between SLCO1B3 expression and cytotoxic potencies of 118 drugs. Positive values of R > 0.3 suggest possible transporter-substrate relationships needed for drug entry into the cells. Abbreviation: DACH-Pt, (trans-l-1,2-diaminocyclo-hexane)platinum. C, Antitumor efficacy of cisplatin in vivo against the NCI60 cell panel, clustered by SLCO1B3 expression level (see Methods for details). Data represent mean of at least 15 observations and error bars represent standard error. The P-value represents statistical comparison of the efficacy compared with no effect.
Table 1.
| Rank | NSC number | Drug name | R-value | P-value |
|---|---|---|---|---|
| 1 | 167780 | Asaley | 0.4567 | 0.0004 |
| 2 | 606172 | Camptothecin,11 formyl (RS) | 0.4251 | 0.0010 |
| 3 | 95441 | Semustine (MeCCNU) | 0.3772 | 0.0038 |
| 4 | 102816 | Azacytidine | 0.3712 | 0.0045 |
| 5 | 142982 | Hycanthone | 0.3490 | 0.0188 |
| 6 | 119875 | Cisplatin | 0.3175 | 0.0161 |
| 7 | 95466 | PCNU | 0.3101 | 0.0189 |
| 8 | 291240 | Carboplatin | 0.3047 | 0.0212 |
| 9 | 271674 | Diaminocyclohexyl-Pt-II | 0.3042 | 0.0214 |
Abbreviation: R, Pearson’s correlation coefficient.
Characterization of platinum transport by OATP1B3
We next evaluated the in vitro transport of cisplatin by OATP1B3 in Xenopus leavis oocytes injected with OATP1B3 cRNA. Compared to water-injected control oocytes, the presence of OATP1B3 was associated with increased uptake of estradiol-D-17β-glucuronide (E2-Glu), a known OATP1B3 substrate, and increased uptake of cisplatin (Fig. 3A), as determined by atomic absorption spectrometry. The uptake of cisplatin was also facilitated in HEK293 cells transfected with OATP1B1 or OATP1B3 (Fig. 3B and 3C). Similar observations were made for OATP1B3 in the case of carboplatin and oxaliplatin, but not for tetraplatin, a platinum analogue that was not identified to be of significance in the COMPARE analysis (Fig. 3D). In agreement with above findings, enhanced accumulation was also observed in OATP1B3-overexpressing HEK293 cells for 14C-carboplatin and 14C-oxaliplatin (Supplementary Fig. S1), as determined by liquid scintillation counting.
Figure 3. In vitro transport studies of platinum chemotherapeutics.
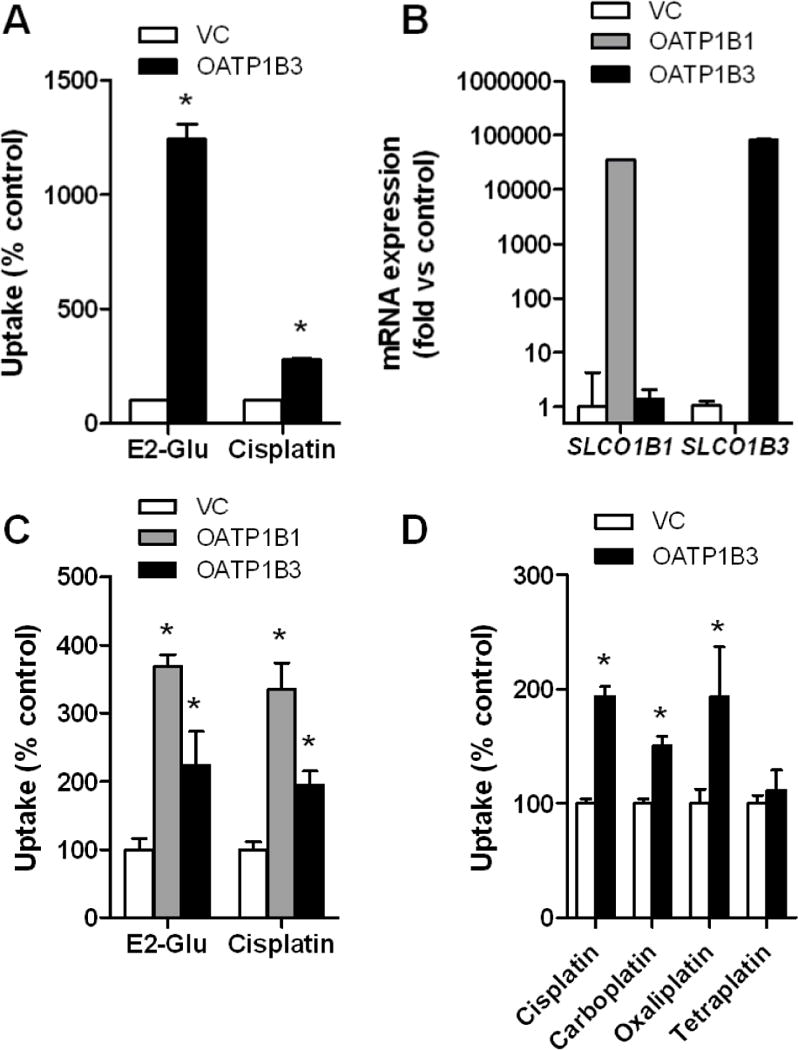
A, Transport of [6,7-3H(N)]estradiol-17β-D-glucuronide (E2-Glu) (2 μM; 60-min incubation), a positive control, and cisplatin (500 μM; 30-min incubation) by human OATP1B3 expressed in Xenopus laevis oocytes. B, mRNA expression of OATP1B1 and OATP1B3 in transfected HEK293 cells. C, Transport of E2-Glu (2 μM; 30-min incubation) and cisplatin (500 μM; 30-min incubation) by human OATP1B1 and OATP1B3 expressed in HEK293 cells. D, Transport of cisplatin, carboplatin, oxaliplatin, and tetraplatin (all 500 μM; 30-min incubation) by human OATP1B3 expressed in HEK293 cells. Data represent the mean of up to 32 observations, and are expressed as the average percent of uptake values (bars) with standard error (error bars) in water-injected cells for oocytes or cells transfected with an empty vector (VC) for HEK293 cells. The star (*) denotes a significant difference from VC (P < 0.05).
Influence of OATP1B-deficiency on cisplatin pharmacokinetics
To assess the role of OATP1B transporters in the disposition properties of cisplatin in vivo, we used mice deficient for the ortholog transporter Oatp1b2 [Oatp1b2(−/−) mice]. These animals were phenotypically normal compared to wildtype mice as determined from a serum chemistry screen with the exception of slightly altered amylase and bilirubin levels (Supplementary Fig. S2), as reported previously (16, 17). Transcriptional profiling of liver samples of wildtype mice did not reveal aberrant gene expression patterns in males versus females beyond a number of genes with known sex-dependent expression (Supplementary Fig. S3A). Furthermore, liver and kidney samples from Oatp1b2(−/−) mice did not have altered expression of genes of putative relevance to the anticancer drugs of interest (Supplementary Figs. S3B–C). Preliminary examination revealed the absence of a sex-dependence in Oatp1b2-mediated cisplatin distribution in the liver (Supplementary Fig. S4), and therefore only male mice were used in subsequent experiments.
Following the administration of cisplatin at a dose of 10 mg/kg (i.p.), hepatic levels of cisplatin were higher in wildtype mice compared to Oatp1b2(−/−) mice, indicating that platinum transport into the livers of these mice is compromised (Fig. 4A). This phenomenon occurred without a substantial influence on circulating concentrations of cisplatin (Fig. 4B). In line with the altered hepatobiliary handling of cisplatin, urinary excretion of platinum was significantly increased in the Oatp1b2(−/−) mice (Fig. 4C).
Figure 4. Influence of Oatp1b2-knockout on cisplatin pharmacokinetics.
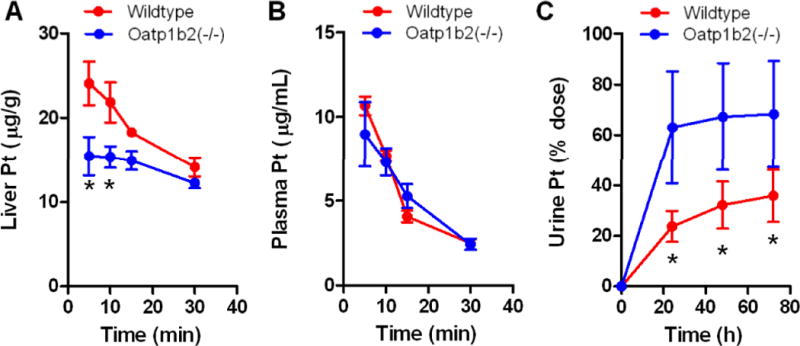
A, Liver concentration-time profile of cisplatin in wildtype and Oatp1b2(−/−) mice (i.p. dose, 10 mg/kg). B, Plasma concentration-time profile of cisplatin in wildtype and Oatp1b2(−/−) mice. C, Urine concentration-time profile of cisplatin in wildtype and Oatp1b2(−/−) mice. Data represent the mean of at least 6 observations per time point, and error bars represent the standard error. The star (*) denotes a significant difference between wildtype and Oatp1b2(−/−) mice (P < 0.05).
Analysis of kidneys from Oatp1b2(−/−) mice revealed that the expression of common drug-metabolizing enzymes, nuclear receptors, ATP-binding cassette transporters, and solute carrier genes were not substantially changed compared with kidneys obtained from wildtype animals, with the exception of an upregulation of Slc14a2 in Oatp1b2(−/−) mice (Supplementary Figs. S5A–B). The Slc14a2 gene encodes the urea transporter UT-B1 that has been implicated in the transport of molecules into urine (18). However, the overexpression of Slc14a2 in the kidney of the Oatp1b2(−/−) mice is unlikely to affect the renal handling of cisplatin since overexpression of UT-B1 or the related transporter UT-A1 (SLC14A1) expressed in HEK293 cells (Supplementary Fig. S5C) did not alter intracellular transport of cisplatin (Supplementary Fig. S5D), which suggests that the drug is not a transported substrate of urea transporters.
DISCUSSION
The current study provides support for a growing body of knowledge that solute carriers belonging to the family of OATPs can have a dramatic impact on cancer cell sensitivity and hepatic accumulation of multiple xenobiotics. Employing an array of in vitro transport assays, including intracellular accumulation studies in multiple transfected model systems, the platinum chemotherapeutics cisplatin, carboplatin, and oxaliplatin were identified as substrates for the human OATP1B1 and OATP1B3 transporters.
Using quantitative real-time PCR, we measured the mRNA expression of SLCO1B3 in the NCI60 cancer cell line panel. By correlating the mRNA expression pattern with the growth inhibitory potential of 118 drugs for which the mechanism of action is largely understood (14), we identified multiple putative compounds for which OATP1B3 is likely to play a dominant role in tumor sensitivity, including several platinum chemotherapeutics. To substantiate some of the OATP1B3-drug pairs for which the transporter was predicted to be sensitizing, follow-up experiments were performed using engineered and characterized models overexpressing OATP1B3. As predicted by the statistical correlations, expression of OATP1B3 in a human cell model resulted in increased cellular uptake of cisplatin, carboplatin, and oxaliplatin. It is important to note that the platinum complexes transported by OATP transporters are unknown. However, Di Pasqua et al have shown that cisplatin and carboplatin can react with carbonate to form negatively charged complexes that are more cytotoxic than parent cisplatin and carboplatin (19). Interestingly, tetraplatin is a platinum (IV) complex and is therefore less reactive, providing support for the concept that these transporters appear to regulate accumulation of selective platinum agents.
Huang et al. previously exploited the NCI60 database to correlate transporter expression data from an oligonucleotide array with the cytotoxicity of the same panel of agents (20). In both these authors’ analyses as in our own, the agent ranked with the highest positive correlation in the SLCO1B3 analysis was Asaley (NSC167780), an L-leucine derivative of melphalan with DNA alkylating activity. Moreover, both cisplatin (R = 0.33; P = 0.0092) and carboplatin (R = 0.37; P = 0.0039) were top-10 ranking compounds in this earlier analysis [see Supplementary data to Huang et al (20)], but these findings were not experimentally confirmed. It should be pointed out that several previous investigations failed to demonstrate indirect (21) or direct transport of cisplatin by OATP1B1 (22), as well as indirect interaction of carboplatin with OATP1B3 (23), or oxaliplatin with OATP1B1 or OATP1B3 (24). The reasons for these discrepant findings are not entirely clear, but may relate to differences in the experimental models and specific conditions applied.
Cisplatin is among the most widely used cytotoxic anticancer agents and has a broad spectrum of activity against various diseases, including endometrial, esophagus, lung, ovarian, gastric, bladder, testicular, and bladder cancers, and we found that the expression of the OATP1B3 gene was particularly high in these tumors, relative to the corresponding healthy tissue. In fact, while expression of OATP1B3 was detectable in colon, endometrium, esophagus, lymph node, ovarian, and bladder was specific to tumors, its expression was completely absent in normal tissue. In this context, it is of note that the effectiveness of platinum-based chemotherapy is directly linked with accumulation into target cells where DNA-mediated damage can then occur. Originally, movement of these agents across the cellular lipid bilayer was thought to occur predominantly by passive diffusion (25, 26). Instead, emerging evidence in the literature indicates that carrier-mediated uptake is likely the major determinant of uptake of these drugs into cancer cells (27).
The mechanism of cellular uptake of cisplatin in tumor cells is not entirely clear and may vary from one cell type to another (28). Nonetheless, the current knowledge as to the role of carrier-mediated platinum transport has been reviewed in great detail (29, 30). Preclinical studies have demonstrated that a high-affinity transporter known as copper transporter 1 (CTR1, encoded by the SLC31A1 gene) plays a role in the uptake of cisplatin in yeast as well as several murine and human tumor cell lines (31). However, the accumulation of cisplatin in human cancer cells expressing this transporter is limited by the fact that cisplatin itself can trigger the down-regulation and proteasomal degradation of CTR1, thereby limiting its own uptake (32). Our current findings support the possibility that the OATP1B3 transporter is another important uptake mechanism for platinum chemotherapeutics in multiple distinct tumors.
Although cisplatin elimination is dependent, in part, on tubular secretion mediated by the organic cation transporter OCT2 (11), in dogs initial levels of platinum are highest in liver and remain significantly elevated over time (33). Likewise, platinum levels are highest in liver in rats (34), mice (35), and are consistently higher than those observed in other organs in humans (36). The high liver uptake of platinum in humans has been confirmed using gamma camera imaging in patients with cancer following a dose of 191Pt-cisplatin (37).
Cisplatin uptake by the liver is not well understood, although our current finding that platinum chemotherapeutics are substrates of OATP1B1 and OATP1B3 sheds new light on the mechanistic basis for the liver uptake process. It should be pointed out that, using rat hepatocytes in primary culture, the kinetics of platinum uptake was previously found to be not saturable up to 400 μM of cisplatin (38). However, this finding does not necessarily rule out the existence of a carrier-mediated process of platinum uptake in the liver. For example, a carrier system with low affinity and high capacity, such as that described here for OATP1B1 and OATP1B3, could be involved in the transfer of cisplatin into the intracellular compartment of hepatocytes.
The rodent Oatp1b2 transporters share approximately 64% amino acid sequence homology to human OATP1B1 and OATP1B3, and on the basis of their shared basolateral localization in hepatocytes and overlapping substrate specificity (39), it is possible that in rodents Oatp1b2 fulfills the same function in the liver as OATP1B1 and OATP1B3 in humans. Based on this premise, we evaluated the pharmacokinetic properties of cisplatin in a mouse model with a genetic deletion of Oatp1b2. One possible limitation of this model is the fact that, unlike in humans, mouse hepatocytes express multiple members of Oatp1a, a related subfamily of transporters that can potentially provide compensatory restoration of function when Oatp1b2 is lost (40). Despite this limitation, compared to wildtype mice, the liver uptake of cisplatin in the Oatp1b2(−/−) mice was significant decreased. Gene expression profiling in liver samples excluded alterations in alternate transport mechanisms or metabolic pathways as a possible cause of this phenotype in Oatp1b2(−/−) mice. These findings suggest that Oatp1b2-mediated transport of cisplatin is an important rate-limiting process in the elimination of this drug in mice. Nonetheless, considering the relatively low amino acid homology between OATP1B1 or OATP1B3 and Oatp1b2, additional investigation is required employing humanized models for these proteins to provide direct evidence for involvement of OATP1B-type carriers in the hepatic uptake of cisplatin.
Interestingly, the altered liver distribution of cisplatin in the Oatp1b2(−/−) mice was associated with a shunting of drug into urine. A similar phenomenon has been reported previously for the anticancer and antirheumatic drug methotrexate, where deficiency of Abcc2 (Mrp2), a transporter regulating biliary secretion of this agent, is associated with increases in the extent of urinary excretion compared with wildtype mice (41). Although additional investigation will be required to assess the pharmacodynamic implications of this phenomenon, the observations made in the mice provide further evidence that hepatic OATP1B-type transporters can affect the pharmacokinetic properties of a remarkably broad range of substrates that include charged organic anions (eg, methotrexate), charged organic cations (eg, imatinib), polar zwitterions (eg, fexofenadine), uncharged hydrophobic agents (eg, taxanes), and chemically-diverse platinum chemotherapeutics.
Collectively, our demonstration that OATP1B-type transporters play an important role in the cellular uptake of platinum chemotherapeutics reveals new tumor and host factors that potentially contribute to enhanced interindividual variation in the efficacy and tolerability to this class of drugs.
Supplementary Material
Acknowledgments
We thank Drs. Herman Burger, David Finkelstein, Ryan Franke, and John Killmar for invaluable assistance with experiments and data analysis, Drs. Richard Kim and Jeffrey Stock for providing the Oatp1b2(−/−) mice, the National Cancer Institute Tumor Repository for providing RNA and DNA from the NCI60 cell lines, and Dr. Sharyn D. Baker for helpful advice and discussions.
Grant Support: Supported in part by the American Lebanese Syrian Associated Charities (ALSAC), and the United States Public Health Service Cancer Center Support Grant 3P30CA021765, and NCI Grant 5R01CA151633 awarded to A. Sparreboom.
Footnotes
Note: This work was previously presented, in part, at the Annual Meeting of the 112th American Society for Clinical Pharmacology and Therapeutics (ASCPT), held March 2–5, 2011, in Dallas, TX.
References
- 1.Franke RM, Sparreboom A. Drug transporters: recent advances and therapeutic applications. Clin Pharmacol Ther. 2010;87:3–7. doi: 10.1038/clpt.2009.239. [DOI] [PubMed] [Google Scholar]
- 2.Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7:205–20. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- 3.Van Winkle LJ. Biomembrane transport. San Diego: Academic Press; 1999. [Google Scholar]
- 4.Smith NF, Figg WD, Sparreboom A. Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination. Expert Opin Drug Metab Toxicol. 2005;1:429–45. doi: 10.1517/17425255.1.3.429. [DOI] [PubMed] [Google Scholar]
- 5.Pressler H, Sissung TM, Venzon D, Price DK, Figg WD. Expression of OATP family members in hormone-related cancers: potential markers of progression. PLoS One. 2011;6:e20372. doi: 10.1371/journal.pone.0020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–8. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- 7.de Graan AJ, Lancaster CS, Obaidat A, Hagenbuch B, Elens L, Friberg LE, et al. Influence of Polymorphic OATP1B-Type Carriers on the Disposition of Docetaxel. Clin Cancer Res. 2012;18:4433–40. doi: 10.1158/1078-0432.CCR-12-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino }methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 9.Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120:1689–99. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- 10.Holbeck SL, Collins JM, Doroshow JH. Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol Cancer Ther. 2010;9:1451–60. doi: 10.1158/1535-7163.MCT-10-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipski KK, Loos WJ, Verweij J, Sparreboom A. Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res. 2008;14:3875–80. doi: 10.1158/1078-0432.CCR-07-4793. [DOI] [PubMed] [Google Scholar]
- 13.Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, et al. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol. 2008;74:320–9. doi: 10.1124/mol.108.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein JN, Kohn KW, Grever MR, Viswanadhan VN, Rubinstein LV, Monks AP, et al. Neural computing in cancer drug development: predicting mechanism of action. Science. 1992;258:447–51. doi: 10.1126/science.1411538. [DOI] [PubMed] [Google Scholar]
- 15.Margiotta N, Marzano C, Gandin V, Osella D, Ravera M, Gabano E, et al. Revisiting [PtCl2(cis-1,4-DACH)]: an underestimated antitumor drug with potential application to the treatment of oxaliplatin-refractory colorectal cancer. J Med Chem. 2012 doi: 10.1021/jm3006838. [DOI] [PubMed] [Google Scholar]
- 16.van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, et al. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120:2942–52. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, et al. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol. 2008;74:320–9. doi: 10.1124/mol.108.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart G. The emerging physiological roles of the SLC14A family of urea transporters. Br J Pharmacol. 2011;164:1780–92. doi: 10.1111/j.1476-5381.2011.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Pasqua AJ, Goodisman J, Kerwood DJ, Toms BB, Dubowy RL, Dabrowiak JC. Role of carbonate in the cytotoxicity of carboplatin. Chem Res Toxicol. 2007;20:896–904. doi: 10.1021/tx700058f. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, et al. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004;64:4294–301. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]
- 21.Okabe M, Szakacs G, Reimers MA, Suzuki T, Hall MD, Abe T, et al. Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther. 2008;7:3081–91. doi: 10.1158/1535-7163.MCT-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, et al. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol. 2002;61:853–60. doi: 10.1124/mol.61.4.853. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, et al. Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett. 2008;260:163–9. doi: 10.1016/j.canlet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 24.Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, et al. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem. 2012;55:4740–63. doi: 10.1021/jm300212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale GR, Morris CR, Atkins LM, Smith AB. Binding of an antitumor platinum compound to cells as influenced by physical factors and pharmacologically active agents. Cancer Res. 1973;33:813–8. [PubMed] [Google Scholar]
- 26.Binks SP, Dobrota M. Kinetics and mechanism of uptake of platinum-based pharmaceuticals by the rat small intestine. Biochem Pharmacol. 1990;40:1329–36. doi: 10.1016/0006-2952(90)90400-f. [DOI] [PubMed] [Google Scholar]
- 27.Dobson PD, Lanthaler K, Oliver SG, Kell DB. Implications of the dominant role of transporters in drug uptake by cells. Curr Top Med Chem. 2009;9:163–81. doi: 10.2174/156802609787521616. [DOI] [PubMed] [Google Scholar]
- 28.Sprowl JA, Mikkelsen TS, Giovinazzo H, Sparreboom A. Contribution of tumoral and host solute carriers to clinical drug response. Drug Resist Updat. 2012;15:5–20. doi: 10.1016/j.drup.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JJ, Lu J, McKeage MJ. Membrane transporters as determinants of the pharmacology of platinum anticancer drugs. Curr Cancer Drug Targets. 2012;12:962–86. doi: 10.2174/156800912803251199. [DOI] [PubMed] [Google Scholar]
- 30.Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- 31.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res. 2009;15:553–60. doi: 10.1158/1078-0432.CCR-08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litterst CL, Gram TE, Dedrick RL, Leroy AF, Guarino AM. Distribution and disposition of platinum following intravenous administration of cis-diamminedichloroplatinum(II) (NSC 119875) to dogs. Cancer Res. 1976;36:2340–4. [PubMed] [Google Scholar]
- 34.Sharma RP, Edwards IR. cis-Platinum: subcellular distribution and binding to cytosolic ligands. Biochem Pharmacol. 1983;32:2665–9. doi: 10.1016/0006-2952(83)90073-4. [DOI] [PubMed] [Google Scholar]
- 35.Junior AD, Mota LG, Nunan EA, Wainstein AJ, Wainstein AP, Leal AS, et al. Tissue distribution evaluation of stealth pH-sensitive liposomal cisplatin versus free cisplatin in Ehrlich tumor-bearing mice. Life Sci. 2007;80:659–64. doi: 10.1016/j.lfs.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Stewart DJ, Benjamin RS, Luna M, Feun L, Caprioli R, Seifert W, et al. Human tissue distribution of platinum after cis-diamminedichloroplatinum. Cancer Chemother Pharmacol. 1982;10:51–4. doi: 10.1007/BF00257239. [DOI] [PubMed] [Google Scholar]
- 37.Areberg J, Bjorkman S, Einarsson L, Frankenberg B, Lundqvist H, Mattsson S, et al. Gamma camera imaging of platinum in tumours and tissues of patients after administration of 191Pt-cisplatin. Acta Oncol. 1999;38:221–8. doi: 10.1080/028418699431654. [DOI] [PubMed] [Google Scholar]
- 38.Marin JJ, Herrera MC, Palomero MF, Macias RI, Monte MJ, El-Mir MY, et al. Rat liver transport and biotransformation of a cytostatic complex of bis-cholylglycinate and platinum (II) J Hepatol. 1998;28:417–25. doi: 10.1016/s0168-8278(98)80315-2. [DOI] [PubMed] [Google Scholar]
- 39.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–87. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iusuf D, van de Steeg E, Schinkel AH. Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol Sci. 2012;33:100–8. doi: 10.1016/j.tips.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Vlaming ML, Pala Z, van Esch A, Wagenaar E, van Tellingen O, de Waart DR, et al. Impact of Abcc2 (Mrp2) and Abcc3 (Mrp3) on the in vivo elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate. Clin Cancer Res. 2008;14:8152–60. doi: 10.1158/1078-0432.CCR-08-1609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


