Abstract
Antibodies against CCR5, the major coreceptor for human immunodeficiency virus type 1 (HIV-1), may have antiviral potential as viral fusion inhibitors. In this study, we generated a virus-like particle (VLP)-based vaccine that effectively breaks B-cell tolerance and elicits autoantibodies against CCR5 in pig-tailed macaques. Initial studies in mice identified a polypeptide comprising the N-terminal domain of pig-tailed macaque CCR5 fused to streptavidin that, when conjugated at high density to bovine papillomavirus major capsid protein L1 VLPs, induced high-titer immunoglobulin G (IgG) that bound to a macaque CCR5-expressing cell line in vitro. In macaques, CCR5 peptide-conjugated VLP preparations induced high-avidity anti-CCR5 IgG autoantibody responses, and all five immunized macaques generated IgG that could block infection of CCR5-tropic simian/human immunodeficiency virus SHIVSF162P3 in vitro. Although the anti-CCR5 IgG titers declined with time, autoantibody levels were boosted upon revaccination. Vaccinated macaques remained healthy for a period of over 3 years after the initial immunization, and no decline in the number of CCR5-expressing T cells was detected. To test the prophylactic efficacy of CCR5 autoantibodies, immunized macaques were challenged with SHIVSF162P3. Although the plasma-associated virus in half of six control macaques declined to undetectable levels, viral loads were lower, declined more rapidly, and eventually became undetectable in all five macaques in which CCR5 autoantibodies had been elicited. In addition, in the four vaccinated macaques with higher autoantibody titers, viral loads and time to control of viremia were significantly decreased relative to controls, indicating the possibility that CCR5 autoantibodies contributed to the control of viral replication.
Primate lentiviruses, such as human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV), use chemokine coreceptors in addition to the CD4 receptor to initiate virus infection (11, 33, 44). While a number of chemokine receptors can function as coreceptors, CCR5 is likely the most physiologically important coreceptor during natural infection. In individuals infected with HIV-1, CCR5-tropic (R5-tropic) viruses are the predominant species isolated during the early stages of viral infection (56), suggesting that these viruses may have a selective advantage during either transmission or the acute phase of disease. Moreover, at least half of all infected individuals harbor only R5 viruses throughout the course of infection (14, 31). Genetic studies of a defective CCR5 allele (Δ32) have demonstrated that homozygous individuals are strongly resistant to HIV-1 infection and that heterozygotes have delayed progression to AIDS (11, 13, 25, 33, 37, 44, 50, 57). Thus, decreasing the availability of coreceptor can have profound effects on viral pathogenesis. Individuals possessing the Δ32 allele are healthy, suggesting that modulation of CCR5 may not strongly affect the normal function of the T cells and macrophages that predominantly express this protein.
Given the important role that it plays during infection, CCR5 is considered an attractive antiviral therapeutic target. In addition, as a cellular protein, CCR5 is genetically stable, unlike viral targets, which may rapidly mutate during the course of infection. Thus, intervention strategies that attempt to inhibit viral replication by either directly blocking virus-coreceptor interactions or decreasing CCR5 expression have been examined. These strategies have employed chemokines and their analogs, small molecular inhibitors, small interfering RNAs, and anti-CCR5 monoclonal antibodies (MAbs) (2, 4, 39, 48, 54).
As an alternate approach, there has been interest in developing a vaccination strategy to induce anti-CCR5 antibodies that can bind native CCR5 and block viral infection in vivo (9). Because CCR5 is continuously exposed to the systemic immune system, effective induction of an anti-CCR5 antibody response is only possible by circumventing the tolerance mechanisms that the immune system has developed to normally block the maturation of B cells specific for central self antigens. Our laboratory and others have shown that immunization with self antigens arrayed at high occupancy on the surface of virus particles can efficiently break B-cell tolerance and induce strong immunoglobulin G (IgG) autoantibody responses (reviewed in reference 51). By using self antigens conjugated to or incorporated into the regular array of papillomavirus-like particles (VLPs), it has been demonstrated in rodents that these immunogens strongly diminish the ability of the humoral immune system to distinguish between self and foreign antigens, resulting in a high-titer, high-avidity IgG autoantibody response (8, 9). The mechanisms responsible for this response have not been completely elucidated, but high self antigen density is a critical factor in enhancing the survival and/or proliferation of autoreactive B cells (7). While the mechanism of B-cell tolerance most likely depends on whether the self antigen is expressed as a soluble or membrane-associated form (19, 22, 38), we have used the conjugated VLP strategy to elicit autoantibody responses against both soluble (tumor necrosis factor alpha [TNF-α]) and cell-associated (CCR5) self antigens in mice. In addition, it has been demonstrated that the induction of autoantibodies has the potential to reduce disease pathologies, as autoantibodies against TNF-α could block or attenuate disease in a TNF-α-dependent mouse model of rheumatoid arthritis (8).
Here, we report the development of a conjugated VLP-based vaccine that induces an anti-CCR5 autoantibody response in primates and examine the magnitude, duration, and consequences of autoantibody induction, as well as the protective effects of vaccination in vivo upon subsequent challenge with an R5-tropic primate retrovirus.
MATERIALS AND METHODS
Preparation of CCR5-conjugated VLPs.
Bovine papillomavirus type 1 (BPV-1) major capsid protein L1 VLPs were generated from recombinant baculovirus-infected Sf9 cell cultures, as described previously (28). Purified VLPs were biotinylated by incubation with NHS-LC-Biotin (Pierce Endogen, Rockford, Ill.) at a 1:2 (wt/wt) ratio (60 min at room temperature), and then purified on a linear sucrose gradient by centrifugation (8). Nucleotide sequences predicted to encode the four extracellular (EC) domains of pig-tailed macaque CCR5 (ptCCR5) were made by using an Applied Biosystems DNA synthesizer (Foster City, Calif.). Each sequence was engineered to contain an EcoRI restriction site at its 5′ end and a stop codon, followed by a BamHI restriction site at its 3′ end. By using the restriction sites, the sequences were cloned as a C-terminal fusion partner with streptavidin (SA) in a modified version of the expression vector pTSA-18F (46). The modified version contains a linker sequence (predicted to encode the amino acids Gly-Gly-Gly-Gly-Ser) between the SA coding sequence and the CCR5 sequence. SA-CCR5 constructs were transformed into lysogen BL21(DE2) (pLysS) (Novagen, Madison, Wis.) for expression, and recombinant protein was subsequently purified from inclusion bodies as previously described (8, 45, 46). The ability of SA fusion proteins to bind biotin was determined by enzyme-linked immunosorbent assay (ELISA) by measuring the ability to bind biotinylated bovine serum albumin (BSA; Pierce Endogen), as described previously (8). Conjugated VLPs were prepared by the incubation of SA fusion proteins with biotinylated BPV-1 L1 VLPs at a 3:1 (wt/wt) ratio and were purified by cross-flow filtration (A/G Technology Corp., Needham, Mass.) as described previously (8).
Mouse inoculations.
Antisera were prepared by inoculating C57BL/6 mice with 5 μg of VLPs conjugated to approximately 10 to 15 μg of SA-EC fusion protein in Freund's adjuvant. Mice were inoculated subcutaneously three times at 2-week intervals. Antigen was diluted 1:1 in complete Freund's adjuvant (Pierce Endogen) for the initial injection and diluted 1:1 in incomplete Freund's adjuvant (Pierce Endogen) for subsequent inoculations. Serum samples were collected 2 weeks after the final boost. Groups of three mice were immunized with each preparation. All animal care was in accordance with the National Institutes of Health guidelines.
Macaque inoculations.
Eleven 3- to 7-year-old pig-tailed macaques were randomized into two groups. One group of five macaques was immunized intramuscularly with approximately 20 to 25 μg of BPV-1 L1 VLPs conjugated to 50 to 75 μg of SA-EC1, in the presence of Titermax Gold adjuvant (CytRx Corp., Atlanta, Ga.) at 20% of the total volume. These macaques were immunized a total of nine times over a period of over 2 years. The second group of six macaques served as controls. Sera were collected at regular intervals. Prior to all subsequent manipulations, macaque sera were heat inactivated for 30 min at 56°C.
ELISA of antibody induction.
Serum antibody specific for CCR5 EC peptides or recombinant SA was detected by ELISA. Peptides corresponding to the four EC domains of ptCCR5 (EC1 to EC4) and containing an additional C-terminal cysteine residue were synthesized (Commonwealth Biotechnologies, Richmond, Va.) and conjugated to activated keyhole limpet hemocyanin (KLH; Pierce Endogen) according to the manufacturer's instructions. Immulon II ELISA plates (Dynex Technologies, Chantilly, Va.) were coated with 500 ng of EC-KLH or 200 ng of recombinant SA per well. Serum was serially diluted in phosphate-buffered saline (PBS)-0.5% milk and applied to wells for 2.5 h at room temperature. Reactivity to target polypeptides was determined by using horseradish peroxidase-labeled goat anti-mouse IgG (for mouse sera) (Boehringer Mannheim Biochemicals Inc., Indianapolis, Ind.) at a dilution of 1:5,000 or horseradish peroxidase-labeled rabbit anti-human IgG (for macaque sera) (Dako, Carpinteria, Calif.) at a dilution of 1:6,000 as a secondary antibody (incubated for 1 h at room temperature). Upon development, the optical densities at 405 nm (OD405) were read by a Thermo Max microplate reader (Molecular Devices Corp., Sunnyvale, Calif.). OD405 values that were greater than twice background were considered positive. The avidity index value of serum antibodies was determined by measuring the resistance of antibody target complexes to 8 M urea by ELISA. Briefly, after the serum incubation, triplicate wells were treated with either PBS or 8 M urea for 5 min. Subsequently, the wells were washed with PBS, and the ELISA was performed as described above. The avidity index value was calculated as the ratio of the mean OD value of urea-treated wells to PBS control wells multiplied by 100, as previously described (23).
Flow cytometry.
Flow cytometry was performed on a FACSCalibur by using the CellQuest software package (BD Biosciences, San Jose, Calif.). Mouse serum IgG binding to native CCR5 was tested by reacting pooled mouse sera with HeLa cells that stably express ptCCR5 (MAGI-ptCCR5 cells; see below). Cells were detached from the monolayer by using 5 mM EDTA and then washed three times in staining buffer (PBS plus 0.5% BSA). Approximately 105 cells were resuspended in 50 μl of staining buffer plus 2 μl of mouse serum for 30 min at 4°C. After being washed three times with staining buffer, cells were resuspended in 50 μl of staining buffer plus 250 ng of fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (Jackson Immunoresearch) and then incubated for 30 min at 4°C. Before analysis, cells were washed an additional three times with staining buffer and resuspended in 0.5 ml of staining buffer. Specific binding was measured relative to cells stained with pooled sera from mice immunized with wild-type SA-conjugated VLPs (VLP:SA) generated previously (8).
The specificity of macaque IgG for native CCR5 expressed on pig-tailed macaque peripheral blood mononuclear cells (PBMCs) was tested by examining the ability of protein A/G (Pierce Biochemical Corp.)-purified macaque IgG to block binding of a CCR5-specific MAb (3A9; BD Biosciences). Fifty microliters of whole blood from a naïve pig-tailed macaque was diluted twofold in staining buffer and then incubated with purified macaque IgG at a final concentration of 1 mg/ml for 15 min at room temperature. Cells were washed twice with staining buffer, resuspended in 100 μl of staining buffer, and then stained with MAbs against CD95 (FITC-labeled; 20 μl) and CCR5 (3A9) (phycoerythrin [PE]-labeled; 1 μl) for 20 min at room temperature. After the cells were washed twice, they were treated with 1 ml of ACK lysis buffer (Cambrex, Walkersville, Md.) for 10 min at room temperature to lyse red blood cells. Cells were washed twice and then analyzed by flow cytometry.
Viral inhibition assays.
The inhibition of infection was measured by using the MAGI-ptCCR5 indicator cell line. These cells and their care were described by Kimata et al. (26). At 24 h prior to infection, 104 cells were plated per well on a 48-well plate. At 30 min prior to infection, dilutions of protein A/G-purified macaque IgG were added to cells in a total volume of 200 μl. Approximately 100 infectious simian/human immunodeficiency virus SHIVSF162P3 virus particles were added to each well in the presence of 10 μg of DEAE-Dextran (Sigma, St. Louis, Mo.) per ml. After 2 h at 37°C, virus and antibody were removed from each well and replaced with 0.5 ml of media. Two days after infection, cells were fixed, washed, and stained for β-galactosidase activity, as described previously (27).
Macaque challenge.
All 11 pig-tailed macaques were challenged with the 50% tissue culture infective dose of SHIVSF162P3, obtained from Janet Harouse and colleagues through the AIDS Research and Reference Reagent Program. This virus stock had been titered in vivo in pig-tailed macaques (Macaca nemestrina) by Nancy Haigwood at the University of Washington, Seattle, Wash. The 50% tissue culture infective dose is equivalent to approximately 100 animal infectious doses (N. L. Haigwood, personal communication). Virus was administered intravenously into the saphenous vein in a total volume of 1 ml. Blood, serum, and plasma were collected from infected animals at regular intervals after infection. Lymphocyte subsets in PBMCs were analyzed by flow cytometry by FAST Systems, Inc. (Gaithersburg, Md.) using a Beckman-Coulter EPICS XL MCL flow cytometer. MAbs used included CD3e-FITC, CD4-PE, CD4-FITC, CD8-peridinin chlorophyll protein, and CCR5-PE (3A9) (all BD Biosciences). Absolute cell counts were determined by staining with fluorescent-labeled MAbs (CD3e-FITC, CD4-PE, and CD8-peridinin chlorophyll protein) along with an internal standard of fluorescent microbeads. The percentage of CD4+ lymphocytes expressing CCR5 was determined by double staining with anti-CD4-FITC and anti-CCR5-PE. Plasma-associated SIV RNA viral loads were determined by using a real-time quantitative reverse transcription-PCR method as described by Lifson et al. (32). Approximately 60 weeks after virus challenge, the 11 macaques were euthanized and necropsied.
Statistical analysis.
Viral loads were transformed to the log base 10 scale since data were approximately normal on that scale. A comparison of the longitudinal profiles between vaccinated and control macaques was done by a permutation test (18). This global test was done by first summing the squared t statistics from individual t tests done at each time point. The observed summed t statistic was compared with a reference distribution obtained by scrambling the group assignments and obtaining the summed squared t statistics for 2,000 random samples. The P value was computed as the proportion of times the reference summed squared t statistics were larger than the observed summed squared t statistic. In addition to the global test, viral loads were compared between vaccinated and control monkeys at each individual time point by using t tests and Wilcoxon rank sum tests. In both cases, a value of 90 was used for viral loads below the level of detection. A log-rank test was used to compare the time to control of detectable viral loads between the two groups. Since the sample size was small, a permutation test was used to compute the P value. The endpoint for this analysis was the time of the first occurrence of three time points below the level of detection. All tests were two-sided.
RESULTS
Induction of ptCCR5-specific antibodies in mice.
Previously, our laboratory generated anti-mouse CCR5 autoantibodies by immunizing mice with chimeric VLPs that contained an EC loop of mouse CCR5 inserted into an immunodominant loop of the papillomavirus major capsid protein, L1 (9). However, our attempts to develop L1-primate CCR5 chimeric VLPs were unsuccessful because the CCR5 sequence interfered with the ability of the L1 protein to self-assemble into VLPs (data not shown). To circumvent this problem, we utilized a more flexible approach in which antigens are conjugated to preformed VLPs by taking advantage of the strong interaction between biotinylated VLPs and SA-target peptide fusion proteins (8).
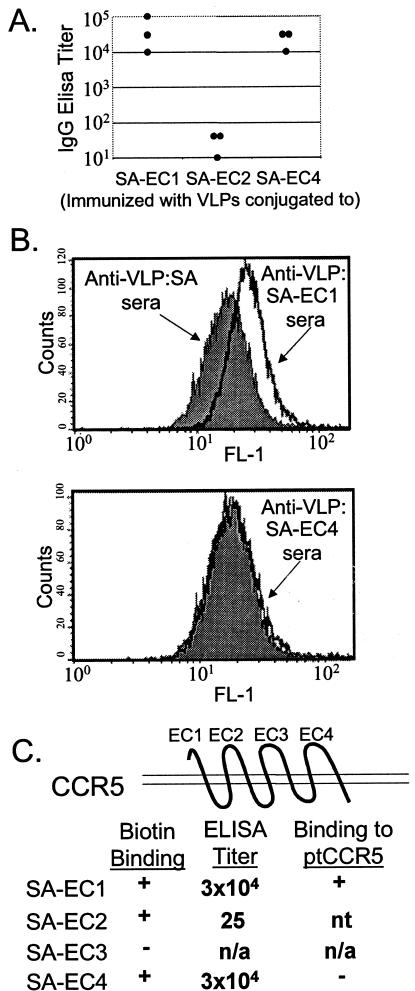
The CCR5 protein is a 7-transmembrane-spanning receptor that has four EC domains. We generated four bacterially expressed fusion proteins in which each of the four individual EC domains of ptCCR5 was added as a C-terminal fusion partner to a truncated version of SA. Each of these chimeric proteins was then characterized individually (as shown in Fig. 1A and B and summarized in C). To determine whether these engineered proteins could be conjugated to biotinylated VLPs, the four fusion proteins (designated SA-EC1, SA-EC2, SA-EC3, and SA-EC4) were tested for biotin-binding activity by ELISA. All except the SA-EC3 fusion protein reacted with immobilized biotinylated BSA on an ELISA plate (data not shown), indicating that for three of the fusion proteins the addition of CCR5 sequences to SA had not impaired the ability of the SA domain to bind biotin. To generate conjugated VLPs, the three SA-EC proteins that bound biotin (SA-EC1, SA-EC2, and SA-EC4) were individually reacted with biotinylated BPV-L1 VLPs. Binding of the SA-EC proteins to the VLPs was confirmed by sucrose gradient centrifugation (data not shown).
FIG. 1.
Analysis of antibody responses in CCR5-EC-conjugated VLP-immunized mice. (A) Serum IgG titers in immunized C57BL/6 mice. Anti-CCR5 EC domain end-point dilution titers in serum from mice immunized with VLP-conjugated EC domains. Titers were measured by ELISA with KLH-conjugated peptides corresponding to EC1, EC2, and EC4. Each data point corresponds to the antibody titer from an individual mouse. (B) Flow cytometric analysis of antibody binding to ptCCR5-expressing HeLa cells (MAGI-ptCCR5). Cells were incubated with sera from mice immunized with VLP:SA-EC1 or VLP:SA-EC4 (dark black line) or, as a control, with sera from mice immunized with VLP:SA (shaded histogram), followed by incubation with an FITC-labeled goat anti-mouse IgG secondary antibody. (C) Summary of data in characterization of SA-EC fusion proteins.
The ability of each conjugated VLP preparation to elicit anti-ptCCR5 antibodies was assessed in mice. Groups of three C57BL/6 mice were vaccinated with VLPs conjugated to SA-EC1, SA-EC2, or SA-EC4. Mice were injected subcutaneously three times at 2-week intervals with 5 μg of VLPs conjugated to ∼10 μg of SA-EC in the presence of Freund's adjuvant. Antibody responses against the EC regions were measured by ELISA, using KLH-conjugated peptides corresponding to the macaque EC domains as the target antigens. Figure 1A shows the end-point dilution IgG ELISA titers of the immunized animals. Strong IgG responses were elicited in animals immunized with VLPs conjugated to SA-EC1 and SA-EC4, but animals immunized with VLP-conjugated SA-EC2 failed to generate EC2-specific antibodies.
Although these data indicated that two of the conjugated VLP preparations elicited antibodies that recognized ptCCR5 peptides, it was possible that these antibodies might not recognize the EC domains in their native conformation when they are part of the membrane-associated ptCCR5. To examine this question, the ability of anti-EC region antibodies to bind to a ptCCR5-expressing cell line was tested by flow cytometry. The binding of sera from VLP:SA-EC-immunized mice to ptCCR5 was tested relative to control (VLP:SA-immunized) sera by using a stable cell line that expresses ptCCR5 (MAGI-ptCCR5) (26) (Fig. 1B). Serum IgG from VLP:SA-EC1-immunized mice bound specifically to this cell line, relative to control sera from VLP:SA-immunized mice, whereas there was no significant binding detected for sera from VLP:SA-EC4-immunized mice. Thus, only the VLP:SA-EC1 immunogen induced antibodies that recognized the native conformation of ptCCR5.
Vaccination of pig-tailed macaques with VLP:SA-EC1.
The EC domains of mouse and macaque CCR5 are approximately 75% homologous. Therefore, induction of anti-ptCCR5 antibodies in mice does not necessarily predict the ability of CCR5-conjugated VLPs to break B-cell tolerance against ptCCR5 in macaques. To test this hypothesis, five macaques were immunized intramuscularly with injections of 20 to 25 μg of BPV-L1 VLPs conjugated to 50 to 75 μg of SA-EC1, in Titermax Gold adjuvant. Antibody responses against the self (EC1) and foreign (SA) components of the vaccine were measured by ELISA, by using KLH-conjugated EC1 peptide or recombinant SA as target antigens, respectively (Fig. 2). All five macaques responded to vaccination by generating anti-EC1 antibodies. By means of an inhibition assay, detailed in the results below, anti-EC1 antibodies were shown to block retroviral infection, strongly suggesting that the anti-EC1 antibodies bound native cell-associated CCR5 and were indeed autoreactive.
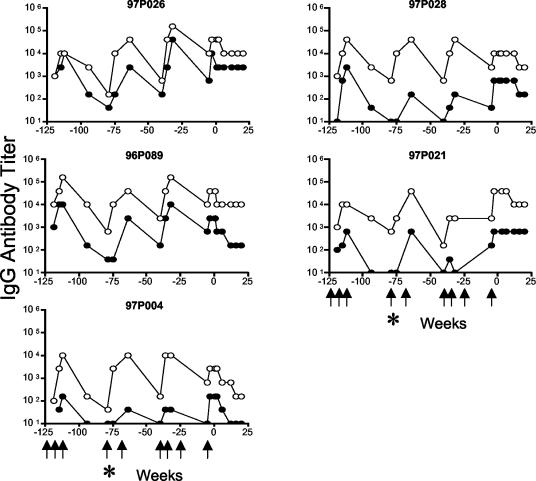
FIG. 2.
Serum IgG antibody titer in immunized pig-tailed macaques. Anti-EC1 (•) and anti-SA (○) end-point dilution titers in serum from individual macaques immunized with VLPs conjugated to SA-EC1 and followed for over 3 years. Time points are relative to the date of virus challenge (week zero). Macaques were immunized at weeks −123, −119, −115, −79, −68, −40, −36, −25, and −5 (indicated by arrows). An asterisk indicates week −79, when all five macaques were mistakenly immunized with VLPs conjugated to wild-type SA.
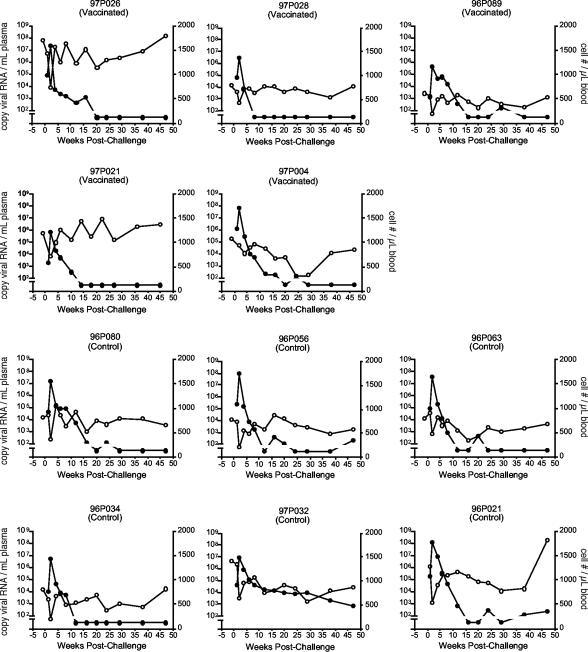
Compared with the responses to EC1 measured in the inbred mice, the magnitude of the antibody responses was more variable in the outbred macaques. Antibody titers against EC1 measured 4 weeks after the third immunization (week −111) were high in two of the macaques (titers of approximately 104), one of the animals had a more intermediate response (titer between 103 and 104), and two monkeys were low responders (titers of <103). The avidities to the EC1 peptide of serum autoantibodies collected at this time point were determined by an ELISA that measures the sensitivity of the antibody-antigen interaction to treatment with 8 M urea. Four of five macaques had serum autoantibody peptide avidity index values above 50%, the standard cutoff for high avidity in this assay (23), and one macaque (97P026) had an intermediate peptide avidity index value (range, 46 to 89%).
Efficiency of autoantibody induction.
To assess the relative efficiency of antibody responses to the self and foreign components of the vaccine, antibodies induced against SA were also measured and compared to the anti-EC responses (Fig. 2). After three inoculations, anti-foreign (SA) antibody titers (range, 1.0 × 104 to 1.6 × 105) were generally about 10-fold higher than anti-self (EC1) antibody titers. The relative titers correspond approximately to the relative sizes of the SA and CCR5 polypeptides in the fusion protein. A similar ratio between anti-foreign and anti-self antibody responses was observed previously in mice inoculated with conjugated VLPs in the presence of Titermax Gold (8). In four of five macaques, the ratio of anti-SA to anti-EC1 antibody titers was ≤12, suggesting that B-cell tolerance had been broken relatively efficiently. However, in the macaque with the lowest anti-EC1 titer (macaque 97P004), the (anti-SA:anti-EC1) IgG ratio was approximately 100, perhaps indicating that autoantibody induction may be less efficient in this macaque. Alternatively, immature B cells with receptors that recognize the relatively short CCR5 peptide might have been underrepresented in the repertoire for this animal at the time of vaccination.
Duration of response and effects of boosting.
Immune responses in the macaques were monitored over a 2-year period to examine the durability of antibody responses, the effectiveness of boosting, and the safety of vaccination. Antibody responses against both EC1 and SA declined over time in roughly a parallel fashion (Fig. 2). For example, in the 36-week period between the third and the fourth immunizations, anti-SA titers declined an average of 85-fold (range, 16- to 256-fold) and anti-EC1 titers declined an average of 170-fold (range, 16- to 256-fold). At three different time points, revaccination was successful at boosting antibody responses against both SA and EC1 to peak levels. The decline in autoantibody titers and the ability to boost autoantibody responses in macaques suggest that exposure of native CCR5 to autoreactive B cells neither restimulated these cells nor acutely anergized them.
Absence of adverse side effects.
A potential concern of autoantibody induction is that these antibodies may lead to adverse consequences for the immunized animals. For example, autoantibodies may be cytotoxic to cells that express CCR5. To examine this possibility, the percentage of T cells expressing measurable CCR5 was monitored over the course of the study. There was no significant difference between the vaccinated group and the group of six age-matched control unvaccinated macaques, suggesting that no such depletion of CCR5-expressing T cells had occurred in the vaccinees. For example, at 1 week prior to SHIVSF162P3 challenge (week −1), the percentage of CD4+ T cells expressing CCR5 was similar in vaccinated (66.9% ± 0.3%) and control (67.3% ± 1.1%) macaques. In addition, we did not observe any depletion of CD4+ T cells within the peripheral blood compartment. One week prior to challenge, the absolute CD4+ T cell counts were similar in the two groups (vaccinated macaques, 1,074 ± 186 cells/ml blood; control macaques, 980 ± 117 cells/ml blood), as was the percentage of peripheral blood cells that were CD4+ (vaccinated macaques, 37.9% ± 2.5%; control macaques, 36.6% ± 2.2%). All of the immunized macaques remained healthy during the more than 2-year period between the initial vaccination and viral challenge and were pathologically normal at necropsy.
Ability of macaque anti-EC1 IgG to bind to ptCCR5.
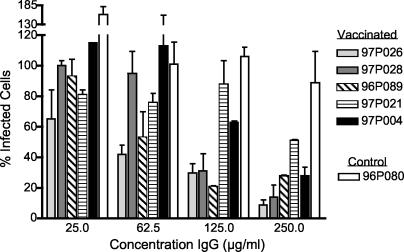
The ability of induced IgG to bind to cell-associated CCR5 was tested in vitro by examining whether anti-EC1 antibodies from the vaccinated macaques blocked the infection of an R5-tropic lentivirus. Serum samples were collected from the five vaccinated macaques and from one control unvaccinated macaque (96P080) 2 weeks after the final boost and 3 weeks prior to challenge. Because the macaque sera had a high level of nonspecific anti-viral inhibitory activity in our infection assay, we tested the activity of protein A/G-purified macaque IgG to block virus infection in vitro and bind to primary lymphocytes. MAGI-ptCCR5 cells were infected with approximately 50 infectious SHIVSF162P3 particles in the presence of macaque IgG. IgG isolated from all five vaccinated macaques had viral inhibitory activity at the highest concentration tested (250 μg/ml), whereas serum IgG from the control animal (96P080) had no detectable inhibitory activity at this concentration (Fig. 3). The extent of inhibition roughly paralleled the anti-EC1 IgG ELISA titers. For example, IgG isolated from the macaques with the highest anti-EC1 ELISA titer (macaques 97P026 and 96P089) exhibited ≥50% inhibition at a low IgG concentration (62.5 μg/ml), whereas serum IgG from the macaques with the lowest titer (macaques 97P021 and 97P004) was least effective at inhibiting viral infection (≥50% inhibition was detected only at the highest concentration, 250 μg/ml).
FIG. 3.
Inhibition of SHIVSF162P3 infection of an indicator cell line (MAGI-ptCCR5) by protein A/G-purified IgG from immunized macaques. IgG was purified from sera taken from all five vaccinated macaques and one control macaque 3 weeks prior to viral challenge (week −3). Cells were incubated with dilutions of purified macaque IgG for 30 min and then infected with approximately 50 infectious SHIVSF162P3 virus particles. Two hours after infection, cells were washed and given fresh media. Two days after infection, cells were stained, and infected cells were scored by counting the number of blue cells in each well. Inhibition of infection was determined by comparing the number of blue (infected) nuclei in the presence of IgG versus the number of blue nuclei in the absence of IgG. The number of infected cells in the absence of IgG is considered 100% infection, and the percent of infected cells in the presence of IgG is represented relative to this number. In some instances this value may be greater than 100%. These results represent the mean inhibition from two experiments. Error bars represent standard error of the means.
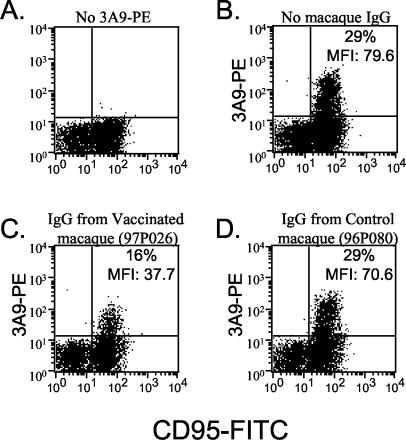
While IgG from vaccinated macaques interfered with CCR5 function as a viral coreceptor in an indicator cell line, the formal possibility existed that these antibodies may not bind native CCR5 as expressed on primary macaque lymphocytes. To rule out this possibility, we examined the ability of IgG from a vaccinated macaque to block the binding of a CCR5-specific MAb (3A9). 3A9 has been demonstrated to bind to a conformational epitope of CCR5 that includes the EC1 domain (29). Whole blood from a naïve pig-tailed macaque was incubated with a MAb against CD95 along with IgG from either a vaccinated (97P026) or a control (96P080) macaque and then reacted with a limiting amount of PE-labeled 3A9. The ability of macaque IgG to block 3A9-PE binding was assessed by flow cytometry, by gating on lymphocytes and examining the CD95+ population (Fig. 4). Incubation with IgG from the vaccinated macaque reduced the percentage of CD95+ lymphocytes that were 3A9 positive approximately twofold (Fig. 4C). In addition, the mean fluorescence index of the cells that remained 3A9 positive was greater than two times lower than that of cells that were not preincubated with macaque IgG. IgG from the vaccinated macaque did not decrease the binding of a control MAb (anti-CD95-FITC) by more than 10% (data not shown), suggesting that the observed inhibition of 3A9 binding was specific. In addition, incubation of lymphocytes with the same concentration of IgG from a control macaque had no effect on 3A9-PE binding (Fig. 4D). Taken together, these results and the virus inhibition data strongly support the conclusion that the vaccination protocol strongly diminished B-cell tolerance to the native conformation of the CCR5 EC1 peptide.
FIG. 4.
Inhibition of binding of a MAb against CCR5 (3A9) to primary pig-tailed macaque lymphocytes by purified macaque IgG. Whole blood from a naïve pig-tailed macaque was incubated with FITC-labeled anti-human CD95 MAb alone or FITC-labeled anti-human CD95 MAb (A) and PE-labeled anti-human CCR5 MAb (3A9) (B), purified IgG from a vaccinated macaque (97P026) and 3A9-PE (C), or purified IgG from a control macaque (96P080) and 3A9-PE (D). Staining was assessed by flow cytometry, and fluorescence was measured after gating on the lymphocyte population. Inhibition of 3A9 binding was determined by two methods: first, by quantitating the percentage of CD95+ cells that were also 3A9-PE positive and, second, by determining the mean fluorescence index (MFI) of CD95+ cells that were bound by 3A9. These values are displayed in the upper-right quadrant of panels B, C, and D.
Macaque challenge with SHIVSF162P3.
Five weeks prior to challenge, the five previously vaccinated macaques were boosted with a final dose of VLP:SA-EC1. Anti-EC1 antibody titers were measured 3 weeks prior to challenge, and in each of the five vaccinated macaques, these titers were within one fourfold dilution of its peak autoantibody titer (Fig. 2). At this time point, the avidity index value of anti-EC1 antibodies from three macaques was high (macaques 97P026, 97P028, and 96P089), one was intermediate (97P021), and one was low (97P004) (Table 1).
TABLE 1.
Comparison of anti-EC1 IgG responses and viral loads in vaccinated macaques
| Macaque | Anti-EC1 IgG titer at wk −3a | Avidity index (%) at wk −3 (relative avidity)b | Peak viral load at wk 2c | Time to control (wk)d |
|---|---|---|---|---|
| 97P026 | 10,240 | 91 (high) | 2.2 × 107 | 20 |
| 97P028 | 640 | 79 (high) | 3.0 × 106 | 8 |
| 97P089 | 2,560 | 94 (high) | 3.9 × 105 | 16 |
| 97P021 | 640 | 34 (intermediate) | 6.3 × 105 | 16 |
| 97P004 | 160 | 20 (low) | 6.0 × 107 | 29 |
Based on data shown in Fig. 2.
The avidity index was determined by ELISA by measuring the relative stability of the KLH-EC1-antibody complex to an 8 M urea wash. Values were calculated by the following equation: (urea-washed wells/PBS-washed wells) × 100. High avidity, values ≥50%; intermediate avidity, values >30% but <50; low avidity, values ≤30%.
Based on data shown in Fig. 5.
Defined as the first time point at which the level of plasma-associated viral RNA was below the level of detection and remained undetectable at the next two subsequent time points.
It is unclear whether SIV isolates use other coreceptors in addition to CCR5 in vivo. Because of this possibility, we selected the SHIVSF162P3 challenge virus to evaluate the effectiveness of this vaccine. SHIVSF162P3 is a chimeric virus that contains the env, tat, and rev genes from HIV-1SF162, a prototypical R5-tropic virus, in the background of SIVmac239 (10, 36). It has been demonstrated that SHIVSF162P3 uses CCR5 as its exclusive coreceptor and does not shift its tropism in vivo (20). In addition, the virus has been reported to be pathogenic in rhesus macaques (20, 21).
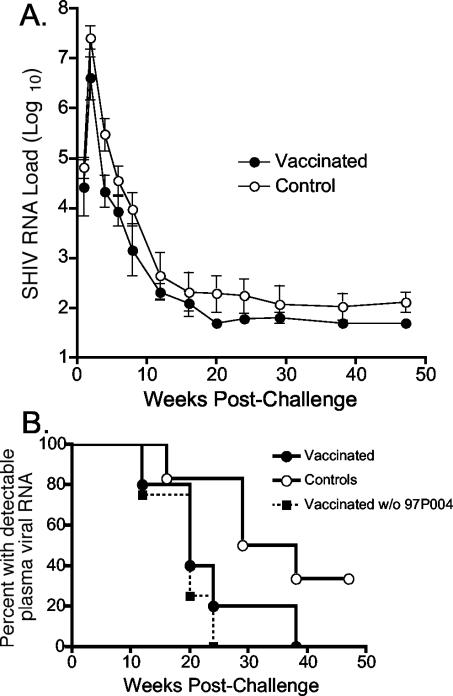
Approximately 2.5 years after the initial vaccination, the five vaccinated macaques along with six naïve control macaques were challenged intravenously with SHIVSF162P3. The course of infection was monitored by measuring plasma viral RNA loads and CD4+-T-cell counts in the infected macaques (Fig. 5). All 11 macaques developed detectable plasma viremia, with transient declines in absolute CD4+-T-cell numbers and peak viral loads at 2 weeks postchallenge. To monitor the effectiveness of vaccination, we compared the geometric mean plasma viral RNA loads of the vaccinated and control groups (Fig. 6A). Over the course of the study, viral RNA loads were consistently lower in the vaccinated macaques than in the control macaques. While the permutation test comparing the two longitudinal profiles over the course of follow-up was not significant (P = 0.15), there were larger differences between the viral loads of the two groups early after challenge, when vaccination, rather than normal macaque immune responses, might be expected to have the greater relative inhibitory effect on viral replication. Between 2 and 8 weeks postchallenge, there were differences of approximately 4- to 14-fold in the geometric mean virus loads of the two groups, and at one time point (week 4) this difference was statistically significant (P = 0.03) (Table 2). However, by 12 weeks after infection, differences in mean viral load between the two groups were less apparent, primarily because the plasma viral loads of several of the control and vaccinated macaques were below the limit of detection. In fact, the majority of both vaccinated and control macaques were able to control viral replication to transiently detectable levels (Fig. 5). Only one macaque (control animal 97P032) had consistently detectable plasma-associated viral RNA. These results were in contrast to data from SHIVSF162P3 challenge studies in rhesus macaques (20, 21) and pig-tailed macaques (N. L. Haigwood, personal communication), in which similar doses established stable viral set points in the majority of infected animals.
FIG. 5.
Intravenous challenge with SHIVSF162P3. Whole blood was collected at defined intervals after challenge and samples were monitored for CD4+ cell number per microliter of whole blood (○) and plasma viremia (•) (copies of viral RNA per milliliter of plasma). To detect viral RNA we used a real-time quantitative RT-PCR method with a sensitivity limit of 100 copies of viral RNA/ml of plasma. Data points plotted below this value indicate that viral RNA was undetectable at this time point.
FIG. 6.
Analysis of viral RNA loads in control and vaccinated groups of macaques. (A) Geometric mean plasma-associated RNA viral loads postinfection. Error bars represent standard error of the means. (B) Control of plasma viremia in control, vaccinated macaques, and vaccinated macaques excluding macaque 97P004. Control of viremia is defined as the first time point at which plasma-associated viral RNA was not detected and remained undetectable at the next two time points. Data are plotted as the percentage of macaques that have controlled detectable plasma viremia by this criterion.
TABLE 2.
Statistical analysis of geometric mean viral loads
| Wks postchallenge | Comparison of the geometric mean viral load of the vaccinated group to the control group
|
|||
|---|---|---|---|---|
| All vaccinees included
|
Excluding macaque 97P004
|
|||
| Ratioa | P valueb | Ratioa | P valueb | |
| 1 | 2.5 | 0.49 | 6.6 | 0.12 |
| 2 | 6.6 | 0.11 | 13.0 | 0.03 |
| 4 | 13.5 | 0.03 | 25.0 | 0.01 |
| 6 | 4.2 | 0.19 | 4.3 | 0.25 |
| 8 | 5.5 | 0.22 | 10.7 | 0.21 |
| 12 | 2.5 | 0.41 | 2.4 | 0.49 |
The ratio of the geometric mean titers (controls:vaccinees) at the indicated time points postinfection. A value of 2.5, for example, indicates that the geometric mean viral load of the control group is 2.5 times higher than that of the vaccinated group.
P values were obtained from two-sample t tests at the indicated time points. Wilcoxon rank sum tests provided similar statistical evidence. Boldface values are statistically significant (P < 0.05).
Despite the control of challenge virus in the majority of the macaques, there was suggestive evidence that detectable plasma-associated virus was cleared more rapidly in the vaccinated macaques. To analyze the rate at which viremia was controlled in the infected macaques, we defined control of viremia as the first time of three consecutive time points in which plasma-associated viral RNA was undetectable (Fig. 6B). By this criterion, all five vaccinated macaques cleared plasma-associated virus by 38 weeks postchallenge, whereas two of six control macaques remained infected at the final time point (47 weeks postchallenge). While this comparison also did not show a statistical difference between the two groups, a log-rank test comparing the relative rate of clearance approached significance (P = 0.10).
Because there was variability in the anti-EC1 responses in the vaccinated macaques, we investigated whether the higher autoantibody titers or avidity were associated with lower viral loads and/or more rapid clearance. To examine this question, we compared the titer and avidity of the autoantibodies prior to challenge with both the peak plasma RNA viral load and the time at which plasma viral RNA became undetectable in the individual vaccinated macaques (Table 1). The macaque with the lowest anti-EC1 titer and lowest autoantibody avidity index value (macaque 97P004) had the highest viral load among the vaccinated animals in the first 4 weeks following virus challenge and had detectable virus through week 24 (with the exception of week 20). Therefore, it is possible that the weak autoantibody response in this animal was responsible for its higher viral loads. When we excluded macaque 97P004 from our statistical analysis, there were stronger statistical differences between the two groups at early time points (Table 2), and the global test comparing the longitudinal profiles over all follow-up between vaccinated and control macaques was significant (P = 0.04). This effect was highly significant (P = 0.008) when we included only measurements from 1 to 8 weeks postchallenge, a period in which autoantibodies might be expected to play a greater role in controlling viral replication than would the normal immune responses directed against the challenge virus. In addition, excluding macaque 97P004 had the effect of making the log-rank test comparing time to control of viremia statistically significant (P = 0.02) (Fig. 6B). These data indicate that viral loads are significantly decreased relative to controls in the four macaques with higher autoantibody titers and avidities.
The four vaccinated macaques with higher autoantibody titers and avidities had variable courses of infection. Macaque 97P028 (intermediate anti-EC1 titer and high avidity) cleared its virus fastest (by 8 weeks postchallenge). The two macaques with the highest autoantibody titers, 97P026 and 96P089, had high and low viral loads, respectively, at 2 weeks postchallenge and controlled viremia between 16 and 20 weeks postinfection, later than macaque 97P028, which had intermediate autoantibody levels. Taken together, these data suggest the possibility that there may be a threshold level required for the autoantibodies to have an inhibitory effect on viral replication. However, there was no evidence that higher anti-EC1 antibody titers or avidities above this threshold level lead to a more rapid decline in plasma viremia.
Stability of V3 domain.
Immunization against CCR5 might, in theory, lead to selection for the evolution of CXCR-4-utilizing or dual-tropic viral variants that are sometimes associated with the onset of disease (41, 49). To examine the possibility that CXCR-4 (X4)-tropic viral variants may have evolved in the macaques, we sequenced envelope variable loop 3 (V3) amplified from proviral DNA isolated from PBMCs 20 weeks after challenge. Specific sequence changes in this region of HIV-1SF162 env have been associated with a shift from R5 to X4 tropism (12). Analysis of multiple clones from the five vaccinated macaques and two control macaques indicated that there were no predicted amino acid changes from the sequence of wild-type HIV-1SF162 env in the V3 region (data not shown). However, this analysis does not exclude the possibility that other regions of env that may potentially modulate coreceptor usage may have accumulated genetic changes.
DISCUSSION
These data provide the first indication that VLP-based vaccines are capable of inducing autoantibody responses in primates. The success of this approach in breaking B-cell tolerance against CCR5 and TNF-α in mice has previously been demonstrated (8, 9). To extend these studies to macaques, we took a comprehensive approach to identifying an M. nemestrina CCR5 peptide that elicits antibodies that bind to native CCR5 and block viral infection. This process identified the N-terminal region of CCR5 (EC1) as a peptide that (i) could be fused to SA and still allow the SA domain to bind biotin and (ii) elicited antibodies that bound to the cognate CCR5 peptide and also bound to native CCR5. Upon immunization of macaques with EC1-conjugated VLPs, anti-CCR5 autoantibodies were elicited in all five vaccinated macaques. Anti-CCR5 IgG responses were relatively durable and, importantly, could be boosted upon subsequent vaccination. IgG autoantibody levels were more variable in monkeys than the levels we have observed in mice. The greater variability in individual macaques could be attributable to the macaques being outbred animals, in contrast to the inbred strains of mice that we have used, to the different adjuvants used for the macaque studies (Titermax Gold) and the mouse studies (Freund's adjuvant), or to other factors. A future goal will be to identify factors that may consistently enhance autoantibody responses in primates.
As with any therapeutic modality, the safety of autoantibody induction by vaccines is an important consideration. In this study, as well as the previous CCR5 study in mice, the induction of autoantibodies against CCR5 was not associated with any gross pathology or peripheral blood abnormalities. Vaccinated macaques remained healthy throughout an observation period of more than 2 years, and there was no apparent loss of CCR5-expressing T cells. Our results are consistent with the observation that a proportion of the human population lacks functional CCR5 (reviewed in reference 6) yet is apparently healthy. Safety concerns will need to be carefully addressed for each individual target self antigen, but it is encouraging to note that a vaccine that induces autoantibodies against human chorionic gonadotropin has been tested in humans as a contraceptive without the report of serious adverse reactions (53).
To assess vaccine efficacy, we sought to infect the vaccinated macaques with a challenge dose of an R5-tropic SHIV virus that would result in a measurable viral set point. Although the dose of SHIVSF162P3 used here achieved this effect upon challenge of rhesus macaques (20, 21) and a group of pig-tailed macaques at the University of Washington (N. L. Haigwood, personal communication), in our study SHIVSF162P3 infection was controlled in half of the control macaques, and only one control macaque had consistently detectable plasma-associated viral RNA throughout the course of the study. This ability of the immunized macaques to control virus infection made it more difficult to assess the effectiveness of vaccination, but several analyses suggested that the vaccine had conferred some degree of protection. These include the observation that vaccinated macaques had consistently lower mean viral loads and cleared detectable plasma-associated viral RNA more rapidly than control macaques. These differences were modest, and it remains possible that the variability of the disease course in individual macaques may account for these differences. However, the trends suggest that vaccination may have decreased viral loads in the peripheral blood. Importantly, the difference between the viral loads in the vaccinated and control macaques was statistically significant if we excluded from our analysis the one macaque with a poor antibody response to vaccination. Such antiviral effects, if confirmed, may be sufficient to confer clinical benefit, as it has been demonstrated in HIV-1-infected individuals that small decreases in viral load associated with set point can have significant effects on time to disease (47, 52). Indeed, even a low-titer anti-CCR5 response may be effective in some instances. For example, low-level anti-CCR5 autoantibodies (with virus inhibitory activity in vitro) have been detected in the seronegative partners of HIV-1-seropositive individuals, suggesting that these autoantibodies may have a role in protection from natural HIV-1 infection (34).
As with any potential antiviral agent, vaccination against CCR5 may lead to the selection of a resistant virus population. This possibility needs to be taken into account in future efforts to improve the efficacy of VLP-based autoantibody-inducing vaccines. Coreceptor switching to CXCR-4 usage remains a formal possibility, but an examination of viral resistance to AD101, a molecular antagonist of CCR5, indicated that resistant viruses did not switch to CXCR-4 but persisted in using CCR5, either through binding to alternative EC domains of CCR5 or by binding to the receptor at a higher affinity (55). Extensive analysis of CCR5 has indicated that the amino terminus of CCR5 plays a dominant role in coreceptor activity (1, 3, 5, 15-17, 24, 35, 40, 42, 43), but the interaction between the HIV-1 envelope and CCR5 is complex and probably involves several other EC regions of CCR5, most notably EC3. Thus, a future challenge of autoantibody induction is to raise antibodies against more complex CCR5 determinants that bind multiple EC domains at high affinity.
Other vaccination strategies to elicit antibodies that bind macaque CCR5 have also been reported. Unlike our study, these approaches have utilized human CCR5 nucleic acid or peptides to elicit cross-reactive anti-human CCR5 antibodies. Using full-length human CCR5 protein or CCR5 peptides, Lehner et al. induced anti-CCR5 antibodies that bound macaque CCR5 and inhibited HIV-1 in vitro (30). That study also identified the N-terminal domain as the most effective at inducing high-titer antibody responses. However, Lehner et al. did not examine the effects of vaccination upon virus challenge. Zuber et al. elicited anti-CCR5 IgG by genetically immunizing macaques with human CCR5 DNA fused to strong T-helper epitopes (58). In that study, immunization was not protective upon intravenous challenge with SIVsm, possibly because of the coreceptor tropism of this viral isolate, which is not well characterized in vivo.
Other anti-CCR5 approaches, including agents such as MAbs, chemokine analogs, small molecular inhibitors, and small interfering RNAs, have shown promise in in vitro studies (2, 4, 39, 48, 54). Our results add evidence to the feasibility of CCR5 inhibitors as therapeutic agents against HIV infection. The induction of prophylactic or therapeutic autoantibodies by vaccination has some theoretical advantages over more passive anti-CCR5 therapeutics, such as MAbs. Induced autoantibodies would be expected to provide a longer duration of response at less cost and be less likely to induce an anti-idiotypic response that might inactivate the CCR5 antibodies. Given our ability to induce autoantibodies in macaques and data suggesting the possibility that the macaques with high-titer autoantibodies may have controlled SHIV infection more rapidly than control macaques, the continued development of CCR5-specific therapy via autoantibodies, and other approaches, warrants future study.
Acknowledgments
We thank Mike Piatak and Jeff Lifson for performing the viral load analyses and Mike Flynn, Bradley Fisher, David Sly, and the staff at the NCI animal sciences branch for their assistance in acquiring and caring for the macaques over the course of these studies. In addition, we thank Arifa Khan for the use of her laboratory's HIV facilities, Nancy Haigwood for sharing unpublished data, Julie Overbaugh and Jason Kimata for the MAGI-ptCCR5 cell line, and members of the laboratory for their support. The SHIVSF162P3 virus was obtained through the AIDS Research and Reference Reagent Program, NIAID, NIH from Janet Harouse, Cecilia Cheng-Mayer, Ranajit Pal, and the DAIDS, NIAID.
This study was funded in part by an NIH Intramural AIDS Targeted Antiviral Program Award and an NCI Intramural Research Award to J.T.S.
REFERENCES
- 1.Alkhatib, G., S. S. Ahuja, D. Light, S. Mummidi, E. A. Berger, and S. K. Ahuja. 1997. CC chemokine receptor 5-mediated signaling and HIV-1 co-receptor activity share common structural determinants. Critical residues in the third extracellular loop support HIV-1 fusion. J. Biol. Chem. 272:19771-19776. [DOI] [PubMed] [Google Scholar]
- 2.Arteaga, H. J., J. Hinkula, I. Van Dijk-Hard, M. S. Dilber, B. Wahren, B. Christensson, A. J. Mohamed, and C. I. Edvard Smith. 2003. Choosing CCR5 or Rev siRNA in HIV-1. Nat. Biotechnol. 21:230-231. [DOI] [PubMed] [Google Scholar]
- 3.Atchison, R. E., J. Gosling, F. S. Monteclaro, C. Franci, L. Digilio, I. F. Charo, and M. A. Goldsmith. 1996. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 274:1924-1926. [DOI] [PubMed] [Google Scholar]
- 4.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanpain, C., B. J. Doranz, J. Vakili, J. Rucker, C. Govaerts, S. S. Baik, O. Lorthioir, I. Migeotte, F. Libert, F. Baleux, G. Vassart, R. W. Doms, and M. Parmentier. 1999. Multiple charged and aromatic residues in CCR5 amino-terminal domain are involved in high affinity binding of both chemokines and HIV-1 Env protein. J. Biol. Chem. 274:34719-34727. [DOI] [PubMed] [Google Scholar]
- 6.Carrington, M., M. Dean, M. P. Martin, and S. J. O'Brien. 1999. Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum. Mol. Genet. 8:1939-1945. [DOI] [PubMed] [Google Scholar]
- 7.Chackerian, B., P. Lenz, D. R. Lowy, and J. T. Schiller. 2002. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J. Immunol. 169:6120-6126. [DOI] [PubMed] [Google Scholar]
- 8.Chackerian, B., D. R. Lowy, and J. T. Schiller. 2001. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J. Clin. Investig. 108:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chackerian, B., D. R. Lowy, and J. T. Schiller. 1999. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc. Natl. Acad. Sci. USA 96:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng-Mayer, C., M. Quiroga, J. W. Tung, D. Dina, and J. A. Levy. 1990. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J. Virol. 64:4390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 12.Dejucq, N., G. Simmons, and P. R. Clapham. 1999. Expanded tropism of primary human immunodeficiency virus type 1 R5 strains to CD4(+) T-cell lines determined by the capacity to exploit low concentrations of CCR5. J. Virol. 73:7842-7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Roda Husman, A. M., M. Koot, M. Cornelissen, I. P. Keet, M. Brouwer, S. M. Broersen, M. Bakker, M. T. Roos, M. Prins, F. de Wolf, R. A. Coutinho, F. Miedema, J. Goudsmit, and H. Schuitemaker. 1997. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann. Intern. Med. 127:882-890. [DOI] [PubMed] [Google Scholar]
- 14.de Roda Husman, A. M., R. P. van Rij, H. Blaak, S. Broersen, and H. Schuitemaker. 1999. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J. Infect. Dis. 180:1106-1115. [DOI] [PubMed] [Google Scholar]
- 15.Doranz, B. J., Z. H. Lu, J. Rucker, T. Y. Zhang, M. Sharron, Y. H. Cen, Z. X. Wang, H. H. Guo, J. G. Du, M. A. Accavitti, R. W. Doms, and S. C. Peiper. 1997. Two distinct CCR5 domains can mediate coreceptor usage by human immunodeficiency virus type 1. J. Virol. 71:6305-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragic, T., A. Trkola, S. W. Lin, K. A. Nagashima, F. Kajumo, L. Zhao, W. C. Olson, L. Wu, C. R. Mackay, G. P. Allaway, T. P. Sakmar, J. P. Moore, and P. J. Maddon. 1998. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J. Virol. 72:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan, M., H. Choe, L. Vaca, K. Martin, Y. Sun, E. Desjardins, N. Ruffing, L. Wu, R. Wyatt, N. Gerard, C. Gerard, and J. Sodroski. 1998. A tyrosine-rich region in the N terminus of CCR5 is important for human immunodeficiency virus type 1 entry and mediates an association between gp120 and CCR5. J. Virol. 72:1160-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good, P. I. 2000. Permutation tests: a practical guide to resampling methods for testing hypotheses, 2nd ed. Springer Verlag, New York, N.Y.
- 19.Goodnow, C. C., J. Crosbie, S. Adelstein, T. B. Lavoie, S. J. Smith-Gill, R. A. Brink, H. Pritchard-Briscoe, J. S. Wotherspoon, R. H. Loblay, K. Raphael, R. J. Trent, and A. Basten. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334:676-682. [DOI] [PubMed] [Google Scholar]
- 20.Harouse, J. M., A. Gettie, T. Eshetu, R. C. Tan, R. Bohm, J. Blanchard, G. Baskin, and C. Cheng-Mayer. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIVSF162P3. J. Virol. 75:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 22.Hartley, S. B., J. Crosbie, R. Brink, A. B. Kantor, A. Basten, and C. C. Goodnow. 1991. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353:765-769. [DOI] [PubMed] [Google Scholar]
- 23.Hedman, K., and I. Seppala. 1988. Recent rubella virus infection indicated by a low avidity of specific IgG. J. Clin. Immunol. 8:214-221. [DOI] [PubMed] [Google Scholar]
- 24.Hill, C. M., D. Kwon, M. Jones, C. B. Davis, S. Marmon, B. L. Daugherty, J. A. DeMartino, M. S. Springer, D. Unutmaz, and D. R. Littman. 1998. The amino terminus of human CCR5 is required for its function as a receptor for diverse human and simian immunodeficiency virus envelope glycoproteins. Virology 248:357-371. [DOI] [PubMed] [Google Scholar]
- 25.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 26.Kimata, J. T., J. J. Gosink, V. N. KewalRamani, L. M. Rudensey, D. R. Littman, and J. Overbaugh. 1999. Coreceptor specificity of temporal variants of simian immunodeficiency virus Mne. J. Virol. 73:1655-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konigs, C., M. J. Rowley, P. Thompson, M. A. Myers, M. Scealy, J. M. Davies, L. Wu, U. Dietrich, C. R. Mackay, and I. R. Mackay. 2000. Monoclonal antibody screening of a phage-displayed random peptide library reveals mimotopes of chemokine receptor CCR5: implications for the tertiary structure of the receptor and for an N-terminal binding site for HIV-1 gp120. Eur. J. Immunol. 30:1162-1171. [DOI] [PubMed] [Google Scholar]
- 30.Lehner, T., C. Doyle, Y. Wang, K. Babaahmady, T. Whittall, L. Tao, L. Bergmeier, and C. Kelly. 2001. Immunogenicity of the extracellular domains of C-C chemokine receptor 5 and the in vitro effects on simian immunodeficiency virus or HIV infectivity. J. Immunol. 166:7446-7455. [DOI] [PubMed] [Google Scholar]
- 31.Li, S., J. Juarez, M. Alali, D. Dwyer, R. Collman, A. Cunningham, and H. M. Naif. 1999. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73:9741-9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. Koup, and N. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 34.Lopalco, L., C. Barassi, C. Pastori, R. Longhi, S. E. Burastero, G. Tambussi, F. Mazzotta, A. Lazzarin, M. Clerici, and A. G. Siccardi. 2000. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 in vitro. J. Immunol. 164:3426-3433. [DOI] [PubMed] [Google Scholar]
- 35.Lu, Z., J. F. Berson, Y. Chen, J. D. Turner, T. Zhang, M. Sharron, M. H. Jenks, Z. Wang, J. Kim, J. Rucker, J. A. Hoxie, S. C. Peiper, and R. W. Doms. 1997. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc. Natl. Acad. Sci. USA 94:6426-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciw, P. A., E. Pratt-Lowe, K. E. Shaw, J. A. Levy, and C. Cheng-Mayer. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc. Natl. Acad. Sci. USA 92:7490-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michael, N. L., G. Chang, L. G. Louie, J. R. Mascola, D. Dondero, D. L. Birx, and H. W. Sheppard. 1997. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat. Med. 3:338-340. [DOI] [PubMed] [Google Scholar]
- 38.Nemazee, D. A., and K. Burki. 1989. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337:562-566. [DOI] [PubMed] [Google Scholar]
- 39.Qin, X. F., D. S. An, I. S. Chen, and D. Baltimore. 2003. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA 100:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabut, G. E., J. A. Konner, F. Kajumo, J. P. Moore, and T. Dragic. 1998. Alanine substitutions of polar and nonpolar residues in the amino-terminal domain of CCR5 differently impair entry of macrophage- and dualtropic isolates of human immunodeficiency virus type 1. J. Virol. 72:3464-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richman, D. D., and S. A. Bozzette. 1994. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J. Infect. Dis. 169:968-974. [DOI] [PubMed] [Google Scholar]
- 42.Ross, T. M., P. D. Bieniasz, and B. R. Cullen. 1998. Multiple residues contribute to the inability of murine CCR-5 to function as a coreceptor for macrophage-tropic human immunodeficiency virus type 1 isolates. J. Virol. 72:1918-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rucker, J., M. Samson, B. J. Doranz, F. Libert, J. F. Berson, Y. Yi, R. J. Smyth, R. G. Collman, C. C. Broder, G. Vassart, R. W. Doms, and M. Parmentier. 1996. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell 87:437-446. [DOI] [PubMed] [Google Scholar]
- 44.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 45.Sano, T., and C. R. Cantor. 1990. Expression of a cloned streptavidin gene in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sano, T., and C. R. Cantor. 1991. Expression vectors for streptavidin-containing chimeric proteins. Biochem. Biophys. Res. Commun. 176:571-577. [DOI] [PubMed] [Google Scholar]
- 47.Schacker, T. W., J. P. Hughes, T. Shea, R. W. Coombs, and L. Corey. 1998. Biological and virologic characteristics of primary HIV infection. Ann. Intern. Med. 128:613-620. [DOI] [PubMed] [Google Scholar]
- 48.Simmons, G., P. R. Clapham, L. Picard, R. E. Offord, M. M. Rosenkilde, T. W. Schwartz, R. Buser, T. N. Wells, and A. E. Proudfoot. 1997. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276-279. [DOI] [PubMed] [Google Scholar]
- 49.Simmons, G., D. Wilkinson, J. D. Reeves, M. T. Dittmar, S. Beddows, J. Weber, G. Carnegie, U. Desselberger, P. W. Gray, R. A. Weiss, and P. R. Clapham. 1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol. 70:8355-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith, M. W., M. Dean, M. Carrington, C. Winkler, G. A. Huttley, D. A. Lomb, J. J. Goedert, T. R. O'Brien, L. P. Jacobson, R. Kaslow, S. Buchbinder, E. Vittinghoff, D. Vlahov, K. Hoots, M. W. Hilgartner, and S. J. O'Brien. 1997. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science 277:959-965. [DOI] [PubMed] [Google Scholar]
- 51.Spohn, G., and M. F. Bachmann. 2003. Therapeutic vaccination to block receptor-ligand interactions. Expert Opin. Biol. Ther. 3:469-476. [DOI] [PubMed] [Google Scholar]
- 52.Sterling, T. R., D. Vlahov, J. Astemborski, D. R. Hoover, J. B. Margolick, and T. C. Quinn. 2001. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N. Engl. J. Med. 344:720-725. [DOI] [PubMed] [Google Scholar]
- 53.Talwar, G. P., O. Singh, R. Pal, N. Chatterjee, P. Sahai, K. Dhall, J. Kaur, S. K. Das, S. Suri, K. Buckshee, L. Saraya, and B. N. Saxena. 1994. A vaccine that prevents pregnancy in women. Proc. Natl. Acad. Sci. USA 91:8532-8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkler, C., W. Modi, M. W. Smith, G. W. Nelson, X. Wu, M. Carrington, M. Dean, T. Honjo, K. Tashiro, D. Yabe, S. Buchbinder, E. Vittinghoff, J. J. Goedert, T. R. O'Brien, L. P. Jacobson, R. Detels, S. Donfield, A. Willoughby, E. Gomperts, D. Vlahov, J. Phair, and S. J. O'Brien. 1998. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science 279:389-393. [DOI] [PubMed] [Google Scholar]
- 58.Zuber, B., J. Hinkula, D. Vodros, P. Lundholm, C. Nilsson, A. Morner, M. Levi, R. Benthin, and B. Wahren. 2000. Induction of immune responses and break of tolerance by DNA against the HIV-1 coreceptor CCR5 but no protection from SIVsm challenge. Virology 278:400-411. [DOI] [PubMed] [Google Scholar]