Abstract
Despite advances in the treatment of diabetic nephropathy (DN), currently available therapies have not prevented the epidemic of progressive chronic kidney disease (CKD). The morbidity of CKD, and the inexorable increase in the prevalence of end-stage renal disease, demands more effective approaches to prevent and treat progressive CKD. We undertook next-generation sequencing in a rat model of diabetic nephropathy to study in depth the pathogenic alterations involved in DN with progressive CKD. We employed the obese, diabetic ZS rat, a model that develops diabetic nephropathy, characterized by progressive CKD, inflammation, and fibrosis, the hallmarks of human disease. We then used RNA-seq to examine the combined effects of renal cells and infiltrating inflammatory cells acting as a pathophysiological unit. The comprehensive systems biology analysis of progressive CKD revealed multiple interactions of altered genes that were integrated into morbid networks. These pathological gene assemblies lead to renal inflammation and promote apoptosis and cell cycle arrest in progressive CKD. Moreover, in what is clearly a major therapeutic challenge, multiple and redundant pathways were found to be linked to renal fibrosis, a major cause of kidney loss. We conclude that systems biology applied to progressive CKD in DN can be used to develop novel therapeutic strategies directed to restore critical anomalies in affected gene networks.
Keywords: diabetic nephropathies, inflammation, renal failure
diabetic nephropathy (dn) is the leading cause of progressive chronic kidney disease (CKD) and end-stage renal disease (ESRD) (41), reflecting a failure of available therapies to protect diabetic patients (41). This alarming situation has reached global proportions, illustrated by a world-wide study showing prevalence for microalbuminuria and macroalbuminuria in 49% and impaired renal function in 22% of Type 2 diabetics who thought were free of renal disease (70). The pathophysiology of progressive CKD in DN is complex, and it remains elusive. Multiple factors, particularly ischemic acute kidney injury, aggravate DN and hasten CKD progression to ESRD (41). We chose a systems biology approach to study the complex pathophysiology of progressive CKD in DN using a rat model: the F1 hybrid Zucker diabetic-fatty/spontaneously hypertensive heart failure (ZS) rat, an obese rat model of Type 2 diabetes. As advocated by the Animal Models of Diabetic Complications Consortium (9, 58), the obese/diabetic ZS rat, unlike many other animal models, is characterized by progressive CKD (21), the hallmark of progressive human DN. Moreover, to better imitate progressive CKD in DN, we subjected one group of animals to a short period of renal ischemia, which is a common clinical element in DN (41). We have reported extensively on salient features of progressive CKD and DN in these rats. For example, under these stressful metabolic and oxidative pressures (19, 21), the renal microvasculature is severely damaged with widespread zones of ischemia (83). The ischemic renal tubules then acquire a proinflammatory phenotype characterized by release of leukocyte chemokines (19) and expression of the adherence molecules intercellular adhesion molecule-1 (ICAM1) and LOX1 (44). Consequently, migrating leukocytes adhere to peritubular capillaries, and clusters of leukocytes bind to renal tubules (20, 44). These inflammatory events are aggravated severely by renal ischemia and are associated with tubular cell death and extensive glomerular and interstitial, i.e., peritubular, fibrosis. We termed this latter entity the postischemic inflammatory syndrome (40, 42), as some of its manifestations are similar to those of straightforward renal ischemia (43). From this earlier work, we concluded that unraveling the pathophysiology of the postischemic inflammatory syndrome in DN would require simultaneous analyses of inflammatory and renal cells incorporated as a pathological and interactive complex system. We reasoned that studying this whole large-scale biological system, as opposed to more reductionist work, might open novel vistas of the pathophysiology of DN (40, 42). Accordingly, we tested the hypothesis that in DN, renal and inflammatory cells form damaging networks that help drive CKD progression. We tested this hypothesis with complete renal transcriptomes from lean and obese ZS rats using high-throughput RNA sequencing (RNA-seq) and high-performance computing and bioinformatics. Advances in genomic profiling make a comprehensive analysis of the transcriptome in renal failure possible. RNA-seq provides a more precise and unbiased quantification of expression than is possible with microarrays and allows analysis of the entire transcriptome (82), with improved sensitivity (17). We found a striking predominance of inflammatory gene pathways among the hundreds of differentially expressed genes. These pathways, based on differential expression of member genes, revealed potential mechanisms of progressive CKD in DN and provide a road map for novel therapies as well as potential diagnostic tools to allow earlier intervention.
MATERIALS AND METHODS
Animals
Lean and obese diabetic male ZS rats (Charles River, Wilmington, MA) were acquired at 8 wk of age and fed Purina diet #5008 with 27% protein, 17% animal fat, and 56% carbohydrate. Their body weights were recorded, and sera plus urine samples were collected for analyses at biweekly intervals until termination. The rats underwent intervention at 10 wk of age as follows: They were anesthetized with pentobarbital (50 mg/kg ip) and placed on a homeothermic table to maintain core body temperature at ∼37°C. After insuring adequate anesthesia, we induced renal ischemia by occluding both renal pedicles for 25 min with microaneurysm clamps in one obese/diabetic group [diabetic/ischemia (DI)] (40, 42). The lean (LS) control group and a second obese/diabetic group [diabetic sham surgery (DS)] were subjected to sham surgery: the kidneys were surgically exposed in an identical manner but not clamped. Changes in renal and metabolic parameters were monitored by sequential measurements of serum creatinine, blood urea nitrogen, urinary protein/creatinine ratios, serum glucose, triglycerides, and cholesterol with the autoanalyzer of the clinical laboratory at the Indianapolis VA Hospital. The rats were terminated at 28 wk of age. Kidneys were removed from anesthetized animals and immediately frozen with liquid nitrogen, and RNA extraction was then performed as below. To examine all renal and inflammatory cells as a unit, we performed no perfusion to avoid removing cells potentially critical in the pathophysiology of diabetic nephropathy.
Histology and Immunohistochemistry
Kidney sections were fixed in 3.8% paraformaldehyde, paraffin embedded, and 4 μM sections obtained for periodic acid-Schiff (PAS) and Leder stains to visualize intrinsic renal cell morphology and neutrophils, respectively. Masson's trichrome was used to stain collagen (20, 44).
RNA-seq and RT-PCR
Total kidney RNA was isolated with a Trizol purification kit as recommended by vendor (Cat. #12183-555; Invitrogen, Grand Island, NY) and cleaned with an RNeasy Mini kit as recommended by vendor (Cat. #74104; Qiagen, Valencia, CA). For RNA-seq, 3 μg were fragmented with RNase III, cDNA libraries constructed with SOLiD adaptors by reverse transcription (RT), and then subjected to RNA sequencing (strand-specific RNA-seq) of short 50 bp reads using the SOLiD 4 platform (Center for Medical Genomics at Indiana University School of Medicine) (16). RNA-seq yielded 12 complete transcriptomes, corresponding to four kidneys randomly chosen from each of the three groups of rats: LS, DS, and DI.
The RNA-seq data analysis includes three major steps: quality control (QC) filtering, sequence alignment, and differential expression analysis.
QC filtering.
We first used SOLiD Instrument Control Software and SOLID Experiment Tracking System software for the read quality recalibration. Sequences containing more than two “n” or wildcards were discarded. Each sequence was scanned for low-quality regions, and if a 5-base sliding window had an average quality score <20, the read was further truncated at that position. Any read with a length of <35 bases was discarded. Our experience suggests that this strategy effectively eliminates low-quality reads while retaining high-quality regions (8, 37, 84).
Sequence alignment.
We used the RNA-seq module in the NGSPipe for RNA-seq analysis. In brief, BFAST (33) was used as our primary alignment algorithm because it has high sensitivity on color-space data for aligning the reads on the loci containing small insertions and deletions comparing with the rat reference genome (m4). We used a TopHat-like strategy (85) to align the sequencing reads that cross splicing junctions. After aligning the sequence reads to a filtering index including repeats, ribosome RNA, and other sequences that are not of interest, we conducted sequence alignment at three levels: genomic, known junctions (UCSC), and novel junctions (based on the enriched regions identified in the genomic alignment). For the subsequent analysis, we used only the uniquely aligned sequences with no more than two mismatches.
Differential expression analysis.
Gene expression was calculated in the form of reads per kilobase exon model per million mapped reads (RPKM), which is a standard method for quantifying gene expression levels from RNA-seq data (10, 63, 82). When calculating RPKM values, we did not consider sequencing reads falling into the genes with highest 25% expression levels; this is done to avoid the biases due to the potential expression changes of highly expressed genes. Among the total of 16,536 annotated rat genes (based on UCSC annotation), 13,453 genes had credible expression level in at least half of samples in at least one of the conditions. To identify the differentially expressed genes, we further conducted Student's t-test on the logarithmically transformed RPKM values, comparing DI vs. LS and DS vs. LS. Pathway analysis was performed with Metacore Software (GeneGo, Carlsbad, CA) using the RPKM expression data for each sample as the input. Motif analysis was performed with the MotifModeler informatics program as described (54).
For RT-PCR, 3 μg of total kidney RNA were used to synthesize cDNA with an AffinityScript QPCR cDNA Synthesis Kit as recommended by vendor (Cat. #600559; Agilent Technologies, Santa Clara, CA). Real-time quantitative PCR was run on a MX3005P Multiples Quantitative PCR System (Agilent) with Brilliant SYBR Green QPCR Master Mix (Agilent), and an RT-PCR primer set for each target gene from Qiagen. Standard curves for each target gene were obtained with the same system before the running of corresponding samples. The samples were always run in quadruplicate. The data were normalized to the mRNA content of β-actin in each sample and are reported as relative amount of mRNA compared with control (LS) samples.
Western Blot
Proteins were separated from renal homogenates on a 12% acrylamide SDS-PAGE gel, electrophoretically transferred to a BIO-RAD Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) at 15 mA, and labeled with anti-α-2-macroglobulin (A2M) goat antibody (Cat. #M-0140; Sigma, St. Louis, MO), anti-collagen 1 rabbit antibody (Cat. #600-401-103s; Rockland, Gilbertsville, PA), anti-acyl-CoA oxidase 2, branched chain (ACOX) goat antibody (Cat. #PA5-18671; Thermo Scientific, Waltham, MA) and anti-actin mouse antibody (Cat. #Ab6276; Abcam, Cambridge, MA). The secondary antibodies were donkey anti-rabbit IRDye 800CW (Cat. #926-32213; donkey anti-goat IRDye 800CW, Cat. #926-32214; and donkey anti-mouse IRDye 680CW, Cat. #926-32222, all of them from LI-COR Biosciences, Lincoln, NE). The membranes were analyzed with a fluorescence scanner (Odyssey Classic, model 9120, LI-Cor Biosciences).
Animal Use Statement
The experiments were conducted in conformity with the American Physiological Society's “Guiding Principles for Research Involving Animals and Human Beings.” The investigations were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine.
Statistics
Results are expressed as means ± 1 SE. Differences in renal and metabolic parameters were determined by one-way ANOVA with subsequent t-tests (when ANOVA indicated statistical significance; GraphPad Prism, La Jolla, CA). Bonferroni correction was used for multiple comparisons. The null hypothesis was rejected at P < 0.05.
RESULTS AND DISCUSSION
Renal/metabolic Phenotype
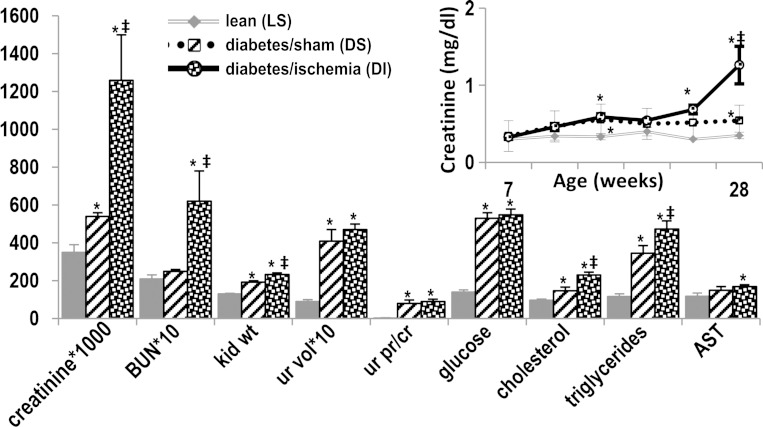
ZS rats were assigned to three study groups: lean sham controls (LS, n = 6) only had their kidneys surgically exposed. The obese-diabetic rats were randomly divided into a sham surgery group (DS, n = 7), which only had their kidneys exposed, and an ischemia/reperfusion surgery group (DI, n = 11), which had both kidney pedicles clamped for 25 min. The rats were acclimatized for 2 wk, and then surgeries were performed at age 10 wk. Serum creatinine levels at 8 wk of age were similar in LS control rats and presurgery DS and DI: 0.30 ± 0.00, and 0.34 ± 0.02, and 0.33 ± 0.03 mg/dl, respectively. Subsequently, 24 h after surgery, mean serum creatinine peaked to 0.71 ± 0.08, returning to presurgery levels at 48 h (0.47 ± 0.06) in DI. Serum creatinine was not altered postoperatively in the LS or DS groups: 0.43 ± 0.04 and 0.37 ± 0.03 for the 24 h time points. However, later on, when DI rats were 20 wk of age, there was a marked and progressive increase in serum creatinine, while in DS the increase in serum creatinine was more gradual and less pronounced (Fig. 1). Mean serum urea nitrogen levels, kidney weights, urine volumes, and urine protein excretion were all higher in DS and DI than in LS (Fig. 1). Initial serum glucose levels were higher in DS, 151 ± 7 mg/dl, and in DI, 142 ± 3, than in their lean litter mates (LS), 125 ± 2, P < 0.01. This difference was magnified over time until the conclusion of the study at 28 wk of age (Fig. 1). Obese-diabetic rats had dyslipidemia: mean initial serum cholesterol levels were 91 ± 21 mg/dl in DS rats, 109 ± 10 in DI, and 61 ± 10 in LS, and increased further in DS and DI (Fig. 1). Mean initial serum triglycerides were 306 ± 18 mg/dl in DS rats, 305 ± 18 in DI, and 129 ± 7.5 in LS, and also increased further in DS and DI. The liver enzyme aspartate aminotransferase was also slightly increased in DS and DI (Fig. 1).
Fig. 1.
Phenotype in experimental diabetic nephropathy. Renal injury in ZS rats is characterized by progressive renal failure (inset), the sine qua non of human disease. The rats also have nephromegaly, polyuria, proteinuria, as well as dyslipidemia. For each parameter, mean values ± SE are presented. The units are mg/dl for creatinine, blood urea nitrogen (BUN), glucose, cholesterol, and triglycerides; mg for kidney weight (kid wt); ml/24 h for urine volume (ur vol); and units/l for aspartate aminotransferase (AST). *P < 0.05 vs. lean; ‡P < 0.05 vs. diabetes/sham.
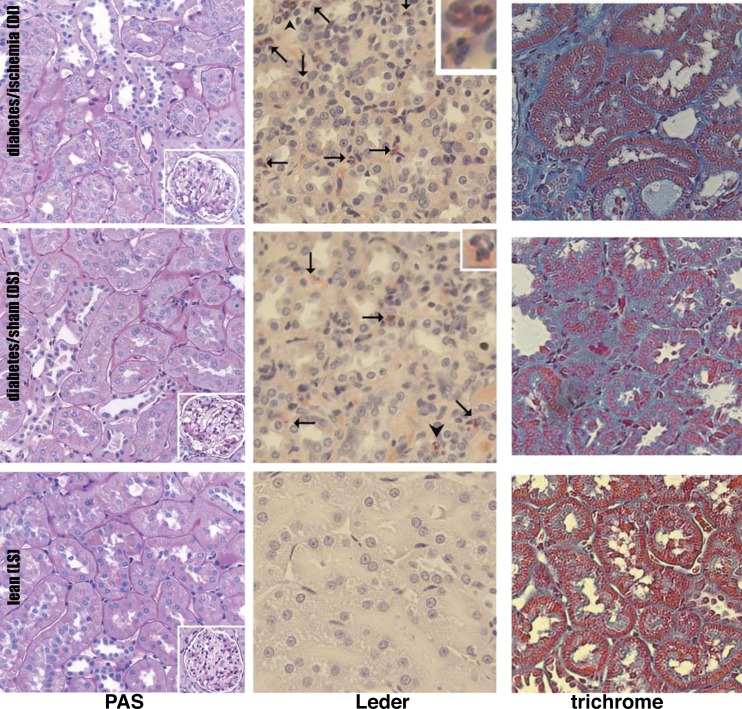
Postmortem renal sections were stained with PAS for a detailed examination of renal histology (20, 21, 40, 83); Leder's stain was used to evaluate renal inflammation represented by infiltrating leukocytes (20, 21, 40, 83), and Masson's trichrome was utilized to determine the extent of renal fibrosis (20, 21, 40, 83) (Fig. 2). The renal pathology in LS, DS, and DI ZS male rats has been described extensively (19–21, 40, 58, 83), and only its main features will be shown here. PAS revealed tubular dilatation, atrophy and cast formation in DS, and more severe tubular and glomerular damage in DI. Leder's stain showed large numbers of leukocytes clustering in the peritubular areas consistent with severe inflammatory changes in DS and DI. The corresponding trichrome stain revealed extensive renal fibrosis in DS, which was even more pronounced in DI (Fig. 2).
Fig. 2.
Structural changes in diabetic nephropathy. The nephropathy in ZS rats is also characterized by tubular atrophy, inflammation, and fibrosis. Representative images are presented: periodic acid Schiff (PAS)-stained sections demonstrate tubular and glomerular injury, Leder-stained images show leukocyte infiltration, and trichrome-stained kidneys show fibrosis in diabetic kidneys. Arrows indicate leukocytes. Insets in Leder-stained images show leukocytes at arrowheads. Insets in PAS-stained images show glomeruli.
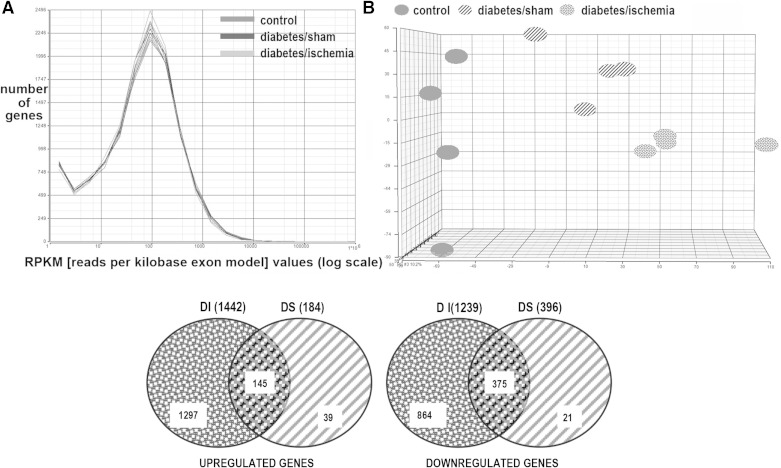
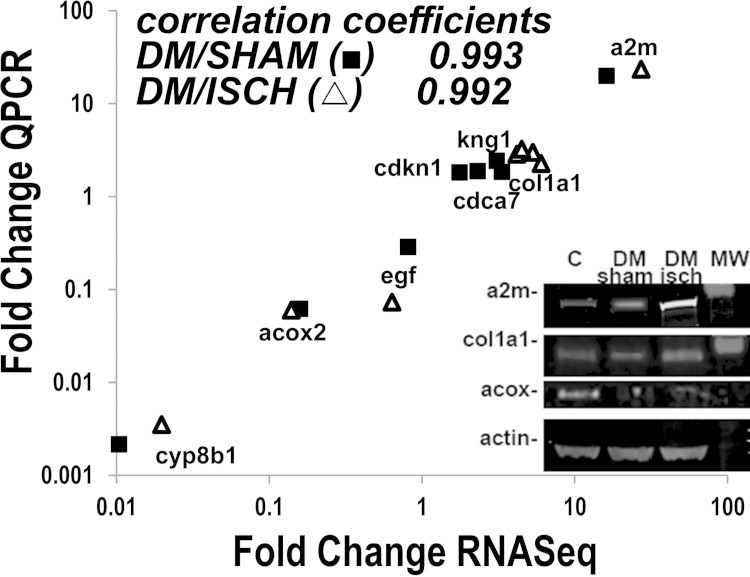
The mechanisms responsible for these and other manifestations of diabetic and ischemic nephropathy are complex, and their understanding is still inadequate to develop effective therapies. Moreover, hypertension, part of the metabolic syndrome phenotype in these obese rats (21) and in humans with Type 2 diabetes (41), was a likely contributor to progressive CKD, although blood pressure was not recorded in this study. In our prior studies (40) systolic blood pressure was significantly elevated (166 ± 9.7) in postischemia diabetic rats compared with 113 ± 9.5 in lean rats and 143 ± 5.6 in the diabetes/sham group. One novel and potentially fruitful approach to this challenging problem is to study the relationships of participating renal genetic elements. Accordingly, we conducted RNA sequencing [RNA-seq, (1, 16)] to capture the interacting genetic elements, renal and nonrenal. Diabetic nephropathy is extremely complex, and renal injury results from the interplay between migrating inflammatory cells and multiple types of stressed renal cells, all included in the analysis. The overall mapping statistics of the RNA-seq experiment are provided in Supplementary Table S1.1 After we removed the genes that are not expressed in any condition, the log transformed RPKM were normally and equally distributed for all groups, which indicated that sequencing data were consistent for all 12 samples contained in the three groups, demonstrating that differences were not secondary to read distribution among the groups (Fig. 3). In addition, principal component analysis (PCA) was conducted to examine the main source of variance in the data (23, 59). Widely used in microarray data analysis, PCA is a dimensionality reduction strategy for visualizing the overall variability among different samples (Fig. 3). PCA verified that the source of variability was mostly biological, i.e., there was greater variability among LS, DS, and DI groups than within group variability of the individual samples in each group (Fig. 3). There were 1,442 upregulated genes in DI compared with LS and 184 upregulated genes in DS compared with LS. In DI, 1,297 genes were uniquely upregulated. In DS 39 genes were uniquely upregulated, and 145 upregulated genes overlapped the two diabetic rat groups (Fig. 3). Moreover, there were 1,239 downregulated genes in DI compared with LS. In DS, 396 genes were downregulated compared with LS. There were also 864 uniquely downregulated genes in DI, 21 uniquely downregulated genes in DS, and 375 downregulated genes overlapped in the two diabetic groups. Hence, about half of the 3,261 differentially expressed genes in DI and DS were upregulated and half downregulated, and 9% of the upregulated genes overlapped in the two diabetic groups, while 30% of the downregulated genes overlapped in the two diabetic groups. (A detailed list of up- and downregulated genes is in Supplementary Table S2.) A random set of eight differentially expressed genes in DI and DS was selected for comparison, and their mRNA levels measured by RT-PCR in the same RNA used for RNA-seq. The changes in the levels of these eight mRNAs were highly correlated with the mRNA levels obtained by RNA-seq (Fig. 4). In addition, Western blots of proteins encoded by selected transcripts showed changes consistent with the RNA-seq and RT-PCR data (Fig. 4).
Fig. 3.
Next-generation sequencing in diabetic nephropathy. RNA-seq data were normally and equally distributed for all kidney samples, indicating that sequencing was consistent in the 3 groups (A). Principal component analysis (PCA) transformed the data into sets of uncorrelated and independent variables. PCA demonstrated that the main basis of variance in the mRNA data is due to biological factors (rather than within groups, B). Venn diagram shows that there were 2,681 differentially expressed genes in diabetes/ischemia (DI), 580 in diabetes/sham (DS) kidneys, of which 520 genes overlapped (C).
Fig. 4.
Verification of RNA-seq data. Upregulated genes, downregulated genes, and genes with unchanged expression in diabetic kidneys were randomly selected, and alterations in expression were evaluated by RT-PCR. The changes observed by RT-PCR were very tightly correlated (log-log transformation) with the results found by next-generation sequencing. Representative immunoblots also show that protein levels for selected transcripts were consistent with RNA-seq and RT-PCR data. CYP8B1: cytochrome p450, family 8, polypeptide 1. ACOX2: acyl-CoA oxidase 2. EGF: epidermal growth factor. CDCa7: cell division cycle associated 7. COL1a1: collagen type 1 alpha 1. KNG1: kininogen 1. CDKN1: cyclin-dependent kinase inhibitor 1. A2M: α-2-macroglobulin.
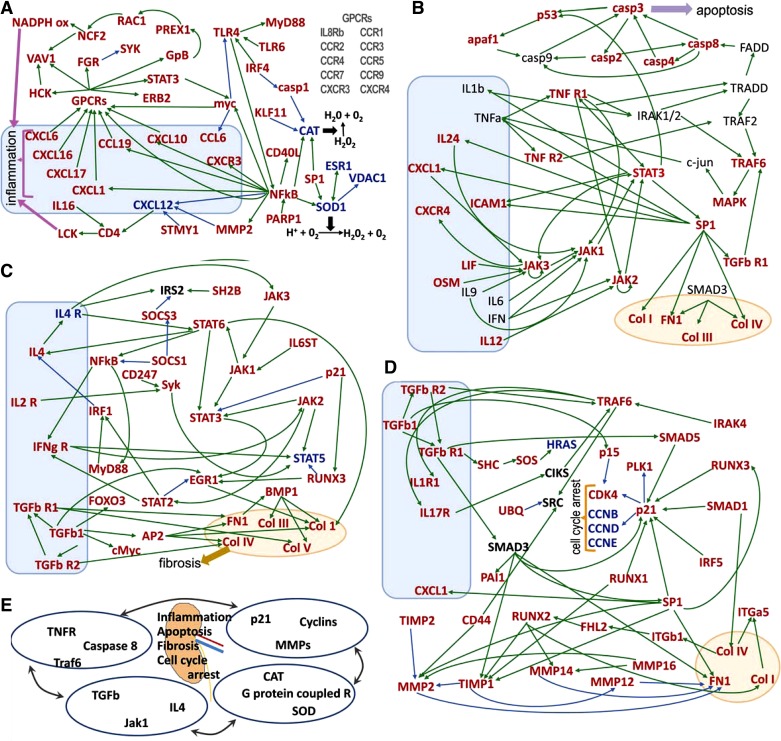
Functional pathways or gene networks (Metacore) were assembled using the differentially expressed gene data sets. The statistical relevance (P < 1 × 10−8 for the pathways presented) of this mapping is calculated as the probability of a chance match between specific genes in our data sets and those existing in the defined pathways (Metacore, http://www.genego.com). This probability is expressed as the P value of the hypergeometric distribution (17, 23). The genes included in the networks (Fig. 5) were those differentially expressed in both conditions, DS and DI, compared with controls (LS). The inflammation pathway is shown in Fig. 5A, and genes in this set are primarily involved in the interaction of renal and inflammatory cells. The illustration includes 35 designated genes plus 10 additional genes encoding G protein-coupled receptors (GPCRs). These 45 genes are upregulated in both DS and DI with respect to LS. The network also includes five genes that were downregulated. In the diabetic kidney, PARP-1 is upregulated and it becomes one of several activators of upregulated NF-κB (30). The NF-κB complex is then seen in positive interaction with upregulated CXCR3, its ligand CXCL10, plus the inflammatory ligands CCL19 and CXCL1. NF-κB is positioned upstream of upregulated atherogenic CD40L (68) and mitogenic myc (25). On the other hand, NF-κB is shown suppressing downregulated proangiogenic CXCL12 (29, 57), also suppressed by activated MMP2 and MMP3 (STMY1) (60). Moreover, the antioxidation genes SOD1 and VDAC1 are attenuated in DS and DI, despite stimulation from activated NF-κB and SP1, an effect consistent with the dependence of VDAC1 on SOD1 activity (11). TLR4 is activated by TLR6 and IRF4, while positively interacting with MyD88 (in Fig. 5C, MyD88 is also seen receiving stimulatory signals from NF-κB) (80) and is potentially involved in cell proliferation via the NF-κB/Myc signaling axis (25). Motif analysis (Table 1) demonstrates a significant level of regulation by NF-κB that involves a total of 82 regulated genes. Other transcription factors with highly significant transcription contribution scores include myc and stat5.
Fig. 5.
Gene pathways revealed by comprehensive genomic profiling. The most significantly (P < 1 × 10−8) altered pathway in RNA-seq data is proinflammatory, and it was divided in 4 parts to facilitate visualization (A–D). Upregulated mRNAs are in red, downregulated in blue, and without significant change in black. Positive interactions are shown in green and negative in blue. Boxes contain proinflammatory transcripts, and ovals profibrotic transcripts. The pathways are interrelated as shown in E. For example, the apoptotic mediator Traf6 is found in B and D; TGF-βR is in B, C, and D. Pathway A shows increases in G protein-coupled receptors that amplify inflammation by increasing damaging mediators, such as neutrophil cytosolic factor 2 (NCF2), which promotes superoxide production. Both catalase and superoxide dismutase are suppressed in our data, which would result in tissue damage. In pathway B, increases in both traditional (TNF receptor 1 and 2) and less-well-investigated (e.g., IL24) proinflammatory mediators are related to apoptosis and fibrosis. TGF-βR upregulation (C) amplifies the inflammatory response (with increases in IFN-γR) and increases in transcription factors that drive collagen synthesis. Alterations in p21 lower cyclin expression that causes cell cycle arrest as shown in pathway D. The alterations in transcription factors (for example SP1) are also related to increases in collagen and fibronectin as well as matrix metalloproteinases that break down connective tissue.
Table 1.
Motif analysis
| Genes, n |
||
|---|---|---|
| Transcription Factor | Diabetes Ischemia | Diabetes Sham |
| AML1a | 220 | 88 |
| AML1 | 220 | 88 |
| E2F | 111 | |
| HNF1 | 55 | 26 |
| E2F-1 | 56 | |
| Xvent-1 | 48 | |
| Rb:EF2-1:DP-1 | 77 | |
| AML | 64 | 25 |
| PU.1 | 67 | 23 |
| Osf2 | 52 | |
| Ets | 82 | |
| PEBP | 63 | |
| Major T-antigen | 71 | |
| E2F | 57 | |
| ETF | 78 | 28 |
| MZF1 | 68 | |
| myc | 50 | |
| c-Ets-2 | 69 | |
| E2F | 81 | |
| Ncx | 57 | |
| STAT5 | 93 | |
| HSF | 73 | |
| PEA3 | 163 | |
| TEF-1 | 198 | |
| MyoD | 66 | 31 |
| c-myc:max | 66 | |
| AP-2rep | 100 | |
| core-binding factor | 57 | 27 |
| IRF1 | 66 | |
| NF-κB | 67 | 32 |
| FOXP3 | 52 | |
Transcription factors with the most significant effects on gene expression (diabetes vs. control) as well as the number of regulated genes are presented.
The network also reports that Myc has negative interaction with TLR4, as reported elsewhere (94), although this conclusion is not shared by others (62). Antioxidant CAT is suppressed, receiving inhibitory signals via casp1 and upregulated KLF11 (24, 75). The inflammation pathway includes interactions of activated CXCL1, 6, 10, 17, and CCL19 with their respective GPCRs and subsequent activation of HCK, VAV1, FGR, STAT3, ERB2, and GpB (G protein β2), followed by positive interaction with PREX1, RAC1, NCF2 (p67phox), which lead to assembly and activation of NADPHox (22). Thus, renal inflammation in DS and DI is associated with combined attenuation of antioxidant systems and activation of pro-oxidant systems.
The apoptosis pathway shown in Fig. 5B includes 39 genes; 27 genes were upregulated in DS and DI with respect to LS. These 27 upregulated genes are interacting with 12 genes not differentially expressed in DS and DI. Figure 5B, top left corner, addresses the intrinsic apoptotic pathway, represented by casp3 acting on p53 and from which released fragments can induce mitochondrial membrane permeabilization (74). p53 also stimulates upregulated Apaf1, which forms the apoptosome with the initiator caspase 9 (7). Figure 5B, top right corner, focuses on the extrinsic apoptotic pathway, illustrated by FADD and its activating relationship with upregulated casp8 and subsequent activation of the execution caspases casp3 and 4 (3). The pathway also portrays positive interaction between upregulated casp3, p53, and Apaf1 (77). Thus, the data point to activation of intrinsic and extrinsic apoptotic pathways in the nephropathy of DS and DI (18). TNFR1 is shown activating IRAK1/2 and then diverging: one branch signals a proapoptotic stimulus via TRADD/FADD/Casp8 axis and the other inflammation via TRAF2/TRAF6/MAPK pathway (89), illustrating one intersection of inflammatory and apoptotic pathways. TNFR1 also interacts positively with STAT3 and JAK2 (92). Activated SP1-dependent transcription is seen acting on transforming growth factor receptor 1 (TGF-βR1), which also stimulates TRAF6, followed by activation of the upregulated MAPK system (92). In addition, SP1 has positive interaction with genes encoding fibrogenic proteins; collagens I, III, IV, and fibronectin (FN-1) (87). SP1 is activated by STAT3, and in turn, SP1 interacts with interleukin (IL)-24, which signals upregulated JAK1, as well as the chemokine CXCL1, which signals JAK3 (31). SP1 is also shown activating upregulated ICAM1 (93), a critical adherence molecule in ischemia and diabetic nephropathy (40, 44). Upregulated LIF (32), OSM (79), and unchanged IL-9 stimulate JAK3 (36), while JAK1 receives positive input from IL-6 (76), and IFN activates upregulated JAK2 (95).
The renal fibrosis pathway depicted in Fig. 5C comprises 33 activated genes, two suppressed genes, and one unchanged gene. In Fig. 5C, bottom left corner, upregulated TGF-β is shown mediating some of its actions via upregulated FOXO3 (26), AP2 (48), and cMyc (96). TGF-β, through activated TGF-βR2 and TGF-βR1, has positive interaction with the extracellular matrix proteins (Col I, III, IV, V; and FN1) (12). The upregulated NF-κB gene complex is shown collaborating with IFN-γR1, presumably via STAT-independent signaling (27). IFN-γR1 also mediates activation of STAT5 (27), through its cognate tyrosine kinase JAK2 (47), whereas upregulated RUNX3 antagonizes STAT5 expression (66). The network also shows the JAK3, JAK1, STAT6 axis stimulating STAT3 (65), with originating signals from the receptors IL6ST and IL4R, although the latter receptor was suppressed in diabetic rats (64, 65, 81). On the other hand, cell cycle inhibitory signals emanating from p21 can inhibit STAT3 signaling (14). Other upregulated targets of STAT6 include Col I (6), NF-κB (52), and IL-4, which in turn activates STAT6 (64) in an activating loop previously reported in inflammatory (50) and epithelial cells (67). IL4 interacts with unchanged IRS2 (88) and upregulated JAK3 (34), which is upstream of activated JAK1, also stimulated by the upregulated receptor IL6ST (gp130) (56). It is noteworthy that upregulation of the JAK-STAT pathway is also seen in human DN but not in mouse models without renal failure or tubulointerstitial fibrosis (90).
The cell cycle arrest network shown in Fig. 5D contains 35 activated genes, four suppressed genes, and three unchanged genes. In Fig. 5D, right center, is the upregulated cyclin-dependent kinase inhibitor p21, the target of upregulated p53 (86), also stimulated by TGF-β1 signaling, including SMAD5, SMAD1, SMAD3 (69) by upregulated IRF5 (4), SP1 (91), RUNX3 (45), and RUNX1 (71). In turn, p21 is shown suppressing the downregulated cyclins CCNB, D, and E, as well as upregulated CDK4 and PLK1 (13). In Fig. 5D, top left corner, upregulated TGF-β1 is seen interacting positively, via a non-SMAD pathway, with the upregulated SHS and SOS axis, although expression of effector signaling molecule HRAS is suppressed in diabetic kidneys (51). In addition, TGF-β1 via TGF-βR1 activates SMAD3, shown interacting positively with upregulated PAI1 (72), TIMP1 (61), and FN1 (55). Furthermore, TGF-β receptors (49) and IRAK4 (39) stimulate upregulated TRAF6, which is shown signaling Src (53), upregulated IL1R1 and upregulated IL17R, the latter a component of a positive feedback loop that includes TRAF6 and unchanged CIKS upstream of the NF-κB complex (not shown) (78). SMAD1 activates collagen genes that link positively to upregulated ITG-α5 and upregulated ITG-β1 (38), which stimulate upregulated RUNX2 via upregulated transcriptional cofactor Fhl2 (28). Figure 5D also illustrates upregulated RUNX3 activating p21, a process mediated by TGF-β signaling (15), as well as RUNX1 (2). RUNX2 is seen interacting in a complex way with genes regulating fibrogenesis: RUNX2 activates profibrotic genes and also has positive interactions with TIMP1 (5) and with MMP14 (35). Activated TIMP1 is shown inhibiting MMP2 and MMP12, and upregulated TIMP2 is shown inhibiting MMP2. Furthermore, the gene encoding the hyaluronic acid (HA) receptor, CD44, is cooperating with MMP2 and SRC (46). The end-result is renal fibrosis, as these interactions seem to overwhelm the role of metalloproteinases. Figure 5D also includes the known association of cell cycle arrest and fibrogenesis (93), exemplified herein by the positive interaction of upregulated TGF-β1 with p15, which inhibits CDK4 (73). It is noteworthy that many of these mediators (including CXCR4, CCL19, CD44, collagens, TIMP1 and FN1) are also increased in human biopsy specimens evaluated by microarray technology (90).
RNA-seq and bioinformatic protocols were used to derive interacting renal gene networks in progressive CKD of DN. This extensive systems biology analysis revealed exceedingly complex interactions among inflammatory and renal cells, a reality made evident even by a single cross-sectional snapshot of the events. For example, it is clear from our data that multiple paths, TGF-β1, IL6ST, BMP1, lead to destructive fibrosis, and we surmise that this redundancy is a major challenge to be considered in antifibrosis drug design. The networks identified other interlinked areas of morbidity and potential opportunity. Thus, the proinflammatory NF-κB/CXCL1 axis, which drives neutrophil migration, was increased 10-fold in both DS and DI kidneys and is a potentially rewarding target. Accordingly, we previously showed that immune suppression with mycophenolate mofetil attenuates upregulated renal CXCL1, improving renal function, inflammation, and fibrosis (19). The networks also revealed critical renal apoptosis is targeted in DN. The networks also revealed potential critical renal apoptosis mechanisms that can be targeted in DN. Another important finding is the pervasive role of p21 in cell cycle arrest, where multiple inputs activate p21, a state that must be considered when trying to restart the arrested cell cycle in DN.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-082739 and a research grant from DCI Paul Teschan Research and Development Fund to K. J. Kelly and VA Merit Review funds to J. H. Dominguez.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.J.K. and J.H.D. conception and design of research; K.J.K., J.Z., and J.H.D. performed experiments; K.J.K., Y.L., J.Z., C.G., and J.H.D. analyzed data; K.J.K., Y.L., J.Z., C.G., H.L., and J.H.D. interpreted results of experiments; K.J.K. prepared figures; K.J.K. and J.H.D. edited and revised manuscript; K.J.K., Y.L., J.Z., C.G., H.L., and J.H.D. approved final version of manuscript; J.H.D. drafted manuscript.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Ayoub AE, Oh S, Xie Y, Leng J, Cotney J, Dominguez MH, Noonan JP, Rakic P. Transcriptional programs in transient embryonic zones of the cerebral cortex defined by high-resolution mRNA sequencing. Proc Natl Acad Sci USA 108: 14950–14955, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakshi R, Hassan MQ, Pratap J, Lian JB, Montecino MA, van Wijnen AJ, Stein JL, Imbalzano AN, Stein GS. The human SWI/SNF complex associates with RUNX1 to control transcription of hematopoietic target genes. J Cell Physiol 225: 569–576, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ 14: 56–65, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barnes BJ, Kellum MJ, Pinder KE, Frisancho JA, Pitha PM. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res 63: 6424–6431, 2003. [PubMed] [Google Scholar]
- 5.Bertrand-Philippe M, Ruddell RG, Arthur MJ, Thomas J, Mungalsingh N, Mann DA. Regulation of tissue inhibitor of metalloproteinase 1 gene transcription by RUNX1 and RUNX2. J Biol Chem 279: 24530–24539, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bhogal RK, Stoica CM, McGaha TL, Bona CA. Molecular aspects of regulation of collagen gene expression in fibrosis. J Clin Immunol 25: 592–603, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci 123: 3209–3214, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breese MR, Liu Y. NGSUtils: a software suite for analyzing and manipulating next-generation sequencing datasets. Bioinformatics 29: 494–496, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breyer MD, Bottinger E, Brosius FC, Coffman TM, Fogo A, Harris RC, Heilig CW, Sharma K. Diabetic nephropathy: of mice and men. Adv Chron Kidn Dis 12: 128–145, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Brooks MJ, Rajasimha HK, Roger JE, Swaroop A. Next-generation sequencing facilitates quantitative analysis of wild-type and Nrl(−/−) retinal transcriptomes. Mol Vis 17: 3034–3054, 2011. [PMC free article] [PubMed] [Google Scholar]
- 11.Budzinska M, Galganska H, Wojtkowska M, Stobienia O, Kmita H. Effects of VDAC isoforms on CuZn-superoxide dismutase activity in the intermembrane space of Saccharomyces cerevisiae mitochondria. Biochem Biophys Res Commun 357: 1065–1070, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Burch ML, Zheng W, Little PJ. Smad linker region phosphorylation in the regulation of extracellular matrix synthesis. Cell Mol Life Sci 68: 97–107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res 704: 12–20, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Chetty C, Dontula R, Ganji PN, Gujrati M, Lakka SS. SPARC expression induces cell cycle arrest via STAT3 signaling pathway in medulloblastoma cells. Biochem Biophys Res Commun 417: 874–879, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, Li QL, Kim HR, Cha EJ, Lee YH, Kaneda A, Ushijima T, Kim WJ, Ito Y, Bae SC. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperation with transforming growth factor beta-activated SMAD. Mol Cell Biol 25: 8097–8107, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirulli ET, Heinzen EL, Dietrich FS, Shianna KV, Singh A, Maia JM, Goedert JJ, Goldstein DB. A whole-genome analysis of premature termination codons. Genomics 98: 337–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen CD, Lindenmeyer MT, Eichinger F, Hahn A, Seifert M, Moll AG, Schmid H, Kiss E, Grone E, Grone HJ, Kretzler M, Werner T, Nelson PJ. Improved elucidation of biological processes linked to diabetic nephropathy by single probe-based microarray data analysis. PLoS One 3: e2937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 17: 1104–1114, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez J, Wu P, Packer CS, Temm C, Kelly KJ. Lipotoxic and inflammatory phenotypes in rats with uncontrolled metabolic syndrome and nephropathy. Am J Physiol Renal Physiol 293: F670–F679, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez JH, Mehta JL, Li D, Wu P, Kelly KJ, Packer CS, Temm C, Goss E, Cheng L, Zhang S, Patterson CE, Hawes JW, Peterson R. Anti-LOX-1 therapy in rats with diabetes and dyslipidemia: ablation of renal vascular and epithelial manifestations. Am J Physiol Renal Physiol 294: F110–F119, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez JH, Wu P, Hawes JW, Deeg M, Walsh J, Packer SC, Nagase M, Temm C, Goss E, Peterson R. Renal injury: similarities and differences in male and female rats with the metabolic syndrome. Kidney Int 69: 1969–1976, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekins S, Nikolsky Y, Bugrim A, Kirillov E, Nikolskaya T. Pathway mapping tools for analysis of high content data. Methods Mol Biol 356: 319–350, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Zapico ME, Mladek A, Ellenrieder V, Folch-Puy E, Miller L, Urrutia R. An mSin3A interaction domain links the transcriptional activity of KLF11 with its role in growth regulation. EMBO J 22: 4748–4758, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerondakis S, Grumont RJ, Banerjee A. Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol 85: 471–475, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J. A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci USA 103: 12747–12752, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev 19: 383–394, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Gunther T, Poli C, Muller JM, Catala-Lehnen P, Schinke T, Yin N, Vomstein S, Amling M, Schule R. Fhl2 deficiency results in osteopenia due to decreased activity of osteoblasts. EMBO J 24: 3049–3056, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamdan R, Zhou Z, Kleinerman ES. SDF-1alpha induces PDGF-B expression and the differentiation of bone marrow cells into pericytes. Mol Cancer Res 9: 1462–1470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, Gersbach M, Imhof R, Hottiger MO. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem 280: 40450–40464, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Henkels KM, Frondorf K, Gonzalez-Mejia ME, Doseff AL, Gomez-Cambronero J. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett 585: 159–166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J 438: 11–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homer N, Merriman B, Nelson SF. BFAST: an alignment tool for large scale genome resequencing. PLoS One 4: e7767, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol 105: 1063–1070, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez MJ, Balbin M, Alvarez J, Komori T, Bianco P, Holmbeck K, Birkedal-Hansen H, Lopez JM, Lopez-Otin C. A regulatory cascade involving retinoic acid, Cbfa1, and matrix metalloproteinases is coupled to the development of a process of perichondrial invasion and osteogenic differentiation during bone formation. J Cell Biol 155: 1333–1344, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston JA, Bacon CM, Riedy MC, O'Shea JJ. Signaling by IL-2 and related cytokines: JAKs, STATs, and relationship to immunodeficiency. J Leukoc Biol 60: 441–452, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Juan L, Wang G, Radovich M, Schneider BP, Clare SE, Wang Y, Liu Y. Potential roles of microRNAs in regulating long intergenic noncoding RNAs. BMC Med Genom 6, Suppl 1: S7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42: 283–323, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Kawagoe T, Sato S, Jung A, Yamamoto M, Matsui K, Kato H, Uematsu S, Takeuchi O, Akira S. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J Exp Med 204: 1013–1024, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly KJ, Burford JL, Dominguez JH. The post-ischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol 297: F923–F931, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Kelly KJ, Dominguez JH. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol 32: 469–475, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Kelly KJ, Dominguez JH. Treatment of the post-ischaemic inflammatory syndrome of diabetic nephropathy. Nephrol Dial Transplant 25: 3204–3212, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutiérrez-Ramos J-C, Bonventre JV. Intercellular adhesion molecule-1 deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly KJ, Wu P, Patterson CE, Temm C, Dominguez JH. LOX-1 and inflammation: a new mechanism for renal injury in obesity and diabetes. Am J Physiol Renal Physiol 294: F1136–F1145, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Choi JK, Cinghu S, Jang JW, Lee YS, Li YH, Goh YM, Chi XZ, Lee KS, Wee H, Bae SC. Jab1/CSN5 induces the cytoplasmic localization and degradation of RUNX3. J Cell Biochem 107: 557–565, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y, Lee YS, Choe J, Lee H, Kim YM, Jeoung D. CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem 283: 22513–22528, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Kirabo A, Oh SP, Kasahara H, Wagner KU, Sayeski PP. Vascular smooth muscle Jak2 deletion prevents angiotensin II-mediated neointima formation following injury in mice. J Mol Cell Cardiol 50: 1026–1034, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koinuma D, Tsutsumi S, Kamimura N, Taniguchi H, Miyazawa K, Sunamura M, Imamura T, Miyazono K, Aburatani H. Chromatin immunoprecipitation on microarray analysis of Smad2/3 binding sites reveals roles of ETS1 and TFAP2A in transforming growth factor beta signaling. Mol Cell Biol 29: 172–186, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landstrom M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol 42: 585–589, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Lederer JA, Perez VL, DesRoches L, Kim SM, Abbas AK, Lichtman AH. Cytokine transcriptional events during helper T cell subset differentiation. J Exp Med 184: 397–406, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J 26: 3957–3967, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G, Yuan K, Yan C, Fox J, 3rd, Gaid M, Breitwieser W, Bansal AK, Zeng H, Gao H, Wu M. 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radic Biol Med 52: 392–401, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu A, Gong P, Hyun SW, Wang KZ, Cates EA, Perkins D, Bannerman DD, Puche AC, Toshchakov VY, Fang S, Auron PE, Vogel SN, Goldblum SE. TRAF6 protein couples Toll-like receptor 4 signaling to Src family kinase activation and opening of paracellular pathway in human lung microvascular endothelia. J Biol Chem 287: 16132–16145, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Taylor MW, Edenberg HJ. Model-based identification of cis-acting elements from microarray data. Genomics 88: 452–461, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Huang XR, Lan HY. Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am J Physiol Renal Physiol 302: F986–F997, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Lupardus PJ, Skiniotis G, Rice AJ, Thomas C, Fischer S, Walz T, Garcia KC. Structural snapshots of full-length Jak1, a transmembrane gp130/IL-6/IL-6Ralpha cytokine receptor complex, and the receptor-Jak1 holocomplex. Structure 19: 45–55, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madge LA, May MJ. The NFkappaB paradox: RelB induces and inhibits gene expression. Cell Cycle 10: 6–7, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Mak RH, Kuo HJ, Cheung WW. Animal models of obesity-associated chronic kidney disease. Adv Chron Kidn Dis 13: 374–385, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Martin JA, Wang Z. Next-generation transcriptome assembly. Nat Rev Genet 12: 671–682, 2011. [DOI] [PubMed] [Google Scholar]
- 60.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem 276: 43503–43508, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 21: 1477–1487, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer-Bahlburg A, Bandaranayake AD, Andrews SF, Rawlings DJ. Reduced c-myc expression levels limit follicular mature B cell cycling in response to TLR signals. J Immunol 182: 4065–4075, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth 5: 621–628, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Moscat J, Rennert P, Diaz-Meco MT. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ 13: 702–711, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Noon-Song EN, Ahmed CM, Dabelic R, Canton J, Johnson HM. Controlling nuclear JAKs and STATs for specific gene activation by IFNgamma. Biochem Biophys Res Commun 410: 648–653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogawa S, Satake M, Ikuta K. Physical and functional interactions between STAT5 and Runx transcription factors. J Biochem 143: 695–709, 2008. [DOI] [PubMed] [Google Scholar]
- 67.Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 108: 18067–18072, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pamukcu B, Lip GY, Snezhitskiy V, Shantsila E. The CD40-CD40L system in cardiovascular disease. Ann Med 43: 331–340, 2011. [DOI] [PubMed] [Google Scholar]
- 69.Pardali K, Kowanetz M, Heldin CH, Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1). J Cell Physiol 204: 260–272, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, DEMAND Investigators. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 69: 2057–2063, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Peterson LF, Lo MC, Okumura AJ, Zhang DE. Inability of RUNX1/AML1 to breach AML1-ETO block of embryonic stem cell definitive hematopoiesis. Blood Cells Mol Dis 39: 321–328, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Samarakoon R, Overstreet JM, Higgins SP, Higgins PJ. TGF-beta1 –> SMAD/p53/USF2 –> PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res 347: 117–128, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandhu C, Garbe J, Bhattacharya N, Daksis J, Pan CH, Yaswen P, Koh J, Slingerland JM, Stampfer MR. Transforming growth factor beta stabilizes p15INK4B protein, increases p15INK4B-cdk4 complexes, and inhibits cyclin D1-cdk4 association in human mammary epithelial cells. Mol Cell Biol 17: 2458–2467, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sayan BS, Sayan AE, Knight RA, Melino G, Cohen GM. p53 is cleaved by caspases generating fragments localizing to mitochondria. J Biol Chem 281: 13566–13573, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 282: 36321–36329, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Shen X, Tian Z, Holtzman MJ, Gao B. Cross-talk between interleukin 1beta (IL-1beta) and IL-6 signalling pathways: IL-1beta selectively inhibits IL-6-activated signal transducer and activator of transcription factor 1 (STAT1) by a proteasome-dependent mechanism. Biochem J 352: 913–919, 2000. [PMC free article] [PubMed] [Google Scholar]
- 77.Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284: 156–159, 1999. [DOI] [PubMed] [Google Scholar]
- 78.Sonder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, Siebenlist U. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem 286: 12881–12890, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song HY, Jeon ES, Jung JS, Kim JH. Oncostatin M induces proliferation of human adipose tissue-derived mesenchymal stem cells. Int J Biochem Cell Biol 37: 2357–2365, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11: 155–161, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH. The transcription factor STAT3 is required for T helper 2 cell development. Immunity 34: 39–49, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su Z, Li Z, Chen T, Li QZ, Fang H, Ding D, Ge W, Ning B, Hong H, Perkins RG, Tong W, Shi L. Comparing next-generation sequencing and microarray technologies in a toxicological study of the effects of aristolochic acid on rat kidneys. Chem Res Toxicol 24: 1486–1493, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Temm C, Dominguez JH. Microcirculation: nexus of comorbidities in diabetes. Am J Physiol Renal Physiol 293: F486–F493, 2007. [DOI] [PubMed] [Google Scholar]
- 84.Todd AG, Lin H, Ebert AD, Liu Y, Androphy EJ. COPI transport complexes bind to specific RNAs in neuronal cells. Hum Mol Genet 22: 729–736, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J Biol Chem 274: 35809–35815, 1999. [DOI] [PubMed] [Google Scholar]
- 87.Venkov C, Plieth D, Ni T, Karmaker A, Bian A, George AL, Jr, Neilson EG. Transcriptional networks in epithelial-mesenchymal transition. PLoS One 6: e25354, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang HY, Zamorano J, Keegan AD. A role for the insulin-interleukin (IL)-4 receptor motif of the IL-4 receptor alpha-chain in regulating activation of the insulin receptor substrate 2 and signal transducer and activator of transcription 6 pathways. Analysis by mutagenesis. J Biol Chem 273: 9898–9905, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol 10: 348–355, 2009. [DOI] [PubMed] [Google Scholar]
- 90.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao H, Hasegawa T, Isobe K. p300 collaborates with Sp1 and Sp3 in p21(waf1/cip1) promoter activation induced by histone deacetylase inhibitor. J Biol Chem 275: 1371–1376, 2000. [DOI] [PubMed] [Google Scholar]
- 92.Xiong Y, Qiu F, Piao W, Song C, Wahl LM, Medvedev AE. Endotoxin tolerance impairs IL-1 receptor-associated kinase (IRAK) 4 and TGF-beta-activated kinase 1 activation, K63-linked polyubiquitination and assembly of IRAK1, TNF receptor-associated factor 6, and IkappaB kinase gamma and increases A20 expression. J Biol Chem 286: 7905–7916, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeller KI, Zhao X, Lee CW, Chiu KP, Yao F, Yustein JT, Ooi HS, Orlov YL, Shahab A, Yong HC, Fu Y, Weng Z, Kuznetsov VA, Sung WK, Ruan Y, Dang CV, Wei CL. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci USA 103: 17834–17839, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol 28: 239–260, 2009. [DOI] [PubMed] [Google Scholar]
- 96.Zhu X, Ozturk F, Liu C, Oakley GG, Nawshad A. Transforming growth factor-beta activates c-Myc to promote palatal growth. J Cell Biochem 113: 3069–3085, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.