Abstract
Most evidence supports the view that ERα is responsible for estrogen (ovarian estradiol, E2)-induced proliferation in the epithelial cells of the mammary gland, but despite this, proliferating epithelial cells do not express ERα. We have examined this apparent paradox by studying the role of ERα and ERβ in E2-induced proliferation in mammary glands (measured by BrdUrd incorporation into DNA) in mice with intact ERβ (WT mice) and those in which the ERβ gene has been inactivated (ERβ-/- mice). On treatment of ERβ-/- mice with E2 or ovariectomized WT mice with E2, tamoxifen, or a specific ERβ agonist (BAG), the number of BrdUrd-labeled cells in mammary glands increased from 3.4% in controls to 28-38% in the treated mice. This indicates that both ERα and ERβ can mediate E2-induced proliferation independently of each other. With specific antibodies, ERβ was found in both epithelial and stromal cells, whereas ERα was strictly epithelial. Within 4 h of a single dose of E2, ERα was lost from the nuclei of epithelial cells. In WT mice, ERα reappeared by 24 h, but in ERβ-/- mice, return to the nucleus was delayed by 24 h. At 4 h after E2, neither ERα nor progesterone receptor was detectable in BrdUrd-labeled nuclei but by 48 h after E2, 29% of the BrdUrd-labeled cells expressed ERα, and 21-38% expressed progesterone receptor. During 3 weeks of continuous E2 treatment, ERβ remained in the nucleus, but there was no detectable ERα. With tamoxifen treatment, ERα remained in the nucleus, but ERβ was lost. From these results, we conclude that ERα receives the proliferation signal from E2, initiates DNA synthesis, and is then lost from cells. The subsequent steps in proliferation can proceed in the absence of either ERα or ERβ. ERβ facilitates the return of ERα to the nucleus and restores responsiveness to E2. By down-regulating ERβ, tamoxifen may prolong refractoriness to E2 in mammary epithelium.
Keywords: progesterone receptor, breast
Cell proliferation in the mammary gland is under multihormonal control. Classical endocrine ablation/hormone replacement studies have demonstrated that ovarian estradiol (E2) is critical for the two major phases of mammary development, ductal elongation during puberty, and lobuloalveolar development during pregnancy (1-3). E2 acts directly on the mammary gland to stimulate ductal morphogenesis during puberty, whereas progesterone is the major stimulator of mammary epithelial DNA synthesis and alveolar development (1, 4). Although E2 elicits proliferation of the mammary gland epithelium and the antiestrogen, tamoxifen inhibits proliferation of ERα-positive breast cancer (5), the mechanism of E2-induced proliferation is a subject of much debate and investigation.
One of the most confounding observations is that in the mammary gland, either normal (4, 6-10) or malignant (11) cells that express proliferation markers do not express ERα. One school of thought holds that the proliferative effects of E2 on epithelium are indirect, i.e., E2 is thought to act on ERα in stromal cells inducing the release of growth factors, which then stimulate proliferation of epithelial cells (12-14). One corollary of this reasoning would be that ERα-containing cells are protected from growth factor-stimulated proliferation. Another hypothesis to explain the dissociation between steroid receptor expression and proliferation in the normal breast is that steroid receptors are normally expressed in fully differentiated resting cells, and it is only in malignancy that proliferating cells express these receptors (6). Recently, it was shown that in mature rats that have had a pregnancy but not in age-matched virgins, ERα does colocalize with proliferation markers (15).
Yet another school of thought maintains that progesterone, not E2, is the proliferative hormone in the mammary epithelium (16-20). The strongest support for this idea is that proliferation in the mammary gland occurs during the luteal phase of the estrus cycle when progesterone levels are high (17). A clear distinction has to be made between lobular growth, which is progesterone-mediated, and ductal growth, which is E2-mediated (21-23). During the estrus cycle and in preparation for pregnancy, it is lobular growth that occurs (17).
The functions of stromal steroid receptors in stimulating epithelial proliferation in mammary gland have been studied in ER knockout mice (14). There is very limited ductal growth in ERα knockout mice (ERα-/-) (12, 24), whereas the mammary glands of virgin ERβ knockout mice (ERβ-/-) are morphologically indistinguishable from those of WT littermates (25). In ERα-/- mice, the mammary gland phenotype results from abnormal pituitary function. A reduction in prolactin secretion from the pituitary leads to reduced mammary gland development, and excessive luteinizing hormone secretion results in hemorrhagic follicles and lack of corpora lutea in ovary (26). Ductal elongation and lobuloalveolar development are restored in intact ERα-/- mice on receipt of a normal pituitary and in ovariectomized ERα-/- mice on estrogen/progesterone treatment (1). These results indicate that the effect of loss of ERα on mammary gland growth is indirect, via the pituitary, and this conclusion is further supported by experiments where tissue recombinants (mammary stromal/epithelial) between WT and ERα-/- mice were used. These experiments showed that epithelial growth occurs when either epithelial or stromal cells are from ERα-/- as long as mice are supplemented with E2 and progesterone (12).
In both rodent and human mammary glands, the dominant ER in the stroma is ERβ, not ERα (3, 4, 7, 27, 28), indicating that E2-stimulated growth factor release from the stroma is very likely ERβ-mediated. This finding is surprising for two reasons: (i) the overwhelming evidence that ERα is the receptor controlling E2-mediated proliferation, and (ii) the apparently normal development of the mammary gland in ERβ-/- mice. Clearly, the mammary epithelium in ERβ-/- mice does not depend on stromal ERβ for E2-stimulated growth. To clarify the roles of the two ERs in E2-induced proliferation, we have examined the effects of E2 and tamoxifen on the mammary glands in WT and ERβ-/- mice and of a selective ERβ agonist in WT mice. We conclude that proliferation in the mammary epithelium is triggered by direct action of E2 on ER in epithelial cells and can be mediated by both ERα and ERβ. Once the proliferation signal is received by the cell, ERα is down-regulated, which is why ERα is never colocalized with proliferation markers.
Materials and Methods
Animals. Animals were used under the Guidelines for Care and Use of Experimental Animals issued by Stockholm Södra Djurförsöksetiska Nämnd. Animals were maintained under standardized environmental conditions, with free access to food and water. WT and ERβ-/- mice were bred from heterozygous male and female mice. Genotyping by PCR was performed on DNA isolated from tails of 2-week-old mice (29). Mice were ovariectomized when they were 12-20 weeks of age. After receiving various treatments, animals were asphyxiated by CO2, and the mammary glands were collected and either frozen in liquid nitrogen for protein preparation or fixed in 4% paraformaldehyde overnight and routinely embedded in paraffin for immunohistochemical staining.
For continuous treatment, WT and ERβ-/- mice were ovariectomized at the age of 14-18 weeks, and at the same time Alzet osmotic pumps (B & K Universal, Sollentuna, Sweden) were put into the abdominal cavity of each mouse. The pump contained either E2 (8.3 μg) or tamoxifen (83 μg) in a total volume of 200 μl. The release rate from the pumps was 0.25 μl/h, which means the mice received 0.25 μg of E2 or 2.5 μg of tamoxifen in 24 h. The control pumps contained vehicle only. There were four mice in each group, and mice were killed 3 weeks after ovariectomy.
Chemicals and Antibodies. 17β-Estradiol and tamoxifen were purchased from Sigma. A selective ERβ agonist, BAG, was provided by Merck. BAG shows a 100-fold selectivity for ERβ over ERα, and, at the doses used in this study, it did not stimulate proliferation in the uterus. BrdUrd was from Roche (Mannheim, Germany), rabbit polyclonal antibodies to mouse ERα (MC20) and progesterone receptor (PR) (C19) were obtained from Santa Cruz Biotechnology, and rabbit polyclonal antibody cyclin D1 was from Lab Vision (Fremont, CA). Mouse monoclonal anti-BrdUrd antibody was from Pharmingen. Rabbit polyclonal anti-ERβ antibody, raised against the ligand-binding domain of human ERβ and chicken polyclonal ERβ 503 IgY, were produced in our laboratory and have been characterized previously (7). Biotinylated secondary antibodies (goat anti-mouse IgG and goat anti-rabbit IgG) and avidin-biotin kits were obtained from Vector Laboratories. FITC-conjugated donkey anti-rabbit, Cy3-conjugated (Amersham Biosciences) donkey anti-mouse, and Cy3-conjugated donkey anti-chicken antibodies were purchased from Jackson ImmunoResearch.
Immunohistochemistry. Paraffin sections (4 μm) were dewaxed in xylene and rehydrated through graduated ethanol to water. Endogenous peroxidase was blocked by incubation for 30 min with a solution of 1% hydrogen peroxide, and antigens were retrieved by microwaving sections in 0.01 M citrate buffer, pH 6.0, for 20 min at 650 W.
Single Antibody Immunostaining. Tissue sections were incubated for 1 h at 4°C with normal goat serum diluted at 1:10 in PBS. Antibodies were diluted individually in PBS containing 3% BSA. Dilution for ERα, PR, and cyclin D1 antibodies were 1:100; and dilution for BrdUrd antibody was 1:150. Sections were incubated with antibodies overnight at 4°C. For negative controls, the primary antibody was replaced with PBS alone or with primary antibody after absorption with the corresponding antigen. Before addition of the secondary antibody, sections were rinsed in PBS. The ABC method was used to visualize the signal according to the manual provided by the manufacturer (Vector). Sections were incubated in biotinylated goat anti-rabbit or goat anti-mouse Ig (1:200 dilution) for 2 h at room temperature, followed by washing with PBS and incubation in avidin-biotin-horseradish peroxidase for 1 h. After thorough washing in PBS, sections were developed with 3,3′-diaminobenzidine tetra-hydrochloride (DAKO), slightly counterstained with Mayer's hematoxylin, and dehydrated through an ethanol series, followed by exposure to xylene and mounting.
The percentage of positively stained cells is an average after counting the stained and the total number of cells from four high-magnification fields with the software image-pro plus (Ver. 4.1, Media Cybernetics, Silver Spring, MD).
Double Antibody Immunostaining. Tissue sections were incubated for 1 h at 4°C with normal donkey serum (Sigma) diluted 1:10 in PBS. This was followed by an overnight incubation at 4°C with a mixture composed of antibodies to either ERα and BrdUrd, ERβ 503 and BrdUrd, or PR and BrdUrd. PBS alone was used in place of these mixtures in the negative controls. Before addition of secondary antibodies, sections were washed with PBS. Slides were incubated for 1 h with a mixture of FITC-conjugated donkey anti-rabbit (1:100) and Cy3-conjugated donkey anti-mouse (1:200) or Cy3-conjugated donkey anti-chicken (1:200) antibodies. After washing with PBS for 30 min, the slides were incubated with 0.1 μg/ml 4′, 6-diamidino-2-phenylindole dihydrochloride in PBS for 30 sec, washed three times in PBS, and mounted with Vectashield (Vector).
Detection of ERα and ERβ Expression by Western Blotting. Frozen tissues were homogenized with a Polytron PT3100 (Kinematica, Littau, Switzerland) for a few seconds in a high-salt buffer (600 mM Tris·HCl/1 mM EDTA, pH 7.4, with 1/10 wt/vol of homogenate). Two tablets of mixture protease inhibitors (Boehringer Mannheim) were added per 50 ml of high-salt buffer before use. The homogenates were centrifuged at 105,000 × g for 1 h at 4°C. Supernatants (cytosol) were aliquoted and kept at -80°C until use. Before Western blotting, protein contents were measured by the Bio-Rad protein assay with BSA as the standard. Equal amounts of protein were loaded onto each lane of an 8% polyacrylamide gel. Western blotting was done according to the protocol described previously (30). Antibody dilutions were 1:1,000 for anti-ERα, 1:3,000 for ERβ, and 1:3,000 for the peroxidase-conjugated goat anti-rabbit IgG.
Evaluation of Proliferation. BrdUrd (5-bromo-2′-deoxyuridine dissolved in 0.9% NaCl) was administered i.p. at a dose of 100 mg/kg of body weight 2 h or 48 h before death. Six randomly selected areas in each sample were counted for BrdUrd-positive cells and total cells in the epithelium. Statistical differences among groups were analyzed with Student's t test by using SPSS (SPSS, Chicago). A value of P < 0.05 was considered significant.
Results
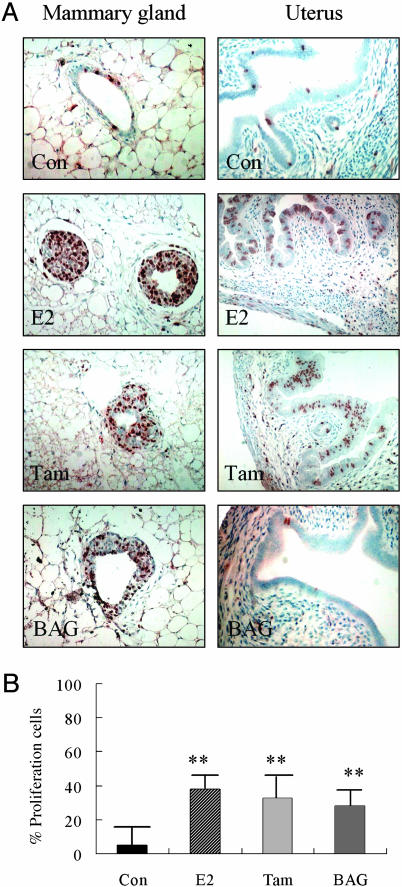
Proliferation Induced by Estrogen, Tamoxifen, or BAG Treatment. Ovariectomized C57BL/6 mice, aged 14-16 weeks, were treated with E2 (20 μg/kg), tamoxifen (0.4 mg/kg), BAG (1 mg/kg), or vehicle for 48 h. E2, tamoxifen, or BAG was dissolved in Intralipid (Pharmacia & Upjohn). BrdUrd (100 mg/kg) was injected i.p. at the same time and repeated 24 h later. There were four mice in each group. As indicated in Fig. 1, cells generated during the treatment period were labeled with BrdUrd. About 1,000 mammary gland epithelial cells in each group were examined. The percentages of BrdUrd-labeled cells were 38%, 28%, and 32% in E2-, BAG-, or tamoxifen-treated mice (Fig. 1 A). There was no statistical difference among the groups. These values were significantly higher than those in the vehicle-treated mice, 3.4% (P < 0.01, Fig. 1B).
Fig. 1.
Proliferation in response to E2, tamoxifen, or BAG. (A) Four-month-old ovariectomized WT mice were treated with E2 (20 μg/kg), tamoxifen (Tam) (0.4 mg/kg), or BAG (1 mg/kg). BrdUrd was injected for 48 h before death. The BrdUrd labeling (brown) indicates cells whose DNA was synthesized during the treatment. (B) In the mammary gland, 38%, 28%, and 32% of the epithelial cells were labeled with BrdUrd in mice receiving E2, BAG, or Tam treatment, respectively, but only 3.4% in mice receiving vehicle (Con). In the uterus, BrdUrd-labeled epithelial, stromal, and myometrial cells were seen in vehicle-treated mice (Con). In mice receiving E2 or Tam, there was a striking increase in the number of BrdUrd-labeled epithelial cells, whereas no significant changes in BrdUrd-labeled cells occurred in mice receiving BAG. The proliferation amount in mammary glands of mice receiving E2, tamoxifen, or BAG was significantly higher than that in the control group (**, P < 0.01).
As a control for the selectivity of BAG for ERβ, proliferation in the uterus was also evaluated. In the uterus, a few epithelial, stromal, or myometrial cells were labeled with BrdUrd in mice receiving vehicle. In both E2- and tamoxifen-treated mice, there were striking increases in the number of BrdUrd-labeled cells in the epithelium. However, in mice receiving BAG treatment, labeling was not different from that in the vehicle-treated mice (Fig. 1 A).
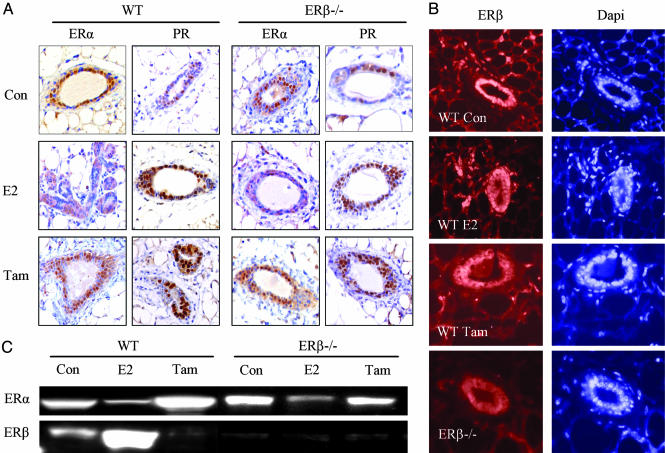
Regulation of ERα and ERβ Expression in Mice Receiving Continuous E2 or Tamoxifen Treatment. The expression of ERα and ERβ was evaluated in mice receiving continuous treatment for 3 weeks. In vehicle-treated mice, 63% of mammary epithelial cell nuclei were positive for ERα in WT mice and 47% in ERβ-/- mice. Very few stromal cells (<0.1%) expressed ERα in mice of either genotype. In mice receiving E2-releasing pumps, there was no nuclear ERα staining but some of the epithelial cells showed cytoplasmic staining. In ERβ-/- and WT mice that received tamoxifen-releasing pumps, nuclear ERα staining was not different from that in vehicle-treated mice (Fig. 2A).
Fig. 2.
ERα and PR expression in mice receiving E2 or tamoxifen treatment for 3 weeks. (A) Ovariectomized adult WT or ERβ-/- mice received a pump releasing estradiol or tamoxifen (Tam) for 3 weeks. ERα and PR were expressed in most of the epithelial cells in the vehicle-treated mice (Con). In neither WT nor ERβ-/- mice was any nuclear ERα staining found after E2 treatment, whereas some cells showed cytoplasmic staining. There was still nuclear ERα staining in mice receiving tamoxifen. Nuclear PR staining was found in both WT and ERβ-/- mice receiving either E2 or tamoxifen. ERβ expression was detected by immunofluorescence. (B) More than 90% epithelial cells and 40% stromal cells in vehicle-treated WT mice expressed ERβ. This pattern was not changed on E2 treatment. In tamoxifen-treated mice, clear down-regulation of nuclear ERβ expression was detected. Some epithelial cells showed cytoplasmic staining of ERβ. In ERβ-/- mice, no ERβ staining was found. The down-regulation of ERα by E2 treatment, but not by tamoxifen, was confirmed by Western blot. (C) ERβ was expressed in WT mice and was up-regulated by E2, but not by tamoxifen treatment. No ERβ was detected in ERβ-/- mice.
In vehicle-treated WT mice, >90% of epithelial cells and 40% stromal cells expressed ERβ. On E2 treatment of WT mice, there was no striking change in ERβ staining in either epithelial or stromal cells. However, in tamoxifen-treated WT mice, nuclear ERβ expression in both epithelial and stromal cells was markedly down-regulated. In about half of the epithelial cells, signals for ERβ were detected in the cytoplasm but not in the nucleus. In ERβ-/- mice, no staining was detected (Fig. 2B).
In vehicle-treated mice, nuclear PR staining was found in 43% of epithelial cells in WT mice and 38% in ERβ-/- mice. No PR was detectable in stromal cells. In mice receiving either E2 or tamoxifen, 69-82% of epithelial cells expressed PR, and there was no obvious difference between WT and ERβ-/- mice (Fig. 2 A).
Because loss of signals on immunohistochemistry can be due to masking of epitopes rather than loss of the whole protein, we also examined the changes of ERα and ERβ expression by Western blotting (Fig. 2C). ERα expression was down-regulated by E2 treatment in both WT and ERβ-/- mice, whereas it remained unchanged after tamoxifen treatment. ERβ was expressed in the mammary glands of WT mice but was not detected in ERβ-/- mice. Levels of ERβ were increased by E2, but down-regulated by tamoxifen.
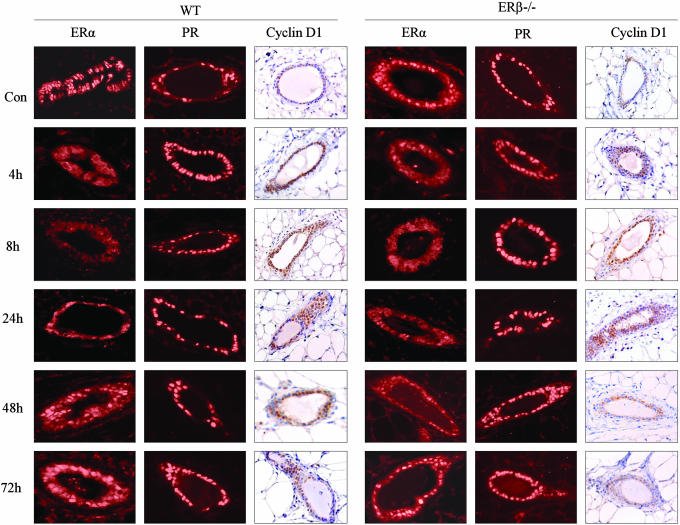
Regulation of ERα, ERβ, PR, and Cyclin D1 After a Single Dose of Estrogen Treatment. WT and ERβ-/- mice aged 12-20 weeks were ovariectomized 2 weeks before treatment. There were three animals in each group, and mice were killed 4, 8, 24, 48, and 72 h after receiving either 20 μg/kg of body weight E2 or vehicle. The expression of ERα, PR, and cyclin D1 after E2 treatment is illustrated in Fig. 3. In the vehicle-treated group, epithelial cell nuclei stained positively for ERα and staining did not change over the time period studied. At 4, 8, and 24 h after E2 treatment, very few epithelial cells expressed nuclear ERα. At 48 and 72 h after E2 treatment, ERα staining returned to the nuclei in many epithelial cells (23% and 51% in WT mice, 9% and 37% in ERβ-/- mice), but there were still some cells showing cytoplasmic staining. Nuclear PR and ERβ in WT mice (not shown here) were expressed in most of the epithelial cells in the vehicle treated mice, and there were no striking differences at any time point studied.
Fig. 3.
Expression of ERα, PR, and cyclin D1 in mice receiving a single dose of E2. Mammary gland tissues were collected at different times from ovariectomized adult WT or ERβ-/- mice that had received a single dose of estradiol (20 μg/kg). In both WT and ERβ-/- mice, ERα expression was down-regulated at 4, 8, and 24 h postinjection, whereas nuclear ERα staining reappeared at 48 h. There were no significant changes in PR expression in either WT or ERβ-/- mice. An increase in the cyclin D1-positive cell population was evident after E2 treatment in both WT and ERβ-/- mice.
In the vehicle-treated group, cyclin D1 was expressed in 36% of the epithelial cells in WT and 27% in ERβ-/- mice. After receiving E2, the number of cells expressing cyclin D1 increased gradually to reach a maximum, ≈3-fold over untreated level, by 24 h in both WT and ERβ-/- mice. At 4, 8, 24, 48, and 72 h, 47%, 57%, 83%, 44%, and 39% of the epithelial cells expressed cyclin D1 in WT mice; in ERβ-/- mice, the corresponding figures were 41%, 49%, 85%, 47%, and 31%, respectively. During the period of accumulation of cyclin D in the nucleus, between 8 and 24 h after E2 administration, ERα was either undetectable or at very low levels in the nucleus. Because there was no significant difference in the time course or extent of change in cyclin D1-positive cells between WT and ERβ-/- mice, it appears that ERβ is not necessary for induction of cyclin D1.
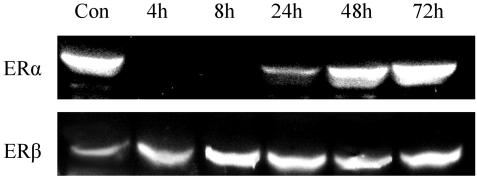
The regulation of ERα and ERβ in WT mice was determined by Western blotting (Fig. 4). In high-salt extracts, ERα was undetectable at 4 and 8 h after E2 treatment. It was about half of the control level at 24 h, and it returned to control level at 8 and 72 h. Instead of being down-regulated, ERβ was significantly up-regulated at 24, 48, and 72 h after E2 treatment.
Fig. 4.
ERα and ERβ expression was detected by Western blotting in WT mice at various times after a single dose of E2. ERα and ERβ were expressed in the control group. There was no detectable ERα band at 4 and 8 h after E2 treatment, but it reappeared at 24 h and was the same as control at 48 and 72 h after E2 treatment. ERβ was up-regulated after E2 treatment.
ERα and PR Expression in Relation to Proliferation. The colocalization of BrdUrd-labeled DNA with either ERα or PR was assessed to determine which cell population was proliferating. In mice that received E2 treatment for 8 h, BrdUrd (100 mg/kg) was injected i. p. 2 h before death. In these mice, 2-5% of epithelial cell nuclei had incorporated BrdUrd, but neither ERα nor PR was present in the labeled cells in WT or ERβ-/- mice (Fig. 5).
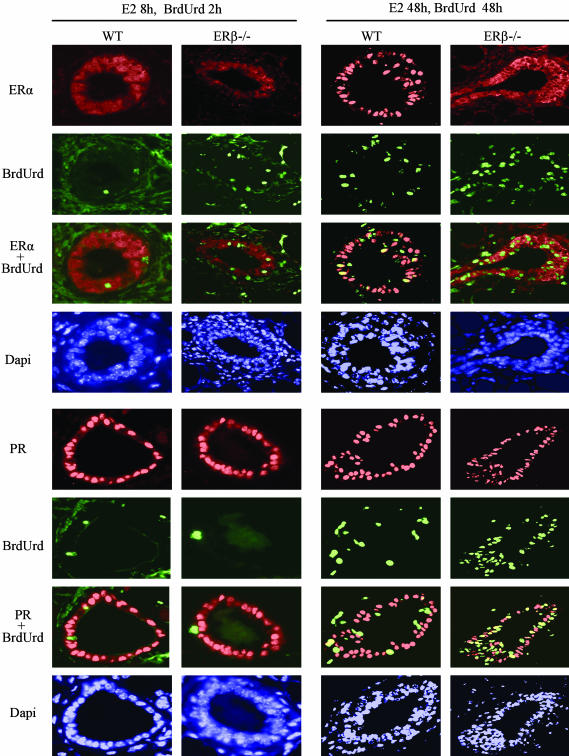
Fig. 5.
Colocalization of BrdUrd with ERα or PR in mice receiving E2 treatment. In both WT and ERβ-/- mice 8 h after a single dose of E2 and 2 h after BrdUrd (100 mg/kg i.p.), there was no ERα, or PR (red) was colocalized with BrdUrd (green) in the mammary gland. However, 48 h after treatment, 29% BrdUrd-labeled cells expressed nuclear ERα in WT mice (yellow indicates colocalization). In the ERβ-/- mice, ERα remained in the cytoplasm at 48 h, and there was no nuclear staining. Twenty-one percent of BrdUrd-labeled cells in WT mice and 38% in ERβ-/- mice expressed PR, but most of the PR-expressing cells were not labeled with BrdUrd.
In mice that were killed 48 h after receiving E2, BrdUrd (100 mg/kg) was injected i.p. twice, 48 and 2 h before sacrifice. In these mice, 27% of epithelial cells were BrdUrd-labeled in WT mice and 38% in ERβ-/- mice. By 48 h, nuclear ERα staining had returned to most of the epithelial cells in WT mice, and 29% BrdUrd-labeled cells were positive for nuclear ERα. In the ERβ-/- mice at this time; however, very few epithelial cells were positive for nuclear ERα, but some cells had cytoplasmic staining. At this time point, most epithelial cells were PR-positive in both WT and ERβ-/- mice. Of the BrdUrd-labeled cells, 21% expressed PR in WT mice and 38% in ERβ-/- mice (Fig. 5). In WT mice, >90% of the epithelial cells expressed ERβ in control and E2-treated mice, and all of the BrdUrd-labeled cells expressed ERβ (data not shown here).
Discussion
Eight years after the discovery of ERβ (31), many questions remain about the role of this receptor in E2-mediated proliferation in breast cancer. ERβ is a weaker transcriptional activator on estrogen response elements (ERE) than is ERα, and it can dimerize with and reduce the activity of ERα, but the role of ERβ is far more complicated than this (32, 33). There is now convincing evidence that proliferative effects of ER are mediated not by ERE but by interactions of ER with AP-1 sites via protein-protein interactions with activator protein 1-binding proteins, fos-jun (34). At these sites, ERα and ERβ have distinctly different actions. Of particular relevance for breast cancer is the fact that ERβ in the presence of hydroxytamoxifen stimulates proliferation, whereas the ERα-tamoxifen complex inhibits proliferation (35). So even though no one is certain about the physiological role of ERβ in the breast, its presence in breast cancer could adversely influence the action of tamoxifen, the most important therapeutic agent used in the treatment (32, 36, 37) and now prevention of breast cancer (37, 38).
Our studies with ovariectomized WT and ERβ-/- mice show that both ERα and ERβ can signal the mammary epithelial cell to proliferate. Proliferation in the mammary gland was elicited by E2 (a ligand for both ERα and ERβ) or by BAG, a selective ERβ ligand. The level of ERβ in the mature uterus is very low, whereas that of ERα is high (39, 40). Unlike E2 or tamoxifen, BAG had no proliferative effect on uterus. This result is taken as a confirmation of the selectivity of BAG for ERβ. We have repeated the BAG study with rats (data not shown here) and found that in immature rats, treatment with BAG or E2 resulted in a similar number of proliferating cells in the mammary gland.
In this study, we found that in mice, >90% of epithelial cell and 40% of stromal cells expressed ERβ. ERα, on the other hand, was expressed in epithelial cells with very few if any positive stromal cells. Because there is very little ERα expression in the stroma, and because E2 caused proliferation in the mammary epithelium in ERβ-/- mice, it can be concluded that E2, acting directly on ERα in the epithelium, induces proliferation. In addition, because the mice had been ovariectomized before E2 treatment, proliferation is occurring in the absence of progesterone. These findings are surprising and have provoked a reevaluation of our ideas about the role of ERα in proliferation. Our new working hypothesis is that E2 does, indeed, initiate proliferation by interacting with ERα or ERβ in the epithelial cells in the mammary gland, but that very shortly after the cell enters the cell cycle, ERα is down-regulated, and this is the reason why ERα is never colocalized in nuclei with proliferation markers.
To test this hypothesis, we have administered E2 to initiate proliferation together with BrdUrd to label proliferating cells. We found that 4 h after E2 administration to mice, ERα levels in cells were significantly reduced and continued to fall over the next 24 h. With a single injection of BrdUrd 2 h before killing the mice, we have shown that 8 h after E2 administration, no BrdUrd-labeled cells express ERα. But when mice are killed 48 h after BrdUrd administration, 29.1% of the BrdUrd-labeled cells express ERα. We conclude that ERα is not expressed during proliferation but is expressed in daughter cells after cell division has occurred. Tamoxifen also caused proliferation in the rat and mouse mammary gland, but ERα was not down-regulated after tamoxifen administration. This result is consistent with a recent in vitro study showing that in MCF7 cells, ERα was down-regulated by E2 and ICI 182780 within 2 h but not by tamoxifen (41).
In the present study, the down-regulation of ERα expression in mammary gland epithelial cells after E2 treatment is similar to what has been reported for uterine epithelial cells, where most cell proliferation is E2-induced. Thus, when cells enter the cell cycle in both mammary gland and uterus, ERα expression is down-regulated. ERα expression in the uterine stroma was up-regulated by E2 (data not shown). However, in the mammary gland, very few ERα-positive stromal cells were found even after E2 treatment. The postulated mechanism of E2-stimulated growth via an indirect pathway, i.e., stimulation of growth factor release from stroma, may apply to the uterus but does not seem to apply to the mammary gland. Unlike ERα, ERβ protein was up-regulated by E2, whereas it was reduced by tamoxifen treatment. Induction of ERβ by E2 has been found in certain brain regions where ERα is thought to regulate ERβ levels (12).
If the data in this paper are of general applicability to proliferation in the mammary gland, i.e., that the presence of ERα is indicative of a nonproliferating cell, one question that arises immediately is why there is so much nuclear ERα in breast cancer and why an antiestrogen blocks proliferation. One obvious response to this question is that ERα is not down-regulated in breast cancer. Evidence that this is the case was recently presented. Henrich et al. (42) found that extracellular signal-regulated kinase 7 (ERK7) is involved in the degradation of ERα, and that there is loss of ERK7 in breast cancer. Furthermore, if ERα is not down-regulated, the cell becomes more responsive to E2. Thus in breast cancer, particularly in ductal grade 1 (38), there is a high level of nuclear ERα and an enhanced sensitivity to the proliferative effects of E2. This is why tamoxifen is so effective in ERα-positive breast cancer.
It is well known that progesterone inhibits proliferation and promotes differentiation in the uterus, whereas in the mammary gland, it induces proliferation. PR-A inhibits proliferation in the uterus, but this action appears to be specific for the uterus and is not observed in the mammary gland (43). PR-B can enhance, rather than inhibit, uterine epithelial cell proliferation (44, 45), and it is the mediator of progesterone-induced proliferation in the mammary gland (46). In the present study, we found that there was an increase in nuclear PR staining in epithelial cells in the mammary gland after E2 treatment, whereas in the uterus, where most of the cells were labeled with BrdUrd, both ERα and PR were lost from epithelium (data not shown here). Thus, in response to E2, the regulation of PR in epithelial cells in mammary gland is different from that in the uterus, whereas the regulation of ERα is similar. The PR regulation observed in the mammary gland in the present study is consistent with the results from Shyamala (3) but not with those from Raafat (47). In both ERα-/- and ERβ-/- mice after ovariectomy, proliferation of mammary epithelial cells could be achieved by estrogen-progesterone treatment, but not by progesterone alone (1, 38). This suggests that induction of PR in the mammary gland can be mediated by both ERα and ERβ.
The main results of this study are that (i) in mice, ERα is mainly expressed in epithelial cells, whereas ERβ is expressed in both epithelial and stromal cells; (ii) both ERα and ERβ in mammary epithelial cells can elicit proliferation; (iii) ERα is rapidly lost from the nuclei of epithelial cells on E2 treatment, but it returns in 24 h; (iv) ERα is not found in cells while they are synthesizing DNA but is expressed in BrdUrd-labeled daughter cells; (v) ERβ but not ERα is expressed in the periductal stroma and therefore may be responsible for E2-induced stromal proliferation; (vi) ERβ is expressed in the proliferating cells; and (vii) ERβ is up-regulated by E2 treatment but down-regulated by tamoxifen. The down-regulation of ERβ by tamoxifen has not been reported previously, but it might be an important consideration in the treatment of breast cancer. Because the ERβ-tamoxifen complex can have effects at activator protein 1 sites that are opposite to those of the ERα-tamoxifen complex, there is the distinct possibility that the presence of ERβ in breast cancer might result in tamoxifen-induced proliferation. Down-regulation of ERβ by tamoxifen would eliminate this unwanted effect. We have previously reported that there is an up-regulation of ERβ in tamoxifen-resistant breast cancer (28). If ERβ is responsible for tamoxifen-mediated proliferation in tamoxifen-resistant breast cancer, this may be one condition where an ERβ antagonist may be of clinical relevance.
Acknowledgments
The patient and skillful assistance of Christina Thulin-Andersson, AnnMarie Witte, and Patricia Humiere is gratefully acknowledged. This research was supported by grants from the Swedish Cancer Fund and by KaroBio.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 30, 2002.
Abbreviations: PR, progesterone receptor; BAG, ERβ agonist; E2, ovarian estradiol.
See accompanying Biography on page 3737.
References
- 1.Bocchinfuso, W. P., Lindzey, J. K., Hewitt, S. C., Clark, J. A., Myers, P. H., Cooper, R. & Korach, K. S. (2000) Endocrinology 141, 2982-2994. [DOI] [PubMed] [Google Scholar]
- 2.Olsson, H., Jernstrom, H., Alm, P., Kreipe, H., Ingvar, C., Jonsson, P. E. & Ryden, S. (1996) Breast Cancer Res. Treat. 40, 187-196. [DOI] [PubMed] [Google Scholar]
- 3.Shyamala, G., Chou, Y. C., Louie, S. G., Guzman, R. C., Smith, G. H. & Nandi, S. (2002) J. Steroid Biochem. Mol. Biol. 80, 137-148. [DOI] [PubMed] [Google Scholar]
- 4.Zeps, N., Bentel, J. M., Papadimitriou, J. M. & Dawkins, H. J. (1999) J. Histochem. Cytochem. 47, 1323-1330. [DOI] [PubMed] [Google Scholar]
- 5.Dorssers, L. C., Van der Flier, S., Brinkman, A., van Agthoven, T., Veldscholte, J., Berns, E. M., Klijn, J. G., Beex, L. V. & Foekens, J. A. (2001) Drugs 61, 1721-1733. [DOI] [PubMed] [Google Scholar]
- 6.Russo, J., Ao, X., Grill, C. & Russo, I. H. (1999) Breast Cancer Res. Treat. 53, 217-227. [DOI] [PubMed] [Google Scholar]
- 7.Saji, S., Jensen, E. V., Nilsson, S., Rylander, T., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soderqvist, G. (1998) Ann. Med. 30, 511-524. [PubMed] [Google Scholar]
- 9.Shoker, B. S., Jarvis, C., Clarke, R. B., Anderson, E., Hewlett, J., Davies, M. P., Sibson, D. R. & Sloane, J. P. (1999) Am. J. Pathol. 155, 1811-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoker, B. S., Jarvis, C., Sibson, D. R., Walker, C. & Sloane, J. P. (1999) J. Pathol. 188, 237-244. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, R. B., Howell, A., Potten, C. S. & Anderson, E. (1997) Cancer Res. 57, 4987-4991. [PubMed] [Google Scholar]
- 12.Mueller, S. O., Clark, J. A., Myers, P. H. & Korach, K. S. (2002) Endocrinology 143, 2357-2365. [DOI] [PubMed] [Google Scholar]
- 13.Koerner, F., Oyama, T., Kurosumi, M. & Maluf, H. (2001) J. Steroid Biochem. Mol. Biol. 78, 285-290. [DOI] [PubMed] [Google Scholar]
- 14.Haslam, S. Z. & Woodward, T. L. (2003) Breast Cancer Res. 5, 208-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivaraman, L., Hilsenbeck, S. G., Zhong, L., Gay, J., Conneely, O. M., Medina, D. & O'Malley, B. W. (2001) J. Endocrinol. 171, 75-83. [DOI] [PubMed] [Google Scholar]
- 16.Graham, J. D. & Clarke, C. L. (1997) Endocr. Rev. 18, 502-519. [DOI] [PubMed] [Google Scholar]
- 17.Clarke, C. L. & Sutherland, R. L. (1990) Endocr. Rev. 11, 266-301. [DOI] [PubMed] [Google Scholar]
- 18.Yang, J., Guzman, R. & Nandi, S. (2001) In Vivo 15, 239-244. [PubMed] [Google Scholar]
- 19.Fata, J. E., Chaudhary, V. & Khokha, R. (2001) Biol. Reprod. 65, 680-688. [DOI] [PubMed] [Google Scholar]
- 20.Hovey, R. C., Trott, J. F. & Vonderhaar, B. K. (2002) J. Mammary Gland. Biol. Neoplasia 7, 17-38. [DOI] [PubMed] [Google Scholar]
- 21.Dunbar, M. E. & Wysolmerski, J. J. (2001) Microsc. Res. Technol. 52, 163-170. [DOI] [PubMed] [Google Scholar]
- 22.Fendrick, J. L., Raafat, A. M. & Haslam, S. Z. (1998) J. Mamm. Gland Biol. Neoplasia 3, 7-22. [DOI] [PubMed] [Google Scholar]
- 23.Silberstein, G. B., Van Horn, K., Shyamala, G. & Daniel, C. W. (1994) Endocrinology 134, 84-90. [DOI] [PubMed] [Google Scholar]
- 24.Couse, J. F. & Korach, K. S. (1999) Endocr. Rev. 20, 358-417. [DOI] [PubMed] [Google Scholar]
- 25.Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J.-Å. & Smithies, O. (1998) Proc. Natl. Acad. Sci. USA 95, 15677-15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couse, J. F., Yates, M. M., Walker, V. R. & Korach, K. S. (2003) Mol. Endocrinol. 17, 1039-1053. [DOI] [PubMed] [Google Scholar]
- 27.Speirs, V., Skliris, G. P., Burdall, S. E. & Carder, P. J. (2002) J. Clin. Pathol. 55, 371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen, E. V., Cheng, G., Palmieri, C., Saji, S., Makela, S., Van Noorden, S., Wahlstrom, T., Warner, M., Coombes, R. C. & Gustafsson, J.-Å. (2001) Proc. Natl. Acad. Sci. USA 98, 15197-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng, G., Weihua, Z., Makinen, S., Makela, S., Saji, S., Warner, M., Gustafsson, J.-Å. & Hovatta, O. (2002) Biol. Reprod. 66, 77-84. [DOI] [PubMed] [Google Scholar]
- 30.Weihua, Z., Makela, S., Andersson, L. C., Salmi, S., Saji, S., Webster, J. I., Jensen, E. V., Nilsson, S., Warner, M. & Gustafsson, J.-Å. (2001) Proc. Natl. Acad. Sci. USA 98, 6330-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J.-Å. (1996) Proc. Natl. Acad. Sci. USA 93, 5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson, S., Makela, S., Treuter, E., Tujague, M., Thomsen, J., Andersson, G., Enmark, E., Pettersson, K., Warner, M. & Gustafsson, J.-Å. (2001) Physiol. Rev. 81, 1535-1565. [DOI] [PubMed] [Google Scholar]
- 33.Pettersson, K. & Gustafsson, J.-Å. (2001) Annu. Rev. Physiol. 63, 165-192. [DOI] [PubMed] [Google Scholar]
- 34.Webb, P., Nguyen, P., Valentine, C., Lopez, G. N., Kwok, G. R., McInerney, E., Katzenellenbogen, B. S., Enmark, E., Gustafsson, J.-Å., Nilsson, S., et al. (1999) Mol. Endocrinol. 13, 1672-1685. [DOI] [PubMed] [Google Scholar]
- 35.Liu, M. M., Albanese, C., Anderson, C. M., Hilty, K., Webb, P., Uht, R. M., Price, R. H., Jr., Pestell, R. G. & Kushner, P. J. (2002) J. Biol. Chem. 277, 24353-24360. [DOI] [PubMed] [Google Scholar]
- 36.Speirs, V. (2002) J. Pathol. 197, 143-147. [DOI] [PubMed] [Google Scholar]
- 37.Gustafsson, J.-Å. & Warner, M. (2000) J. Steroid Biochem. Mol. Biol. 74, 245-248. [DOI] [PubMed] [Google Scholar]
- 38.Palmieri, C., Cheng, G. J., Saji, S., Zelada-Hedman, M., Warri, A., Weihua, Z., Van Noorden, S., Wahlstrom, T., Coombes, R. C., Warner, M., et al. (2002) Endocr. Relat. Cancer 9, 1-13. [DOI] [PubMed] [Google Scholar]
- 39.Weihua, Z., Saji, S., Makinen, S., Cheng, G., Jensen, E. V., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 5936-5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weihua, Z., Ekman, J., Almkvist, A., Saji, S., Wang, L., Warner, M. & Gustafsson, J.-Å. (2002) Biol. Reprod. 67, 616-623. [DOI] [PubMed] [Google Scholar]
- 41.Giamarchi, C., Chailleux, C., Callige, M., Rochaix, P., Trouche, D. & Richard-Foy, H. (2002) Biochim. Biophys. Acta 1578, 12-20. [DOI] [PubMed] [Google Scholar]
- 42.Henrich, L. M., Smith, J. A., Kitt, D., Errington, T. M., Nguyen, B., Traish, A. M. & Lannigan, D. A. (2003) Mol. Cell. Biol. 23, 5979-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conneely, O. M., Mulac-Jericevic, B., DeMayo, F., Lydon, J. P. & O'Malley, B. W. (2002) Recent Prog. Horm. Res. 57, 339-355. [DOI] [PubMed] [Google Scholar]
- 44.Conneely, O. M., Mulac-Jericevic, B., Lydon, J. P. & De Mayo, F. J. (2001) Mol. Cell Endocrinol. 179, 97-103. [DOI] [PubMed] [Google Scholar]
- 45.Mulac-Jericevic, B., Mullinax, R. A., DeMayo, F. J., Lydon, J. P. & Conneely, O. M. (2000) Science 289, 1751-1754. [DOI] [PubMed] [Google Scholar]
- 46.Mulac-Jericevic, B., Lydon, J. P., DeMayo, F. J. & Conneely, O. M. (2003) Proc. Natl. Acad. Sci. USA 100, 9744-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raafat, A. M., Hofseth, L. J., Li, S., Bennett, J. M. & Haslam, S. Z. (1999) Endocrinology 140, 2570-2580. [DOI] [PubMed] [Google Scholar]