Abstract
The kidneys play a major role in glucose homeostasis through its utilization, gluconeogenesis, and reabsorption via sodium glucose cotransporters (SGLTs). The defective renal glucose handling from upregulation of SGLTs, mainly the SGLT2, plays a fundamental role in the pathogenesis of type 2 diabetes mellitus. Genetic mutations in a SGLT2 isoform that results in benign renal glycosuria, as well as clinical studies with SGLT2 inhibitors in type 2 diabetes support the potential of this approach. These studies indicate that inducing glycosuria by suppressing SGLT2 can reduce plasma glucose and A1c levels, as well as decrease weight, resulting in improved β-cell function and enhanced insulin sensitivity in liver and muscle. Because the mechanism of SGLT2 inhibition is independent of insulin secretion and sensitivity, these agents can be combined with other antidiabetic agents, including exogenous insulin. This class represents a novel therapeutic approach with potential for the treatment of both type 2 and type 1 diabetes.
Keywords: Glucose homeostasis, glycosuria, renal glucose handling, Sodium–glucose co-transporter 2 inhibitors, sodium glucose cotransporters
INTRODUCTION
Diabetes has prevailed through many civilizations as a devastating and deadly disease before the discovery of insulin and still an incurable disease. Insulin resistance in muscles, liver and adipose tissue has been long considered as the fundamental pathology in type 2 diabetes mellitus (T2DM).[1] Besides the contribution of kidney in glucose homeostasis through gluconeogenesis and glucose utilization, abnormal renal glucose reabsorption is yet another key abnormality in pathogenesis of T2DM, defined as the septicidal septet in the ominous octet by Ralph. DeFronzo in his Banting Lecture. The other seven players in the ominous octet are muscle, liver, fat cells, pancreatic β-cells, pancreatic α-cells, gastrointestinal tract, and brain.[2] The sodium glucose cotransporter (SGLT) 2 and SGLT1 are the sites of reclamation of all the filtered glucose, which are maladaptive in the kidneys of type 2 diabetics. SGLT2 inhibitors are the emerging treatment targeting this defective renal glucose reabsorption.[3,4]
THE FATE OF FILTERED GLUCOSE IN KIDNEY
The major role of kidney in human physiology is to maintain intravascular volume and acid-base and electrolyte balance through filtration, secretion and selective reabsorption of vital minerals sodium, potassium, and chloride; hydrogen and bicarbonate ions. Glucose is also filtered and reabsorbed in similar fashion in order to retain energy essential for physiological functioning between meals. With a daily glomerular filtration rate of 180 L, approximately 162 g (180 L/day × 90 mg/dL) of glucose must be reabsorbed each day to maintain a normal fasting plasma glucose concentration of 5.6 mmol/L (101 mg/dL). Reabsorption of glucose occurs mainly in the proximal tubule and is mediated by 2 different transport proteins, SGLT1 and SGLT2. SGLT1, which are found in the straight section of the proximal tubule (S3), are responsible for approximately 10% of glucose reabsorption. The other 90% of filtered glucose is reabsorbed through by SGLT2, which are located in the convoluted section on the proximal tubule (S1)[5] [Table 1].
Table 1.
A comparison of selected characteristics of SGLT1 and SGLT2

The amount of glucose reabsorbed by the kidneys is equivalent to the amount entering the filtration system. The reabsorption increases with increase in glucose concentration up to approximately 11 mmol/L (198 mg/dL). At this threshold, the system becomes saturated and the maximal resabsorption rate, the glucose transport maximum (TmG), is reached. No more glucose can be absorbed, and the kidneys begin excreting it in the urine, the beginning of glycosuria.[6,7] The TmG varies among individuals but it has an average value of approximately 375 mg/min for healthy subjects. Although 11 mmol/L (198 mg/dL) represents the theoretical threshold glucose concentration, the actual concentration varies due to nephron heterogeneity, resulting in slight differences in actual glucose reabsorption levels and TmG values between individual tubules. Thus, the actual threshold is not a single point but a curve, where excretion begins to occur at plasma glucose levels of ~10 mmol/L (180 mg/dL), and increases more gradually rather than abruptly. As the reabsorption approaches the TmG, it tails off and becomes parallel to the glucose concentration threshold. The difference between the actual and theoretical TmG creates the splay[6,7,8] [Figure 1].
Figure 1.

TmG, Transport maximum reabsorption of glucose. N, Normal plasma glucose concentration; T, plasma (renal) threshold
The SGLT2 are located on the luminal side of the early proximal tubule S1 segment. Absorption of sodium across the cell membrane creates an energy gradient that in turn allows glucose to be absorbed. On the other side of the cell, sodium is reabsorbed through sodium-potassium ATPase pump into the bloodstream. The concentration gradient within the cell, resulting from this exchange drives glucose reabsorption into the bloodstream via the Glucose transporter (GLUT) 2 [Figure 2]. The GLUT2 is present at many different sites including red blood cells, brain, and other tissues and is therefore not an appropriate site for pharmacological intervention. In contrast, SGLT2 is specific to the proximal tubule, so that pharmacological inhibition will affect glucose reabsorption in the kidney but not in other tissues[9] [Figure 2, Table 1].
Figure 2.
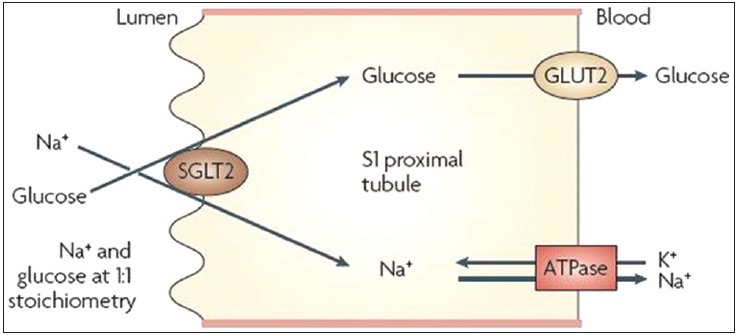
Sodium–glucose co-transporter 2 (SGLT2) catalyses the active transport of glucose (against a concentration gradient) across the luminal membrane by coupling it with the downhill transport of Na+. The inward Na+ gradient across the luminal epithelium is maintained by active extrusion of Na+ across the basolateral (anti-luminal) surface into the intercellular fluid, which is in equilibrium with the blood. Glucose passively diffuses out of the cell down a concentration gradient by basolateral facilitative transporters: Glucose transporter type 2 (GLUT2) and GLUT1. Adapted with permission from Ref[10]
Familial renal glycosuria is a benign genetic disorder worth mentioning in this discussion, as it serves as a model for the effects of SGLT2 inhibition. Patients with this condition are asymptomatic but have impaired functioning of their SGLT2 proteins and they excrete between a few to 100 g/day of glucose in their urine. These individuals are otherwise normal. Blood glucose concentration is usually within the normal range, and blood volume remains essentially normal due to sodium reabsorption through other channels. Kidney and bladder function remain unaffected, and they do not show any increased tendency towards kidney disease, diabetes, or urinary tract infections (UTI).[5,11]
Renal glucose handling in diabetes
Conservation of glucose through renal reabsorption is an adaptive response to help meet energy needs between meals. This process unfortunately becomes maladaptive in diabetes, and glycosuria is not observed until the plasma glucose concentration is substantially higher than 11 mmol/l, the glucose concentration threshold in nondiabetics. Increased SGLT2 transporters maintain the elevated plasma glucose concentration, instead of allowing the kidney to excrete the excess filtered glucose in the urine and correct the hyperglycemia.[7] The renal glucose reabsorption may be augmented in absolute terms by an increase in the renal Tm for glucose. In both type 2 and type 1 diabetes, the TmG is increased by approximately 20%.[12,13] Human exfoliated proximal tubular epithelial cells (HEPTECs), which can be isolated from urine, have been used to study the expression of a variety of proximal tubular markers, including SGLT2. In a study, HEPTECs isolated from individuals with normal glucose tolerance (NGT) and T2DM were cultured in a hyperglycemic environment. The cells from T2DM patients expressed significantly more SGLT2 and GLUT2 proteins than cells from NGT individuals. In addition, renal glucose uptake, measured using the glucose analogue methyl-α-D-[U14C]-glucopyranoside, a nonmetabolizable glucose analogue, was significantly greater in the T2DM HEPTECs than the NGT cells.[14] These results suggest that chronic hyperglycemia upregulates SGLT2/GLUT2 transport expression and activity.
THE PROSPECT OF SGLT2 INHIBITION
Diabetes is a highly prevalent disease affecting more than 150 million people worldwide.[15] The 20-year follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) has shown that improved blood glucose control in newly diagnosed patients significantly decreases the long-term risk of both microvascular and macrovascular complications.[16] In contrast, three large trials of intensive glucose control in patients with long duration diabetes have failed to show a macrovascular benefit.[17,18,19] All these studies point towards the importance of early therapeutic intervention to preserve β-cell function, increase insulin sensitivity and prevent vascular complications.[20] The current agents for T2DM are often limited by their potential to induce significant adverse effects. Moreover, glycemic control can be difficult to attain, even with a combination of multiple oral agents, and with insulin added. Long-term blood glucose control becomes difficult when the treatment is accompanied by weight gain during the therapeutic process.[21] The poor glycemic control leaves diabetics susceptible to developing both microvascular and macrovascular complications that increase morbidity and mortality. The search for new therapeutic agents with different mechanisms of action will ever continue, as novel pathophysiologic mechanisms responsible for T2DM are being understood. As kidney plays a key role in regulating glucose levels through SGLTs, blocking SGLT2 has emerged, in the past few years, as a potential therapeutic option in the treatment of diabetes.
SGLT2 inhibition increases glucose excretion rate by decreasing TmG. In normal animals, SGLT2 inhibition has no effect on plasma glucose concentration, because the liver increases glucose production to compensate for glycosuria. In diabetic animals, however, administration of an SGLT2 inhibitor produces both dose-dependent glycosuria and a significant reduction in plasma glucose concentrations.[7] Inhibition of SGLT2 transport resets the system by lowering the threshold for glycosuria [Figure 3], eventually correcting the hyperglycemia. This decreases insulin resistance in muscle by augmenting insulin signalling, GLUT4 and glycogen synthase activity.[23,24] In the liver, correction of hyperglycemia decreases glucose- 6-phosphatase and phospho enol pyruvate carboxykinase activity, which results in decreased gluconeogenesis and total hepatic glucose production, with a resulting decrease in fasting plasma glucose levels.[25,26] Correction of hyperglycemia reverses the glucotoxicity and also improves β ?cell function-cell function.[23,27] As glycosuria results in loss of calories through urine, SGLT2 inhibition would be expected to cause weight loss and eventually increase insulin sensitivity. Since SGLT2 inhibitors block transcellular glucose flux in renal proximal tubular cells and therefore reduces intracellular oxidative stress by preventing activation of cytokines and advanced glycation products, they may help in preventing the development of albuminuria, mesangial expansion, and progression of diabetic nephropathy.[28] The diuretic effect observed with SGLT2 inhibition may be useful in controlling hypertension, an associated finding in patients with T2DM. SGLT2 inhibitors also induce weight loss, which can be effective in the treatment of obesity and metabolic syndrome. Although the early weight loss is due to mild osmotic diuresis, the long-term weight reduction is due to a reduction of the fat mass, which is attributed to the loss of energy through glycosuria. This class of drugs may be useful in type 1 diabetes as well, as the mechanism of action is independent of insulin, both secretion and sensitivity.[29]
Figure 3.
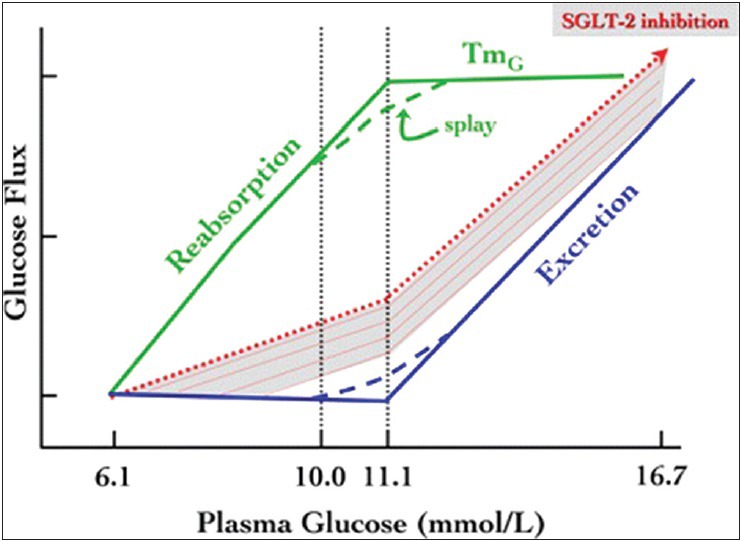
Renal glucose handling. Scheme of normal renal glucose handling, with reabsorption (in green) being complete, and excretion nil (in blue), up to a splayed threshold of plasma glucose concentration. The dotted red line and the underlying shaded area represent the hypothetical displacement of the excretion function when glucose reabsorption is reduced by SGLT2 inhibition. TmG, maximal transport rate for glucose. Adapted with permission from Ref[22]
Although failing to get Food and Drug Administration (FDA) approval, dapagliflozin, among the first of these compounds is currently the most widely studied SGLT2 inhibitor in phase III trial, therefore discussion of effects of SGLT2 inhibition is mostly based on its study. In the Zucker diabetic fatty rats study, treatment with dapagliflozin induced renal glucose excretion and significant decrease in FPG in both normal and diabetic rats.[30] The same study also evaluated insulin-stimulated glucose disposal and hepatic glucose output. The insulin-stimulated glucose disposal significantly increased after 2 weeks of treatment with dapagliflozin, while the hepatic glucose output significantly decreased (P < 0.01 versus controls for both).[30] In a phase II, randomized, double-blind, placebo-controlled, dose-ranging study, 12 weeks of dapagliflozin treatment significantly reduced HbA1c in patients with T2DM with baseline HbA1c ranging from 7.7% to 8.0%. Placebo-subtracted HbA1c reductions ranged from 0.5% to 0.7% and were similar to that achieved with metformin XR (−0.6%; however, no statistical comparisons were made among the active treatments).[3] The same study also demonstrated significant improvements in a variety of metabolic parameters. Dapagliflozin increased mean glycosuria by 52-85 g/day, which in turn reduced mean FPG by 16-30 mg/dL (0.9-1.7 mmol/L) and mean postprandial glucose by 23-29 mg/dL (1.3-1.6 mmol/L). Mean body weight also declined by 2.2-3.2 kg compared with a 1.7 kg loss in the metformin group. A small increases in urine volume of 107-470 mL per day also occurred.[3] In a 52-week study by Nauck et al. comparing the efficacy of dapagliflozin and glipizide in metformin-treated patients with T2DM and a starting HbA1c of 7.7%, the decrement in the HbA1c was identical (−0.52%) in both treatment groups. Dapagliflozin-treated subjects lost on average 3.2 kg, while glipizide-treated subjects gained 1.4 kg (P < 0.0001).[31] Wilding et al. randomized insulin-treated patients with T2DM who were also receiving an insulin sensitizer (metformin and/or thiazolidinedione) to add on therapy with dapagliflozin or placebo. Although the insulin dose was reduced by 50% at the start of therapy (the insulin sensitizer dose was unchanged), after 12 weeks of dapagliflozin therapy, the HbA1c declined by 0.70-0.78% (P < 0.01 vs. placebo). The placebo-subtracted reductions in body weight were 2.6 and 2.4 kg, respectively (P < 0.01 vs. placebo). Both the increase in glycosuria and reduction in insulin dose could have contributed to the weight loss in dapagliflozin-treated subjects.[32] Zhang et al. compared late stage (diabetes duration = 11 years) and early stage (diabetes duration = 1 year) patients with T2DM randomly assigned to receive of dapagliflozin for 12 weeks. The decline in HbA1c (0.5-0.7% vs. 0.6-0.8%, respectively) was similar in late and early stage patients with diabetes.[29] This is explained by the unique mechanism of action of dapagliflozin on the kidney that is independent of the severity of insulin resistance or β-cell failure.
THE FUTURE OF SGLT2 INHIBITORS
Initial evidence that augmenting renal glucose excretion could lead to improved glycemic control has come from animal studies using phlorizin, a molecule originally isolated from the bark of apple tree, which inhibits both the SGLT2/1 (and therefore has not been studied for use in humans). In partially pancreatectomized diabetic rats, phlorizin induced glycosuria and normalized plasma glucose concentrations. Withdrawal of phlorizin was associated with a return to the diabetic state.[33,34] Subsequently many selective SGLT2 inhibitors like Dapagliflozin, Canagliflozin, Sergliflozin, Remogliflozin, ASP-1941, BI-10773 and BI-44847 are in development and ongoing trials. Dapagliflozin is approximately 1,200 times more selective for SGLT2 over SGLT1. An in vitro study revealed that dapagliflozin exhibited around 30 times greater potency against SGLT2 in humans than phlorizin, and approximately 4 fold less potency versus phlorizin against human SGLT1.[29,30] It has demonstrated promise as monotherapy and as synergistic combination therapy with the currently available agents.[35] It has also shown to reduce total body weight, predominantly by reducing fat mass, visceral and subcutaneous adipose tissue in inadequately controlled T2DM; and therefore improve glycemic control, stabilize insulin dosing, and reduce weight without increasing major hypoglycemic episodes.[36,37] Prevention of obesity-associated hyperglycemia, improved glucose intolerance, and increased glucose-stimulated insulin secretion support SGLT2 inhibition as a viable insulin-independent treatment and prevention of T2DM, and perhaps T1DM.[38,39] However, the safety issue remains the most important parameter determining the future of any drug in development. By virtue of their nature, SGLT2 inhibitors cause glycosuria that can lead to urinary tract and genital infections, electrolyte imbalances, and increased urinary frequency. The most frequently reported adverse events in phase II and III trials include constipation, diarrhea, nausea, urinary frequency, and genitourinary infections involving UTIs and vulvovaginal infections.[3] Although dapagliflozin appeared to be safe and well tolerated in trials until recently, it has not yet attained FDA approval, due to unanswered questions regarding safety. In July, 2011, an FDA advisory committee voted against approving dapagliflozin citing rates of breast and bladder cancer in the treatment arm.[40] The U.S. Food and Drug Administration has recently approved Canagliflozin, used with diet and exercise, to improve glycemic control in adults with type 2 diabetes.[41] Canagliflozin treatment improved glycemic control, reduced body weight and was generally well tolerated in subjects with T2DM inadequately controlled with diet and exercise.[42] A randomized, placebo-controlled study has shown that Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion.[43] Canagliflozin has also improved glycemic control and was generally well tolerated in subjects with T2DM and Stage 3 CKD.[44]
SUMMARY
The flame of hope was lit at the discovery of insulin. As a symbol of hope, the flame will burn until a cure for diabetes is found. We are still not in a position to predict when or even if the Flame of hope could ever be extinguished. Far from cure, the disease is still difficult to treat efficiently in the long run. However, with discovery of new pathogenesis in diabetes, hope and possibility of treatments arise. Understanding kidney as a major organ in glucose homeostasis has given rise to a new paradigm for intervention. Increased renal glucose reabsorption through SGLT2 upregulation has recently been identified in type 2 diabetes. SGLT2 inhibition revisits the various unmet needs of diabetes treatment through a novel mechanism independent of insulin pathways. This unique mechanism of action complements those of other antidiabetic agents, making them suitable for combination therapy. It promotes weight loss by increasing glycosuria and stimulates breakdown of fat cells for fuel. Because their function is independent of insulin, SGLT2 inhibitors do not increase the risk of hypoglycemia, a major concern with other diabetes treatment. The improvements in weight and glycemia achieved with SGLT2 inhibition will also reduce cardiovascular risk. Many SGLT2 inhibitors are in clinical trials and Canagliflozin has recently passed through the safety issues and has become the first one to be FDA approved, We can hope more SGLT2 inhibitors to come up with both efficacy and safety in the future. As the flame of hope continues, hope for diabetes will also continue.
ACKNOWLEDGMENT
I would like to express my gratitude to Prof. PK Shrestha, and my friends who helped me to complete this work. I have furthermore to thank Dr. Sanjay Kalra who gives me constant encouragement to write.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.DeFronzo RA. Lilly lecture 1987.The triumvirate: Beta-cell, muscle, liver: A collusion responsible for NIDDM. Diabetes. 1988;37:667–8. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 2.Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose co-transport inhibition with dapagliflozin in type 2 diabetes mellitus. Diabetes Care. 2009;32:650–7. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447:510–8. doi: 10.1007/s00424-003-1063-6. [DOI] [PubMed] [Google Scholar]
- 5.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 6.Ganong WF. Renal function and micturition. In: Ganong WF, editor. Review of medical physiology. 21st ed. New York: McGraw-Hill Companies; 2003. pp. 702–30. [Google Scholar]
- 7.Abdul-Ghani MA. Inhibition of renal glucose absorption: A novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract. 2008;14:782–90. doi: 10.4158/EP.14.6.782. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: A new path towards normalizingglycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 9.Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- 10.Chao EC, Henry RR. SGLT2 inhibition: A novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–9. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 11.Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol. 2003;14:2873–82. doi: 10.1097/01.asn.0000092790.89332.d2. [DOI] [PubMed] [Google Scholar]
- 12.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest. 1951;30:125–9. doi: 10.1172/JCI102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest. 1971;28:101–9. doi: 10.3109/00365517109090668. [DOI] [PubMed] [Google Scholar]
- 14.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–34. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 15.Mohler ML, He Y, Wu Z, Hwang DJ, Miller DD. Recent andemerging anti-diabetes targets. Med Res Rev. 2009;29:125–95. doi: 10.1002/med.20142. [DOI] [PubMed] [Google Scholar]
- 16.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. UKPDS 80. N Engl J Med. 2008;359:1565–76. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 19.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 20.Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia. 2009;52:1219–26. doi: 10.1007/s00125-009-1352-5. [DOI] [PubMed] [Google Scholar]
- 21.Katsuno K, Fujimori Y, Ishikawa-Takemura Y, Isaji M. Long-term treatment with sergliflozinetabonate improves disturbed glucose metabolism in KK-A (y) mice. Eur J Pharmacol. 2009;618:98–104. doi: 10.1016/j.ejphar.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Ferrannini E. Sodium–glucosetransporter-2 inhibition as an antidiabetic therapy. Nephrol Dial Transplant. 2010;25:2041–3. doi: 10.1093/ndt/gfq249. [DOI] [PubMed] [Google Scholar]
- 23.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–30. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 24.Kahn BB, Rossetti L, Lodish HF, Charron MJ. Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest. 1991;87:2197–206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mevorach M, Giacca A, Aharon Y, Hawkins M, Shamoon H, Rossetti L. Regulation of endogenous glucose production by glucose per se is impaired in type 2 diabetes mellitus. J Clin Invest. 1998;102:744–53. doi: 10.1172/JCI2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oku A, Ueta K, Arakawa K, Ishihara T, Nawano M, Kuronuma Y, et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes. 1999;48:1794–800. doi: 10.2337/diabetes.48.9.1794. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka K, Kuzuya T, Akanuma Y, Hagura R. Increase in insulin response after treatment of overt maturity-onset diabetes is independent of the mode of treatment. Diabetologia. 1980;18:23–8. doi: 10.1007/BF01228297. [DOI] [PubMed] [Google Scholar]
- 28.Arakawa K, Ishihara T, Oku A, Nawano M, Ueta K, Kitamura K, et al. Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na(+)-glucose cotransporter inhibitor T-1095. Br J Pharmacol. 2001;132:578–86. doi: 10.1038/sj.bjp.0703829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: Effects on glycaemic control and body weight. Diabetes Obes Metab. 2010;12:510–6. doi: 10.1111/j.1463-1326.2010.01216.x. [DOI] [PubMed] [Google Scholar]
- 30.Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57:1723–9. doi: 10.2337/db07-1472. [DOI] [PubMed] [Google Scholar]
- 31.Nauck M, Del Prato S, Rohwedder K, Elze M, Parikh S. Dapagliflozin vs glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin: 52-week results of a double-blind, randomised, controlled trial. Diabetologia. 2010;53:S1–556. [Google Scholar]
- 32.Wilding JP, Norwood P, T’Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: Applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–62. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–5. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: A review. Diabetes Metab Res Rev. 2005;21:31–8. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 35.Anderson SL, Marrs JC. Dapagliflozin for the treatment of type 2 diabetes. Ann Pharmacother. 2012;46:590–8. doi: 10.1345/aph.1Q538. [DOI] [PubMed] [Google Scholar]
- 36.Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–31. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 37.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Dapagliflozin 006 Study Group. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: A randomized trial. Ann Intern Med. 2012;156:405–15. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 38.Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, et al. SGLT2 deletion improves glucose homeostasis andpreserves pancreatic beta-cell function. Diabetes. 2011;60:890–8. doi: 10.2337/db10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao EC. A paradigm shift in diabetes therapy: Dapagliflozin and other SGLT2 inhibitors. Discov Med. 2011;11:255–63. [PubMed] [Google Scholar]
- 40.Burki TK. FDA rejects novel diabetes drug over safety fears. Lancet. 2012;379:507. doi: 10.1016/s0140-6736(12)60216-5. [DOI] [PubMed] [Google Scholar]
- 41.Liscinsky M. Fda.gov [Internet] Silver Spring (MD): U.S. Food and Drug Administration; c2010. [updated 2013 Mar 29, cited 2013 Apr 2]. FDA approves Invokana to treat type 2 diabetes: First in a new class of diabetes drugs. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345848.htm . [Google Scholar]
- 42.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–82. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, et al. Canagliflozin Lowers Postprandial Glucose and Insulin by Delaying Intestinal Glucose Absorption in Addition to Increasing Urinary Glucose Excretion: Results of a randomized, placebo-controlled study. Diabetes Care. 2013 Feb 14; doi: 10.2337/dc12-2391. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]


