Abstract
B lymphocytes contribute to physiological immunity through organogenesis of secondary lymphoid organs, presentation of antigen to T cells, production of antibodies, and secretion of cytokines. Their role in several autoimmune diseases, mainly as producers of pathogenic antibodies, is also well known. However, certain subsets of B cells are emerging as the important regulatory cell populations in both mouse and human. The regulatory functions of B cells have been demonstrated in a variety of mouse models of autoimmune diseases including collagen-induced arthritis (CIA), experiment autoimmune encephalomyelitis (EAE), anterior chamber-associated immune deviation (ACAID), diabetes, contact hypersensitivity (CHS), and intestinal mucosal inflammation. Accumulating evidence from both mouse and human studies confirms the existence of regulatory B cells, and is beginning to define their mechanisms of action. In this article, we first review the history of B cells with regulatory function in autoimmune diseases, and summarize the current understanding about the characterizations of such B-cell subsets. We then discuss the possible regulatory mechanisms of B cells, and specifically define the role of regulatory B cells in immune homeostasis in the intestine.
Keywords: Regulatory B cells, immunoregulation, autoimmune diseases, molecular mechanism, mucosal homeostasis
History of regulatory B cells in autoimmune diseases
In 1974, Katz et al. [1] provided the first evidence that B cells might harbor the ability of immunoregulation. They found that depletion of B cells from splenocyte preparation abolished their ability of inhibiting inflammatory reaction in the skin of the recipient mice. Their pioneer studies introduced the concept of suppressive B cells in immunoregulation of T-cell function. Since then, B cells with regulatory functions have been demonstrated in a variety of mouse models of autoimmune disease (Figure 1), suggesting that in parallel with the occurrence of autoimmune T- and B-cell reactivity in host, certain B cells with regulatory function are also generated as a compensatory mechanism to inhibit immune-mediated inflammation. A few reviews have summarized the current knowledge of regulatory B cells [2–4]. In this article, we add the latest advancement in research of regulatory B cells, and discuss the clinical implications from mouse models to human diseases.
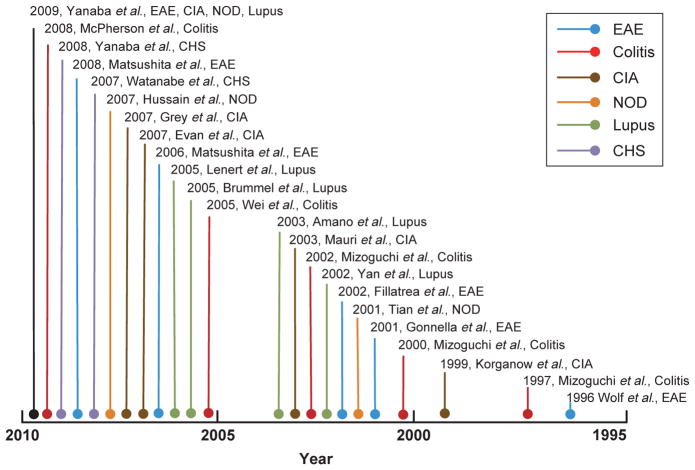
Figure 1.
A summary of the featured publications from 1995 to present on regulatory B cells in autoimmune diseases. The scheme indicates the trend of increased publications on the regulatory function of B cells in a variety of mouse model of autoimmune diseases during the past 15 years.
Collagen-induced arthritis
CIA is a murine model mimicking the immunopathogenesis of human rheumatoid arthritis [5]. Chronic arthritis develops after immunization of DBA/1-TcR-β-Tg mice with type II collagen in complete Freund’s adjuvant. Although B cells are required for the disease initiation and progression [6,7], regulatory B cells have also been identified in CIA [8–10].
Mauri et al. [8] observed the in vitro activation of splenic arthritogenic B cells, with CD40 monoclonal antibody (mAb) and collagen resulted in an increased IL-10 production. Transfer of these B cells into CIA mice inhibited T helper cell type 1 (Th1) cell differentiation, prevented arthritis development, and displayed therapeutic effects on the established disease. A major IL-10-producing B subset, marginal zone (MZ) B cell, and its precursor, transitional stage 2 (T2-MZP) B cell, were increased during the remission phase of arthritis. Adoptive transfer of T2-MZP B cells to the CIA mice significantly prevented disease development and ameliorated established disease [9]. The suppressive effects on arthritis were paralleled by an inhibition of antigen (Ag)-specific T-cell activation and a reduction in cells exhibiting Th1 type of immune responses. The authors further demonstrated that this regulatory B subset displayed its suppression through the secretion of suppressive cytokines, but not by cell–cell contact.
Gray et al. [10] reported that administration of apoptotic cells (AC) could protect mice from autoimmune joint inflammation by induction of regulatory B cells. AC treatment increased the production of IL-10 by activated splenic B cells. Also, passive transfer of B cells from AC-treated mice provided significant protection from CIA. The IL-10-producing B cells were able to skew the cytokine profile of effector T cells toward an immunosuppressive phenotype [10]. These data demonstrate that AC exert profound influence on adaptive immune response by acting as endogenous Ags through the generation of IL-10-producing regulatory B cells, which in turn are able to influence T-cell functioning. Although the mechanism about how AC induce regulatory B cells remains unclear, it reveals the possibility that breakdown of this negative feedback loop may contribute to the pathogenesis of autoimmunity.
Experimental autoimmune encephalomyelitis
Experimental autoimmune encephalomyelitis (EAE) in mouse is an autoimmune CD4+ T-cell-mediated inflammatory disease affecting the central nervous system with clinical symptoms similar to multiple sclerosis (MS) in human [11]. Whether B cell plays a protective or pathological role in EAE or MS has been a matter of debate. Although B-cell depletion with rituximab (anti-CD20 mAb) has shown therapeutic effects in patients with relapsing–remitting MS [12], more and more evidence suggests that the B cells may also carry out protective functions.
Wolf and colleagues induced acute EAE in μMT (B-cell-deficient) mice with myelin oligodendrocyte glycoprotein peptide to test whether the absence of B cells was capable of preventing the induction of the pathogenic autoimmune responses [13]. Unexpectedly, μMT developed much more severe disease, suggesting that B cells negatively regulated inflammatory response in EAE. Following this study, Gonnella and co-workers [14] found that the major difference in EAE process between the μMT and wild-type (WT) mice was characterized by different cytokine profiles in the gut-associated lymphoid tissue (GALT). An upregulation of B-cell-derived IL-4, IL-10, and TGF-β was detected in WT but not in μMT mice both in vivo and in vitro. The importance of B-cell-derived IL-10 was further confirmed by an adoptive transfer study [15]. Specifically, the adoptive transfer of WT B cells, but not that of IL-10−/− B cells, normalized EAE severity in μMT mice [15].
Accumulating evidence suggested that B cells might play distinguished roles in different disease stages of EAE development. Indeed, transfer of regulatory B cells was maximally effective during early EAE initiation, but had no obvious effect in late stage. On the contrary, B-cell depletion during EAE disease progression dramatically suppressed symptoms [16]. These results demonstrate dynamic regulatory roles for B cells in EAE initiation and progression, which indicates that the timing of B-cell depletion treatment is critical in clinics.
Non-obese diabetes
Non-obese diabetes (NOD) mice have a polygenic susceptibility to spontaneous development of autoimmune, type I diabetes [17], which is a Th1-mediated autoimmune disease. Although B cells are a necessary component for the initiation of spontaneous T-cell autoimmunity to β cells by presenting Ags in NOD mice, a regulatory role of activated B cells in the autoimmune process has also been observed.
Lipopolysaccharide (LPS)-activated B cells have an increased expression of Fas ligand and secretion of TGF-β [18]. Transfusion of activated B cells, but not untreated control B cells, into pre-diabetic NOD mice inhibited spontaneous Th1 autoimmunity. Co-transfer of activated B cells with diabetogenic splenic T cells prevented the type I diabetes mellitus (T1DM) in NOD mice. In a separate study, Hussain and colleagues found an increased production of IL-10 by spleen B cells upon B-cell receptor (BCR) stimulation. Transfusion of those IL-10-producing B cells significantly delayed disease onset as well as incidence of disease in an IL-10-dependent manner in NOD mice [19]. However, the same treatment of mice at later stage only delayed the disease onset, but had no effect on the occurrence of disease.
Therefore, it is speculated that in NOD mice, there are multiple subsets of regulatory B cells that develop through the stimulation of different signaling pathways and also exhibit different functional phenotypes. One important possibility raised by these studies is that intravenous transfusion of regulatory B cells may be used therapeutically to protect human subjects at risk for T1DM. Preventative treatment before disease onset is critical for the protective effect.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by auto-Ab production associated with a wide range of clinical manifestations. Patients with SLE and murine lupus models generate high titers of affinity-matured, isotype-switched auto-Abs featured as T-cell-dependent immune responses. An important target of autoimmune responses of the disease is a group of small nuclear ribonucleoprotein particles (snRNP) designated U1 and Sm [20,21].
The first evidence of the existence of regulatory B cells in SLE was from Yan and co-workers [22], who demonstrated that anti-snRNP B cells from normal mice tolerated T cells in the periphery and did not secrete auto-Ab. The accelerated development of SLE in BXSB male mice is associated with the presence of a mutant gene, Yaa (Y-linked autoimmune acceleration). To explore whether the Yaa mutation would affect the differentiation of B cells, thereby contributing to the acceleration of the disease, Amano and co-workers showed that both BXSB and C57BL/6 Yaa mutant mice displayed an impaired development of MZ B cells early in life. The lack of selective expansion of MZ B cells in BXSB Yaa lupus mice strongly argues against a major role of MZ B cells in the generation of pathogenic auto-Abs in the BXSB model of SLE, but rather a suppressive role in disease progression [23].
Recent studies have shown that CpG oligodeox-ynucleotides (ODNs) can stimulate a subset of B cells from lupus mice to increase their IL-10 secretion [24,25]. Vice versa, the abnormal IL-10 response in lupus mice could be down-regulated with inhibitory ODNs [24,25]. In conclusion, B cells have dual functions in the pathogenesis of lupus, and their functions are decided by the surrounding environments. They promote disease progression by presenting self-Ags as antigen-presenting cells (APC), and by producing auto-Ab as effector cells. Meanwhile, when encountered by specific innate stimulations, they exert suppressive role by secreting IL-10.
Characterization of regulatory B cells
Studies with animal models as described above have suggested that B cells may play different roles in the development of autoimmune diseases. Although some B cells are pathogenic at certain stage of disease process, some of them may also be protective. The origins of regulatory B cells and how they develop remain unclear. Using IL-10 transcriptional reporter mice, Madan et al. [26] found an unexpected predominance of B cells among IL-10-expressing cells in peripheral lymphoid tissues in both naïve and immune system-activated mice, suggesting that negative regulation is a general property of B cells induced as a consequence of normal B-cell activation.
Identification of regulatory B cells
Distinguished phenotypes of regulatory B cells have been described in numerous studies. It is most likely that regulatory B cells have multiple subsets with defined phenotypes and biological functions.
Peritoneal CD5+ B-1a cells are known to produce IL-10 [27,28]. Spleen CD5+ B cells also produce IL-10 after IL-12 stimulation, whereas CD5−B cells do not [29]. Specifically, spleen B cells with a CD21+ CD23− MZ phenotype from lupus mice can produce IL-10 in response to CpG stimulation [25]. Spleen CD1dhiCD21+ CD23+IgM+ B cells with a T2-MZP phenotype also produce IL-10 and can inhibit CIA [9]. More recently, the IL-10-producing CD1dhiCD5+ B cells (B10) in the spleen identified by Matsushita et al. [16] appear to share some phenotypic markers with both CD1dhiCD21+ CD23+ T2-MZP B cells and CD5+ CD19hiB220lo B-1a cells. However, the frequency of B10 cells in WT mice was significantly lower than the frequencies of MZ B cells in the spleen. It is still debatable whether B10 cells represent a novel subset of functional B cells or just different activated state of MZ or B-1a cells.
Conversely, a B-2-like phenotype (CD5− CD11b− IgD+) of IL-10-producing regulatory B cells, which were only detectable after IL-7 stimulation, was also documented [4]. IL-7 favors the development of B-2 B cell but not B-1 B subset. Consistent with the above finding, Booth and co-workers newly identified that a population derived from CD5− CD11c− CD21+ B cells in Peyer’s patches (PPs) spontaneously secretes high levels of IL-10. Neutralization of the IL-10 or depletion of CD21+ B cells results in a significant increase in CpG-induced interferon response in PPs, suggesting that IL-10 from B cells negatively regulates innate responses in PPs. These IL-10-secreting PP B cells may represent a novel subset of the recently proposed regulatory B cells in the intestine [30].
Activation signaling for regulatory B cells
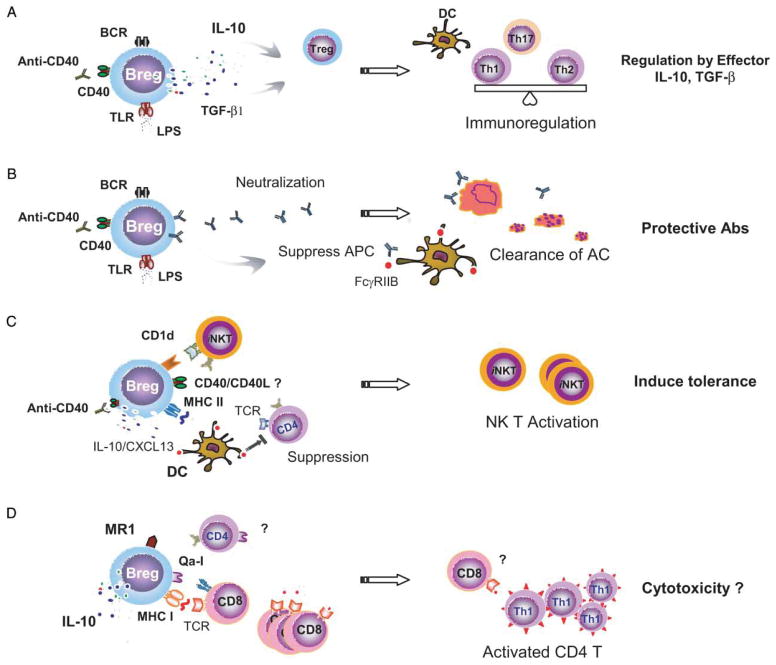
The pathways in generating such regulatory B-cell population have been summarized in an earlier review [3]. Three pathways appear to be crucial for this process: signals from the BCR, signals from CD40, and signals from toll-like receptors (TLR) (Figure 2). The involvement of all these three pathways in the development of regulatory B cells raises a question: how do the non-specific activating signals trigger the specific regulatory function of B cells? A few models have been proposed to address this question. Mizoguchi and Bhan postulated that activation of follicular B cells through BCR and CD40 by ligation with self-Ags leads to the development of a population of regulatory B cells, whereas activation of MZ B cells through TLR pathways by ligation with bacterial products matures a different population of regulatory B cells [4]. Fillatreau and co-workers proposed that the suppressive function of B cells is determined by its condition of activation and, in particular, by the different types of TLR agonists available [3].
Figure 2.
Regulatory mechanisms of B cells in immunoregulation. (A) B-cell-derived effector molecules, IL-10 and TGF-β that are produced in response to the stimulations via CD40, TLRs, or BCR, play important roles in suppressing inflammatory T cells. (B) Antibodies may participate in protective immunoregulation through several pathways by binding to FcγRIIB on DC and suppressing APC function, or neutralizing self-reactive Ags or labeling AC to diminish autoimmune responses. (C) Regulatory B cells suppress APC function by producing IL-10 or CXCL13, or down-regulating CD4+ T-cell responses via engagement with TCR expression on effector CD4+ T cells. Regulatory B cells can also activate iNKT cells through an increased CD1d expression and up-regulate iNKT-cell function and induce immune tolerance. (D) Regulatory B cells from MALT present antigenic peptides through MHC I or non-classic MHC I molecules, Qa-1 or MR1, to mediate the expansion of cytolytic CD8+ T cells in the intestine which monitor the activated CD4+ T cells and suppress mucosal inflammation.
In addition to BCR, CD40, and TLR signaling, the role of CD19, a B-cell-specific surface molecule that defines BCR signaling thresholds, has also been found important in the development of regulatory B cells in various autoimmune models [31–33]. CD19-deficient mice are hyposensitive to a variety of transmembrane signals. However, deficiency of CD19 results in an increased and prolonged inflammatory reaction in mice prone to autoimmune diseases, suggesting an overall anti-inflammatory role of CD19. Overexpression of CD19 expanded the population of regulatory B cells in CHS mice suggesting that CD19 plays an important role in the developmental stage of regulatory B cells [33], potentially through BCR signaling. However, adoptive transfer experiments revealed that CD19 expression in recipient mice is required for optimal suppression of inflammatory response in the CHS model, indicating another possible role of CD19 that it might also be important for the elicitation phase of the suppressor functions [32].
Interestingly, the effector cytokine IL-10 of regulatory B cells is also required in the generation of the regulatory B cells [8]. B cells cultured with Ag and anti-CD40 in the presence of neutralizing anti-IL-10R fail to exert the regulatory functions in recipient mice with CIA [8]. It is not known what the sources of the IL-10 are in the development of regulatory B cells. It is possible that CD4+ T cells regulate B cells via the release of soluble cytokines, or B cells have a positive feedback system.
Molecular mechanisms of regulatory B cells
It has been shown that regulatory B cells can modulate a variety of immune cell populations, including CD4+ effector T cells (Th1/Th2 response), regulatory T cells (FOXP3+ T cells, CD8+ T cells, and invariant NKT cells (iNKT)), and dendritic cells (DCs). By studying murine models of autoimmune diseases, several effector and interaction molecules have been demonstrated to participate in B-cell-mediated immune regulations. Here, we briefly summarize the recent understandings in the suppressive mechanisms mediated by regulatory B cells (Figure 2).
Regulation by effector molecules
Role of IL-10
The role of B-cell-derived IL-10 in autoimmune diseases has been nicely summarized and reviewed by Aja Rieger and Fillatreau [3,34]. In general, IL-10 suppresses both Th1 and Th2 polarization, and inhibits Ag presentation and pro-inflammatory cytokine production by monocytes and macrophages. It also has potent activity in limiting DC function in secreting IL-6 and IL-12, and thereby inhibits Th17 cells [35]. Furthermore, a transient reduction in naturally occurring FOXP3+ regulatory T cells can be induced in mice when B cells cannot secrete IL-10, suggesting that B-cell-derived IL-10 is required for the induction of regulatory T cells.
B-cell-derived IL-10 is essential for the regulatory function of B cells in most autoimmune disease models, as B-cell population isolated from IL-10 knockout mice failed to mediate the protective function of regulatory B cells in CIA [8], EAE [15], NOD [19], and inflammatory bowel diseases (IBD) [36].
Role of TGF-β
Additionally, several B-cell subsets have been found to be capable of producing TGF-β in different disease models [13,18,37,38]. TGF-β is known to be a multifunctional cytokine, which exerts its anti-inflammatory function by inducing apoptosis in the effector cells, suppressing cytokine production of T cells, and inhibiting functions of APC. It also serves as a potent negative regulator of mucosal inflammation in the intestine [37].
Wolf and colleagues [13] first suggested that regulatory B cells might suppress inflammatory responses by secreting TGF-β in addition to IL-10. Tian and colleagues showed that LPS-activated B cells specifically inhibited spontaneous Th1 autoimmunity in pre-diabetic NOD mice by secreting TGF-β [18]. Their study proved that activated B cells could down-regulate pathogenic inflammatory response through the induction of the apoptosis in Th1 cells and/or inhibition of Ag presentation activity in APC by the secretion of TGF-β. Similarly, Parekh and co-workers discovered that B cells activated by LPS, but not by anti-Ig and anti-CD40 Ab, induced energy in CD8+ T cells by the expression of TGF-β but not by IL-10 [38].
Role of anti-inflammatory Ab
B cells are known for their ability to produce Abs. Evidence has been provided that Ab not only can amplify the inflammatory response in early stages of the immune response to an infectious agent or in the development of autoimmune disease, but also down-regulate the inflammatory response. [39–43]. Consistent with this concept, administration of passive Ab was associated with reversal of the inflammatory response of B-cell-deficient mice to pro-inflammatory conditions [39,41,44].
Although it is not fully understood how and which Ab isotope functions as a regulator in autoimmunity, an appealing observation is that IgG is elevated under multiple inflammatory conditions [44]. IgG has the capability of binding to inhibitory receptor FcγRIIB on DC, which provides an important mechanism to maintain immune tolerance [45,46]. Administration of IgG has been noted to have beneficial effects in Ab-mediated autoimmune diseases [47]. Taken together, B cells might control inflammatory responses in certain situations by the production of specific IgG, which might exert an anti-inflammatory effect by promoting clearance of microbial Ags, down-regulating pro-inflammatory cytokine secretion by effector cells, promoting release of anti-inflammatory cytokines, and inducing DC tolerance [48] (Figure 2).
Regulation by cell–cell interaction
Role of CD1d
The CD1 glycoproteins are family members of major histocompatibility complex (MHC) class I-like molecules conserved in mammalian species [49]. Although human expresses multiple CD1 molecules including CD1a, b, c, d, and e, CD1d is the only molecule in mice and rats that expressed widely on cells of multiple hematopoietic lineages, including B and T cells, macrophages, and DCs. CD1d presents lipid/glycolipid Ags of host or microbial origin to a subset of T cells, iNKT cells, which is an important innate-like lymphocyte population in immune system [50–56]. Deficiency of CD1d-reactive iNKT cells has been associated with the pathogenesis of autoimmune diseases.
Intriguingly, several studies have characterized regulatory B-cell subsets with high expression of CD1d [28,33,57], suggesting a potential regulatory mechanism that involved regulatory B cells and iNKT cell interaction through CD1d. Study by Mizoguchi et al has shown that the upregulation of CD1d on GALT B cells is associated with the B-cell-mediated protection against intestinal mucosal inflammation [28]. CD1d and iNKT cell interaction participated in the suppression of intestinal inflammatory progression, as CD1d-deficient TCRα double-knockout mice developed colitis spontaneously [28]. Sonoda and Stein-Streilein also concluded that the same anti-inflammatory role of CD1d and iNKT cell interaction using an immune tolerance model was associated with anterior chamber-associated immune deviation [57].
Role of MR1
Like CD1, MR1 is a non-polymorphic MHC class Ib molecule [58–60], probably specialized for lipid Ag presentation [61–63]. T cells specific for MR1 (mucosal-associated invariant T, MAIT) reside primarily in the gut lamina propria, and their post-thymic expansion depends on both commensal microbiota and B lymphocytes [64–66]. MAIT cells are oligoclonal, predominated by Vα19 and Vα7.2-Jα33 in mouse and human, respectively [62–64,66,67]. Studies thus far indicate that MAIT cells include distinct sets of cytokine-producing subpopulations [63,64,67], and are associated with human MS lesions [67]. Thus, while their effector and regulatory roles are just emerging, MAIT cells represent a highly B-cell-dependent population of T cells, likely to participate in mucosal homeostasis and autoimmunity.
Role of Qa-1/HLA-E
Qa-1 is another MHC class Ib molecule expressed by B cells, DC, and T cells in mouse [68]. Its equivalent molecule in human is HLA-E. Qa-1 mediates an important immuno-regulatory function in suppression of activated CD4+ T cells by a subset of Qa-1-restricted invariant CD8+ T cells [69]. Qa-1-restricted CD8+ T cells control autoimmune response and protect mice from the induction of EAE [70]. In our studies of mice bearing an altered microbial community, RF microbiota caused expansion of CD8+ T cells and depletion of selective immune cell types –plasmacytoid DCs (pDC) and MZ B-cell subsets via a cytolytic CD8+ T-cell mechanism [71,72].
The role of Qa-1 on B cells or other APC such as pDC was particularly interesting, as it demonstrated that enteric microbiota acted by inducing CD8+ invariant Qa-1 T cells, which target a conserved nonapeptide encoded by the host stress molecule Hsp60, and by GroEL-related prokaryote homologues expressed by certain enteric bacteria. Further investigation into this concept would establish some mechanistic links between enteric microbiota, mucosal immunoregulation in the intestine, and the pathological biology of IBD (Figure 3).
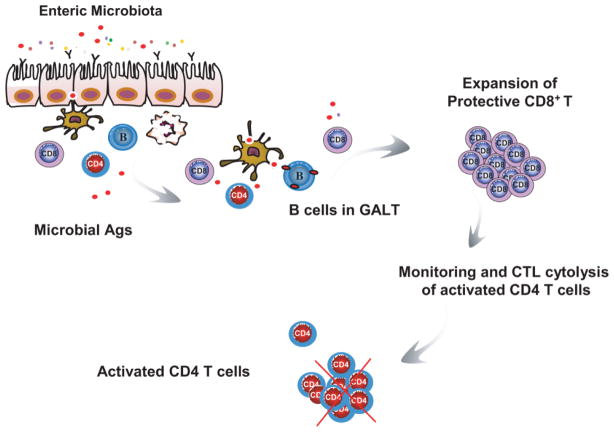
Figure 3.
Proposed model of regulatory B-cell-mediated immunoregulation in mucosal homeostasis. Regulatory B cells from mucosal-associated lymphoid tissue induce the expansion of protective CD8+ T cells in the intestine by presenting antigenic peptides from enteric microbiota or self-stress responses in an immunogenic setting. These expanded CD8+ T cells are cytolytic and play a role of monitoring immune responses in the intestine by directly targeting and killing activated CD4+ T cells. If validated, this concept would establish mechanistic links between bacterial commensalism, featured intestinal mucosal immune system, and the biology of IBD susceptibility.
Role of CD40/CD40L
CD40 is a member of the tumor necrosis factor superfamily expressing mainly on B cells and DCs as well as on other cell populations [73]. The interaction between CD40 on B cells and CD40L (CD154) on activated T cells is important for the activation and differentiation of both B cells and T cells and maintenance of immune tolerance [74]. As described above, signal through CD40 is required for the development of regulatory B cells. In addition, CD40 may also be involved in the regulatory mechanisms of B cells. First, B cells themselves not only express CD40, but also CD40L, allowing possible B-cell intrinsic control of IL-10 production. Indeed, the production of IL-10 is restricted to CD40L+ B cells and correlates with their CD40L expression levels in the EAE model [33,75].
Second, CD40L is also expressed on CD1d or MR1-restricted invariant T cells and effector T cells, in which CD1d and MR1-restricted invariant T cells convey protection against autoimmune diseases in mice [76–78]. Therefore, it is possible that regulatory B cells can directly regulate their functioning through CD40/CD40L engagement. Moreover, B cells suppress spontaneous chronic colonic inflammation by inhibiting proliferation of effector T cells via CD40/CD40L interaction [36].
However, it is worth to mention that we have observed some controversial results in a co-transfer model system of Gαi2−/− CD4+ T cells and regulatory B cells from mesenteric lymph nodes (MLN). Gαi2 protein is one subunit of the trimeric G protein family of signaling molecules involved in the regulation of cellular physiological function. Deficiency of Gαi2 in mouse results in spontaneous mucosal inflammation characterized by polarized Th1 reactivity [79,80] and increased Th17 cells [81].
Transfer of isolated CD4+ T cells from colitis-prone Gαi2−/− mice to immunodeficient Rag II−/− mice induces severe mucosal inflammation with clinical symptoms similar to Gαi2−/− mice. Notably, IL-10-producing B cells (T2-MZP B- and B-1a cells) are also selectively deficient in Gαi2−/− mice [82]. This and later studies [83] have demonstrated that B cells play an important immune regulatory role in mucosal homeostasis and IBD resistance. Co-transfer of regulatory MLN B cells with Gαi2−/− CD4+ T cells in immunodeficient mice allows us to address the mechanisms of B-cell-mediated immunoregulation. In this T- and B-cell co-transfer system, MLN B cells from CD40−/− mice could still provide protection against colitigenic CD4+ T-cell-induced mucosal inflammation [84].
CD40−/− MLN B cells were equally effective as WT MLN B cells in suppression of colitigenic Gαi2−/− CD4+ T-cell expansion and their production of pro-inflammatory cytokines as well as mucosal damages in recipient mice. These findings indicate that regulatory B-cell-mediated protection did not require CD40 expression that is typically required for B cells to interact with CD4+ T cells. The sufficiency of B cells to regulate disease without co-stimulatory molecules such as CD40 also suggests that B cells may not regulate colitis through production of immunoglobulin in this model since Ab production by B cells is a CD40-dependent process.
Regulatory role of B cells in mucosal immune homeostasis
Gastrointestinal tract is one of the most complex functional systems in human. Mucosal immune homeostasis in the intestine involves the dynamic interaction of enteric microbiota, the intestinal epithelium, and the mucosal immune system. Dysregulation of mucosal lymphocyte homeostasis, particularly the reactivity of CD4+ T cells against commensal microbiota, has been identified as the major pathogenic features in mucosal inflammatory disorders in the intestine. The mouse models of IBD have been employed to investigate the host–microbe interactions at intestinal mucosal interfaces and the regulatory mechanisms in mucosal immune homeostasis due to their unique biological features involving microbiota, mucosal barrier integrity, and immune regulation.
Regulatory CD4+ T cells and a variety of mucosal-associated regulatory T-cell populations have been the focus regarding to the immune regulation in mucosal homeostasis, partly due to their capability of producing TGF-β and IL-10 [85]. However, a series of studies by Atul Bhan group have revealed a role for B cells in suppressing Th2 T-cell-mediated mucosal inflammation in TCRα−/− mice [28,86]. The protective function of B cells is dependent on their capacity to express CD1d and produce IL-10 [44]. Similarly, in transfer models of colitis induced by colitigenic Th1 or Th17 cells (CD4+ CD45RBhi T or Gαi2−/− T cells as described above), we have demonstrated that B cells from MLN conferred protection which also requires the IL-10 sufficiency [81,83].
However, an impressive observation from our studies was that the transferred protective B cells did not undergo proliferation and expansion during the colitis protection. We actually rarely observed the significant expansion of protective B cells in the recipients, particularly in the GALTs including intra-epithelium, lamina propria, and MLN (data not shown). Instead, B cells provided protection to CD4+ T-cell-induced mucosal inflammation through the induction of CD8+ T-cell population systemically and locally in the intestines. This surprising finding prompts us to address the cellular mechanism of B cells in their regulatory responses to the development of mucosal inflammation.
In TCR-α−/− mice, B-cell protection was independent of MHC II on B cells, suggesting the absence of cognate interaction with CD4+ T cells. In mouse models of colitis induced by transferring colitigenic CD4+ CD45RBhi T and Gαi2−/− T cells, we have observed that B-cell protection required the presence of CD8+ T cells [81]. Regulatory MLN B cells participate in intestinal immunoregulation by recruiting mucosal-associated CD8+ T cells. These findings point to a protective mechanism in mucosal inflammation distinct from regulatory CD4+ T cells. Notably, CD8+ T cells are the predominant immune cell population in the intestine. Human studies have identified regulatory CD8+ T cells induced by intestinal epithelial cells and MHC class Ib molecules, and defects in this interaction are a feature of some patients with IBD [87,88]. Therefore, we further used genetic approaches to examine the requirements for B-cell immunoregulation in Gαi2−/− T-cell-transferred colitis model.
Analysis of the genetic requirements for B cells and CD8+ T cells in the immune regulation of CD4+ T-cell inflammation has indicated that the protective function of B cells in Gαi2−/− T-cell inflammation requires genetic sufficiency of IL-10, MHC I, and TAP1, but B cells deficient in B7.1/B7.2, CD40, MHC II (Abb), or native BCR (MD4) were competent for colitis protection [84]. Notably, TAP-deficient B cells failed to protect, suggesting a requirement for peptide MHC I presentation. CD8+ T cells deficient in native TCR repertoire (OT-1) or defective in cytolysis (perforin−/−) also failed to confer protection. These findings reveal an integrated role for Ag-specific and perforin-dependent CD8+ Immunor-egulation T-cell cytotoxicity in colitis immunoregulation, and its efficient induction by a subset of mucosal B lymphocytes.
These results suggest that B cells function primarily as class I APC to induce Ag-specific CD8+ regulatory T cells that act via a cytotoxic mechanism to participate in the immunoregulation. The presence of such a regulatory mechanism is also supported by the work from Malin and co-workers [89]. They observed that both impairment of B-cell activation and decrease in CD8+ T cells was associated with the exacerbation of chronic intestinal inflammation observed in Gαi2−/− mice. Therefore, we believe that regulatory B cells also serve as the mediators, interacting with certain regulatory CD8+ T-cell subsets to control immune responses.
Taken together, we have observed a partnership relationship between regulatory B cells and CD8+T-cell subsets from a serial of studies. We have understood that MLNs regulatory B cells, mainly the B cells from MLNs characterized as CD19hiIgMhiCD1dhi, co-ordinate with CD8+ T cell to perform their protective function. Nevertheless, (i) Not all the B cells are protective. Similar to regulatory T cells, only certain subset of B cells can modulate the pathogenic CD4+ T-cell-mediated mucosal and systemic inflammation and provide protection. (ii) In our experimental system, we have demonstrated that B cells from MLNs mediate protection to colitigenic CD4+ T-cell-induced mucosal inflammation. Regulatory B cells co-ordinate with regulatory CD8+ T cells to play an important role in mucosal immune homeostasis. (iii) Our work also indicates that cytotoxicity is a protective trait of regulatory CD8+ T cells in the intestine.
Conclusions and future directions
Much evidence now establishes that B cells contribute to the regulation of immune effector function. There appear to be two functional modes of such regulation: (a) direct regulation by B cells polarized for direct expression of secreted or cell interaction regulatory products; and (b) indirect regulation by B cells bearing MHC class Ib (CD1, Qa-1, and possibly MR1) which promote expansion and activation of MHC-cognate regulatory T cells. Several important and interesting mechanistic and translational questions lie ahead. For directly regulatory B cells, we need to understand what developmental pathway(s) lead to their formation, in what settings do they substantially contribute to immune homeostasis, and by what genetic or environmental factors may their deficiency contribute to chronic or autoimmune inflammatory disease. For B cells interacting with MHC class Ib-restricted regulatory T cells, what local or developmental processes augment their MHC Ib and Ag uptake/loading, why do they predominate over other APC types, and in what human disease settings does their activity promote (or deficiency impair) control of chronic inflammation and prevention of autoimmu-nity? The resolution of these issues will provide new perspectives on the pathways of B-cell development, and new modes of immune regulation that might provide targets for the prevention and treatment of diverse chronic inflammatory diseases.
Acknowledgments
This study was supported by grants from the Crohn’s and Colitis Foundation of America, and NIH PO1-DK4673 and RO1-DK069434.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251(5475):550. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- 2.Bouaziz J-D, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224(1):201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 3.Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8(5):391. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- 4.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176(2):705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 5.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korganow A-S, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali J-L, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10(4):451. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 7.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: Arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179(2):1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 8.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JG, Chavez-Rueda KV, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 10.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104(35):14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauro CDC, Roger WM, Byung SK, Stephen DM. Two models of multiple sclerosis: Experimental allergic encepha-lomyelitis (EAE) and theiler’s murine encephalomyelitis virus (TMEV) infection. A pathological and immunological comparison. Microsc Res Tech. 1995;32(3):215–229. doi: 10.1002/jemt.1070320305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH HERMES Trial Group. B-cell depletion with Rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 13.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184(6):2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonnella PA, Waldner HP, Weiner HL. B cell-deficient ({micro}MT) mice have alterations in the cytokine micro-environment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J Immunol. 2001;166(7):4456–4464. doi: 10.4049/jimmunol.166.7.4456. [DOI] [PubMed] [Google Scholar]
- 15.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118(10):3420. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Ann Rev Immunol. 2005;23(1):447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 18.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167(2):1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 19.Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol. 2007;179(11):7225–7232. doi: 10.4049/jimmunol.179.11.7225. [DOI] [PubMed] [Google Scholar]
- 20.Pettersson I, Hinterberger M, Mimori T, Gottlieb E, Steitz JA. The structure of mammalian small nuclear ribonucleopro-teins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984;259(9):5907–5914. [PubMed] [Google Scholar]
- 21.Fatenejad S, Brooks W, Schwartz A, Craft J. Pattern of anti-small nuclear ribonucleoprotein antibodies in MRL/Mp-lpr/lpr mice suggests that the intact U1 snRNP particle is their autoimmunogenic target. J Immunol. 1994;152(11):5523–5531. [PubMed] [Google Scholar]
- 22.Yan J, Mamula MJ. B and T cell tolerance and autoimmunity in autoantibody transgenic mice. Int Immunol. 2002;14(8):963–971. doi: 10.1093/intimm/dxf064. [DOI] [PubMed] [Google Scholar]
- 23.Amano H, Amano E, Moll T, Marinkovic D, Ibnou-Zekri N, Martinez-Soria E, Semac I, Wirth T, Nitschke L, Izui S. The Yaa mutation promoting murine lupus causes defective development of marginal zone B cells. J Immunol. 2003;170(5):2293–2301. doi: 10.4049/jimmunol.170.5.2293. [DOI] [PubMed] [Google Scholar]
- 24.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25(1):29. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 25.Brummel R, Lenert P. Activation of marginal zone B cells from lupus mice with type A(D) CpG-oligodeoxynucleotides. J Immunol. 2005;174(4):2429–2434. doi: 10.4049/jimmunol.174.4.2429. [DOI] [PubMed] [Google Scholar]
- 26.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183(4):2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1(6):475. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 28.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 29.Spencer NF, Daynes RA. IL-12 directly stimulates expression of IL-10 by CD5+ B cells and IL-6 by both CD5+ and CD5− B cells: Possible involvement in age-associated cytokine dysregulation. Int Immunol. 1997;9(5):745–754. doi: 10.1093/intimm/9.5.745. [DOI] [PubMed] [Google Scholar]
- 30.Booth JS, Griebel PJ, Babiuk LA, Mutwiri GK. A novel regulatory B-cell population in sheep Peyer’s patches spontaneously secretes IL-10 and downregulates TLR9-induced IFN{alpha} responses. Mucosal Immunol. 2009;2(3):265. doi: 10.1038/mi.2009.6. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, Sato S. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomye-litis by regulating cytokine response. Am J Pathol. 2006;168(3):812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, Yazawa N, Okochi H, Sato S, Tedder TF, Tamaki K. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007;171(2):560–570. doi: 10.2353/ajpath.2007.061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Riegert A, Bar-Or A. B-cell-derived interleukin-10 in autoimmune disease: Regulating the regulators. Nat Rev Immunol. 2008;8(6):486. doi: 10.1038/nri2315-c1. [DOI] [PubMed] [Google Scholar]
- 35.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: Increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192(4):475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol. 2000;12(5):597–605. doi: 10.1093/intimm/12.5.597. [DOI] [PubMed] [Google Scholar]
- 37.Takenoshita S, Fukushima T, Kumamoto K, Iwadate M. The role of TGF-β in digestive organ disease. J Gastroenterol. 2002;37(12):991. doi: 10.1007/s005350200168. [DOI] [PubMed] [Google Scholar]
- 38.Parekh VV, Prasad DVR, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: Role of TGF-{beta}1. J Immunol. 2003;170(12):5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 39.Deshpande SP, Zheng M, Daheshia M, Rouse BT. Pathogenesis of Herpes Simplex virus-induced ocular immunoinflam-matory lesions in B-cell-deficient mice. J Virol. 2000;74(8):3517–3524. doi: 10.1128/jvi.74.8.3517-3524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-{gamma}, TNF-{alpha}, and inducible nitric oxide synthase. J Immunol. 2000;164(5):2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 41.Smelt SC, Cotterell SEJ, Engwerda CR, Kaye PM. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164(7):3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 42.Buendia AJ, Del Rio L, Ortega N, Sanchez J, Gallego MC, Caro MR, Navarro JA, Cuello F, Salinas J. B-cell-deficient mice show an exacerbated inflammatory response in a model of Chlamydophila abortus infection. Infect Immun. 2002;70(12):6911–6918. doi: 10.1128/IAI.70.12.6911-6918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77(4):2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor {alpha} mutant mice. J Exp Med. 1997;186(10):1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fc {gamma} receptors on dendritic cells. J Exp Med. 2002;195(12):1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196(2):217–227. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siragam V, Brinc D, Crow AR, Song S, Freedman J, Lazarus AH. Can antibodies with specificity for soluble antigens mimic the therapeutic effects of intravenous IgG in the treatment of autoimmune disease? J Clin Invest. 2005;115(1):155. doi: 10.1172/JCI22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casadevall A, Pirofski L-A. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24(9):474. doi: 10.1016/s1471-4906(03)00228-x. [DOI] [PubMed] [Google Scholar]
- 49.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Ann Rev Immunol. 2004;22(1):817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald HR. NK1.1+ T cell receptor-alpha/beta+ cells: New clues to their origin, specificity, and function. J Exp Med. 1995;182(3):633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283(5399):225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 52.Chiu Y-H, Park S-H, Benlagha K, Forestier C, Jayawardena-Wolf J, Savage PB, Teyton L, Bendelac A. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nat Immunol. 2002;3(1):55. doi: 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- 53.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu Y-P, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 54.Kinjo Y, Wu D, Kim G, Xing G-W, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong C-H, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 55.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 56.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 57.Koh-Hei S, Joan S-S. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. Eur J Immunol. 2002;32(3):848–857. doi: 10.1002/1521-4141(200203)32:3<848::AID-IMMU848>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 58.Miley MJ, Truscott SM, Yu YYL, Gilfillan S, Fremont DH, Hansen TH, Lybarger L. Biochemical features of the MHC-related protein 1 consistent with an immunological function. J Immunol. 2003;170(12):6090–6098. doi: 10.4049/jimmunol.170.12.6090. [DOI] [PubMed] [Google Scholar]
- 59.Parra-Cuadrado J, del Moral M, García-Pavía P, Setién F, Martínez-Naves E. Characterization of the MHC class I-related MR1 locus in nonhuman primates. Immunogenetics. 2001;53(8):643. doi: 10.1007/s00251-001-0380-1. [DOI] [PubMed] [Google Scholar]
- 60.Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol. 1998;161(8):4066–4077. [PubMed] [Google Scholar]
- 61.Hansen TH, Huang S, Arnold PL, Fremont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8(6):563. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- 62.Schümann J, De Libero G. MR1-restricted Valpha19i T cells -a second population recognizing lipid antigens? Eur J Immunol. 2007;37(7):1724–1726. doi: 10.1002/eji.200737509. [DOI] [PubMed] [Google Scholar]
- 63.Shimamura M, Huang Y-Y, Okamoto N, Suzuki N, Yasuoka J, Morita K, Nishiyama A, Amano Y, Mishina T. Modulation of Valpha19 NKT cell immune responses by alpha-mannosyl ceramide derivatives consisting of a series of modified sphingosines. Eur J Immunol. 2007;37(7):1836–1844. doi: 10.1002/eji.200636689. [DOI] [PubMed] [Google Scholar]
- 64.Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1-Restricted V{alpha}19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol. 2006;176(3):1618–1627. doi: 10.4049/jimmunol.176.3.1618. [DOI] [PubMed] [Google Scholar]
- 65.Middendorp S, Nieuwenhuis EES. NKT cells in mucosal immunity. Mucosal Immunol. 2009;2(5):393. doi: 10.1038/mi.2009.99. [DOI] [PubMed] [Google Scholar]
- 66.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 67.Illes Z, Shimamura M, Newcombe J, Oka N, Yamamura T. Accumulation of V{alpha}7.2-J{alpha}33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int Immunol. 2004;6(2):223–230. doi: 10.1093/intimm/dxh018. [DOI] [PubMed] [Google Scholar]
- 68.Nell LJ, Kastner DL, Rich RR. Qa-1-associated antigens. III. Distribution of Qa-1 region antigens on lymphoid subpopu-lations. J Immunol. 1980;125(6):2597–2603. [PubMed] [Google Scholar]
- 69.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5(5):516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 70.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci USA. 2001;98(11):6301–6306. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei B, Su TT, Dalwadi H, Stephan RP, Fujiwara D, Huang TT, Brewer S, Chen L, Arditi M, Borneman J, Rawlings DJ, Braun J. Resident enteric microbiota and CD8 + T cells shape the abundance of marginal zone B cells. Eur J Immunol. 2008;38(12):3411–3425. doi: 10.1002/eji.200838432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujiwara D, Wei B, Presley LL, Brewer S, McPherson M, Lewinski MA, Borneman J, Braun J. Systemic control of plasmacytoid dendritic cells by CD8 + T cells and commensal microbiota. J Immunol. 2008;180(9):5843–5852. doi: 10.4049/jimmunol.180.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Ann Rev Immunol. 1998;16(1):111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 74.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Ann Rev Immunol. 2004;22(1):307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 75.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97(9):2063. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J-F, Monteiro RC. Overexpression of natural killer T cells protects V{alpha}14-J{alpha}281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188(10):1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413(6855):531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 78.Croxford JL, Miyake S, Huang Y-Y, Shimamura M, Yamamura T. Invariant Valpha19i T cells regulate auto-immune inflammation. Nat Immunol. 2006;7(9):987. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- 79.Hornquist CE, Lu X, Rogers-Fani PM, Rudolph U, Shappell S, Birnbaumer L, Harriman GR. G{alpha}i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines. J Immunol. 1997;158(3):1068–1077. [PubMed] [Google Scholar]
- 80.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G{alpha}i2-deficient mice. Nat Genet. 1995;10(2):143. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 81.Wei B, McPherson M, Turovskaya O, Velazquez P, Fujiwara D, Brewer S, Braun J. Integration of B cells and CD8 + T in the protective regulation of systemic epithelial inflammation. Clin Immunol. 2008;127(3):303. doi: 10.1016/j.clim.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dalwadi H, Wei B, Schrage M, Spicher K, Su TT, Birnbaumer L, Rawlings DJ, Braun J. B cell developmental requirement for the G{alpha}i2 gene. J Immunol. 2003;170(4):1707–1715. doi: 10.4049/jimmunol.170.4.1707. [DOI] [PubMed] [Google Scholar]
- 83.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci USA. 2005;102(6):2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McPherson M, Wei B, Turovskaya O, Fujiwara D, Brewer S, Braun J. Colitis immunoregulation by CD8+ T cell requires T cell cytotoxicity and B cell peptide antigen presentation. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G485–G492. doi: 10.1152/ajpgi.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izcue A, Coombes J, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212(1):256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 86.Ken S, Atsuhiro O, Yasuyo S, Kiyotaka N, Atsushi M, Atul KB. Inducible IL-12-producing B cells regulate Th2-mediated intestinal inflammation. Gastroenterology. 2007;133(1):124. doi: 10.1053/j.gastro.2007.03.112. [DOI] [PubMed] [Google Scholar]
- 87.Matthieu A, Jens B, Iris D, Lloyd M. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123(5):1516. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 88.Lilani P, Ling S, Anjlee P, Kelly E, Bertrand M, Richard B, Daniel G, Veronika G, Thomas S, Bana J, Lloyd M. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm Bowel Dis. 2007;13(3):298–307. doi: 10.1002/ibd.20026. [DOI] [PubMed] [Google Scholar]
- 89.Malin B, Paul WB, Roger W, Hultgren HE. Long-term treatment with anti-α4 integrin antibodies aggravates colitis in G{alpha}i2-deficient mice. Eur J Immunol. 2005;35(8):2274–2283. doi: 10.1002/eji.200526022. [DOI] [PubMed] [Google Scholar]