Abstract
UV-induced DNA damage gives rise to mutations and skin cancer. We have previously reported that treatment of skin cells in vitro with thymidine dinucleotide (pTT) activates p53 and increases the ability of cells to repair subsequent UV-induced DNA damage by enhancing endogenous DNA repair capacity. Here we show that topical pTT pretreatment enhances the rate of DNA photoproduct removal, decreases UV-induced mutations, and reduces photocarcinogenesis in UV-irradiated hairless WT repair-proficient and Xpc+/- heterozygous partially repair-deficient mice, both transgenic for the lacZ/pUR288 mutation-indicator gene. These data support the existence of inducible mammalian DNA damage responses that increase DNA repair capacity after DNA damage and hence reduce the impact of future exposures to environmental carcinogens. The ability of topically applied pTT to induce protective physiologic responses that normally result from DNA damage suggests a previously undescribed means of reducing skin cancer in high-risk individuals.
Skin cancer accounts for at least 40% of all human malignancies, >1,000,000 cases annually in the U.S. (1, 2). Incidence is clearly linked to UV exposure and increases exponentially with age (1, 3). Skin cancer risk is greatly increased in the rare disease xeroderma pigmentosum (XP), because of mutation in one of several DNA repair enzymes responsible for nucleotide excision repair (NER) (4-6). Development of a hairless mouse model (7, 8) and more recently hairless XP gene knockout mice that mimic the human cancer susceptibility (4) has greatly facilitated studies of photocarcinogenesis. In particular, low-dose daily UV irradiation of XP group C (Xpc)-/- mice leads to the development of skin cancer with a short latency time (80-100 days) and 100% prevalence, and partially repair deficient Xpc+/- mice are also more prone to UV-induced skin cancer than their WT counterparts (9). Finally, to study DNA mutations induced by physical and chemical agents in tissues of higher eukaryotes, transgenic mice carrying multiple copies of a lacZ/pUR288 mutation-indicator reporter plasmid have been generated (10) and crossed into WT and repair-deficient mice strains (11).
Our laboratory has shown that many protective responses triggered by UV irradiation are duplicated by treatment of cells in vitro and in vivo with thymidine dinucleotide (pTT) (12, 13), originally selected for study because it is the obligate substrate for formation of most UV-induced DNA photoproducts (14). These effects include tanning, activation of the p53 transcription factor and tumor suppressor protein (12, 15-17), transient cell cycle arrest (17, 18), and immunosuppression mediated in part by tumor necrosis factor α (19) and IL-10 (20). Of relevance to the present study, pTT up-regulates several gene products involved in DNA repair, some but not all of which are known to be p53-regulated, and increases the rate of DNA repair after UV irradiation as measured in multiple assay systems (13, 17, 21). As anticipated from the fact that carcinogenic chemical adducts are generally removed by the same NER pathway as DNA photoproducts, pTT treatment also accelerates removal of highly mutagenic benzo(a)pyrene adducts (16).
Efforts to dissect the mechanism of pTT's protective UV-mimetic effects on cultured human cells and rodent skin revealed that several other oligonucleotides have comparable effects (13, 22) and are rapidly taken up into the nucleus (22). Ultimately, we determined that only oligonucleotides sharing substantial sequence homology with the mammalian telomere repeat sequence 5′-TTAGGG-3′ (termed T-oligos) produce the same effects as pTT, a 100% homolog for one-third of the sequence, and that their molar efficacy is roughly proportional to their length and degree of homology (23). Furthermore, we demonstrated that T-oligos induce their protective responses without shortening telomeres or causing digestion of the genomic 5′-TTAGGG-3′ telomeric overhang in treated cells (23).
The de Lange group has established that telomeres are normally in a loop configuration (24), stabilized by sequestration of the 75- to 300-base single-stranded 3′ overhang within the proximal double-stranded telomere (25), and that disruption of this loop structure leads to activation of p53 and subsequent apoptosis or a senescent phenotype, depending on cell type (26). Based on these results and our recent work using an 11-base T-oligo (27, 28), we hypothesize that exposure of the 3′ single-stranded telomere overhang sequence is a physiologic signal that initiates multiple DNA damage responses, all of which reduce the probability of mutation and malignant conversion of damaged cells (23). We further hypothesize that exposure of the 5′-TTAGGG-3′ sequence occurs not only after experimental telomere loop disruption but also after acute DNA damage or critical telomere shortening during aging (27, 28), and that providing cells or intact skin with oligonucleotides homologous to this sequence evokes the same protective responses in the absence of DNA damage and/or telomere loop disruption (23).
We now report that topical pTT treatment of WT and Xpc+/- mice enhances DNA repair capacity, decreases mutation frequency (MF) in a transgenic lacZ/pUR288 reporter plasmid, and reduces and delays carcinogenesis after UV irradiation. This approach has therapeutic implications and documents the existence of inducible mammalian DNA damage responses capable of protecting against environmental carcinogen-induced DNA damage.
Experimental Procedures
Xpc Mice. Hairless WT and Xpc+/-/lacZ+ mice aged 4-6 weeks at the start of experiments were housed in a room illuminated only with yellow fluorescent tubes (Philips TL40W/16, Eindhoven, The Netherlands) 12 h/day. All animal protocols were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine. Mice were fed regular chow, and water access was not limited.
Oligonucleotide Preparation. pTT was obtained from Midland Certified Reagent (Midland, TX), kept as a stock solution of 2 mM, and diluted to 100 μM for experiments in a mixture of propylene glycol 75%/DMSO 25% previously used to deliver a biologically active concentration of pTT to guinea pig skin (29). To evaluate penetration of pTT through intact mouse stratum corneum, FITC-labeled pTT was applied in the vehicle and epidermal distribution after 2 and 4 h was evaluated by confocal microscopy.
UV Irradiation Protocols. Mice were irradiated with six fluorescent American Phillips F40 sunlamps permanently mounted above the animal cages. Irradiance was metered with a research radiometer fitted with a UV probe, at 285 ± 5 nm (model IL1700 A, International Light, Newburyport, MA), as described (17, 21, 29). Mice in the acute irradiation protocol received a single dose of UV (30 mJ/cm2) after daily application of pTT 100 μM for 10 days. We killed mice before (no UV), immediately after (time 0), and 24 and 72 h after exposure. To evaluate the effect of pTT on UV-induced MF in vivo, we pretreated mice with pTT or diluent for 10 days and then sham or UV irradiated (30 mJ/cm2) and killed them after 14 days.
Mice in the chronic UV irradiation protocol were pretreated with pTT 100 μM for 5 days (Monday through Friday) then sham or UV irradiated for the next 3 weeks (Monday through Friday) with 30 mJ/cm2 as described (9). Five cycles of 1 week of daily pTT applications followed by 3 weeks of irradiations constituted 75 days of intermittent UV irradiation. The first 18 mice (nine of each genotype) were irradiated with 100 mJ/cm2 daily for 3 days, but when all mice experienced marked inflammatory responses, the daily UV dose was reduced to 30 mJ/cm2 for the remainder of the experiment.
Tissue Specimens. After irradiation, the back skin was removed and bisected. Skin was snap frozen and kept at -80°C for immunohistologic detection of cyclobutane pyrimidine dimers (CPDs). For mutational analysis or Western blotting, irradiated back skin was bisected, and epidermis was separated from dermis by incubating skin in 6 M NaBr for 1 hour.
Mutation Analysis. We performed mutation analysis using coded samples from at least four to six mice per condition, 14 days after a single UV exposure, and after 24 weeks of chronic intermittent UV irradiation. Genomic DNA was isolated by using a commercially available kit (Qiagen, Valencia, CA). We isolated 30-40 μg of genomic DNA from each 1 × 2-cm tissue sample, enough to perform several mutation analyses of the integrated lacZ gene as described by Boerrigter et al. (10) (see Supporting Methods, which is published as supporting information on the PNAS web site, for further details).
Quantification and Classification of Tumors. Starting from week 6, mice were checked weekly for neoplasms. Prevalence and multiplicity of neoplasms were evaluated according to standard criteria (9). Mice were killed 24 weeks after the first UV irradiation. All neoplasms in both vehicle- and pTT-treated sham-irradiated skin samples were carefully bisected and processed for histology. Diagnosis was performed by a blinded dermatopathologist. Representative sections were photographed.
Western Blot Analysis. We obtained mouse epidermis as described above. Epidermis was instantly frozen (-80°C) and then homogenized by using a homogenizer (PRO 200, PRO Scientific, Monroe, CT). We then isolated total protein and processed lysates for Western blotting (21) using antibodies specific for total p53 (p53-DO1, Santa Cruz Biotechnology) and serine-15 phosphorylated p53 (Cell Signaling, Beverly, MA).
Immunohistology. We fixed 5-μm sections of frozen tissue on slides with ice-cold methanol-acetone (1:1) for 10 min at -20°C then air dried them. For immunohistological detection of CPDs, we incubated acetone-fixed sections with CPD-specific antibodies (kind gift from Toshio Mori and Nobuhiko Kobayashi, Nara Medical University, Kashihara, Japan) overnight, then used the AEC mouse tissue detection system (LabVision, Fremont, CA), as described in the manufacturer's protocol. We delineated ≈10-μm × 1-mm areas using computer-assisted image analysis and counted CPD (+) nuclei in the epidermis. For each time point, we analyzed 10 randomly selected visual fields of CPD-stained epidermis of three to five animals per treatment condition. We set the average number of CPD (+) nuclei at time 0 for each condition at 100%, and the 24- and 72-h time points were recalculated as percent remaining CPDs.
Statistical Analysis. We analyzed differences in the rate of removal of UV-induced CPDs, MF in the lacZ gene, and tumor multiplicity in pTT-treated vs. control mice by using the ANOVA post hoc analysis. We also analyzed neoplasm data for prevalence over time using the statistical method first described by Kaplan and Meier (30), as performed by the statview program (SAS Institute, Cary, NC). Groups were considered different when P < 0.05.
Results
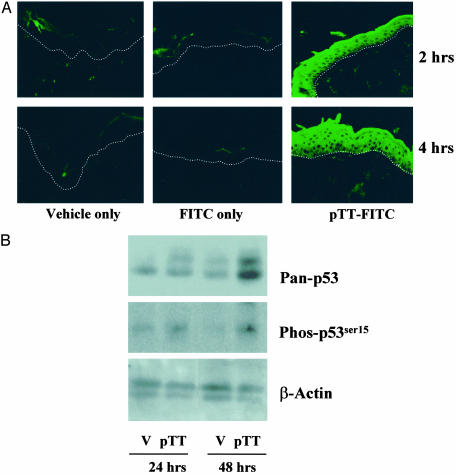
pTT Penetrates Intact Mouse Skin. To determine whether pTT penetrates through intact stratum corneum in hairless mice, we topically applied FITC-labeled pTT (100 μM; 50 μl/3 cm2) and subsequently examined skin specimens by confocal microscopy. FITC-labeled pTT was visible throughout the epidermis within 2 h and for at least 4 h after topical application (Fig. 1A). Animals treated with vehicle alone or FITC alone showed only autofluorescence or retained label in the stratum corneum.
Fig. 1.
pTT penetrates intact stratum corneum and up-regulates and activates p53. (A) Vehicle alone, FITC alone in vehicle, or FITC-labeled pTT (100 μM) was applied once topically (50 μl/3cm2) to the backs of hairless mice. We processed mouse skin 2 and 4 h after application for immunofluorecent analysis. The dotted white line indicates the dermoepidermal junction as visualized by light microscopy. (B) We pretreated the dorsal skin of hairless mice (three per condition) with topical pTT or vehicle alone (V) once per day for 3 days. We killed the animals 24 or 48 h after treatment and processed epidermal sheets for Western blotting as described in Experimental Procedures. Protein loading was assessed by probing the membrane with β-actin. Densitometric analysis of blots from two independent studies revealed up-regulation of total p53 and of phospho-53 to 420 ± 75% and 375 ± 68% of control levels at 48 h.
pTT Treatment Up-Regulates and Activates p53. To determine whether pTT can induce p53 protein level and Ser-15 phosphorylation indicative of p53 activation (31) in vivo, as previously observed in vitro (13, 17, 21, 23, 27, 28), we performed Western blot analysis on total cellular protein from epidermal homogenates of mouse skin treated with pTT or vehicle alone once daily for up to 3 days. After 24 h (one application) and more notably after 48 h (two applications), pTT up-regulated both p53 protein level and Ser-15 phosphorylation 3- to 4-fold (Fig. 1B).
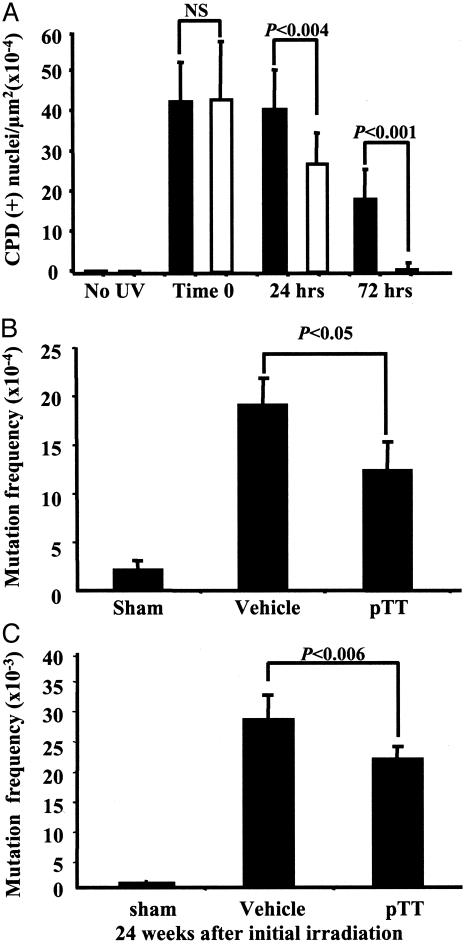
pTT Pretreatment Accelerates the Removal of CPDs in Murine Skin. To determine whether pTT treatment accelerates the removal of the major DNA photoproduct, CPDs, we treated mice as described in Experimental Procedures and processed tissue for immunohistology using CPD-specific antibodies. We observed no CPD-positive (+) nuclei in unirradiated samples and the highest number of CPDs, comparable in pTT and vehicle pretreated skin at time 0, immediately after UV (Fig. 5, which is published as supporting information on the PNAS web site). CPDs decreased with time, but by 72 h, abundant CPD (+) nuclei remained in vehicle-treated epidermis, whereas only occasional CPD (+) nuclei remained in pTT-treated epidermis (Fig. 5). Immediately after UV (time 0), we counted equal number of CPDs (+) nuclei per μm2 in vehicle-treated vs. pTT-treated epidermis: 42.4 ± 10.4 vs. 43 ± 14.5 (×10-4) respectively, confirming that pTT does not serve as a sunscreen. By 24 h, 95% vs. 63% of CPDs (+) nuclei remained in vehicle-treated vs. pTT-treated mouse epidermis (40.2 ± 10 vs. 26.7 ± 7.5, P < 0.004), and by 72 h, almost no CPDs remained in the epidermis of pTT-treated mice, whereas in vehicle-treated animals 40% of CPDs (+) nuclei remained (17.6 ± 7.7 vs. 0.56 ± 1.3, P < 0.001) (Fig. 2A).
Fig. 2.
pTT accelerates the removal of CPDs and reduces mutations in murine skin after acute and chronic UV irradiation. (A) Quantification of CPD (+) nuclei in UV-irradiated skin. All samples were coded, then evaluated by a single blinded observer to eliminate bias and interobserver variability. CPD (+) nuclei were counted in at least 8-10 randomly selected areas of 10,000 μm2 of epidermis in at least three to five animals per treatment condition for each time point. A total of 168 images were analyzed. The number of initial CPDs (time 0) was similar in all groups and designated as 100%. CPD (+) nuclei after 24 and 72 h are shown for vehicle-treated (black bars) vs. pTT-treated (open bars) epidermis. (B) Hairless mice also transgenic for the lacZ gene were treated as described in Experimental Procedures. Two weeks after a single UV dose, we evaluated MF in the lacZ gene (four to six mice per treatment condition). Plotted values are MF per transgene in the harvested epidermis. (C) pTT treatment reduces mutations in chronically irradiated mouse skin. At week 24 after the initial UVB exposures, we killed the mice (four to six per treatment condition) and processed the epidermis for evaluation of MF in the lacZ transgene.
Severely UV-damaged epidermal keratinocytes are known to undergo apoptosis (32) and, as demonstrated above and in previous studies (16, 17, 21), pTT activates p53, a mediator of apoptosis in such cells. UV irradiation also causes a dose-dependent arrest of epidermal proliferation, followed by a period of hyperplasia (33). We therefore stained sections from the same skin samples for markers of apoptosis [TdT-dUTP terminal nick-end labeling (TUNEL)] and proliferation (Ki67). Sham-irradiated samples showed no TUNEL staining and occasional Ki67-positive cells in the basal layer, as expected. In all other samples (24, 48, and 72 h postUV) we only rarely observed TUNEL-positive presumptively apoptotic cells or Ki67-positive presumptively proliferating cells (data not shown). The only apparent difference between pTT-treated and control samples was observed at 72 h, when Ki67-positive cells were slightly more numerous in control skin, consistent with the thickened epidermis noted at that time (Fig. 5). These data suggest that the effect of pTT was largely the consequence of more rapid DNA repair rather than of greater loss of damaged cells through apoptosis or the dilution of CPD-bearing cells by greater proliferation of surrounding less damaged cells in pTT-treated skin. Indeed, the expected UV-induced epidermal growth arrest may have been somewhat prolonged in pTT-treated skin, as a result of the cell cycle arrest previously demonstrated in vitro after treatment with pTT or other T-oligos (18, 23, 27, 28).
pTT Pretreatment Reduces Mutations in Murine Skin After a Single UV Exposure. Because pTT pretreatment accelerated the removal of UV-induced CPDs, we wanted to determine whether pTT-pretreatment also reduces mutations in mouse skin in vivo, as anticipated from the known reciprocal relationship between these parameters (34). Sham-irradiated WT and Xpc+/- animals had only a background level of mutations (2.3 ± 0.6 × 10-4), as reported previously for this assay (8), and this level increased nearly 10-fold after a single UV exposure (Fig. 2B). Compared to vehicle control, pTT pretreatment decreased UV-induced mutations by ≈38% (19.3 ± 1.9 × 10-4 vs. 12.0 ± 2.5 × 10-4, vehicle vs. pTT, P < 0.05) when assessed 2 weeks after the single UV exposure (Fig. 2B).
Intermittent pTT Treatment Decreases Prevalence of Mutations and Neoplasms After Chronic UV Irradiation. We next subjected the mice to a known carcinogenic 24-week irradiation protocol, preceding each 3-week period of daily UV exposures with 1 week of daily topical pTT or vehicle applications to determine whether intermittent pTT treatment can reduce photocarcinogenesis. None of the sham-irradiated mice developed any neoplasms, as expected, and after chronic UV irradiation, tumors occurred predominantly in vehicle-treated animals (Fig. 6, which is published as supporting information on the PNAS web site). That none of the pTT-treated sham-irradiated animals developed neoplasms or other apparent skin changes within the 24-week study period indicates that pTT is well tolerated and not carcinogenic or otherwise harmful, at least within the sensitivity of the experiment.
After 24 weeks of sham irradiation, animals had only a slightly higher background level of mutations (0.3 ± 0.06 × 10-3) than at 2 weeks after a single sham irradiation, as reported previously for this assay (8). UV irradiation produced an ≈100-fold increase in mutations of the lacZ gene, but MF was 21% lower in pTT-treated than in vehicle-treated WT and Xpc+/- mice (Fig. 2C): 21.5 ± 1.9 × 10-3 vs. 27.2 ± 3.2 × 10-3 (P < 0.006). Note that compared to a single UVB irradiation, chronic irradiation produced a >13-fold increase in the MF in the integrated lacZ gene (Figs. 2 B and C), mutating 2-3% of all transgene plasmids.
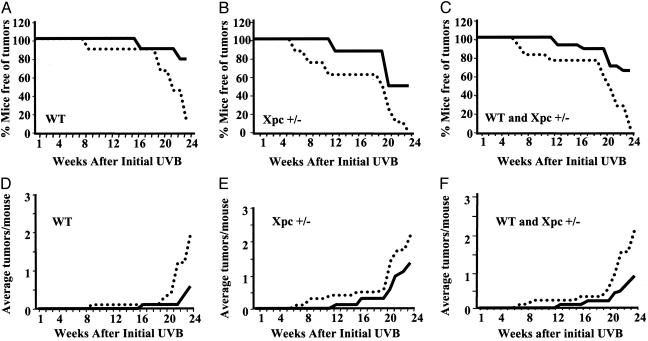
Vehicle-treated WT mice began to develop UV-induced neoplasms within 9 weeks of the first irradiation and by week 24, only 12% of the vehicle-treated animals were free of tumors. In contrast, pTT-treated mice were completely free of neoplasms through 16 weeks, and 78% remained tumor-free at week 24 (Fig. 3A). In vehicle-treated Xpc+/- mice, neoplasms appeared by week 7, and by week 24, all of the mice had UV-induced neoplasms, consistent with their reported slightly increased UV susceptibility (9), whereas pTT-treated Xpc+/- animals were neoplasm-free through week 12, and 50% remained neoplasm free at week 24 (Fig. 3B), indicating that pTT protects against UV-induced carcinogenesis in partially repair-deficient as well as WT mice. The differences in tumor prevalence were statistically significant as determined by Kaplan-Meier Survival Analysis for WT (P < 0.05), Xpc+/- (P < 0.02), and combined (P < 0.03, Fig. 3C) groups. Multiplicity of neoplasms was evaluated weekly beginning 6 weeks after the first UV exposure. In WT mice, by week 24, the average number of tumors per mouse was 2.0 ± 0.4 vs. 0.6 ± 0.3 (P < 0.0001) in mice treated with vehicle vs. pTT (Fig. 3D), and in Xpc+/- mice was 2.3 ± 0.6 vs. 1.4 ± 0.6 (P < 0.03) (Fig. 3E), reflecting 70% and 40% decreases in UV-induced tumor multiplicity in pTT-treated UV-irradiated WT and Xpc+/- mice, respectively. In the combined groups, there was a 57% reduction in number of tumors per mouse for pTT-treated vs. control animals: 2.1 ± 0.3 vs. 0.9 ± 0.3 (P < 0.0001), with progressive separation between the groups evident after week 20 (Fig. 3F).
Fig. 3.
pTT pretreatment decreases prevalence and multiplicity of neoplasms after chronic UV irradiation. All neoplasms ≥1 mm in diameter were measured weekly beginning with week 6, and the prevalence and multiplicity of neoplasms (average number of neoplasms per mouse) were calculated on a weekly basis through week 24 after initial UVB exposure. The solid line indicates intermittent pTT treatment, and the dotted line indicates vehicle treatment. (A) WT mice (nine per treatment group). (B) Xpc+/- mice (eight per treatment group). (C) Combined groups (17 per treatment group). (D) Graphed values are for multiplicity of neoplasms. WT mice (nine per treatment group) treated with pTT had fewer neoplasms than controls in weeks 21 and 24 after initial UVB (P < 0.03 and P < 0.06, respectively). (E) XPC+/- mice (eight per treatment group) treated with pTT had fewer neoplasms than controls each week beginning in week 22 after initial UVB (P < 0.0001). (F) Combined groups (17 per treatment group) treated with pTT had fewer neoplasms each week beginning in week 21 (P < 0.01, 0.0001, 0.0008, and 0.0001 in weeks 21-24, respectively).
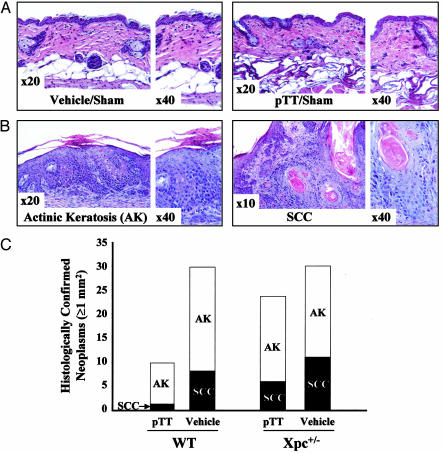
pTT Protects Against both Premalignant and Malignant Skin Neoplasms. To evaluate the effect of long-term pTT application to mouse skin, we processed vehicle or pTT-treated sham-irradiated back skin for routine histology. In both WT and Xpc+/- mice, sham-irradiated skin treated intermittently with pTT for >5 months looked similar to vehicle-treated mouse skin (Fig. 4A). Nontumor-bearing skin of the irradiated animals similarly could not be distinguished morphologically by treatment group or genotype, although we would not have detected subtle differences in epidermal thickening or average severity of histologic photodamage in our screening inspection.
Fig. 4.
pTT protects against both premalignant and malignant neoplasms. (A) Lack of histologic pTT effect on sham-irradiated skin after 24 weeks. We examined skin from at least six mice per treatment group. (B) Representative sections of tumor-bearing skin. A single dermatopathologist evaluated all hematoxylin/eosin-stained sections of neoplasms >1mm2 in surface area (140 neoplasms from 46 mice), after coding to eliminate possible bias. Classic features included loss of polarity and nuclear atypia (AKs) and dermal invasion with keratin “pearls” (SCCs), but individual neoplasms in vehicle-treated vs. pTT-treated could not be distinguished from each other. (C) Number of AKs and SCCs in WT vs. Xpc+/- mice.
We histologically evaluated all tumors >1mm2 in surface area 24 weeks after the initial UV exposure. Nearly half (48%) were premalignant actinic keratoses (AKs), and 19% were invasive squamous cell carcinomas (SCCs) (Fig. 4B), as expected in chronically UV-irradiated hairless mice at this time point. The remaining clinically apparent lesions were classified histologically as scars or crusted ulcerations. There were no apparent differences in individual AKs or SCCs arising in pTT-treated vs. vehicle-treated skin or in WT vs. Xpc+/- mice. However, compared to controls, pTT-treated mice overall had 33% and 63% decreases in AKs and SCCs (Fig. 4C). pTT-treated WT mice had 57% and 87% decreases in AKs and SCCs, whereas pTT-treated Xpc+/- mice had 6% and 45% decreases, respectively.
Discussion
UV irradiation is the major environmental carcinogen for human skin. The shorter more biologically active UV wavelengths are mostly absorbed in the epidermis, where UV directly damages DNA via production of mutagenic photoproducts (1, 3, 7). The present study demonstrates that topical application of pTT to murine skin up-regulates and activates p53 and after subsequent UV exposure enhances DNA repair rate and decreases mutation rate and photocarcinogenesis. We observed protective effects in both relatively repair-proficient WT and relatively repair-deficient Xpc+/- mice, modeling the spectrum of UV vulnerability in a human population. Specifically, our results demonstrate that treatment with pTT before and intermittently during a protocol of repeated UV exposures decreases and delays tumor development. The effect of pTT treatment on prevalence of neoplasms was already evident by week 8, and the separation of the curves between pTT and vehicle-treated skin for both WT and Xpc+/- animals increased over time through week 24.
Because UV irradiation itself induces and activates p53 and can increase subsequent DNA repair rate (35, 36), we speculate that pTT may have had an even more dramatic effect on photocarcinogenesis if applied before each irradiation in a protocol using a single exposure or infrequent widely spaced exposures rather than blocks of daily exposures 3 of 4 weeks each month. However, the expected slower rate of development and lower final prevalence of tumors in the irradiated control animals in such a protocol would require a far longer time and larger experimental groups to demonstrate these anticipated relatively larger benefits. Similarly, we anticipate the protective effect of pTT might have been greater in an animal capable of tanning, unlike mice that lack epidermal melanocytes. UV-induced increased melanogenesis is the major recognized protective response of human skin against subsequent photodamage, at least in part because of the ability of melanin to absorb damaging photons that otherwise target DNA (3, 12), and among its other protective responses in guinea pigs whose skin contains epidermal melanocytes pTT causes tanning that is histologically identical to UV-induced tanning (12) and highly photoprotective (37).
NER is principally responsible for correction of the DNA damage resulting from sun exposure (5). Much evidence supports the concept that rate and extent of DNA repair determine MF and ultimately cancer risk (34, 38). For example, an inverse relationship has been observed both in older individuals (34) and in patients with XP (4). Further, enhancing NER rate by providing an exogenous DNA repair enzyme, T4 endonucleaseV, decreases photocarcinogenesis in animals (39) and in XP patients (40). Our results strongly support this presumptive role of unrepaired or incorrectly repaired CPDs in the induction of skin neoplasms (38).
Inducible DNA repair was first recognized in bacteria. This so-called SOS response (41) is initiated by partially processed damaged DNA and mediated by transcriptional up-regulation of genes encoding DNA repair enzymes. Interestingly, several recent reports have suggested that NER is also inducible in mammalian cells. For example, carcinogen treatment (42) and low-dose UV irradiation (43, 44) enhance the repair rate for subsequent DNA damage in repair-proficient as well as repair-deficient mammalian cells. We have shown that pTT and other DNA oligonucleotides partially or totally homologous to the 3′ telomere overhang also induce protective effects (12, 13, 17, 22, 23, 27, 28), presumably by mimicking a physiologic signal generated during the course of DNA damage or its repair (17, 23), and in any case mimicking the cellular response to telomere loop disruption and overhang exposure (23, 27, 28), a presumptive DNA damage signal (26).
Telomere homolog oligonucleotides appear to exert their protective effects by activating the ATM kinase (27) and its downstream effectors, including p95/Nbs-1 and p53 (17, 23, 27), as well as by up-regulating a variety of other gene products not known to be regulated through ATM, p53, or p95/Nbs-1, such as RPA, ERCC1, XPA, p16INK4a, p33, and p27 (17, 21, 23, 28).
Conclusion
Our findings suggest that telomere-based inducible DNA damage responses are important mechanisms by which cells manage recurrent DNA insults throughout life. Our data further suggest that topically applied pTT may enhance DNA repair capacity in human skin, in the absence of actual DNA damage that normally induces this protective response (17, 23), and may thus reduce the carcinogenic risk from subsequent solar UV irradiation in individuals at high risk because of fair skin, other genetic predisposition, or advanced age.
Supplementary Material
Acknowledgments
We are grateful to Drs. Mark Eller, Jan Vijg, Mary Khlgatian, and Heidi Giess for assistance with early attempts to assess mutagenesis in pTT-treated cells and animals; to Drs. Nobuhiko Kobayashi and Toshio Mori for providing CPD antibodies; and to Kathleen Huard for assistance in the preparation of the manuscript. This work was supported by research grants from the Skin Cancer Foundation, the Dermatology Foundation, the American Cancer Society, and the American Federation for Aging Research (to D.A.G.) and the Herzog Foundation (to B.A.G.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: pTT, thymidine dinucleotide; XP, xeroderma pigmentosum; Xpc, XP group C; MF, mutation frequency; CPD, cyclobutane pyrimidine dimers; AK, actinic keratoses; SCC, squamous cell carcinoma; NER, nucleotide excision repair.
References
- 1.Woodhead, A. D., Setlow, R. B. & Tanaka, M. (1999) J. Epidemiol. 9, S102-S114. [DOI] [PubMed] [Google Scholar]
- 2.Scotto, J., Fears, T. & Fraumeni, J. (1983) in DHEW Publication no. (NIH) 83-2433 (Natl. Cancer Inst., Bethesda), pp. 43-45.
- 3.Kochevar, I. E. & Taylor, C. (2003) in Dermatology in General Medicine, eds. Freedberg, I., Eisen, A., Wolff, K., Austen, K., Goldsmith, L., Katz, S. & Fitzpatrick, T. (McGraw-Hill, New York), pp. 1267-1274.
- 4.Kraemer, K. H., Lee, M. M. & Scotto, J. (1987) Arch. Dermatol. 123, 241-250. [DOI] [PubMed] [Google Scholar]
- 5.Sancar, A. (1996) Annu. Rev. Biochem. 65, 43-81. [DOI] [PubMed] [Google Scholar]
- 6.Cleaver, J. E. (1969) Proc. Natl. Acad. Sci. USA 63, 428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Gruijl, F. R., Sterenborg, H. J., Forbes, P. D., Davies, R. E., Cole, C., Kelfkens, G., van Weelden, H., Slaper, H. & van der Leun, J. C. (1993) Cancer Res. 53, 53-60. [PubMed] [Google Scholar]
- 8.van Steeg, H., Mullenders, L. H. & Vijg, J. (2000) Mutat Res. 450, 167-180. [DOI] [PubMed] [Google Scholar]
- 9.Cheo, D. L., Meira, L. B., Burns, D. K., Reis, A. M., Issac, T. & Friedberg, E. C. (2000) Cancer Res. 60, 1580-1584. [PubMed] [Google Scholar]
- 10.Boerrigter, M. E., Dolle, M. E., Martus, H. J., Gossen, J. A. & Vijg, J. (1995) Nature 377, 657-659. [DOI] [PubMed] [Google Scholar]
- 11.de Vries, A., Dolle, M. E., Broekhof, J. L., Muller, J. J., Kroese, E. D., van Kreijl, C. F., Capel, P. J., Vijg, J. & van Steeg, H. (1997) Carcinogenesis 18, 2327-2332. [DOI] [PubMed] [Google Scholar]
- 12.Eller, M. S., Yaar, M. & Gilchrest, B. A. (1994) Nature 372, 413-414. [DOI] [PubMed] [Google Scholar]
- 13.Goukassian, D. A., Bagheri, S., el-Keeb, L., Eller, M. S. & Gilchrest, B. A. (2002) FASEB J. 16, 754-756. [DOI] [PubMed] [Google Scholar]
- 14.Setlow, R. B. & Carrier, W. L. (1966) J. Mol. Biol. 17, 237-254. [DOI] [PubMed] [Google Scholar]
- 15.Eller, M. S., Ostrom, K. & Gilchrest, B. A. (1996) Proc. Natl. Acad. Sci. USA 93, 1087-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda, T., Eller, M. S., Hedayati, M., Grossman, L. & Gilchrest, B. A. (1999) Mutat. Res. 433, 137-145. [DOI] [PubMed] [Google Scholar]
- 17.Eller, M. S., Maeda, T., Magnoni, C., Atwal, D. & Gilchrest, B. A. (1997) Proc. Natl. Acad. Sci. USA 94, 12627-12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedeux, R., Al-Irani, N., Marteau, C., Pellicier, F., Branche, R., Ozturk, M., Ranchi, J. & Dore, J. F. (1998) J. Invest. Dermatol. 111, 472-477. [DOI] [PubMed] [Google Scholar]
- 19.Cruz, P. D., Jr., Leverkus, M., Dougherty, I., Gleason, M. J., Eller, M., Yaar, M. & Gilchrest, B. A. (2000) J. Invest. Dermatol. 114, 253-258. [DOI] [PubMed] [Google Scholar]
- 20.Curiel-Lewandrowski, C., Venna, S. S., Eller, M. S., Cruikshank, W., Dougherty, I., Cruz, P. D. & Gilchrest, B. A. (2003) Exp. Dermatol. 12, 145-152. [DOI] [PubMed] [Google Scholar]
- 21.Goukassian, D. A., Eller, M. S., Yaar, M. & Gilchrest, B. A. (1999) J. Invest. Dermatol. 112, 25-31. [DOI] [PubMed] [Google Scholar]
- 22.Hadshiew, I. M., Eller, M. S., Gasparro, F. P. & Gilchrest, B. A. (2001) J. Dermatol. Sci. 25, 127-138. [DOI] [PubMed] [Google Scholar]
- 23.Eller, M. S., Puri, N., Hadshiew, I. M., Venna, S. S. & Gilchrest, B. A. (2002) Exp. Cell Res. 276, 185-193. [DOI] [PubMed] [Google Scholar]
- 24.Griffith, J. D., Comeau, L., Rosenfield, S., Stansel, R. M., Bianchi, A., Moss, H. & de Lange, T. (1999) Cell 97, 503-514. [DOI] [PubMed] [Google Scholar]
- 25.Stansel, R. M., de Lange, T. & Griffith, J. D. (2001) EMBO J. 20, 5532-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlseder, J., Broccoli, D., Dai, Y., Hardy, S. & de Lange, T. (1999) Science 283, 1321-1325. [DOI] [PubMed] [Google Scholar]
- 27.Eller, M. S., Li, G. Z., Firoozabadi, R., Puri, N. & Gilchrest, B. A. (2003) FASEB J. 17, 152-162. [DOI] [PubMed] [Google Scholar]
- 28.Li, G. Z., Eller, M. S., Firoozabadi, R. & Gilchrest, B. A. (2003) Proc. Natl. Acad. Sci. USA 100, 527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allan, A. E., Archambault, M., Messana, E. & Gilchrest, B. A. (1995) J. Invest. Dermatol. 105, 687-692. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan, E. L. & Meier, P. (1958) J. Am. Stat. Accoc. 53, 203-224. [Google Scholar]
- 31.Banin, S., Moyal, L., Shieh, S., Taya, Y., Anderson, C. W., Chessa, L., Smorodinsky, N. I., Prives, C., Reiss, Y., Shiloh, Y. & Ziv, Y. (1998) Science 281, 1674-1677. [DOI] [PubMed] [Google Scholar]
- 32.Baba, T., Hanada, K. & Hashimoto, I. (1996) J. Dermatol. Sci. 12, 18-23. [DOI] [PubMed] [Google Scholar]
- 33.Walker, S. L., Hawk, J. L. & Young, A. R. (2003) in Dermatology in General Medicine, eds. Freedberg, I., Eisen, A., Wolff, K., Austen, K., Goldsmith, L., Katz, S. & Fitzpatrick, T. (McGraw-Hill, New York), pp. 1275-1282.
- 34.Moriwaki, S., Ray, S., Tarone, R. E., Kraemer, K. H. & Grossman, L. (1996) Mutat. Res. 364, 117-123. [DOI] [PubMed] [Google Scholar]
- 35.Smith, M. L. & Fornace, A. J., Jr. (1997) Proc. Natl. Acad. Sci. USA 94, 12255-12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tron, V. A., Trotter, M. J., Ishikawa, T., Ho, V. C. & Li, G. (1998) J. Cutan. Med. Surg. 3, 16-20. [DOI] [PubMed] [Google Scholar]
- 37.Gilchrest, B. A. & Eller, M. S. (1999) J. Invest. Dermatol. Symp. Proc. 4, 35-40. [DOI] [PubMed] [Google Scholar]
- 38.You, Y. H., Lee, D. H., Yoon, J. H., Nakajima, S., Yasui, A. & Pfeifer, G. P. (2001) J. Biol. Chem. 276, 44688-44694. [DOI] [PubMed] [Google Scholar]
- 39.Yarosh, D., Alas, L. G., Yee, V., Oberyszyn, A., Kibitel, J. T., Mitchell, D., Rosenstein, R., Spinowitz, A. & Citron, M. (1992) Cancer Res. 52, 4227-4231. [PubMed] [Google Scholar]
- 40.Yarosh, D., Klein, J., O'Connor, A., Hawk, J., Rafal, E. & Wolf, P. (2001) Lancet 357, 926-929. [DOI] [PubMed] [Google Scholar]
- 41.Radman, M. (1975) Basic Life Sci. 5A, 355-367. [DOI] [PubMed] [Google Scholar]
- 42.Protic, M., Roilides, E., Levine, A. S. & Dixon, K. (1988) Cell Mol. Genet. 14, 351-357. [DOI] [PubMed] [Google Scholar]
- 43.Jeeves, W. P. & Rainbow, A. J. (1983) Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. 43, 625-647. [DOI] [PubMed] [Google Scholar]
- 44.Francis, M. A. & Rainbow, A. J. (1999) Carcinogenesis 1, 19-26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.