Abstract
Chronic restraint stress, psychosocial stress, as well as systemic or oral administration of the stress-hormone corticosterone induces a morphological reorganization in the rat hippocampus, in which adrenal steroids and excitatory amino acids mediate a reversible remodeling of apical dendrites on CA3 pyramidal cell neurons of the hippocampus. This stress-induced neuronal remodeling is accompanied also by behavioral changes, some of which can be prevented with selective antidepressant and anticonvulsive drug treatments. Lithium is an effective treatment for mood disorders and has neuroprotective effects, which may contribute to its therapeutic properties. Thus, we wanted to determine whether lithium treatment could prevent the effects of chronic stress on CA3 pyramidal cell neuroarchitecture and the associated molecular and behavioral measures. Chronic lithium treatment prevented the stress-induced decrease in dendritic length, as well as the stress-induced increase in glial glutamate transporter 1 (GLT-1) mRNA expression and the phosphorylation of cAMP-response element binding in the hippocampus. Lithium treatment, however, did not prevent stress effects on behavior in the open field or the plus-maze. These data demonstrate that chronic treatment with lithium can protect the hippocampus from potentially deleterious effects of chronic stress on glutamatergic activation, which may be relevant to its therapeutic efficacy in the treatment of major depressive disorder and bipolar disorder.
Mood disorders, such as major depressive disorder (MDD) and bipolar disorder, are leading causes of disability and are recognized as chronic illnesses with significant morbidity and mortality (1). Although multiple factors, such as stress, are implicated in the onset and time course of psychiatric illness, specific changes in brain structure and function have been identified also. For example, depression is associated with a decrease in hippocampal volume (2-4), whereas patients with bipolar disorder are reported to have an enlarged amygdala relative to normal controls (5, 6).
Animal models of chronic stress, such as 3 weeks of chronic restraint stress (CRS) or corticosterone (CORT) treatment, induce neuronal remodeling in the hippocampus (7), amygdala (8), and prefrontal cortex (9). In the hippocampus, CRS significantly decreases dendritic length and branch points on pyramidal cell neurons in the CA3 region (10), and it suppresses neurogenesis in the granule cell layer of the dentate gyrus (DG) (11). Under chronic conditions, stressful experiences and MDD both can lead to neuronal atrophy in hippocampus (2, 10). In fact, treatment with antidepressants can block or reverse some measures of chronic stress and depression, influencing neuronal architecture, cell survival, and function (12-14).
CRS triggers various molecular changes (15, 16), such as alterations in glutamate receptor subunits (17) and increased glial glutamate transporter 1 (GLT-1) expression (18), and it may potentially lead to excitotoxicity by means of increased synaptic glutamate levels (19). The dendritic shrinkage in CA3 pyramidal cell neurons after CRS can be blocked by treatment with the anticonvulsant drug, phenytoin, and the antidepressant tianeptine, both of which have been shown to block excitotoxicity (20-22). In addition, excitotoxic increases in glutamate lead to phosphorylation of cAMP-response element binding (CREB) (23). Moreover, the transcription factor CREB is implicated in stress effects and the pathology of MDD (24), and CREB protein mRNA levels are increased by chronic antidepressant treatment (25). Lithium is a clinically effective treatment of mood disorders (26), and it has been neuroprotective under various conditions, including glutamate excitotoxicity (27, 28). Therefore, in the present study, we tested whether chronic lithium treatment (CLT) could prevent or reduce the well characterized stress-induced neuronal remodeling of CA3 pyramidal cell neurons in the hippocampus or alter these potential molecular markers of CRS.
Methods
Animals. Adult male Sprague-Dawley rats (Charles River Breeding Laboratories) weighing 250-290 g were housed in groups of three rats per cage. Animals had unlimited access to food and water except during restraint sessions. Control rats were housed in a room separately from stressed rats, and all animals were maintained on a 12:12 h light/dark cycle, with lights on from 7:00 a.m. to 7:00 p.m. Rats were divided into four groups that include 21 days of CRS versus no stress control conditions. Rats had access to regular rat chow or chow containing Li2CO3 (0.24%, wt; Harlan Teklad, Madison, WI) with supplemental saline (0.9%), beginning 14 days before the onset of stress to mimic clinical conditions (29). All experiments were performed during the lights-on period and were approved by the institutional Animal Care Committee at the Rockefeller University, or the Canadian Council on Animal Care at McMaster University.
Restraint Stress. Animals were subjected to 21 days of CRS (6 h/day) in the homecage, as described (30). Each day, rats entered wire-mesh tubes with protective rubberized edges and were restrained for as minimal movement as possible throughout the stress period.
Single-Section Golgi-Impregnation Technique. At the end of the study, rats were perfused transcardially with a phosphate buffer or with 4% paraformaldehyde, and post-fixed brains were cut into 100 μm-thick sections, processed, and analyzed for dendritic length and branch number according to a modified version of the rapid Golgi method described in ref. 31. Only pyramidal cells that (i) were located within the CA3c subregion; (ii) had dark and consistent impregnation throughout the dendrites; (iii) were relative isolation from neighboring cells; and (iv) had cell bodies in middle-third of section were analyzed. For each brain, 6-12 pyramidal cells from the dorsal hippocampus (Paxinos Atlas: interaural 6.88-4.70) were traced by using a camera-lucida drawing tube and a ×400 objective and analyzed. Additional Sholl analysis was performed on these cells, examining each 20-μm segment of the dendritic tree by using an interactive digitizing system (Zeiss).
In Situ Hybridization. Fresh frozen brains were cut into 10-μm coronal sections from rostral to caudal hippocampus (Paxinos Atlas: interaural 4.18-0 mm). Tissue was prepared for in situ hybridization for the GLT-1 as described (32) (see Supporting Methods, which is published as supporting information on the PNAS web site).
Tissue Preparation and Immunohistochemical Staining. Rats were killed with pentobarbital (60 mg/kg) and perfused transcardially with 250 ml of 4% paraformaldehyde containing 0.125% glutaraldehyde. Brains were dissected, post-fixed, rinsed in phosphate buffer, sunk in sucrose, and cut on a freezing microtome into 40-μm coronal sections that were stored at -20°C in cryoprotectant. Immunohistochemistry was carried out with an Ab that recognizes the phosphorylated CREB (pCREB) (Ser-133) (Cell Signaling Technology, Beverly, MA) by using the ABC Elite kit and following the streptavidin-peroxidase method (Vector Laboratories) (33). The peroxidase reaction was carried out with a 3,3′-diaminobenzidine tetrahydrochloride solution according to the manufacturer's instructions (Sigma), and sections were mounted for analysis (see Supporting Methods).
Analysis of pCREB Staining. pCREB-labeled cells were identified and counted by using a ×100 1.4 numerical aperture objective and a 1.4 numerical aperture auxiliary condenser lens (Nikon) to achieve optimal optical sectioning of the tissue. Cells appearing in the upper focal plane were omitted to prevent counting cell caps. Total granule cell numbers were estimated in a 1:12 series of sections at ×100 by using the optical-fractionator method in which 1% of the area of the granule cell layer on each section was sampled through 5 μm of the section thickness by using a stack of five 1-μm dissectors and a 15 × 15-μm dissector frame (34). The volume of the granule cell layer was measured in each animal on the same series of sections by the Cavalieri method by using a counting grid with an area associated with the counting points of 2,500 μm2 at ×10 magnification.
Open-Field Exploration. The day after the last stress treatment (day 22), exploratory behavior of stress and control rats was measured in an open-field apparatus, for one 5-min trial. Rats were placed in a novel square arena (70 × 70 × 40 cm) made of black Plexiglas with a video camera mounted 1 m above the maze (see Supporting Methods).
Plus-Maze Exploration. On day 23, rats were placed in the plus-maze, and behavioral responses were videotaped for 5 min. Behavior was assessed in a novel elevated plus-shaped Plexiglas apparatus, consisting of two open arms and two enclosed arms (each arm 50 × 10 cm). The plus-maze is an effective measure for determining the efficacy of several anxiolytic and anxiogenic drugs (35) (see Supporting Methods).
Results
The Effect of Chronic Stress and Lithium Treatment on CA3 Pyramidal Cell Neurons. We found a significant decrease in apical length and branch points on pyramidal neurons of the CA3 subregion of the hippocampus in animals exposed to CRS. Specifically, there was a significant effect of stress on the length of apical dendrites [F(1,26) = 14.75; P < 0.001]. CLT prevented the stress-induced reductions in dendritic length in the apical dendrites in the CA3 region, but it had no significant effect on its own (Figs. 1 and 2). Thus, there was a significant interaction between stress and lithium treatment [F(1,26) = 4.44; P < 0.05], such that lithium treatment prevented the stress-induced reduction in apical dendritic length. Stress significantly altered the number of branch points on the pyramidal cell apical dendrites [F(1,26) = 4.91; P < 0.05], with marginal effects on branch points reported in the basal dendrites as well [F(1,26) = 4.12; P = 0.052; Fig. 2]. There were no significant effects of chronic stress or lithium treatment on the dendritic length of basal dendrites [F(1,26) = 2.08; P = 0.16; and F(1,26) = 1.39; P = 0.25, respectively].
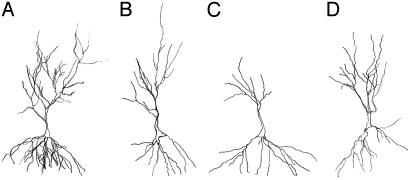
Fig. 1.
Drawings of pyramidal cell neurons representing each experimental group under conditions of stress versus no stress, administered either lithium impregnated or regular chow. (A) No stress with lithium. (B) No stress. (C) Stress. (D) Stress with lithium. Drawings were completed at ×400 magnification and do not necessarily depict dendritic thickness.
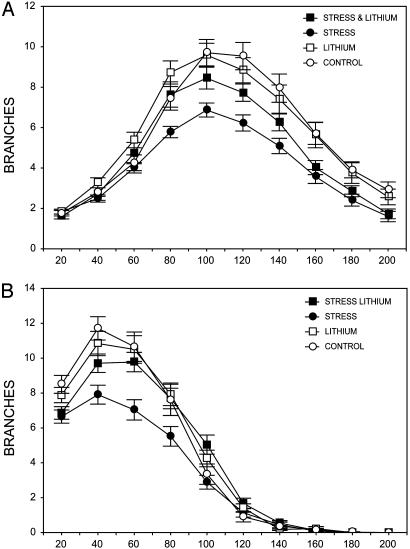
Fig. 2.
Effect of chronic stress and lithium treatment on CA3 pyramidal cell dendritic length and branch points in each experimental group. White bars, control chow (n = 7); light gray bars, lithium control (n = 7); black bars, stress (n = 9); and hatched bars, stress with lithium (n = 7). Chronic stress significantly reduced the length of apical (A), but not basal (B), dendrites. Chronic stress significantly reduced the number of branch points on the apical dendrites (C), and it had marginal effects on basal dendrites (D). CLT prevented the stress-induced decrease in apical dendritic length but did not have significant effects on its own or prevent stress effects on the number of branch points.
To examine the effects of chronic stress and lithium treatment more thoroughly, we performed a Sholl analysis to determine the extent of these effects along the length of the dendritic tree. Our results indicate that there is a significant effect of stress and lithium, depending on the segment of the cell analyzed. There was an interaction between stress and lithium treatment over segments in the apical [F(9,1,611) = 1.89; P < 0.05] and basal [F(9,1,611) = 2.45; P < 0.05] dendrites (Fig. 3). Planned comparisons indicate that there is an interaction between stress and lithium treatment in the apical dendritic tree (peaking at 120 μm away from the cell body) as well as sections of the basal dendritic tree (40-60 μm away from the cell body).
Fig. 3.
The effects of chronic stress and lithium treatment along the length of the pyramidal cells analyzed for length and number of branch points. Chronic stress and lithium treatment significantly altered specific areas of the apical (A) and basal (B) dendritic tree. Additionally, Sholl analysis indicates a significant interaction between stress and lithium, such that lithium treatment prevented the effect of stress in selected regions of the dendritic tree.
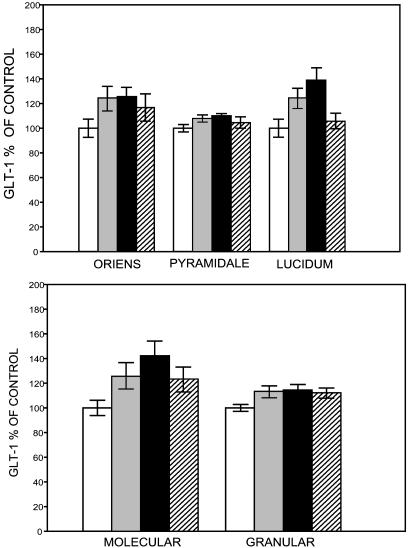
The Effect of Chronic Stress and Lithium Treatment on GLT-1 Expression. GLT-1 mRNA expression may reflect an elevation in extracellular glutamate levels as a result of CRS (18). The effect of 21 days of CRS and lithium treatment on GLT-1 mRNA expression is evident throughout many of the regions that we measured. CRS reliably caused an increase in GLT-1 mRNA expression in several regions of the hippocampus relative to the controls: CA3, DG, and the hilus (Fig. 4, and see Table 1, which is published as supporting information on the PNAS web site). There was a stress by lithium treatment interaction, in which lithium prevented the effects of stress in the CA3c [F(1,39) = 7.70; P < 0.01] and molecular and granule layers of the DG [F(1,41) = 5.49; P < 0.05] and the hilus [F(1,41) = 5.09; P < 0.05], with no significant effects in the cortex [F(1,41) = 0.23; P = 0.64]. We report that lithium treatment prevented the chronic stress-induced reduction of dendritic length on pyramidal cells. Planned comparisons of GLT-1 mRNA expression measures in these regions indicate that lithium prevents the effects of stress in most layers of the CA3c: the stratum oriens [F(1,39) = 3.23; P = 0.08], the stratum pyramidale [F(1,39) = 4.33; P < 0.05], and the stratum lucidum [F(1,39) = 11.67; P < 0.01].
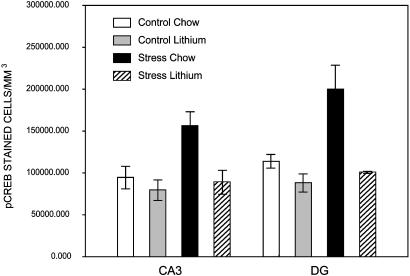
The Effect of Chronic Stress on pCREB Staining. As reported (23), CREB phosphorylation reflects elevated glutamatergic activity in the hippocampus. There was a significant effect of experimental group on pCREB-stained cell density in the CA3 [F(3,28) 6.21; P < 0.01] and the DG [F(3,28) 8.39; P < 0.001]. Post hoc analysis using a Newman-Keuls test indicates that stressed animals had a significantly higher density of pCREB staining than controls in the CA3 (P < 0.01) and DG (P < 0.01). Moreover, CLT prevented the stress-induced increase in pCREB-stained cell density, which did not differ from controls in the CA3 (P = 0.78) or the DG (P = 0.59). The effects of stress were apparent without altering the total volume of the CA3 [F(3,28) = 2.17; P = 0.11] or the DG [F(3,28) = 0.34; P = 0.79]. Furthermore, CLT prevented the CRS-induced increase in pCREB expression but had no significant effects on pCREB cell density on its own in the CA3 (P = 0.73) or DG (P = 0.55).
Behavioral Observations. Chronic stress significantly altered all measures in the open field and the plus-maze; however, CLT did not prevent those behavioral effects (see Figs. 6 and 7 and Table 2, which are published as supporting information on the PNAS web site). The following effects of stress were apparent on behavior in the open field: measures of activity and exploration, i.e., total grid crosses [F(1,39) = 14.0; P < 0.01] and rearing [F(1,39) = 6.72; P < 0.05]; and measures of anxiety or fear, i.e., latency to enter the six center grids, [F(1,39) = 4.19; P = 0.05] and the ratio of inner/total grid crosses [F(1,39) = 4.78; P < 0.05]. The following behavioral measures in the plus-maze are thought to reflect levels of anxiety, and stress significantly altered all of them: total arm entries [F(1,35) = 14.1; P < 0.001]; latency to move from the starting position and explore the rest of maze [F(1, 35) = 6.58; P < 0.05]; entries into exposed or open arms [F(1, 35) = 3.99; P < 0.05]; percentage of time in open and closed arms [F(1,35) = 3.89; P = 0.05]; and the percentage of open arm and closed arm entries [F(1,35) = 4.11; P < 0.05]. CLT did not prevent the behavioral effects of stress.
Plasma CORT Levels, Lithium Levels, and Body Weights. A total of 21 days of CRS and 36 days of CLT did not significantly alter mean CORT levels at the time of killing [F(1,40) = 2.29; P = 0.14]. These data support previous reports that the male hypothal-amic-pituitary-adrenal axis habituates to the stressor during the 21-day period and is not significantly altered by CLT. Final body weights were carefully monitored and were not significantly altered by CRS or CLT because lithium-fed animals were supplemented with 0.9% saline and a rat-chow pellet daily. The final body weights were not significantly altered by stress [F(1,30) = 0.34; P = 0.56], lithium [F(1,30) = 1.70; P = 0.20], or stress-plus-lithium [F(1,30) = 0.65; P = 0.43] treatments. The mean plasma levels for both the lithium chow and lithium-plus-stress groups were within the therapeutic range (0.61 mmol/liter and 0.82 mmol/liter, respectively).
Discussion
Here, we report that the decrease in CA3 pyramidal apical dendritic length induced by CRS was prevented in animals treated with lithium, even though lithium administration did not prevent stress-induced changes in branch points or have significant effects on its own (Figs. 1, 2, 3). Chronic stress increased two molecular markers of glutamatergic activity (GLT-1 mRNA and pCREB immunoreactivity) significantly in the CA3 region and DG (Figs. 4 and 5, respectively). Lithium treatment prevented these stress-induced cellular and molecular changes, which depend on glutamatergic activity.
Fig. 4.
The effects of chronic stress and lithium treatment on GLT-1 mRNA expression in each experimental group. White bars, control chow; light gray bars, lithium control; black bars, stress; and hatched bars, stress with lithium. The number of subjects per group were 12, 12, 11, and 8 in CA3 (Upper) and 12, 12, 10, and 11 in the DG (Lower). GLT-1 mRNA optical density in the individual layers of the CA3 (stratum oriens, pyramidale, and lucidum) is shown, as well as the molecular and granule layers of the DG. Lithium prevented the stress-induced increase in GLT-1 mRNA expression in the CA3 region and in layers of the DG. There were no significant effects on the cortex (see Table 1).
Fig. 5.
Effect of chronic stress and lithium treatment on the density of pCREB staining in the CA3 and the DG. Chronic stress increases pCREB staining significantly in both regions. CLT prevented the stress-induced increase in pCREB-stained cell density, which did not differ from controls.
The Neuroprotective Effects of Lithium Treatment. Lithium is an effective treatment for patients with mood disorders. Lithium treatment has mood-stabilizing effects and reduces the risk of suicide (36). The specific mechanisms by which lithium treatment stabilizes mood disorders, however, are not fully understood. CLT has been proven to be neuroprotective under various conditions such as excitotoxicity (37, 38), status epilepticus (29), and hypoxia (39) in cellular and intact animal models. CLT has also been shown to increase neurogenesis in the DG of the rodent brain (40).
Lithium treatment has various effects on signal transduction, including signaling pathways that are implicated in neuroprotective mechanisms (27, 38). Lithium treatment mediates the expression of several critical target genes downstream of these signal-transduction pathways by, for example, increasing levels of the antiapoptotic factor Bcl-2 (41) and decreasing levels of the proapoptotic factor p53 (37). These data suggest that lithium treatment could be neuroprotective by preventing or reversing neuronal damage in an animal model relevant to the pathophysiology of mood disorders.
The Effect of Chronic Stress and Lithium on Glutamate Transporters. CRS increased levels of GLT-1 mRNA expression in the hippocampus ≈40% in the stratum lucidum of the CA3c and the molecular layer of the DG (Fig. 4). CLT prevents the stress-induced changes in GLT-1 mRNA expression, although it has no significant effects on its own. We have observed that CRS increases GLT-1 mRNA in the DG and Ammon's horn and GLT-1 protein levels in the CA3c region, increases that were reportedly (18) prevented by chronic treatment with the antidepressant tianeptine. The CRS-induced increase in GLT-1 protein expression in the CA3 suggests that the increase in extracellular concentrations of glutamate observed during stress (19, 42) may elicit a compensatory increase in GLT-1 expression. The ability of lithium to prevent this stress-induced increase is likely due to its ability to stabilize glutamate activity, including changes in transporter activity.
The Effect of Chronic Stress and Lithium Treatment on the pCREB. CRS significantly up-regulates pCREB expression in the hippocampus, whereas CLT prevented the effects of the stressor on pCREB expression (Fig. 5). Patients with mood disorders are reported to exhibit abnormalities in brain intercellular signal transduction (43). Specifically, altered cAMP signaling has been implicated in bipolar disorder, MDD, and suicide victims, and it is a target for antidepressant and mood-stabilizing treatments (44). Also, CLT is reported to decrease pCREB immunoreactivity in rat cerebral cortex and in the CA1 and CA3 regions of the hippocampus, whereas CREB immunoreactivity was unchanged (33). However, there is evidence to suggest that the Ab used here has nonspecific effects (H. Manji, personal communication).
The expression of pCREB is induced by diverse extracellular stimuli by means of protein kinase A, extracellular signal-related protein kinase, and CaMK (45), and it is implicated in activity-dependent neuronal plasticity and neurotrophin-mediated neuronal survival (46, 47). Transient pCREB and subsequent CRE-mediated gene transcription occur after exposure of glutamate in cultured neurons and after ischemic insult in adult gerbil hippocampal neurons (23). This increase in pCREB expression has been interpreted as an N-methyl-d-aspartate-type receptor-dependent protective response against metabolic stresses (23). Given that the neuronal remodeling induced after CRS can be prevented by blocking the actions of excitatory amino acids using or N-methyl-d-aspartate receptor inhibitors (10), a similar role for pCREB is implicated in neuronal remodeling.
The Effect of Chronic Stress and Lithium Treatment on Behavior. CRS is reported to induce deficits in hippocampal-dependent tasks such as spatial learning and memory performance (48), which, like neuronal remodeling, can be prevented by inhibiting glucocorticoid secretion (10). Some of these hippocampal-dependent stress effects have been prevented with antidepressant treatment (49). In contrast to the reversible dendritic atrophy of hippocampal pyramidal neurons, chronic immobilization stress, which is similar to CRS, results in a dendritic hypertrophy in the amygdala (50), a region implicated in the expression and neuroendocrine control of emotion (51). Furthermore, CRS is reported to increase aggression between cagemates (30) and facilitate conditioned fear and fearfulness in the open field (52, 53).
Measures of fear and anxiety in the open field and the plus-maze were altered significantly by stress, but CLT did not prevent these effects of stress (Table 2 and Fig. 5, see Fig. 8, which is published as supporting information on the PNAS web site). It is possible that lithium prevents stress-induced effects on the hippocampus but not the amygdala. Given the neuroprotective effects of lithium treatment on hippocampal neurons, a hippocampal-dependent task (e.g., contextual or classical conditioning) may be more sensitive to the protective effects of lithium treatment against CRS. Our behavioral-anxiety measures conflict with other reports (54) that lithium prevented swim stress-induced impairments on open field behavior; it is possible that by using a much larger, elevated open field for a significantly longer test trial would have been more sensitive to the effects of lithium here as well.
Many of the behavioral measures noted above are altered by stress-induced changes in CORT levels. The hypothalamic-pituitary-adrenal axis also has a critical role in neuronal atrophy in the hippocampus (55, 56), but CORT plasma levels are known to habituate to the stressor quickly by the end of the 21-day treatment (10). It has been reported (57) that clinically sufficient lithium prophylaxis does not necessarily prevent intermittent hypothalamic-pituitary-adrenal axis dysregulation after stressful experiences; thus, lithium treatment was not expected to disrupt CORT levels here, although a shift in hypothalamic-pituitary-adrenal axis activity earlier in the stress treatment cannot be eliminated. These data support the postulation that there are specific downstream effects of chronic stress, some of which are associated with changes in glutamate, may be mediated by lithium treatment, and may underlie changes in morphology, whereas others are not.
Chronic Stress and the DG-CA3 Circuit. The proximal segments of the apical dendrites of CA3 pyramidal cell neurons are associated with complex spines, or excrescences, which receive mossy-fiber input from granule cell neurons of the DG (58). Neural activity from the entorhinal cortex activates mossy-fiber projections from the DG to the CA3 pyramidal region and is proposed to mediate dendritic remodeling in that region (7). The DG-CA3 pathway is the primary excitatory afferent to the hippocampal region inferior where glutamate is the major neurotransmitter (59). In addition, CA3 pyramidal cells excite other CA3 neurons by means of recurrent projections (60, 61).
Restraint stress has been reported to elevate extracellular glutamate levels in hippocampus (19), which may be the stimulus for elevated GLT-1 mRNA expression (18). The data presented here indicate a significant interaction between CRS and CLT on dendritic morphology in the CA3, as well as GLT-1 and pCREB expression in the CA3 and DG. The cells in the CA3 region are reported to synapse back onto neurons in the DG and produce an excitatory bursting of activity known as sharp waves, which are thought to drive the reorganization of synaptic vesicles within the mossy-fiber terminals, excitatory input onto the CA3 pyramidal cell neurons, and subsequent neuronal remodeling (62). Lithium prevented the stress-induced neuronal remodeling, as well as the increase in GLT-1 mRNA expression and pCREB expression in both the CA3 region and the DG. Together with the lithium effects on seizure-induced mossy-fiber sprouting (29), these results suggest that the CA3-DG feedback loop may be a primary site of action for the therapeutic effects of lithium.
The Effect of Chronic Stress and Lithium Treatment on CA3 Dendritic Morphology: The Excitotoxic Hypothesis of Neuronal Remodeling. Our data indicate that CRS significantly alters apical dendritic length as well as branch number in both the apical and basal dendrites of CA3 pyramidal cell neurons. The neuronal remodeling of CA3c pyramidal cell dendrites during CRS, glucocorticoid exposure, or psychosocial stress have all proven to depend on excitatory amino acid activity (10, 21). It is interesting to note that the effects of glutamate activity parallel the effects of stress on learning in the literature. Mild stress exposure is reported to facilitate many types of learning, but it can lead to learning deficits under extreme conditions, presumably because of its effects in the hippocampus and the amygdala (63). This inverted “U” relationship may be tied to a similar relationship between glutamate and excitotoxic effects in limbic regions. During learning, glutamate accumulates in the synapse, binds to receptors, activates N-methyl-d-aspartate-type receptors (which mobilizes free cytosolic calcium and allows it to enter the neuron), and activates long-term changes in synaptic plasticity associated with learning and memory (64). Lee et al. (64) suggest that, at high concentrations, glutamate no longer functions as an excitatory neurotransmitter but rather as an excitotoxin, potentially resulting in more permanent damage to neurons. The therapeutic actions of CLT on the dendritic tree may depend on the ability of lithium to stabilize glutamate and, thus, prevent the neuronal remodeling of dendrites and the dysregulation of glutamate receptors. The ability of CLT to up-regulate and stabilize glutamate uptake has been demonstrated in presynaptic nerve endings in mouse cerebral cortex (27). The specific mechanism activated here by CRS and CLT remains a point of interest and examination.
Summary and Conclusions. These results emphasize the neuroprotective role of lithium treatment in selective hippocampal regions and the relevance of this animal model to the pathophysiology of mood disorders. Mood disorders are associated with cell loss in specific layers, and such a reduction in hippocampal volume may be important for the outcome of these disorders. CLT may, therefore, have specific effects that block dendritic remodeling, an ability that is shared with a few agents, such as tianeptine and phenytoin. These data provide supportive evidence of a link between the neuroprotective and therapeutic properties of CLT and advocate the usefulness of the CRS model in the examination of the pathophysiology of mood disorders.
Supplementary Material
Acknowledgments
We thank Dr. Husseini Manji for helpful editorial comments, as well as Katherine Hew, Eileen Guilfoyle, and Adelaide Acquaviva for their contribution to this effort. This research was funded in part by National Institute of Mental Health Grants MH41256 (to B.S.M.) and MH15125 (to G.E.W.); Canadian Institutes of Health Research Grant MOP-14998; and Stanley Medical Research Institute Grant 98-307 (to L.T.Y.).
Abbreviations: CORT, corticosterone; CLT, chronic lithium treatment; GLT-1, glial glutamate transporter 1; CREB, cAMP-response element binding; pCREB, phosphorylated CREB; MDD, major depressive disorder; CRS, chronic restraint stress; DG, dentate gyrus.
References
- 1.Lopez, A. D. & Murray, C. C. (1998) Nat. Med. 4, 1241-1243. [DOI] [PubMed] [Google Scholar]
- 2.Sheline, Y. I. (1996) Mol. Psychiatry 1, 298-299. [PubMed] [Google Scholar]
- 3.Sheline, Y. I., Sanghavi, M., Mintun, M. A. & Gado, M. H. (1999) J. Neurosci. 19, 5034-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacQueen, G. M., Campbell, S., McEwen, B. S., Macdonald, K., Amano, S., Joffe, R. T., Nahmias, C. & Young, L. T. (2003) Proc. Natl. Acad. Sci. USA 100, 1387-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altshuler, L. L., Bartzokis, G., Grieder, T., Curran, J. & Mintz, J. (1998) Arch. Gen. Psychiatry 55, 663-664. [DOI] [PubMed] [Google Scholar]
- 6.Strakowski, S. M., DelBello, M. P., Sax, K. W., Zimmerman, M. E., Shear, P. K., Hawkins, J. M. & Larson, E. R. (1999) Arch. Gen. Psychiatry 56, 254-260. [DOI] [PubMed] [Google Scholar]
- 7.McEwen, B. S. (1999) Annu. Rev. Neurosci. 22, 105-122. [DOI] [PubMed] [Google Scholar]
- 8.Vyas, A., Mitra, R., Rao, B. S. S. & Chattarji, S. (2002) J. Neurosci. 22, 6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wellman, C. L. (2001) J. Neurobiol. 49, 245-253. [DOI] [PubMed] [Google Scholar]
- 10.Magarinos, A. M. & McEwen, B. S. (1995) Neuroscience 69, 89-98. [DOI] [PubMed] [Google Scholar]
- 11.Pham, K., Nacher, J., Hof, P. R. & McEwen, B. S. (2003) Eur. J. Neurosci. 17, 879-886. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya, V. A. & Duman, R. S. (2001) Br. Med. Bull. 57, 61-79. [DOI] [PubMed] [Google Scholar]
- 13.Czeh, B., Michaelis, T., Watanabe, T., Frahm, J., de Biurrun, G., van Kampen, M., Bartolomucci, A. & Fuchs, E. (2001) Proc. Natl. Acad. Sci. USA 98, 12796-12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Hart, M. G. C., Czeh, B., de Biurrun, G., Michaelis, T., Watanabe, T., Natt, O., Frahm, J. & Fuchs, E. (2002) Mol. Psychiatry 7, 933-941. [DOI] [PubMed] [Google Scholar]
- 15.Magarinos, A. M., Orchinik, M. & McEwen, B. S. (1998) Brain Res. 809, 314-318. [DOI] [PubMed] [Google Scholar]
- 16.Woolley, C. S., Gould, E. & McEwen, B. S. (1990) Brain Res. 531, 225-231. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe, Y., Weiland, N. G. & McEwen, B. S. (1995) Brain Res. 680, 217-225. [DOI] [PubMed] [Google Scholar]
- 18.Reagan, L. P., Rosell, D. R., Wood, G. E., Spedding, M., Mundóz, C., Rothstein, J. & McEwen, B. S. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 19.Lowy, M. T., Gault, L. & Yamamoto, B. K. (1993) J. Neurochem. 61, 1957-1960. [DOI] [PubMed] [Google Scholar]
- 20.Magarinos, A. M., Deslandes, A. & McEwen, B. S. (1999) Eur. J. Pharmacol. 371, 113-122. [DOI] [PubMed] [Google Scholar]
- 21.Magarinos, A. M., McEwen, B. S., Flugge, G. & Fuchs, E. (1996) J. Neurosci. 16, 3534-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe, Y., Gould, E., Cameron, H., Daniels, D. & McEwen, B. S. (1992) Eur. J. Pharmacol. 222, 157-162. [DOI] [PubMed] [Google Scholar]
- 23.Mabuchi, T., Kitagawa, K., Kuwabara, K., Takasawa, K., Ohtsuki, T., Xia, Z., Storm, D., Yanagihara, T., Hori, M. & Matsumoto, M. (2001) J. Neurosci. 21, 9204-9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowlatshahi, D., MacQueen, G. M., Wang, J. F. & Young, L. T. (1998) Lancet 352, 1754-1755. [DOI] [PubMed] [Google Scholar]
- 25.Nibuya, M., Nestler, E. J. & Duman, R. S. (1996) J. Neurosci. 16, 2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poolsup, N., Li Wan Po, A. & de Oliveira, I. R. (2000) J. Clin. Pharm. Ther. 25, 139-156. [DOI] [PubMed] [Google Scholar]
- 27.Dixon, J. F. & Hokin, L. E. (1998) Proc. Natl. Acad. Sci. 95, 8363-8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nonaka, S. & Chuang, D. M. (1998) NeuroReport 9, 2081-2084. [DOI] [PubMed] [Google Scholar]
- 29.Cadotte, D. W., Xu, B., Racine, R. J., MacQueen, G. M., Wang, J. F., McEwen, B. & Young, L. T. (2003) Neuropsychopharmacology 28, 1448-1453. [DOI] [PubMed] [Google Scholar]
- 30.Wood, G. E., Young, L. T., Reagan, L. P. & McEwen, B. S. (2003) Horm. Behav. 43, 205-213. [DOI] [PubMed] [Google Scholar]
- 31.Magarinos, A. M. & McEwen, B. S. (1995) Neuroscience 69, 83-88. [DOI] [PubMed] [Google Scholar]
- 32.Reagan, L. P., McKittrick, C. R. & McEwen, B. S. (1999) Neuroscience 91, 211-219. [DOI] [PubMed] [Google Scholar]
- 33.Chen, B., Wang, J. F., Hill, B. C. & Young, L. T. (1999) Brain Res. Mol. Brain Res. 70, 45-53. [DOI] [PubMed] [Google Scholar]
- 34.West, M. J., Slomianka, L. & Gundersen, H. J. G. (1991) Anat. Rec. 231, 482-497. [DOI] [PubMed] [Google Scholar]
- 35.File, S. E. (1993) Behav. Brain Res. 58, 199-202. [DOI] [PubMed] [Google Scholar]
- 36.Grof, P. (2003) J. Clin. Psychiatry 64, 53-61. [PubMed] [Google Scholar]
- 37.Chen, G. & Chuang, D. M. (1999) J. Biol. Chem. 274, 6039-6042. [DOI] [PubMed] [Google Scholar]
- 38.Coyle, J. T. & Duman, R. S. (2003) Neuron 38, 157-160. [DOI] [PubMed] [Google Scholar]
- 39.Ren, M., Senatorov, V. V., Chen, R. W. & Chuang, D. M. (2003) Proc. Nat. Acad. Sci. 100, 6210-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen, G., Rajkowska, G., Du, F., Seraji-Bozorgzad, N. & Manji, H. K. (2000) J. Neurochem. 75, 1729-1734. [DOI] [PubMed] [Google Scholar]
- 41.Chen, G., Zeng, W. Z., Yuan, P. X., Huang, L. D., Jiang, Y. M., Zhao, Z. H. & Manji, H. K. (1999) J. Biol. Chem. 72, 879-882. [DOI] [PubMed] [Google Scholar]
- 42.Lowy, M. T., Wittenberg, L. & Yamamoto, B. K. (1995) J. Neurochem. 65, 268-274. [DOI] [PubMed] [Google Scholar]
- 43.Wang, J. F., Li, P. P., Warsh, J. J. & Young, L. T. (1997) in Bipolar Disorder: Biological Models and Their Clinical Application., eds. Young, L. T. & Joffe, R. T. (Marcel Dekker, New York), pp. 41-49.
- 44.Dowlatshahi, D., MacQueen, G. M., Wang, J. F., Reiach, J. S. & Young, L. T. (1999) J. Neurochem. 73, 1121-1126. [DOI] [PubMed] [Google Scholar]
- 45.Finkbeiner, S. (2000) Neuron 25, 11-14. [DOI] [PubMed] [Google Scholar]
- 46.Bonni, A., Brunet, A., West, A. E., Datta, S. R., Takasu, M. A. & Greenberg, M. E. (1999) Science 286, 1358-1362. [DOI] [PubMed] [Google Scholar]
- 47.Riccio, A., Ahn, S., Davenport, C. M., Blendy, J. A. & Ginty, D. D. (1999) Science 286, 2358-2361. [DOI] [PubMed] [Google Scholar]
- 48.Luine, V. (2002) Stress (Amsterdam, The Netherlands) 5, 205-216. [DOI] [PubMed] [Google Scholar]
- 49.Conrad, C. D., Galea, L. A. M., Kuroda, Y. & McEwen, B. S. (1996) Behav. Neurosci. 110, 1321-1334. [DOI] [PubMed] [Google Scholar]
- 50.Vyas, A., Mitra, R. & Chattarji, S. (2003) Ann. N.Y. Acad. Sci. 985, 554-555. [Google Scholar]
- 51.Lupien, S. J. & McEwen, B. S. (1997) Brain Res. Rev. 24, 1-27. [DOI] [PubMed] [Google Scholar]
- 52.Conrad, C. D., Magarinos, A. M., LeDoux, J. E. & McEwen, B. S. (1999) Behav. Neurosci. 113, 902-913. [DOI] [PubMed] [Google Scholar]
- 53.Sandi, C., Merino, J. J., Cordero, M. I., Touyarot, K. & Venero, C. (2001) Neuroscience 102, 329-339. [DOI] [PubMed] [Google Scholar]
- 54.Einat, H., Yuan, P., Gould, T. D., Li, J., Du, J., Zhang, L., Manji, H. K. & Chen, G. (2003) J. Neurosci. 23, 7311-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEwen, B. S. (1999) Integr. Med. 1, 135-141. [Google Scholar]
- 56.Sapolsky, R. M. (2000) in Coping with the Environment: Neural and Endocrine Mechanisms (Oxford Univ. Press, New York), pp. 517-532.
- 57.Deshauer, D., Grof, E., Alda, M. & Grof, P. (1999) Biol. Psychiatry 48, 1023-1029. [DOI] [PubMed] [Google Scholar]
- 58.Blackstad, T. W. & Kjarheim, A. (1961) J. Comp. Neurol. 11, 133-146. [DOI] [PubMed] [Google Scholar]
- 59.Storm-Mathisen, J., Leknes, A. K., Bore, A. T., Valand, J. L., Edminson, P., Haug, F. S. & Ottersen, O. P. (1983) Nature 301, 517-520. [DOI] [PubMed] [Google Scholar]
- 60.Ishizuka, N., Weber, J. & Amaral, D. G. (1990) J. Comp. Neurol. 295, 580-623. [DOI] [PubMed] [Google Scholar]
- 61.Li, X. G., Somogyi, P., Ylinen, A. & Buzsaki, G. (1994) J. Comp. Neurol. 339, 181-208. [DOI] [PubMed] [Google Scholar]
- 62.McEwen, B. S. & Magarinos, A. M. (2001) Hum. Psychopharmacol. 16, S7-S19. [DOI] [PubMed] [Google Scholar]
- 63.Roozendaal, B., Nguyen, B. T., Power, A. E. & McGaugh, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 11642-11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee, A. L., Ogle, W. O. & Sapolsky, R. M. (2002) Bipolar Disord. 4, 117-128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.