Abstract
Background: Qualitative aspects of diet influence eating behavior, but the physiologic mechanisms for these calorie-independent effects remain speculative.
Objective: We examined effects of the glycemic index (GI) on brain activity in the late postprandial period after a typical intermeal interval.
Design: With the use of a randomized, blinded, crossover design, 12 overweight or obese men aged 18–35 y consumed high- and low-GI meals controlled for calories, macronutrients, and palatability on 2 occasions. The primary outcome was cerebral blood flow as a measure of resting brain activity, which was assessed by using arterial spin-labeling functional magnetic resonance imaging 4 h after test meals. We hypothesized that brain activity would be greater after the high-GI meal in prespecified regions involved in eating behavior, reward, and craving.
Results: Incremental venous plasma glucose (2-h area under the curve) was 2.4-fold greater after the high- than the low-GI meal (P = 0.0001). Plasma glucose was lower (mean ± SE: 4.7 ± 0.14 compared with 5.3 ± 0.16 mmol/L; P = 0.005) and reported hunger was greater (P = 0.04) 4 h after the high- than the low-GI meal. At this time, the high-GI meal elicited greater brain activity centered in the right nucleus accumbens (a prespecified area; P = 0.0006 with adjustment for multiple comparisons) that spread to other areas of the right striatum and to the olfactory area.
Conclusions: Compared with an isocaloric low-GI meal, a high-GI meal decreased plasma glucose, increased hunger, and selectively stimulated brain regions associated with reward and craving in the late postprandial period, which is a time with special significance to eating behavior at the next meal. This trial was registered at clinicaltrials.gov as NCT01064778.
INTRODUCTION
The mesolimbic dopaminergic system of the brain, which converges on the nucleus accumbens (part of the striatum), plays a central role in reward and craving, and this system appears to mediate hedonic food responses (1–3). In rodent studies, extracellular concentrations of dopamine and its metabolites in the nucleus accumbens increased more after the consumption of highly palatable food than standard rodent feed pellets (4). Furthermore, microinjections of opiate into the nucleus accumbens increased food intake and the reward value of food (5). Clinical studies that used functional brain imaging have reported greater activation in the nucleus accumbens or other regions of the striatum in obese than lean individuals after they viewed or consumed palatable, high-calorie food (6–11). Of particular interest, striatal dopamine D2 receptor availability was significantly lower in obese individuals than in nonobese matched controls (11), which raised the possibility that overeating may compensate for low dopaminergic activity. However, these cross-sectional comparisons between groups of lean and obese people could not assess the causal direction.
Physiologic observations regarding the glycemic index (GI)5 provide a mechanism for understanding how a specific dietary factor, other than palatability, might elicit food craving and overeating. The GI describes how carbohydrate-containing foods affect blood glucose in the postprandial state (12, 13). As previously described in obese adolescents (13, 14), the consumption of a high- compared with low-GI meal resulted in higher blood glucose and insulin in the early postprandial period (0–2 h), which were followed by lower blood glucose in the late postprandial period (3–5 h). The decrease in blood glucose, which often falls below fasting concentrations by 4 h after a high-GI meal, may lead to excessive hunger, overeating, and a preference for foods that rapidly restore blood glucose to normal (ie, high GI) (15–17), propagating cycles of overeating. Indeed, in a study of lean and obese adults, a mean insulin-induced decrease in blood glucose concentrations from 4.9 to 3.7 mmol/L increased the food-stimulus activation of the striatum and desire for high-calorie foods (18). To explore these mechanisms, we compared effects of high- and low-GI test meals controlled for calories, macronutrient contents, ingredient sources, and palatability during the late postprandial period by using functional brain imaging of reward circuitry implicated in food motivation and energy balance.
SUBJECTS AND METHODS
We conducted a randomized, blinded, crossover study in healthy overweight and obese young men and compared the effects of high- and low-GI test meals on 2 d separated by 2–8 wk. The primary outcome was cerebral blood flow as a measure of resting brain activity, which was determined by using arterial spin labeling (ASL) fMRI 4 h after the test meal. We hypothesized that the high-GI meal would increase the activity in the striatum, hypothalamus, amygdala, hippocampus, cingulate, orbitofrontal cortex, and insular cortex, which are brain regions involved in eating behavior, reward, and addiction (6–11). Secondary endpoints included plasma glucose, serum insulin, and reported hunger throughout the 5-h postprandial period. The palatability of the test meals was also assessed by using a 10-cm visual analog scale (VAS). Statistical treatments included the prespecification of brain regions of interest and correction for multiple comparisons. The protocol was conducted at and received ethical review from the Beth Israel Deaconess Medical Center (Boston, MA). The trial was registered at clinicaltrials.gov as NCT01064778, and participants provided written informed consent. Data were collected between 24 April 2010 and 25 February 2011.
Participants
Participants were recruited with fliers and posters distributed in the Boston metropolitan area and Internet listings. Inclusion criteria were male sex, age between 18 and 35 y, and BMI (in kg/m2) ≥25. Women were not included in this initial study to avoid the confounding that might have arisen from the menstrual cycle (19). Exclusion criteria were any major medical problem, use of a medication that affected appetite or body weight, smoking or recreational drug use, high levels of physical activity, current participation in a weight-loss program or change in body weight >5% in the preceding 6 mo, allergies to or intolerance of test meals, and any contraindication to the MRI procedure [eg, contraindicated metallic implants, weight >300 lb (136 kg)]. Eligibility was assessed by telephone screening followed by an in-person evaluation session. At the evaluation session, we obtained anthropometric measures and conducted an oral glucose tolerance test. In addition, participants sampled test meals and underwent an MRI sequence to ascertain ability to tolerate the procedure.
Enrolled participants were entered sequentially onto a list of random assignments (prepared by the Clinical Research Center at Boston Children's Hospital) for the order of test meals by using randomly permuted blocks of 4. Liquid test meals were supplied to participants by study staff in paper cups. Both test meals had a similar appearance, smell, and taste. All participants and research staff involved in data collection were masked to the intervention sequence. Participants received $250 for completing the protocol.
Test meals
Test meals were modified from Botero et al (20) to achieve similar sweetness and palatability in taste tests that involved study staff. As shown in Table 1, both test meals were composed of similar ingredients and had the same macronutrient distribution (ProNutra Software, version 3.3.0.10; Viocare Technologies Inc). The predicted GI of the high- and low-GI test meals were 84% and 37%, respectively, by using glucose as the reference standard. The calorie content of test meals was individually determined to provide each participant with 25% of daily energy requirements on the basis of an estimate of resting energy expenditure (21) and an activity factor of 1.2.
TABLE 1.
Test-meal composition1
| High GI | Low GI | |
| Ingredients2 | ||
| Cornstarch (g) | — | 57.2 |
| Light corn syrup (g) | 79.0 | — |
| Vanilla extract (g) | 7.3 | 7.3 |
| Milk, 1% fat (g) | — | 260.0 |
| Lactaid milk, 1% fat (g) | 254.8 | — |
| Egg white, dried (g) | 13.0 | 11.4 |
| Equal3 (Merisant Co) (g) | 2.1 | 3.1 |
| Olive oil (g) | 11.1 | 10.9 |
| Nutrient composition | ||
| Energy (kcal) | 500 | 500 |
| Carbohydrate (g) | 68.9 | 68.7 |
| Fat (g) | 13.7 | 13.5 |
| Protein (g) | 18.1 | 17.9 |
| Calculated GI4 | 84 | 37 |
GI, glycemic index.
Ingredient amounts were scaled so that the meal energy content corresponded to 25% of estimated daily energy requirements. Each participant's high- and low-GI test meals had the same caloric content.
Equal is an artificial sweetener containing aspartame, acesulfame potassium, dextrose, and maltodextrin.
The GI of each meal was calculated by using glucose as a reference standard.
Procedures
At the evaluation session, height and weight were measured, baseline descriptive data (including self-reported ethnicity and race) were collected, and serum thyroid-stimulating hormone (to screen for hypothyroidism) was obtained. Participants received a 75-g oral glucose tolerance test (beverage 10-O-75; Azer Scientific) with the sampling of plasma glucose and serum insulin at 0, 30, 60, 90, and 120 min.
Test sessions were separated by 2–8 wk. Participants were instructed to avoid changes in habitual diet and physical activity level for 2 d before each test session and maintain body weight within 2.5% of baseline throughout the study. Participants arrived for both test sessions between 0800 and 0930 having fasted ≥12 h and abstained from alcohol since the previous evening. At the beginning of each session, interval health was assessed, the duration of fast was confirmed, and weight and blood pressure were measured. A 20-gauge intravenous catheter was placed for serial blood sampling. After a 30-min acclimatization period, the randomly determined test meal was consumed in its entirety within 5 min. Blood samples and hunger ratings were obtained before and every 30 min after the start of the test meal during the 5-h postprandial period. We were unable to use a metallic hand-warming device to arterialize venous blood near the fMRI machine, and the stress involved in repeated finger sticks for capillary blood could have confounded the primary study outcome. The use of venous blood could have caused an error in the measurement of arterial blood glucose concentrations above and below fasting concentrations, especially for the high-GI meal, which comprised a study limitation (22). Palatability was assessed after completion of the test meal, and neuroimaging was performed after 4 h.
Measurements
Weight was measured in a hospital gown and light undergarments with a calibrated electronic scale (Scaletronix). Height was measured with a calibrated stadiometer (Holtman Ltd). BMI was calculated by dividing weight in kilograms by the square of height in meters. Blood pressure was obtained with an automated system (IntelliVue monitor; Phillips Healthcare) with the participant seated quietly for 5 min. Plasma glucose and thyroid-stimulating hormone were measured with Clinical Laboratory Improvement Amendments-approved methods (Labcorp). Serum was prepared by centrifugation and stored at −80°C for the measurement of insulin in one batch at the end of the study (Harvard Catalyst Central Laboratory).
Palatability was assessed with the question “How tasty was this meal?” Participants were instructed to make a vertical mark on a 10-cm VAS with verbal anchors that ranged from “not at all tasty” (0 cm) to “extremely tasty” (10 cm). Hunger was assessed similarly, with the question “How hungry are you right now?” and verbal anchors that ranged from “not hungry at all” to “extremely hungry” (14).
Neuroimaging was performed at 4 h after the test meal, when the blood glucose nadir after the high-GI meal was expected (14), by using a GE 3Tesla whole-body scanner (GE Healthcare). Cerebral blood flow was determined by using ASL, which is an MRI-based method that uses externally applied magnetic fields to transiently label inflowing arterial blood water for use as a diffusible tracer. A 3-plane localizer scan was obtained, followed by a T1-weighted dataset for anatomic correlation (Modified Driven Equilibrium Fourier Transform) (23), with a repetition time of 7.9 ms, echo time of 3.2 ms, 32-kHz bandwidth coronal acquisition plane, 24 × 19 field of view, 1-mm in-plane resolution, and 1.6-mm slices. The preparation time was 1100 ms with repeated saturation at the beginning of the preparation period and an adiabatic inversion pulse 500 ms before imaging. After these sequences, an ASL scan was obtained following previously described methods (24). The sequence used pseudocontinuous labeling with background suppression to minimize motion artifacts, a 3-dimensional multishot stack of spiral imaging, an image resolution of 3.8 mm in plane, and forty-four 4-mm slices per single volume. Pseudocontinuous labeling for 1.5 s with a 1.5-s postlabeling delay before image acquisition (25) was performed 1 cm below the base of the cerebellum (4 averages of label and control and 2 unsuppressed images for cerebral blood flow quantification were acquired). Cerebral blood flow was quantified with customized software as previously reported (24–26).
Statistical analyses
The study was designed to provide 80% power by using a 5% type I error rate to detect a difference in cerebral blood flow of 11.8%, assuming a sample size of 12 participants, residual SD of 11% for a single measurement, and intrasubject correlation of 0.6. The attained sample of 11 participants with useable data provided 80% power to detect a difference of 12.4%, with all other assumptions remaining.
Analyses of neuroimaging data were performed within the Statistical Parametric Mapping statistical image analysis environment (SPM5; Wellcome Department of Cognitive Neurology). Cerebral blood flow images were realigned to the first image and transformed to a standard anatomic space (Montreal Neurologic Institute/International Consortium for Brain Mapping) (27) by using the registration variables derived from the SPM5 Normalization algorithm. Images were smoothed with an 8-mm full width at half maximum kernel in preparation for the statistical analysis.
We examined stereotactic space by using templates within the WFU Pickatlas toolkit (28). Of a total 334 nonredundant anatomic regions throughout the brain, prespecified areas of interest encompassed 25 separate regions (see Supplemental Table 1 under “Supplemental data” in the online issue). To test our primary hypothesis, we compared the difference in mean regional blood flow (high-GI meal minus low-GI meal) by using paired, 2-tailed t tests adjusted for order effect and with Bonferroni correction for multiple comparisons (raw P value multiplied by 25). To depict the spatial distribution of cerebral blood flow differences, we conducted a voxel-by-voxel analysis by using algorithms of the general linear model (29) and a statistical threshold of P ≤ 0.002.
Incremental AUCs for plasma glucose (0–2 h), serum insulin (0–2 h), and hunger (0–5 h) were calculated by using the trapezoidal method. These areas and values for these outcomes at 4 h (the prespecified time point of primary interest) were analyzed for the test meal effect by using a 2-sided, paired t test with SAS software (version 9.2; SAS Institute Inc). Adjustment for the order effect did not materially affect these outcomes. To examine the relation between physiologic variables and brain activation, general linear model analyses were performed with blood flow in the right nucleus accumbens as a dependent variable and the participant number and respective metabolic variables as independent variables. Data are presented as means and, where indicated, SEs.
RESULTS
Study participants
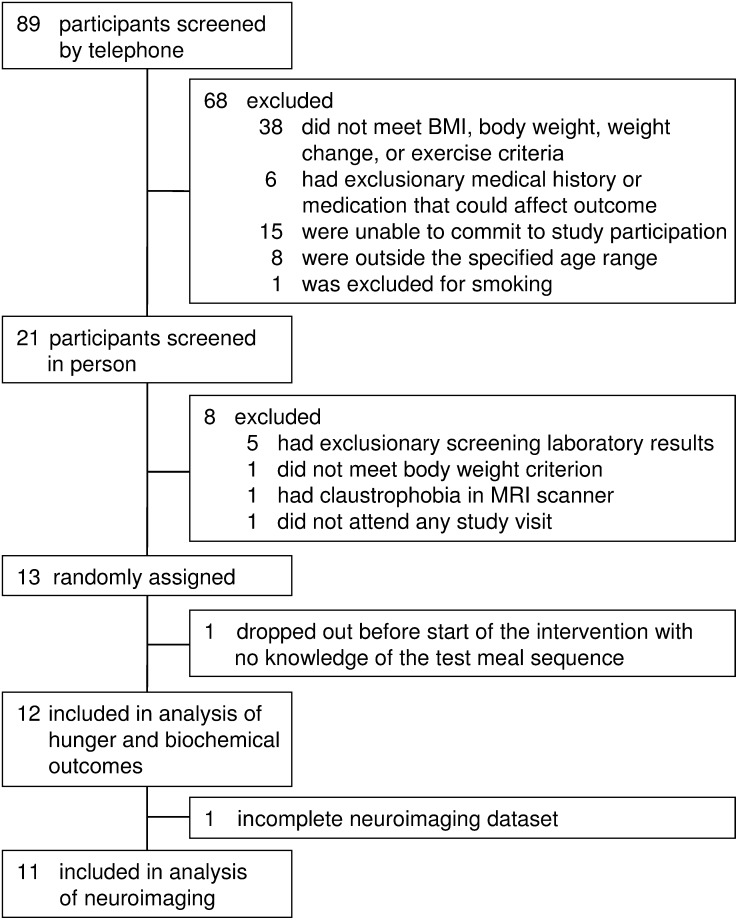
Of 89 individuals screened, we enrolled 13 men, with 1 dropout before administration of the first test meal (Figure 1). The remaining 12 participants included 2 Hispanics, 3 non-Hispanic blacks, and 7 non-Hispanic whites. The mean age was 29.1 y (range: 20–35 y), BMI was 32.9 (range: 26–41), fasting plasma glucose concentration was 4.9 mmol/L (range: 3.6–6.2 mmol/L), and fasting insulin concentration was 10.3 μU/mL (range: 0.8–25.5 μU/mL). Imaging data for one participant were incomplete because of a data-storage error; the other participants completed the protocol uneventfully.
FIGURE 1.
Participant flow diagram.
Subjective and biochemical responses to test meals
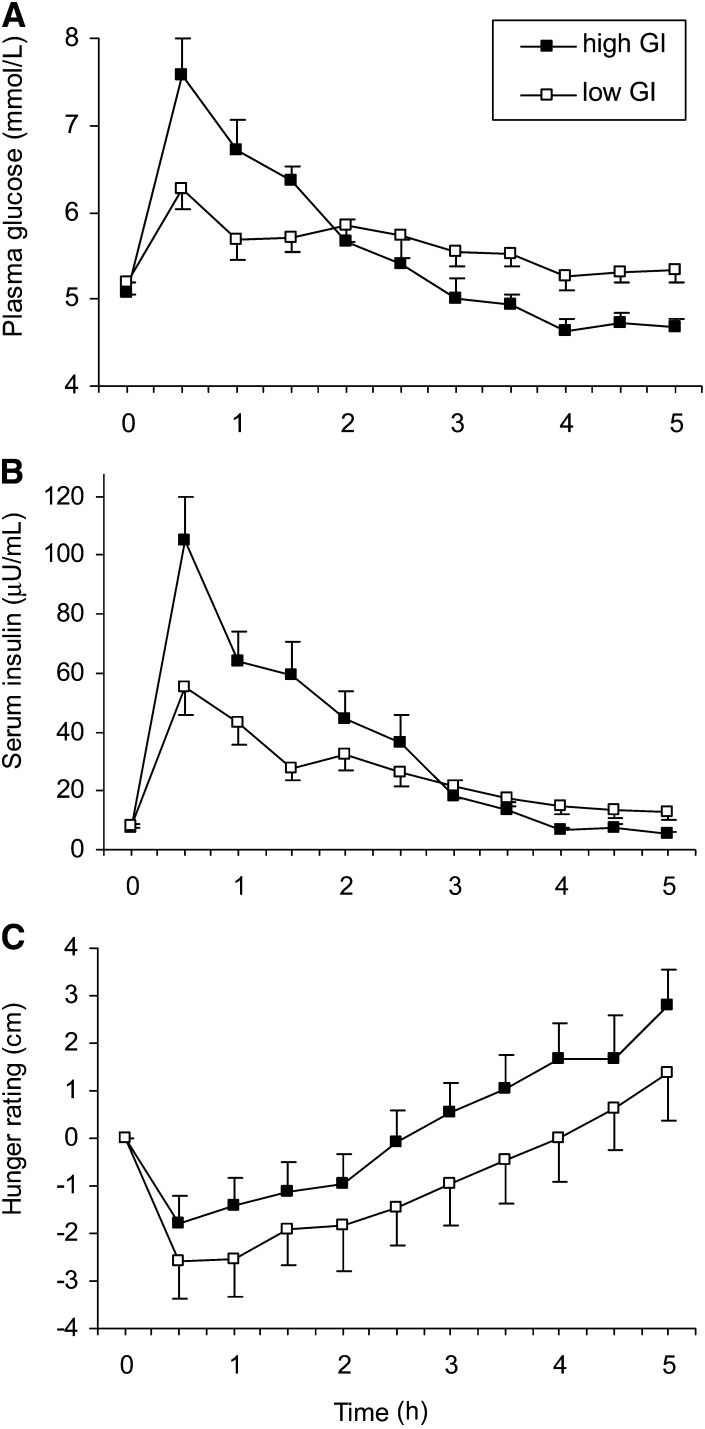
The palatability of high- and low-GI test meals did not differ according to responses on the 10-cm VAS (5.5 ± 0.67 compared with 5.3 ± 0.65 cm, respectively; P = 0.7). Consistent with the predicted GI (Table 1), the incremental 2-h AUC for glucose was 2.4-fold greater after the high- than low-GI test meal (2.9 ± 0.36 compared with 1.2 ± 0.27 mmol · h/L, respectively; P = 0.0001) (Figure 2). The incremental 2-h AUC for insulin (127.1 ± 18.1 compared with 72.8 ± 9.78 μU · h/mL; P = 0.003) and incremental 5-h AUC for hunger (0.45 ± 2.75 compared with −5.2 ± 3.73 cm · h; P = 0.04) were also greater after the high- than low-GI test meal, respectively. At 4 h into the postprandial period, the blood glucose concentration was lower (4.7 ± 0.14 compared with 5.3 ± 0.16 mmol/L, P = 0.005), and the change in hunger from baseline was greater (1.65 ± 0.79 compared with −0.01 cm ± 0.92; P = 0.04) after the high- than low-GI test meal, respectively.
FIGURE 2.
Mean ± SE changes in plasma glucose (A), serum insulin (B), and hunger (C) after test meals. Differences between high- and low-GI meals were significant at 4 h (the time point of interest) for all 3 outcomes by using paired t tests. n = 12. GI, glycemic index.
Brain imaging
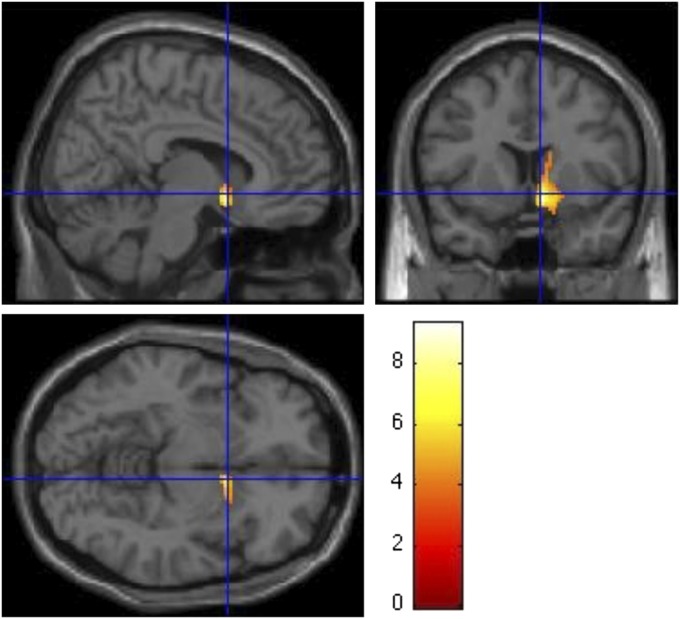
Cerebral blood flow was greater 4 h after the high- than low-GI meal in the right nucleus accumbens (mean difference: 4.4 ± 0.56 mL · 100 g−1 · min−1; range: 2.1–7.3 mL · 100 g−1 · min−1; an 8.2% relative difference). This difference remained significant after Bonferroni correction for the 25 prespecified anatomic regions of interest (P = 0.0006) and after correction for all 334 nonredundant brain regions (P = 0.009). An image-based analysis showed a single region in the right nucleus accumbens at Montreal Neurologic Institute/International Consortium for Brain Mapping coordinates 8, 8, −10 (peak t = 9.34) and another local maximum at coordinates 12, 12, 2 (t = 5.16), which spread to other areas of the right striatum (caudate, putamen, and globus pallidus) and olfactory area (Figure 3). We did not observe differences in the contralateral striatum or other prespecified regions of interest.
FIGURE 3.
Regions with significantly different cerebral blood flow 4 h after test meals (P ≤ 0.002). The color scale represents the value of the t statistic for the comparison between meals (n = 11) by using general linear model analyses as described in Subjects and Methods. For all areas depicted, the blood flow was greater after the high- than after the low-GI meal. GI, glycemic index.
The relation between metabolic variables and blood flow in the right nucleus accumbens is shown in Table 2. All variables related to plasma glucose, serum insulin, and hunger were significantly related to blood flow in the right nucleus accumbens, whereas the palatability of meals was not.
TABLE 2.
Relation between physiologic variables and blood flow in the right nucleus accumbens1
| Physiologic variables | F | P |
| Plasma glucose AUC 0–2 h | 14.46 | 0.003 |
| Plasma glucose at 4 h | 8.33 | 0.02 |
| Serum insulin AUC 0–2 h | 16.93 | 0.002 |
| Serum insulin at 4 h | 12.87 | 0.005 |
| Hunger AUC 0–5 h | 10.03 | 0.01 |
| Hunger at 4 h | 7.72 | 0.02 |
| Palatability | 0.35 | 0.56 |
General linear model analyses as described in Subjects and Methods (n = 11).
DISCUSSION
Food intake is regulated by hedonic and homeostatic systems (3) that historically served to maintain mean BMI within a healthy range under widely varying environmental conditions. However, coincident with the obesity epidemic, the food supply has changed radically, with the rapidly increasing consumption of highly processed food products derived primarily from grain commodities. As a consequence, the glycemic load (the multiplicative product of GI and carbohydrate amount) (30) of the US diet has risen substantially in the past half century, and this secular trend may adversely affect both systems that regulate food intake. The decline in blood glucose (and other metabolic fuels) (13, 14) in the late postprandial period after a high-GI meal would not only constitute a powerful homeostatic hunger signal (15) but also increase the hedonic value of food through striatal activation (18). This combination of physiologic events may foster food cravings with a special preference for high-GI carbohydrates (16, 17), thereby propagating cycles of overeating. In addition, the recurrent activation of the striatum may downregulate dopamine receptor availability and further heighten the drive to overeat (11).
This study had several strengths. First, we used ASL, which is a novel imaging technique that provides a quantitative measure of cerebral blood flow. The conventional method (blood oxygenation level–dependent fMRI) assesses acute changes in brain activity, not absolute differences, which typically limits observations to a few minutes after a physiologic perturbation. With ASL, we were able to examine persistent effects of test meals without superimposed stimuli (eg, pictures of high-calorie foods). Second, we used a crossover intervention rather than a cross-sectional comparison between groups (eg, lean compared with obese), which provided increased statistical power and evidence for causal direction. Third, we focused on a specific dietary factor by controlling for calorie content, macronutrient composition, ingredient sources, and food form, instead of comparing grossly different foods (eg, cheesecake compared with vegetables) (6, 10, 31, 32). Fourth, the 2 test meals were designed and documented to have a similar palatability, which helped to disentangle metabolic effects from immediate hedonic responses. Fifth, we examined the late postprandial period, which is a time with special significance to eating behavior at the next meal. Previous studies have typically limited the duration of observation to ≤1 h after food consumption, when glucose absorption peaks and a high-GI meal may transiently appear to provide benefits to brain function (33). Sixth, we used mixed meals with a macronutrient composition and dietary glycemic load within prevailing ranges. Thus, the findings have relevance to high-GI breakfasts commonly consumed in the United States (eg, a bagel and fat-free cream cheese) (12).
Main study limitations included the small size and an exclusive focus on overweight and obese men. Small studies limit generalizability and increase risk of a false-negative (but not false-positive) finding. Our study, despite its size, had robust power to test the a priori hypothesis with adjustment for multiple comparisons. Additional studies with lean control subjects, women, and obese individuals pre– and post–weight loss would be informative. We did not assess hedonic responses to the meals or food cravings directly, and therefore, we could not explore the relation between these subjective values and brain activation. In addition, the liquid form of the test meals limited the generalizability of findings to solid meals.
Several other interpretive issues warrant consideration. We did not anticipate an effect of the GI on the brain limited to the right hemisphere, although laterality has been previously implicated in neurobehavioral disorders that involve reward circuitry. Indeed, a study that compared insulin-sensitive compared with insulin-resistant men showed a differential effect of systemic insulin administration on glucose metabolism for the right, but not left, ventral striatum (34). We also did not observe differences in other prespecified brain regions, either because our study lacked power to see less robust effects or because such effects did not occur at the 4-h time point. Nevertheless, the chemical manipulation of the nucleus accumbens in rats resulted in the stimulation of orexigenic neurons and inhibition of anorexigenic neurons in the hypothalamus (35), which illustrated the influence of the striatum on other brain areas involved in feeding.
Beyond reward and craving, the nucleus accumbens is crucially involved in substance abuse and dependence (36–38), raising the question as to whether certain foods might be addictive. Indeed, the notion of food addiction has received extensive popular attention through diet books and anecdotal reports and is increasingly the subject of scholarly investigation. Recent studies that used the conventional blood oxygenation level–dependent fMRI have shown selective overactivity in the nucleus accumbens and related brain areas in obese compared with lean individuals when shown imagines of highly palatable food (6–11) and in subjects who scored high on a measure of food addiction (39). However, it might be argued that this pleasure response involving food does not fundamentally differ from the enjoyment of a golfer viewing pictures of a putting green or an audiophile hearing beautiful music (40). In contrast to previous research, our study used test meals of similar palatability and ASL methods to examine unstimulated brain activity after 4 h. Nevertheless, the validity of the concept of food addiction remains vigorously debated (41–47). Unlike drugs of abuse, food is necessary for survival, and some individuals can habitually consume large amounts of high-GI (and high-calorie, highly processed) food products with no apparent adverse physical or psychological consequences. Thus, the application of the concept of addiction to food warrants additional mechanistically-oriented interventional and observational study.
In conclusion, we showed that the consumption of a high- compared with a low-GI test meal increased the activity in brain regions related to food intake, reward, and craving in the late postprandial period, which was coincident with lower blood glucose and greater hunger. These neurophysiologic findings, together with longer feeding studies of weight-loss maintenance (48, 49), suggest that a reduced consumption of high-GI carbohydrates (specifically, highly processed grain products, potatoes, and concentrated sugar) may ameliorate overeating and facilitate maintenance of a healthy weight in overweight and obese individuals.
Supplementary Material
Acknowledgments
We thank Dorota Pawlak, Simon Warfield, and Phillip Pizzo for stimulating discussions and advice; Joanna Radziejowska for help with test-meal formulation and provision; and Henry Feldman for statistical advice. None of these individuals received compensation for their contributions.
The authors’ responsibilities were as follows—DCA, CBE, JMG, LMH, BSL, DSL, and ES: provided the study concept and design; DCA and BSL: acquired data and provided statistical expertise; DCA, JMG, LMH, BSL, and DSL: analyzed and interpreted data; BSL and DSL: drafted the manuscript; DCA, CBE, JMG, LMH, RR, and ES: critically revised the manuscript; RR: provided technical support; DCA, BSL, and DSL: obtained funding; DCA and DSL: provided supervision; and DSL: as principal investigator, had full access to all of the data in the study and took responsibility for the integrity of the data and accuracy of the data analysis. DCA received grants from the NIH and GE Healthcare, which is an MRI vendor, for imaging technique development and applications and royalties through his current and former academic institutions for inventions related to the ASL techniques used in this study. DSL received grants from the NIH and foundations for obesity-related research, mentoring, and patient care and royalties from a book about childhood obesity. BSL, LMH, ES, RR, CBE, and JMG reported no conflicts of interest.
Footnotes
Abbreviations used: ASL, arterial spin labeling; GI, glycemic index; VAS, visual analog scale.
REFERENCES
- 1.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav 2009;97:537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagher A. Functional brain imaging of appetite. Trends Endocrinol Metab 2012;23:250–60. [DOI] [PubMed] [Google Scholar]
- 3.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 2009;139:629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav 1996;53:221–6. [DOI] [PubMed] [Google Scholar]
- 5.Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking’ for food: map based on microinjection Fos plumes. Brain Res 2000;863:71–86. [DOI] [PubMed] [Google Scholar]
- 6.Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 2010;34:1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, Brooks WM, Butler MG, Zarcone JR, Goldstein JM. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes (Lond) 2012;36:638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007;37:410–21. [DOI] [PubMed] [Google Scholar]
- 9.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117:924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–47. [DOI] [PubMed] [Google Scholar]
- 11.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet 2001;357:354–7. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics 1999;103:E26. [DOI] [PubMed] [Google Scholar]
- 15.Campfield LA, Smith FJ, Rosenbaum M, Hirsch J. Human eating: evidence for a physiological basis using a modified paradigm. Neurosci Biobehav Rev 1996;20:133–7. [DOI] [PubMed] [Google Scholar]
- 16.Thompson DA, Campbell RG. Hunger in humans induced by 2-deoxy-D-glucose: glucoprivic control of taste preference and food intake. Science 1977;198:1065–8. [DOI] [PubMed] [Google Scholar]
- 17.Strachan MW, Ewing FM, Frier BM, Harper A, Deary IJ. Food cravings during acute hypoglycaemia in adults with Type 1 diabetes. Physiol Behav 2004;80:675–82. [DOI] [PubMed] [Google Scholar]
- 18.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest 2011;121:4161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 2010;1363:81–92. [DOI] [PubMed] [Google Scholar]
- 20.Botero D, Ebbeling CB, Blumberg JB, Ribaya-Mercado JD, Creager MA, Swain JF, Feldman HA, Ludwig DS. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity (Silver Spring) 2009;17:1664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 22.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev 2005;18:145–71. [DOI] [PubMed] [Google Scholar]
- 23.Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage 2004;21:757–67. [DOI] [PubMed] [Google Scholar]
- 24.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60:1488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 1996;16:1236–49. [DOI] [PubMed] [Google Scholar]
- 26.Järnum H, Steffensen EG, Knutsson L, Frund ET, Simonsen CW, Lundbye-Christensen S, Shankaranarayanan A, Alsop DC, Jensen FT, Larsson EM. Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 2010;52:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007;28:1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–9. [DOI] [PubMed] [Google Scholar]
- 29.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 1996;4:223–35. [DOI] [PubMed] [Google Scholar]
- 30.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50. [DOI] [PubMed] [Google Scholar]
- 31.Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012;58:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 2012;59:2709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013;309:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 2006;55:2986–92. [DOI] [PubMed] [Google Scholar]
- 35.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol 2003;284:R1436–44. [DOI] [PubMed] [Google Scholar]
- 36.Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci 1999;877:461–85. [DOI] [PubMed] [Google Scholar]
- 37.Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol 2008;154:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162:1403–13. [DOI] [PubMed] [Google Scholar]
- 39.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry 2011;68:808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salimpoor VN, van den Bosch I, Kovacevic N, McIntosh AR, Dagher A, Zatorre RJ. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 2013;340:216–9. [DOI] [PubMed] [Google Scholar]
- 41.Benton D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin Nutr 2010;29:288–303. [DOI] [PubMed] [Google Scholar]
- 42.Blumenthal DM, Gold MS. Neurobiology of food addiction. Curr Opin Clin Nutr Metab Care 2010;13:359–65. [DOI] [PubMed] [Google Scholar]
- 43.Corwin RL, Grigson PS. Symposium overview–food addiction: fact or fiction? J Nutr 2009;139:617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno C, Tandon R. Should overeating and obesity be classified as an addictive disorder in DSM-5? Curr Pharm Des 2011;17:1128–31. [DOI] [PubMed] [Google Scholar]
- 45.Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol Behav 2011;104:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelchat ML. Food addiction in humans. J Nutr 2009;139:620–2. [DOI] [PubMed] [Google Scholar]
- 47.Toornvliet AC, Pijl H, Tuinenburg JC, Elte-de Wever BM, Pieters MS, Frolich M, Onkenhout W, Meinders AE. Psychological and metabolic responses of carbohydrate craving obese patients to carbohydrate, fat and protein-rich meals. Int J Obes Relat Metab Disord 1997;21:860–4. [DOI] [PubMed] [Google Scholar]
- 48.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunesova M, Pihlsgard M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010;363:2102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012;307:2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.