Abstract
The endothelial cell adhesion molecules, including the integrin alpha v beta 3 (αvβ3) and E-selectin, are involved in the process of angiogenesis required for tumour growth, cell migration and metastasis. The purpose of this study was to assess and compare widely used tumour models to select the ones most suitable for angiogenesis research. Fifteen murine tumours were selected including melanoma (B16), colon (C26, C38, C51), mammary (MA13, MA16, MA16/Adr, MA17, MA17/Adr, MA25, MA44), pancreatic (PO2, PO3), Glasgow osteogenic sarcoma (GOS) and Lewis lung carcinoma (LLC). The tumour vascular density, assessed using the platelet endothelial cell adhesion molecule 1 (PECAM-1; CD31) immunostaining, revealed that B16 melanoma was poorly vascularized (<5%), whereas the colon and mammary tumours were well vascularized (5–15%). The most vascularized tumours (>15%) were the pancreatic tumours (PO2 and PO3), the sarcoma (GOS) and the lung tumour (LLC). The integrin αvβ3 and E-selectin evaluated by immunohistology, showed that 7/15 tumours expressed the αvβ3 integrin which was homogeneously distributed on all tumour sections (B16, C26, MA17/Adr, MA25, MA44, PO2, LLC). E-selectin was expressed in 4/15 tumours and its expression was restricted to the tumour periphery. Only 2/15 tumours (B16 and C26) were shown to express both integrin αvβ3 and E-selectin. In conclusion, these data not only contribute to a better understanding of the tumour biology of murine tumours, but can also guide the choice of appropriate models for antiangiogenic therapy, for selective drug delivery to tumours and the validation of tumour imaging modalities targeting these endothelial cell adhesion molecules.
Keywords: Animals; Cell Adhesion Molecules; metabolism; Cell Line, Tumor; E-Selectin; metabolism; Endothelial Cells; metabolism; Female; Integrin alphaVbeta3; metabolism; Mice; Neoplasms, Experimental; blood supply; metabolism; Neovascularization, Pathologic; metabolism
Keywords: mouse tumors, vascularization, integrin alpha v beta 3, E-selectin, melanoma, colon, mammary, pancreas, sarcoma, lung
Introduction
Angiogenesis, which is required for the growth of solid tumours and the metastatic dissemination of tumour cells, is probably the most important parameter for the control of cancer progression [1]. Angiogenesis is the formation of new capillaries from pre-existing microvessels and consists in a multi-step process, in which endothelial cells degrade their extracellular matrix, migrate into the perivascular spaces, proliferate and align to form cell-cell contacts to construct patent blood vessels (reviewed in [2]). This process is mediated by endothelial cell adhesion molecules (CAMs) represented by four principal classes: the selectins, which are transmembrane proteins subdivided in three groups E, L and P, expressed by endothelial cells, leucocytes and platelets, respectively; the integrins, which are transmembrane glycoproteins composed of two subunits α and β; the membrane proteins immunoglobulin superfamily; and, the cadherins, mediators of cell-cell interactions [3]. These CAMs also play an important role in the process of metastasis development and migration [4, 5]. Compared to normal tissues, the expression pattern of the different CAMs vary widely between tumour types [6]. Therefore, the knowledge of specific CAM expression in tumours is of importance because it can allow a better understanding of the biology of tumour metastasis and also contribute to better predict the tumour evolution in vivo.
Among the CAMs, two proteins appear more particularly involved in the angiogenesis process, i.e., the integrin alpha v beta 3 (αvβ3) and the E-selectin {Varner, 1996 10294/id;Kraling, 1996 9166/id}. The integrin αvβ3 is not (or weakly) constitutively expressed in normal vascular endothelial cells, whereas it is selectively expressed on proliferating vascular endothelial cells [9] and on vascular cells in tumours [10–12]. Several studies have indeed reported the requirement of integrin αvβ3 for angiogenesis {Brooks, 1994 10300/id;Eliceiri, 1999 10301/id;Weis, 2011 10351/id}. Similarly, E-selectin is also detected at very low levels in normal adult blood vessels, but is highly expressed in newly formed tumour capillaries [15] and in activated endothelium [4, 8]. In addition, several investigators have shown that E-selectin is involved in angiogenesis [8, 16, 17]. By their angiogenic and adhesion properties, both CAMs are involved in tumour growth and metastasis and can therefore influence cancer prognosis and/or response to antiangiogenic therapy [4, 5, 18, 19].
Although the importance of angiogenesis has become central to a better understanding of the cancer process, as well as to the search for more specific treatments, it is surprising that most preclinical murine models are poorly characterized with regard to their vascular density and their expression of the principal CAMs. This information could be helpful in order to select the best tumour models for angiogenic studies and to follow experimental therapeutic approaches.
The aim of the present study was to evaluate the vascular density of 15 frequently employed murine solid tumours using PECAM-1 (a constitutively expressed CAM; CD31), and to characterize the expression pattern of the integrin αvβ3 and E-selectin using immunological methods. Our results show that each tumour type possesses a unique expression pattern of CAMs that could influence the tumour biology and response to therapy.
Materials and methods
Mouse tumours
Mouse tumours were obtained from the Aventis Oncology Department (now Sanofi, Vitry sur Seine, France) and were maintained in the mouse strain of origin. Briefly, tumour fragments (30–60 mm3) were implanted subcutaneously using a 12 gauge trocar into the flanks of 6-week-old female mice of the appropriate mouse strain (reviewed in [20]) for a given tumour, as listed hereafter: B16 melanoma was implanted in C57Bl/6 mice [21]; colon carcinoma C26 and colon adenocarcinoma C51 in Balb/C, and C38 in C57Bl/6 [22, 23]; mammary adenocarcinomas MA13 in Balb/C, MA16/C [24], MA16/C/ADR [25], MA17, and MA17/Adr in C3H/HeN mice [26], and MA25 and MA44 in C57Bl/6; pancreatic ductal adenocarcinomas PO2 and PO3 in C57Bl/6 mice [27]; Glasgow osteogenic sarcoma GOS in C57Bl/6 mice [28]; and, Lewis lung carcinoma LLC in C57Bl/6 mice [29]. Mice were sacrificed by cervical dislocation when the tumour size reached 200 mm3, i.e., 10–20 days post-inoculation depending on tumour type. Samples of tumours (5×5×5 mm) were embedded frozen in isopentane immersed in liquid nitrogen and stored at −70°C until cutting on microtome and prepared for immunohistological analysis.
Primary antibodies used for immunostaining
Monoclonal rat antibodies anti-PECAM-1 (CD31, clone: MEC13.3), anti-E-selectin (CD62E, clone: 10E9.6) and hamster antibody anti-integrin αvβ3 (CD61, clone: 2C9.G2, directed against sub-unit β3) were obtained from BDBiosciences (Le Pont de Claix, France). These antibodies were shown to cross-react with the corresponding mouse antigens. The working dilutions for immunostaining were 50-fold for anti-PECAM-1 (15.6 μg/mL) and 20-fold for anti-E-selectin (62.5 μg/mL) and anti-integrin αvβ3 (250 μg/mL). Omission of the primary antibody was used for negative controls.
Immunohistological analysis
Ten-micron frozen tissue sections were placed on Superfrost Plus slides. Immunostaining of the CAMs was performed using a three-step procedure as previously described [8]. Endogenous peroxidase was inactivated with 0.3% H2O2 in phosphate-buffered saline (PBS) for 30 min. Non-specific binding sites were blocked with 10% serum of the species used for the secondary antibody in PBS for 30 min. Slides were then incubated for 1 h (CD31, anti-PECAM-1) or 2 h (CD62E, anti-E-selectin and CD61, anti-integrin αvβ3) at 37°C in a humidified chamber with primary antibody. After 3 rinses of 5 min each, slides were incubated for 30 min with the corresponding biotinylated-secondary antibody (dilution 1/400 for the goat anti-rat antibody (0.5 mg/mL) and 1/200 (125 μg/mL) for the mouse anti-hamster antibody). After 3 rinses, slides were re-incubated with the streptavidin-conjugated peroxidase (Sigma-Aldrich, St. Quentin Fallavier, France) according to the manufacturer’s instructions (dilution 1/400). The DAB (3,3′-diamino-benzidine, Sigma-Aldrich) substrate was then added for 5 to 7 min until a brown precipitate was visible. Tissues were counterstained with Gill’s hematoxylin (Sigma-Aldrich) and treated with 30 mmol/L NH4OH to generate a blue nuclear stain. Slides were dehydrated in graded ethanol solutions and xylene and mounted with Eukitt® (Sigma-Aldrich).
Immunostaining semi-quantitative analysis
The density of CAMs was expressed as a percent range of total cells, as follows: -, no staining detected; <1%, few positive cells; 1–5%, some positive cells in all tumour sections; 5–15%, positive cells observed in all fields; >15%, high density of positive cells in all fields. The immunostaining was assessed by two independent investigators in a blinded manner.
Results
Choice of tumour types
In this study, we investigated 15 solid murine tumours to evaluate their vascular density and to assess their expression of important molecular markers involved in angiogenesis. The tumours were chosen among the most frequently used mouse tumour models, and also to be representative of the most frequent human tumour types. The murine tumours examined are listed in Table 1 and included 1 melanoma, 3 colon carcinomas, 7 mammary adenocarcinomas, 2 pancreatic tumours, 1 osteosarcoma and 1 lung carcinoma. Along with the tumour code names are presented the mouse strain used for propagation and the tumour main characteristics including doubling time, histological type and metastasis potential (invasiveness).
Table 1.
Principal characteristics of the murine tumours employed in this study
| Tumour code name | Mouse strain | Tda (days) | Histological type | Invasivenessb | References |
|---|---|---|---|---|---|
| B16 | C57BL/6 | 1.25–3.0 | Epidermoid melanoma | Highly metastatic: >70% to lungs | [20, 21, 54–56] |
| C26 colon | Balb/C | 1.7–2 | Colon carcinoma, undifferentiated | Highly metastatic: >90% to lungs | [20, 22, 23, 57] |
| C38 colon | C57Bl/6 | 2.5–4.0 | Colon adenocarcinoma | Moderately metastatic: <30% to lungs | [22, 23, 57] |
| C51 colon | Balb/C | 2.5–4.3 | Colon adenocarcinoma, mucinous, drug insensitive | Moderately to highly metastatic: >80% to lungs and to lymph nodes | [22, 23, 26, 57] |
| MA13/C | Balb/C | 3.4 | Mammary adenocarcinoma | [24, 55] | |
| MA16/C | C3H/HeN | 1.3–2.0 | Mammary adenocarcinoma | Highly metastatic: >80% to lungs and >30% to lymph nodes | [20, 24, 57] |
| MA16/C/Adr | C3H/HeN | 1.1 | Mammary adenocarcinoma, multidrug resistant, PgP c negative | [25] | |
| MA17 | C3H/HeN | 1.0 | Mammary adenocarcinoma | [26] | |
| MA17/Adr | C3H/HeN | 1.0 | Mammary adenocarcinoma, multidrug resistant, PgP c positive | [26] | |
| MA25 | Balb/C | Mammary adenocarcinoma | [58] | ||
| MA44 | C3H/HeN | 2.0 | Mammary adenocarcinoma | Highly metastatic: >90% to lungs | [20, 55] |
| PO2 | C57BL/6 | 2.2 | Pancreatic ductal adenocarcinoma, drug insensitive | Highly metastatic | [27] |
| PO3 | C57BL/6 | 2.2–3.5 | Pancreatic ductal adenocarcinoma, drug insensitive | Metastatic | [27] |
| GOS | C57BL/6 | 1.9 | Undifferentiated osteogenic sarcoma (Glasgow) | Moderately metastatic: <15% to lungs | [28, 55, 57] |
| LLC | C57BL/6 | 1–1.7 | Lung carcinoma (Lewis) | Metastatic to lungs (mostly) and kidneys | [20, 29, 59] |
Td, tumour doubling time in days.
Metastases from subcutaneously implanted tumour fragment.
PgP, P-glycoprotein.
Tumour vascular density
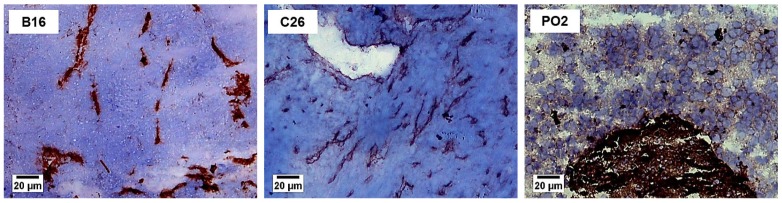
Medium sized tumours of approximately 200 mm3 were harvested and analyzed for the endothelial expression of PECAM-1 (CD31), which correlates with vascularization. Figure 1 illustrates the three different types of vascular density observed. The B16 melanoma presented a poor vascular density (<5%), whereas colon C26 (5–15%), and pancreatic adenocarcinoma PO2 (>15%) were well and highly vascularized, respectively. Table 2 presents the comparison of the vascular density for the 15 tumours investigated. It is noteworthy that only the B16 melanoma was poorly vascularized, whereas all the colon and the mammary tumours were well vascularized (5–15%). The pancreatic adenocarcinomas (PO2, PO3), sarcoma (GOS) and the lung carcinoma (LLC) were the most vascularized tumours in our study (>15%).
Figure 1. Vascular density in representative mouse tumours.
B16 melanoma, poorly vascularized (<5%); C26 colon carcinoma, well vascularized (5–15%); PO2 pancreatic adenocarcinoma, highly vascularized (>15%). Mouse tumour slices of 10 μm were prepared and stained using PECAM-1 (CD31) antibody as described in Materials and Methods. Original magnification ×100, bar 20 μm.
Table 2.
Murine tumour models vascular density and expression of endothelial cell adhesion molecules.a
| Tumour type and name | Vascular density b | Vascular distribution | Integrin αvβ3c | E-selectin d |
|---|---|---|---|---|
| Melanoma | ||||
| B16 | <5% | Some small spots showing numerous vessels | <5% | <5% |
| Colon | ||||
| C26 | 5–15% | Homogeneous distribution of vessels, some small spots with numerous vessels | <5% | 5–15% |
| C38 | 5–15% | - | - | |
| C51 | 5–15% | - | <5% | |
| Mammary | ||||
| MA13/C | 5–15% | Heterogeneous vessels distribution | - | - |
| MA16/C | 5–15% | - | - | |
| MA16/C/Adr | 5–15% | - | - | |
| MA17 | 5–15% | - | - | |
| MA17/Adr | 5–15% | <5% | - | |
| MA25 | 5–15% | <5% | - | |
| MA44 | 5–15% | <5% | - | |
| Pancreas | ||||
| PO2 | >15% | Homogeneous vessels distribution, some small spots with numerous vessels | 5–15% | - |
| PO3 | >15% | - | <5% | |
| Sarcoma | ||||
| GOS | >15% | Homogeneous vessels distribution | - | - |
| Lung | ||||
| LLC | >15% | Homogeneous vessels distribution | 5–15% | - |
Three independent samples were evaluated for each tumour type by 2 independent investigators, giving similar results. Tumour volumes were approximately 200 mm3.
The vascular density was assessed using PECAM-1 immunostaining (CD31 antibody).
The expression of αvβ3 was assessed using CD61 antibody.
E-selectin was assessed using CD62E antibody.
Tumour vascular distribution
The vascular distribution pattern of each tumour type is presented in Table 2. Most tumours showed a homogeneous and regular distribution of blood vessels (colon, pancreas, sarcoma and lung). However, two tumour types presented a different profile: the B16 melanoma presented small spots with numerous vessels; and, all the mammary tumours showed a heterogeneous distribution of blood vessels.
Expression of integrin αvβ3 in tumours
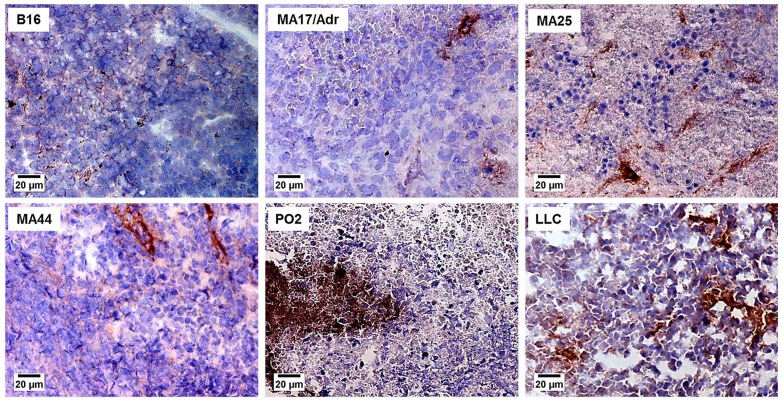
The percent expression of positive vessels for integrin αvβ3 is presented in Table 2. Seven tumours expressed the αvβ3 integrin, although at various levels. The B16 melanoma, one colon tumour (C26), 3 mammary tumours (MA17/Adr, MA25, MA44), one pancreatic tumour (PO2) and the Lewis lung carcinoma expressed this integrin. However the αvβ3 integrin was expressed at higher levels in the Lewis lung carcinoma and the PO2 pancreatic adenocarcinoma. Representative photographs depicting a low expression (<5%) of αvβ3 in B16 melanoma, MA17/Adr, MA25 and MA44 mammary adenocarcinoma are shown in Fig. 2, whereas high expression (5–15%) is shown for the PO2 pancreatic adenocarcinoma and LLC Lewis lung carcinoma.
Figure 2. Expression of the αvβ3 integrin in murine tumours.
B16 melanoma, MA17/Adr, MA25 and MA44 mammary adenocarcinoma show a low expression of the αvβ3 integrin (<5%), whereas PO2 pancreatic adenocarcinoma and LLC Lewis lung carcinoma presented a high expression (5–15%). Mouse tumours slices of 10 μm were stained using CD61 antibody, as described in Materials and Methods. Original magnification ×100, bar 20 μm.
The distribution pattern of this integrin was however different for the expressing tumours. The MA17/Adr, MA25 and MA44 mammary adenocarcinomas expressed the αvβ3 integrin on some vessels distributed all over the tumour section, whereas the C26 colon carcinoma and B16 melanoma showed αvβ3 restricted to some areas localized at the tumour periphery. The high expression of this integrin in the LLC lung carcinoma and the PO2 pancreatic adenocarcinoma was however distributed differently: in the LLC, αvβ3 was homogeneously distributed, whereas in PO2, the expressing cells formed clusters.
Expression of E-selectin in tumours
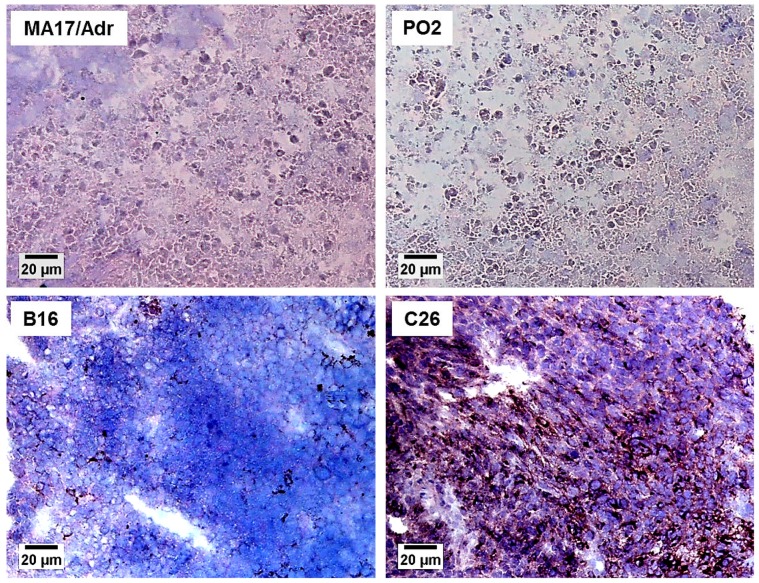
The E-selectin expression in the various tumours is presented in Table 2. E-selectin was detected in only four tumours (B16, C26, C51, PO3). Representative photographs are presented in Fig. 3, where MA17/Adr and PO2 did not show detectable levels of E-selectin. In B16 melanoma, only few positive cells showed E-selectin expression (Fig. 3), similarly to the C51 colon adenocarcinoma and the PO3 pancreatic adenocarcinoma which also showed some isolated vessels expressing E-selectin. The C26 colon carcinoma expressed E-selectin to a high degree (5–15%), with numerous vessels expressing this selectin, although with a heterogeneous distribution, as depicted in Fig. 3.
Figure 3. E-selectin expression in representative mouse tumours.
MA17/Adr mammary adenocarcinoma and PO2 pancreatic adenocarcinoma representing undetectable expression; B16 melanoma, poor expression (<5%); C26 colon carcinoma, high expression (5–15%). Mouse tumour slices of 10 μm were stained using CD62E antibody, as described in Materials and Methods. Original magnification ×100, bar 20 μm.
Co-expression of integrin αvβ3 and E-selectin in tumours
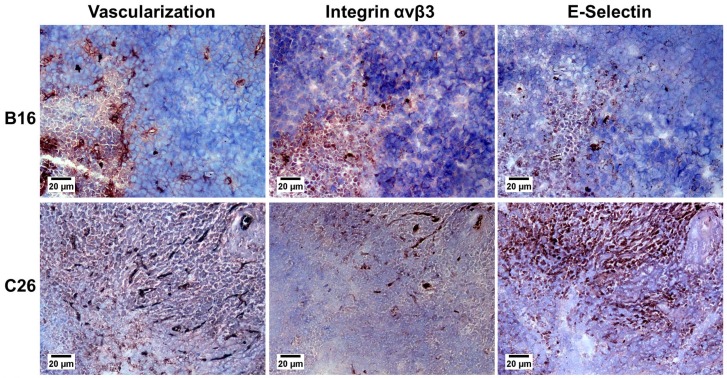
To study the localization of αvβ3 and E-selectin in the two tumours that co-expressed these CAMs, i.e., B16 and C26, we used adjacent histological sections presented in Fig. 4. The co-expression of αvβ3 and E-selectin was not homogeneously distributed and was only observed at the tumour periphery for B16 and C26 tumours. In addition, we noted that the detection of PECAM-1 expression exhibited a denser vascularization in these areas.
Figure 4. Co-expression of αvβ3 integrin and E-selectin in colon carcinoma C26 and B16 melanoma.
Adjacent slices showing the vascularization (PECAM-1, CD31), the integrin αvβ3 (CD61) expression and the E-selectin (CD62E) expression in B16 melanoma and C26 colon tumour. Adjacent slices were processed with the different antibodies as described in Materials and Methods. Original magnification ×100, bar 20 μm.
Discussion
The aim of this study was to better characterize the vascular density and the CAMs expression in murine solid tumours frequently employed in preclinical research. These data could facilitate the choice of the best tumour models for testing therapeutic approaches aiming at blocking angiogenesis or destroying existing tumour vascularization.
Concerning the vascular density, assessed with the staining of a CAM constitutively expressed by endothelial cells (PECAM-1, CD31), we found significant differences between the various tumours examined. Indeed, the least vascularized tumour was the B16 melanoma, whereas the colon and mammary tumours were all well vascularized. Also of note, the pancreatic tumours, the sarcoma (GOS) and the lung (LLC) tumours were found to be the most vascularized tumours in our series. There was apparently no correlation between the vascular density and the metastatic potential, because some poorly vascularized tumours are known to be highly metastatic (e.g., B16; compare Tables 1 and 2), whereas other tumours were highly vascularized and are moderately metastatic (e.g., GOS). Although these results may appear to contradict observations showing that high vascularization often correlates with metastatic potential within the same tumour type (reviewed in [30]), it should be noted that in our study, different tumours from various tissue origins were compared, and not at various growth stages for the same tumour type. Also, the results showing poor vascularization for the B16 melanoma could support the hypothesis that a tumour hypoxic environment could favour metastasis as previously reported [31].
The vascular distribution pattern in tumours was also of interest, because it revealed a well-organized vascularization in all subcutaneously transplanted tumour models. Most tumour types presented a regular and homogeneous distribution of blood vessels (colon, pancreas, sarcoma and lung). However, in the B16 melanoma and the mammary tumours, the blood vessel distribution was found heterogeneous. For the mammary tumours, it was noteworthy that tumours of a same organ origin exhibited a similar vascularization pattern, even if they were transplanted in different mouse strains.
In humans, high tumour vessel density has been shown to be associated with metastasis, tumour progression and decreased survival time in several tumour types, including breast, lung, melanoma, colon, cervix, prostate and bladder [32, 33]. Although increased vascularity could be anticipated to favor oxygenation and drug delivery to tumours, it is possible that the more vascularized tumours metastasize to distant sites and therefore become more difficult to treat because of their dissemination throughout the organism [33].
In this study, we have also assessed the expression of two endothelial CAMs involved in angiogenesis in frequently employed mouse tumour models. These CAMs, including the integrin αvβ3 and the E-selectin, are particularly important molecules because they are involved in the process of angiogenesis which is required for tumour growth, cell migration and metastasis {Weis, 2011 10351/id}.
Integrin αvβ3 is not constitutively expressed by normal endothelial cells and is therefore considered diagnostic of proliferative endothelial cells in neovascularized areas [9]. In the present study, the αvβ3 integrin was expressed in 7/15 of the tumours examined (B16, C26, MA17/Adr, MA25, MA44, PO2, LLC). It is noteworthy that there was no correlation between the tumour type and the expression of this integrin because only 1/3 of colon tumours and 3/7 of mammary tumours expressed this integrin. For the distribution pattern of this integrin in expressing tumours, it was interesting to observe that mammary and lung tumours have a homogenous distribution, whereas C26 and B16 melanoma presented a peripheral distribution. Similar results obtained by magnetic resonance imaging have recently been published for the B16 melanoma where it was also observed that αvβ3 is peripherally distributed [34]. Similarly to mouse tumours, the integrin αvβ3 has also been found expressed in the endothelium of several human tumours including melanoma, breast, colon, prostate, cervix, brain, and pancreas, colon, and lung [11, 19, 35, 36].
E-selectin was expressed in only 4 mouse tumours, including B16 melanoma, C26 and C51 colon tumours and pancreatic PO3 tumours. In the C26 colon tumour and B16 melanoma, E-selectin expression was restricted to areas localized at the tumour periphery, which suggests that these areas correspond to tumour neovascularization zones which also expressed the αvβ3 integrin. This localized expression of E-selectin at tumour periphery has already been observed in previous studies [37, 38]. Like integrin αvβ3 E-selectin is not expressed by normal endothelial cells but was found on microvessels that showed proliferating endothelial cells [8]. With regard to the E-selectin clinical applications, antagonists have been developed to target cellular interactions with this CAM including antibodies, ligand inhibitors and metabolic carbohydrate mimetics [39]. E-selectin has also recently been used as a target for drug delivery [40].
We noticed that only half of the tumours expressed integrin αvβ3 and a quarter expressed the E-selectin. Previous studies have also reported heterogeneity in CAMs expression by tumour endothelium, i.e., some tumours are deficient while others highly express CAMs [6]. The deficient CAMs expression could be due to the fact that CAMs are also involved in the inflammatory responses and recruitment processes. A reduced endothelial CAM expression in tumour microvessels could facilitate tumour progression in avoiding the patrolling by circulating lymphocytes [6]. In addition, the heterogeneity of CAMs expression has been shown to depend on tumour type, implantation mode, growth site and host strain [41, 42]. In the present study, subcutaneously implanted tumours have been used and these models may not be the exact reflection of in situ tumours, for which more complex interactions between the different CAMs appear to regulate tumour angiogenesis [43].
As previously mentioned, tumour viability, growth and metastasis depend on tumour angiogenesis. Integrin αvβ3 and E-selectin mediate the processes of microvessel neoformation, and detection of the expression of both CAMs allows to determine whether angiogenesis occurs in a tumour. Indeed, several studies have reported the use of specific angiogenesis specific markers as targeting ligands for systemic drug or gene delivery to cancer [44–46] or to other vascular diseases [47].
The expression of these CAMs in tumours appears to be shared by murine and human tumours as well. For example, αvβ3 has indeed been found expressed in several human tumours, e.g., melanoma, breast, prostate, cervix, brain and pancreas [19, 35, 36]. E-selectin has also been identified in human melanoma as a novel target for inhibition of melanoma angiogenesis and tumour growth [53].
These CAMs can be the target of antiangiogenic therapy by using inhibitors of integrin αvβ3 [48, 49] or of E-selectin [16]. Indeed, a better knowledge of the CAMs expressed in tumours has already allowed the development of several therapeutic approaches. For example, integrin antagonists, including the αvβ3 and αvβ5 inhibitor cilengitide, have demonstrated encouraging activity in clinical trials [50, 51]. With regard to the E-selectin, antagonists have been developed to target cellular interactions with this CAM including antibodies, ligand inhibitors and metabolic carbohydrate mimetics [39]. E-selectin has also recently been used as a target for drug delivery [40].
In addition to therapeutic applications, the identification of these CAMs in tumours has also permitted the use of this knowledge for molecular imaging. The integrin αvβ3 has been targeted for imaging purposes with near-infrared fluorescent dye-RGD peptide conjugates, their multivalent analogs, and nanoparticle conjugates [50, 52]. E-selectin has also been used as a target for molecular imaging [40].
In conclusion, the assessment of the vascular density and the expression of the important integrin αvβ3 and E-selectin in a series of widely used murine solid tumour models has allowed the identification of several tumours expressing these CAMs. We have also identified two tumours expressing both αvβ3 and E-selectin (B16 and C26). These data may prove useful for the choice of appropriate tumour models for the study of the biology of tumour angiogenesis, the evaluation of antiangiogenic therapies and the validation of tumour imaging modalities targeting these CAMs.
Acknowledgments
We are grateful to the Oncology Department of Aventis Pharma, S.A. (now Sanofi, S.A.), for providing the murine tumour samples. This work was supported in part by Gencell S.A., the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Ecole Nationale Supérieure de Chimie Paris (ENSCP). We also thank the Institut National du Cancer for grant support to GGC (INCa, Boulogne Billancourt, France).
Abbreviation used
- CAM
endothelial cell adhesion molecule
References
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 2.Nussenbaum F, Herman IM. Tumor angiogenesis: insights and innovations. J Oncol. 2010;2010:132641. doi: 10.1155/2010/132641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff J. Cell adhesion and angiogenesis. J Clin Invest. 1997;100:S37–S39. [PubMed] [Google Scholar]
- 4.OI, Otvos L, Kieber-Emmons T, Blaszczyk-Thurin M. Role of SA-Le(a) and E-selectin in metastasis assessed with peptide antagonist. Peptides. 2002;23:999–1010. doi: 10.1016/s0196-9781(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 5.Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–e35. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Langley RR, Russell J, Eppihimer MJ, Alexander SJ, Gerritsen M, Specian RD, Granger DN. Quantification of murine endothelial cell adhesion molecules in solid tumors. Am J Physiol. 1999;277:H1156–H1166. doi: 10.1152/ajpheart.1999.277.3.H1156. [DOI] [PubMed] [Google Scholar]
- 7.Varner JA, Cheresh DA. Tumor angiogenesis and the role of vascular cell integrin alpha v beta 3. Important Adv Oncol. 1996:69–87. [PubMed] [Google Scholar]
- 8.Kraling BM, Razon MJ, Boon LM, Zurakowski D, Seachord C, Darveau RP, Mulliken JB, Corless CL, Bischoff J. E-selectin is present in proliferating endothelial cells in human hemangiomas. Am J Pathol. 1996;148:1181–1191. [PMC free article] [PubMed] [Google Scholar]
- 9.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 11.Max R, Gerritsen RR, Nooijen PT, Goodman SL, Sutter A, Keilholz U, Ruiter DJ, De Waal RM. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71:320–324. doi: 10.1002/(sici)1097-0215(19970502)71:3<320::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 13.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weis SM, Cheresh DA. Alpha v Integrins in Angiogenesis and Cancer. Cold Spring Harb Perspect Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haviv YS, Curiel DT. Conditional gene targeting for cancer gene therapy. Adv Drug Deliv Rev. 2001;53:135–154. doi: 10.1016/s0169-409x(01)00225-3. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen M, Eilber FR, Defrees S. Novel synthetic analogs of sialyl Lewis X can inhibit angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 1996;228:716–723. doi: 10.1006/bbrc.1996.1722. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda M, Shimizu S, Ohhinata K, Naito S, Tokuyama S, Mori Y, Kiuchi Y, Yamamoto T. Differential roles of ICAM-1 and E-selectin in polymorphonuclear leukocyte-induced angiogenesis. Am J Physiol Cell Physiol. 2002;282:C917–C925. doi: 10.1152/ajpcell.00223.2001. [DOI] [PubMed] [Google Scholar]
- 18.Mannori G, Santoro D, Carter L, Corless C, Nelson RM, Bevilacqua MP. Inhibition of colon carcinoma cell lung colony formation by a soluble form of E-selectin. Am J Pathol. 1997;151:233–243. [PMC free article] [PubMed] [Google Scholar]
- 19.Vonlaufen A, Wiedle G, Borisch B, Birrer S, Luder P, Imhof BA. Integrin alpha(v)beta(3) expression in colon carcinoma correlates with survival. Mod Pathol. 2001;14:1126–1132. doi: 10.1038/modpathol.3880447. [DOI] [PubMed] [Google Scholar]
- 20.Polin L, Corbett TH, Roberts BJ, Lawson AJ, Leopold WR, III, White K, Kushner J, Hazeldine S, Moore R, Rake J, Horwitz JP. Transplantable syngeneic rodent tumors: Solid tumors of mice. In: Teicher BA, editor. Tumors models in cancer research, Cancer Drug Discovery and Development. New York: Springer Science & Business Media LLC; 2011. pp. 43–78. [Google Scholar]
- 21.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–224. [PubMed] [Google Scholar]
- 22.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
- 23.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Evaluation of single agents and combinations of chemotherapeutic agents in mouse colon carcinomas. Cancer. 1977;40:2660–2680. doi: 10.1002/1097-0142(197711)40:5+<2660::aid-cncr2820400940>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Biology and therapeutic response of a mouse mammary adenocarcinoma (16/C) and its potential as a model for surgical adjuvant chemotherapy. Cancer Treat Rep. 1978;62:1471–1488. [PubMed] [Google Scholar]
- 25.Schabel FM, Jr, Skipper HE, Trader MW, Laster WR, Jr, Griswold DP, Jr, Corbett TH. Establishment of cross-resistance profiles for new agents. Cancer Treat Rep. 1983;67:905–922. [PubMed] [Google Scholar]
- 26.Liang J, Moore RE, Moher ED, Munroe JE, Alawar RS, Hay DA, Varie DL, Zhang TY, Aikins JA, Martinelli MJ, Shih C, Ray JE, Gibson LL, Vasudevan V, Polin L, White K, Kushner J, Simpson C, Pugh S, Corbett TH. Cryptophycins-309, 249 and other cryptophycin analogs: preclinical efficacy studies with mouse and human tumors. Invest New Drugs. 2005;23:213–224. doi: 10.1007/s10637-005-6729-9. [DOI] [PubMed] [Google Scholar]
- 27.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Jr, Schabel FM., Jr Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–726. [PubMed] [Google Scholar]
- 28.Glasgow LA, Crane JL, Jr, Kern ER, Youngner JS. Antitumor activity of interferon against murine osteogenic sarcoma in vitro and in vivo. Cancer Treat Rep. 1978;62:1881–1888. [PubMed] [Google Scholar]
- 29.Bertram JS, Janik P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980;11:63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- 30.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 31.Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer. 1999;80:1697–1707. doi: 10.1038/sj.bjc.6690586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strohmeyer D. Pathophysiology of tumor angiogenesis and its relevance in renal cell cancer. Anticancer Res. 1999;19:1557–1561. [PubMed] [Google Scholar]
- 33.Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boles KS, Schmieder AH, Koch AW, Carano RA, Wu Y, Caruthers SD, Tong RK, Stawicki S, Hu G, Scott MJ, Zhang H, Reynolds BA, Wickline SA, Lanza GM. MR angiogenesis imaging with Robo4- vs. alphaVbeta3-targeted nanoparticles in a B16/F10 mouse melanoma model. FASEB J. 2010;24:4262–4270. doi: 10.1096/fj.10-157933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felding-Habermann B, Fransvea E, O’Toole TE, Manzuk L, Faha B, Hensler M. Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin Exp Metastasis. 2002;19:427–436. doi: 10.1023/a:1016377114119. [DOI] [PubMed] [Google Scholar]
- 36.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson H, Ramsey PS, Donohue JH, Wold LE. Cell adhesion molecule expression within the microvasculature of human colorectal malignancies. Clin Immunol Immunopathol. 1994;72:129–136. doi: 10.1006/clin.1994.1116. [DOI] [PubMed] [Google Scholar]
- 38.Fox SB, Turner GD, Gatter KC, Harris AL. The increased expression of adhesion molecules ICAM-3, E- and P-selectins on breast cancer endothelium. J Pathol. 1995;177:369–376. doi: 10.1002/path.1711770407. [DOI] [PubMed] [Google Scholar]
- 39.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jubeli E, Moine L, Vergnaud-Gauduchon J, Barratt G. E-selectin as a target for drug delivery and molecular imaging. J Control Release. 2012;158:194–206. doi: 10.1016/j.jconrel.2011.09.084. [DOI] [PubMed] [Google Scholar]
- 41.Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 43.Tucker GC. Inhibitors of integrins. Curr Opin Pharmacol. 2002;2:394–402. doi: 10.1016/s1471-4892(02)00175-3. [DOI] [PubMed] [Google Scholar]
- 44.Parkes RJ, Hart SL. Adhesion molecules and gene transfer. Adv Drug Deliv Rev. 2000;44:135–152. doi: 10.1016/s0169-409x(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 45.Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, Li H, Parameshwar M, Vickers SM, Jaffee EM, Huibregtse K, Curiel DT, Dmitriev I. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- 46.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 47.Spragg DD, Alford DR, Greferath R, Larsen CE, Lee KD, Gurtner GC, Cybulsky MI, Tosi PF, Nicolau C, Gimbrone MA., Jr Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc Natl Acad Sci U S A. 1997;94:8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 49.Kumar CC, Malkowski M, Yin Z, Tanghetti E, Yaremko B, Nechuta T, Varner J, Liu M, Smith EM, Neustadt B, Presta M, Armstrong L. Inhibition of angiogenesis and tumor growth by SCH221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer Res. 2001;61:2232–2238. [PubMed] [Google Scholar]
- 50.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10:753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye Y, Chen X. Integrin targeting for tumor optical imaging. Theranostics. 2011;1:102–126. doi: 10.7150/thno/v01p0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu ZJ, Tian R, Li Y, An W, Zhuge Y, Livingstone AS, Velazquez OC. Inhibition of tumor angiogenesis and melanoma growth by targeting vascular E-selectin. Ann Surg. 2011;254:450–456. doi: 10.1097/SLA.0b013e31822a72dc. [DOI] [PubMed] [Google Scholar]
- 54.Formelli F, Rossi C, Supino R, Parmiani G. In vivo characterization of a doxorubicin resistant B16 melanoma cell line. Br J Cancer. 1986;54:223–233. doi: 10.1038/bjc.1986.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bissery MC, Nguyen CH, Bisagni E, Vrignaud P, Lavelle F. Antitumor activity of intoplicine (RP 60475, NSC 645008), a new benzo-pyrido-indole: evaluation against solid tumors and leukemias in mice. Invest New Drugs. 1993;11:263–277. doi: 10.1007/BF00874425. [DOI] [PubMed] [Google Scholar]
- 56.Bissery MC, Guenard D, Gueritte-Voegelein F, Lavelle F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991;51:4845–4852. [PubMed] [Google Scholar]
- 57.Corbett TH, Leopold WR, Dykes DJ, Roberts BJ, Griswold DP, Jr, Schabel FM., Jr Toxicity and anticancer activity of a new triazine antifolate (NSC 127755) Cancer Res. 1982;42:1707–1715. [PubMed] [Google Scholar]
- 58.LoRusso PM, Demchik L, Foster B, Knight J, Bissery MC, Polin LM, Leopold WR, III, Corbett TH. Preclinical antitumor activity of CI-994. Invest New Drugs. 1996;14:349–356. doi: 10.1007/BF00180810. [DOI] [PubMed] [Google Scholar]
- 59.DeWys WD. Studies correlating the growth rate of a tumor and its metastases and providing evidence for tumor-related systemic growth-retarding factors. Cancer Res. 1972;32:374–379. [PubMed] [Google Scholar]