Abstract
Accumulating evidence suggests that adolescence represents a sensitive period during which social stressors may serve to influence adult behavior and stress reactivity. However, relatively little is known about the impact of social stress in adolescence on behaviors or stress reactivity in females. In this study, we exposed adolescent or adult female rats to the repeated social stress of defeat for 7 consecutive days. Repeated defeat resulted in distinctly different behavioral repertoires during defeat in adolescent compared to adult female rats. Adolescent females exhibited more play and avoidant behaviors and adult females exhibited more active and aggressive behaviors toward the resident female. Examination of the short-term effects of social defeat using the Porsolt-forced swim test (FST) indicated that adolescents, regardless of their exposure to social defeat, showed increased time immobile and decreased time swimming compared to adults. Adolescent rats exposed to defeat also exhibited increased climbing compared to their age–matched naïve counterparts. These effects dissipated with age. Interestingly, no effects of defeat were observed in adult females, however, when these females were re-assessed in the FST 30 days after the end of defeat, we observed increased swimming at the expense of climbing. Using exposure to a novel restraint to assess stress reactivity, we found that stress during adolescence and adulthood led to lower basal ACTH concentrations and that both stressed and control adolescent groups exhibited a delay in recovery in adulthood compared to stressed and control adult groups. Fos protein analysis further suggested that cortical/thalamic structures serve as potential substrates that mediate these long-term impacts of stress during adolescence. Thus, repeated social stress during adolescence produces different patterns of effects as compared with repeated social stress during adulthood.
Keywords: social stress, resident intruder, adolescent, Porsolt forced swim test, restraint
1. INTRODUCTION
Exposure to stressful life events is a risk factor for the onset of a variety of disorders during adulthood (McEwen, 2003); (Wood et al., 2010). Adolescence, which is a time of rapid cerebral development and organization most notably in the hippocampus, prefrontal cortex, and amygdala (Romeo et al., 2006); (Spear, 2000), may be a particularly vulnerable time for the effects of stress. Repeated social stress has been shown to impact the brain norepinephrine system that regulates defensive strategies (Bingham et al., 2011), increasing adolescents’ susceptibility to anxiety and depression (Garber, 2006); (Romeo et al., 2006); (Weintraub et al., 2010). Passive coping in response to stress has been associated with greater activation of the hypothalamic-pituitary-adrenal (HPA) stress axis (Walker et al., 2009), which may increase the risk of depression, whereas proactive coping in response to stress has been associated with higher sympathetic activity and resiliency (Billings et al., 1984). Differential effects of stress in adolescent as compared with adult animals suggest a window of potential plasticity in the HPA stress response and imply that the impact of stress may not be the same at all ages of development (Foilb et al., 2011); (Jankord et al., 2011); (McCormick and Matthews, 2010); (Romeo, 2010). Similarly, prior stress history may increase or decrease the magnitude of physiological stress responses (Grissom et al., 2008), and different experimental stressors may result in different behavioral outcomes as well as different HPA and neural reactivity profiles (Grissom et al., 2007).
Studies in animals have also suggested that stress during adolescence has a particularly strong effect in female rats, with adult females isolated in adolescence exhibiting increased stress reactivity (Weintraub et al., 2010). Adult female rats exposed to social stress in adolescence exhibited increased startle reflex suggesting an anxiety-like phenotype (Bourke and Neigh, 2012) whereas adult female rats subjected to chronic mixed-modality stress did not exhibit an affected startle response compared to age-matched controls (Bourke and Neigh, 2011). Social isolation stress has also been shown to produce an active coping behavioral phenotype in adult female rats but not in adult male rats assessed in the forced swim test (Hong et al., 2011). Evidence from studies using physical restraint stress have shown a pattern of delayed recovery for female relative to male rats (Romeo, 2010) and have suggested that females are more vulnerable than males to the physiological effects of chronic restraint (Campbell et al., 2003). However little is known about the impact of repeated social defeat during adolescence, whether it produces different outcomes in the short- and long-term and how its effects are different than social defeat experienced in adulthood.
The resident-intruder model of defeat (Miczek, 1979) has been shown to be a useful model for studying the impact of repeated social stress in rats. In this model a rat (intruder) is placed in the home cage of a larger, aggressive rat (resident) and subjected to repeated threatening encounters. Adult male rats exposed to this type of social defeat stress have been shown to exhibit decreased motivation (Becker et al., 2008) and to experience altered HPA function (Bhatnagar and Vining, 2003), which may promote depressive-like behaviors (Miczek et al., 2004). Although many modifications to the resident-intruder paradigm have been implemented, repeated resident-intruder stress during adolescence seems to increase proactive responses in the forced swim test for early adolescent male rats (Bingham et al., 2011), whereas it tends to induce a shift from active to passive coping behavior in adult female rats (Bourke and Neigh, 2011). It is not known whether social defeat stress alone can impact the development of affect-related behaviors different in the immediate term (during adolescence) compared to the long-term (adulthood) in female rats. In this study, we examined the impact of chronic social defeat stress on behavior during defeat in adolescent or adult females, and assessed the short and long-term effects on behavioral coping, HPA reactivity, and neural activation in these animals.
2. EXPERIMENTAL PROCEDURES
2.1 Animals and housing
The experimental animals consisted of female Sprague-Dawley rats (n=49) that were purchased from Charles River Laboratories (Wilmington, MA) at PND 29–31 (early adolescence) or at PND 69–71 (adulthood). Animals were randomly assigned to stress (experimental) and naïve (control) groups and run in 4 separate cohorts (final n’s of adolescent stress=15; adolescent naïve=7; adult stress=19; adult naïve=8). The specific group sizes for each measure are provided in the figure legends and can vary from measure to measure particularly in the neuroendocrine/neural measures due to lack of sample or elimination of outliers (samples more than two standard deviations from the mean). All animals were pair-housed within same-age, same-treatment sub-groups in standard 26 cm × 46 cm clear polypropylene cages with ad libitum access to food and water. Animals were allowed to acclimate to a 12-hour light/dark cycle (lights on at 06:00 AM), temperature-controlled standard colony room for approximately one week prior to study initiation. A group of 10 Sprague-Dawley lactating adult rats was housed separately for use as resident animals in the resident-intruder test to induce the stress of defeat. These females were housed in cages that were 48cm × 35cm × 20 cm (width × depth × height) in size. All procedures were conducted between 9:00–11:00 AM. The experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Children’s Hospital of Philadelphia Research Institute’s Animal Care and Use Committee.
2.2 Resident-intruder social stress paradigm
The resident-intruder social stress paradigm ((Miczek, 1979) was used with the modification that lactating female rats were used as resident animals because lactating females are likely to defeat other female rats (Flannelly and Flannelly, 1987). On each of seven consecutive days, adolescent or adult experimental animals (intruders) were subjected to 30-min episodes of social defeat stress. Intruders were individually placed in the home-cage of a novel lactating female (resident) whose pups had been removed immediately prior. Following the intruder’s placement in the resident’s cage, resident and intruder were allowed to interact until one of two possible criteria was met: (a) the intruder exhibited a submissive defeat posture (>2 seconds frozen in a supine position), or (b) 15 min. elapsed (as in our previous study with adult male rats (Wood et al., 2010)). Upon reaching one of these criteria, the animals were separated by a wire barrier, allowing only auditory, olfactory and visual contact for the remainder of the 30-minute test period. Intruders were then returned to their home-cages and lactating mothers (residents) were reunited with their pups. The social defeat occurred at the same time each day with the intruder being randomly placed into the cage of a different lactating female each day. Naïve animals remained in their home-cages in the colony room for the duration of the 30-min test.
All 30-min social defeat episodes were videotaped and later scored by two trained investigators who were blind to the experimental conditions. Videotapes were evaluated to record the timing of each attack on the intruder, the number of attacks received, and latency to assume a submissive posture or defeat (if applicable). In addition, to document the development of submission in intruder animals, frequencies of each of the following six behaviors were tallied: ‘boxing’ (intruder assumed posture on hindlegs while in physical contact and attentive to the resident); ‘upright posture’ (intruder assumed posture on hindlegs while attentive toward the resident but with no physical contact); ‘under the cover’ (intruder was underneath the resident); ‘grooming by resident’ (intruder was aggressively groomed by the resident), ‘freezing/crouching posture’ (intruder assumed a completely still and crouching posture in response to resident’s presence); “self-grooming’ (intruder groomed itself during the interaction time).
2.3 Short-term and long-term behavioral effects of repeated social stress
To assess the immediate and long-term behavioral responses to repeated social defeat stress, all experimental animals were individually subjected to the Porsolt forced swim test (FST) (26) on two occasions. Based on the work of Lucki (1997), the two-day FST involved a 15-min forced swim (on day 1), followed 24 hours later by a 5-min forced swim test (on day 2). The 5-min swim test was videotaped from directly above the clear glass cylinder [46 cm in height × 20 cm in diameter], filled to 35 cm with water at a temperature of 25°C (±1°C). The first Porsolt FST occurred on the two days following the seventh social defeat episode (day 8–9), after which rats were removed from the water, towel-dried, placed back into their home-cages and left undisturbed for 30 days. The cylinder was cleaned and the water was changed before testing of the next subject. To assess the long-term behavioral effects of repeated social defeat, experimental animals were again subjected to a Porsolt FST 30 days post stress (day 38–39). Identical procedures were followed on days 8–9 and 38–39 to assess naïve animals’ responses during the Porsolt FST.
Following the procedures developed by Detke, et al. (1995), two trained observers viewed the videotaped 5-min swim tests and categorized the rat’s behavior every 5 s for one of the following three behaviors: immobility—floating in the water without struggling and using only small movements to keep the head above water; swimming —moving limbs in an active manner causing movement around the cylinder (more than required to keep head above water); and climbing—making active movements with the forepaws in and out of the water, usually directed against the wall.
2.4 Response to acute restraint
On day 40, the day following the second 5-min swim test, each animal was placed in a novel cage in a well-ventilated, size-adjusted Plexiglas restraint tube and subjected to a 30-min restraint. This relatively brief form of restraint is commonly used as a mild stressor designed to stimulate the HPA axis (Bhatnagar and Vining, 2003). Following the 30-min restraint, each animal was returned to its home-cage before being sacrificed at 60-min (30 min after termination of restraint). The HPA response was assessed from blood collected at 0, 15, 30, and 60 minutes and later assayed for ACTH and corticosterone. Plasma collected at 60 min was also assayed for estradiol. Following sacrifice at 60 min post-stress, rat brains were isolated and immediately flash frozen in 2-methyl butane.
Plasma corticosterone and ACTH
Blood was collected in eppendorf tubes at 0, 15, and 30 min during restraint by snipping 2–3 mm of the tip of the tail vein at 0min and re-sampling at 15 and 30min and immediately placing the samples on ice. At 60 min (30min post-restraint), trunk blood was collected in 15 ml conical tubes containing EDTA to prevent coagulation. Whole blood was centrifuged at 3000 rpm for 15 min and the plasma was isolated and kept frozen at −20°C. An RIA kit from MP Biomedical (Orangeburg, NY) was used to assay the plasma for corticosterone and ACTH. Plasma estradiol was also assessed using plasma from the trunk blood collected at the 60min timepoint. At time of sacrifice, a vaginal smear was also collected to assess estrous cyclicity. A 17B-Estradiol (E2) Double Antibody- 125I RIA Kit was used to assay the plasma for estradiol.
C-Fos protein
Brains were stored at −80 °C until collection of tissue punches for Western blot analysis. 1mm diameter punches of five regions of each rat brain were collected. These were: prelimbic prefrontal cortex (PFC), Infralimbic PFC, cingulate cortex, paraventricular nucleus of the hypothalamus (PVN), and paraventricular nucleus of the thalamus (PVT). Total protein was extracted and the presence of the C-Fos protein was determined by Western Blot analysis using a rabbit polyclonal antibody at 1:1000 dilution (sc-52, Santa Cruz, CA).
Western Blots
A subset of the 8 animals from each of the four groups was randomly selected for examination of C-Fos protein in response to acute restraint. Tissue was homogenized in 125uL of Hypotonic Lysis Buffer containing 1M Tris pH 8, HALT protease inhibitor cocktail (Thermo Scientific), EDTA (Thermo Scientific), phosphosafe extraction reagent, and Nanopure H2O. Protein concentration was determined using Pierce/Thermo/Fischer BCA Assay on a 96 well plate. The absorbance was read on a Magellan plate reader. 5ug of sample was run on a Bio-rad ready gel (Bio-Rad 161-1158. Hercules, CA) and transferred to Immobilon transfer membranes (Millipore, USA). Blots were placed in blocking buffer (1:3 Odyssey Blocking buffer (Licor) to TBS). Blots were incubated in primary antibodies for 16h (overnight) at 4° Celsius. Following washes, blots were incubated with Licor secondary antibodies, Donkey anti-rabbit IRDye 680 and Donkey anti-mouse IRDye 800cw (1:5000 dilution in 1:3 Odyssey blocking buffer: TBS between 20) for 1 hour at room temperature. The proteins were quantified as a percent of total β-actin (Sigma, St. Louis, MO) in their respective fractions with the Odyssey program for all analyses.
2.5 Statistical analysis
GraphPad Prism (GraphPad Software, La Jolla, CA) was used to conduct statistical analyses. T-tests (two-tailed) were used to identify significant differences in average frequencies of behaviors during social defeat observed over the 7-day defeat period. Two way (Age × Day) ANOVAs were conducted in order to assess potential differences over the 7-day defeat period due to Age (adolescent vs. adult) and Day (defeat days 1–7). Two-way (Age × Stress) ANOVAs were conducted in order to assess potential differences due to Age (adolescent vs. adult) and Stress (defeat vs. naïve) for the forced swim test, and for c-Fos protein quantification. These two way ANOVAs were also conducted at the 4 different timepoints in analysis of ACTH and corticosterone responses to restraint. Bonferroni post hoc tests were used to assess individual group differences following two-way ANOVAs. The p-value was set to 0.05.
3. RESULTS
3.1 Behavior during Social Defeat
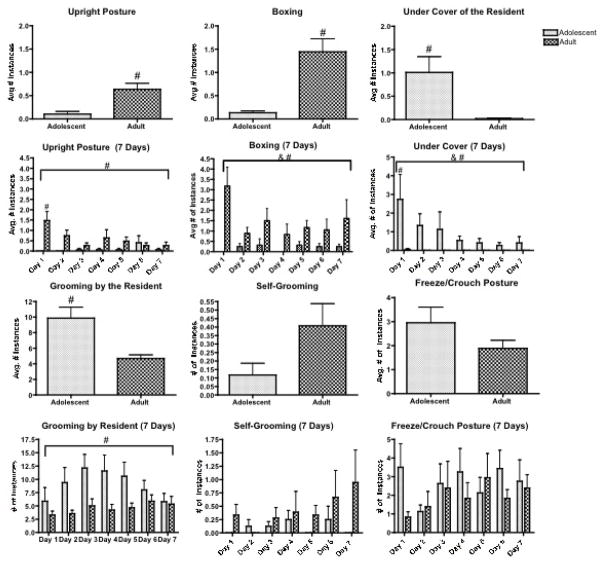
As indicated in Figure 1, adolescent rats spent significantly less time than adult rats in an upright position (t(32)=3.50, p<0.001) and demonstrated lower frequencies of boxing behavior (t(32)=4.14, p< 0.001) than adults averaged across the 7 days of stress. Furthermore, adolescents were under the cover of (t(32)=3.32, p< 0.01) and being aggressively groomed by (t(32)=3.85, p<0.001) the lactating female resident more often than adults. Although not significant, adolescents tended to exhibit the freeze/crouch posture and more frequently than adult rats and instances of self-grooming less frequently (p<0.12 and 0.08, respectively) than did adult rats exposed to the resident female.
Figure 1.
Behaviors Exhibited During Repeated Social Defeat. Adult female rats (d69–71 of age; n=19) and adolescent female rats (d29–31 of age; n=15) were subjected to 7 days of repeated social defeat with an aggressive lactating female resident. Adolescent and adult rats exhibited distinct behavioral repertoires in response to an aggressive conspecific. Over the 7 days, adolescent rats exhibited significantly fewer numbers of upright postures and boxing behaviors and fewer instances of under the cover of and being aggressively groomed by the lactating female resident compared to adults. Self-grooming and freezing/crouching behaviors were also assessed but not significantly different between adolescent and adult female rats. The average of these behaviors over the 7 days are shown in the first and third rows and the second and fourth rows shows these behaviors on each of the 7 days.
# p≤ .05 indicates a significant Age effect with a significant difference between adolescent and adult female rats
& p≤ .05 indicates a significant Day effect with a significant difference across the 7 days, regardless of age
Two-way (Age × Day) ANOVAs were conducted to assess how these specific behaviors changed over the course of the seven days (Figure 2). For upright position, there was a significant Age effect (F(1,192)=12.24, p<0.001) with post hoc tests indicating that adolescent rats exhibited overall lower instances of upright postures across the 7 days compared with adults. A significant interaction (F(6,192)=2.52, p<0.05) further indicated a significantly lower instance of upright postures in adolescent compared to adult female rats on day 1. For boxing behavior, there was a significant Day effect (F(6,192)=16.98, p<0.01) with post hocs indicating that adolescent rats exhibited decreased frequency of boxing postures overall throughout the week of exposure to the resident female. There was also a significant age effect in the two-way ANOVA with adult rats demonstrating a higher frequency of boxing behavior compared to adolescents over the 7-day defeat period (F(1,192)= 16.98, p<0.001).
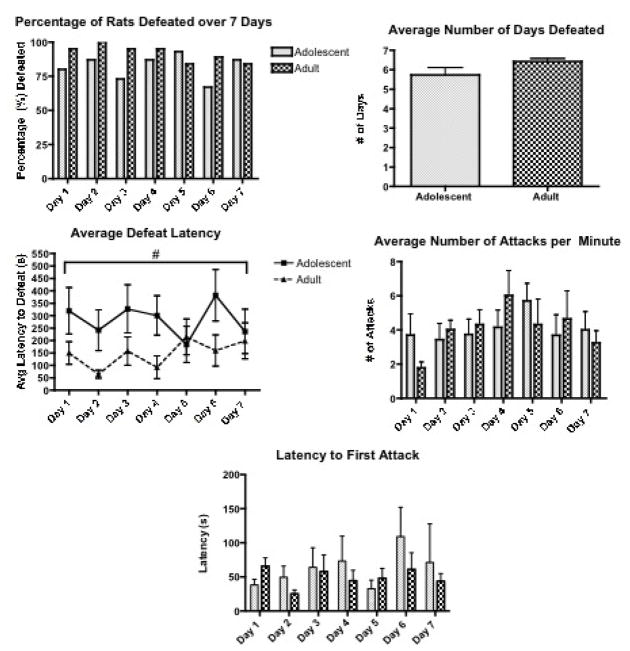
Figure 2.
Behaviors over 7 days. Adult females (n=19) and adolescent females (n=15) were subjected to 7 days of repeated social defeat with an aggressive lactating female resident. Following the intruder’s placement in the resident’s cage, resident and intruder were allowed to interact until one of two possible criteria was met: (a) the intruder exhibited a submissive defeat posture (>2 seconds frozen in a supine position), or (b) 15 min. elapsed without defeat. On each of the 7 days, the latency to be defeated was measured. The percentage of rats being defeated on each of the 7 days is shown in the top left and the average number of days that adolescents compared to adults were defeated is shown in the top right. Adolescent rats exhibited an overall significantly longer latency to defeat over the 7 days than did adult rats, shown in the middle left. Similar number of attacks occurred across the 7 days for both age groups, shown in the middle right graph. On each of the seven days of defeat, the latency to first attack was recorded, which is shown in the bottom graph.
# p≤ .05 indicates a significant Age effect with a significant difference between adolescent and adult female rats
For under the cover, there were significant effects of Age (F(1,192)=10.57, p<0.01), and Day (F(6,192)=2,44, p<0.03), as well as a significant interaction (F(6,192)=2.25, p<0.04) effect. Post hoc tests indicated that adolescent rats demonstrated increased instances of under the cover behavior overall across the seven days as compared with adults. Furthermore, there was significantly more time spent under the cover on the first day in adolescent compared to adult rats. For grooming by the resident, there was a significant Age effect (F(1,192)=13.69, p<0.01) with post hocs indicating significantly higher instances of grooming by the resident towards the adolescent compared to the adult female intruder. There were no significant effects on self-grooming or freezing/crouching across the seven days.
There were no significant effects in the percentage of rats defeated over 7 days and in the average number of days that defeat was exhibited between adult and adolescent rats (Figure 2). Results of a two-way ANOVA (Age × Day) for the latency to be defeated over the 7 days (Figure 2, bottom left graph) indicated a significant main effect of Age (F(1,192)=7.00, p<0.01) but no significant effect of Day and no interaction effects. Post hoc tests indicated that adolescent rats exhibited an overall significantly longer latency to be defeated over the 7 days of social stress than did adult rats. A two-way ANOVA (Age × Day) assessing the number of attacks received from the lactating female for adolescent and adult rats over the 7 days (Figure 2, bottom right) showed no overall effect of Age, or Day and no interaction effects. These results indicate that although there were no significant differences between adolescent and adult females in terms of how many rats were defeated across the 7 days and how many times rats were defeated, adolescent rats did exhibit a longer latency to be defeated overall despite experiencing a similar number of attacks by the resident females. Furthermore, a two-way ANOVA (Age × Day) indicated no significant differences in the latency to first attack by the resident female, indicating that the resident female treated both adolescent and adult rats that entered her cage as comparable threats.
3.2 Short-term effects of social defeat on behavior in the Porsolt forced swim test (FST)
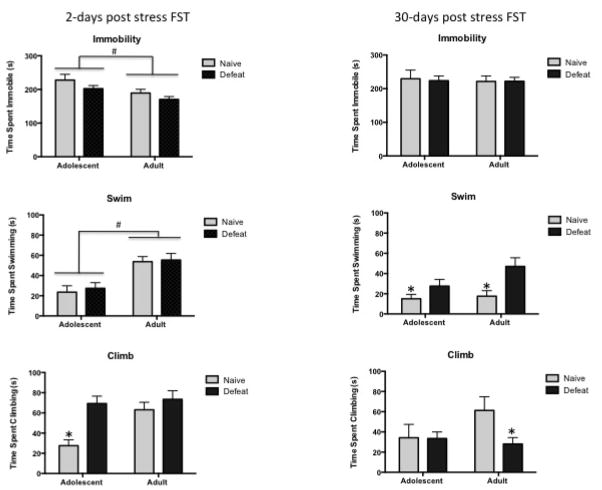
Following the seven days of repeated social defeat, behavior was assessed on the subsequent two days in the Porsolt FST. Results of the two-way (Age × Stress) ANOVA indicated an overall effect of Age (F(1,44)= 9.43, p< 0.01) on immobility behavior indicating that adolescent rats spent significantly more time immobile than did adult rats regardless of stress condition (Figure 3, left column). There was a trend toward a Stress effect (p< 0.06) suggesting that socially defeated animals tended to spend less time immobile compared with their non-stress naïve counterparts. In time spent swimming, there was a significant Age effect (F(1,45)=16.34, p< 0.001) indicating that adolescent female rats spent less time swimming than adult female rats. Results of a two-way ANOVA for climbing indicated a significant Age effect (F(1,43)= 4.17, p< 0.05) with adults climbing more than adolescents, as well as a significant Stress effect (F(1,43)= 7.15, p≤ 0.01) with a post hoc tests indicating that defeated adolescents spent more time climbing than did their naïve counterparts. Overall, the results indicated significant differences in each behavior across age and that repeated defeat in adolescence increases climbing behavior but does not have any other effects in the immediate period after the end of defeat.
Figure 3.
Short-term and long-term effects of social defeat on the Porsolt forced swim test. Defeated animals were subject to 7 days of social defeat, whereas naïve animals were left untouched in their homecage for the 7 days. The Porsolt FST was used to assess the immediate and long-term behavioral response to repeated social defeat stress. Animals (Adolescent Naïve: n=7, Adolescent defeat: n=15, Adult Naïve: n=8, Adult Defeat: n=19) were individually subjected to the Porsolt FST on two occasions: 2 days and 30 days after the end of the 7 days of social defeat or in naïve controls. Time spent in immobility (top graphs), swimming (middle graphs) and climbing (bottom graphs) during the FST administered two days after the end of repeated stress are shown in the left column and the right column shows the results of the FST administered 30 days after the end of repeated social defeat or control manipulation.
# p≤ .05 indicates a significant Age effect with a significant difference between adolescent and adult female rats
* p ≤ .05 indicates a significant Stress effect with a significant difference between defeated rats compared to age matched counterparts
@ p≤ .09 indicates a trend towards a significant Stress effect when compared to age matched counterparts
3.3 Long-term effects of social defeat on behavior in the FST
Results of a two-way ANOVA (Age × Stress) on behavior in the FST conducted 30 days after the end of defeat in either adolescence or earlier in adulthood are shown in Figure 3, right column. No significant effects were observed on immobility. There was a significant Stress effect (F(1,42=5.84), p<0.05) for time spent swimming with post-hoc tests indicating that defeated rats at both ages exhibited increased time spent swimming compared to naïve rats. In climbing, there was a trend toward a significant interaction effect (p<0.09) with adult defeated rats exhibiting a trend towards less time spent climbing than adult naïve rats. Overall, these results indicated that defeat, regardless of age that it is experienced, produces a long lasting increase in swim behavior and tends to decrease climbing in animals that are defeated earlier in adulthood.
3.4 Response to acute restraint
Adult rats that had been defeated during adolescence or earlier in adulthood were exposed to a 30min period of acute restraint on the day after the second forced swim test (Figure 4).
Figure 4.
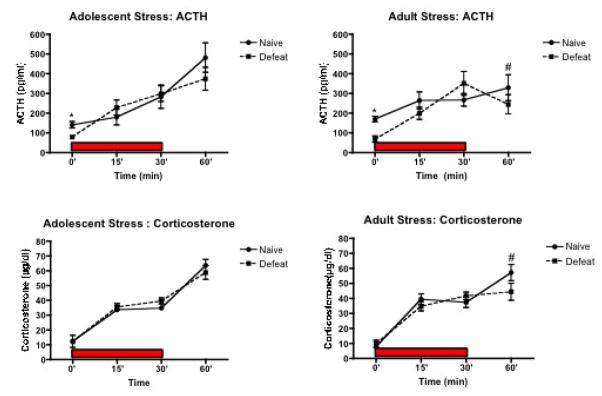
Neuroendocrine responses to acute restraint. The day following the second FST, a subset of rats was placed in a well-ventilated, size-adjusted Plexiglas restraint tube and subjected to a 30-min restraint (shown in the dark bar). Results for ACTH indicated a significant effect of stress at baseline (0 min) with defeated rats showing lower levels of ACTH compared to naïve rats regardless of the age at which they were stressed. There was a significant effect at 60 min with rats stressed earlier in adulthood displaying lower concentrations of ACTH and corticosterone compared to rats stressed as adolescents. Group sizes for ACTH: Adolescent Naïve: n=6–7; Adolescent Defeat: n=9–10; Adult Naïve: n=6–7; Adult Defeat: n=10–11. Group sizes for Corticosterone: Adolescent Naïve: n=6–7, Adolescent Defeat: n=8–11, Adult Naïve: n=7–8, Adult Defeat: n=10–11.
* p ≤ .05 indicates a significant Stress effect with a significant difference between defeated rats compared to age matched counterparts
# p≤ .05 indicates a significant Age effect with a significant difference between adolescent and adult female rats
Neuroendocrine responses to restraint
Results from a two-way ANOVA for ACTH at 0 min indicated a significant effect of Stress (F(1,30)= 29.56, p< 0.001) with defeated rats showing lower levels of ACTH compared to naïve rats regardless of the age at which they were stressed. There were no significant effects on ACTH at 15 and 30min. At 60min, there was a significant effect of Age (F(1,30)=5.05, p<0.03) with rats that were stressed or that served as controls in adulthood exhibiting lower ACTH compared with rats that were stressed or that served as controls during adolescence. For corticosterone, there was a significant Time × Stress effect (F(3,75)=4.57, p<0.005). Relevant post-hocs indicated that naie rats exhibited greater corticosterone than defeated rats there were no significant effects at 0, 15 or 30min but there was a significant Age effect at 60 min (F(1,33)=3.84, p<0.05). At 60min, rats that were stressed or that served as control as adults displayed a significantly lower corticosterone concentration than did rats that were stressed or that served as controls as adolescents. Thus, repeated defeat decreased baseline ACTH in rats stressed as adolescents as well as in adult rats stressed 30 days earlier compared to their naïve counterparts. Furthermore, adult rats that had been stressed or that served as controls earlier in adulthood displayed lower ACTH and corticosterone at recovery from restraint than rats that were stressed or that served as controls in adolescence.
Results from a two-way (Stress × Age) ANOVA assessing estradiol at 60 min indicated no significant effects on plasma estradiol (Adolescent Naïve: 179.794 ± 77.50 ng/ml; Adolescent Defeat: 219.002 ± 49.53 ng/ml; Adult Naïve: 177.126 ± 22.71 ng/ml; Adult Defeat: 120.035 ± 10.03 ng/ml). Furthermore, assessment of the vaginal smears indicated no differences in the numbers of rats in estrous and proestrous between any groups. Adolescent defeat animals: 53.3% proestrus, 40% estrus, 6.7% diestrus. Adolescent Naïve animals: 28.6% proestrus, 14.3% estrus, 28.6% diestrus, 28.6% undetermined. Adult defeat: 47.4% proestrus, 31.6% estrus, 5.3% diestrus, 15.8% undetermined. Adult Naïve: 75% proestrus, 25% estrus.
Neuronal activity
The cFos protein was assessed by Western blots as an index of neuronal activity in response to restraint (Figure 5). Results from two-way (Age × Stress) ANOVAs indicated that for the prelimbic PFC, overall Age (F(1,26)=10.84, p<0.01) and Stress (F(1,26)=4.37, p<0.05) effects were found in response to restraint, with adult rats showing a higher percentage of cFos than adolescents and rats that were defeated earlier as adults showing a lower cFos protein than their non-stressed counterparts. For the infralimbic PFC, cingulate cortex, and PVT, significant Age effects indicated that rats stressed or not earlier in adulthood showed a higher expression of the cFos protein than rats stressed as adolescents ((F(1,27)=7.58, p< 0.01); F(1,25)=5.49, p<0.05; and F(1,19)=8.01, p<0.01), respectively). In the PVN, there was an overall Stress effect (F(1,24)=8.80, p<0.01), with defeated rats showing lower expression of the Fos protein than naive rats regardless of the age at which they were stressed.
Figure 5.
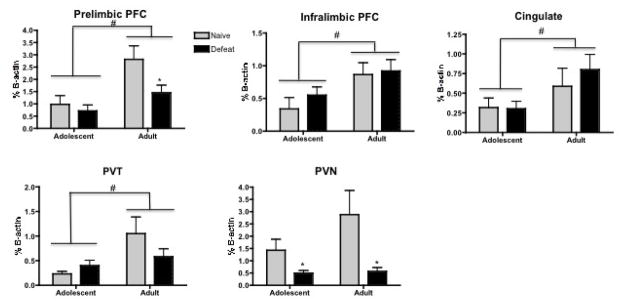
Neuronal activity in select brain regions in response to restraint. Rats were either exposed to a seven day defeat paradigm (defeat), or left untouched in their homecage (naïve). The brain regions examined were the prelimbic prefrontal cortex (Prelimbic PFC), infralimbic prefrontal cortex (Infralimbic PFC), cingulate, paraventricular nucleus of the thalamus (PVT) and the Paraventricular nucleus of the hypothalamus (PVN). Graphs show the quantification of c-Fos as a percent of β-actin. In the prelimbic PFC, infralimbic PFC, cingulate, and PVT, rats that were stressed or naïve earlier in adulthood exhibit elevated neural activation as assessed by c-Fos when compared to rats stressed as adolescents. In the PVN, rats exposed to defeat exhibit less neural activation after restraint than naïve animals regardless of the age that they were stressed. Group sizes were Adolescent Naïve: n=6–7, Adolescent Defeat: n=7–8, Adult Naïve: n=5–9; Adult Defeat:n-5–8).
* p ≤ .05 indicates a significant Stress effect with a significant difference between defeated rats compared to age matched counterparts
# p≤ .05 indicates a significant Age effect with a significant difference between adolescent and adult female rats
4. DISCUSSION
We examined the behaviors exhibited by adolescent and adult female rats during repeated exposure to the social stress of defeat and assessed the immediate and long-term consequences of this stress experience. We found that adolescent and adult female rats exhibited distinctly different behavioral repertoires in response to the aggressive resident. Adult females exhibited increased numbers of upright postures and boxing postures compared with adolescent rats. In contrast, adolescent rats exhibited increased numbers of instances in which they went under the resident (termed under the cover of the resident) and instances in which they were groomed by the resident female rat. Furthermore, there was a tendency of adolescent rats to freeze/crouch more and to self-groom less than adult rats. In sum, the behaviors of adolescent rats during defeat appeared more passive, play-related and/or avoidant when compared with the behaviors of adult rats. However, adolescent rats exhibited increased latency to be defeated across the 7 days perhaps because of these avoidant/play behaviors. This increased latency occurred despite similar numbers of attacks received on each day and latencies to be attacked on each day, as compared to adults, suggesting that the differences in latency to be defeated were not due to lack of aggressiveness of the resident female towards the adolescent rats. Together, these results indicate that adolescent females exhibit a more passive, play/avoidant behavioral repertoire compared to the adult females resulting in an increased latency to be defeated. This pattern of behavior develops into a more active repertoire characterized by upright and boxing postures in adulthood. This developmental pattern of behavior in response to conspecific aggression is similar to that observed in male rats (Bingham et al., 2011), indicating that the behavioral response to conspecific aggression develops similarly in male and female rats, becoming more active in the transition from adolescence to adulthood.
Two consequences of repeated social defeat were examined. The first was behavior in the forced swim test, which was examined twice. Behavior was assessed at two days after the end of defeat to assess the immediate impact of defeat during adolescence or adulthood. Behavior was then re-assessed 30 days after end of defeat primarily to determine whether defeat in adolescence altered behavior in adulthood. In the short-term, adolescents, regardless of whether they were exposed to social defeat or not, show increased time immobile and decreased time swimming compared to adults. In addition, adolescent rats exposed to defeat exhibited increased climbing compared to their age–matched naïve counterparts. No effects of defeat were observed in the adult females. Thus, repeated defeat in adolescent females favored increased climbing whereas it did not have a significant impact on adult females suggesting an impact of repeated defeat on noradrenergic system function (Detke et al., 1995) in adolescent female rats. When behavior was examined 30 days after the end of defeat, adult females that had been exposed to defeat as adolescents did not exhibit any behavioral differences compared to naïve females suggesting that there was not a long lasting impact of social defeat in adolescence in female rats. However, social defeat in adulthood increased swimming behavior at the expense of climbing suggesting an impact of adult defeat on both serotonergic and noradrenergic system function. The lack of effect of repeated defeat on immobility in either adolescent or adult females does not necessarily indicate a lack of effect of defeat at these ages on depression-related behavior. Others have shown that the repeated stress of defeat alternating with restraint increases pro-depressive or passive behavior in the FST and in the sucrose preference test (Bourke and Neigh, 2011). We used social defeat as the sole stressor and only had the forced swim test as a test of behavior so the use of additional tests, such as the sucrose preference test or novelty-induced hypophagia (Hodes et al., 2010), would be valuable in fully determining the effects of repeated defeat on depressive-type behavior. Furthermore, exposure to the forced swim test on the two days after the end of defeat may have obscured an impact of defeat on behavior in this test 30 days later. Overall, females stressed in adolescence tended to be more passive but these effects were transitory and were not observed when these animals became adults. On the other hand, there were no significant effects of defeat in adults that were immediate but there was a long-lasting impact on swimming and climbing. Therefore, the main finding on the forced swim test indicates an immediate and transient impact if stress occurs in adolescence but a long- term impact if stress occurs in adulthood.
We examined the hypothalamic pituitary adrenal (HPA) response to acute restraint in adult rats that had been defeated as adolescents or defeated earlier in adulthood. At baseline (0 min pre-stress timepoint), ACTH was significantly lower in the defeated rats, regardless of the age at which they were stressed, compared to naïve controls. No accompanying significant effects in baseline corticosterone were observed. Thus, repeated defeat, regardless of whether it was experienced in adolescence or earlier adulthood, lowered the baseline ACTH concentrations. The lack of effect on baseline corticosterone could reflect an increased adrenal sensitivity to ACTH in the defeated females. Indeed, early life manipulations like prenatal alcohol alter adrenal sensitivity (Osborn et al., 2000). The effect at the pituitary suggests a plastic change occurring at least at the PVN to inhibit basal pituitary function. Furthermore, females exposed to defeat or that were naïve controls in adolescence exhibited higher ACTH and corticosterone at recovery from restraint (60min timepoint) than females that were stressed or were naïve controls during adulthood. Thus, repeated defeat or handling/behavioral testing of control rats in adolescence had long-lasting effects on HPA activity, delaying recovery of both ACTH and corticosterone from restraint compared to females that had been stressed or were handled/behaviorally tested during adulthood. This may be because both stress and control groups were exposed to the forced swim test in adolescence and behavioral testing experience may have been sufficient to induce a state of stress significant enough to alter later responses to novel stress. Alternatively, it is possible this result occurred because HPA reactivity was tested in adult rats stressed in adolescence compared to adult rats tested 40 days earlier in adulthood. Thus, although all rats are adults at the time of restraint, the rats that were manipulated in adulthood were 40 days older at the time of restraint than the rats that were manipulated in adolescence and this age difference could account for the delayed recovery effects. Finally, these results cannot easily be explained by differences in estrous cyclicity since there were no significant effects on plasma estradiol. Nonetheless, differences in estrous cyclicity impacting the stress response cannot be ruled out because a systematic assessment of cyclicity throughout the study was not carried out. Overall, these results indicate that stress experienced in either adolescence or adulthood has a long lasting effect to lower basal ACTH concentrations and that stressful or other adverse experiences (like behavioral testing) produce a long-lasting impact on subsequent stress responses, delaying recovery of the HPA response to novel stressors.
We examined the neural substrates engaged by restraint in adulthood in rats exposed to defeat in adolescence or earlier in adulthood by determining density of the Fos protein in select stress-regulatory brain regions. In 4 of the 5 regions examined, females defeated in adolescence showed decreased neuronal activation when compared to females who were defeated earlier in adulthood. These regions were the prelimbic prefrontal cortex, infralimbic prefrontal cortex, cingulate cortex and the paraventricular nucleus of the thalamus. This decreased activation in adult animals stressed in adolescence was accompanied by higher ACTH and corticosterone concentrations during recovery from restraint. Interestingly, PVT and cingulate cortex regions are primarily inhibitory to HPA activity (Diorio, 1993); (Bhatnagar, 2002) (Jaferi and Bhatnagar, 2006). The prelimbic and infralimbic portions of the medial prefrontal cortex have different roles in regulating stress responses (Radley et al., 2006; Herman et al., 2005) although neuronal activity was similar in both regions in this study. Thus, it is possible that the more delayed recovery from restraint in adult females stressed in adolescence may be a result of decreased activation of inhibitory cortical and thalamic structures. In the PVN, animals that had experienced defeat, either in adolescence or in adulthood, exhibited lower neuronal activity compared to naïve animals. This was an unexpected finding as neuronal activity at 60min should reflect the response to restraint. However, it is possible that neuronal activity in the PVN in these females is on a different timecourse than other regions and is reflecting baseline activity consistent with lower ACTH concentrations in these groups at baseline. Alternatively, the discordant response in the PVN compared to the pituitary/adrenal could reflect a disproportionate activation of one cell population over another (ex. autonomic compared to neuroendocrine), an option that could not be evaluated using the Western blot approach used here. Overall, the results suggest that the cortical/thalamic structures are potential substrates that mediate the long-term impact of stress occurring in adolescence.
In sum, the results show that the behavioral repertoire of an adolescent vs. an adult female in response to an aggressive conspecific is different. Adolescent females exhibit more play and avoidant behaviors and adult females exhibit more active and aggressive behaviors toward the resident female, indicating that behaviors toward aggressive conspecifics are significantly remodeled from adolescence to adulthood. Age-dependent effects are observed in the forced swim test in which adolescent females exhibit increased immobility and decreased swim but defeat in adolescent females produces increased climbing. Thirty days after the end of defeat, the effects of defeat in adolescence on behavior in the forced swim test have dissipated but the effects of defeat earlier in adulthood are revealed in increased swimming and decreased climbing. Finally, animals defeated and/or behaviorally tested in adulthood exhibited enhanced recovery from restraint compared to animals defeated and/or behaviorally tested in adolescence. These results could be due to differences in adult ages at time of testing or could suggest a long-term deleterious effect of adolescent stress or other adverse experiences on recovery from novel stress. This delay in recovery was associated with lower activity in some neural structures that generally inhibit HPA activity, and these structures are potential substrates underlying the long-term effects of stress in adolescence. Together, these results suggest that repeated social stress during adolescence produces a different pattern of effects, particularly in the short-term, than does repeated social stress in adulthood.
Highlights.
behavioral response to defeat more active in transition from adolescence to adulthood
immediate impact of stress in adolescence but long-term impact of stress in adulthood
cortical/thalamic regions engaged by social defeat in adolescence
Acknowledgments
Studies were funded by MH090420 to Bhatnagar and MH093981 to Bhatnagar and R.J. Valentino.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat- induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;12:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Billings, Andrew G, Moos, Rudolf H. Coping, stress, and social resources among adults with unipolar depression. J Pers Soc Psychol. 1984;46:877–891. doi: 10.1037//0022-3514.46.4.877. [DOI] [PubMed] [Google Scholar]
- Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, Valentino R. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology. 2011;36:896–909. doi: 10.1038/npp.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Exposure to repeated maternal aggression induces depressive-like behavior and increases startle in adult female rats. Behav Brain Res. 2012;227:270–275. doi: 10.1016/j.bbr.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T, Lin S, DeVries C, Lambert K. Coping strategies in male and female rats exposed to multiple stressors. Physiol Behav. 2003;78:495–504. doi: 10.1016/s0031-9384(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meany MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannelly KJ, Flannelly L. Time course of postpartum aggression in rats (Rattus norvegicus) Journal of Comparative Psychology. 1987;101:101–103. [Google Scholar]
- Foilb AR, Lui P, Romeo RD. The transformation of hormonal stress responses throughout puberty and adolescence. J Endocrinol. 2011;210:391–398. doi: 10.1530/JOE-11-0206. [DOI] [PubMed] [Google Scholar]
- Garber J. Depression in Children and Adolescents Linking Risk Research and Prevention. Am J Prev Med. 2006;31:104–125. doi: 10.1016/j.amepre.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. Horm Behav. 2007;110:21232–21237. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Grissom N, Kerr W, Bhatnagar S. Struggling behavior during restraint is regulated by stress experience. Behav Brain Res. 2008;191:219–226. doi: 10.1016/j.bbr.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G, Hill-Smith TE, Lucki I. Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett. 2010;52:244–253. doi: 10.1016/j.neulet.2010.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Flashner B, Chiu M, Ver Hoeve E, Luz S, Bhatnagar S. Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not in male rats. Physiol Behav. 2011;105:269–275. doi: 10.1016/j.physbeh.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McEwen B. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo S, De Almeida RM, Bannai M, Fish EW, Debold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Osborn J, Yu C, Stelzl GE, Weinberg J. Effects of fetal ethanol exposure pituitary-adrenal sensitivity to secretagogues. Alcohol Clin Exp Res. 2000;24:1110–1119. [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Romeo R. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo R, Bellani R, Karatsoreos I, Chhua N, Vernov M, Conrad C, McEwen B. Stress History and Pubertal Development Interact to Shape Hypothalamic-Pituitary-Adrenal Axis Plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Walker FR, Masters LM, Dielenberg RA, Day TA. Coping with defeat: acute glucocorticoid and forebrain responses to social defeat vary with defeat episode behaviour. Neurosci Biobehav Rev. 2009;162:244–253. doi: 10.1016/j.neuroscience.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]