Summary
Temporal lobe epilepsy is the most common and often devastating form of human epilepsy. The molecular mechanism underlying the development of temporal lobe epilepsy remains largely unknown. Emerging evidence suggests that activation of the BDNF receptor, TrkB, promotes epileptogenesis caused by status epilepticus. We investigated a mouse model in which a brief episode of status epilepticus results in chronic recurrent seizures, anxiety-like behavior, and destruction of hippocampal neurons. We used a chemical-genetic approach to selectively inhibit activation of TrkB. We demonstrate that inhibition of TrkB commencing after status epilepticus and continued for two weeks prevents recurrent seizures, ameliorates anxiety-like behavior, and limits loss of hippocampal neurons when tested weeks to months later. That transient inhibition commencing after status epilepticus can prevent these long-lasting devastating consequences establishes TrkB signaling as an attractive target for developing preventive treatments of epilepsy in humans.
Introduction
The epilepsies are one of the most common serious disorders of the CNS. Among the epilepsies, temporal lobe epilepsy (TLE) is the most common form and is often devastating both because of its resistance to anticonvulsants and its associated behavioral disorders (Engel et al., 1998). Retrospective studies of patients with medically refractory TLE revealed that the majority experienced an episode of continuous seizure activity (status epilepticus [SE]) years earlier (French et al., 1993). Longitudinal studies reveal that almost half of individuals experiencing de novo SE develop recurrent seizures (epilepsy) after a seizure-free latent period of variable duration (Annegers et al., 1987; Tsai et al., 2009). Because induction of SE alone is sufficient to induce TLE in diverse mammalian species ranging from mice to subhuman primates (Pitkanen, 2010), the occurrence of de novo SE is thought to contribute to development of TLE in humans.
Insight into the molecular mechanisms by which SE transforms a normal brain into an epileptic brain may reveal novel targets for development of preventive therapies. It has been widely hypothesized that the brain-derived neurotrophic factor (BDNF) receptor, TrkB, is required for SE-induced TLE (Boulle et al., 2012; but see Paradiso et al., 2009); however, off-target effects of TrkB inhibitors together with inadequate temporal control afforded by genetically modified animals have precluded testing this idea. We therefore sought a method to selectively inhibit TrkB following SE. Here we use a chemical-genetic method (Chen et al., 2005) and demonstrate that inhibition of TrkB signaling for two weeks following SE prevents development of TLE and ameliorates comorbid anxiety-like behavior and destruction of hippocampal neurons.
Results
Activation of TrkB following SE
We first sought to confirm that SE induction enhanced activation of TrkB. A major pathway by which SE can be induced in hippocampus and related temporal lobe structures involves activation of neurons in the amygdala by chemical or electrical methods (Goddard et al., 1969; Mouri et al., 2008). Infusion of the chemical convulsant, kainic acid (KA), into the right amygdala of an awake wild type (WT) mouse induced SE (Ben-ari et al., 1980; Mouri et al., 2008) (Fig S1A,B, Fig S3, Fig S4). Mice were euthanized either immediately (0) or at 3, 6, 24, or 96 h later. Mice infused with vehicle (PBS) served as controls. Using p-TrkB (pY816 and pY705/706) immunoreactivity as surrogate measures of activation (Segal et al., 1996), we detected increased activation of TrkB in the hippocampus ipsilateral to the infused amygdala immediately upon termination of SE and at each of the subsequent time-points relative to the vehicle controls (p<0.01) (Fig S2A).
Chemical-genetic approach enables selective inhibition of TrkB kinase
We next sought to verify that we could selectively inhibit TrkB activation using a chemical-genetic approach. A genetic modification of mice in the TrkB locus (TrkBF616A) in which alanine is substituted for phenylalanine at residue 616 within kinase subdomain V renders TrkB sensitive to inhibition by a blood-brain barrier and membrane-permeable, small-molecule, 1-(1,1-dimethylethyl)-3-(1-naphthalenylmethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (1NMPP1; henceforth, the terms 1NMPP1 and inhibitor will be used interchangeably). Importantly, in the absence of 1NMPP1, no differences in TrkB kinase activity or overt behavior are detectable in TrkBF616A compared to WT mice (Chen et al., 2005). We infused the amygdala of TrkBF616A mice either with PBS or KA and then administered vehicle or 1NMPP1, respectively (see Experimental Procedures and Fig S1B). We detected enhanced p-TrkB (pY816) immunoreactivity in Western blots of lysates from the hippocampus ipsilateral to the infused amygdala in vehicle-treated WT (3 h post-SE, p<0.001) and TrkBF616A mice (3 h post-SE, p<0.001; 24 h post-SE, p<0.01) compared to their vehicle-treated PBS-infused controls (Fig S2B,C,D). Importantly, 1NMPP1 treatment inhibited the increase in p-TrkB (pY816) after SE in TrkBF616A (3 h, p<0.001; 24 h, p<0.01) but not in WT mice (Fig S2B,C,D). Similar results were obtained with an additional antibody directed to pY705/706 (Fig S2B,C,D). These results provide direct biochemical evidence that systemic treatment with 1NMPP1 can selectively inhibit SE-induced TrkB activation in TrkBF616A mice and validate our chemical-genetic method.
Transient inhibition of TrkB kinase commencing after SE prevents development of TLE
The ability to effectively and selectively inhibit activation of TrkB induced by SE enabled us to further determine whether inhibition of TrkB kinase following SE could prevent the development of chronic, spontaneous recurrent seizures (SRS). We maintained animals on 1NMPP1 for a period of 2 weeks (Fig S1B and Experimental Procedures) because this approach ensured inhibition of TrkB kinase for the duration of the SE-induced elevation (Fig S2). To minimize its effects on KA-induced SE, we withheld treatment with 1NMPP1 until diazepam was administered following 40 min of SE. Importantly, behavioral (Fig S3A,B) and electrographic (Fig S3C and Fig S4) seizures during SE prior to treatment with diazepam were similar in the vehicle and 1NMPP1 treated TrkBF616A mice. Moreover, assessment of electrographic seizure number or duration in hippocampal EEG recordings during the 1 h interval between diazepam and lorazepam or during the 1 h following treatment with lorazepam by a blinded observer revealed no significant differences between vehicle and 1NMPP1-treated TrkBF616A mice (Fig S3F, G respectively). These results of visually inspected EEG were corroborated by quantitative measures of EEG power which revealed no significant differences between vehicle and 1NMPP1-treated TrkBF616A mice during the 1 h intervals following treatment with diazepam or lorazepam (Fig S3D, E and Fig S4).
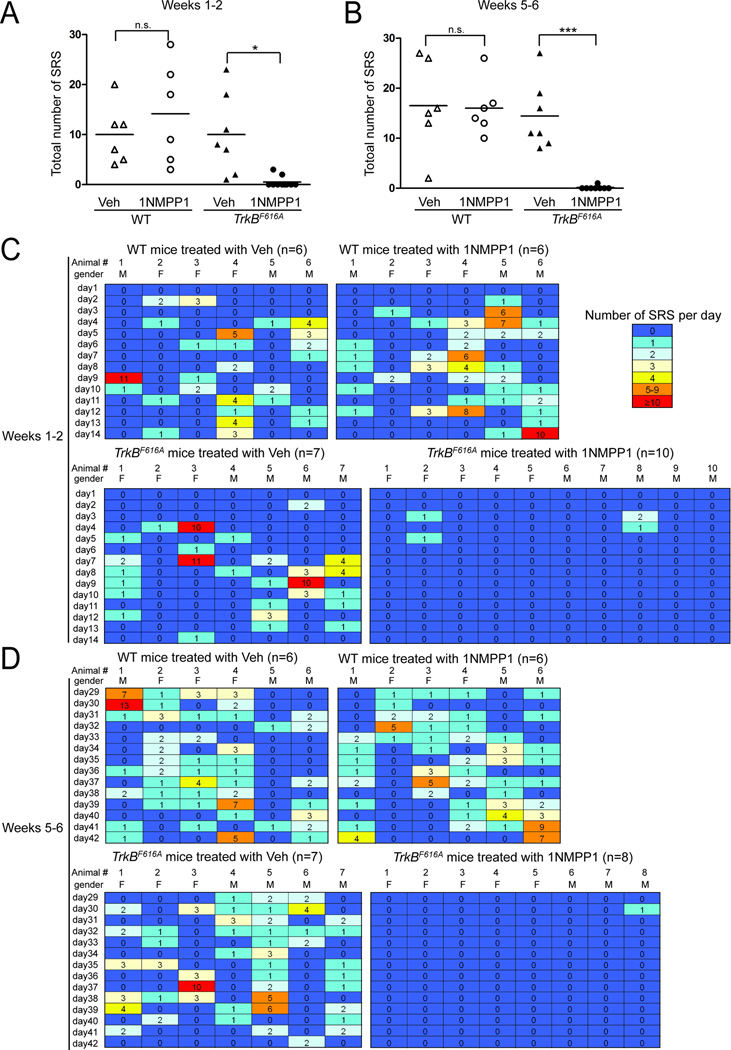
We first asked whether SRS can be suppressed during the 2 weeks of 1NMPP1 treatment and subsequently (i.e., weeks 5–6) whether SRS are eliminated following termination of 1NMPP1 treatment of TrkBF616A mice. Despite displaying SE with behavioral and EEG features similar to those of vehicle-treated TrkBF616A mice (Fig S3, Fig S4), no seizures were detected in 8 of the 10 1NMPP1-treated TrkBF616A mice during the 2 weeks following SE (Fig 1A,C). Of the 2 1NMPP1-treated TrkBF616Amice that exhibited seizures, a limited number of seizures (2 and 3 respectively) were detected within 3 to 5 days after SE, whereas no seizures were observed during days 6–14 after SE (Fig. 1C). By contrast, analyses of continuous video-EEG during weeks 1–2 following SE revealed that SE-induced SRS commenced several days thereafter in all vehicle-treated TrkBF616A mice and in all WT mice treated with either vehicle or 1NMPP1 (Fig 1A,C). There was a striking reduction in the number of SRS per 1NMPP1-treated TrkBF616A mouse (0.5 ± 0.3) compared to the vehicle-treated TrkBF616A group (10.0 ± 3.1; p<0.05) (Fig 1A). Importantly, 1NMPP1 treatment did not reduce the occurrence of SRS in WT mice in comparison to their vehicle-treated controls (p=0.57), thereby demonstrating the specificity of 1NMPP1 inhibition. The seizures that did occur in the two 1NMPP1-treated TrkBF616A mice were of similar duration (p=0.66, Student’s t-test) and behavioral class (p=0.71, Student’s t-test) to those observed in vehicle-treated TrkBF616A mice. Importantly, no seizures were detected in control mice receiving infusion of PBS into amygdala (data not shown). Thus continuous infusion of the TrkB kinase inhibitor, 1NMPP1, for 2 weeks commencing after SE markedly reduces the SE-induced SRS.
Figure 1. Transient inhibition of TrkB kinase prevents SRS following SE.
(A) Total number of SRS detected during weeks 1–2 post SE during treatment with vehicle or 1NMPP1 in WT or TrkBF616A mice (heat map in panel C). Occurrence of SRS was significantly reduced by 1NMPP1 treatment in TrkBF616A (*p<0.05) but not WT mice compared to their vehicle-treated controls. (B) Total number of SRS detected during weeks 5–6 post-SE (heat map in panel D); video-EEG analyses were initiated in these mice approximately 2 weeks after terminating treatment with 1NMPP1. Among the ten 1NMPP1-treated TrkBF616A mice monitored during 1–2 weeks after SE, eight of them (the left first eight columns on the bottom right panel of Fig 1C) were monitored during weeks 5–6 after SE (Fig 1D). Occurrence of SRS was prevented in these eight 1NMPP1-treated TrkBF616A mice (***p<0.001), but not in WT mice compared to their vehicle-treated controls. (C) and (D) Number of SRS detected each day during weeks 1–2 (C) and weeks 5–6 (D) after SE are presented as heat maps (one mouse per column). Note that SRS were detected in only two of ten 1NMPP1-treated TrkBF616A mice during weeks 1–2 (C) and one of eight 1NMPP1-treated TrkBF616A mice during weeks 5–6 (D). By contrast, all vehicle-treated TrkBF616A mice (7 of 7) and all WT mice treated with either vehicle (6 of 6) or 1NMPP1 (6 of 6) exhibited SRS during weeks 1–2 (C) and weeks 5–6 (D) post-SE. Statistics were performed using two-way ANOVA with Bonferroni post hoc tests, n=6–10. M: male and F: female. Animal #s correspond to #s in Figures S3 and S4 in supplementary information.
If inhibition of TrkB kinase activity prevented development of epilepsy, then a reduction of SRS should persist following termination of the inhibitor (1NMPP1). Following discontinuation of 1NMPP1 treatment, animals were housed in home cages for 2 weeks (i.e., weeks 3–4) before video-EEG monitoring was resumed during weeks 5–6. Among eight TrkBF616A mice that had undergone 1NMPP1 treatment during weeks 1–2 after SE, no seizures were detected in seven of them during weeks 5–6 and only a single seizure was detected in the eighth mouse (Fig 1B,D). By contrast, all vehicle-treated TrkBF616A mice and all WT mice treated with either vehicle or 1NMPP1 exhibited SRS during this same time (Fig 1B,D). Indeed the epilepsy appeared to worsen, in that the percentage of days with seizure during weeks 5–6 increased in comparison to weeks 1–2 in each of these three control groups (p<0.001, paired Student’s t-test, n=19). Consistent with the worsening, when all three groups were considered together, a significant increase (38%) in the total number of seizures was found during weeks 5–6 compared to weeks 1–2 (p<0.05, paired Student’s t-test, n=19). In contrast to TrkBF616A mice, 1NMPP1 treatment did not reduce the frequency of SRS in WT mice relative to the vehicle controls (p>0.99) (Fig 1B,D). Importantly, the reduction of SRS in 1NMPP1-treated TrkBF616A mice during weeks 5–6 (p<0.001) (Fig 1B,D) was not due to residual inhibition of TrkB kinase because an evoked seizure induced similar amounts of pTrk immunoreactivity in TrkBF616A mice when examined 1 week after terminating 1NMPP1 treatment compared to the vehicle alone (G.L. unpublished data), a finding consistent with a half-life of 1NMPP1 of less than 1 h (Wang et al., 2003). In sum, the striking reduction of seizures in 1NMPP1-treated TrkBF616A mice following termination of 1NMPP1 treatment demonstrates that transient inhibition of TrkB kinase following SE prevents SE-induced chronic, recurrent seizures (TLE).
Transient inhibition of TrkB kinase ameliorates SE-induced anxiety-like behavior
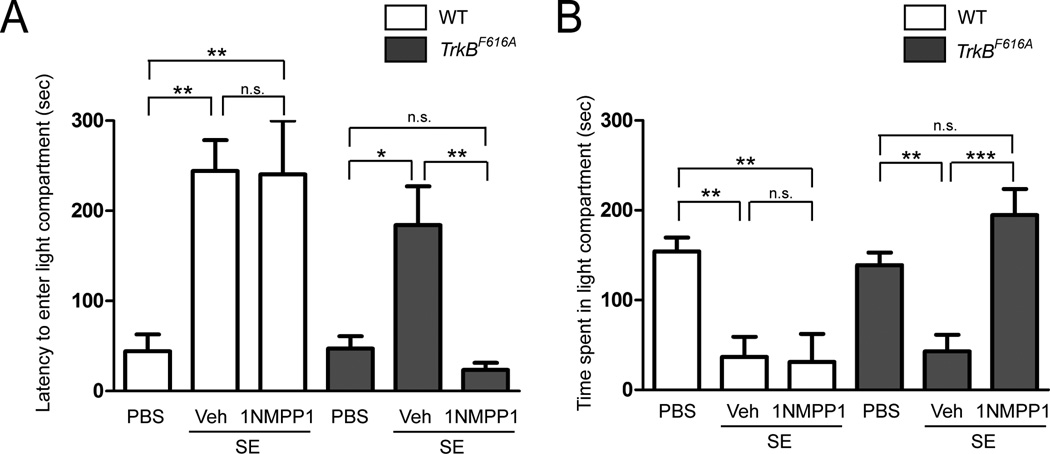
Increased levels of anxiety have been reported in humans with TLE and anxiety-like behavior has been documented in animal models of TLE (Beyenburg et al., 2005; Groticke et al., 2007). We sought to determine whether SE-induced anxiety-like behavior in animals was present and, if so, whether this SE-induced behavioral abnormality can be prevented by the transient inhibition of TrkB kinase activity. Following completion of video-EEG recording during weeks 5–6, anxiety-like behavior was assessed using the light-dark emergence test (Bourin and Hascoet, 2003). In comparison to controls (n=9) in which PBS was infused into the amygdala, WT and TrkBF616A mice undergoing SE followed by treatment with vehicle exhibited a prolonged latency to enter the lighted compartment (WT: p<0.01; TrkBF616A: p<0.05) (Fig 2A) and both groups spent less time in lighted compartment (WT: p<0.01; TrkBF616A: p<0.01) (Fig 2B). Notably, similar results were observed following SE in WT animals treated with vehicle or 1NMPP1 and in TrkBF616A mice treated with vehicle. By comparison to the vehicle-treated TrkBF616A mice, TrkBF616A mice given 1NMPP1 for 2 weeks following SE exhibited a significantly reduced latency to enter the lighted compartment (p<0.01) and they spent increased time in the lighted compartment (p<0.001) (Fig 2). Similarities in locomotor activity in an open field among all four groups undergoing SE excluded differences in spontaneous activity as a confounding variable in the light-dark emergence results (data not shown) (Bourin and Hascoet, 2003). Collectively, these results demonstrate that transient inhibition of TrkB kinase activity prevents SE-induced anxiety-like behavior.
Figure 2. SE-induced anxiety-like behavior is reduced by inhibition of TrkB kinase.
(A) Both WT and TrkBF616A mice undergoing SE and treated with vehicle thereafter exhibited prolonged latency to exit the darkened and enter the lighted compartment compared to PBS controls (WT: **P<0.01; TrkBF616A: *p<0.05). 1NMPP1 treatment reduced this latency in TrkBF616A mice (**p<0.01), but not in WT mice. (B) Both WT and TrkBF616A mice undergoing SE and treated with vehicle thereafter spent less time in lighted compartment compared to PBS controls (WT: **p<0.01; TrkBF616A: **p<0.01). 1NMPP1 treatment increased the time spent in the lighted compartment in TrkBF616A mice (***p<0.001), but not in WT mice. Data are presented as mean ± SEM and analyzed using two-way ANOVA with Bonferroni corrections, n=5–9.
Neuroprotective effects of inhibition of TrkB kinase following SE
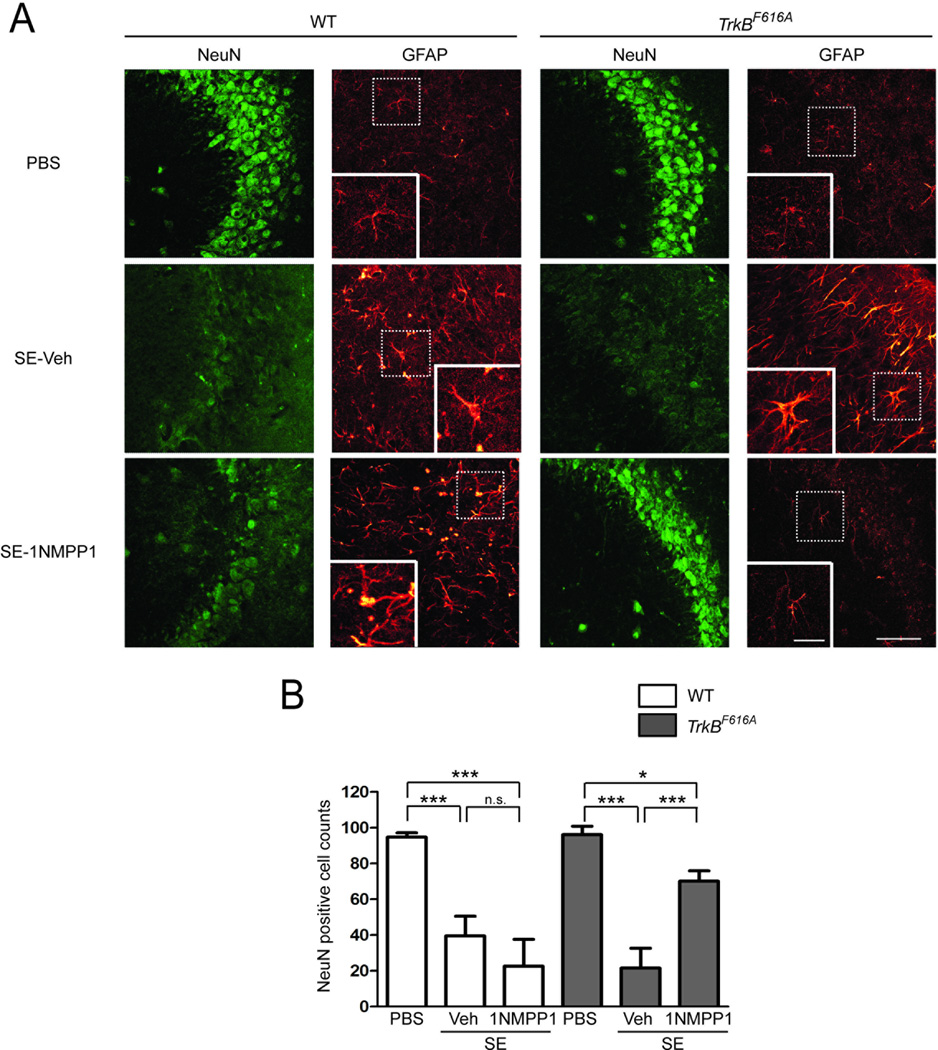
Death of hippocampal neurons and reactive gliosis are well recognized neuropathological features of TLE in humans (Mathern et al., 1998) and similar features have been identified in the hippocampus ipsilateral to the KA-infused amygdala 2 weeks following SE (Mouri et al., 2008). Histological analyses of a subset of WT mice given vehicle after SE and euthanized 2–3 months thereafter revealed ~60% reduction of neurons (NeuN immunoreactive cells) in CA3b hippocampus compared to control WT animals undergoing PBS infusion into amygdala (Fig 3A compare images in top and middle rows on far left column, and Fig 3B, p<0.001), confirming results of Mouri et al. (2008). Significant reductions of similar magnitude were observed following SE in 1NMPP1-treated WT and vehicle-treated TrkBF616A mice (Fig 3A,B). A significant yet notably less marked reduction (27%) of neurons was detected in 1NMPP1-treated TrkBF616A mice following SE compared to control TrkBF616A mice undergoing infusion of PBS into the amygdala (Fig 3A,B; p<0.05). Reactive gliosis evidenced by enlarged GFAP-immunoreactive cells with thickened processes in CA3b of hippocampus were observed following SE in WT animals treated with either vehicle or 1NMPP1 and in TrkBF616A mice treated with vehicle (Fig 3A), confirming a previous report of Mouri et al. (2008). Importantly, these abnormalities were attenuated by 1NMPP1 treatment following SE in the TrkBF616A mice (Fig 3A).
Figure 3. SE-induced hippocampal damage is attenuated by inhibition of TrkB kinase.
(A) Representative images of immunostaining of NeuN and GFAP in the hippocampal CA3b region ipsilateral to the infusion site in WT and TrkBF616A mice in respective PBS controls, SE-vehicle treated, and SE-1NMPP1 treated mice, scale bar = 50 µM. Insets: GFAP positive cells exhibited enhanced immunoreactivity and enlarged cell bodies and braches in SE-vehicle or 1NMPP1 treated WT mice and in SE-vehicle treated TrkBF616A mice, scale bar = 20 µM. (B) Number of NeuN positive cells within ipsilateral CA3b hippocampus was reduced in both WT and TrkBF616A mice undergoing SE and treated with vehicle thereafter compared to PBS controls (WT: ***p<0.001; TrkBF616A: ***p<0.001). 1NMPP1 treatment inhibited loss of NeuN positive cells in TrkBF616A mice (***p<0.001), but not in WT mice. Data are presented as mean ± SEM and analyzed using two-way ANOVA with Bonferroni post hoc tests, n=3–6.
Discussion
We hypothesized that transient inhibition of TrkB kinase commencing after SE should prevent the subsequent expression of chronic, recurrent seizures. We used biochemical, electrophysiological, and pharmacological studies of WT and TrkBF616A mice to test this hypothesis. A brief (40 min) epoch of SE was followed by recovery and a seizure-free latent period of several days after which a devastating condition characterized by recurrent seizures with progressively increasing frequency, anxiety-like behavior, and destruction of hippocampal neurons ensued. Biochemical studies revealed increased activation of TrkB in hippocampal membranes that was detectable shortly after onset of SE and persisted for several days. Inhibition of TrkB kinase initiated following SE and continued for just 2 weeks prevented the development of TLE and anxiety-like behavior, and limited destruction of hippocampal neurons when tested weeks to months thereafter. These findings establish TrkB signaling as an appealing target for therapies aimed at preventing development of epilepsy and associated behavioral disorders following SE.
The seizure-free latent period following SE is recognized clinically (Annegers et al., 1987; French et al., 1993; Tsai et al., 2009) and provides an opportunity to intervene with therapy to prevent chronic recurrent seizures, a finding that has fostered intensive study of the molecular mechanisms by which a brief episode of SE induces lifelong epilepsy. Activation of mammalian target of rapamycin (mTOR) signaling by SE has provided an attractive mechanism because continuous treatment with an mTOR inhibitor (rapamycin), initiated after SE, reduced the frequency of epileptic seizures (Wong, 2010). Disappointingly, the epileptic seizures emerged following discontinuation of rapamycin, implying that rapamycin suppressed seizures rather than targeting the mechanisms underlying their development (Huang et al., 2010). Administration of decoy oliognucleotides limiting the transcriptional repressor, NRSF, initiated after SE resulted in a 70% reduction in the number of spontaneous seizures during the ensuing two weeks (McClelland et al., 2011). However, it is presently unclear whether the reduced frequency of seizures will persist following discontinuation of decoy oligonucleotide therapy. Likewise, pharmacological depletion of a microRNA, miR-134, initiated after SE reduced the occurrence of spontaneous seizures when tested weeks later. Nevertheless, whether this treatment was preventive requires additional study because reductions of miR-134 persisted (Jimenez-Mateos et al., 2012). Treatment with atipamezole, an α2-adrenergic receptor antagonist, after SE reduced the frequency of seizures but failed to prevent epilepsy or behavioral impairments (Pitkanen et al., 2004). In the context of these studies, the present findings are notable both with respect to the magnitude of inhibition of the disease process and its time-course. Whereas multiple spontaneous recurrent seizures were detected during weeks 5–6 following SE in each of the 19 control animals undergoing SE, no seizures were detected in 7 of 8 animals in which TrkB kinase was inhibited for just two weeks following KA-SE and only a single seizure was detected in the eighth animal. Importantly, the short half-life of 1NMPP1 (less than 1 h) (Wang et al., 2003) together with direct biochemical evidence excluding persistent inhibition (G.L. unpublished data) establish the transient nature of the kinase inhibition. The virtual elimination of spontaneous recurrent seizures and associated anxiety-like behavior were evident long after discontinuation of TrkB kinase inhibition, demonstrating a truly preventive effect of this intervention.
That SE induces loss of hippocampal neurons is evident from both histological and magnetic resonance imaging studies of humans with severe TLE (Cascino, 1998; Mathern et al., 1998). The control animals undergoing SE in the present study exhibited neuronal loss predominantly in the hippocampal CA3 region ipsilateral to the KA injection, as well as, increased GFAP immunoreactivity typical of reactive gliosis, resembling the pathology in humans and confirming previous reports (Mouri et al., 2008). This pathology was significantly attenuated, but not eliminated, by transient inhibition of TrkB kinase commencing after SE. Because activation of TrkB signaling would be expected to protect neurons from death (Huang and Reichardt, 2003), the reduction in neuronal death following inhibition of TrkB kinase is surprising. One possibility is that the loss of hippocampal neurons in animals undergoing SE followed by inhibition of TrkB kinase is due to injury sustained during SE itself. If so, the greater loss of hippocampal neurons in the control groups may be due both to SE and to the many isolated seizures that ensued over a couple of months prior to death. The fact that many isolated seizures result in destruction of hippocampal neurons (Kotloski et al., 2002) supports this idea.
A diversity of behavioral disorders have been identified in patients with epilepsy with a greater frequency than in other chronic diseases, impairing the quality of life (Torta and Keller, 1999). Anxiety disorders are the most common behavioral conditions found in patients with epilepsy (Beyenburg et al., 2005). Animals undergoing SE in the current study exhibited a striking reluctance to enter the lighted compartment in the light-dark emergence test. Based on the innate aversion of rodents to brightly illuminated areas and on the spontaneous exploratory behavior of rodents in response to mild stressors (novel environment and light), the reluctance of mice undergoing SE to enter the lighted compartment is a response thought to reflect anxiety. Notably, this reluctance was eliminated in animals undergoing TrkB kinase inhibition. Thus enhanced TrkB kinase signaling induced by SE not only results in recurrent seizures, but it also renders the subject vulnerable to expressing anxiety-like behavior. Together these findings raise the interesting possibility that experience-driven activation of TrkB kinase activity may contribute to other CNS illnesses that, like epilepsy, can be induced by an episode of pathological neuronal activity. A traumatic emotional experience inducing a lifelong anxiety disorder would be one possibility. Evidence implicating TrkB signaling in the induction of contextual fear conditioning (Rattiner et al., 2004), an animal model mimicking some features of post-traumatic stress disorder, supports this idea.
The nature of the cellular consequences of enhanced TrkB activation that underlies the pathological consequences of the brief epoch of SE are presently unclear. Determining the cellular and subcellular locale of the activated TrkB is a critical first step to elucidating the cellular consequences, a determination that can be made using high resolution microscopy methods to localize pTrkB (Helgager et al., 2013).
The present findings provide proof of concept evidence that activation of TrkB kinase is required for the induction of chronic, recurrent seizures and anxiety-like behavior following SE. This result provides a strong rationale for developing selective inhibitors of TrkB kinase for clinical use. That commencing TrkB kinase inhibition following SE was effective together with the short latency of access to emergency medical care of many patients with SE (Alldredge et al., 2001) enhance the feasibility of this approach to preventive therapy. The fact that just two weeks of treatment was sufficient to prevent TLE could minimize potential unwanted effects inherent in long-term exposure to preventive therapy. In sum, TrkB signaling provides an appealing target for developing drugs aimed at prevention of TLE.
Experimental Procedures
Animals
TrkBF616A and WT mice in a C57BL/6 background (Charles River) were housed under a 12-hour light/dark cycle with food and water provided ad libitum. Animals were handled according to the National Institutes of Health Guide for the Care and Use of the Laboratory Animals and the experiments were conducted under an approved protocol by the Duke University Animal Care and Use Committee.
Surgery and Amygdala Kainic Acid (KA) Microinfusion
Adult mice were anesthetized and a guide cannula was inserted above the right amygdala and a bipolar electrode was inserted into the left hippocampus under stereotaxic guidance (Fig S1A). After a 7-day postoperative recovery, either KA (0.3 µg in 0.5 µl phosphate-buffered saline, PBS) or vehicle (0.5 µl of PBS) was infused into the right basolateral amygdala in an awake, gently restrained animal. Hippocampal EEG telemetry (Grass Instrument Co.) and time-locked video-monitoring were performed using Harmonie software (Stellate Systems). Monitoring started at least 5 min before amygdala KA infusion for recording baseline EEG and behavioral activity. SE was typically evident electrographically and behaviorally (Mouri et al., 2008) 8–12 min after KA infusion (Fig S3A and Fig S4A). Forty minutes after onset of KA-induced SE, diazepam (10 mg/kg, i.p.) was administered to suppress SE and this was followed by lorazepam (6 mg/kg, i.p.) 1 h later. To assure similarity of SE intensity, behavioral and EEG seizures were quantified following infusion of KA and for 1 h intervals following treatment with diazepam and lorazepam in both vehicle and 1NMPP1-treated TrkBF616A mice (Fig S3 and S4). The EEG recording electrode was placed in the left hippocampus so as not to confound histological analyses of the hippocampus ipsilateral to the infused (right) amygdala; the extensive commissural connections between the hippocampi notwithstanding, it is possible that electrographic seizure activity localized to the right hippocampus occurred and escaped detection.
Unless specified otherwise, following SE animals underwent continuous video-EEG monitoring 24 h/day, 7 days/week during weeks 1–2 and weeks 5–6 post-SE. Spontaneous recurrent seizures (SRS) were identified by review of video-EEG files by two independent trained readers blinded to both genotype and treatment of mice. Behavioral seizures were classified according to a modification of the Racine scale for mice (Borges et al., 2003). All EEG SRS were confirmed by corresponding behavioral seizures documented by time-locked video review. Quantitative analysis of EEG energy content was performed as described (Lehmkuhle et al., 2009) (Fig S3 and S4).
Treatment
In experiments examining effects of 1NMPP1 treatment on SE-induced spontaneous recurrent seizures, the first dose of 1NMPP1 (16.6 µg/g, i.p.) was injected immediately after giving diazepam and a second dose of 1NMPP1 (16.6 ng/g) immediately after administration of lorazepam (Fig S1B). A third dose of 1NMPP1 (16.6 µg/g, i.p.) was injected approximately 12 h post-SE after which 1NMPP1 was administered daily (16.6 µg/g, i.p.) and also included in drinking water (25 µM) for the ensuing two weeks at which point it was tapered and discontinued. WT mice and TrkBF616A mice injected under the same regimen with vehicle (i.p. and in drinking water) served as controls.
Western Blotting
Animals were euthanized and decapitated. Crude membranes were prepared from hippocampi and subjected to SDS-PAGE. Following transfer, Western blotting was conducted as described in Supplementary Information.
Behavioral Tests
After EEG and behavioral monitoring, KA-infused mice were examined for spontaneous activity in the open field and anxiety-like behavior in the light/dark box at 8 weeks post-SE as described in Supplementary Information. PBS-infused (amygdala) WT or TrkBF616A mice treated with vehicle or 1NMPP1 were tested at 8 weeks post-infusion and served as controls.
Neuropathology
At 10 weeks post-SE, mice were anesthetized and perfused with heparinized PBS followed by 4% paraformaldehyde and brains prepared for immunofluorescent study of neurons and astrocytes as described byMouri et al. (2008). NeuN-positive cell counting was performed by an investigator blinded to the genotype and treatment conditions with ImageJ software (Ferreira and Rasband, 2011) as described in Supplementary Information.
Data Analysis
All data are presented as the mean ± standard error of the mean (SEM). Unless otherwise noted, comparisons between two groups were analyzed using unpaired Student’s t-tests, while multi-group comparisons were analyzed using two-way ANOVA followed by Bonferroni post-hoc tests. A p< 0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank Dr. David Ginty for providing TrkBF616A mice. We thank Drs. J. Victor Nadler and Richard D. Mooney for critical discussions and reading of the manuscript. Daniella Cordero assisted in EEG and video reading. Wei-Hua Qian assisted in animal breeding and genotyping. This work was supported by NINDS grants NS56217 and NS060728 (J.O.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O'Neil N, Neuhaus JM, Segal MR, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- Ben-ari Y, Tremblay E, Ottersen OP. Injections of Kainic Acid into the Amygdaloid Complex of the Rat - an Electrographic, Clinical and Histological Study in Relation to the Pathology of Epilepsy. Neuroscience. 1980;5:515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- Beyenburg S, Mitchell AJ, Schmidt D, Elger CE, Reuber M. Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav. 2005;7:161–171. doi: 10.1016/j.yebeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Boulle F, Kenis G, Cazorla M, Hamon M, Steinbusch HW, Lanfumey L, van den Hove DL. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog Neurobiol. 2012;98:197–206. doi: 10.1016/j.pneurobio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Cascino GD. Structural Brain Imaging. In: Engel J, Pedley TA, editors. Epilepsy : a comprehensive textbook. Philadelphia: Lippincott-Raven; 1998. pp. 937–946. [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Engel J, Williamson P, Wieser H-G. Mesial Temporal Lobe Epilepsy. In: Engel J, Pedley TA, editors. Epilepsy : a comprehensive textbook. Philadelphia: Lippincott-Raven; 1998. pp. 2417–2426. [Google Scholar]
- Ferreira T, Rasband W. The ImageJ User Guide. 2011 [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Groticke I, Hoffmann K, Loscher W. Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp Neurol. 2007;207:329–349. doi: 10.1016/j.expneurol.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Helgager J, Liu G, McNamara JO. The cellular and synaptic location of activated TrkB in mouse hippocampus during limbic epileptogenesis. J Comp Neuro. 2013;521:499–521. doi: 10.1002/cne.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, Sano T, O'Tuathaigh C, Waddington JL, Prenter S, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18:1087–1094. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95–110. doi: 10.1016/S0079-6123(02)35010-6. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle MJ, Thomson KE, Scheerlinck P, Pouliot W, Greger B, Dudek FE. A simple quantitative method for analyzing electrographic status epilepticus in rats. J Neurophysiol. 2009;101:1660–1670. doi: 10.1152/jn.91062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Armstrong DL. Hippocampal Sclerosis. In: Engel J, Pedley TA, editors. Epilepsy : a comprehensive textbook. Philadelphia: Lippincott-Raven; 1998. pp. 133–156. [Google Scholar]
- McClelland S, Flynn C, Dube C, Richichi C, Zha Q, Ghestem A, Esclapez M, Bernard C, Baram TZ. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011;70:454–464. doi: 10.1002/ana.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri G, Jimenez-Mateos E, Engel T, Dunleavy M, Hatazaki S, Paucard A, Matsushima S, Taki W, Henshall DC. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–151. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Paradiso B, Marconi P, Zucchini S, Berto E, Binaschi A, Bozac A, Buzzi A, Mazzuferi M, Magri E, Navarro Mora G, et al. Localized delivery of fibroblast growth factor-2 and brain-derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proc Natl Acad Sci U S A. 2009;106:7191–7196. doi: 10.1073/pnas.0810710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Narkilahti S, Bezvenyuk Z, Haapalinna A, Nissinen J. Atipamezole, an alpha(2)-adrenoceptor antagonist, has disease modifying effects on epileptogenesis in rats. Epilepsy Res. 2004;61:119–140. doi: 10.1016/j.eplepsyres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Therapeutic approaches to epileptogenesis--hope on the horizon. Epilepsia. 2010;51(Suppl 3):2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Bhattacharyya A, Rua LA, Alberta JA, Stephens RM, Kaplan DR, Stiles CD. Differential utilization of Trk autophosphorylation sites. J Biol Chem. 1996;271:20175–20181. doi: 10.1074/jbc.271.33.20175. [DOI] [PubMed] [Google Scholar]
- Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(Suppl 10):S2–S20. doi: 10.1111/j.1528-1157.1999.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Tsai MH, Chuang YC, Chang HW, Chang WN, Lai SL, Huang CR, Tsai NW, Wang HC, Lin YJ, Lu CH. Factors predictive of outcome in patients with de novo status epilepticus. QJM. 2009;102:57–62. doi: 10.1093/qjmed/hcn149. [DOI] [PubMed] [Google Scholar]
- Wang H, Shimizu E, Tang YP, Cho M, Kyin M, Zuo W, Robinson DA, Alaimo PJ, Zhang C, Morimoto H, et al. Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc Natl Acad Sci U S A. 2003;100:4287–4292. doi: 10.1073/pnas.0636870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.