Abstract
Ever since the discovery of sirtuins a decade ago, interest in this family of NAD-dependent deacetylases has exploded, generating multiple lines of evidence implicating sirtuins as evolutionarily conserved regulators of lifespan. In mammals, it has been established that sirtuins regulate physiological responses to metabolism and stress, two key factors that affect the process of aging. Further investigation into the intimate connection among sirtuins, metabolism, and aging has implicated the activation of SIRT1 as both preventative and therapeutic measures against multiple age-associated disorders including type 2 diabetes and Alzheimer’s disease. SIRT1 activation has clear potential to not only prevent age-associated diseases but also to extend healthspan and perhaps lifespan. Sirtuin activating compounds and NAD intermediates are two promising ways to achieve these elusive goals.
Keywords: Age-associated disorders, Aging, Lifespan, NAD intermediates, NAD world, Nicotinamide adenine dinucleotide, Nicotinamide mononucleotide, SIRT1, Sirtuins, Sirtuin activating compounds
1 Introduction
The silent information regulator 2(SIR2) family of proteins, also called sirtuins, are evolutionarily conserved nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylases/ADP-ribosyltransferases. Now classified as class III histone deacetylases (HDACs), the founder SIR2 gene was originally identified as one of the genes that regulate the mating types of budding yeast, Saccharomyces cerevisiae (Klar and Fogel 1979). The biochemical function of SIR2 proteins had been a mystery for 20 years when the Salmonella typhimurium SIR2-like protein, CobB, was found to catalyze the reaction in the late step of cobalamin biosynthesis, transferring phosphoribose from nicotinic acid mononucleotide to dimethylbenzimidazole (Tsang and Escalante-Semerena 1998). This first important clue for the enzymatic activity of SIR2 proteins suggested that they might catalyze a related pyridine nucleotide transfer reaction. Indeed, both bacterial and mammalian SIR2 proteins were reported to transfer 32P from [32P]NAD to bovine serum albumin (Frye 1999). Subsequently, it was proposed that the ADP-ribosyltransferase activity of SIR2 was essential for gene silencing in budding yeast (Tanny et al. 1999). Furthermore, it was found that acetylated amino-terminal tails of histone H3 or H4 could specifically accept 32P from [32P]NAD in the reactions mediated by recombinant yeast and mammalian SIR2 proteins (Imai et al. 2000). Surprisingly, analysis of the reaction products by mass spectrometry revealed that SIR2 proteins across evolution could specifically deacetylate lysine 16 of H4 in an NAD-dependent manner, strongly indicating that this novel and unique deacetylase activity of SIR2 proteins plays a critical role in establishing silenced chromatin structures in vivo (Imai et al. 2000). The absolute requirement of NAD for the SIR2 deacetylase activity suggested that SIR2 and its closely related homologs function as sensors of the cellular energy status represented by NAD. Following this breakthrough, reports appeared showing that both SIR2 and a yeast SIR2 homolog, HST2, catalyze the NAD-nicotinamide exchange reaction and NAD-dependent deacetylation (Landry et al. 2000; Smith et al. 2000).
Further studies have demonstrated that sirtuins play an important role in the regulation of lifespan. For example, in yeast, an extra copy of the SIR2 gene increases replicative lifespan up to 30%, while its deletion or mutation shortens lifespan to ∼50% (Kaeberlein et al. 1999). In C. elegans, increasing the dosage of sir-2.1, the ortholog of yeast SIR2, extends lifespan by up to 50% (Tissenbaum and Guarente 2001). An increase in the dosage of Drosophila Sir2(dSir2) also extends lifespan, whereas a decrease in the dosage of dSir2 shortens lifespan and blocks the lifespan extension by caloric restriction (CR) (Rogina and Helfand 2004). CR extends lifespan in diverse organisms, from yeast (Lin et al. 2000), worms (Lakowski and Hekimi 1998), and flies (Chapman and Partridge 1996) to rodents (McCay et al. 1989; Weindruch and Walford 1982) and primates (Colman et al. 2009). In certain genetic backgrounds, sirtuins are also required for CR-induced lifespan extension (Anderson et al. 2003; Boily et al. 2008; Lin et al. 2000, 2002; Rogina and Helfand 2004; Wang and Tissenbaum 2006). Collectively, these findings implicate sirtuins as evolutionary conserved regulators of lifespan. In mammals, it has been established that sirtuins regulate metabolic and stress responses, two important components that affect the process of aging. In this chapter, we will focus on the metabolic functions of mammalian sirtuins as well as mechanisms regulating their activity. We will further discuss the potential connections among sirtuin biology, age-associated diseases, and the aging process, and finally introduce the manipulation of sirtuin function as a pharmacological approach to remedy and prevent age-associated pathophysiological changes.
2 The Catalytic Reaction of Sirtuins
The principal factor distinguishing sirtuins from other HDACs is that sirtuins require the cosubstrate NAD to perform their lysine deacetylase reaction (Fig. 1) (Imai et al. 2000; Landry et al. 2000; Smith et al. 2000). In addition to this dependency on NAD, sirtuin activity can be pharmacologically separated from that of other HDACs by their insensitivity to a potent HDAC inhibitor, Trichostatin A (Dali-Youcef et al. 2007). All sirtuin family members contain a highly conserved, 250- to 270-residue catalytic domain consisting of a large domain containing a reverse Rossmann fold and a smaller domain composed of a zinc ribbon and a flexible helical subdomain (Finnin et al. 2001; Min et al. 2001). The cleft between these two domains forms a tunnel lined with hydrophobic residues in which catalysis occurs. The Rossmann fold contains a Gly–X–Gly sequence important for binding of the phosphate of NAD as well as a small pocket and charged residues to bind the two ribose groups of NAD. The zinc ribbon is composed of a three-stranded antiparallel β sheet, an α helix, and a zinc atom bound and stabilized by two pairs of cysteine residues. To commence catalysis, sirtuins require both the acetylated lysine substrate and NAD to respectively bind the cleft and the Rossmann fold, forming a ternary complex (Borra et al. 2005). Substrate binding triggers a conformational change that buries the acetyl-lysine of the substrate in the hydrophobic tunnel (Avalos et al. 2002). This conformational change also forms an enzyme-substrate β sheet as well as generates a charge destabilization that promotes productive binding of NAD (Avalos et al. 2002, 2004). Subsequent to these events, sirtuins cleave the glycosidic bond separating nicotinamide and ribose moieties of NAD, forming nicotinamide and an enzyme–ADP–ribose intermediate (Landry et al. 2000). Sirtuins then transfer the acetyl group from the acetylated substrate to the ADP–ribose portion of NAD, generating 2′-O-acetyl-ADP-ribose (Borra et al. 2005; Zhao et al. 2004). Next, nicotinamide is released, followed by the 2′-O-acetyl-ADP-ribose and the deacetylated lysine.
Fig. 1.
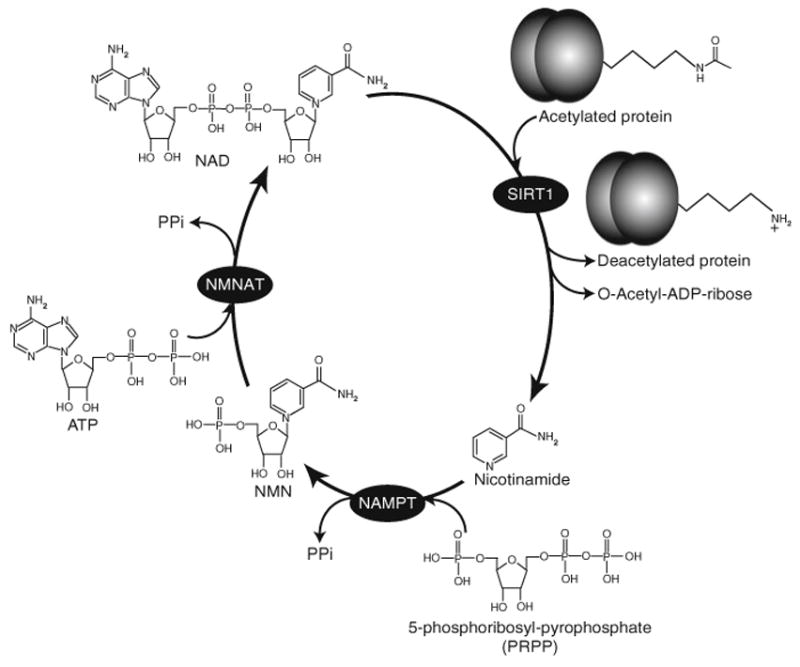
The deacetylation reaction of sirtuins. Upon the binding of both an acetylated lysine substrate and NAD, sirtuins cleave the glycosidic bond separating NAD into its nicotinamide and ribose moieties, producing nicotinamide, 2′-O-acetyl-ADP-ribose, and a deacetylated substrate
In contrast to the high degree of structural conservation across family members in this catalytic domain (Finnin et al. 2001; Min et al. 2001), the N- and C-terminal regions flanking it are highly divergent (Zhao et al. 2003). Interestingly, the X-ray crystal structure of a yeast sirtuin family member, HST2, provides evidence for the involvement of the N- and C-terminal regions in catalysis, potentially indicating that the sequence divergence in the regions among family members may play a role in their substrate binding differences and/or biological activities. The number of sirtuin family members tends to increase with organismal complexity (except for budding yeast having five): prokaryotes express one to two family members, fission yeast expresses three, worms express four, flies express five, and mammals express seven (Blander and Guarente 2004). The seven mammalian family members, SIRT1 through SIRT7, have slightly different enzymatic activities, localizations, and functions (Table 1). Among them, all except SIRT4 exhibit deacetylase activity (Imai and Guarente 2010). For SIRT4, only ADP-ribosyltransferase activity has been reported (Imai and Guarente 2010). SIRT1 and SIRT2 can be cytoplasmic or nuclear proteins, while SIRT3, SIRT4, and SIRT5 are localized to the mitochondria, and SIRT6 and SIRT7 reside in the nucleus or nucleolus, respectively (Finkel et al. 2009; Imai and Guarente 2010). Of these seven members, SIRT1 is the ortholog of yeast SIR2 (Finkel et al. 2009), and its function has been characterized most extensively. Therefore, the following sections will mainly focus on SIRT1 function.
Table 1.
The enzymatic activity and localization of mammalian sirtuins
| Enzymatic activity
|
Localization | ||
|---|---|---|---|
| Deacetylase | ADP-ribosyltransferase | ||
| Sirt1 | ✓ | Nucleus, cytoplasm | |
| Sirt2 | ✓ | Cytoplasm, nucleus | |
| Sirt3 | ✓ | Mitochondria | |
| Sirt4 | ✓ | Mitochondria | |
| Sirt5 | ✓ | Mitochondria | |
| Sirt6 | ✓ | ✓ | Nucleus |
| Sirt7 | ✓ | Nucleolus | |
3 Metabolic Regulations by Mammalian Sirtuins
Numerous studies now show an intricate connection exists between metabolism and aging (Conti et al. 2006; Hakimi et al. 2007; Harrison et al. 2009; Pawlikowska et al. 2009; Selman et al. 2009; Tatar et al. 2003). It has been well established that mammalian sirtuins regulate metabolic responses to nutritional input in multiple tissues/organs. In this section, we will outline the functions of SIRT1 in the liver, skeletal muscle, adipose tissue, pancreatic β-cells, and hypothalamus (Fig. 2).
Fig. 2.
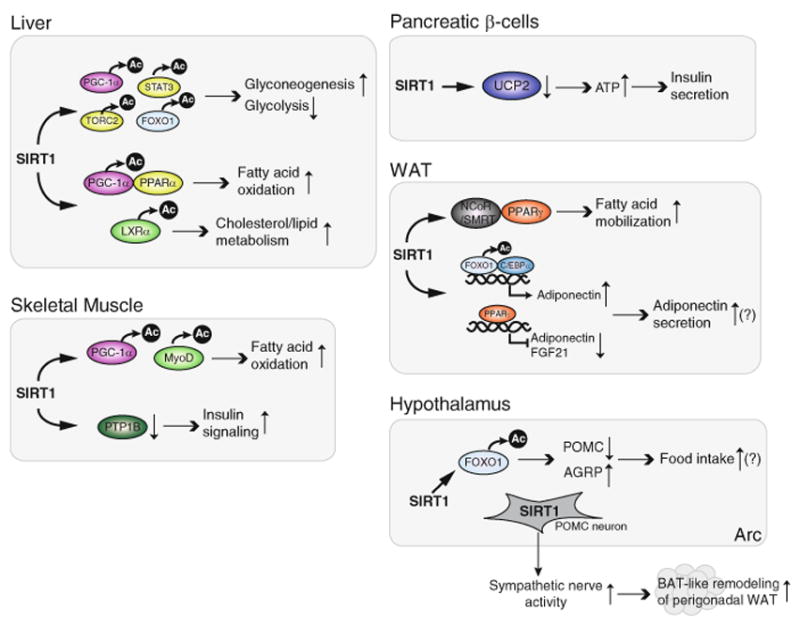
The metabolic regulation of SIRT1 in the liver, skeletal muscle, white adipose tissue (WAT), and hypothalamus. In the liver, SIRT1 regulates gluconeogenesis by deacetylating PGC-1α, TORC2, FOXO1, and STAT3. SIRT1 promotes fatty acid oxidation by deacetylating PGC-1α, promoting an interaction between SIRT1 and PPARα. SIRT1 also regulates cholesterol homeostasis by positively regulating the function of LXRα. In skeletal muscle, SIRT1 enhances mitochondrial fatty acid oxidation by deacetylating PGC-1α and MyoD. SIRT1 also improves insulin sensitivity by inhibiting the transcription of PTP1B. In WAT, SIRT1 enhances fatty acid mobilization by repressing the transcriptional activation of PPARγ by binding to NCoR/SMRT complex. SIRT1 regulates the production/secretion of adiponectin by deacetylating FOXO1, and possibly by inhibiting PPARγ. In pancreatic β-cells, SIRT1 enhances glucose-stimulated insulin secretion and improves glucose tolerance, at least in part, by repressing the expression of UCP-2. In the hypothalamus, SIRT1 in POMC neurons prevents the pathology of diet-induced obesity by reducing sympathetic nerve activity and BAT-like remodeling of perigonadal WAT. SIRT1 also regulates food intake and feeding behavior by decreasing and increasing the protein levels of AgRP and POMC, respectively
3.1 Liver
The liver plays an important role in the regulation of glycolysis, gluconeogenesis, and lipid metabolism, such as fatty acid oxidation. Under nutrient deprivation, gluconeogenesis increases while glycolysis decreases in the liver in order to maintain plasma glucose levels. Meanwhile, fatty acid oxidation is enhanced in response to pancreatic glucagon and adrenal cortisol, promoting gluconeogenesis (Bhathena 2000; McGarry and Foster 1980). The liver also plays a vital role in the excretion of ammonia through the urea cycle (Haussinger 1990).
SIRT1 is important for the positive regulation of gluconeogenesis and fatty acid oxidation under nutrient deprivation. In the case of gluconeogenesis, SIRT1 deacetylates and activates peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) (Rodgers et al. 2005), stimulating hepatic gluconeogenic gene expression while inhibiting glycolytic genes, as well as multiple targets including CREB-regulated transcription coactivator 2 (CRTC2, also known as TORC2), forkhead transcription factor O1 (FOXO1), and signal transducer and activator of transcription 3 (STAT3) (Frescas et al. 2005; Liu et al. 2008b; Nie et al. 2009). To up-regulate fatty acid oxidation, SIRT1 deacetylates PGC-1α, promoting an interaction between SIRT1 and peroxisome proliferator-activated receptor α (PPARα). This complex transcriptionally induces PPARα target genes, which in turn enhances fatty acid oxidation (Purushotham et al. 2009).
It has also been reported that SIRT1 positively regulates the function of liver X receptor α (LXRα), a nuclear receptor that functions as cholesterol sensor and regulates cholesterol and lipid homeostasis (Zelcer and Tontonoz 2006). SIRT1 interacts with and deacetylates LXRα, activating the transcription of the LXRα target gene ABCA1, a transporter that mediates HDL synthesis and HDL-mediated reverse cholesterol transport (RCT) (Li et al. 2007). Consistent with this finding, Sirt1-deficient mice display lower levels of HDL cholesterol in plasma and accumulate hepatic cholesterol (Li et al. 2007). However, total plasma cholesterol levels are reduced in both Sirt1-deficient mice (Li et al. 2007) and Sirt1-overexpressing transgenic mice (Bordone et al. 2007). Thus, further study is required to fully understand the function of SIRT1 in cholesterol homeostasis.
Similar to SIRT1, two other sirtuin family members, SIRT5 and SIRT6, also play important roles in metabolic regulation in the liver. SIRT5 regulates urea cycle by deacetylating carbamoyl phosphate synthase1 (CPS1), an enzyme that catalyzes the initial step of the urea cycle for ammonia detoxification and disposal, and up-regulating its activity (Nakagawa et al. 2009; Schapira 2011). On the other hand, SIRT6 down-regulates glycolysis by interacting with and deacetylating/inactivating hypoxia-inducible factor-1α (HIF1α), a transcription factor that modulates multiple genes to activate glycolysis (Zhong et al. 2010; Mahajan et al. 2011). The evidence that liver-specific Sirt6-deficient mice results in hepatic steatosis suggests that increasing SIRT6 activity in the liver would, at least in part, prevent the liver dysfunction caused by hepatic steatosis (Kim et al. 2010).
3.2 Skeletal Muscle
In skeletal muscle, mitochondrial fatty acid oxidation is vital for preserving glycogen stores and blood glucose levels upon nutrient deprivation and after exercise. SIRT1 enhances mitochondrial fatty acid oxidation by binding to and deacetylating PGC-1α, which increases its activity on its promoter through an interaction with myogenic determining factor (MyoD) (Amat et al. 2009; Canto et al. 2010). Given that dysregulation of skeletal muscle fatty acid oxidation is associated with insulin resistance and obesity (Kelley et al. 1999; Newgard et al. 2009), SIRT1 might also improve the insulin sensitivity of skeletal muscle. Indeed, in myotube cells, SIRT1 also inhibits the transcription of protein tyrosine phosphatase 1B (PTP1B). PTP1B dephosphorylates the insulin receptor and negatively regulates insulin signaling (Sun et al. 2007). In fact, Ptp1b-deficient mice display higher susceptibility to insulin when fed a high-fat diet (HFD) and resistance to diet-induced obesity (Elchebly et al. 1999). Moreover, a decrease in SIRT1 protein levels in the gastrocnemius muscle under HFD is accompanied by an increase in PTP1B levels (Sun et al. 2007). On the other hand, during fasting, SIRT1 protein levels increase while PTP1B protein levels decrease (Sun et al. 2007). Together, these findings suggest that SIRT1 plays an important role in the maintenance of skeletal muscle insulin sensitivity.
3.3 Adipose Tissue
A primary function of white adipose tissue (WAT) is to produce and store fatty acids as an energy reserve to allow their mobilization into the blood upon nutrient deprivation. WAT also plays an important role as an endocrine organ for adipokines, including leptin, adiponectin, and tumor necrosis factor-α (TNF-α) that modulate insulin resistance, hepatic lipoprotein production, and vascular inflammation (Mohamed-Ali et al. 1998).
SIRT1 can enhance fatty acid mobilization from WAT. Upon fasting, SIRT1 is recruited by PPARγ to DNA-binding sites in the aP2 promoter, promoting adipogenesis and fat storage (Lehrke and Lazar 2005). SIRT1 binds to PPARγ and represses the transcriptional activation of PPARγ by binding to nuclear receptor coreceptor/silencing mediator of retinoid and thyroid hormone receptor (NCoR/SMRT) complex, resulting in the reduction of fat accumulation in WAT and higher level of free fatty acids in blood (Picard et al. 2004).
On the other hand, whether or not SIRT1 regulates adipokine secretion from WAT is still debatable. Whole-body bacterial artificial chromosome (BAC)-driven Sirt1-overexpressing transgenic mice have increased levels of plasma adiponectin (Banks et al. 2008). Adiponectin is known as antidiabetic and antiatherogenic adipokine by exerting a potent insulin sensitizing effect (Kadowaki et al. 2006). In contrast, suppression of SIRT1 enhances the expression of endoplasmic reticulum oxidoreductase Ero1-Lα, a membrane-associated oxidoreductase that generates disulfide bonds (Frand and Kaiser 1998), and stimulates adiponectin secretion in adipocytes (Qiang et al. 2007). By binding to and deacetylating FOXO1, SIRT1 enhances the formation of FOXO1-CCAAT/enhancer-binding protein α (C/EBPα) transcription complex in the adipocytes, increasing the transcription of adiponectin (Qiao and Shao 2006). However, it has also been reported that SIRT1 suppresses the expression of adiponectin and fibroblast growth factor 21 (FGF21, an activator of glucose uptake in adipocytes), possibly by inhibiting of PPARγ in adipocytes (Qiang et al. 2007; Wang et al. 2008). Since it has been reported that SIRT1 upregulates the expression of adiponectin in adipocytes (Qiang et al. 2007; Qiao and Shao 2006; Wang et al. 2008), and the direction of the adiponectin secretion is not conclusive between in vivo and in vitro (Banks et al. 2008; Qiang et al. 2007), further work is required to understand adiponectin secretion.
While SIRT1 is predominantly a nuclear protein, SIRT2 is predominantly a cytoplasmic one (Michishita et al. 2005; North et al. 2003). In fact, SIRT2 is the most abundant sirtuin in adipocytes, where it plays an important role for adipocyte differentiation by deacetylating FOXO1 (Jing et al. 2007). Thus, it is possible that SIRT2 targets FOXO1 in the cytoplasm, while SIRT1 catalyzes FOXO1 deacetylation in the nucleus. It is also possible that SIRT1 and SIRT2 target different molecules, giving them different physiological or pathological functions in WAT. Further in vivo studies using genetically modified SIRT2 mice are required to address this possibility.
Brown adipose tissue (BAT) is a crucial regulator of energy expenditure through mitochondrial uncoupling protein-1 (UCP-1), thus protecting against obesity and diet-induced insulin resistance in mammals (Cederberg et al. 2001; Kopecky et al. 1995; Nedergaard et al. 2007; Seale et al. 2008). Although it has been reported that SIRT3 is highly expressed in BAT and plays an important role in adaptive thermo-genesis by activating mitochondrial function (Scher et al. 2007; Schapira 2011), the metabolic regulatory function of SIRT1 in BAT is still unknown. It is reported that SIRT1 might regulate brown adipocyte differentiation based on microarray gene expression study (Timmons et al. 2007). Since adult humans have morphologically distinguishable BAT and increasing brown adipocyte differentiation may offer a new therapeutic treatment for obesity and type 2 diabetes (Cypess et al. 2009; Kajimura et al. 2010; Lidell and Enerback 2010; Virtanen et al. 2009), it will be of great interest to further investigate the role of SIRT1 in the brown adipocyte differentiation.
3.4 Pancreatic β-Cells
Pancreatic β-cells play a major role in the regulation of glucose homeostasis by secreting insulin in response to elevated blood glucose. In pancreatic β-cells, SIRT1 enhances glucose-stimulated insulin secretion and improves glucose tolerance, at least in part, by repressing the expression of uncoupling protein 2 (UCP-2), an inner mitochondrial membrane protein (Bordone et al. 2006; Moynihan et al. 2005). UCP-2 functions as a proton transporter, whose activity has the effect of uncoupling the electron transport chain and ATP biosynthesis. Suppression of UCP-2 by SIRT1 increases ATP production, inducing glucose-stimulated insulin secretion. Indeed, islets isolated from beta cell-specific Sirt1-overexpressing (BESTO) transgenic mice exhibit increased ATP production in response to glucose. Interestingly, both pancreata and islets of BESTO mice show the enhancement of insulin secretion not only by glucose, but also by KCl-induced depolarization, suggesting that SIRT1 might also regulate insulin secretion downstream of β-cell depolarization, independently of UCP-2 (Moynihan et al. 2005).
β-cell dysfunction can be caused by a variety of cellular injuries, including glucolipotoxicity (Fontes et al. 2010). SIRT1 is able to prevent β-cell dysfunction against cellular injury. BESTO mice maintain glucose tolerance under a long-term HFD by increasing glucose-stimulated insulin secretion (Ramsey et al. 2008). The finding that BESTO mice exposed to HFD-induced stress display better β-cell functionality than wildtype littermates suggests that increasing SIRT1 activity in pancreatic β-cells might be able to sustain β-cell function under hyperlipidemic, diabetogenic conditions. Indeed, by deacetylating FOXO1, SIRT1 induces the expression of NeuroD and MafA, which are both transcriptional regulators of insulin 2 (Ins2) gene expression and protect β-cells under conditions that could cause apoptosis and promote β-cell senescence (Kitamura et al. 2005). Furthermore, SIRT1 overexpression completely prevents IL-1β and/or IFNγ-mediated cellular injury by inhibiting NF-κB signaling and subsequently repressing the expression of inducible nitric oxide synthase (iNOS) (Lee et al. 2009). Together, these findings strongly suggest that SIRT1 activity can prevent pancreatic β-cell dysfunction and thus type 2 diabetes.
Contrary to the function of SIRT1 in pancreatic β-cells, SIRT4 suppresses insulin secretion in response to amino acids and glucose (Schapira 2011). SIRT4 ADP-ribosylates and represses the activity of glutamate dehydrogenase (GDH), an enzyme that converts glutamate to α-ketoglutamate in mitochondria, thereby decreasing amino acid-stimulated insulin secretion (Haigis et al. 2006). Furthermore, depletion of SIRT4 increases glucose-stimulated insulin secretion through with the effects on ATP/ADP translocase (ANT), an ADP/ATP carrier protein, and insulin-degrading enzyme (IDE) (Ahuja et al. 2007). It is of great interest to investigate how SIRT1 and SIRT4 work together to regulate insulin secretion under various metabolic conditions such as HFD.
3.5 Hypothalamus
The hypothalamus regulates feeding behaviors in response to metabolic signals from peripheral tissues by way of hormones such as insulin, leptin, and ghrelin (Elmquist 2001). Neurons in diverse nuclei of the hypothalamus including arcuate and paraventricular nuclei (Arc and PVN, respectively) and the ventromedial, dorsomedial, and lateral hypothalamic nuclei (VMH, DMH, and LH, respectively) produce and secrete multiple neuropeptides and neurohormones that play important roles in the regulation of metabolism, body temperature, food intake, arousal, circadian rhythm, and the secretion of pituitary hormones (Cone 2005; Elmquist 2001; Green et al. 2008; Morrison et al. 2008; Morton et al. 2006; Sakurai 2007).
Hypothalamic SIRT1 appears to regulate food intake and feeding behavior, but the direction of regulation currently remains controversial. Intracerebroventricular administration of EX527, a selective inhibitor of SIRT1, or siRNA-mediated knockdown of Sirt1 in the Arc inhibits feeding behavior in rats through decrease in augouti-related protein (AgRP) and increase in pro-opiomelanocortin (POMC) protein (Cakir et al. 2009). In contrast, it has been reported that intracerebroventricular administration of adenovirus expressing Sirt1 in the mediobasal hypothalamus suppresses food intake (Sasaki et al. 2010). Since each hypothalamic nucleus has a distinct role in systemic metabolic regulation, neuron-specific or nuclei-specific modification of Sirt1 by genetic or stereotactic manipulation is necessary to elucidate the function of SIRT1 in the hypothalamus.
Numerous experimental and clinical findings suggest that hypothalamic dysfunction might be one of the underlying causes of abnormal glucose and lipid metabolism that occurs in type 2 diabetes and diet-induced obesity (Rosmond and Bjorntorp 2000; Schwartz and Porte 2005). Hypothalamic SIRT1 is reported to prevent the pathology of diet-induced obesity. Deletion of SIRT1 in POMC neurons results in weight gain and reduced energy expenditure by reducing sympathetic nerve activity and BAT-like remodeling of perigonadal WAT under HFD (Ramadori et al. 2010). BAT-like remodeling of perigonadal WAT increases mitochondrial content and UCP-1 expression under HFD, resulting in increased energy expenditure against obesity and insulin resistance (Plum et al. 2007). These results suggest that SIRT1 in POMC neurons is required for normal autonomic adaptation to diet-induced obesity and possibly insulin resistance.
4 The Regulation of Sirtuin Function
Given the importance and widespread effects of SIRT1 activity, it is important to understand factors that regulate its activity and expression. These regulatory factors can be broken down into four categories: NAD, protein regulators of SIRT1 activity, nucleocytoplasmic shuttling of SIRT1, and regulators of SIRT1 expression (Fig. 3).
Fig. 3.
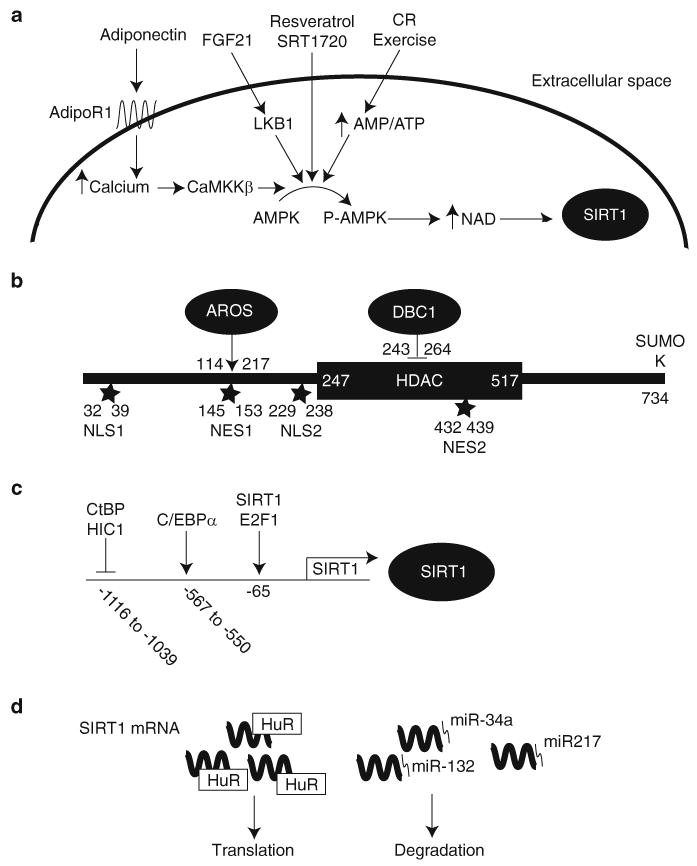
Regulation of SIRT1. SIRT1 activity is regulated by (A) NAD levels, (B) protein–protein interactions and posttranslational modifications, (C) factors that modulate its transcription, and (D) factors that modulate its translation. (A) SIRT1 activity is primarily regulated by NAD levels. NAD levels are regulated by AMPK-mediated up-regulation of NAMPT expression. AMPK can be activated by FGF21 and adiponectin. FGF21 induces LKB1, one of two major AMPK activators, to phosphorylate AMPK. Adiponectin induces calcium influx through its receptor adiponectin receptor 1 (adipoR1) and this calcium activates the other major AMPK kinase, calcium/calmodulin-dependent protein kinase kinase β (CaMKK β). (B) SIRT1 is activated by direct binding of AROS and sumoylation at Lysine734. Conversely, SIRT1 is inhibited by direct binding of DBC1. (C) p53 and HIC1 repress transcription of SIRT1 while C/EBPα and E2F1 enhance it. (D) Binding of HuR to SIRT1 mRNA increases its half-life. In contrast, binding of miR-34a, miR-132, or miR-217 results in translational repression of SIRT1 mRNA
4.1 NAD
Perhaps the most important regulator of sirtuin activity is cellular NAD (Imai 2009). NAD is specifically required for the sirtuin deacetylase reaction and cannot be substituted by NADH, NADP, and NADPH (Imai et al. 2000). However, NAD is required for many vital cellular processes besides the sirtuin reaction. For example, it acts as a cofactor in redox reactions of glycolysis, the trichloroacetic acid cycle, and the catabolism of carbohydrates, fats, proteins, and alcohols and also participates in DNA repair, G-protein coupled signaling, intracellular calcium signaling, and transcription (Bogan and Brenner 2008; Garten et al. 2009). As a result, the pool of intracellular NAD is limiting for sirtuin activity despite its seemingly adequate concentration of 300–400 μM (Bogan and Brenner 2008; Canto et al. 2010; Houtkooper et al. 2010; Koltai et al. 2010; Penberthy and Tsunoda 2009; Rodgers et al. 2005; Sauve 2008; Yang et al. 2007a). In addition to affecting SIRT1 activity, high levels of NAD can augment SIRT1 expression over twofold in multiple tissues (Hayashida et al. 2010; Hwang et al. 2009; Qin et al. 2006; Rodgers et al. 2005), whereas depletion of NAD had the reverse effect (Borradaile and Pickering 2009; de Kreutzenberg et al. 2010; Liu et al. 2008a). Since high concentrations of NADH inhibit sirtuin activity, there is some debate as to whether absolute NAD levels or the NAD/NADH ratio is more important to sirtuin function (Bogan and Brenner 2008; Lin et al. 2004). With the IC50 for NADH at 11–28 mM and the amount of NADH in the cytosol less than 1% of total free NAD and NADH, it is unlikely for NADH levels to become high enough to inhibit SIRT1 activity (Schmidt et al. 2004). Thus, NAD levels are expected to be a more critical indicator of SIRT1 activity.
Four different substrates can be used to generate NAD: tryptophan (Trp), nicotinamide (NAM), nicotinic acid (NA), and nicotinamide riboside (NR) (Fig. 4) (Bogan and Brenner 2008). Of these substrates, the pathway starting from nicotinamide is the predominant source of NAD in mammals (Houtkooper et al. 2010). In this pathway, homodimeric nicotinamide phosphoribosyltransferase (NAMPT) converts NAM to nicotinamide mononucleotide (NMN), and nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) converts NMN to NAD (Houtkooper et al. 2010; Revollo et al. 2004, 2007). Because NAMPT functions as the rate-limiting enzyme in this NAD biosynthetic pathway, NAMPT is perhaps the most important component for the regulation of mammalian NAD biosynthesis. Expression levels of NAMPT are varied throughout the body: high in the liver, kidney, and BAT; intermediate in the heart; and low in the skeletal muscle, brain, pancreas, WAT, lung, spleen, and testis in mice (Revollo et al. 2007). Overexpression of NAMPT increases NAD levels, while overexpression of NMNAT has no effect (Araki et al. 2004; Revollo et al. 2004). The fact that intracellular NAD levels increase with NAMPT overexpression in multiple, diverse cell types (Borradaile and Pickering 2009; Fulco et al. 2008; Hsu et al. 2009; Pillai et al. 2005; Revollo et al. 2004; Rongvaux et al. 2008; Song et al. 2008; van der Veer et al. 2005) suggests that NAMPT expression is sufficient to induce NAD biosynthesis. As pharmacological inhibition of NAMPT activity by the highly specific inhibitor FK866 reduces NAD levels 50–95% (Billington et al. 2008; Bruzzone et al. 2009; Hasmann and Schemainda 2003; Revollo et al. 2007; Rongvaux et al. 2008), indicating that NAMPT is necessary for NAD biosynthesis. NAMPT is required for survival, with lack of NAMPT causing embryonic lethality prior to day 10.5 (Revollo et al. 2007). In fact, NAMPT function is so vital that reducing NAMPT activity hampers cellular viability up to 90% (Billington et al. 2008; Dahl et al. 2010; Jia et al. 2004; Rongvaux et al. 2008; van der Veer et al. 2005). Thus, the factors that regulate NAMPT activity and expression should also be potent regulators of mammalian sirtuins.
Fig. 4.
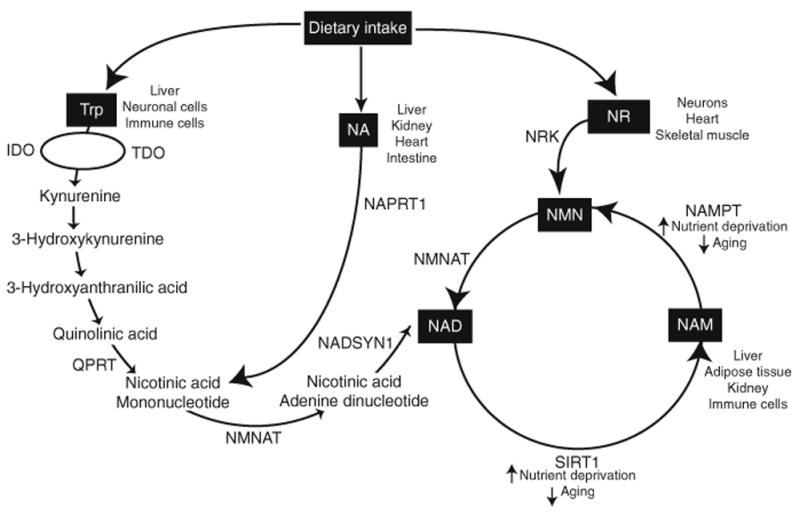
Pathways of mammalian NAD biosynthesis. Four dietary metabolites can be used to generate NAD: tryptophan (Trp), nicotinamide (NAM), nicotinic acid (NA), and nicotinamide riboside (NR). De novo NAD biosynthesis occurs from Trp via the eight-step Kynurenine pathway. The first and rate-limiting step in this pathway is shared by tryptophan dioxygenase (TDO) and indoleamine-2,3-dioxygenase (IDO), with TDO acting in the liver and the brain and IDO acting in the immune system. NA generates NAD through the Preiss–Handler pathway (PHP). In this pathway, nicotinic acid phosphoribosyltransferase (NAPRT1) forms nicotinic acid mononucleotide (NaMN), which is converted to NAD by the sequential actions of glutamine-dependent NAD synthetase (NADSYN1) and Nmnat. In the salvage pathway, homodimeric nicotinamide phosphoribosyltransferase (NAMPT) converts NAM to nicotinamide mononucleotide (NMN) and nicotinamide nucleotide adenylyltransferase (Nmnat) converts NMN to NAD. NR generates NAD by way of nicotinamide riboside kinase (Nrk) or purine nucleoside phosphorylase (Pnp) and nicotinamide salvage
One of these factors is AMP-activated protein kinase (AMPK). As knockdown of SIRT1 in myocytes impairs the ability of AMPK to regulate the expression of genes related to mitochondrial metabolism and fatty acid oxidation and to increase lipid oxidation-driven O2 consumption (Canto et al. 2009), SIRT1 is clearly a primary downstream component of AMPK signaling. AMPK activates SIRT1 to deacetylate FOXO1, FOXO3a, and PGC-1α by increasing cellular NAD levels with no effect on SIRT1 protein levels, its protein–protein interactions, or its phosphorylation status (Canto et al. 2009; Chau et al. 2010). Thus, conditions that activate AMPK, such as nutrient deprivation and exercise, are associated with higher NAD levels and SIRT1 activity (Canto et al. 2010; Fulco et al. 2008). While the mechanism by which AMPK increases NAD levels is not fully elucidated, it appears to be, at least in part, by up-regulating expression of NAMPT (Canto et al. 2010; Costford et al. 2010; Fulco et al. 2008). Interestingly, an increase in mitochondrial β-oxidation appears to be required for AMPK to increase NAD levels and PGC-1α deacetylation. FGF21 and adiponectin have been reported to modulate SIRT1 activity by activating AMPK. FGF21 regulates energy homeostasis in adipocytes by inducing LKB1 that phosphorylates AMPK (Chau et al. 2010). Indeed, an increase in levels of phosphorylated AMPK by FGF21 triggers SIRT1 to deacetylate PGC-1α in ob/ob mice, resulting in enhanced mitochondrial function and reduced body weight (Chau et al. 2010). In myocytes, adiponectin has a similar effect. Adiponectin induces calcium influx through its receptor adiponectin receptor 1 (adipoR1), and this calcium activates the other major AMPK kinase, calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ) (Iwabu et al. 2010). CaMKKβ-mediated activation of AMPK increased SIRT1-mediated deacetylation of PGC-1α and increased mitochondrial biogenesis. Accordingly, skeletal muscle-specific AdipoR1 knockout mice exhibited decreased SIRT1 activity, PGC-1α expression, PGC-1α deacetylation, and mitochondrial biogenesis. Supporting the notions that FGF21 and adiponectin act upstream of AMPK, both factors also increase NAD levels (Chau et al. 2010; Iwabu et al. 2010).
4.2 Protein Regulators of SIRT1 Activity
Two proteins have been identified that regulate SIRT1 activity via a direct interaction: active regulator of SIRT1 (AROS) and deleted in breast cancer 1 (DBC1). Binding of the nuclear protein AROS to SIRT1 enhanced SIRT1-mediated p53 deacetylation, thus inhibiting the transcriptional activity of p53 and preventing p53-induced cell cycle arrest and apoptosis in response to DNA damage (Kim et al. 2007b). Opposite to AROS, binding of DBC1 to the catalytic domain of SIRT1 inhibits its activity, preventing SIRT1 from deacetylating p53 and FOXO3 following cellular stress, with the result of up-regulating p53 and FOXO-mediated apoptosis (Kim et al. 2008, 2009; Zhao et al. 2008). The activity of SIRT1 has been shown to be regulated by posttranslational modifications: phosphorylation and sumoylation (Yang et al. 2007b). At least 14 residues in SIRT1 are phosphorylated in vivo, with phosphorylation increasing the activity of SIRT1 (Guo et al. 2010; Nasrin et al. 2009; Sasaki et al. 2008). The kinases responsible for this phosphorylation are known to include CyclinB/Cdk1, cJun N-terminal kinase (JNK1), and two dual specificity tyrosine phosphorylation-regulated kinases, DYRK1A and DYRK3. It will be interesting to determine what other SIRT1 kinases exist. Sumoylation of human SIRT1 has also been reported to enhance its ability to deacetylate p53 sufficiently to prevent stress-induced apoptosis, although the sumoylation site is not evolutionarily conserved. Thirdly, two proteins have been identified which enhance the activity of SIRT1 by binding SIRT1 substrates. Four-and-a-half LIM2 (FHL2) enhances SIRT1-mediated deacetylation of FOXO1 in prostate cancer cells by binding FOXO1 in a manner that promotes the interaction between SIRT1 and FOXO1 (Yang et al. 2005). Since deacetylation inactivates FOXO1, FHL2 activity decreases the levels of FOXO target genes and FOXO1-induced apoptosis. Similarly, in postmitotic neurons, necdin, a melanoma antigen family protein, interacts with SIRT1 and p53, potentiating SIRT1-mediated deacetylation of p53, and thus, protecting neurons from DNA damage-induced apoptosis (Hasegawa and Yoshikawa 2008).
4.3 Nucleocytoplasmic Shuttling of SIRT1
Finally, nucleocytoplasmic shuttling may modulate SIRT1 activity. Cytoplasmic localization of SIRT1 is higher in adulthood than during embryonic development in the heart (Tanno et al. 2007) and during postnatal development in brain (Li et al. 2008). The cellular localization of SIRT1 is also affected by differentiation and apoptosis. In myoblasts, SIRT1 is a nuclear protein until differentiation, at which point SIRT1 translocates to the cytoplasm (Tanno et al. 2007). In neural precursor cells, SIRT1 translocates from the cytoplasm into the nucleus for differentiation and then returns to the cytoplasm (Hisahara et al. 2008). SIRT1 also translocates from the nucleus to the cytoplasm during apoptosis (Jin et al. 2007; Ohsawa and Miura 2006). Despite these events, the significance of cytoplasmic SIRT1 remains unclear. As only nuclearly localized SIRT1 can engender direct transcriptional events (Tanno et al. 2007), cytoplasmic shuttling may be a way to down-regulate SIRT1 activity. However, SIRT1 deacetylase activity could still be highly relevant in the cytoplasm, as many substrates of SIRT1, such as p53, FOXOs, and NF-κB, also shuttle between compartments (Kwon and Ott 2008). Some evidence even suggests that cytoplasmically localized SIRT1 may promote apoptosis (Cohen et al. 2004; Jin et al. 2007; Tanno et al. 2007; Zhang 2007).
4.4 Regulators of SIRT1 Expression
Four transcriptional regulators have been reported to modulate of SIRT1 expression: p53, hypermethylated in cancer 1 (HIC1), C/EBPα, and E2F1. The first three may play a role in up-regulating SIRT1 expression under CR. Under basal nutritional conditions, p53 binds the SIRT1 promoter with the effect of repressing transcription of SIRT1 (Nemoto et al. 2004). However, upon starvation, the association of p53 with the SIRT1 promoter is prevented by the binding of FOXO3α to p53, thus up-regulating SIRT1 expression. HIC1 forms a transcriptional repression complex with SIRT1, the transcriptional corepressors CtBP, and class I HDACs that binds to the 5′ end of the SIRT1 promoter CpG island (Chen et al. 2005b). The association of CtBP with HIC1 is reduced upon glycolytic blockade, thus increasing SIRT1 expression in response to nutrient deprivation (Jin et al. 2010; Zhang 2007). SIRT1 expression is also enhanced upon nutrient deprivation by increased binding of C/EBPα to the SIRT1 promoter (Jin et al. 2010). While not linked to metabolic state, the cell-cycle regulator E2F1 may connect SIRT1 expression with replicative aging, as it activates SIRT1 transcription when cells begin to enter S phase (Wang et al. 2006).
Translationally, at least one protein and three microRNAs have been shown to regulate SIRT1 protein levels by binding the 3′ untranslated region of SIRT1’s mRNA: Hu antigen R (HuR), miR-34a, miR-132, and miR-217. HuR is an mRNA-binding protein whose binding increases the half-life of SIRT1 mRNA from 1.2 to 8 hours (Abdelmohsen et al. 2007). miR-34a decreases SIRT1 protein levels in multiple, diverse cell types, with the effect of increasing acetylation levels of p53 and FOXO (Lee et al. 2010; Tarantino et al. 2010; Yamakuchi et al. 2008; Zhao et al. 2010). miR-132 decreased SIRT1-mediated deacetylation of p65 in adipocytes, leading to activation of NF-κB (Strum et al. 2009). In endothelial cells, inhibition of SIRT1 by miR-217 induced premature senescence due to high levels of FOXO1 and nitric oxide synthase acetylation (Menghini et al. 2009). It will be interesting to see future work delineating the relevance of these molecular mechanisms to SIRT1 activity in vivo.
5 Sirtuin in Age-Associated Disorders
To achieve healthy aging, it is important to decrease the incidence and delay the onset of age-associated disorders. Accumulating bodies of evidence indicate that SIRT1 prevents age-associated disorders including type-2 diabetes (T2D) and Alzheimer’s disease (AD) (Imai and Guarente 2010; Wang et al. 2010). Therefore, SIRT1 is an important therapeutic target to prevent age-associated diseases and extend healthspan (Fig. 5).
Fig. 5.
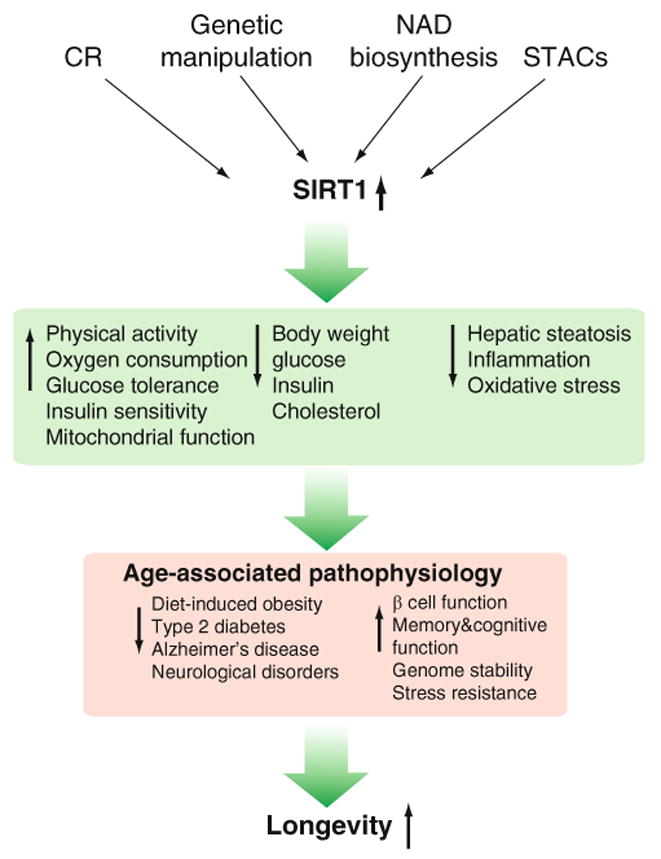
Manipulation of SIRT1 function. Caloric restriction (CR), genetic manipulation, NAD biosynthesis, and sirtuin activating compounds (STACs) increase the dosage/activity of SIRT1, providing beneficial metabolic responses against numerous age-associated physiology and thereby promoting longevity
5.1 SIRT1 and T2D
T2D has a complex pathology consisting of defects in insulin secretion and action that together result in hyperglycemia (Bell and Polonsky 2001; Cavaghan et al. 2000). SIRT1 plays an important role in promoting insulin secretion by pancreatic β-cells and protecting against insulin resistance in the liver, skeletal muscle, and adipose tissue. These findings support the notion that SIRT1 is important for the maintenance of glucose homeostasis and the prevention of T2D. Indeed, under diabetogenic HFD conditions (Kraegen et al. 1991; Kubota et al. 1999; Surwit et al. 1988), the activity and/or protein expression of SIRT1 are reduced in several tissues (Deng et al. 2007; Escande et al. 2010; Qiao and Shao 2006). Moreover, increasing SIRT1 by genetic manipulation prevents metabolic disorders induced by HFD feeding. It has been reported that whole-body Sirt1-overexpressing transgenic mice improves glucose tolerance by decreasing hepatic glucose production without changes in body weight or fat composition in mice on a HFD (Banks et al. 2008). Similarly, another line of whole-body Sirt1-overexpressing transgenic mice show improvement of glucose tolerance, reduced lipid-induced inflammation, and protection against HFD-induced hepatic steatosis (Pfluger et al. 2008). In the kidney, which is highly susceptible to diabetic nephropathy, SIRT1 inhibits oxidative stress by inducing cyclooxygenase-2 (COX-2) expression (He et al. 2010b). It has also been reported that kidney-specific overexpression of SIRT1 protects cisplatin-induced acute kidney injury, likely by maintaining peroxisome number, upregulating catalase, and reducing renal reactive oxygen species (Hasegawa et al. 2010). Furthermore, SIRT1 genetic variation influences survival in the subjects with T2D (Zillikens et al. 2009a, b). These findings strongly suggest that SIRT1 could be a potential therapeutic target to improve insulin resistance and to protect against cell damage due to T2D.
Supporting the idea that SIRT1 could be a potential therapeutic target to combat metabolic disorders, SIRT1 also plays an important role in circadian rhythm. SIRT1 regulates CLOCK/BMAL1, the key transcription factor complex that controls the expression of clock genes (Nakahata et al. 2009). It has also been reported that SIRT1 directly regulates PER2 (Asher et al. 2008). The facts that Clock mutant mice and Bmal1 mutant mice display metabolic disorders (Oishi et al. 2006; Turek et al. 2005) and that diet-induced obesity disrupts circadian behavior and circadian clock genes (Kohsaka et al. 2007) suggest that the maintenance of circadian rhythm is vital to prevent metabolic disorders. Interestingly, it has also been demonstrated that CLOCK:BMAL1 complex produces the circadian oscillation of NAMPT and NAD levels in vivo, comprising a novel circadian clock feedback loop involving NAMPT/NAD and SIRT1/CLOCK:BMAL1 (Nakahata et al. 2009; Ramsey et al. 2009). These findings strongly suggest that up-regulation of SIRT1 activity by NAMPT-mediated NAD biosynthesis could maintain proper circadian rhythm and prevent metabolic disorders.
5.2 SIRT1, AD, and Other Age-Associated Neurological Disorders
AD is chronic neurodegenerative disorder leading to synaptic dysfunction and neuronal cell loss (Haass and Selkoe 2007). Increasing lines of evidence suggest that SIRT1 provides protection from AD. Injection of lentivirus carrying Sirt1in the CA1 region of the hippocampus protects against neurodegeneration in the p25 transgenic mouse model of AD (Cruz et al. 2006; Kim et al. 2007a). In an AD mouse model encoding the human APPswe and PSEN1 dE9 alleles, which exhibits strong β-amyloid (Aβ) plaque formation, SIRT1 suppresses the production of Aβ and plaques by deacetylating retinoic acid receptor β (RARβ) and thereby activating the transcription of ADAM10 encoding α-secretase (Donmez et al. 2010). α-secretases cleave amyloid precursor protein (APP), a type I transmembrane glycoprotein and the precursor of Aβ, preventing Aβ formation (He et al. 2010a). As a result, the overexpression of ADAM10 prevents amyloid plaque formation and hippocampal behavioral defects in AD mice (Postina et al. 2004). These findings potentially indicate that increasing SIRT1 dosage can prevent AD. Interestingly, NAD treatment attenuates Aβ generation, by activating SIRT1 in primary hippocampal neuronal cultures (Qin et al. 2006). Epidemiological studies have shown a strong association between diabetes and AD (Biessels and Kappelle 2005; Janson et al. 2004), and risk of AD is increased with development of T2D (Leibson et al. 1997; Ott et al. 1999; Takeda et al. 2010). Although it is still uncertain whether or not SIRT1 has a unifying pathophysiological role in T2D and AD, it is of great interest to investigate whether NAMPT-mediated systemic NAD biosynthesis plays an important role in AD pathophysiology as well as T2D.
SIRT1 has also been shown to be required for basal neural functions such as synaptic plasticity, cognition, and memory. In the hippocampus, SIRT1 modulates synaptic plasticity and memory formation by miR-134, a brain-specific microRNA, through the up-regulation of the expression of cAMP response element binding protein (CREB) and brain-delivered neurotrophic factor (BDNF) (Gao et al. 2010), which have critical roles in synaptic plasticity and synapse formation (Frank and Greenberg 1994; Kang and Schuman 1995). Moreover, Sirt1-deficient mice have impaired cognitive abilities such as deficits in immediate memory, classical conditioning, and spatial learning due to defects in synaptic plasticity (Michan et al. 2010). These findings highlight the benefits of increasing SIRT1 activity in preserving brain function and preventing age-associated neurological disorders.
6 Sirtuins in the Aging Process
Although it is still unclear whether sirtuins also play an important role in the regulation of mammalian lifespan, as it does in lower eukaryotes, recent results suggest that SIRT1 is important in the regulation of age-induced physiological changes (Fig. 5). For example, SIRT1 is involved in DNA damage-induced chromatin reorganization that promotes genome stability and causes the reduction in silencing of SIRT1 target genes in mouse embryonic stem (ES) cells (Oberdoerffer et al. 2008; Oberdoerffer and Sinclair 2007). This damage-induced SIRT1 redistribution requires DNA damage signaling through a mammalian PI3-kinase ATM and histone H2AX, one of the targets of ATM. Importantly, these SIRT1-bound target genes that are derepressed by oxidative stress in mouse ES cells are also derepressed in aged mouse brain, and SIRT1 overexpression can suppress these age-associated changes (Oberdoerffer et al. 2008), suggesting that the damage-induced SIRT1 redistribution might trigger certain age-associated physiological changes in the brain.
The activity and expression of SIRT1 declines with age in several tissues. For example, in the kidney, mRNA and protein levels of SIRT1 significantly decline with age. Aged mice show the reduction of the interaction between SIRT1 and FOXO3, its transcriptional activity is regulated by SIRT1. Thus, SIRT1 activity is decreased in the aged kidney (Kume et al. 2010). In pancreatic β cells, SIRT1 activity appears to be reduced in aged mice due to a decline in systemic NAD biosynthesis. Aged BESTO mice lose the phenotype (see Sect. 3.4) that was shown in young BESTO mice, displays reduced level of Ucp2 consistent with the lack of a difference in ATP content (Ramsey et al. 2008). Furthermore, the protein levels of SIRT1 are declined in several murine disease or accelerated aging models. (Pallas et al. 2008; Sommer et al. 2006), as well as during replicative senescence in normal human fibroblasts (Michishita et al. 2005). These findings strongly suggest that the maintenance of expression and/or activity of SIRT1 during senescence prevents against age-associated disorders.
Investigation into the intimate connection among sirtuins, metabolism, and aging has implicated that SIRT1 as a key mediator for physiological responses to CR in mammals. CR has been employed in aging research as an antiaging dietary intervention that shows protective effects on age-associated pathology of metabolic disorders and lifespan extension (Bronson and Lipman 1991; Colman et al. 2009; Fontana et al. 2004; Weindruch et al. 1986). Interestingly, CR-induced elevation of physical activity is abrogated in Sirt1-deficient mice (Chen et al. 2005a). Sirt1-deficient mice also exhibit dramatically reduced oxygen consumption under CR (Boily et al. 2008) and are resistant to CR-mediated improvement in the accumulation of damaged mitochondria under hypoxia in the kidney (Kume et al. 2010). Additionally, Sirt1-overexpressing transgenic mice display phenotypes similar to CR mice, including reduced body weight, improved glucose tolerance, reduced blood cholesterol, insulin, and fasted glucose levels, increased oxygen consumption, better performance on a rotarod challenge, and a delay in reproduction (Bordone et al. 2007). Additionally, two other systemic Sirt1-overexpressing transgenic mice have delayed the onset of age-associated disorder including obesity and T2D (Banks et al. 2008; Pfluger et al. 2008). Furthermore, SIRT1 functions as a key regulator of cell defenses and survival in response to stress, promoting CR-induced cell survival (Cohen et al. 2004). These findings suggest that manipulation of SIRT1 activity might mimic beneficial effects of CR on reducing age-associated disorders and possibly increasing longevity in mammals.
However, because SIRT1 has a divergent role in the regulation of metabolism in multiple tissues in mammals, it is conceivable that simply increasing SIRT1 activity systemically might not retard aging and show lifespan extension as well as CR. Indeed, it has recently been reported that whole-body SIRT1-overexpressing transgenic mice do not show lifespan extension (Herranz et al. 2010). Additionally, while most tissues show increases in SIRT1 protein levels under CR (Cohen et al. 2004; Qin et al. 2006), some other tissues show decreases or no change (Chen et al. 2008a; Cohen et al. 2009). In the brain, the effects of CR on SIRT1 expression are highly regional (Chen et al. 2008b). Therefore, increasing SIRT1 in a tissue/organ-specific manner by genetic manipulation might be important to retard aging and extend lifespan.
In this regard, the function of SIRT1 in the brain is interesting. In the hypothalamus, SIRT1 protein levels increase in the DMH, LH, and SCN (Satoh et al. 2010) under CR. Increasing SIRT1 in the brain of transgenic mice (brain-specific Sirt1-overexpressing transgenic mice: BRASTO mice) enhances neural activity in the DMH and LH, maintains body temperature, and promotes physical activity by increasing the expression of orexin type-2 receptor, a G protein-coupled receptor that binds to the neuropeptide hormone orexin. Moreover, BRASTO mice are more responsive to ghrelin, a gut hormone whose plasma level is increased under CR, than wildtype littermate, suggesting the function of SIRT1 in the hypothalamus, particularly in the DMH and LH, as a key mediator of the central adaptive response to CR (Satoh et al. 2010). These results support the idea that increasing hypothalamic SIRT1 activity mediates neurobehavioral adaptation to CR. Additionally, it has been reported that SIRT1 in the brain is a link between somatotropic signaling and CR in mammals (Cohen et al. 2009). Brain-specific Sirt1 knockout (BSKO) mice induce dwarfism, reduce somatotropic signaling such as levels of growth hormone (GH) and insulin-like growth factor 1 (IGF-1) in plasma, displaying similar phenotypes to those in long-lived mutant mice (Chen et al. 2010), while BSKO mice do not increase their physical activity in response to CR as well as whole-body Sirt1-deficient mice (Chen et al. 2005a) and develop severe glucose intolerance with age (Cohen et al. 2009).
Other sirtuins might also play an important role for the regulation of aging and longevity. For example, SIRT3 has been linked to human longevity (Rose et al. 2003; Schapira 2011). Sirt6-deficient mice display the phenotype of premature senescence such as severe lymphopenia, loss of subcutaneous fat, osteopenia, and metabolic disorders, dying at around 4 weeks of age (Mostoslavsky et al. 2006). SIRT6 modulates telomeric chromatin by deacetylating lysine 9 of histone H3, preventing telomere dysfunction and cellular senescence (Michishita et al. 2008). Moreover, SIRT6 attenuates NF-κB signaling by deacetylating lysine 9 of histone H3 at chromatin, inhibiting apoptosis and cellular senescence (Kawahara et al. 2009; Mahajan et al. 2011). These findings provide the idea that increasing SIRT6 might prevent against age-induced physiological changes, thus possibly extend lifespan. It has been recently reported that SIRT1 is involved in maintaining SIRT6 expression under nutrient deprivation in both in vivo and in vitro (Kim et al. 2010). It should be addressed whether SIRT6 and other mammalian sirtuins serve to maintain physiological responses to CR.
7 Therapeutic Manipulation of Sirtuin Function
Considering the physiological effects of SIRT1 activity, SIRT1 activation has clear potential to combat numerous age-associated diseases, including metabolic syndrome, type 2 diabetes, cardiovascular disease, and neurodegenerative diseases (Borradaile and Pickering 2009; Ginsberg and MacCallum 2009; Hwang et al. 2009; Liu et al. 2008b; Milne et al. 2007; Picard et al. 2004; Qin et al. 2006; Ramsey et al. 2008; Sun et al. 2007). Augmentation of SIRT1 activity may also combat functional declines that occur with normal aging (Marton et al. 2010; Revollo et al. 2007). With this degree of potential, small-molecule sirtuin activating compounds (STACs) have been searched for and developed.
The STAC that coined the phrase is the polyphenolic compound, resveratrol, naturally found in grape skins (Howitz et al. 2003). First identified in a screen of small molecule libraries for hits which enhanced the deacetylase activity of human SIRT1 towards a synthetic, fluorophore-conjugated p53 peptide substrate, resveratrol increases deacetylation 2.0- to 2.6-fold, with 35- and 5-fold decreases in the Km of SIRT1 towards its substrate and NAD, respectively (Feige et al. 2008; Howitz et al. 2003; Milne et al. 2007; Pacholec et al. 2010). Supporting these biochemical assays, resveratrol can only convey its effects in cells expressing SIRT1 (Howitz et al. 2003; Lagouge et al. 2006). In mice, resveratrol (22 mg/kg/day) can significantly ameliorate or prevent HFD-induced impairments in insulin sensitivity, glucose tolerance, and motor function (Baur et al. 2006; Lagouge et al. 2006; Smith et al. 2009; Um et al. 2010). At higher doses (400 mg/kg/day), resveratrol can also protect HFD-fed mice against diet-induced weight gain. Furthermore, it has been reported that resveratrol prevents the HFD-induced decrease in lifespan (25–31%) (Baur et al. 2006; Pearson et al. 2008).
However, use of resveratrol is limited by two significant confounding factors. Firstly, resveratrol has many off-target effects (Baur 2010). Besides SIRT1, it can modulate AMPK, the estrogen receptor, the aryl hydrocarbon receptor, a cannabinoid receptor, and quinine reductase 2. Secondly, resveratrol has such low bioavailability that it is difficult to achieve serum levels in vivo high enough to activate SIRT1. For example, long-term administration of resveratrol (200 or 400 mg/kg/day) in rodents only generated nanomolar plasma concentrations (Lagouge et al. 2006). Furthermore, a volunteer trial in healthy humans found that even high doses of resveratrol could not elicit systemic levels high enough to be beneficial (Chaudhary and Pfluger 2009). To find compounds with higher potency and systemic retention, another small molecule screen was conducted, also using deacetylation of the fluorophore-conjugated p53 substrate to assess SIRT1 activity (Milne et al. 2007). The most potent molecule identified by this screen was SRT1720, which activates SIRT1 7.4-to 8.7-fold (Feige et al. 2008; Milne et al. 2007; Pacholec et al. 2010). Similar to resveratrol, treating HFD-fed mice or rodent models of diabetes with SRT1720 (100 mg/kg/day) improved glucose tolerance and insulin sensitivity in the liver, adipose tissue, and skeletal muscle as well as increased mitochondrial biogenesis (Feige et al. 2008; Milne et al. 2007; Smith et al. 2009). A higher dosage of SRT1720 (500 mg/kg/day) prevented diet-induced weight gain and reduced triglyceride and cholesterol levels in HFD-fed mice (Feige et al. 2008). It also strongly promoted fatty acid oxidation and fat consumption in the skeletal muscle, liver, and BAT. Both molecules appear to induce these physiological effects by stimulating the transcription of genes involved in oxidative phosphorylation and mitochondrial biogenesis while decreasing those involved in inflammatory NF-κB signaling (Baur et al. 2006; Lagouge et al. 2006; Smith et al. 2009).
Even though these molecules were shown to activate SIRT1 in vitro (Baur et al. 2006; Feige et al. 2008; Howitz et al. 2003; Lagouge et al. 2006; Milne et al. 2007), recent work has cast doubt on the notion that resveratrol, SRT1720, and other STACs are direct activators of SIRT1 (Borra et al. 2005; Kaeberlein et al. 2005; Pacholec et al. 2010). The in vitro assays originally used to identify all of these compounds were shown to be dependent upon the fluorophore placed on the substrate peptide. One explanation for the fluorophore dependency of SIRT1 activity is that the fluorophore mimics a hydrophobic residue or pocket found on the native, full-length substrate and/or endogenous regulators that promote a higher affinity for SIRT1. Another potentially reconciling explanation is that these compounds do affect SIRT1 in vitro but indirectly do so through AMPK-mediated increases in cellular NAD levels in vivo. Both resveratrol and SRT1720 have been shown to activate AMPK in multiple organs, and resveratrol almost doubles NAD levels in myotubes in an AMPK-dependent manner (Baur et al. 2006; Dasgupta and Milbrandt 2007; Feige et al. 2008; Um et al. 2010). In fact, resveratrol (400 mg/kg/day) cannot affect any of the aforementioned physiological processes in mice lacking functional AMPK (Um et al. 2010). In other words, while the downstream effects of STACs show clear promise, further enhancement of their specificity and bioavailability are required.
While these STACs currently provide hope for sirtuin-targeted pharmaceutical interventions, several potential problems exist. Firstly, as SIRT1 activity is limited by NAD availability and as NAD levels decline with age, attempts to increase SIRT1 activity long term may prove difficult. Increasing SIRT1 activity without increasing the NAD pool may even be deleterious, as unnaturally high and/or prolonged SIRT1 activity can deplete NAD levels (Liu et al. 2008a). Secondly, attempts to generate highly specific sirtuin activators may prevent concurrent, beneficial activation of other sirtuin family members. Finally, although STACs can protect against or ameliorate disease pathology, they have minimal effects on metabolism and aging in regular chow fed rodents (Barger et al. 2008; Baur et al. 2006; Feige et al. 2008; Milne et al. 2007; Pearson et al. 2008).
One approach that may solve all these problems is to augment NAD levels through dietary supplementation of NAD substrates and intermediates. As these compounds are natural biological entities, they could be taken in the absence of disease pathology, potentially enhancing normal bodily functions throughout life. As evidence for this idea, pharmacologically increasing NADH oxidation in vivo increases SIRT1 activity with the effect of dramatically ameliorating all aspects of metabolic syndrome in a rodent model of the condition (Hwang et al. 2009). Systemic administration of NAD intermediates has further benefits of supplying NAD to the tissues/organs in which NAMPT expression is low, such as pancreatic β-cells and neurons (Imai 2009). These cell types likely depend upon extracellular NAD intermediates. Indeed, the existence of an extracellular form of NAMPT (eNAMPT) has led us to generate the concept of the NAD World, in which systemic NAD biosynthesis mediated by intra- and extracellular NAMPT intricately links metabolic status throughout the body. Based on this concept, systemic administration of NAD intermediates would result in a coordinated adjustment of a variety of metabolic functions.
Interestingly, multiple studies have shown that SIRT1 activity directly depends upon that of NAMPT. For example, NAMPT activity augments SIRT1-mediated transcriptional effects (Revollo et al. 2004; Zhang et al. 2009) and cellular survival upon insult (Araki et al. 2004; Pillai et al. 2005). NAMPT has also been shown to activate SIRT1 to lengthen replicative lifespan, delay senescence (Ho et al. 2009), promote cellular maturation (van der Veer et al. 2007), and induce differentiation (Skokowa et al. 2009). NAMPT can also stimulate SIRT1 to enhance the ability of smooth muscle cells to develop blood vessels (van der Veer et al. 2005), chrondrocytes to produce cartilage (Dvir-Ginzberg et al. 2008), osteoblasts to produce type I collagen (Xie et al. 2007), THP-1 monocytes to induce matrix metalloproteinase-9 activity, peripheral blood mononuclear cells to relate IL-8 and TNF-α (Dahl et al. 2007), and aortic endothelial cells to form endothelial tube networks (Borradaile and Pickering 2009). Since NAMPT produces NMN, administration of NMN should have similar effects. As increased dosage of NAMPT has similar effects on gene expression profiles as increasing the dosage of SIRT1 (Revollo et al. 2004), it appears that SIRT1 is the main transcription factor downstream of NAMPT, and thus the primary target of NMN. Consistent with this idea, systemic administration of NMN can correct defects in insulin secretion and glucose tolerance caused by aging (Ramsey et al. 2008). NMN may even be able to reduce plasma cholesterol and increase insulin sensitivity in the liver and WAT, effects were seen upon injection of a NAMPT expression vector into HFD rats (Sun et al. 2009). Thus far, no side effects of NMN administration have been reported, making NMN supplementation an ideal way to optimize SIRT1 function through the enhancement of NAD biosynthesis. NR is another therapeutic possibility as it can maintain NAD levels and improves SIR2-dependent functions in yeast (Belenky et al. 2007; Bieganowski and Brenner 2004). In mammals, NR generates NAD by way of nicotinamide riboside kinase (Nrk) or purine nucleoside phosphorylase (Pnp) and nicotinamide salvage (Belenky et al. 2009; Bieganowski and Brenner 2004). However, this newly discovered compound has yet to be applied therapeutically in mammalian studies (Houtkooper et al. 2010; Sauve 2008). Thus, more studies will be required to understand the effects of NR.
8 Conclusions
In the past 10 years, interest in the field of sirtuin biology has exploded, generating countless investigations into the functions of mammalian sirtuins. These findings have clearly established that sirtuins are critical mediators of physiological responses to nutritional availability and also that SIRT1 activity has beneficial effects against numerous age-associated disorders, especially those accompanied by metabolic complications. As SIRT1 functionality declines in metabolic syndrome, age-associated disorders, and the aging process, we hypothesize that augmentation of SIRT1 activity will positively affect mammalian metabolism, aging, and longevity. This notion also gives clear therapeutic appeal to long-term augmentation of SIRT1 activity. Currently, using STACs and NAD intermediates are two promising ways to achieve this potentially valuable goal. Nevertheless, the path before us is still long. Answering the following critical questions in the near future will greatly further our progress:
Which tissue(s)/organ(s) play the most critical roles in, and thus are the most relevant therapeutic targets for sirtuin-mediated physiological responses to metabolic state and aging?
How is the activity and/or expression of SIRT1 regulated in each tissue in response to metabolic state and aging?
How is NAD biosynthesis regulated in each subcellular compartment, and how does that regulation impact on each sirtuin?
Which NAD intermediates most effectively increase SIRT1 activity in each tissue/organ, and what are the pharmacokinetics of each NAD intermediate?
Can chronic supplementation of STACs or NAD intermediates augment SIRT1 activity and combat the symptoms of metabolic syndrome and the aging process in humans?
Future work addressing these questions will greatly enrich our understanding of how sirtuin function can be fine-tuned throughout our body and surely provide insights into pharmaceutical and nutriceutical interventions that will promote healthy aging for mankind.
Acknowledgments
We apologize to those whose work is not cited due to space limitations. L.S. is supported by the NIH training program in cellular and molecular biology (5T32GM007067-36). S.I. is supported by grants from the National Institute of Health (AG024150 and HL097817), Ellison Medical Foundation, and Longer Life Foundation. S.I. serves as a scientific advisory board member for and has a sponsored research agreement with Sirtris pharmaceuticals, a GSK company.
References
- Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Amat R, Planavila A, Chen SL, Iglesias R, Giralt M, Villarroya F. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J Biol Chem. 2009;284:21872–21880. doi: 10.1074/jbc.M109.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Avalos JL, Celic I, Muhammad S, Cosgrove MS, Boeke JD, Wolberger C. Structure of a Sir2 enzyme bound to an acetylated p53 peptide. Mol Cell. 2002;10:523–535. doi: 10.1016/s1097-2765(02)00628-7. [DOI] [PubMed] [Google Scholar]
- Avalos JL, Boeke JD, Wolberger C. Structural basis for the mechanism and regulation of Sir2 enzymes. Mol Cell. 2004;13:639–648. doi: 10.1016/s1097-2765(04)00082-6. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804:1626–1634. doi: 10.1016/j.bbapap.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Belenky P, Christensen KC, Gazzaniga F, Pletnev AA, Brenner C. Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J Biol Chem. 2009;284:158–164. doi: 10.1074/jbc.M807976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature. 2001;414:788–791. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- Bhathena SJ. Relationship between fatty acids and the endocrine system. Biofactors. 2000;13:35–39. doi: 10.1002/biof.5520130107. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kappelle LJ. Increased risk of Alzheimer’s disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans. 2005;33:1041–1044. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- Billington RA, Travelli C, Ercolano E, Galli U, Roman CB, Grolla AA, Canonico PL, Condorelli F, Genazzani AA. Characterization of NAD uptake in mammalian cells. J Biol Chem. 2008;283:6367–6374. doi: 10.1074/jbc.M706204200. [DOI] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell. 2009;8:100–112. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- Bronson RT, Lipman RD. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- Bruzzone S, Fruscione F, Morando S, Ferrando T, Poggi A, Garuti A, D’Urso A, Selmo M, Benvenuto F, Cea M, Zoppoli G, Moran E, Soncini D, Ballestrero A, Sordat B, Patrone F, Mostoslavsky R, Uccelli A, Nencioni A. Catastrophic NAD+ depletion in activated T lymphocytes through Nampt inhibition reduces demyelination and disability in EAE. PLoS One. 2009;4:e7897. doi: 10.1371/journal.pone.0007897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 2009;4:e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest. 2000;106:329–333. doi: 10.1172/JCI10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci USA. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care. 2009;12:431–437. doi: 10.1097/MCO.0b013e32832cdaae. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005a;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005b;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008a;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Hutter G, Bruno J, Govindarajan A, Easlon E, Lin SJ, Aguzzi A, Lindquist S, Guarente L. The role of calorie restriction and SIRT1 in prion-mediated neurodegeneration. Exp Gerontol. 2008b;43:1086–1093. doi: 10.1016/j.exger.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Wu CY, Kao CH, Tsai TF. Longevity and lifespan control in mammals: lessons from the mouse. Ageing Res Rev. 2010;9(suppl 1):s28–s35. doi: 10.1016/j.arr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298:E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J Neurosci. 2006;26:10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl TB, Yndestad A, Skjelland M, Oie E, Dahl A, Michelsen A, Damas JK, Tunheim SH, Ueland T, Smith C, Bendz B, Tonstad S, Gullestad L, Froland SS, Krohg-Sorensen K, Russell D, Aukrust P, Halvorsen B. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- Dahl TB, Haukeland JW, Yndestad A, Ranheim T, Gladhaug IP, Damas JK, Haaland T, Loberg EM, Arntsen B, Birkeland K, Bjoro K, Ulven SM, Konopski Z, Nebb HI, Aukrust P, Halvorsen B. Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down-regulated in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:3039–3047. doi: 10.1210/jc.2009-2148. [DOI] [PubMed] [Google Scholar]
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP, Avogaro A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59:1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708–715. doi: 10.1111/j.1478-3231.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav. 2001;74:703–708. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- Escande C, Chini CC, Nin V, Dykhouse KM, Novak CM, Levine J, van Deursen J, Gores GJ, Chen J, Lou Z, Chini EN. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120:545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Pavletich NP. Structure of the histone deacetylase SIRT2. Nat Struct Biol. 2001;8:621–625. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, Moore PC, Prentki M, Rhodes CJ, Jetton TL, Poitout V. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia. 2010;53(11):2369–2379. doi: 10.1007/s00125-010-1850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand AR, Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell. 1998;1:161–170. doi: 10.1016/s1097-2765(00)80017-9. [DOI] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2009;4:113–119. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Williams JG, Schug TT, Li X. DYRK1A and DYRK3 promote cell survival through phosphorylation and activation of SIRT1. J Biol Chem. 2010;285:13223–13232. doi: 10.1074/jbc.M110.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem. 2007;282:32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Yoshikawa K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J Neurosci. 2008;28:8772–8784. doi: 10.1523/JNEUROSCI.3052-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M, Skorecki K, Hayashi K, Itoh H. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010;285:13045–13056. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- Haussinger D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem J. 1990;267:281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida S, Arimoto A, Kuramoto Y, Kozako T, Honda S, Shimeno H, Soeda S. Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARalpha in mice. Mol Cell Biochem. 2010;339:285–292. doi: 10.1007/s11010-010-0391-z. [DOI] [PubMed] [Google Scholar]
- He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010a;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010b;120:1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci USA. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C, van der Veer E, Akawi O, Pickering JG. SIRT1 markedly extends replicative lifespan if the NAD+ salvage pathway is enhanced. FEBS Lett. 2009;583:3081–3085. doi: 10.1016/j.febslet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, Yoo SK, Park MK, Kwak TH, Kho YL, Han J, Choi HS, Lee SH, Kim JM, Lee I, Kyung T, Jang C, Chung J, Kweon GR, Shong M. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58:965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S. The NAD World: a new systemic regulatory network for metabolism and aging–Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- Jin Q, Zhang F, Yan T, Liu Z, Wang C, Ge X, Zhai Q. C/EBPalpha regulates SIRT1 expression during adipogenesis. Cell Res. 2010;20:470–479. doi: 10.1038/cr.2010.24. [DOI] [PubMed] [Google Scholar]
- Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007a;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007b;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chen J, Lou Z. p30 DBC is a potential regulator of tumorigenesis. Cell Cycle. 2009;8:2932–2935. doi: 10.4161/cc.8.18.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Klar AJ, Fogel S. Activation of mating type genes by transposition in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:4539–4543. doi: 10.1073/pnas.76.9.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008;33:517–525. doi: 10.1016/j.tibs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, Wang L, Kemper JK. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010;285:12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann N Y Acad Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Enerback S. Brown adipose tissue–a new role in humans? Nat Rev Endocrinol. 2010;6:319–325. doi: 10.1038/nrendo.2010.64. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Pitta M, Mattson MP. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann N Y Acad Sci. 2008a;1147:275–282. doi: 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, 3rd, Olefsky J, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008b;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SS, Leko V, Simon JA, Bedalov A. Sirtuin modulators. In: Yao T-P, editor. Histone deacetylases: the biology and clinical implication Handbook of experimental pharmacology. Vol. 206. Springer, Heidelberg: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton O, Koltai E, Nyakas C, Bakonyi T, Zenteno-Savin T, Kumagai S, Goto S, Radak Z. Aging and exercise affect the level of protein acetylation and SIRT1 activity in cerebellum of male rats. Biogerontology. 2010;11(6):679–686. doi: 10.1007/s10522-010-9279-2. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935 Nutrition. 1989;5:155–171. discussion 172. [PubMed] [Google Scholar]
- McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Landry J, Sternglanz R, Xu RM. Crystal structure of a SIR2 homolog-NAD complex. Cell. 2001;105:269–279. doi: 10.1016/s0092-8674(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, Gao Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007;8:692–702. doi: 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S, Miura M. Caspase-mediated changes in Sir2alpha during apoptosis. FEBS Lett. 2006;580:5875–5879. doi: 10.1016/j.febslet.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006;580:127–130. doi: 10.1016/j.febslet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, Pizarro JG, Gutierrez-Cuesta J, Crespo-Biel N, Alvira D, Tajes M, Yeste-Velasco M, Folch J, Canudas AM, Sureda FX, Ferrer I, Camins A. Modulation of SIRT1 expression in different neurodegenerative models and human pathologies. Neuroscience. 2008;154:1388–1397. doi: 10.1016/j.neuroscience.2008.04.065. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy WT, Tsunoda I. The importance of NAD in multiple sclerosis. Curr Pharm Des. 2009;15:64–99. doi: 10.2174/138161209787185751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Galli M, Denanglaire S, Van Gool F, Dreze PL, Szpirer C, Bureau F, Andris F, Leo O. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, BonaFe M, Franceschi C, Tan Q, Boiko S, Yashin AI, De Benedictis G. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kim HJ, Kobayashi M, Kitamura YI, Yokota-Hashimoto H, Shiuchi T, Minokoshi Y, Kitamura T. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology. 2010;151:2556–2566. doi: 10.1210/en.2009-1319. [DOI] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther. 2008;324:883–893. doi: 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- Schapira M. Structural biology of human metal-dependent histone deacetylases. In: Yao T-P, editor. Histone deacetylases: the biology and clinical implication Handbook of experimental pharmacology. Vol. 206. Springer, Heidelberg: 2011. [DOI] [PubMed] [Google Scholar]
- Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21:920–928. doi: 10.1101/gad.1527307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MT, Smith BC, Jackson MD, Denu JM. Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation. J Biol Chem. 2004;279:40122–40129. doi: 10.1074/jbc.M407484200. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, Brechlin AM, Schambach A, Hinrichsen L, Meyer G, Gaestel M, Stanulla M, Tong Q, Welte K. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Poliak N, Upadhyay S, Ratovitski E, Nelkin BD, Donehower LA, Sidransky D. DeltaNp63alpha overexpression induces downregulation of Sirt1 and an accelerated aging phenotype in the mouse. Cell Cycle. 2006;5:2005–2011. doi: 10.4161/cc.5.17.3194. [DOI] [PubMed] [Google Scholar]
- Song HK, Lee MH, Kim BK, Park YG, Ko GJ, Kang YS, Han JY, Han SY, Han KH, Kim HK, Cha DR. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1485–F1494. doi: 10.1152/ajprenal.90231.2008. [DOI] [PubMed] [Google Scholar]
- Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sun Q, Li L, Li R, Yang M, Liu H, Nowicki MJ, Zong H, Xu J, Yang G. Overexpression of visfatin/PBEF/Nampt alters whole-body insulin sensitivity and lipid profile in rats. Ann Med. 2009;41:311–320. doi: 10.1080/07853890902729760. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via The Role of Mammalian Sirtuins in the Regulation of Metabolism, Aging, and Longevity 161 cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–745. doi: 10.1016/s0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- Tarantino C, Paolella G, Cozzuto L, Minopoli G, Pastore L, Parisi S, Russo T. miRNA 34a, 100, and 137 modulate differentiation of mouse embryonic stem cells. FASEB J. 2010;24:3255–3263. doi: 10.1096/fj.09-152207. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Tsang AW, Escalante-Semerena JC. CobB, a new member of the SIR2 family of eucaryotic regulatory proteins, is required to compensate for the lack of nicotinate mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase activity in cobT mutants during cobalamin biosynthesis in Salmonella typhimurium LT2. J Biol Chem. 1998;273:31788–31794. doi: 10.1074/jbc.273.48.31788. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD, Chen J. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fivecoat H, Ho L, Pan Y, Ling E, Pasinetti GM. The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer’s disease neuropathology. Biochim Biophys Acta. 2010;1804:1690–1694. doi: 10.1016/j.bbapap.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, Zhou HD, Wu XP, Liao EY. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007a;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007b;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate2 tyrosine phosphorylation. J Biol Chem. 2007;282:34356–34364. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]
- Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009;284:20408–20417. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Chai X, Clements A, Marmorstein R. Structure and autoregulation of the yeast Hst2 homolog of Sir2. Nat Struct Biol. 2003;10:864–871. doi: 10.1038/nsb978. [DOI] [PubMed] [Google Scholar]
- Zhao K, Harshaw R, Chai X, Marmorstein R. Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases. Proc Natl Acad Sci USA. 2004;101:8563–8568. doi: 10.1073/pnas.0401057101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab. 2010;299:E110–E116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillikens MC, van Meurs JB, Rivadeneira F, Amin N, Hofman A, Oostra BA, Sijbrands EJ, Witteman JC, Pols HA, van Duijn CM, Uitterlinden AG. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes. 2009a;58:2828–2834. doi: 10.2337/db09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillikens MC, van Meurs JB, Sijbrands EJ, Rivadeneira F, Dehghan A, van Leeuwen JP, Hofman A, van Duijn CM, Witteman JC, Uitterlinden AG, Pols HA. SIRT1 genetic variation and mortality in type 2 diabetes: interaction with smoking and dietary niacin. Free Radic Biol Med. 2009b;46:836–841. doi: 10.1016/j.freeradbiomed.2008.12.022. [DOI] [PubMed] [Google Scholar]


