Abstract
While it is commonly held that the capacity to learn is greatest in the young, there have been few direct comparisons of the response to training across age groups. Here, adolescents (11–17 years, n = 20) and adults (≥18 years, n = 11) practiced detecting a backward-masked tone for ∼1 h/day for 10 days. Nearly every adult, but only half of the adolescents improved across sessions, and the adolescents who learned did so more slowly than adults. Nevertheless, the adolescent and adult learners showed the same generalization pattern, improving on untrained backward- but not forward- or simultaneous-masking conditions. Another subset of adolescents (n = 6) actually got worse on the trained condition. This worsening, unlike learning, generalized to an untrained forward-masking, but not backward-masking condition. Within sessions, both age groups got worse, but the worsening was greater for adolescents. These maturational changes in the response to training largely followed those previously reported for temporal-interval discrimination. Overall, the results suggest that late-maturing processes affect the response to perceptual training and that some of these processes may be shared between tasks. Further, the different developmental rates for learning and generalization, and different generalization patterns for learning and worsening imply that learning, generalization, and worsening may have different origins.
INTRODUCTION
Sensory perception can be improved with training during both childhood (e.g., Banai et al., 2011; Halliday et al., 2012; Dorfberger et al., 2012) and adulthood (for reviews, see Wright and Zhang, 2009, auditory learning; Sagi, 2011, visual learning), but little is known about the maturation of the response to perceptual training. We recently reported differences in how adolescents and adults responded to the same perceptual training regimen on a basic auditory perceptual task (temporal-interval discrimination), suggesting that the processes involved in perceptual learning on this task can continue to develop well into adolescence (Huyck and Wright, 2011). Here, we asked whether signs of immaturity in the response to training are also present for a different auditory task (backward masking) and whether those immaturities extend to generalization. In doing so, we sought to gain insight into the relative contributions of task-specific and more global processes to developmental differences in the response to perceptual training, and the relationships between different responses to perceptual training (learning and worsening) and generalization during adolescence.
In our previous study, we trained 11-yr-olds, 14-yr-olds, and adults on a temporal-interval discrimination task (standard stimulus: two 15-ms, 1-kHz tones separated by 100 ms) using the same training regimen for all groups (900 trials per session for 10 sessions; Huyck and Wright, 2011). The adolescents demonstrated an immature response to perceptual training, as indicated by four key differences between adolescents and adults. First, all of the adults learned (six out of six), but only half of the 14-yr-olds (four out of eight) and none of the 11-yr-olds (zero out of five) learned. Second, among the adolescents who learned, the rate of improvement across training sessions appeared to be slower than it was for the adults. Third, one third of the adolescents (five out of thirteen) actually got worse across sessions on the trained condition. Finally, regardless of their across-session performance, all of the adolescents but none of the adults got worse within sessions.
Here we begin to explore the nature of the immaturities that give rise to these developmental differences in the response to perceptual training, focusing on whether these immaturities only affect learning on the temporal-interval discrimination task (task specific), affect perceptual learning on multiple tasks (global), or some combination of the two. Both task-specific and global processes are thought to contribute to perceptual learning, and there is evidence from investigations of naive (untrained) performance that both types of process can have a prolonged maturational course. On the one hand, the involvement of task-specific processes in perceptual learning is inferred from demonstrations that behavioral learning patterns (for reviews, see Wright and Zhang, 2009; Sagi, 2011) and the physiological loci affected by training (e.g., Kilgard, et al., 2002; Polley et al., 2006) differ across tasks. For example, in the case of temporal-interval discrimination, training may affect the actual encoding of the elapsed time between two events. The late maturation of some task-specific processes is implicated by the presence of maturational changes in naive performance on some, but not other, tasks during adolescence (e.g., Hartley et al., 2000; Skoczenski and Norcia, 2002; Wright and Zecker, 2004; Bertone, et al., 2010; Banai et al., 2011). On the other hand, the involvement of more general processes in perceptual learning is supported by the observation that perceptual learning appears to follow some of the same principles across multiple tasks and sensory modalities. For example, top-down processes such as attention or reward appear to be necessary for perceptual learning on many tasks (Karni and Sagi, 1991; Ahissar and Hochstein, 1993; Li et al., 2004; Polley et al., 2006; Seitz and Dinse, 2007; Fahle, 2009; Wright, et al., 2010a). These same top-down processes have been shown to develop well into adolescence (Booth et al., 2003; Rosso et al., 2004; van Duijvenvoorde et al., 2008; Geier and Luna, 2009).
To take a step toward understanding the extent to which the immaturities underlying developmental differences in the response to perceptual training are task specific, global, or both, we trained adolescents and adults on a different auditory task than before and compared the results between studies. Specifically, we trained younger adolescents (∼11.5 yr), older adolescents (∼14.5 yr), and adults (≥18 yr) on an auditory backward-masking task in which they were asked to detect a brief tone immediately preceding a bandpass noise. The training regimen was the same for all three age groups. It was already known to be successful in adults (Wright, 1998) and was similar to the regimen in the temporal-interval discrimination study in terms of trial structure, number of trials per session, and number of daily training sessions (Huyck and Wright, 2011). While any difference in the response to training between the adolescents and adults would indicate that the younger age groups were still maturing, we were particularly interested in whether adolescents would demonstrate one or more of the markers of immature perceptual learning that we had observed previously: a smaller proportion of learners than in the adult group, slower learning among adolescent learners than among adults, across-session worsening in some adolescents, and within-session worsening in adolescents, but not adults. Differences in the behavioral patterns of maturation between the two studies (two tasks) would imply that task-specific processes contribute to developmental changes in the response to training. Similarities could arise from either the development of global processes, or the coincident development of task-specific processes, with the likelihood of global-process involvement increasing as the number of similarities increase.
In addition, to further investigate which aspects of the response to perceptual training are immature during adolescence, we also examined how training affected adolescents' and adults' performance on conditions that were not encountered during training (generalization). We did not investigate this aspect of the response to perceptual training previously, and while others have assessed the generalization of skill learning in children (e.g., Dorfberger et al., 2012; Halliday et al., 2012), we are not aware of any direct comparison of generalization between children or adolescents and adults. Therefore, before and after training, we assessed performance on the trained backward-masking condition as well as on several untrained tone-in-noise detection conditions. We were particularly interested in whether adolescents and adults who learn on the trained condition show improvements on different untrained conditions than one another, thus indicating an immaturity in generalization. We were also interested in whether worsening, if observed, generalizes differently than learning, as this outcome would suggest that these two responses to perceptual training arise from at least partially separate processes.
METHOD
Listeners
Forty-eight 11- to 17-yr-olds and 30 adults (≥18 years of age) served as paid listeners. The adolescents were evenly divided into two groups (n = 24 per group) based on whether they were younger (range: 11 years, 0 months to 13 years, 2 months; mean age: 11 years, 8.3 months) or older (range: 13 years, 4 months to 17 years, 1 month; mean age: 14 years, 8.5 months) than the mean age of all adolescents tested (mean = median = 13 years, 2.4 months). All listeners had normal hearing (thresholds < 25 dB hearing level) in the test (left) ear at standard audiometric frequencies between 250 and 8000 Hz, reported no history of language or learning problems, and had no prior experience with psychoacoustic tasks. All data were collected in accordance with Northwestern University policies on the conduct of research with human subjects and with approval of the Institutional Review Board. Written informed consent was obtained from listeners (if over age 18) or their parents or guardians (if under age 18) prior to the experiment. Written informed assent was also obtained from all listeners under 18 years of age. Group mean data from the 11 trained adults (see Sec. 2B) were reported previously in a book chapter (Wright, 1998).
General protocol
The experiment consisted of three phases: a pre-test, training phase, and post-test. During the pre- and post-tests, all 78 listeners were tested on the condition used in training as well as 5 other conditions. The order of the conditions was randomized across listeners but fixed between the pre- and post-tests for each individual listener. Listeners were encouraged to take brief breaks between conditions (typically 5–10 min). Subgroups of younger adolescents (n = 15), older adolescents (n = 13), and adults (n = 19), referred to as controls, participated in the pre- and post-tests but not the training phase. The performance of the controls provided an estimate of the magnitude of threshold change between the pre- and post-tests that could be attributed to pre-test exposure alone. The remaining listeners (9 younger adolescents, 11 older adolescents, and 11 adults), referred to as trained listeners, completed the pre-test, ∼10 training sessions on separate days, and the post-test. During each training session, the trained listeners practiced the trained backward-masking condition for 1 to 1.5 h. Listeners were required to take one 5–10 min break midway through each training session and were encouraged to take other breaks if needed. To keep every session approximately equal in duration (1 to 1.5 h), the pre- and post-tests each were split over two consecutive days, with three conditions completed on each day. The second day of the pre-test and first day of the post-test were separated by an average of 14.6 days [standard deviation (SD) = 3.6] for the controls and 17.1 days (SD = 2.8) for the trained listeners.
Trained condition
The trained task was tone detection in backward masking. Listeners were asked to detect a 10-ms, 1-kHz signal tone presented immediately before a 300-ms bandpass masker (0.2–1.8 kHz) centered on the signal frequency. In each two-presentation forced-choice trial, one presentation was randomly selected to be the signal interval while the other was the standard interval. Both the signal and the masker were present in the signal interval, but only the masker was present in the standard interval. Listeners were required to indicate which of the two randomly selected presentations contained the signal.
During each training session, the adolescents completed 900 trials of the trained backward-masking condition, as did one subgroup of adults (n = 6). Another subgroup of adults (n = 5) completed 720 trials of the trained backward-masking condition during 7 training sessions, and during the other 3 sessions completed 360 consecutive trials of the trained condition followed by 360 trials of the same backward-masking task, but with maskers that had spectral notches centered at the signal frequency (notch width varied from 0 kHz to 0.8 kHz across conditions). We excluded from analyses the data from the second half of each training session in which the notched maskers were used. As noted in Sec. 2D, there were no statistical differences in performance on the trained condition between adults who completed the standard training and those who participated in the three slightly altered sessions.
Untrained conditions
During the pre- and post-tests, all listeners completed ∼300 trials of each of 5 conditions in addition to the trained backward-masking condition. Two of these were common to all listeners. One was a backward-masking condition identical to the trained one except that the frequency of the signal tone was 3.8 kHz and the masker ranged from 3–4.6 kHz. The other was a forward-masking condition that differed from the trained condition only in that the signal immediately followed rather than preceded the noise.
The other three untrained conditions differed across subgroups of listeners. For half of the listeners (n = 39 of 78 total listeners), these were masking conditions that differed from the trained condition only in the temporal relationship between the signal and masker. One was a backward-masking condition in which the onset of the 10-ms signal tone preceded the onset of the masker by 20 ms. The other two were simultaneous-masking conditions in which the signal and masker either shared the same onset or the signal began 200 ms after masker onset. In lieu of these conditions, some of the adolescents (n = 11 trained listeners and n = 15 controls) completed three temporal-interval discrimination conditions and some of the adults (n = 5 trained listeners who practiced 720 trials per session and n = 8 controls) completed three backward-masking conditions with notched-noise maskers (as described in Sec. 2C). The variation in the conditions included in the pre- and post-tests occurred because, in order to increase power, we pooled data across several different training experiments. Importantly, this practice did not appear to affect the outcomes reported here. On each of the three conditions common to all listeners (the trained condition and two untrained conditions), the pre- to post-test changes in performance of trained listeners vs controls did not differ significantly between listeners of the same age who completed different sets of pre- and post-test conditions [2 groups (trained vs control) × 2 set analyses of covariance (ANCOVAs) done separately for each age group and condition; interactions: all non-significant (n.s.), main effect of testing set: all n.s.]. Because we sought to examine the effects of perceptual training in adolescence vs adulthood, we only report results from the untrained conditions on which there were data from both adolescents and adults.
Procedure
Detection thresholds were estimated by adaptively varying the signal level across the forced-choice trials using the maximum-likelihood method (Green, 1990). The two presentations in each trial were separated by 800 ms, as measured from masker onset in one interval to masker onset in the next. Listeners pressed a key on a computer keyboard to select the signal presentation. During every session, each condition was described verbally and ∼4–20 single-interval sample trials (labeled as to whether the signal was present or absent) were provided immediately prior to beginning testing on that condition. Throughout the experiment, all listeners received visual feedback as to whether each of their responses was correct or incorrect. Threshold was defined as the signal level corresponding to the 94.2% correct point on the most likely of 60 psychometric functions after 30 trials. The set of possible psychometric functions covered a 60-dB range in 1-dB steps. Each was a logistic function that increased from 55% to 95% correct in about 10 dB. It was reasonable to assume the same psychometric function slope for all listeners because the maximum likelihood method yields similar threshold values to those based on a listener's actual psychometric function, even when the assumed function is quite different from the actual one (Green, 1990). Threshold estimates were excluded if the listener did not respond correctly on the first trial in a 30-trial block because the selection of the most likely psychometric function was less accurate under those circumstances. This procedure differed from that used in the comparison temporal-interval discrimination experiment (Huyck and Wright, 2011) in which the temporal interval was adjusted using a three-down/one-up adaptive rule that yielded an estimate of the 79.4% correct point on the psychometric function based on 60-trial blocks.
Stimulus generation
All stimuli were generated digitally. Each signal tone was presented in random phase. The masker spectrum level was always 40 dB SPL. The signal and masker durations included 5-ms raised-cosine on/off ramps. The level of the tonal signal was calibrated using a continuous tone. Both the signal and the masker were generated using a digital-signal-processing board [Tucker Davis Technologies, Alachua, FL (TDT) AP2], played through a 16-bit digital-to-analog converter (TDT DD1), followed by an anti-aliasing filter set to low-pass at 8.5 kHz (TDT FT5), a programmable attenuator (TDT PA4), a sound mixer (TDT SM3), and a headphone driver (TDT HB6). The sounds were then presented to the left ear using Sennheiser (Wennebostel, Germany) HD265 headphones.
RESULTS
Multiple session training
Across-session performance
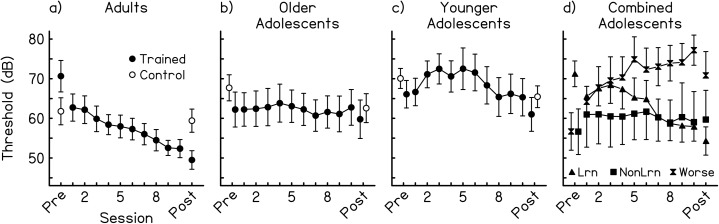
At the group level, the multiple-session training was effective at improving performance on the trained condition for the adults but not the adolescents [Figs. 1a, 1b, 1c, filled circles]. The effect of training differed significantly between the trained adults and each of the two trained adolescent groups [3 age × 12 session analysis of variance (ANOVA) interaction: F(22,308) = 3.26, p < 0.01; 2 × 12 interactions: younger adolescents vs adults, F(11,198) = 4.02, p = 0.01; older adolescents vs adults, F(11,220) = 5.063, p = 0.01; younger vs older adolescents, n.s.]. The adults improved across all 12 sessions (pre-test, training, and post-test), as indicated by a linear regression of the daily mean thresholds for each listener on the session number that yielded a line with a significant and negative slope [slope = −1.50 dB/session, t(130) = −6.33, p < 0.01]. In contrast, the two adolescent groups did not learn across sessions (younger: slope = −0.51 dB/session, older: slope = −0.17 dB/session, both n.s.).
Figure 1.
Across-session performance on the trained backward-masking condition. (a)–(c) Mean results by age group: mean signal detection thresholds (dB for 94.2% correct) for the trained (filled circles) and control (open circles) groups at the pre- and post-tests, and for the trained groups during the training phase. Results are shown separately for the (a) adults (≥18 years of age), (b) older adolescents (13 years, 4 months–17 years, 1 month), and (c) younger adolescents (11 years, 0 months–13 years, 2 months). Trained groups: adults, n = 11; older adolescents, n = 11; younger adolescents, n = 9. Control groups: adults, n = 19; older adolescents, n = 13; younger adolescents, n = 15. (d) Learners vs non-learners: mean signal detection thresholds for the combined group of younger and older adolescents, divided into learners (filled triangles, n = 10), worseners (filled hourglasses, n = 6), and non-learners (filled squares, n = 4) based on each listener's performance across all 12 sessions. Error bars indicate +/− one standard error. Only the upper or lower error bar is shown when the error bars overlapped between groups.
Because there were no differences in the effects of multiple-session training between the younger and older adolescent groups, we decided to re-group the adolescents based on the response to training, rather than the age, of each individual. An individual listener was said to have learned if the linear regression of all of that listener's threshold estimates on the session number was statistically significant and had a negative slope. Based on this analysis, 10 of the 11 trained adults improved on the trained condition across all sessions [adult learners: range of slopes = −0.65 to −3.11 dB/session, all t(213 to 318) ≤ −3.36, all p < 0.01, group mean: −1.45 dB/session; non-learner: slope = 0.22 dB/session, t(318) = 1.24, p = 0.21; this non-learner had the lowest pre-test threshold among the trained adults (42.70 dB)].
Among the 20 adolescents, only 10 demonstrated across-session learning [adolescent learners: range of slopes = −0.46 to −2.47 dB/session, all t(254 to 318) ≤ −2.02, all p ≤ 0.04; group mean = −1.26 dB/session; Fig. 1d, filled triangles], and this learning appeared to occur more slowly than in adults. The adults' performance improved both over the first six [slope = −2.68, t(58) = −3.53, p < 0.01] and over the last six [slope = −1.38, t(58) = −225, p = 0.03] sessions, indicating that their learning began early in the training phase and continued throughout it. The performance of the adolescent learners, however, did not improve during the first half of training (slope = −0.68, n.s.) and only showed a non-significant trend toward improvement during the second half [slope = −1.73, t(58) = −1.79, p = 0.08], suggesting that the adolescents started to learn later in training than did the adults.
Of the ten adolescents who did not learn, more than half actually got worse across sessions. For six individual adolescents, the linear regression of threshold on session number was statistically significant and had a positive slope, indicating a worsening in performance as training progressed [adolescent worseners: range of slopes = 0.63 to 1.51 dB/session, all t(283 to 318) ≥ 2.45, all p ≤ 0.02; group mean = 1.17 dB/session; Fig. 1d, filled hourglasses]. Finally, the four remaining adolescents did not seem to be affected by the training [adolescent non-learners: range of slopes = −0.33 to 0.19 dB/session, all n.s.; group mean = −0.07 dB/session; Fig. 1d, filled squares].
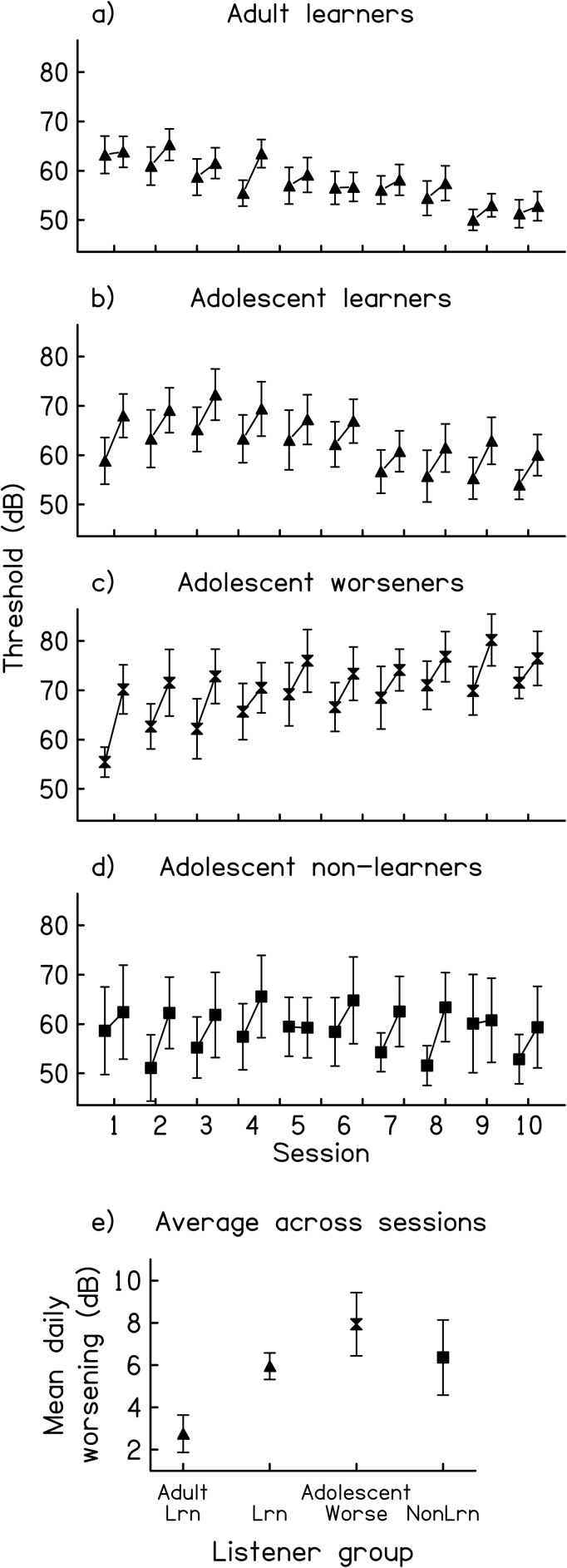
Within-session performance
In addition to the developmental differences in across-session performance described in Sec. 3A1. Across-session performance, there were also differences between the adults and the adolescents in terms of their within-session performance during the training phase. Within-session performance (Fig. 2) was assessed by comparing the thresholds obtained early to those obtained late in each training session using 2 time (early: mean of first six thresholds per session vs late: mean of the 19th through 24th thresholds) × 10 session ANOVAs with repeated measures on time.1 All four trained groups (adult learners, as well as adolescent learners, worseners, and non-learners) demonstrated within-session performance deterioration during each session according to separate 2 time × 10 session ANOVAs for each group [main effects of time: all F(1,27 to 81) ≥ 9.86, all p < 0.04; time × session interactions: all n.s.]. However, the magnitude of this worsening differed across the groups [4 group × 2 time × 10 session ANOVA; group × time interaction: F(3,238) = 4.36, p = 0.01, all other interactions n.s.]. The magnitude of worsening was smaller for the adult learners [Fig. 2a] than for each of the three adolescent groups [Figs. 2b, 2c, 2d; 2 group × 2 time interactions: F(1,102 to 162) ≥ 7.85, p ≤ 0.01], but did not differ among the adolescent groups [Figs. 2b–2d; 3 group × 2 time interaction: n.s.]. The worsening of the adults averaged ∼2.8 dB within each session, while that of the adolescents averaged ∼6.6 dB [Fig. 2e].
Figure 2.
Within-session performance on the trained backward-masking condition. (a)–(d) Mean threshold values from early (estimates 1–6) and late (estimates 19–24) during each training session. Results are shown separately for the (a) adult learners (n = 10) and each group of adolescents: (b) learners (n = 10), (c) worseners (n = 6), and (d) non-learners (n = 4). (e) Change in threshold (late minus early threshold estimates) averaged across the training sessions for each of the four trained groups. Error bars indicate +/− one standard error.
Note that the presence of within-session worsening did not prevent improvement across sessions because the adults and adolescents who learned across sessions got worse within sessions. It also did not appear to mask across-session improvement in the remaining groups. The performance of all but one of the adolescent worseners still deteriorated and that of all of the adolescent non-learners still stayed the same even when the across-session analyses were computed only on the threshold estimates obtained early in each session, before much within-session deterioration had occurred [linear regression of the daily mean thresholds for each listener on the session number; five out of six worseners: range of slopes = 1.23 to 1.94 dB/session, all t(54 to 59) ≥ 2.99, p < 0.01; remaining worsener: slope = 0.92, t(59) = 1.63, p = 0.11; group mean for worseners = 1.48 dB/session; non-learners: range of slopes = −1.04 to 0.15 dB/session, all n.s., group mean = −0.47 dB/session].
Pre- to post-test assessments
We also compared performance on each of the conditions in the pre- and post-tests between the trained groups and same-age controls as well as between the different age groups. Because we had re-grouped the trained adolescents according to their training phase performance instead of their age, we combined the younger and older adolescent controls into a single group for these analyses (adolescent controls). Larger changes in pre- to post-test thresholds for trained listeners than controls indicate that learning or worsening (for the trained condition), or generalization (for the untrained conditions), could be attributed to the multiple-session training and not simply to exposure to the trained and untrained conditions during the pre-test. The comparison between adolescents and adults allowed for an assessment of age differences in the response to training.
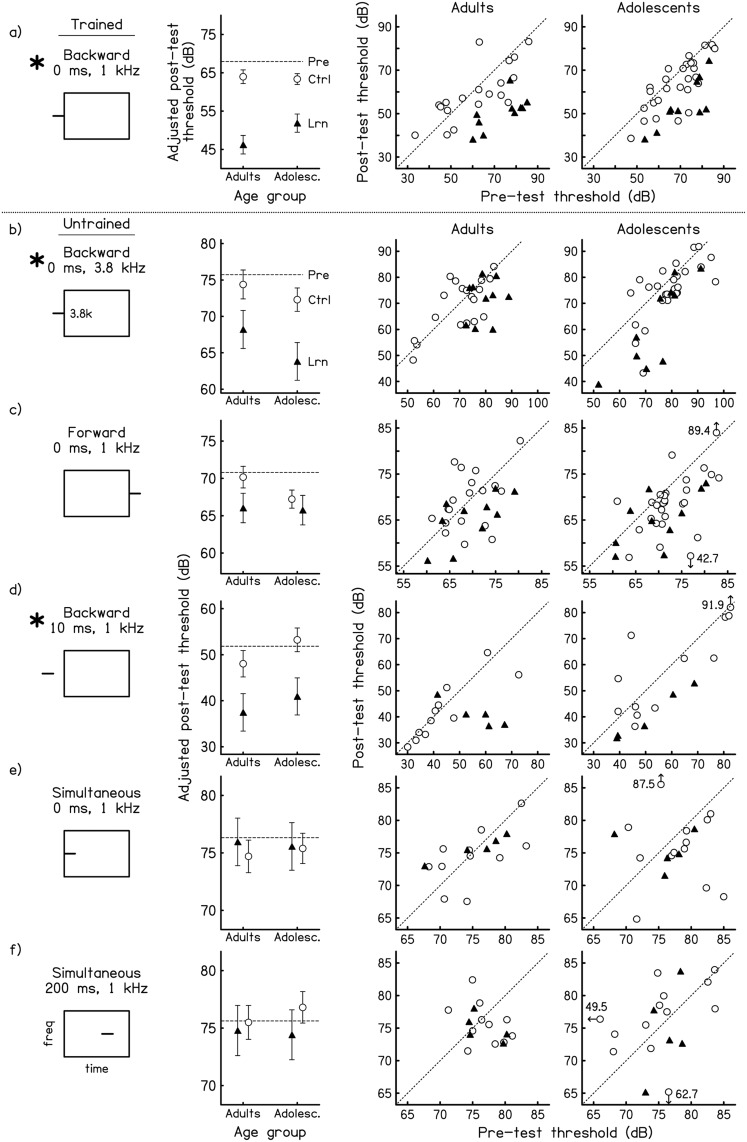
Learning on the trained condition
For the trained backward-masking condition, the outcomes of the pre- to post-test analyses were consistent with the results from the training phase reported above. The adolescent and adult learners did not differ from one another in the amount of performance improvement relative to same-age controls [Fig. 3a; ANCOVA on post-test thresholds with pre-test threshold as the covariate; 2 age (adolescent vs adult) × 2 group (trained vs control) interaction: n.s.], with both groups demonstrating better post-test performance than their respective control groups [main effect of group: F(1,62) = 48.72, p < 0.01; post hoc paired comparisons: adolescent learners vs adolescent controls, p < 0.01; adult learners vs adult controls, p < 0.01]. The pre- to post-test assessments for the adolescent worseners and non-learners were performed only within and not between age groups because only one adult failed to learn on the trained task [Fig. 4a]. The across-session deterioration shown by the adolescent worseners was large enough to differentiate the worseners from the adolescent controls [one-way ANCOVA, main effect of group: F(1,31) = 18.34, p < 0.01] and the adolescent non-learners showed no difference in performance on the trained condition relative to controls (n.s.). Aside from the proportion of learners in each age group, the only apparent age difference on this condition was that the adult controls did not improve significantly on the trained condition between the pre- and post-tests [planned comparison paired t-test: t(18) = 1.04, p = 0.31], while the adolescent controls did [t(27) = 3.49, p < 0.01].
Figure 3.
Pre- and post-test performance of learners and controls on the trained and untrained conditions. Signal detection thresholds (dB for 94.2% correct) for adult and adolescent learners (filled triangles) and controls (open circles) on (a) the trained backward-masking condition and (b)–(f) the five untrained masking conditions. Note that due to the variability in thresholds across conditions, the range of the axes is different for each condition. Condition labels and schematics are shown in the 1st column. Asterisks mark the conditions on which the learners improved more than same-age controls. Mean data (2nd column): Post-test thresholds for each group after adjusting for pre-test thresholds using IBM-SPSS Statistics 20.3 Error bars indicate +/− one standard error and the dashed horizontal line indicates the average pre-test threshold across all four groups. Individual data (3rd and 4th columns): pre- and post-test thresholds for individual listeners. The dotted diagonal line in each panel has a slope of 1, indicating equivalent performance on the pre- and post-tests. Points below this line therefore represent individuals who improved while points above this line represent individuals who got worse.
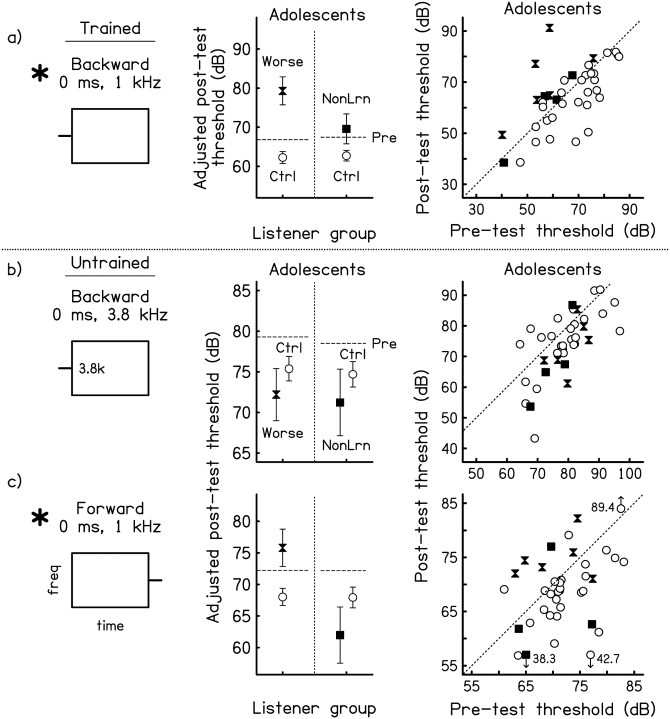
Figure 4.
Pre- and post-test performance for worseners, non-learners, and controls on the trained and untrained conditions. As in Fig. 3, but for the three conditions completed by all of the adolescent worseners (filled hourglasses), non-learners (filled squares), and controls (open circles). Asterisks mark the conditions on which the post-test thresholds of the worseners were significantly higher than those of controls. Note that the range of values on the axes varies across conditions.
Generalization to untrained conditions
The adolescent and adult learners showed the same generalization pattern (constellation of conditions to which learning did and did not generalize) across the five untrained conditions. Of the two untrained conditions completed by all listeners, learning generalized to the backward-masking condition with an untrained frequency region [3.8 kHz vs 1 kHz; Fig. 3b], but not to the forward-masking condition [Fig. 3c] at both ages [separate 2 age × 2 group ANCOVAs on post-test thresholds with pre-test threshold as the covariate; backward 3.8 kHz: group, F(1,61) = 10.97, p < 0.01, age and age × group, n.s.; forward: all n.s.]. Just as for the trained condition, the only difference between age groups on these conditions was that the adolescent controls improved between the pre- and post-tests [backward 3.8 kHz: t(26) = 2.53, p = 0.02; forward: t(27) = 2.99, p = 0.01], but the adult controls did not (both n.s.). Of the three conditions that were completed by half of the listeners, learning generalized to the backward-masking condition with an untrained signal delay [10 vs 0 ms; Fig. 3d; ANOVA: p = 0.03], but not to the two simultaneous-masking conditions for both age groups [Figs. 3e and 3f; ANCOVAs: all n.s.]. Neither the adolescent nor adult controls improved on any of these three conditions (all n.s.).
The adolescent worseners (there were no adult worseners) generalized their worsening to forward masking [Fig. 4c; F(1,31) = 5.63, p = 0.02] but not to backward masking at a higher frequency [Fig. 4b; all n.s],2 the opposite pattern to that seen for the generalization of learning for these two conditions. The adolescent non-learners (there was only one adult non-learner) did not differ from controls on either the backward-masking 3.8-kHz condition [Fig. 4b] or the forward-masking condition [Fig. 4c; all n.s.].2 Performance was analyzed only for these two untrained conditions because they were the only ones completed by all six worseners and all four non-learners. The remaining untrained conditions were completed by only one of the worseners and three of the non-learners.
DISCUSSION
Summary of results
The present training regimen yielded across-session learning on the trained backward-masking condition in nearly all of the adults, but in only half of the adolescents. While adolescents who learned on the trained condition appeared to improve at a slower rate than did adults, their pattern of generalization to untrained conditions was similar to that of adults. Among the adolescents who did not learn on the trained condition, more than half actually became worse across sessions. This worsening generalized in a different pattern than did learning for the two conditions assessed: worsening generalized to the condition to which learning did not, and vice versa. Finally, both adults and adolescents demonstrated within-session worsening, although the magnitude of this worsening was greater for adolescents than for adults.
Comparison across tasks
There are several notable similarities between the age differences in the response to training observed here for backward masking and those observed previously for temporal-interval discrimination (Huyck and Wright, 2011). In both cases, no more than half of the trained adolescents benefited from a training regimen that reliably yielded across-session learning in adults, the adolescents who did learn across sessions did so at a slower rate than did adults, and a subset of adolescents, including some who were 14 years of age or older, actually got worse at the trained condition across training sessions. These trends are remarkably similar to those recently reported for juvenile vs adult gerbils trained on auditory amplitude modulation detection (Sarro and Sanes, 2010). Further, regardless of their across-session performance, the adolescents in both human studies showed a greater magnitude of within-session worsening than did adults.
These between-task similarities suggest that common or global processes may be involved in the maturation of the response to multiple-session perceptual training. Though it is possible that these similarities could reflect the coincident development of independent task-specific processes, given the number of similarities observed here it seems more probable that some of the processes involved may be the same for backward-masking and temporal-interval discrimination. As mentioned in the Introduction, these shared processes could include top-down influences such as selective attention and internal reward that are known to change during adolescence (Booth et al., 2003; Rosso et al., 2004; van Duijvenvoorde et al., 2008; Geier and Luna, 2009) and are thought to be necessary for perceptual learning on most tasks (Ahissar and Hochstein, 1993; Li et al., 2004; Polley et al., 2006; Seitz and Dinse, 2007; Fahle, 2009; Wright et al., 2010a). These shared processes are most likely to affect the acquisition phase of learning, but immaturities in other, as yet unknown, processes may affect the consolidation phase (e.g., Dorfberger et al., 2007). Previously we concluded that both phases might differ between adolescents and adults (for a detailed discussion, see Huyck and Wright, 2011).
While the response to training was quite similar between the backward masking and temporal-interval discrimination tasks in adolescents, there were several differences in learning patterns between tasks in the adults. For example, the adult learners improved across all ten training sessions on backward masking, but learned only over the first five of ten sessions on temporal-interval discrimination. In addition, within the training sessions, the performance of the adults who practiced backward masking got worse during each session while that of the adults who practiced temporal-interval discrimination got better during the first session and stayed relatively constant thereafter. Such differences in learning patterns between tasks have been interpreted previously as reflecting the contribution of task-specific processes to behavioral improvement (Wright and Zhang, 2009; Sagi, 2011). The only notable difference in learning patterns between tasks during adolescence was that, on an individual level, learning emerged at a younger age for backward masking than for temporal-interval discrimination, at least for the current sample. Five of the ten learners on backward masking were less than 12 years of age while all of the learners on temporal-interval discrimination were over 14 years old. Thus, there was some hint of task-specific differences in learning in adolescents, but those differences were not nearly as pronounced as in adults. One possible account for this overall set of similarities and differences in the response to training on backward masking and temporal-interval discrimination is that both shared and task-specific processes are involved in learning at all ages, but that the behavioral response to training is dominated by shared factors during adolescence (as suggested by the similarities in learning patterns between these tasks in the adolescents) and task-specific factors in adulthood (as suggested by the differences in learning patterns between these tasks in adults).
Generalization of learning
Both the adolescent learners and the adults generalized their learning to the backward-masking conditions with an untrained signal delay, and an untrained signal frequency, but not to the untrained simultaneous or forward-masking conditions. This pattern implies that the neural substrates affected by backward-masking training in these listeners responded to a range of temporal delays between the signal and masker, and were not selective for the frequency used in training. It also suggests that the modified substrates were separate from those involved in detecting a tone presented after or during a noise, consistent with the idea that some of the processes underlying backward masking may differ from those involved in simultaneous or forward masking (Elliott, 1962; Duifhuis, 1972; Weber and Green, 1979; Hartley et al., 2000). The lack of generalization to the simultaneous-masking conditions replicates a previous finding that adults who learned on backward masking with training did not improve more than controls on a simultaneous-masking condition with the same signal tone and masker (Roth et al., 2001).
It appears from the present results that the pattern in which learning generalizes could be largely mature even before the rate of learning on the trained condition is adult-like. The adolescents who learned showed the same pattern of generalization as adults across all four untrained conditions for which generalization was assessed. At the same time, these adolescents improved on the trained condition more slowly across sessions than did adults. These results in combination raise the possibility that the maturation of learning rate and of generalization patterns have different time courses. Note, however, that the conclusion that generalization was adult-like in these adolescents is based only on the generalization pattern, and that other facets of generalization that we did not assess could have been immature (e.g., the time course of generalization, see Wright et al., 2010b). As mentioned in the Introduction, others have evaluated the generalization of skill learning in children (e.g., Dorfberger et al., 2012; Halliday et al., 2012) but, to our knowledge, we are the first to directly compare generalization patterns between children or adolescents and adults.
If learning and generalization do indeed become mature at different ages, this finding would add to other recent data suggesting that there may be some functional separation between these two outcomes of training. In adults, skill learning (perceptual and motor) and its generalization can follow different trajectories over the course of multiple-session training, with generalization either lagging behind learning (Wright et al., 2010b) or decreasing as learning increases (Hikosaka et al., 1999; Korman et al., 2003; Park and Shea, 2005; Jeter et al., 2010). In addition, the neural correlates of improvement can differ between trained and untrained conditions, as indicated by functional magnetic resonance imaging (fMRI) studies of single-session motor learning (Seidler and Noll, 2008; Seidler, 2010). Thus, the processes involved in learning a trained condition may differ at least partially from those involved in generalization.
Across- and within-session worsening
Finally, it is particularly striking that worsening across and within sessions was a prominent component of the immature response to training for both backward masking (here) and temporal-interval discrimination (Huyck and Wright, 2011). (For an example of worsening arising from perceptual training in juvenile gerbils, see Sarro and Sanes, 2010.) There are two indications in the present data that different processes may be involved in learning and worsening across sessions. First, the generalization pattern for worsening differed from that for learning. Second, while perceptual learning generally endures over long periods of time (e.g., Karni and Sagi, 1993; Mossbridge et al., 2006), the across-session worsening observed here did not appear to be long-lasting, at least based on data from two individuals. When one of the adolescent worseners was assessed 11 months post-training, he demonstrated performance that was ∼11 dB better than his pre-test thresholds. One of the adolescent worseners in the temporal-interval discrimination study also showed improvement relative to her pre-test performance when tested one year later. Therefore, it seems that training-induced across-session worsening may not be simply “negative learning,” but may rather have its own set of characteristics and constraints. For example, learning and worsening might differ in their susceptibility to interference from intervening events (see Seitz et al., 2005; Dorfberger et al., 2007; Been et al., 2011) or in the number of daily trials required to generate the effect (see Wright and Sabin, 2007; Aberg et al., 2009). It is worth noting, however, that there have been cases of “negative generalization” in adults where learning on a trained condition has been associated with worsening on an untrained condition, suggesting a shared mechanism for learning and worsening in that context (Fitzgerald and Wright, 2005; Sabin et al., 2012).
Within-session worsening also appears to be separable from across-session learning. In the present study, within-session worsening was observed among the adolescents and adults who learned across sessions as well as the adolescents who stayed the same or got worse across sessions. This pattern suggests that within-session deterioration neither prevents nor facilitates across-session improvement on backward masking.
Intuitively, it seems that fatigue, waning attention, or dwindling motivation with increased training could contribute to either across- or within-session worsening. Indeed, in the only study of which we are aware in which the processes contributing to across-session worsening were mentioned (Rowan and Lutman, 2006), it was assumed that the worsening was the result of these general factors. However, it seems unlikely that these factors caused either the across- or within-session deterioration observed on backward masking and temporal-interval discrimination. Personal observations from both studies suggest that the adolescents who got worse across sessions were no more or less fatigued, attentive, or motivated than their peers who learned. Further, if the across-session worsening was caused by general lapses in attention or motivation, worsening would be expected to generalize to all untrained conditions, but it did not. Arguing against the contribution of general factors to within-session worsening, neither manipulations of motivation, task difficulty, and attention nor the degree of self-reported sleepiness appeared to affect within-session perceptual deterioration on a visual texture-discrimination task, at least in adults (Mednick et al., 2002; Mednick et al., 2008). Within-session decrements also did not generalize to untrained portions of the visual field tested during the same day (Mednick et al., 2002). Based on these experiments, it appears that the within-session worsening among adults in the present study is unlikely to be caused by motivation, fatigue, or other general factors. Still, it remains possible that these factors added to within-session worsening among the adolescents, thus yielding the greater magnitude of worsening in adolescents than in adults for backward masking (here) and the presence of within-session worsening among adolescents, but not adults for temporal-interval discrimination (Huyck and Wright, 2011).
A more likely contributor to across- and within-session worsening is over-stimulation of the neural circuitry responsible for performance on the trained condition. The training we provided may have over-taxed the neural circuitry of some listeners such that this circuitry could no longer be used to distinguish between stimuli that had previously been perceivable as distinct from one another. This type of adverse effect might be analogous to the focal dystonia observed in musicians and typists who practice too intensely (for a review, see Hinckley et al., 2009). Recent evidence suggests that stimulus-driven over-stimulation may be the primary cause of within-day worsening in adults trained on a visual texture-discrimination task (Mednick et al., 2008). Performance deterioration across multiple sessions within the same day was correlated with a decreased hemodynamic response in primary visual cortex (V1) as measured using fMRI. This decrease was attributed to stimulus-driven fatigue with increased training because it was observed only in V1 and not in any higher visual areas, and occurred even when the stimulus was not attended. Thus, bottom-up over-stimulation may have contributed to the deterioration observed in the present backward-masking study and in the previous temporal-interval discrimination study. If over-stimulation is the only contributor to worsening, it appears that adolescents are more vulnerable to it than adults given the same number of trials and training sessions. Given that across- and within-session worsening appear to function separately of one another, the influence of over-stimulation may differ in its nature or location between these two time frames.
Conclusions
We trained adolescents and adults on an auditory perceptual task (tone detection in backward masking) using the same multiple-session training regimen for both age groups. There were four key results. First, the response to training on the trained condition itself differed markedly between the adolescents and adults, indicating that the processes that affect the response to this training regimen continue to develop well into the teenage years. Second, the maturational trends in the response to training on backward masking (here) largely matched those previously obtained with the same training regimen on a different task (temporal-interval discrimination), implying that the contributors to this development may be shared between tasks. Third, the pattern of generalization appeared to mature earlier than the rate of learning, consistent with other recent evidence that learning and generalization can be dissociated. Fourth, the pattern of generalization differed between learning and worsening, suggesting that learning and worsening may arise from different substrates. These data thus predict that the functional neural markers of training-induced learning in adults will be absent in many adolescents and that the optimal training regimens for refining or remediating a perceptual skill will differ dramatically with age. Overall, they challenge the common assumption that plasticity is greatest in the young.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health [Grant No. 1F31DC08250 to J.J.H., Grant No. 5R01DC004452 to B.A.W.]. Ann Bradlow, Nicole Marrone, Andrew Sabin, Steven Zecker provided insightful comments on previous drafts of this manuscript, Matthew Fitzgerald, Paul Johnston, and Miriam Reid helped with data collection from the adults, and the Office of Catholic Schools in Chicago assisted with listener recruitment.
Portions of this work were published in Wright, B. A. (1998). “Specific language impairment: Abnormal auditory masking and the potential for its remediation through training,” in Psychophysical and Physiological Advances in Hearing, edited by A. R. Palmer, A. Rees, Q. Summerfield and R. Medis (Whurr, London), pp. 604–610.
Footnotes
These within-session analyses excluded the data from the training sessions that employed backward maskers with spectral notches of various widths (data from three sessions for each of five adults).
Given the small number of worseners (n = 6) and non-learners (n = 4), comparisons between these listeners and the controls were conducted both using parametric statistics (main text) and non-parametric statistics (here). Consistent with the parametric analyses, independent samples Mann Whitney U tests on the change between the pre-test and post-test thresholds (pre minus post) indicated that the worseners showed deterioration on the trained backward-masking condition relative to controls (p < 0.01), did not show the improvement on the untrained forward-masking condition that was present among controls (p = 0.01), and showed similar improvement to controls on the untrained backward-masking condition at 3.8 kHz (n.s.). Contrary to the parametric analyses, a Mann Whitney U test indicated that the non-learners failed to show the pre- to post-test improvement demonstrated by the controls on the trained condition (p = 0.03). However, both the parametric and non-parametric statistics showed that the adolescent non-learners did not differ from controls on either of the two conditions on which generalization was assessed (both n.s.).
ANCOVA on the untrained-delay backward-masking condition [Fig. 3d] was precluded due to a significant heterogeneity of regression between the groups [F(3,34) = 2.99, p = 0.05]. Therefore, the conclusion that learning generalized to this condition is based on a significant 2 group (learner vs control) × 2 test time (pre vs post) interaction in a 2 age × 2 group × 2 test time ANOVA on learner-control pairs [F(1,16) = 5.54, p = 0.03; all other interactions, n.s.]. These pairs were matched for starting performance (pre-test values between 41 and 73 dB for adults and either between 38 and 40 dB or between 49 and 77 dB for adolescents).
References
- Aberg, K. C., Tartaglia, E. M., and Herzog, M. H. (2009). “ Perceptual learning with Chevrons requires a minimal number of trials, transfers to untrained directions, but does not require sleep,” Vision Res. 49, 2087–2089. 10.1016/j.visres.2009.05.020 [DOI] [PubMed] [Google Scholar]
- Ahissar, M., and Hochstein, S. (1993). “ Attentional control of early perceptual learning,” Proc. Natl. Acad. Sci. U.S.A. 90, 5718–5722. 10.1073/pnas.90.12.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai, K., Sabin, A. T., and Wright, B. A. (2011). “ Separable developmental trajectories for the abilities to detect auditory amplitude and frequency modulation,” Hear. Res. 280, 219–227. 10.1016/j.heares.2011.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been, M., Jans, B., and De Weerd, P. (2011). “ Time-limited consolidation and task interference: No direct link,” J. Neurosci. 31, 14944–14951. 10.1523/JNEUROSCI.1046-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone, A., Hanck, J., Guy, J., and Cornish, K. (2010). “ The development of luminance- and texture-defined form perception during the school-aged years,” Neuropsychologia 48, 3080–3085. 10.1016/j.neuropsychologia.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Booth, J. R., Burman, D. D., Meyer, J. R., Lei, Z., Trommer, B. L., Davenport, N. D., Li, W., Parrish, T. B., Gitelman, D. R., and Mesulam, M. M. (2003). “ Neural development of selective attention and response inhibition,” Neuroimage 20, 737–751. 10.1016/S1053-8119(03)00404-X [DOI] [PubMed] [Google Scholar]
- Dorfberger, S., Adi-Japha, E., and Karni, A. (2007). “ Reduced susceptibility to interference in the consolidation of motor memory before adolescence,” PLoS ONE 2, e240. 10.1371/journal.pone.0000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfberger, S., Adi-Japha, E., and Karni, A. (2012). “ Sequence specific motor performance gains after memory consolidation in children and adolescents,” PLoS ONE 7, e28673. 10.1371/journal.pone.0028673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duifhuis, H. (1972). “ Consequences of peripheral frequency selectivity for nonsimultaneous masking,” J. Acoust. Soc. Am. 54, 1471–1488. [DOI] [PubMed] [Google Scholar]
- Elliott, L. L. (1962). “ Backward masking: Monotonic and dichotic conditions,” J. Acoust. Soc. Am. 34, 1108–1115. 10.1121/1.1918253 [DOI] [Google Scholar]
- Fahle, M. (2009). “ Perceptual learning and sensorimotor flexibility: Cortical plasticity under attentional control?” Philos. Trans. R. Soc. London, Ser. B. 364, 313–319. 10.1098/rstb.2008.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, M. B., and Wright, B. A. (2005). “ A perceptual learning investigation of the pitch elicited by amplitude-modulated noise,” J. Acoust. Soc. Am. 118, 3794–3803. 10.1121/1.2074687 [DOI] [PubMed] [Google Scholar]
- Geier, C., and Luna, B. (2009). “ The maturation of incentive processing and cognitive control,” Pharmacol., Biochem. Behav. 93, 212–221. 10.1016/j.pbb.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D. M. (1990). “ Stimulus selection in adaptive psychophysical procedures,” J. Acoust. Soc. Am. 97, 2662–2674. [DOI] [PubMed] [Google Scholar]
- Halliday, L. F., Taylor, J. L., Millward, K. E., and Moore, D. E. (2012). “ Lack of generalization of auditory learning in typically developing children,” J. Speech Lang. Hear. Res. 55, 168–181. 10.1044/1092-4388(2011/09-0213) [DOI] [PubMed] [Google Scholar]
- Hartley, D. E. H., Wright, B. A., Hogan, S. C., and Moore, D. R. (2000). “ Age-related improvements in auditory backward and simultaneous masking in 6- to 10-year-old children,” J. Speech Lang. Hear. Res. 43, 1402–1415. [DOI] [PubMed] [Google Scholar]
- Hikosaka, O., Nakahara, H., Rand, M. K., Sakai, K., Lu, X., Nakamura, K., Miyachi, S., and Doya, K. (1999). “ Parallel neural networks for learning sequential procedures,” Trends Neurosci. 22, 464–471. 10.1016/S0166-2236(99)01439-3 [DOI] [PubMed] [Google Scholar]
- Hinckley, L. B. N., Webster, R. L., Byl, N. N., and Nagarajan, S. S. (2009). “ Neuroimaging characteristics of patients with focal hand dystonia,” J. Hand Ther. 22, 125–134. 10.1016/j.jht.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck, J. J., and Wright, B. A. (2011). “ Late maturation of perceptual learning,” Dev. Sci. 14, 614–621. 10.1111/j.1467-7687.2010.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter, P. E., Dosher, B. A., Liu, S. H., and Lu, Z. L. (2010). “ Specificity of perceptual learning increases with increased training,” Vision Res. 50, 1928–1940. 10.1016/j.visres.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, A., and Sagi, D. (1991). “ Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity,” Proc. Natl. Acad. Sci. U.S.A. 88, 4966–4970. 10.1073/pnas.88.11.4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, A., and Sagi, D. (1993). “ The time course of learning a visual skill,” Nature 365, 250–252. 10.1038/365250a0 [DOI] [PubMed] [Google Scholar]
- Kilgard, M. P., Pandya, P. K., Engineer, N. D., and Moucha, R. (2002). “ Cortical network reorganization guided by sensory input features,” Biol. Cybern. 87, 333–343. 10.1007/s00422-002-0352-z [DOI] [PubMed] [Google Scholar]
- Korman, M., Raz, N., Flash, T., and Karni, A. (2003). “ Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance,” Proc. Natl. Acad. Sci. U.S.A. 100, 12492–12497. 10.1073/pnas.2035019100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. W., Levi, D. M., and Klein, S. A. (2004). “ Perceptual learning improves efficiency by re-tuning the decision ‘template’ for position discrimination,” Nat. Neurosci. 7, 178–183. 10.1038/nn1183 [DOI] [PubMed] [Google Scholar]
- Mednick, S. C., Drummond, S. P., Arman, A. C., and Boynton, G. M. (2008). “ Perceptual deterioration is reflected in the neural response: fMRI study of nappers and non-nappers,” Perception 37, 1086–1097. 10.1068/p5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick, S. C., Nakayama, K., Cantero, J. L., Atienza, M., Levin, A. A., Pathak, N., and Stickgold, R. (2002). “ The restorative effect of naps on perceptual deterioration,” Nat. Neurosci. 5, 677–681. [DOI] [PubMed] [Google Scholar]
- Mossbridge, J. A., Fitzgerald, M. B., O'Connor, E. S., and Wright, B. A. (2006). “ Perceptual-learning evidence for separate processing of asynchrony and order tasks,” J. Neurosci. 26, 12708–12716. 10.1523/JNEUROSCI.2254-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. H., and Shea, C. H. (2005). “ Sequence learning: Response structure and effector transfer,” Q. J. Exp. Psychol. A. 58, 387–419. [DOI] [PubMed] [Google Scholar]
- Polley, D. B., Steinberg, E. E., and Merzenich, M. M. (2006). “ Perceptual learning directs auditory cortical map reorganization through top-down influences,” J. Neurosci. 26, 4970–4982. 10.1523/JNEUROSCI.3771-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso, I. M., Young, A. D., Femia, L. A., and Yurgelun-Todd, D. A. (2004). “ Cognitive and emotional components of frontal lobe functioning in childhood and adolescence,” Ann. N. Y. Acad. Sci. 1021, 355–362. 10.1196/annals.1308.045 [DOI] [PubMed] [Google Scholar]
- Roth, D. A., Kishon-Rabin, L., and Hildesheimer, M. (2001). “ Auditory backward masking and the effect of training in normal hearing adults,” J. Basic Clin. Physiol. Pharmacol. 12, 145–159. [DOI] [PubMed] [Google Scholar]
- Rowan, D., and Lutman, M. E. (2006). “ Learning to discriminate interaural time differences: An exploratory study with amplitude-modulated stimuli,” Int. J. Audiol. 45, 513–520. 10.1080/14992020600801434 [DOI] [PubMed] [Google Scholar]
- Sabin, A. T., Eddins, D. A., and Wright, B. A. (2012). “ Perceptual learning evidence for tuning to spectrotemporal modulation in the human auditory system,” J. Neurosci. 32, 6542–6549. 10.1523/JNEUROSCI.5732-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi, D. (2011). “ Perceptual learning in Vision Research,” Vision Res. 51, 1552–1566. 10.1016/j.visres.2010.10.019 [DOI] [PubMed] [Google Scholar]
- Sarro, E. C., and Sanes, D. H. (2010). “ Prolonged maturation of auditory perception and learning in gerbils,” Dev. Neurobiol. 70, 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler, R. D. (2010). “ Neural correlates of motor learning, transfer of learning, and learning to learn,” Exerc. Sport Sci. Rev. 38, 3–9. 10.1097/JES.0b013e3181c5cce7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler, R. D., and Noll, D. C. (2008). “ Neuroanatomical correlates of motor acquisition and motor transfer,” J. Neurophysiol. 99, 1836–1845. 10.1152/jn.01187.2007 [DOI] [PubMed] [Google Scholar]
- Seitz, A. R., and Dinse, H. R. (2007). “ A common framework for perceptual learning,” Curr. Opin. Neurobiol. 17, 148–153. 10.1016/j.conb.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Seitz, A. R., Yamagishi, N., Werner, B., Naokzu, G., Kawato, M., and Watanabe, T. (2005). “ Task-specific disruption of perceptual learning,” Proc. Natl. Acad. Sci. U.S.A. 102, 14895–14900. 10.1073/pnas.0505765102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoczenski, A. M., and Norcia, A. M. (2002). “ Late maturation of visual hyperacuity,” Psychol. Sci. 13, 537–541. 10.1111/1467-9280.00494 [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde, A. C., Zanolie, K., Rombouts, S. A., Raijmakers, M. E., and Crone, E. A. (2008). “ Evaluating the negative or valuing the positive? Neural mechanisms supporting feedback-based learning across development,” J. Neurosci. 28, 9495–9503. 10.1523/JNEUROSCI.1485-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, D. L., and Green, D. M. (1979). “ Suppression effects in backward and forward masking,” J. Acoust. Soc. Am. 65, 1258–1267. 10.1121/1.382793 [DOI] [PubMed] [Google Scholar]
- Wright, B. A. (1998). “ Specific language impairment: Abnormal auditory masking and the potential for its remediation through training,” in Psychophysical and Physiological Advances in Hearing, edited by Palmer A. R., Rees A., Summerfield Q., and Medis R. (Whurr, London: ), pp. 604–610. [Google Scholar]
- Wright, B. A., and Sabin, A. T. (2007). “ Perceptual learning: how much daily training is enough?,” Exp. Brain Res. 180, 727–736. 10.1007/s00221-007-0898-z [DOI] [PubMed] [Google Scholar]
- Wright, B. A., and Zecker, S. G. (2004). “ Learning problems, delayed development, and puberty,” Proc. Natl. Acad. Sci. U.S.A. 101, 9942–9946. 10.1073/pnas.0401825101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, B. A., and Zhang, Y. (2009). “ Insights into human auditory processing gained from perceptual learning,” in The Cognitive Neurosciences IV, edited by Gazzangiga M. S. (MIT Press, Cambridge: ), pp. 353–366. [Google Scholar]
- Wright, B. A., Sabin, A. T., Zhang, Y., Marrone, N. M., and Fitzgerald, M. B. (2010a). “ Enhancing perceptual learning by combining practice with periods of additional sensory stimulation,” J. Neurosci. 30, 12868–12877. 10.1523/JNEUROSCI.0487-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, B. A., Wilson, R. M., and Sabin, A. T. (2010b). “ Generalization lags behind learning on an auditory perceptual task,” J. Neurosci. 30, 11635–11639. 10.1523/JNEUROSCI.1441-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]