Abstract
Background:
Bone mineral densiy (BMD) is known to be affected by serum 25-hydroxyvitamin D (25(OH) D) levels, intact parathyroid hormone (iPTH) levels. Indian data pertinent to above observation is scant. Our study aimed to investigate the relationships between serum 25-hydroxyvitamin D (25(OH) D) levels, intact parathyroid hormone (iPTH) levels and bone mineral density (BMD) in a cohort of Indian patients.
Materials and Methods:
Adults with or without fragility fractures with low BMD at the hip or lumbar spine were evaluated clinically along with laboratory investigations. T-scores of the hip and spine were derived from BMD-DEXA (dual-energy X-ray absorptiometry). Multivariate regression models were used to investigate the relationships between serum 25(OH) D, iPTH and BMD.
Results:
Total of 102 patients (male:female = 38:64) with a mean age of 62.5 ± 6.4 years were included in the study. Forty-four patients had osteopenia. Osteoporosis was present in 58 patients. The mean values for serum 25(OH) D and iPTH levels were 21.3 ± 0.5 ng/ml and 53.1 ± 22.3 pg/ml, respectively. In 84.3% of patients, serum 25(OH) D levels were below 30 ng/ml (Normal = 30-74 ng/ml), confirming vitamin D deficiency. There was no association between 25(OH) D levels and BMD at the hip or lumbar spine (P = 0.473 and 0.353, respectively). Both at the hip and lumbar spine; iPTH levels, male gender, body mass index (BMI) and age were found to be significant predictors of BMD. Patients with higher BMI had significantly lower BMD and T-score. At levels <30 ng/ml, 25(OH) D was negatively associated with iPTH (P = 0.041).
Conclusion:
Among our cohort of patients with low BMD, no direct relationship between serum 25(OH) D levels and BMD was observed. However, a negative correlation between iPTH and 25(OH) D at serum 25(OH) D concentrations <30 ng/ml. Serum iPTH levels showed a significant negative association with BMD at the hip and lumbar spine. Our findings underscore the critical role of parathyroid hormone in bone metabolism and health.
Keywords: Bone mineral density, osteoporosis, parathyroid hormone, vitamin D
INTRODUCTION
Osteoporosis is a widely prevalent public health problem with substantial morbidity and mortality. Poor nutrition, inadequate exposure to sunlight and low vitamin D status contribute to severe osteoporosis in India.1,2
In adults, severe vitamin D deficiency can cause osteomalacia, which is characterized by inadequate mineralization of the newly formed osteoid. Vitamin D deficiency is believed to cause secondary hyperparathyroidism, leading to an increase in the bone turnover and bone loss.3,4 A negative correlation between serum intact parathyroid hormone (iPTH) (the whole PTH molecule consisting of the N-terminal PTH; midmolecule PTH and C-terminal PTH) and serum 25-hydroxyvitamin D (25(OH) D) has been noted in both Caucasian and non Caucasian populations.3,5,6,7,8 However, from several studies it is has become evident that not all patients with hypovitaminosis D develop secondary hyperparathyroidism.9,10 It is hypothesized that low 25(OH) D concentrations by virtue of development of secondary hyperparathyroidism could be a contributing factor to the low BMD.
Current standard approach for diagnosing osteoporosis is the estimation of bone mineral density (BMD) using dual energy X-ray absorptiometry (DEXA). Low serum 25(OH) D concentrations have been reported in community-dwelling southeast Asian and far east Asian women with no previous history of osteoporosis. Varying effects of 25(OH) D concentrations on bone mineral density (BMD) were reported in these studies.5,6,7,11 However, very few studies have investigated the status of vitamin D in adults with prevalent low BMD and different rates of vitamin D insufficiency have been reported in these investigations.12,13,14,15 Another such study on southeast Asians included very few Indian subjects (3.1% of total study subjects).16
The objectives of this study were (1) to estimate the distribution of serum 25(OH) D levels and (2) to assess the correlation among 25(OH) D levels, iPTH levels and BMD in a population of Indian patients presenting for the evaluation of low BMD.
MATERIALS AND METHODS
All patients presenting with symptoms or risk factors of osteoporosis were subjected to DEXA scanning for evaluation of osteopenia or osteoporosis. The study population included patients seen primarily at our OPD and patients referred by the department of orthopedics and general medicine. The study was duly approved by the institutional ethics committee. Patients were included into the study after obtaining an informed consent. A social and medical history, features of osteoporosis including fragility fracture, intake of medications and supplements, personal history and family history of fractures, loss of height, bone pains and other complaints were elicited. Other important assessments included dietary intake of calcium and exposure to sunlight per day. BMI was calculated as weight in kilograms divided by square of height in meters. The standard deviation (SD) was expressed as T-score. It was calculated as a difference between the measured bone mineral density (BMD) of the patients and the expected bone density value in a normal young person (YN) divided by the population standard deviation (SD).17 Osteopenia was defined with T-score between −1 and −2.5 and osteoporosis was defined with T-score less than −2.5.17
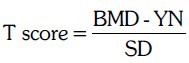
Patient selection
Inclusion criteria
All patients who had a low BMD defined as a T-score (determined by DEXA) of < −1.0 SD at the hip or lumbar spine. (Patients with or without fragility fractures were included).
Exclusion criteria
(1) Diagnosis of primary hyperparathyroidism by elevated serum calcium with unsuppressed iPTH levels. (2) Patients with conditions associated with malabsorption of vitamin D, such as inflammatory bowel disease, chronic pancreatitis, or a history of gastric or small bowel resections. (3) Those patients taking any medication (s) that could adversely affect bone metabolism and thus contribute to a decreased BMD by causing vitamin D deficiency like (rifampicin, ketoconazole, phenytoin, valproic acid, corticosteroid, orlistat) etc. (4) Patients with a creatinine clearance ≤50 ml/min. Below this level, there is impaired hydroxylation of 25(OH) D to 1,25-dihydroxyvitamin D.18 (5) Patients with secondary osteoporosis, prolonged glucocorticoid intake (defined as use of prednisolone in a dosage of more than 5 mg/d for at least 3 months), or significant hepatic or thyroid dysfunction as measured from liver function tests and thyroid profile.
All participants in the study were nonsmokers, denied alcohol consumption, were ambulatory and were not receiving antiosteoporosis agents like (bisphosphonates, calcitonin, hormone replacement therapy, selective estrogen receptor modulators, strontium, or teriparatide).
Biochemical measurements
Laboratory tests included serum creatinine, albumin, total calcium, inorganic phosphate, liver enzymes, iPTH and 25(OH) D. Serum calcium and serum phosphorus were determined by spectrophotometric analysis; albumin-corrected calcium was calculated by using the following formula: Corrected calcium (in mg/dL) = measured total calcium (in mg/dL) + 0.8 [4.0- serum albumin (in g/dL)] (4.0 represents the average serum albumin level (in g/dL). Serum 25(OH) D was measured by radioimmunoassay (DiaSorin Inc., Stillwater, Minnesota) after extraction with acetonitrile. Serum iPTH was measured by chemiluminiscence method on the Beckman Coulter, Unicel Dxl 800 Immunoassay System (Beckman Coulter Inc., Brea, California) using a 2-site sandwich immunoenzymatic chemiluminescent assay. The assay uses a monoclonal anti-PTH antibody conjugated to alkaline phosphatase and paramagnetic particles coated with a goat polyclonal anti-PTH antibody.
Bone mineral density
The T-score, bone mineral content and BMD were determined at two anatomic sites – namely the hip and the lumbar spine, using the DEXA (Lunar Corp., Madison, Wisconsin; CV 0.64%). Values of the BMD at the lumbar spine were expressed as the mean of those at the L1 through L4 vertebrae. The T-scores and Z-scores were calculated on the basis of normal reference values for age- and sex-matched subjects (provided by the DEXA manufacturer). The estimation of BMD was done with use of the same machine for all patients participating in the study. Osteopenia was defined T-score < −1 and osteoporosis as T-score ≤ −2.5.
Statistics
The summary data are expressed as mean values ± SD and comparisons between the groups were done using multivariate regression analysis method. Demographics and other characteristics were summarized using descriptive statistics. The independent t-test was used to assess the difference in serum 25(OH) D concentrations between patients with and those without fragility fractures. For investigation of the factors affecting iPTH and BMD, multivariate analysis with the linear mixed model was used. The analysis on iPTH levels was performed separately on two groupings of 25(OH) D levels of <30 ng/ml and ≥30 ng/ml. The factors evaluated for their effect on iPTH were age, sex, race, total calcium intake, duration of calcium intake and continuous values of 25(OH) D level <30 ng/ml or ≥30 ng/ml. For BMD at the femoral neck, hip and lumbar spine; age, sex, race, body mass index (BMI), continuous values of 25(OH) D level as well as groupings of 25(OH) D levels <30 ng/ml or ≥30 ng/ml, iPTH level, total calcium intake and groupings of exposure to sunlight (<15 min daily or ≥15 min daily) were assessed. P values were reported for all statistical tests and values <0.05 were considered significant.
RESULTS
A total of 102 consecutive patients were selected taking into account the inclusion and exclusion criteria. Baseline demographic characteristics are mentioned in Table 1. The multiple regression model [Table 2] showed that there was no statistically significant associations between serum 25(OH) D concentrations and BMD at the hip (P = 0.473) and lumbar spine (P = 0.353) after adjustments were made for age, sex, race, BMI, iPTH level, total calcium intake and groupings of exposure to sunlight (<15 min daily or ≥15 min daily). At the hip; iPTH levels, male gender, BMI and age were found to be significant predictors of BMD. Similarly at the lumbar spine; iPTH levels, BMI, age and male gender were independently predictive of BMD. There was no statistically significant difference in mean serum 25(OH) D levels between patients with and those without fragility fractures (P = 0.495), nor were prevalent 25(OH) D levels predictive of fragility fractures after adjustments were made for age, sex, race, BMI, total calcium intake and iPTH levels (P = 0.461).
Table 1.
Baseline demography and biochemical data
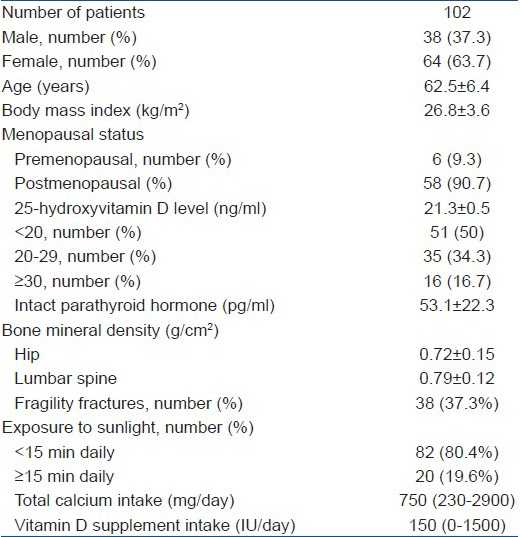
Table 2.
Multiple regression analysis of the impact of different variables on bone mineral density at lumbar spine and hip
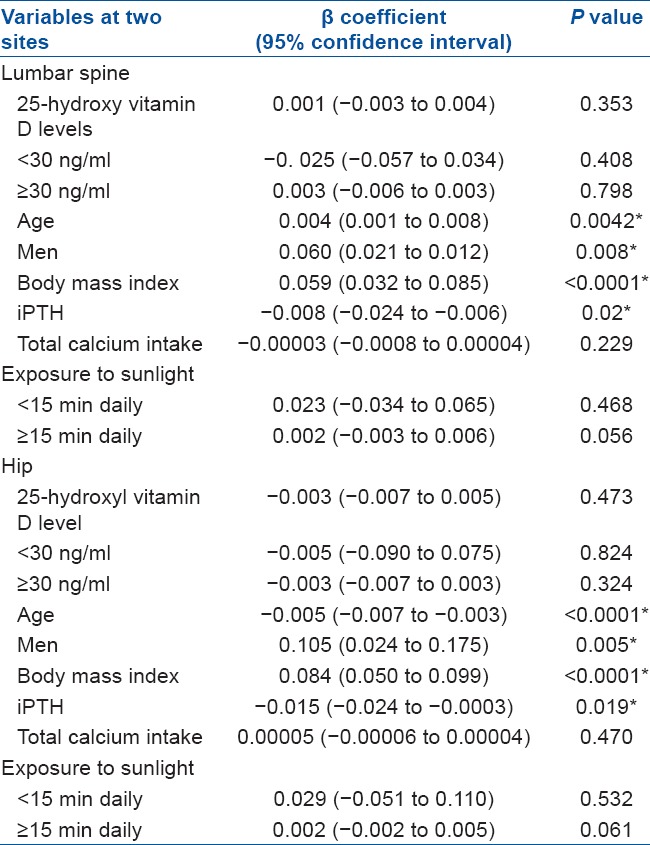
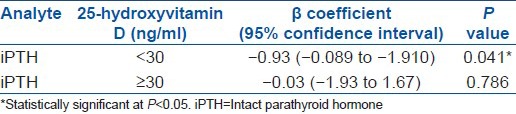
Forty-four (M:F = 20:24) patients had osteopenia and the rest of the 58 patients (M:F = 18: 40) had osteoporosis. Table 3 depicts the characteristics of the patients stratified as per T-score. Relationship between 25(OH) D and iPTH level is shown in Table 4. For serum 25(OH) D concentrations <30 ng/ml, we found that for every increase in serum 25(OH) D of 1 ng/ml, there was a 1.03 pg/ml (95% CI, 0.089 to 1.1902; P = 0.041) decrease in serum iPTH level after adjustments were made for gender, race, age, total calcium intake and duration of calcium intake. This relationship was not observed at 25(OH) D levels ≥30 ng/ml.
Table 3.
Clinical and biochemical characteristics of study patients stratified as per their T score
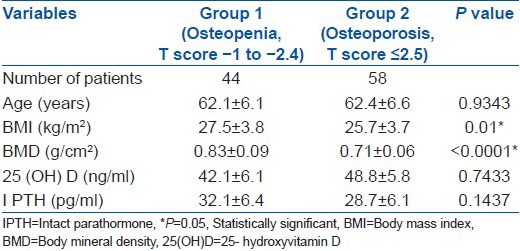
Table 4.
Relationship between serum intact parathyroid hormone and 25-hydroxyvitamin D levels
DISCUSSION
We investigated the association amongst prevalent 25(OH) D levels, BMD and iPTH levels in patients with osteopenia and osteoporosis. Several studies have documented hypovitaminosis D in people living in countries with abundant sunshine.2,5,12,19,20 Reasons attributed for the same are, indoor activities with inadequate sun exposure, improper duration and timing of sun exposure, poor dietary intake and genetic factors. Prevalence of vitamin D deficiency in India is alarmingly high.2,21 Vitamin D deficiency is considered to be present when serum 25(OH) D levels are <20 ng/ml.22 Harinarayan et al.,23 have studied 25(OH) D and BMD in women of reproductive (WR) age group and postmenopausal women (PMW) in south India. They have reported vitamin D deficiency in 76% in WR, 70% in PMW and insufficiency in 16.5% in WR and 23% in PMW. Osteoporosis was seen at hip, 15 and 28%; forearm, nil and 11%; lumbar spine antero-posterior (LSAP), 6 and 22%; and LS lateral, 0 and 23% among WR and PMW; respectively. BMD did not correlate with any of the biochemical indices but correlated with BMD at other sites.23 Marwaha et al.,24 have reported vitamin D deficiency in healthy Indians above the age of 50 years from north India. Vitamin D deficiency was present in 91.2% and insufficiency in 6.8% cases. However, there was no correlation of BMD with 25(OH) D in this study.24 Previous studies have shown that 25(OH) D concentrations of 30-32 ng/ml are associated with maximal suppression of iPTH.25,26 Hypovitaminosis D is gaining importance over the fact that it is shown to be associated with many chronic infectious and noninfectious illnesses.27,28 In our study, prevalence of hypovitaminosis D was 84.3%.
Existing data point to lower average areal BMD values in Asian populations,17,19,27,28,29 The rates of osteoporotic fractures, in urbanized nations in Asia, approach the rates observed in Caucasian populations.30 The age-adjusted rates for men and women (per 100,000) are as follows: Hong Kong, 180 and 459; Singapore, 164 and 442; Malaysia, 88 and 218; Thailand, 114 and 289 this is in contrast with US White rates of 187 in men and 535 in women.30 Vitamin D deficiency is an important risk factor for the development of osteoporosis.25,26 Some studies have reported a positive correlation between 25(OH) D levels and measured BMD at all sites.31 Others have found a correlation at the femoral neck but not at other sites.3,7,32,33 Although some studies have not found any direct correlation between the two at any of the sites.6,12,34 All these studies were performed in subjects without a known diagnosis of bone loss. When patients with osteoporosis were included, a positive correlation between 25(OH) D levels and BMD at the femoral neck (but not at the lumbar spine) was found in one study;35 An association between serum 25(OH) D and BMD at the trochanter only was found in one study13 In a similar study from southeast Asia including patients with low BMD revealed no association between 25(OH) D levels and BMD at the femoral neck, hip, or lumbar spine.16 Though, we did not measure femoral neck BMD, we too observed lack of association between 25(OH) D levels and BMD at the hip or lumbar spine, however the reason for this lack of association remains unexplained.
In our study population, serum 25(OH) D levels were not directly correlated with BMD. The relationship between serum 25(OH) D concentrations and BMD has been shown to differ between black and white races in population-based studies.6,12,34,35,36,37 Although African Americans have lower vitamin D levels than do white Americans, they have a lower prevalence of osteoporosis.38 We did not find an association between prevalent serum 25(OH) D levels and BMD at any anatomic site measured in our patients. Presence of very few cases with extremely low levels of 25(OH) D <10 ng/ml, could account for the observed association.
Bone density tends to decline in older people because of age-related bone loss. The exact age of attainment of peak bone density at various skeletal sites remains controversial, as does the age at which age-related bone loss begins at each site. In comparison with women, men have a greater bone cross-sectional area and hence a higher BMD.39,40 We demonstrated BMI, age, male gender and PTH as significant predictors of BMD at hip and lumbar spine. Observations by the study from Singapore were similar with the exception that male gender was not a significant predictor of BMD at the lumbar spine. BMD is shown to be positively correlated with BMI,41 and the high BMD of overweight individuals has been attributed to several causes, including the permissive effect of weight on the skeleton42 and vitamin D deficiency causing secondary hyperparathyroidism.43 BMI in our group of patients with osteopenia was significantly higher than patients with osteoporosis. Our data supports the widely known fact about the protective effect of obesity in preventing osteoporosis. In postmenopausal women, increased body weight has various effects on mature bone and extra skeletal collagen-containing tissues, resulting in increased bone mineral content.44 Recently, however, an interesting study by Zhao et al.,45 showed that an increased fat mass may not have a beneficial effect on bone if controlled for the mechanical loading effects.
Several studies have shown suppression of iPTH at serum 25(OH) D levels at or around 30 ng/ml,3,8,9,22,46 with an inverse relationship between iPTH and 25(OH) D at serum 25(OH) D concentrations below 30 ng/ml. Secondary hyperparathyroidism has been proposed as the principal mechanism whereby vitamin D deficiency could contribute to bone loss. The increased serum iPTH level causes an increase in bone turnover, which is associated with primarily cortical but also trabecular bone loss.47,48 As previously noted, not all patients with hypovitaminosis D develop secondary hyperparathyroidism. Patients with hypovitaminosis D and a blunted PTH response have shown a lower serum calcium concentration, a reduction in bone turnover and protection of bone density in comparison with those who have hypovitaminosis D and secondary hyperparathyroidism.10 Nevertheless our study observed that levels of 25(OH) D <30 ng/ml were predictive of iPTH levels and increasing PTH levels were associated with lower BMD at the hip and lumbar spine. This leads to the suggestion that secondary hyperparathyroidism is a potential mediator of bone loss in hypovitaminosis D.
Limitations of our study are a small sample size leading to inadequate power (0.62, normal ≥0.8), single center experience with study population not reflective of the Indian population at large and lack of randomization in the recruitment of patients as most of our subjects were hospital based. The calcium intake and exposure to sunlight were calculated according to the participants’ own assertion. However, we still feel that our single center experience would pave way for a longitudinal multicenter study involving larger number of patients to confirm or negate our observations.
To conclude though a direct relationship did not appear to exist between serum 25(OH) D levels and BMD, the significant associations observed between iPTH levels and BMD at the hip and lumbar spine emphasize the critical role of this hormone in bone metabolism and bone health. Advancing age, male gender and BMI are other significant predictors for BMD both at the hip and lumbar spine.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Malhotra N, Mithal A. Osteoporosis in Indians. Indian J Med Res. 2008;127:263–8. [PubMed] [Google Scholar]
- 2.Londhey V. Vitamin D deficiency: Indian scenario. J Assoc Physicians India. 2011;59:695–6. [PubMed] [Google Scholar]
- 3.Ooms ME, Lips P, Roos JC, van der Vijgh WJ, Popp-Snijders C, Bezemer PD, et al. Vitamin D status and sex hormone binding globulin: Determinants of bone turnover and bone mineral density in elderly women. J Bone Miner Res. 1995;10:1177–84. doi: 10.1002/jbmr.5650100806. [DOI] [PubMed] [Google Scholar]
- 4.Dawson-Hughes B. Calcium, vitamin D and vitamin D metabolites. In: Papapoulos SE, Lips P, Pols HA, Johnston CC, Delmas PD, editors. Osteoporosis 1996: Proceedings of the 1996 World Congress on Osteoporosis. Excerpt Med Int Congr Ser 118. Amsterdam, the Netherlands: Elsevier; 1996. pp. 299–303. [Google Scholar]
- 5.Rahman SA, Chee WS, Yassin Z, Chan SP. Vitamin D status among postmenopausal Malaysian women. Asia Pac J Clin Nutr. 2004;13:255–60. [PubMed] [Google Scholar]
- 6.Wat WZ, Leung JY, Tam S, Kung AW. Prevalence and impact of vitamin D insufficiency in southern Chinese adults. Ann Nutr Metab. 2007;51:59–64. doi: 10.1159/000100822. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Tsugawa N, Saito T, Ishikawa M, Tsuchiya Y, Hyodo K, et al. Vitamin D status, bone mass and bone metabolism in home-dwelling postmenopausal Japanese women: Yokogoshi Study. Bone. 2008;42:271–7. doi: 10.1016/j.bone.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 9.Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–50. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 10.Sahota O, Mundey MK, San P, Godber IM, Lawson N, Hosking DJ. The relationship between vitamin D and parathyroid hormone: Calcium homeostasis, bone turnover and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004;35:312–9. doi: 10.1016/j.bone.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Chailurkit LO, Kruavit A, Rajatanavin R. Vitamin D status and bone health in healthy Thai elderly women. Nutrition. 2011;27:160–4. doi: 10.1016/j.nut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Raso AA, Navarra SV, Li-Yu J, Torralba TP. Survey of vitamin D levels among postmenopausal Filipino women with osteoporosis. Int J Rheum Dis. 2009;12:225–9. doi: 10.1111/j.1756-185X.2009.01414.x. [DOI] [PubMed] [Google Scholar]
- 13.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: Baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–21. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 14.Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover and BMD in postmenopausal women with osteoporosis: Global perspective. J Bone Miner Res. 2009;24:693–701. doi: 10.1359/jbmr.081209. [DOI] [PubMed] [Google Scholar]
- 15.Serhan E, Newton P, Ali HA, Walford S, Singh BM. Prevalence of hypovitaminosis D in Indo-Asian patients attending a rheumatology clinic. Bone. 1999;5:609–11. doi: 10.1016/s8756-3282(99)00209-4. [DOI] [PubMed] [Google Scholar]
- 16.Walts NB. T-scores and Osteoporosis. Menopause Med. 2002;10:1–4. [Google Scholar]
- 17.Chandran M, Hoeck HC, Wong HC, Zhang RF, Dimai HP. Vitamin D status and its relationship with bone mineral density and parathyroid hormone in Southeast Asian adults with low bone density. Endocr Pract. 2011;17:226–34. doi: 10.4158/EP10202.OR. [DOI] [PubMed] [Google Scholar]
- 18.Francis RM, Peacock M, Barkworth SA. Renal impairment and its effects on calcium metabolism in elderly women. Age Ageing. 1984;13:14–20. doi: 10.1093/ageing/13.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Arya V, Bhambri R, Godbole MM, Mithal A. Vitamin D status and its relationship with bone mineral density in healthy Asian Indians. Osteoporos Int. 2004;15:56–61. doi: 10.1007/s00198-003-1491-3. [DOI] [PubMed] [Google Scholar]
- 20.Fuleihan GE, Deeb M. Hypovitaminosis D in a sunny country. N Engl J Med. 1999;340:1840–1. doi: 10.1056/NEJM199906103402316. [DOI] [PubMed] [Google Scholar]
- 21.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–20. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 23.Harinarayan CV, Sachan A, Reddy PA, Satish KM, Prasad UV, Srivani P. Vitamin D status and bone mineral density in women of reproductive and postmenopausal age groups: A cross-sectional study from south India. J Assoc Physicians India. 2011;59:698–704. [PubMed] [Google Scholar]
- 24.Marwaha RK, Tandon N, Garg MK, Kanwar R, Narang A, Sastry A, et al. Vitamin D status in healthy Indians aged 50 years and above. J Assoc Physicians India. 2011;59:706–9. [PubMed] [Google Scholar]
- 25.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. McCollum Award Lecture, 1994: Vitamin D-new horizons for the 21st century. Am J Clin Nutr. 1994;60:619–30. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 27.Kota SK, Kota SK, Jammula S, Meher LK, Panda S, Tripathy PR, et al. Renin-angiotensin system activity in vitamin D deficient, obese individuals with hypertension: An urban Indian study. Indian J Endocrinol Metab. 2011;15(Suppl 4):S395–401. doi: 10.4103/2230-8210.86985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kota SK, Jammula S, Kota SK, Tripathy PR, Panda S, Modi KD. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diab Met Syndr: Clin Res Rev. 2011;5:85–9. doi: 10.1016/j.dsx.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Thoo FL, Chng SM, Lam KS, Lee JB, Tan MC, Teh HS, et al. To establish the normal bone mineral density reference database for the Singapore male. Ann Acad Med Singapore. 2002;31:21–5. [PubMed] [Google Scholar]
- 30.Leong KH, Feng PH. Bone mineral density measurements using the Hologic QD2000 in 175 Singaporean women aged 20-80. Singapore Med J. 1997;38:25–6. [PubMed] [Google Scholar]
- 31.Wu XP, Liao EY, Zhang H, Dai RC, Shan PF, Cao XZ, et al. Determination of age-specific bone mineral density and comparison of diagnosis and prevalence of primary osteoporosis in Chinese women based on both Chinese and World Health Organization criteria. J Bone Miner Metab. 2004;22:382–91. doi: 10.1007/s00774-004-0499-x. [DOI] [PubMed] [Google Scholar]
- 32.Lau EM, Lee JK, Suriwongpaisal P, Saw SM, Das De S, Khir A, et al. The incidence of hip fracture in four Asian countries: the Asian Osteoporosis Study (AOS) Osteoporos Int. 2001;12:239–43. doi: 10.1007/s001980170135. [DOI] [PubMed] [Google Scholar]
- 33.Roy DK, Berry JL, Pye SR, Adams JE, Swarbrick CM, King Y, et al. Vitamin D status and bone mass in UK South Asian women. Bone. 2007;40:200–4. doi: 10.1016/j.bone.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 35.Khaw KT, Sneyd MJ, Compston J. Bone density parathyroid hormone and 25-hydroxyvitamin D concentrations in middle aged women. BMJ. 1992;305:273–7. doi: 10.1136/bmj.305.6848.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saadi HF, Nagelkerke N, Benedict S, Qazaq HS, Zilahi E, Mohamadiyeh MK, et al. Predictors and relationships of serum 25 hydroxyvitamin D concentration with bone turnover markers, bone mineral density and vitamin D receptor genotype in Emirati women. Bone. 2006;39:1136–43. doi: 10.1016/j.bone.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Aguado P, del Campo MT, Garcés MV, González-Casaús ML, Bernad M, Gijón-Baños J, et al. Low vitamin D levels in outpatient postmenopausal women from a rheumatology clinic in Madrid, Spain: Their relationship with bone mineral density. Osteoporos Int. 2000;11:739–44. doi: 10.1007/s001980070052. [DOI] [PubMed] [Google Scholar]
- 38.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults. Am J Med. 2004;116:634–9. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–53. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–9. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 41.Melton LJ, 3rd, Khosla S, Achenbach SJ, O’Connor MK, O’Fallon WM, Riggs BL. Effects of body size and skeletal site on the estimated prevalence of osteoporosis in women and men. Osteoporosis Int. 2000;11:977–83. doi: 10.1007/s001980070037. [DOI] [PubMed] [Google Scholar]
- 42.Seeman E. Clinical review 137: Sexual dimorphism in skeletal size, density and strength. J Clin Endocrinol Metab. 2001;86:4576–84. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 43.Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Yamasaki H, et al. Difference in the effect of adiposity on bone density between pre- and postmenopausal women. Maturitas. 2000;34:261–6. doi: 10.1016/s0378-5122(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 44.Slemenda CW. Body composition and skeletal density: Mechanical loading or something more? J Clin Endocrinol Metab. 1995;80:1761–3. doi: 10.1210/jcem.80.6.7775618. [DOI] [PubMed] [Google Scholar]
- 45.Liel Y, Edwards J, Shary J, Spicer KM, Gordon L, Bell NH. The effects of race and body habitus on bone mineral density of the radius, hip and spine in premenopausal women. J Clin Endocrinol Metab. 1988;66:1247–50. doi: 10.1210/jcem-66-6-1247. [DOI] [PubMed] [Google Scholar]
- 46.Papakitsou EF, Margioris AN, Dretakis KE, Trovas G, Zoras U, Lyritis G, et al. Body mass index (BMI) and parameters of bone formation and resorption in postmenopausal women. Maturitas. 2004;47:185–93. doi: 10.1016/S0378-5122(03)00282-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–6. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krall EA, Sahyoun N, Tannenbaum S, Dallal GE, Dawson-Hughes B. Effect of vitamin D intake on seasonal variations in parathyroid hormone secretion in postmenopausal women. N Engl J Med. 1989;321:1777–83. doi: 10.1056/NEJM198912283212602. [DOI] [PubMed] [Google Scholar]


