Abstract
Streptozotocin (STZ)-induced diabetes is associated with reductions in the electrical response of the outer retina and retinal pigment epithelium (RPE) to light. Aldose reductase (AR) is the first enzyme required in the polyol-mediated metabolism of glucose, and AR inhibitors have been shown to improve diabetes-induced electroretinogram (ERG) defects. Here, we used control and AR−/− mice to determine if genetic inactivation of this enzyme likewise inhibits retinal electrophysiological defects observed in a mouse model of type 1 diabetes. STZ was used to induce hyperglycemia and type 1 diabetes. Diabetic and age-matched nondiabetic controls of each genotype were maintained for 22 weeks, after which ERGs were used to measure the light-evoked components of the RPE (dc-ERG) and the neural retina (a-wave, b-wave). In comparison to their nondiabetic controls, wildtype (WT) and AR−/− diabetic mice displayed significant decreases in the c-wave, fast oscillation, and off response components of the dc-ERG but not in the light peak response. Nondiabetic AR−/− mice displayed larger ERG component amplitudes than did nondiabetic WT mice; however, the amplitude of dc-ERG components in diabetic AR−/− animals were similar to WT diabetics. ERG a-wave amplitudes were not reduced in either diabetic group, but b-wave amplitudes were lower in WT and AR−/− diabetic mice. These findings demonstrate that the light-induced responses of the RPE and outer retina are disrupted in diabetic mice, but these defects are not due to photoreceptor dysfunction, nor are they ameliorated by deletion of AR. This latter finding suggests that benefits observed in other studies utilizing pharmacological inhibitors of AR might have been secondary to off-target effects of the drugs.
Keywords: Electroretinogram, Aldose reductase, Diabetes, Retinal pigment epithelium, Diabetic retinopathy, c-wave, Outer retina
Introduction
The ocular complications associated with diabetes are the leading cause of new cases of blindness in adults between the ages of 20 and 74 years in the United States (Centers for Disease Control and Prevention, 2011). The characteristic diabetic pathologies of the retina, namely macular edema and neovascularization, are typically found after protracted periods, whereas electrophysiological defects of the retina associated with diabetes have been identified soon following the onset of hyperglycemia (Shirao & Kawasaki, 1998). Thus, it has been suggested that defects in the electroretinogram (ERG) may be useful in diagnosing and predicting progression of diabetic retinopathy (Bresnick et al., 1984).
Across 50 years of research in patient populations and animal models of diabetes, studies define dysfunction in the inner retina by delayed timing and amplitude of oscillatory potentials (OPs) and reductions in b-wave amplitude (Yonemura, 1978; Levin et al., 1982; Bresnick et al., 1984; Bresnick & Palta, 1987; Coupland, 1987; Lovasik & Spafford, 1988; Juen & Kieselbach, 1990; Hardy et al., 1995; Shirao & Kawasaki, 1998). Altered function of the outer retina has also been identified by changes in activation kinetics of the a-wave (Phipps et al., 2006). Similarly, retinal pigment epithelium (RPE) dysfunction has been observed through reduced c-wave amplitudes (Pautler & Ennis, 1980; MacGregor & Matschinsky, 1986; Holopigian et al., 1992; Phipps et al., 2004; Phipps & Feener, 2008; Wong et al., 2011). These findings indicate that nearly every cell type in the outer retina is affected by diabetes (reviewed in Shirao & Kawasaki, 1998; Tzekov & Arden, 1999). Notably, the electrophysiological defects appear early and progress throughout the duration of the disease, classifying them as one of the first characteristics of diabetes identifiable by an objective in vivo measure.
Aldose reductase (AR) is the primary enzyme in the polyol metabolism pathway whereby glucose is converted to sorbitol (Hers, 1962). Under normal blood glucose concentrations, AR has low substrate affinity for glucose compared to hexokinase. However, under hyperglycemic conditions, AR activity is elevated (Barski et al., 2008). As a byproduct of this reaction, nicotinamide adenine dinucleotide phosphate (NADPH) is converted to NADP+, reducing the availability of NADPH. Both animal studies and clinical trials have been conducted to determine the efficacy of AR inhibitors in reducing the occurrence of diabetes-related visual defects. Findings have, however, been mixed. A number of studies demonstrated positive outcomes in ERG testing as a result of pharmacological inhibition of AR (MacGregor & Matschinsky, 1985; Funada et al., 1987), while others report no improvement in diabetes-induced alterations in retinal electrophysiology after treatment (Biersdorf et al., 1988; Matsui et al., 1994; Hotta, 1995; Hotta et al., 1995a,b, 1997; Ashizawa et al., 1997).
In this study, we addressed both the impact of diabetes on the ERG and the role of AR in the pathogenesis of diabetes-induced defects in the ERG. In contrast to previous studies, which attempted to target AR by pharmacological inhibition, we directly assessed the contribution of AR by genetic inactivation of the enzyme. Streptozotocin (STZ) was utilized to induce type 1 diabetes in wildtype (WT) and AR−/− (ARKO) C57BL/6J mice. Diabetic and nondiabetic control mice were maintained for 6 months, and ERG analyses were subsequently performed to document the defects induced by diabetes and to determine if the absence of AR prevents the ERG reductions observed in diabetic animals.
Materials and methods
Animals
Male C57BL/6J WT and AR−/− mice (Ho et al., 2000) were randomly assigned to diabetic or nondiabetic groups, with half of each group designated for ERG analysis. Diabetes was induced by five sequential daily intraperitoneal injections of a freshly prepared solution of STZ in 0.1 M citrate buffer (pH 4.5) at 60 mg/kg body weight. Insulin was given as needed to prevent ketosis without preventing hyperglycemia and glucosuria (0−0.2 units of neutral protamine Hagedorn (NPH) insulin, subcutaneously, 0–3 times per week). Between 10 and 12 animals were assigned to each group. Survival rates varied between 83.3 and 100% (WT nondiabetic: 10/10; WT diabetic: 10/12; AR−/− nondiabetic: 10/11; AR−/− diabetic: 10/12) up to 6 months. Glycohemoglobin was measured by Bio-Rad Total Glycated Hemoglobin Assay (Bio-Rad Laboratories, Hercules, CA) every 2–3 months and just before animals were sacrificed. Food consumption and body weight were measured weekly. Treatment of animals was in compliance with the ARVO Resolution on Treatment of Animals in Research, as well as institutional guidelines. Animals were maintained for 6 months in order to investigate the long-term effects on electrophysiological changes associated with diabetes.
ERG testing
After overnight dark adaptation, mice were anesthetized (ketamine, 80 mg/kg; xylazine, 16 mg/kg), the cornea was anesthetized (1% proparacaine HCl), and the pupils were dilated (1% tropicamide, 2.5% phenylephrine HCl, 1% cyclopentolate). Mice were placed on a temperature-regulated heating pad throughout each recording session. The protocols used to record ERG components generated by the outer neural retina or the RPE have been previously described (Samuels et al., 2010). In brief, responses of the outer retina were recorded with a stainless steel electrode referenced to a needle electrode placed in the cheek in response to strobe flash stimuli (ranging from −3.6 to 2.1 log cd s/m2) presented in the dark. Photopic stimuli (−0.8 to 1.9 log cd s/m2) were superimposed on a steady rod-desensitizing adapting field (20 cd/m2) after 7 min of light adaptation. Responses were differentially amplified (0.3–1500 Hz), averaged, and stored using a UTAS E-3000 signal averaging system (LKC Technologies, Gaithersburg, MD). The amplitude of the a-wave was measured 8 ms after flash onset from the prestimulus baseline. The amplitude of the b-wave was measured from the a-wave trough to the peak of the b-wave or, if no a-wave was present, from the prestimulus baseline. OPs were filtered from the averaged responses at 4.3 cds/m2, and peak amplitudes and latencies were measured as previously described (Hancock & Kraft, 2004).
Components of the dc-ERG, generated by the RPE, were recorded with an Ag/AgCl electrode bridged to the corneal surface with Hanks' balanced salt solution in response to a single light stimulus (2.4 log cd/m2) presented for 7 min. Responses were differentially amplified at dc-100 Hz; gain = 1000× (DP-301; Warner Instruments, Hamden, CT), digitized at 20 Hz, and stored using LabScribe Data Recording Software (iWorx, Dover, NH). Approximately 10 min of recording included a 30-s baseline, the four light-evoked waveform components, and 2 min of poststimulus recording time (see Fig. 2A). The amplitude of the c-wave was measured from the prestimulus baseline to the peak of the c-wave. The amplitude of the fast oscillation (FO) was measured from the c-wave peak to the trough of the FO. The amplitude of the light peak (LP) was measured from the FO trough to the asymptotic value. The amplitude of the off response was measured from the LP asymptote to the peak of the off response.
Fig. 2.
Loss of AR does not inhibit diabetes-induced reductions in components of the dc-ERG. (A) Representative dc-ERG tracings for each group recorded in response to a 7-min light stimulus. (B–E) Amplitude of the major components of the dc-ERG. For each group, n ≥ 4. Statistical analysis was performed with one-way ANOVA and post hoc Tukey’s test. *P ≤ 0.01, ***P ≤ 0.0001.
Results
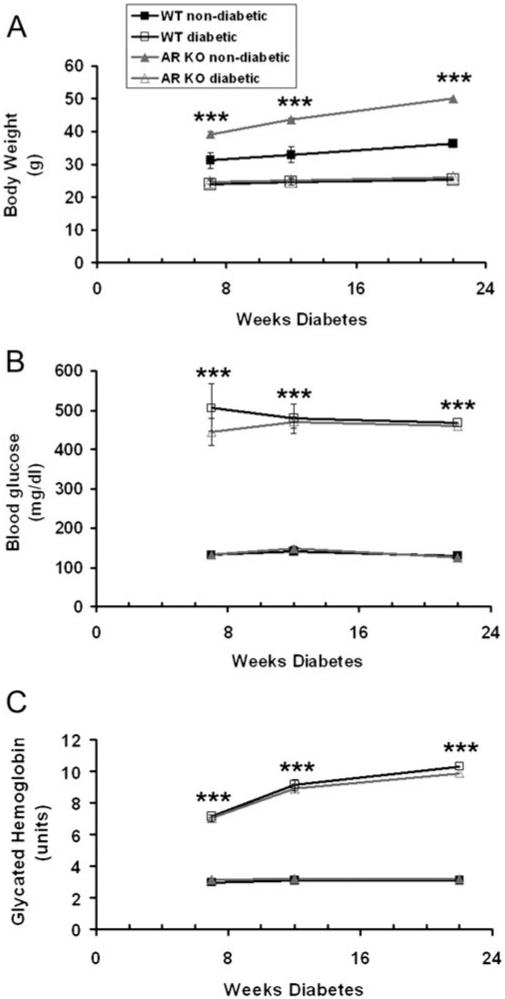
All nondiabetic mice had blood glucose levels below 150 mg/dl (Fig. 1B). STZ-induced diabetes affected WT and AR−/− mice similarly, both in blood glucose and glycated hemoglobin levels. Diabetic mice were hyperglycemic for the duration of the 6-month experiments and displayed significantly higher blood glucose and glycated hemoglobin levels at all time points (Fig. 1B and 1C). Both genotype and diabetes status had a significant effect on body weight. We found that nondiabetic AR−/− mice were significantly larger than WT mice throughout the duration of the experiment (Fig. 1A), and both groups of diabetic mice weighed significantly less than their nondiabetic counterparts (Fig. 1A). However, neither group lost weight during the course of the experiment.
Fig. 1.
Loss of AR does not significantly alter glycemia. Body weight (A), blood glucose (B), and glycated A1c hemoglobin (C) were measured in each group of mice at 7, 14, and 22 weeks following treatment (nondiabetic = citrate buffer; diabetic = STZ). For each group, n ≥ 4. Statistical analysis was performed with repeated measures ANOVA and post hoc Bonferroni test. ***P ≤ 0.0001.
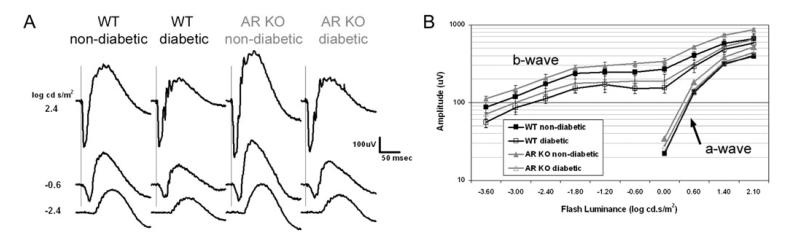
After 22 weeks of diabetes, ERG studies were conducted. In WT nondiabetic mice, the 7-min light stimulus evoked all four major components of the dc-ERG (Fig. 2A1), each of which was reduced in amplitude in WT diabetic mice (Fig. 2A2). AR−/− nondiabetic mice (Fig. 2A3) generated consistently larger dc-ERGs as compared to WT nondiabetic mice. Similar to WT diabetic mice, AR−/− diabetic mice also displayed reductions in each dc-ERG waveform (Fig. 2A4). Although AR−/− nondiabetic responses were larger, we did not find a significant difference in amplitude for any component when comparing WT and AR−/− nondiabetics. Importantly, there were no significant differences between WT diabetics and AR−/− diabetics. The amplitude of the c-wave was significantly decreased in WT diabetic as compared to WT nondiabetic mice (P > 0.001) and in AR−/− diabetic as compared to AR−/− nondiabetic mice (Fig. 2B; P > 0.001). We similarly found significant amplitude reductions in the FO and off response from WT diabetic compared to the WT nondiabetic mice (Fig. 2C and 2E). These amplitude reductions were also seen in AR−/− diabetic as compared to AR−/− nondiabetic animals. Our recordings revealed a general trend for reductions in the LP amplitude of the diabetic groups as compared to the nondiabetics; however, these reductions were not statistically significant (Fig. 2D).
Strobe flash ERGs were recorded to determine how the neural retina is affected by loss of AR and to ensure that reductions in the dc-ERG waveforms are not due to reduced photoreceptor activity (Samuels et al., 2010). As seen in Fig. 3A, characteristic strobe flash ERGs were generated by mice of all genotypes and diabetic states. The amplitude of the a-wave was not significantly affected by either diabetic status or genotype (Fig. 3B). In comparison, b-waves of both WT and AR−/− diabetic mice were smaller in amplitude as compared to their nondiabetic controls (Fig. 3B). Nondiabetic AR−/− mice generated slightly greater ERG responses as compared to WT nondiabetics; however, responses from AR−/− diabetic mice were reduced to a similar extent as WT diabetics. Interestingly, we did not observe a significant change in b-wave latency [data not shown, P = 0.13 by one-way analysis of variance (ANOVA)]. These data are consistent with previous findings demonstrating reductions in b-wave but not a-wave parameters (Phipps et al., 2006).
Fig. 3.
Loss of AR does not alter the differences in strobe flash ERG responses between normal and diabetic mice. (A) Averaged strobe flash ERG responses recorded at increasing light intensities for each group. (B) Luminance response function of a- and b-wave amplitude. For each group, n ≥ 4. Statistical analysis was performed using a one-way ANOVA followed by post hoc Tukey’s test. *P ≤ 0.01, **P ≤ 0.001.
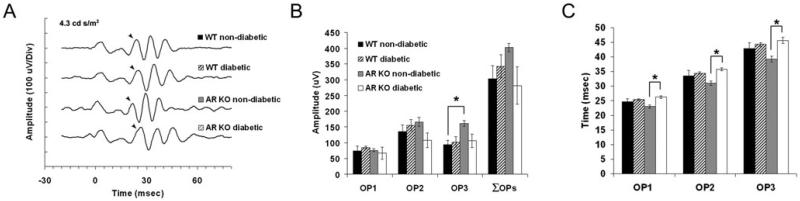
We digitally filtered OPs from the strobe flash ERGs and assessed peak amplitude and latency for each OP wavelet at 4.3 cd s/m2 (Fig. 4A). Reductions in OP amplitude and prolonged latencies are the most common ERG anomaly found as a result of diabetes (Yonemura et al., 1962; Hancock & Kraft, 2004; Phipps et al., 2004) and have been proposed to be predictive of progression to retinopathy in humans (Bresnick et al., 1984). However, most animal studies have focused on rat models of diabetes, and only a few reports have found any significant differences in these parameters in diabetic mice (Barile et al., 2005; Zheng et al., 2007). In our analysis, we did not observe significant differences in either peak latency or amplitude between WT diabetic or AR−/− diabetic mice and their corresponding control. The only significant difference identified between any group was found in the AR−/− nondiabetic as compared to its diabetic counterpart. Specifically, the AR−/− wavelets peaked faster (Fig. 4C) and were of greater amplitude (OP2) than the AR−/− diabetic (Fig. 4B). This finding matches the larger amplitudes found in both the dc-ERG components and the b-wave amplitude. These findings indicate that in this model of diabetes, the OP generators retain normal function at this time point.
Fig. 4.
Diabetes does not significantly affect OP wavelets of WT or AR KO mice at 22 weeks. (A) Representative OP wavelets in response to a 4.3 cd s/m2 strobe flash. The peak of the first wavelet is denoted by a closed arrow. (B) Peak amplitude of each wavelet and the summed amplitudes (ΣOP). (C) Latency of each wavelet. For each group, n ≥ 4. *P ≤ 0.05.
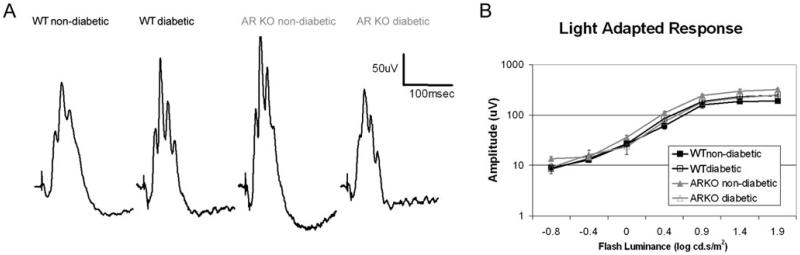
We also recorded the cone ERG. Despite a slightly greater amplitude observed in the AR−/− nondiabetic group, cone-dominant light-adapted ERG responses were not significantly different between groups (Fig. 5), indicating that diabetes did not affect cone function at this time point. Cone responses have been shown to exhibit reductions in hyperglycemic zebrafish (Alvarez et al., 2010); however, no single animal model of type 1 diabetes recapitulates all aspects of the disorder identified in patients, and we do not observe significant defects in either rod or cone function within this model of type 1 diabetes.
Fig. 5.
Loss of AR does not alter differences in light-adapted ERG responses between normal and diabetic mice. (A) Representative ERG tracings recorded in response to a 1.9 log cds/m2 strobe flash superimposed on a steady rod-desensitizing (20 cd/m2) adaptation field. (B) Intensity response function for each group (for each group, n ≥ 4).
Discussion
This study presents two novel findings. It is the first comprehensive investigation of in vivo RPE electrophysiology in an animal model of diabetes. Our work demonstrates that after 6 months of diabetes, mice exhibit profound defects in outer retinal function manifested by reductions in most components of the dc-ERG. We also confirm reports of disrupted inner retina processing evidenced by reductions in b-wave amplitude. In addition, our findings indicate that AR does not play a key role in the mechanisms underlying these diabetes-induced retinal electrophysiology abnormalities. Diabetic mice lacking AR had decreases in bipolar cell and RPE function comparable to those in WT diabetic mice. In fact, control AR−/− mice generally displayed larger amplitude ERG waveforms than WT mice, yet ERG waveform amplitudes in diabetic AR−/− mice were reduced to similar levels as WT diabetics. As no change in a-wave amplitude or latency was found between any groups, this data set suggests that defects observed in the dc-ERG components of diabetic mice (RPE function) are not secondary to photoreceptor dysfunction.
Reductions in c-wave amplitude have been observed in diabetic rats as early as 2 weeks following the onset of diabetes (Pautler & Ennis, 1980; MacGregor & Matschinsky, 1985). The later dc-ERG components had not previously been measured in diabetic animals. Importantly, the amplitudes of the FO, dark peak voltage and LP voltage of the electrooculogram (EOG), the clinical correlate of the dc-ERG, are all known to be reduced in diabetic patients as compared to controls (Schneck et al., 2008), and these parameters are affected independent of retinopathy status. Schneck et al. (2000, 2008) also reported that glucose severely impacts the FO component of the EOG in both diabetic and normoglycemic individuals. The EOG demonstrates a change in potential across the eye in response to light, and much like the dc-ERG, it measures the functional status of the RPE-photoreceptor complex in humans (Arden & Constable, 2006).
A preponderance of data exists to support the idea that a structural insult to the RPE as a result of hyperglycemia may underlie alterations observed in the ERG of diabetic mice (reviewed in Simo & Hernandez, 2009; Xu & Le, 2011). The RPE is a polarized monolayer that acts to separate the fenestrated choriocapillaris from the neural retina. It maintains this barrier function by the formation of tight junctions and adherent junctions, both of which can be altered in diabetes (Vinores et al., 1999; Wang et al., 2010; Xu & Le, 2011; Xu et al., 2011). Studies demonstrating the overexpression of tight junction proteins, increased transepithelial resistance (TER), and decreased dextran diffusion across the RPE of animal models of diabetes suggest that the RPE may employ a buffering mechanism to combat diabetic hyperglycemia (Villarroel et al., 2009a,b). Thus, stress to the RPE and its structural integrity in response to diabetes might underlie our observed reductions in the dc-ERG. Experiments using in vitro cell culture systems reveal that high glucose, chronic oxidative stress, and cytokine treatment each lead to decreases in the TER and reductions in tight junction proteins (Xu et al., 2011).
While establishment of a membrane potential and maintenance of the outer blood–retinal barrier is a key component of RPE function, another critical role of the RPE is to buffer the ion composition of the subretinal space, a requirement for proper photoreceptor function (Steinberg, 1985; Strauss, 2005). Each dc-ERG component is generated by the movement of ions across the polarized epithelium, as a secondary response to photoreceptor activity (Wu et al., 2004b). The c-wave is generated by hyperpolarization of the apical membrane (Steinberg et al., 1970; Schmidt & Steinberg, 1971), which occurs in response to the decrease in subretinal [K+] resulting from photoreceptor activation by light (Steinberg, 1985; Wu et al., 2004a,b) and a negative polarity slow PIII [resulting from Kir4.1 activity in Muller glia (Steinberg & Miller, 1973; Witkovsky et al., 1975; Oakley & Green, 1976; Kofuji et al., 2000)]. The FO is generated by a restoration in subretinal [K+] and the delayed hyperpolarization of the basal RPE membrane mediated by a [Cl−] conductance (Linsenmeier & Steinberg, 1982; Griff & Steinberg, 1984). The LP reflects the depolarization of the RPE basal membrane by a [Cl−]-based conductance (Linsenmeier & Steinberg, 1982; Gallemore & Steinberg, 1989, 1993; Fujii et al., 1992). The off response is generated at light offset by an unknown mechanism, which, in the mouse, changes polarity in a luminance-dependent fashion (Wu et al., 2004b; Samuels et al., 2010). These findings imply that alterations in expression, localization, and/or activity of a variety of ion channels can underlie the diabetes-induced RPE dysfunction observed here. While specific ion conductances definitively altered by diabetes and underlying the reductions in the dc-ERG have not yet been elucidated, as noted above, Schneck et al. (2000) have demonstrated that increased blood glucose levels strongly affected FO amplitude of patient EOGs, which is generated by photoreceptor-driven changes in RPE cell [Cl−] concentration.
Structural and/or functional damage to the RPE likely factors into our findings of reductions in dc-ERG components. It is likely that other metabolic abnormalities may also contribute. Alterations in timing and amplitude of the b-wave and OPs associated with diabetes have been shown to be reversible by pharmacological treatments including docosahexaenoic acid (DHA)/lutein (Arnal et al., 2009; Yee et al., 2010) and acetyl-l-carnitine (Lowitt et al., 1993). Other treatments such as lipoic acid (Johnsen-Soriano et al., 2008), topical ciliary neurotrophic factor (CNTF) (Aizu et al., 2003), propionyl-l-carnitine (Hotta et al., 1996), angiotensin-converting-enzyme (ACE) inhibition (Bui et al., 2003), protein kinase C (PKC) inhibitors (Nakamura et al., 1999), and induction of heat shock proteins (Biro et al., 1998; Nanasi & Jednakovits, 2001) have each also been shown to prevent delays and reductions in the OPs but not the b-wave. Comparison of results obtained using pharmacological therapies to those obtained using genetic deletion of the same enzyme likely will prove informative to identify the mechanisms by which therapies do and do not act.
With regard to AR, it was previously reported that administration of the AR inhibitor, sorbinil, or dietary supplementation with myo-inositol arrested the progressive deterioration of the c-wave in diabetes; however, these treatments did not prevent the initial c-wave defect (Pautler & Ennis, 1980; MacGregor & Matschinsky, 1985; MacGregor et al., 1986). These results suggest that two distinct insults may occur; an early reduction in c-wave amplitude, followed by a further decline if intervention is not taken. The reports evaluated the ERG at early time points following the onset of diabetes (2–19 weeks) in rat and rabbit models of diabetes. In comparison, our study assessed the mouse ERG at a single time point, 6 months following the onset of diabetes. Notably, the c-wave reduction noted here is comparable to the initial defect observed in the previous reports (Pautler & Ennis, 1980; MacGregor & Matschinsky, 1985; MacGregor et al., 1986) and the later decline is absent. This disparity may reflect differences between experimental protocols (species, method of diabetes induction). An alternate possibility is that the secondary reduction in c-wave amplitude in diabetic rats is due in part to formation of anterior cataracts (Pautler & Ennis, 1980), which is greatly lessened in diabetic mice.
A major clinical interest in ERG stems from the possibility that changes in retinal function might predict susceptibility to diabetic retinopathy. That remains an exciting area of clinical and translational research. We have shown here that dc-ERG defects are easily identified in diabetic mice, and current studies will define the time course over which these defects developed. We have also shown that AR does not appear to contribute to the ERG changes associated with diabetes. Our results, coupled with previous studies that demonstrated positive effects of AR inhibitors on retinal function, raise a possibility that these benefits might have been mediated by off-target effects of the drugs.
Acknowledgments
We would like to thank Gwen M. Sturgill-Short for technical assistance and reading of the manuscript. This study was supported by the Department of Veterans Affairs CY10-035 (I.S.S.), Foundation Fighting Blindness, Research to Prevent Blindness, National Institute of Heath grants R01 EY005856 (J.M.P.), and R01 EY00300 (T.S.K.).
References
- Aizu Y, Katayama H, Takahama S, Hu J, Nakagawa H, Oyanagi K. Topical instillation of ciliary neurotrophic factor inhibits retinal degeneration in streptozotocin-induced diabetic rats. Neuroreport. 2003;14:2067–2071. doi: 10.1097/00001756-200311140-00012. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Chen K, Reynolds AL, Waghorne N, O’Connor JJ, Kennedy BN. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Disease Models and Mechanisms. 2010;3:236–245. doi: 10.1242/dmm.003772. [DOI] [PubMed] [Google Scholar]
- Arden GB, Constable PA. The electro-oculogram. Progress in Retinal and Eye Research. 2006;25:207–248. doi: 10.1016/j.preteyeres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Arnal E, Miranda M, Johnsen-Soriano S, Alvarez-Nolting R, Diaz-Llopis M, Araiz J, Cervera E, Bosch-Morell F, Romero FJ. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Current Eye Research. 2009;34:928–938. doi: 10.3109/02713680903205238. [DOI] [PubMed] [Google Scholar]
- Ashizawa N, Yoshida M, Sugiyama Y, Akaike N, Ohbayashi S, Aotsuka T, Abe N, Fukushima K, Matsuura A. Effects of a novel potent aldose reductase inhibitor, GP-1447, on aldose reductase activity in vitro and on diabetic neuropathy and cataract formation in rats. Japanese Journal of Pharmacology. 1997;73:133–144. doi: 10.1254/jjp.73.133. [DOI] [PubMed] [Google Scholar]
- Barile GR, Rachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Investigative Ophthalmology and Visual Science. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metabolism Reviews. 2008;40:553–624. doi: 10.1080/03602530802431439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biersdorf WR, Malone JI, Pavan PR, Lowitt S. Cone electroretinograms and visual acuities of diabetic patients on sorbinil treatment. Documenta Ophthalmologica. 1988;69:247–254. doi: 10.1007/BF00154405. [DOI] [PubMed] [Google Scholar]
- Biro K, Palhalmi J, Toth AJ, Kukorelli T, Juhasz G. Bimoclomol improves early electrophysiological signs of retinopathy in diabetic rats. Neuroreport. 1998;9:2029–2033. doi: 10.1097/00001756-199806220-00022. [DOI] [PubMed] [Google Scholar]
- Bresnick GH, Korth K, Groo A, Palta M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Archives of Ophthalmology. 1984;102:1307–1311. doi: 10.1001/archopht.1984.01040031057023. [DOI] [PubMed] [Google Scholar]
- Bresnick GH, Palta M. Oscillatory potential amplitudes. Relation to severity of diabetic retinopathy. Archives of Ophthalmology. 1987;105:929–933. doi: 10.1001/archopht.1987.01060070065030. [DOI] [PubMed] [Google Scholar]
- Bui BV, Armitage JA, Tolcos M, Cooper ME, Vingrys AJ. ACE inhibition salvages the visual loss caused by diabetes. Diabetologia. 2003;46:401–408. doi: 10.1007/s00125-003-1042-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- Coupland SG. A comparison of oscillatory potential and pattern electroretinogram measures in diabetic retinopathy. Documenta Ophthalmologica. 1987;66:207–218. doi: 10.1007/BF00145234. [DOI] [PubMed] [Google Scholar]
- Fujii S, Gallemore RP, Hughes BA, Steinberg RH. Direct evidence for a basolateral membrane Cl− conductance in toad retinal pigment epithelium. The American Journal of Physiology. 1992;262:C374–383. doi: 10.1152/ajpcell.1992.262.2.C374. [DOI] [PubMed] [Google Scholar]
- Funada M, Okamoto I, Fujinaga Y, Yamana T. Effects of aldose reductase inhibitor (M79175) on ERG oscillatory potential abnormalities in streptozotocin fructose-induced diabetes in rats. Japanese Journal of Ophthalmology. 1987;31:305–314. [PubMed] [Google Scholar]
- Gallemore RP, Steinberg RH. Effects of DIDS on the chick retinal pigment epithelium. II. Mechanism of the light peak and other responses originating at the basal membrane. The Journal of Neuroscience. 1989;9:1977–1984. doi: 10.1523/JNEUROSCI.09-06-01977.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallemore RP, Steinberg RH. Light-evoked modulation of basolateral membrane Cl− conductance in chick retinal pigment epithelium: The light peak and fast oscillation. Journal of Neurophysiology. 1993;70:1669–1680. doi: 10.1152/jn.1993.70.4.1669. [DOI] [PubMed] [Google Scholar]
- Griff ER, Steinberg RH. Changes in apical [K+] produce delayed basal membrane responses of the retinal pigment epithelium in the gecko. The Journal of General Physiology. 1984;83:193–211. doi: 10.1085/jgp.83.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock HA, Kraft TW. Oscillatory potential analysis and ERGs of normal and diabetic rats. Investigative Ophthalmology and Visual Science. 2004;45:1002–1008. doi: 10.1167/iovs.03-1080. [DOI] [PubMed] [Google Scholar]
- Hardy KJ, Fisher C, Heath P, Foster DH, Scarpello JH. Comparison of colour discrimination and electroretinography in evaluation of visual pathway dysfunction in aretinopathic IDDM patients. The British Journal of Ophthalmology. 1995;79:35–37. doi: 10.1136/bjo.79.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers HG. [Increase of activity of glucose-6-phosphatase in intolerance to fructose] Revue Internationale d’Hepatologie. 1962;12:777–782. [PubMed] [Google Scholar]
- Ho HT, Chung SK, Law JW, Ko BC, Tam SC, Brooks HL, Knepper MA, Chung SS. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Molecular and Cellular Biology. 2000;20:5840–5846. doi: 10.1128/mcb.20.16.5840-5846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopigian K, Seiple W, Lorenzo M, Carr R. A comparison of photopic and scotopic electroretinographic changes in early diabetic retinopathy. Investigative Ophthalmology and Visual Science. 1992;33:2773–2780. [PubMed] [Google Scholar]
- Hotta N. New approaches for treatment in diabetes: Aldose reductase inhibitors. Biomedicine and Pharmacotherapy. 1995;49:232–243. doi: 10.1016/0753-3322(96)82629-1. [DOI] [PubMed] [Google Scholar]
- Hotta N, Koh N, Sakakibara F, Nakamura J, Hamada Y, Hara T, Fukasawa H, Kakuta H, Sakamoto N. Effect of propionyl-L-carnitine on oscillatory potentials in electroretinogram in streptozotocin-diabetic rats. European Journal of Pharmacology. 1996;311:199–206. doi: 10.1016/0014-2999(96)00420-7. [DOI] [PubMed] [Google Scholar]
- Hotta N, Koh N, Sakakibara F, Nakamura J, Hamada Y, Hara T, Takeuchi N, Inukai S, Kasama N, Fukasawa H, Kakuta H. An aldose reductase inhibitor, TAT, prevents electroretinographic abnormalities and ADP-induced hyperaggregability in streptozotocin-induced diabetic rats. European Journal of Clinical Investigation. 1995a;25:948–954. doi: 10.1111/j.1365-2362.1995.tb01972.x. [DOI] [PubMed] [Google Scholar]
- Hotta N, Koh N, Sakakibara F, Nakamura J, Hamada Y, Naruse K, Sasaki H, Mizuno K, Matsubara A, Kakuta H, Fukasawa H, Sakamoto N. Effect of an aldose reductase inhibitor, SNK-860, on deficits in the electroretinogram of diabetic rats. Experimental Physiology. 1995b;80:981–989. doi: 10.1113/expphysiol.1995.sp003909. [DOI] [PubMed] [Google Scholar]
- Hotta N, Koh N, Sakakibara F, Nakamura J, Hara T, Hamada Y, Fukasawa H, Kakuta H, Sakamoto N. Effect of an aldose reductase inhibitor on abnormalities of electroretinogram and vascular factors in diabetic rats. European Journal of Pharmacology. 1997;326:45–51. doi: 10.1016/s0014-2999(97)00135-0. [DOI] [PubMed] [Google Scholar]
- Johnsen-Soriano S, Garcia-Pous M, Arnal E, Sancho-Tello M, Garcia-Delpech S, Miranda M, Bosch-Morell F, Diaz-Llopis M, Navea A, Romero FJ. Early lipoic acid intake protects retina of diabetic mice. Free Radical Research. 2008;42:613–617. doi: 10.1080/10715760802206791. [DOI] [PubMed] [Google Scholar]
- Juen S, Kieselbach GF. Electrophysiological changes in juvenile diabetics without retinopathy. Archives of Ophthalmology. 1990;108:372–375. doi: 10.1001/archopht.1990.01070050070033. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: Phenotypic impact in retina. The Journal of Neuroscience. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RD, Kwaan HC, Dobbie JG, Fetkenhour CL, Traisman HS, Kramer C. Studies of retinopathy and the plasma cofactor of platelet hyperaggregation in type 1 (insulin-dependent) diabetic children. Diabetologia. 1982;22:445–449. doi: 10.1007/BF00282588. [DOI] [PubMed] [Google Scholar]
- Linsenmeier RA, Steinberg RH. Origin and sensitivity of the light peak in the intact cat eye. The Journal of Physiology. 1982;331:653–673. doi: 10.1113/jphysiol.1982.sp014396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovasik JV, Spafford MM. An electrophysiological investigation of visual function in juvenile insulin-dependent diabetes mellitus. American Journal of Optometry and Physiological Optics. 1988;65:236–253. doi: 10.1097/00006324-198804000-00002. [DOI] [PubMed] [Google Scholar]
- Lowitt S, Malone JI, Salem A, Kozak WM, Orfalian Z. Acetyl-L-carnitine corrects electroretinographic deficits in experimental diabetes. Diabetes. 1993;42:1115–1118. doi: 10.2337/diab.42.8.1115. [DOI] [PubMed] [Google Scholar]
- MacGregor LC, Matschinsky FM. Treatment with aldose reductase inhibitor or with myo-inositol arrests deterioration of the electroretinogram of diabetic rats. The Journal of Clinical Investigation. 1985;76:887–889. doi: 10.1172/JCI112048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor LC, Matschinsky FM. Experimental diabetes mellitus impairs the function of the retinal pigmented epithelium. Metabolism: Clinical and Experimental. 1986;35:28–34. doi: 10.1016/0026-0495(86)90184-8. [DOI] [PubMed] [Google Scholar]
- MacGregor LC, Rosecan LR, Laties AM, Matschinsky FM. Altered retinal metabolism in diabetes. I. Microanalysis of lipid, glucose, sorbitol, and myo-inositol in the choroid and in the individual layers of the rabbit retina. The Journal of Biological Chemistry. 1986;261:4046–4051. [PubMed] [Google Scholar]
- Matsui T, Nakamura Y, Ishikawa H, Matsuura A, Kobayashi F. Pharmacological profiles of a novel aldose reductase inhibitor, SPR-210, and its effects on streptozotocin-induced diabetic rats. Japanese Journal of Pharmacology. 1994;64:115–124. doi: 10.1254/jjp.64.115. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Kato K, Hamada Y, Nakayama M, Chaya S, Nakashima E, Naruse K, Kasuya Y, Mizubayashi R, Miwa K, Yasuda Y, Kamiya H, Ienaga K, Sakakibara F, Koh N, Hotta N. A protein kinase C-beta-selective inhibitor ameliorates neural dysfunction in streptozotocin-induced diabetic rats. Diabetes. 1999;48:2090–2095. doi: 10.2337/diabetes.48.10.2090. [DOI] [PubMed] [Google Scholar]
- Nanasi PP, Jednakovits A. Multilateral in vivo and in vitro protective effects of the novel heat shock protein coinducer, bimoclomol: Results of preclinical studies. Cardiovascular Drug Reviews. 2001;19:133–151. doi: 10.1111/j.1527-3466.2001.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Oakley B, II, Green DG. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. Journal of Neurophysiology. 1976;39:1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Pautler EL, Ennis SR. The effect of induced diabetes on the electroretinogram components of the pigmented rat. Investigative Ophthalmology and Visual Science. 1980;19:702–705. [PubMed] [Google Scholar]
- Phipps JA, Feener EP. The kallikrein-kinin system in diabetic retinopathy: Lessons for the kidney. Kidney International. 2008;73:1114–1119. doi: 10.1038/ki.2008.9. [DOI] [PubMed] [Google Scholar]
- Phipps JA, Fletcher EL, Vingrys AJ. Paired-flash identification of rod and cone dysfunction in the diabetic rat. Investigative Ophthalmology and Visual Science. 2004;45:4592–4600. doi: 10.1167/iovs.04-0842. [DOI] [PubMed] [Google Scholar]
- Phipps JA, Yee P, Fletcher EL, Vingrys AJ. Rod photoreceptor dysfunction in diabetes: Activation, deactivation, and dark adaptation. Investigative Ophthalmology and Visual Science. 2006;47:3187–3194. doi: 10.1167/iovs.05-1493. [DOI] [PubMed] [Google Scholar]
- Samuels IS, Sturgill GM, Grossman GH, Rayborn ME, Hollyfield JG, Peachey NS. Light-evoked responses of the retinal pigment epithelium: Changes accompanying photoreceptor loss in the mouse. Journal of Neurophysiology. 2010;104:391–402. doi: 10.1152/jn.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Steinberg RH. Rod-dependent intracellular responses to light recorded from the pigment epithelium of the cat retina. The Journal of Physiology. 1971;217:71–91. doi: 10.1113/jphysiol.1971.sp009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck ME, Fortune B, Adams AJ. The fast oscillation of the electrooculogram reveals sensitivity of the human outer retina/retinal pigment epithelium to glucose level. Vision Research. 2000;40:3447–3453. doi: 10.1016/s0042-6989(00)00173-5. [DOI] [PubMed] [Google Scholar]
- Schneck ME, Shupenko L, Adams AJ. The fast oscillation of the EOG in diabetes with and without mild retinopathy. Documenta Ophthalmologica. 2008;116:231–236. doi: 10.1007/s10633-007-9088-3. [DOI] [PubMed] [Google Scholar]
- Shirao Y, Kawasaki K. Electrical responses from diabetic retina. Progress in Retinal and Eye Research. 1998;17:59–76. doi: 10.1016/s1350-9462(97)00005-0. [DOI] [PubMed] [Google Scholar]
- Simo R, Hernandez C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care. 2009;32:1556–1562. doi: 10.2337/dc09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RH. Interactions between the retinal pigment epithelium and the neural retina. Documenta Ophthalmologica. 1985;60:327–346. doi: 10.1007/BF00158922. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Miller S. Aspects of electrolyte transport in frog pigment epithelium. Experimental Eye Research. 1973;16:365–372. doi: 10.1016/0014-4835(73)90130-9. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Schmidt R, Brown KT. Intracellular responses to light from cat pigment epithelium: Origin of the electroretinogram c-wave. Nature. 1970;227:728–730. doi: 10.1038/227728a0. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiological Reviews. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Survey of Ophthalmology. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Experimental Eye Research. 2009a;89:913–920. doi: 10.1016/j.exer.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. High glucose concentration leads to differential expression of tight junction proteins in human retinal pigment epithelial cells. Endocrinologia y Nutricion. 2009b;56:53–58. doi: 10.1016/S1575-0922(09)70552-2. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Documenta Ophthalmologica. 1999;97:217–228. doi: 10.1023/a:1002136712070. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Dudek FE, Ripps H. Slow PIII component of the carp electroretinogram. The Journal of General Physiology. 1975;65:119–134. doi: 10.1085/jgp.65.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VH, Bui BV, Vingrys AJ. Clinical and experimental links between diabetes and glaucoma. Clinical and Experimental Optometry. 2011;94:4–23. doi: 10.1111/j.1444-0938.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Kofuji P, Peachey NS. Contribution of Kir4.1 to the mouse electroretinogram. Molecular Vision. 2004a;10:650–654. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Peachey NS, Marmorstein AD. Light-evoked responses of the mouse retinal pigment epithelium. Journal of Neurophysiology. 2004b;91:1134–1142. doi: 10.1152/jn.00958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Investigative Ophthalmology and Visual Science. 2011;52:2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H-Z, Song Z, Fu S, Zhu M, Le Y-Z. RPE barrier breakdown in diabetic retinopathy: Seeing is believing. Journal of Ocular Biology, Diseases, and Informatics. 2011;4:83–92. doi: 10.1007/s12177-011-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee P, Weymouth AE, Fletcher EL, Vingrys AJ. A role for omega-3 polyunsaturated fatty acid supplements in diabetic neuropathy. Investigative Ophthalmology and Visual Science. 2010;51:1755–1764. doi: 10.1167/iovs.09-3792. [DOI] [PubMed] [Google Scholar]
- Yonemura D. Electrophysiological study on activities of neuronal and non-neuronal retinal elements in man with reference to its clinical application. Japanese Journal of Ophthalmology. 1978;22:195–213. [PubMed] [Google Scholar]
- Yonemura D, Aoki T, Tsuzuki K. Electroretinogram in diabetic retinopathy. Archives of Ophthalmology. 1962;68:19–24. doi: 10.1001/archopht.1962.00960030023005. [DOI] [PubMed] [Google Scholar]
- Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Kern TS, Ball S, Berkowitz BA. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]