Abstract
Ex ovo omnia—all animals come from eggs—this statement made in 1651 by the English physician William Harvey marks a seminal break with the doctrine that all essential characteristics of offspring are contributed by their fathers, while mothers contribute only a material substrate. More than 360 years later, we now have a comprehensive understanding of how haploid gametes are generated during meiosis to allow the formation of diploid offspring when sperm and egg cells fuse. In most species, immature oocytes are arrested in prophase I and this arrest is maintained for few days (fruit flies) or for decades (humans). After completion of the first meiotic division, most vertebrate eggs arrest again at metaphase of meiosis II. Upon fertilization, this second meiotic arrest point is released and embryos enter highly specialized early embryonic divisions. In this review, we discuss how the standard somatic cell cycle is modulated to meet the specific requirements of different developmental stages. Specifically, we focus on cell cycle regulation in mature vertebrate eggs arrested at metaphase II (MII-arrest), the first mitotic cell cycle, and early embryonic divisions.
Keywords: APC/C, cell cycle, early embryo, meiosis, MPF
See the Glossary for abbreviations used in this article.
Glossary.
- APC/C
anaphase-promoting complex/cyclosome
- CAK
CDK-activating kinase
- Cdk1
cyclin-dependent kinase 1
- CKI
cyclin-dependent kinase inhibitor
- CPE
cytoplasmic polyadenylation element
- CPEB
cytoplasmic polyadenylation element binding protein
- D-box
destruction box
- ICM
inner cell mass
- MII
meiosis II
- MII-arrest
arrest in metaphase of meiosis II
- MBT
mid-blastula transition
- MPF
maturation-promoting factor
- p90Rsk
ribosomal S6 kinase
- Plk1
Polo-like kinase 1
- PKA
protein kinase A
- PP2A
protein phosphatase 2 A
- RL-tail
C-terminal arginine-leucine dipeptide in XErp1/Emi2
- SAC
spindle assembly checkpoint
- UPS
ubiquitin proteasome system
- ZBR
zinc-binding region in XErp1/Emi2 and Emi1
- ZGA
zygotic genome activation
Introduction
Metaphase II arrest
The arrest in metaphase of meiosis II (MII-arrest) of mature vertebrate eggs is characterized biochemically by high activity of the cyclin-dependent kinase 1 (Cdk1) and morphologically by chromosomes aligned on a bipolar spindle. This arrest point serves to coordinate completion of meiosis with fertilization (Figure 1). To meet this challenge, the MII-arrest has to be highly robust to prevent parthenogenesis, while at the same time being highly responsive to quickly allow sister chromatid segregation upon fertilization.
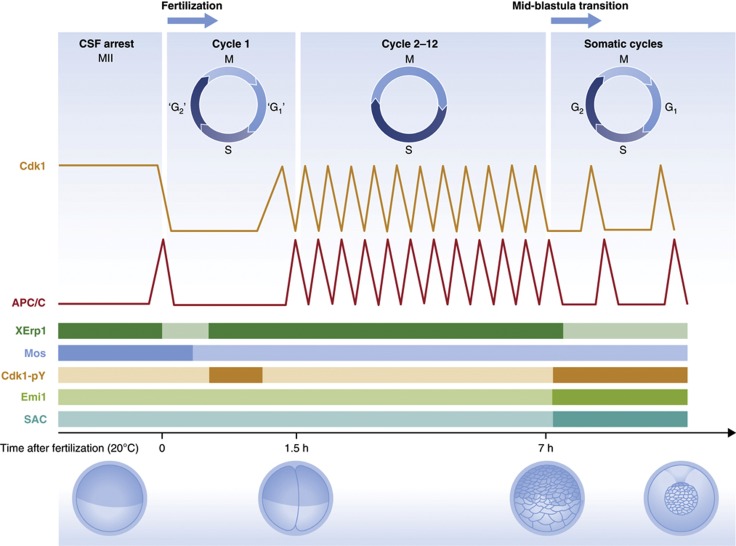
Figure 1.
Schematic representation of cell cycle regulation during Xenopus early development. Illustrated are specialized cell cycle types, major developmental transitions and oocyte/embryo stages, as well as oscillations of Cdk1–cyclin B and APC/C activity. Bars in the lower half depict activity levels for XErp1/Emi2, c-Mos/MAPK, Emi1 and the spindle assembly checkpoint (SAC), as well as inhibitory Thr-14/Tyr-15 phosphorylation of Cdk1 (Cdk1-pY). See text for details.
The first embryonic division cycle
The first mitotic division in sea urchin, nematodes, frogs and mice embryos is characterized by its prolonged duration, as compared to subsequent divisions (Figure 1). Except for sea urchin, where MII is already accomplished in the unfertilized egg, this extra time seems to be required to allow completion of the second meiotic division and decondensation of the newly received sperm chromatin. Furthermore, male and female pronuclei have to fuse during the first division to form the diploid genome. In sea urchin, Caenorhabditis elegans, and Xenopus laevis, this fusion happens in interphase prior to the first mitosis (Longo and Anderson, 1968; Strome and Wood, 1983; Ubbels et al, 1983), while in mammals the two pronuclei independently undergo DNA replication and nuclear envelope breakdown before their chromosomes eventually intermingle during the first mitosis (Das and Barker, 1976; Ciemerych and Czolowska, 1993; Mayer et al, 2000; Bomar et al, 2002).
Early embryonic divisions
After the prolonged first mitotic cell cycle, embryos enter a series of rapid cleavage cycles, during which cell numbers increase in the absence of significant cell growth (Figure 1). These divisions can be asynchronous—as in mammals and nematodes—but in most metazoans are highly synchronous. These rapid cell cycles are specific for the early embryo, and in most metazoans result in the formation of the blastula, or blastocyst in the case of mammals (Newport and Kirschner, 1982a; Boiani and Scholer, 2005).
Despite the differences in their setup, all these divisions have in common that entry into M-phase is driven by the activity of maturation-promoting factor (MPF), whose core components are Cdk1 and cyclin B. Thus, in order to adjust the timing of Cdk1 activation and inactivation to specific developmental stages, the regulatory circuits acting on Cdk1/cyclin B need to be modulated.
Regulatory circuits acting on Cdk1
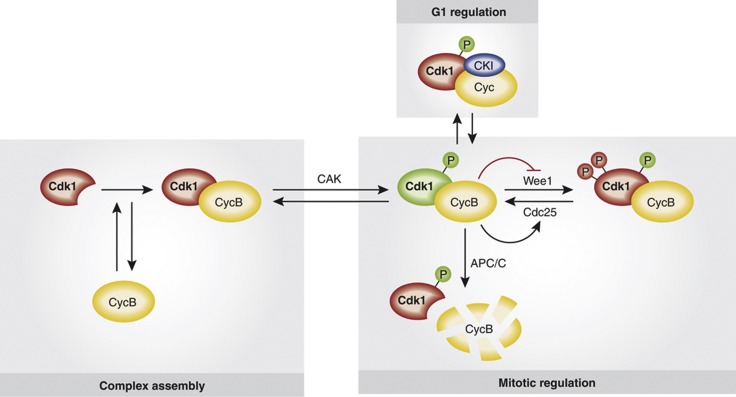
In M-phase, Cdk1 activity depends on association with cyclin B (Figure 2). Cyclin B levels are themselves controlled by regulated cyclin B synthesis and degradation. Exit from M-phase is triggered by ubiquitin-mediated cyclin B proteolysis, which is controlled by the E3 ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C) and its substrate-binding activators Cdc20 or Cdh1 (Peters, 2006; Primorac and Musacchio, 2013). In addition to cyclin B association, full Cdk1 activation also involves phosphorylation events. Stimulating phosphorylation of a conserved threonine in the activation loop (Krek and Nigg, 1992) by Cdk-activating kinases occurs in an unregulated, constitutive manner (Fisher and Morgan, 1994; Tassan et al, 1994). On the other hand, phosphorylation of Cdk1 in the ATP-binding pocket (Thr-14 and Tyr-15) by vertebrate Wee1 and Myt1 kinases results in inactive MPF, and this negative effect on Cdk1 activation is antagonized by the Cdc25 family of phosphatases (Dunphy and Kumagai, 1991) (Figure 2). Following exit from M-phase, Cdk1 activity remains suppressed during G1 phase by the association with Cdk-inhibitory proteins, a regulatory mechanism that will not be discussed further here (for a review see Vidal and Koff, 2000).
Figure 2.
Cdk1 regulatory mechanisms. Red background colour indicates inactive Cdk1 or inhibitory phosphorylation (P), while green colour denotes active Cdk1 or activating phosphorylation. CycB and Cyc denote cyclin B and any cyclin, respectively; CKI, Cdk inhibitory protein; CAK, Cdk-activating kinase. See text for details.
M-phase onset requires both MPF activation and inactivation of MPF-antagonizing phosphatases
At the onset of M-phase, Cdk1 is activated in a switch-like manner via an auto-amplification loop, in which Cdk1 inactivates its inhibitors (the Wee1 and Myt1 kinases) and activates it’s the counteracting Cdc25 phosphatases (Figure 2). In addition to swift MPF activation, timely entry into mitosis, however, also requires effective inactivation of phosphatases that would counteract Cdk1-mediated phosphorylation events. In particular, recent studies revealed that Cdk1 itself mediates inactivation of its antagonist protein phosphatase 2A (PP2A). Specifically, Cdk1 activates Greatwall kinase, which in turn phosphorylates and primes Ensa/Arpp19, a protein inhibitor of the B55 subtype of PP2A (Yu et al, 2006; Mochida et al, 2009; Vigneron et al, 2009; Gharbi-Ayachi et al, 2010; Mochida et al, 2010). Thus, Cdk1 activation ultimately flips the switch from a state of MPF substrate hypophosphorylation to one where MPF substrates are maximally phosphorylated.
MII-arrest: Putting the cell cycle on hold
The proto-oncogene c-Mos and the MAP kinase pathway
MII-arrest of mature vertebrate eggs depends on cytostatic factor (CSF), an activity initially described as being present in the cytoplasm of mature Rana pipiens eggs and capable of inducing a metaphase-like arrest when injected into two-cell embryos (Masui and Markert, 1971). Based on their studies, Masui and Markert (1971) framed three criteria that any candidate factors proposed to be CSF would have to fulfill the following conditions: (i) accumulation during oocyte maturation, with maximal levels in MII, (ii) ability to cause a metaphase-like arrest when injected into embryonic blastomeres and (iii) inactivation upon fertilization.
The first factor found to meet all three criteria was the proto-oncogene c-Mos, which is newly synthesized in maturing Xenopus oocytes, causes a CSF-like arrest when expressed in embryos, and is degraded shortly after fertilization (Sagata et al, 1989a,b, 109). Further elegant biochemical and pharmacological studies demonstrated that the serine–threonine protein kinase c-Mos triggers a MEK/MAP kinase (MAPK) signalling cascade that is essential for both the establishment and maintenance of MII-arrest in Xenopus oocytes (Sagata et al, 1988; Haccard et al, 1993; Kosako et al, 1994; Abrieu et al, 1996; Bhatt and Ferrell, 1999). Subsequent work identified the p90 ribosomal S6 kinase (p90Rsk) as the key MAPK substrate relevant for CSF arrest, indicating that MII-arrest is mediated by a strictly linear c-Mos/MEK/MAPK/p90Rsk signalling cascade in Xenopus eggs (Gross et al, 1999). The situation seems to be more complex in mice, since c-Mos-deficient eggs, although eventually undergoing parthenogenic activation, initially manage to establish a transient CSF arrest (Colledge et al, 1994; Hashimoto et al, 1994; Choi et al, 1996; Verlhac et al, 1996). Here, c-Mos may, therefore, not be strictly essential for establishment of the second meiotic arrest, but may only be required for its maintenance. Furthermore, mouse oocytes lacking all three mammalian homologues of p90Rsk exhibit a normal CSF arrest (Dumont et al, 2005), suggesting that mouse c-Mos functions to impose a robust MII-arrest in a manner independent of p90Rsk kinases. Instead, mitogen- and stress-activated protein kinase 1 (Msk1) may be the relevant downstream MAPK target in mouse oocytes (Miyagaki et al, 2011).
The APC/C inhibitor XErp1/Emi2 and the c-Mos/MAPK/p90Rsk signalling cascade
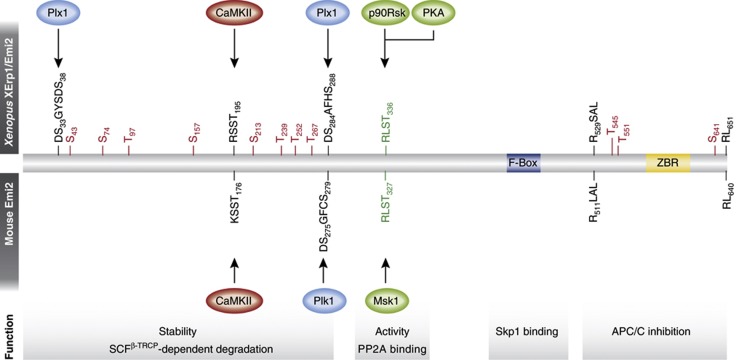
How then does the c-Mos/MAPK pathway transmit its inhibitory signal to the APC/C? Data from Xenopus implicated components of the spindle assembly checkpoint (SAC) as links between p90Rsk and the APC/C, since the SAC kinase Bub1 was identified as a p90Rsk substrate, and since depletion of Bub1 as well as SAC components Mad1 and Mad2 appeared to interfere with the CSF arrest (Schwab et al, 2001; Tunquist et al, 2002). However, how the SAC would mechanistically mediate CSF arrest remained elusive, since (i) there is no conclusive explanation of how SAC silencing could be coordinated with fertilization, (ii) a robust CSF arrest can be mounted in Xenopus egg extract lacking DNA and, therefore, also lacking kinetochores from which SAC signals could originate and (iii) the SAC is completely dispensable for the MII-arrest in mouse oocytes (Tsurumi et al, 2004). The gap between the c-Mos/MAPK/p90Rsk pathway and the APC/C was filled by the identification of XErp1 (Xenopus Emi1-related protein 1) or Emi2, whose function requires activation by p90Rsk (Figure 3). XErp1/Emi2, a highly conserved F-box protein, accumulates in maturing oocytes (CSF criterion I), mediates the MII-arrest in mature eggs by directly inhibiting the APC/C, and causes a cell cycle arrest when injected into Xenopus embryos (CSF criterion II) (Schmidt et al, 2005). XErp1/Emi2 is phosphorylated by p90Rsk on Ser-335, Thr-336, Ser-342 and Ser-344, and subsequently associates with the B’56 subtype of PP2A (PP2A-B’56), which in turn removes Cdk1-mediated inhibitory phosphorylation from amino- and carboxy-terminal regions of XErp1/Emi2 (Inoue et al, 2007; Nishiyama et al, 2007a; Wu et al, 2007a,b, 136; Isoda et al, 2011) (Figure 3). Phosphorylation of the XErp1/Emi2 C-terminal region interferes with its ability to bind and inhibit the APC/C (Figure 4A) (Wu et al, 2007a), similar to the situation of the activator Cdc20 that can also only associate with the APC/C when dephosphorylated (Labit et al, 2012). Phosphorylation in the amino-terminal Cdk1 site cluster, on the other hand, controls XErp1/Emi2 stability (Figure 4A), by serving as priming event for the recruitment of Polo-like kinase 1 (Plk1). Further phosphorylation by this kinase creates a phosphorylation-dependent recognition motif (or phosphodegron) for the ubiquitin ligase SCFβ-TRCP, which then ubiquitylates and targets XErp1/Emi2 for proteasomal degradation (Wu et al, 2007a; Isoda et al, 2011). Thus, p90Rsk-mediated recruitment of PP2A-B’56 downstream of the c-Mos/MAPK pathway activates XErp1/Emi2, both by stabilizing it and by directly impinging on its APC/C inhibitory properties.
Figure 3.
XErp1/Emi2 regulation in Xenopus and mouse. Depicted is the primary sequence structure of frog (upper) and mouse (lower) XErp1/Emi2, with functional elements and sites of regulatory modifications spaced according to the complete Xenopus protein. Red and green letters denote sites with negative and positive effects on XErp1’s function, respectively. Red S/T sites in Xenopus XErp1/Emi2 are targets for inhibitory phosphorylation by Cdk1, while ‘DS…’ sequences denote phosphodegrons regulating XErp1/Emi2 stability. Plx1, Xenopus Polo-like kinase 1; ZBR, zinc-binding region. See text for details.
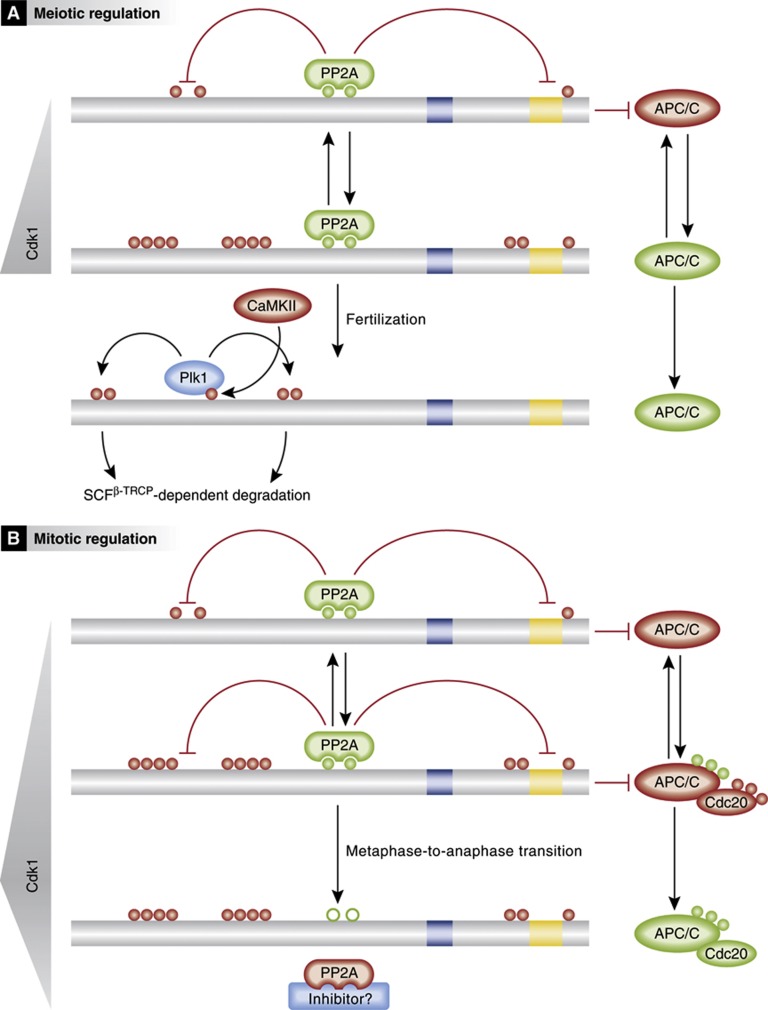
Figure 4.
XErp1/Emi2 and APC/C regulation in meiosis (A) and mitosis (B). Bars indicate XErp1/Emi2 primary structure and sequence elements as in Figure 3. Inhibitory and activating phosphorylation is depicted by red and green circles, respectively. Inactive and active APC/C or PP2A are indicated by red and green background colours, respectively. See text for details.
Although these data explain the essential CSF-arrest role of c-Mos, it would appear somewhat paradoxical that Cdk1 inactivates CSF, a factor that serves to impose robust MII-arrest in the presence of high Cdk1 activity. However, the cybernetic regulation of Cdk1-mediated XErp1/Emi2 inactivation (APC/C activation) and PP2A-B’56-mediated XErp1/Emi2 activation (APC/C inactivation) provides a mechanism to maintain Cdk1 activity at a high but constant level during MII-arrest, despite continuous cyclin B synthesis (Kubiak et al, 1993; Ledan et al, 2001; Yamamoto et al, 2005); once cyclin B levels exceed a certain upper threshold, Cdk1 transiently activates cyclin B degradation by the APC/C, to decrease cyclin B amounts to an optimal plateau with sufficiently strong MPF activity for mounting a stable CSF arrest and sufficiently low cyclin B levels to allow rapid release upon fertilization (Figure 4A). In mice, the essential function of both Emi2 and PP2A for CSF arrest seems conserved, as their knockdown or pharmacological inhibition results in parthenogenesis (Madgwick et al, 2006; Shoji et al, 2006; Chang et al, 2011). Consistent with the dispensability of mouse p90Rsk kinases for MII-arrest, Msk1 appears to be the key c-Mos/MAPK pathway downstream target for Emi2 activation in mouse oocytes (Figure 3) (Miyagaki et al, 2011).
Inhibition of the APC/C by XErp1/Emi2
The molecular mechanism of APC/C inhibition by XErp1/Emi2 is still not fully understood. Three motifs located in the C terminus of XErp1/Emi2 have been identified as being essential for APC/C inhibition: a destruction box (D-box), the zinc-binding region (ZBR) and the two C-terminal amino acids arginine and leucine (‘RL-tail’) (Schmidt et al, 2005; Ohe et al, 2010; Tang et al, 2010). The interaction between XErp1/Emi2 and the APC/C seems to be primarily mediated via the RL-tail, which binds to the APC/C at a site distinct from the one interacting with the related IR-tail of the APC/C activator Cdc20 (Ohe et al, 2010; Tang et al, 2010). Based on the observation that the APC/C inhibitors Emi1 (Miller et al, 2006), Acm1 (Choi et al, 2008; Ostapenko et al, 2008; Burton et al, 2011) and BubR1 (Burton and Solomon, 2007; Malureanu et al, 2009)—all of which contain D-box-like motifs—act by competing with substrates for APC/C binding, XErp1/Emi2 may also function as a pseudosubstrate inhibitor. Recent work, however, suggests that even Emi1 only weakly competes with substrates for APC/C binding, but rather blocks ubiquitin chain elongation by the E2 enzymes UbcH10 and Ube2S (Wang and Kirschner, 2013). Intriguingly, the ZBR motif of Emi1 prevents polyubiquitylation by UbcH10, while the C-terminal tail antagonizes Ube2S-mediated polyubiquitylation by competitively preventing the association of this E2 to APC/C. These Emi1 inhibitory domains are highly conserved in XErp1/Emi2 and essential for its inhibitory activity as well; therefore, it is likely that Emi1 and XErp1/Emi2 employ similar modes of APC/C inhibition. However, it remains puzzling how substoichiometric amounts of XErp1/Emi2 could efficiently prevent substrate polyubiquitylation. A possible explanation could be that only a part of the APC/C is in an active configuration (i.e., activator-bound, core subunit-phosphorylated) and XErp1/Emi2 can efficiently inhibit this fraction of active complexes. Alternatively, XErp1/Emi2 could have additional functions to inhibit the APC/C. It has been speculated that XErp1/Emi2 itself might act, in a ZBR-dependent manner, as a ubiquitin ligase to catalytically inhibit ubiquitin transfer to APC/C-bound substrates (Tang et al, 2010). However, it remains to be determined whether the observed ubiquitylation of XErp1/Emi2 is indeed a reflection of XErp1/Emi2 auto-ubiquitylation activity (Tang et al, 2010) or simply mediated by the APC/C (Hörmanseder et al, 2011).
While CSF-mediated APC/C inhibition is the central component of MII-arrest, additional mechanisms operate to support high MPF activity in mature eggs. In Xenopus, depletion of Cdc25C phosphatase (the only Cdc25 isoform present in CSF extract) results in inhibitory Thr-14/Tyr-15 phosphorylation of Cdk1, as well as in dephosphorylation of MPF substrates, such as Cdc27/Apc3 and XErp1/Emi2 (Lorca et al, 2010). As described above, this relieve of XErp1/Emi2 from Cdk1-mediated inhibitory effects efficiently inhibits the APC/C (Wu et al, 2007b), leading to loss of the meiotic state in the absence of cyclin B destruction under these conditions. These data suggest that during the MII-arrest in mature Xenopus eggs, Cdc25 activity is continuously required to maintain Cdk1 in its active state (Gautier et al, 1991; Izumi et al, 1992; Smythe and Newport, 1992). High Cdk1 activity in turn ensures maximal overall phosphorylation of MPF substrates by inhibiting PP2A-B55 via the Greatwall kinase-Ensa/Arpp19 pathway (Yu et al, 2006; Gharbi-Ayachi et al, 2010; Mochida et al, 2010).
Inactivation of XErp1/Emi2 at fertilization
The reproductive capacity of a mature egg depends not only on robust MII-arrest, but also on the ability to quickly escape from this arrest point upon fertilization. Therefore, sperm entry and CSF inactivation need to be precisely coordinated. Fertilization triggers a single (Xenopus) or multiple (mammals) calcium waves that quickly sweep across the egg (Kubota et al, 1987; Lawrence et al, 1997; Runft et al, 1999; Nixon et al, 2002). The calcium-sensitive signalling molecule calmodulin then transmits this transient calcium peak towards two independent signalling pathways. In one of them, activation of the phosphatase PP2B/calcineurin initiates global dephosphorylation of MPF substrates, such as the APC/C subunit Cdc27/Apc3, and its activator Cdc20 (Mochida and Hunt, 2007; Nishiyama et al, 2007b). As calcineurin inhibition delays calcium-triggered cyclin B degradation in Xenopus egg extract, the removal of inhibitory Cdc20 phosphorylation may directly contribute to APC/C activation (Labit et al, 2012). On the other hand, calcineurin appears to be dispensable for exit from the MII-arrest in mice (Suzuki et al, 2010) and, therefore, further studies are required to dissect how global dephosphorylation of MPF substrates is initiated in fertilized mouse oocytes.
The second calcium-triggered event is activation of calcium-/calmodulin-dependent kinase II (CaMKII) (Lorca et al, 1993, 1994; Dupont, 1998; Markoulaki et al, 2003), which itself initiates two independent processes, namely meiotic spindle depolymerization at anaphase onset via microtubule stability reduction (Reber et al, 2008), and CSF arrest release by targeting XErp1/Emi2 for degradation (Figure 4A). Specifically, CaMKII phosphorylation of XErp1/Emi2 on Thr-195 serves as a priming event for subsequent Plk1 docking and XErp1/Emi2 phosphorylation at Ser-33 and Ser-38 to again create a phosphodregron for SCFβ-TRCP-mediated ubiquitylation (Figure 3) (Liu and Maller, 2005; Rauh et al, 2005). In this case, fertilization triggers the complete and efficient destruction of XErp1/Emi2 via SCFβ-TRCP, in contrast to the homeostatic XErp1/Emi2 inactivation described above in response to transiently elevated Cdk1 activity levels during MII-arrest. In this way, XErp1/Emi2 also conforms to the third CSF criterion of Masui and Markert (1971), that is, inactivation at fertilization. Notably, XErp1/Emi2 is also subject to ubiquitylation by the APC/C (Hörmanseder et al, 2011). Unlike the SCFβ-TRCP-mediated ubiquitylation, APC/C in concert with its E2 UbcX/UbcH10 catalyses a non-proteolytic ubiquitylation of XErp1/Emi2, which interferes with its ability to inhibit the APC/C. This positive feedback loop could accelerate APC/C activation once calcium initiates the liberation of the APC/C from XErp1/Emi2-mediated inhibition and, thereby, contribute to switch-like onset of anaphase on fertilization.
Results from mouse oocytes suggest that the principle of calcium-induced Emi2 destruction is conserved between frogs and mammals (Liu andand Maller, 2005; Rauh et al, 2005; Madgwick et al, 2006; Shoji et al, 2006; Jones, 2007). However, the molecular mechanism seems to be more complex, because mouse oocytes—unlike Xenopus eggs—do not exit from the MII-arrest upon expression of constitutively active CaMKII (Suzuki et al, 2010). Nevertheless, mouse eggs deficient for CaMKIIγ fail to exit from the CSF arrest, suggesting that CaMKII is necessary but not sufficient for meiosis resumption upon fertilization (Chang et al, 2009; Backs et al, 2010). Identification of additionally required factors is therefore expected to provide important insights into how the MII-arrest is released in mammals.
The first embryonic division
In most metazoan organisms, the first cell cycle following fertilization is characterized by its prolonged duration compared to subsequent divisions. In Xenopus, the first cell cycle takes about 90 min, whereas the following 11 cleavage cycles each last for only about 30 min (Hara et al, 1980; Newport and Kirschner, 1982a). In mouse, the length of the first and second mitotic division is comparable, but metaphase duration is shortened from 120 min in the first cell cycle to 70 min in the second one (Ciemerych et al, 1999). An explanation for this extension may lie in the fact that the first division cycle has to fulfill an array of specialized functions. Among them is the completion of the female genome’s second meiotic division, accompanied by extrusion of the second polar body. Additionally, the specialized chromatin of the incoming sperm has to be adjusted to support embryonic development. The process starts with incorporation of the sperm nucleus into the egg, and the breakdown of its nuclear envelope. Following decondensation of the highly compact sperm chromatin, associated with replacement of protamine by somatic histones (Rodman et al, 1981; Santos et al, 2002), the nuclear envelope reassembles to form swollen male pronuclei (Longo, 1985; Katagiri and Ohsumi, 1994). DNA replication eventually initiates after decondensation of both male and female chromatin (Luthardt and Donahue, 1973; Bouniol-Baly et al, 1997; Ferreira and Carmo-Fonseca, 1997).
Inhibitory phosphorylation of Cdk1
While XErp1/Emi2, which following its complete degradation upon fertilization re-accumulates during the first mitotic division cycle, could in principle be well-placed to contribute to its increased length, there are currently no experimental data to support this hypothesis. Instead, the extended duration of the first division in Xenopus can at least in part be attributed to delayed activation of Cdk1 caused by inhibitory Tyr-14/Thr-15 phosphorylation. During the first mitotic division, the responsible inhibitory kinases Wee1 and Myt1 appear not to be constantly antagonized by Cdc25 phosphatases (Figure 2), this shift in the balance possibly due to an active c-Mos/MAPK pathway in the first cell cycle (Murakami and Vande Woude, 1998). The c-Mos/MAPK pathway has been reported to activate Wee1 (Murakami and Vande Woude, 1998; Murakami et al, 1999; Walter et al, 2000), and strong MAPK pathway activation can also trigger destabilization of Cdc25A by targeting it for SCFβ-TRCP-dependent degradation (Isoda et al, 2009). Since the c-Mos/MAPK pathway remains partially active during the first cycle (until Xenopus c-Mos degradation ∼30 min after fertilization), it is therefore possible that it mediates both Cdc25A degradation and Wee1 activation, with the net result of prolonged inhibitory phosphorylation of Cdk1 and an increased length of the first cell cycle. As the first division progresses and c-Mos levels decline, the balance could then tip in favour of Cdk1 activation, a process further promoted by Plx, which is able to activate Cdc25 (Abrieu et al, 1998; Qian et al, 1998; Toyoshima-Morimoto et al, 2002) as well as to—specifically during embryonic M-phase—bind and inhibit Myt1 (Inoue and Sagata, 2005). Thus, Plx and Cdk1 in concert are able to start the autoamplification loop of Cdk1 activation.
A full mitosis-competent state further depends on inactivation of the Cdk1-antagonizing PP2A phosphatase. It is likely that the mechanism of Cdk1-mediated inactivation of its antagonist PP2A-B55 via the Greatwall-Ensa/Arpp19 pathway (Gharbi-Ayachi et al, 2010; Mochida et al, 2010) acts not only in MII-arrested eggs (Yu et al, 2006; Hara et al, 2012) and during somatic divisions (Burgess et al, 2010; Voets and Wolthuis, 2010), but also functions during early embryonic divisions.
In mouse embryos, Cdk1 activation during the first mitotic division does not appear to be significantly delayed; on the other hand, mouse embryos exhibit prolongation of the first mitosis compared to the second division cycle (Ciemerych et al, 1999). This mitotic delay is however not mediated by the SAC (Sikora-Polaczek et al, 2006), and further studies are therefore required to elucidate the mechanisms impeding timely Cdk1 inactivation in the first mitotic cell cycle in mice.
Early embryonic cell division cycles
After the prolonged first cell division, embryos enter a series of specialized early embryonic cell division cycles. Their length varies between species, from 15 min per cycle in zebrafish to 30 min in frogs and 12 h in mice. Early cell divisions in mammalian embryos are set apart not only by their extended duration, but also by their marked asynchrony between sister cells, resulting in frequent stages with odd numbers of cells instead of exponential cell number increases (from two- to four- to eight-cell stages) (Piko and Clegg, 1982). In Echinoderms Xenopus and zebrafish, these early embryonic cell cycles last until the so-called mid-blastula transition (MBT) towards somatic cell cycles, which is initiated after 13 cycles in Xenopus and is marked by switching of gene expression from maternally supplied to newly transcribed zygotic mRNAs, referred to as zygotic genome activation (ZGA). In Xenopus, ZGA is initiated after 13 cell cycles (Kimelman et al, 1987), in zebrafish after 10 divisions and in mouse at the two-cell stage (Tadros and Lipshitz, 2009). Unlike MII-arrest and the first prolonged division, the subsequent early embryonic divisions display a high degree of variation between species, likely reflecting the divergent metazoan strategies for generating offspring. Indeed, embryos released into the environment without parental protection, like those of Xenopus, display faster early embryonic cell cycles than, for example, mammalian embryos protected in the womb (Strathmann et al, 2002). Rapid divisions allow fast developmental progression and may therefore be favoured in less predictable environmental conditions, while slow divisions might be generally more favourable for the proper embryo development as long as parental protection ensures decreased effects of environmental stress. Therefore, adaptive cell cycle mechanisms could have evolved to adjust cell division length to the developmental strategy of a given species.
Cdc25 phosphatases
The rapid early embryonic cell divisions of Xenopus embryos lack gap phases, surveillance mechanisms such as DNA replication-, DNA damage- and spindle assembly checkpoints (described in Musacchio and Salmon, 2007, Zegerman and Diffley, 2009), as well as the APC/C inhibitor Emi1, and are hence referred to as ‘minimal cell cycles’ (Figure 1, S–M cycles) (Masui and Wang, 1998). The pacemaker underlying the highly synchronous divisions in early Xenopus embryos is again the Cdk1 activity, which accordingly has to peak and drop every 30 min. In contrast to the lengthened first mitotic division, there is no inhibitory Thr-14/Tyr-15 phosphorylation on Cdk1 during these rapid divisions (Ferrell et al, 1991; Hartley et al, 1996), which could be due to either decreased Wee1/Myt1 kinase activity or increased Cdc25 phosphatase activity. In support of the latter possibility, embryos express Cdc25A in addition to the Cdc25C isoform from fertilization until MBT (Kim et al, 1999), which may be sufficient to constantly keep the balance tipped towards unphosphorylated active Cdk1 and thus could contribute to the rapidness of early divisions.
Regulated translation of cyclin B
In the absence of direct Cdk1 regulation via posttranslational modifications, regulated synthesis and destruction of cyclin B assumes the key role for early embryonic cell cycle regulation. Since no transcription takes place during the first 12 divisions in Xenopus embryos, gene expression is solely regulated at the posttranscriptional level (Newport and Kirschner, 1982b). Indeed, sequence elements present in the 3′-untranslated region of cyclin B mRNA and other maternally inherited mRNAs seem to regulate their stage-specific and/or a cell cycle-dependent translation. One of these elements is the cytoplasmic polyadenylation element (CPE), which recruits the CPE-binding protein (CPEB) that in turn mediates polyadenylation and translation of the mRNA. Consequently, injection of inhibitory CPEB antibodies into one-cell embryos results in decreased cyclin B protein levels, defective cell cycle progression and aberrant cell divisions (Groisman et al, 2000). Cyclin B translation is coordinated with the cell cycle phase by activating phosphorylation on CPEB, mediated by the cell cycle kinase Aurora A (Mendez et al, 2000; Groisman et al, 2001, 2002). Furthermore, cyclin B expression is silenced upon exit from mitosis by the protein maskin, which binds CPEB to inhibit mRNA translation. Consistently, maskin protein levels have been found to oscillate in a cell cycle-dependent manner in cycling Xenopus egg extracts (Groisman et al, 2002), although the underlying mechanism as well as the full extent of cyclin B translation control via maskin specifically in embryonic interphase remain to be determined.
Regulated cyclin B degradation
Regulated cyclin B synthesis is only half the story underlying oscillating cyclin B levels during the cell cycle, equally important is the regulated degradation of cyclin B by the APC/C. Xenopus early embryos lack APC/C inhibitory components, such as the SAC or Emi1 (Gerhart et al, 1984; Ohsumi et al, 2004), and the only APC/C inhibitor present in early dividing Xenopus embryos is XErp1/Emi2. Its depletion causes the untimely destruction of APC/C substrates, ultimately resulting in embryonic lethality (Tischer et al, 2012). Before undergoing apoptosis, XErp1/Emi2-depleted embryos exhibit a notable increase in cell cycle length, suggesting that the APC/C inhibitory activity of XErp1/Emi2 contributes to the short periodicity of early embryonic divisions, a notion confirmed also by recent mathematical modelling studies (Vinod et al, 2013). XErp1/Emi2 levels decline at the MBT, when cell cycle length increases and gap phases first become apparent, and its function as APC/C inhibitor is taken over by Emi1, the SAC (Clute and Masui, 1995) and additional regulatory mechanisms (Figure 1).
Control of XErp1/Emi2 activity during early embryonic divisions
In the mature egg, XErp1/Emi2 mediates cell cycle arrest until fertilization triggers its complete destruction and, thus, anaphase onset. During the first mitotic interphase, XErp1/Emi2 is rapidly resynthesized and its levels remain constant until MBT, and the XErp1/Emi2 activity during pre-MBT divisions is regulated on the posttranslational level via Cdk1 (negatively) and PP2A-B’56 (positively). A current simplified model posits that entry into mitosis is facilitated by XErp1/Emi2-mediated APC/C inhibition. PP2A-B’56 prevails over Cdk1 in early mitosis, resulting in sustained APC/C inhibition and hence increasing cyclin B levels. Once the Cdk1 activity reaches a certain threshold, the balance tips towards XErp1/Emi2 hyperphosphorylation and subsequent inactivation, allowing APC/C activation, cyclin B degradation and ultimately exit from M-phase (Figure 4B). Therefore, the antagonistic regulation of XErp1/Emi2 by Cdk1 and PP2A-B’56 in early Xenopus embryos reflects the regulatory mechanism active during meiotic MII-arrest, however, with two crucial adaptations: first, PP2A-B’56 recruitment to XErp1/Emi2 is not mediated by p90Rsk, whose upstream regulator c-Mos disappears about 30 min after fertilization and does not reappear during subsequent embryonic divisions (Sagata et al, 1988, 1989b), but instead by protein kinase A (PKA) in Xenopus. Intriguingly, PKA phosphorylates the same residues in XErp1/Emi2 (Ser-335, Thr-336, Ser-342 and Ser-344) that are also targeted by p90Rsk during the CSF arrest. Consequently, embryos depleted of endogenous XErp1/Emi2 (which undergo apoptosis at the time point of gastrulation) cannot be rescued by the expression of XErp1/Emi2 versions that fail to recruit PP2A-B’56 due to mutations in these phosphorylation sites, nor by XErp1/Emi2 mutants unable to inhibit APC/C.
The second major adaptation of the Cdk1/PP2A-B’56 antagonism is related its objective; while this mechanism serves the role of a rheostat that balances continuous cyclin B synthesis with transient APC/C activation during MII-arrest, its key function during early embryonic divisions is as a switch that controls entry into and exit from M-phase. The demand for a switch-like regulation is especially evident at exit from M-phase, as any XErp1/Emi2 reactivation upon decreasing Cdk1 activity would interfere with the complete destruction of cyclin B required for mitotic exit. An additional layer of cell cycle-dependent control could be imposed by the XErp1/Emi2 activator PKA, whose activity has been found to oscillate in cycling egg extracts (Grieco et al, 1994, 1996); however, studies in dividing embryos found global PKA activity levels to remain constant throughout early divisions (Tischer et al, 2012). In this respect, cycling extract may not faithfully recapitulate early embryonic divisions, but rather mimic the situation of the first mitotic division, a notion also supported by the observation that XErp1/Emi2 itself is dispensable for mitotic progression in cycling extracts (Liu et al, 2006). Therefore, it is unlikely that PKA regulation helps to prevent XErp1/Emi2 reactivation when Cdk1 activity declines during M-phase exit. As an alternative scenario, we speculate that maximal Cdk1 activity in late metaphase might trigger PP2A-B’56 inactivation, similar to Cdk1-induced PP2A-B55δ via the Greatwall-Ensa/Arpp19 pathway at the entry into M-phase (Gharbi-Ayachi et al, 2010; Mochida et al, 2010), thereby causing permanent XErp1/Emi2 inactivation. Should such a mechanism indeed exist, it would have to be explained how XErp1/Emi2 is reactivated in the subsequent cell cycle and how PP2A-B’56 activity could be kept constant during CSF arrest in the face of high Cdk1 activity.
APC/C Cdc20 activity regulation by Cdk1-mediated phosphorylation
While XErp1/Emi2-mediated APC/C inhibition is central to cell cycle regulation in early Xenopus embryos, it is likely that additional regulatory mechanisms are active during pre-MBT divisions to reinforce faithful cell cycle progression. Experiments in somatic cells and Xenopus cycling egg extract suggested the existence of a negative feedback loop, in which Cdk1-cyclin B complexes activate their own antagonist APC/C by phosphorylating several APC/C core subunits (Kraft et al, 2003). Indeed, the APC/C subunit Cdc27/APC3 is phosphorylated in a cell-cycle-dependent manner in Xenopus embryos. This positive Cdk1 effect on APC/C activity is however opposed by the inhibitory effect on the APC/C activator Cdc20 (Yudkovsky et al, 2000; D'Angiolella et al, 2003; Labit et al, 2012), whose Cdk1-mediated phosphorylation reduces its affinity for the APC/C. Consequently, Cdc20 mutated at five Cdk1 sites bound efficiently to and activated the APC/C even in the presence of high Cdk1 activity. Degradation of APC/C substrates at anaphase onset therefore requires Cdc20 dephosphorylation, which may be mediated by PP2A in early dividing embryos (Labit et al, 2012). While more research is required to fully dissect the underlying molecular mechanisms and their physiological relevance for early embryonic divisions, it seems likely that differences in the phosphorylation and dephosphorylation kinetics of Cdc20, APC/C and XErp1/Emi2 may all contribute to transitions from phases where the APC/C is inactive—due to XErp1/Emi2 hypophosphorylation and Cdc20 hyperphosphorylation—to phases where the APC/C is fully active—due to APC/C and XErp1/Emi2 hyperphosphorylation and Cdc20 hypophosphorylation (Vinod et al, 2013).
Cell cycle adaptations in mouse early embryos
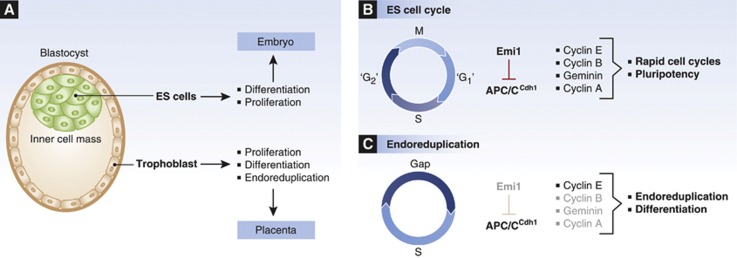
In mice, early embryos divide asynchronously and asymmetrically to give rise to the blastocyst, comprising the inner cell mass (ICM), which will subsequently form the embryo proper, and the trophoblast, a surrounding layer of cells that will form a major part of the placenta (Figure 5). Similar to the situation in amphibians, mammalian early embryonic cells exhibit specialized cell cycles and are subject to cell cycle control mechanisms distinct from those operating in somatic cells (Yang et al, 2012). Specifically, trophoblast cells differentiate and undergo endoreduplication to amplify their genomes more than 500-fold (Yang et al, 2012). In contrast, embryonic stem (ES) cells derived from the pluripotent cells of the ICM exhibit, like their cell of origin, rapid cell cycles without fully accentuated G1 and G2 phases, reminiscent of Xenopus early pre-MBT divisions (McAulay et al, 1993; Stead et al, 2002). Apparently, constitutively high Cdk2 activity and elevated levels of both cyclin A and E underlie these rapid divisions with truncated gap phases (Stead et al, 2002), with cell-cycle-dependent Cdk regulation restricted to Cdk1–cyclin B and primarily achieved by inhibitory Tyr-15 phosphorylation of Cdk1 as well as oscillating cyclin B levels (Stead et al, 2002). Another reported characteristic of mouse ES cells is Emi1-mediated suppression of APC/CCdh1 activity during late M- and early G1-phase (Ballabeni et al, 2011; Yang et al, 2011). Emi1 is highly homologous to XErp1/Emi2 and similarly is able to directly inhibit the APC/C. Consequently, ES cells display elevated levels of interphase APC/C substrates, such as cyclins and geminin (Fujii-Yamamoto et al, 2005; Yang et al, 2011), and depletion of Emi1 leads to geminin and cyclin A degradation due to unopposed APC/C activity. Interestingly, Emi1 depletion in ES cells does not only result in DNA re-replication, as seen in somatic cells (Di Fiore and Pines, 2007, Machida and Dutta, 2007), but also in differentiation and giant cell formation (Yang et al, 2011, 2012). This phenotype may be linked to the dual ES cell functions of the APC/CCdh1 target and replication licensing inhibitor geminin, which is important for the inhibition of endoreduplication and for the maintenance of pluripotency alike (Yang et al, 2011) (Figure 5). Consistently, geminin mutant embryos fail to form the pluripotent cells of the ICM, but commit to the trophoblast cell lineage (Gonzalez et al, 2006). Likewise, wild-type trophoblast cells exhibit low geminin levels, allowing them to undergo endoreduplication, that is, multiple rounds of replication in the absence of cell division, and differentiation (Gonzalez et al, 2006). Collectively, these adaptations of the early embryonic cell cycle are crucial, both for embryonic development and placenta formation in mice.
Figure 5.
APC/CCdh1 regulation in mouse embryonic cell cycles and trophoblast endoreduplication cycles. (A) Mouse blastocyst with the ICM surrounded by the trophoblast. ES cells are derived from the ICM, and ES cells as well as the cells of the ICM can proliferate and differentiate to form the embryo. Cells of the trophoblast initially proliferate, then stop and undergo endoreduplication and differentiation to give rise to the placenta. (B) Pluripotent ES cell cycles are rapid and lack accentuated G1 and G2 phases (please note that ‘G1’ and ‘G2’ phases are not drawn to scale here). Emi1 inhibits APC/C activity for an extended duration, leading to increased cyclin levels that drive rapid cell cycle progression. Geminin stabilization in ES cells prevents DNA re-replication and maintains pluripotency. (C) Endoreduplicating cells of the trophoblast lineage show high APC/CCdh1 activity due to low levels of Emi1, resulting in the degradation of geminin, cyclin A and cyclin B. Cyclin E as the only remaining S-phase-promoting Cdk partner drives endoreduplication, facilitated by degradation of the replication re-licensing inhibitor geminin.
Beyond the essential Emi1 role in protecting geminin from degradation in ES cells, it remains to be determined whether Emi1-regulated APC/C activity also matters for entry into and exit from mitosis in these cells. Emi1 is inactivated in prometaphase in somatic cells via sequential phosphorylation by Cdk1–cyclin B and Plk1, and subsequent SCFβ-TRCP-mediated targeting to the ubiquitin–proteasome system (Hansen et al, 2004; Moshe et al, 2004, 2011), but preliminary data suggest that such degradation may not occur during ES cell mitosis (Yang et al, 2011). On the other hand, Emi1 activity in somatic cells is subject to regulation by Cdk1 and possibly PP2A (Moshe et al, 2011), as also seen for XErp1/Emi2. Specifically, the fact that Emi1 stabilized upon Plk1 inhibition does not result in the expected mitotic arrest (Di Fiore and Pines, 2007) has lead to the proposal that Emi1 may, similar to XErp1/Emi2, additionally be inactivated by Cdk1-mediated phosphorylation decreasing its affinity for the APC/C (Moshe et al, 2011). It is therefore tempting to speculate that similar Emi1 regulatory mechanisms operate in mouse embryonic cell cycles to allow progression through mitosis.
Conclusion
There is a remarkable degree of plasticity underlying the basic mechanisms of cell cycle regulation, which allows adjusting the control and timing of cell cycle progression to the specific requirements of distinct developmental stages in different species. This flexibility is based on the modular organization of cell cycle regulation, where individual modules such as inhibitory Cdk1 phosphorylation, amplification loops or stage-specific APC/C inhibitors can be activated, attenuated or completely inactivated. Clearly, future research efforts are required to understand the regulatory circuits of cell cycle regulation at the molecular level. Especially, elucidation of the molecular network underlying early embryonic divisions with their much higher degree of interspecies variations as compared to MII-arrest and the first mitotic division remains a significant challenge, which will likely require multidisciplinary approaches combining biochemistry, cell biology, live cell microscopy and mathematical modelling.
Acknowledgments
We apologize to all colleagues whose work was not cited due to space constraints. We thank JB Gurdon, R Laskey and the members of their groups, as well as the Mayer lab, for critical comments on the manuscript and support. This work was supported by the Collaborative Research Center 969 of the German Research Foundation (DFG).
Footnotes
The authors declare that they have no conflict of interest.
References
- Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC, Doree M (1998) The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci 111(Pt 12): 1751–1757 [DOI] [PubMed] [Google Scholar]
- Abrieu A, Lorca T, Labbe JC, Morin N, Keyse S, Doree M (1996) MAP kinase does not inactivate, but rather prevents the cyclin degradation pathway from being turned on in Xenopus egg extracts. J Cell Sci 109((Pt 1)): 239–246 [DOI] [PubMed] [Google Scholar]
- Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN (2010) The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci USA 107: 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabeni A, Park IH, Zhao R, Wang W, Lerou PH, Daley GQ, Kirschner MW (2011) Cell cycle adaptations of embryonic stem cells. Proc Natl Acad Sci USA 108: 19252–19257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt RR, Ferrell JE Jr. (1999) The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science 286: 1362–1365 [DOI] [PubMed] [Google Scholar]
- Boiani M, Scholer HR (2005) Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 6: 872–884 [DOI] [PubMed] [Google Scholar]
- Bomar J, Moreira P, Balise JJ, Collas P (2002) Differential regulation of maternal and paternal chromosome condensation in mitotic zygotes. J Cell Sci 115: 2931–2940 [DOI] [PubMed] [Google Scholar]
- Bouniol-Baly C, Nguyen E, Besombes D, Debey P (1997) Dynamic organization of DNA replication in one-cell mouse embryos: relationship to transcriptional activation. Exp Cell Res 236: 201–211 [DOI] [PubMed] [Google Scholar]
- Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A (2010) Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA 107: 12564–12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JL, Solomon MJ (2007) Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev 21: 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JL, Xiong Y, Solomon MJ (2011) Mechanisms of pseudosubstrate inhibition of the anaphase promoting complex by Acm1. EMBO J 30: 1818–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Jennings PC, Stewart J, Verrills NM, Jones KT (2011) Essential role of protein phosphatase 2A in metaphase II arrest and activation of mouse eggs shown by okadaic acid, dominant negative protein phosphatase 2A, and FTY720. J Biol Chem 286: 14705–14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Minahan K, Merriman JA, Jones KT (2009) Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development 136: 4077–4081 [DOI] [PubMed] [Google Scholar]
- Choi E, Dial JM, Jeong DE, Hall MC (2008) Unique D box and KEN box sequences limit ubiquitination of Acm1 and promote pseudosubstrate inhibition of the anaphase-promoting complex. J Biol Chem 283: 23701–23710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF (1996) The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA 93: 7032–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciemerych MA, Czolowska R (1993) Differential chromatin condensation of female and male pronuclei in mouse zygotes. Mol Reprod Dev 34: 73–80 [DOI] [PubMed] [Google Scholar]
- Ciemerych MA, Maro B, Kubiak JZ (1999) Control of duration of the first two mitoses in a mouse embryo. Zygote 7: 293–300 [DOI] [PubMed] [Google Scholar]
- Clute P, Masui Y (1995) Regulation of the appearance of division asynchrony and microtubule-dependent chromosome cycles in Xenopus laevis embryos. Dev Biol 171: 273–285 [DOI] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ (1994) Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 370: 65–68 [DOI] [PubMed] [Google Scholar]
- D'Angiolella V, Mari C, Nocera D, Rametti L, Grieco D (2003) The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev 17: 2520–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das NK, Barker C (1976) Mitotic chromosome condensation in the sperm nucleus during postfertilization maturation division in Urechis eggs. J Cell Biol 68: 155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore B, Pines J (2007) Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J Cell Biol 177: 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J, Umbhauer M, Rassinier P, Hanauer A, Verlhac MH (2005) p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J Cell Biol 169: 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A (1991) The cdc25 protein contains an intrinsic phosphatase activity. Cell 67: 189–196 [DOI] [PubMed] [Google Scholar]
- Dupont G (1998) Link between fertilization-induced Ca2+ oscillations and relief from metaphase II arrest in mammalian eggs: a model based on calmodulin-dependent kinase II activation. Biophys Chem 72: 153–167 [DOI] [PubMed] [Google Scholar]
- Ferreira J, Carmo-Fonseca M (1997) Genome replication in early mouse embryos follows a defined temporal and spatial order. J Cell Sci 110(Pt 7): 889–897 [DOI] [PubMed] [Google Scholar]
- Ferrell JE Jr., Wu M, Gerhart JC, Martin GS (1991) Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol 11: 1965–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RP, Morgan DO (1994) A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 78: 713–724 [DOI] [PubMed] [Google Scholar]
- Fujii-Yamamoto H, Kim JM, Arai K, Masai H (2005) Cell cycle and developmental regulations of replication factors in mouse embryonic stem cells. J Biol Chem 280: 12976–12987 [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW (1991) cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67: 197–211 [DOI] [PubMed] [Google Scholar]
- Gerhart J, Wu M, Kirschner M (1984) Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol 98: 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T (2010) The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330: 1673–1677 [DOI] [PubMed] [Google Scholar]
- Gonzalez MA, Tachibana KE, Adams DJ, van der Weyden L, Hemberger M, Coleman N, Bradley A, Laskey RA (2006) Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes Dev 20: 1880–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco D, Avvedimento EV, Gottesman ME (1994) A role for cAMP-dependent protein kinase in early embryonic divisions. Proc Natl Acad Sci USA 91: 9896–9900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco D, Porcellini A, Avvedimento EV, Gottesman ME (1996) Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science 271: 1718–1723 [DOI] [PubMed] [Google Scholar]
- Groisman I, Huang YS, Mendez R, Cao Q, Richter JD (2001) Translational control of embryonic cell division by CPEB and maskin. Cold Spring Harb Symp Quant Biol 66: 345–351 [DOI] [PubMed] [Google Scholar]
- Groisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter JD (2000) CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 103: 435–447 [DOI] [PubMed] [Google Scholar]
- Groisman I, Jung MY, Sarkissian M, Cao Q, Richter JD (2002) Translational control of the embryonic cell cycle. Cell 109: 473–483 [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Lewellyn AL, Maller JL (1999) Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science 286: 1365–1367 [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL (1993) Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science 262: 1262–1265 [DOI] [PubMed] [Google Scholar]
- Hansen DV, Loktev AV, Ban KH, Jackson PK (2004) Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC Inhibitor Emi1. Mol Biol Cell 15: 5623–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Tydeman P, Kirschner M (1980) A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc Natl Acad Sci USA 77: 462–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Abe Y, Tanaka T, Yamamoto T, Okumura E, Kishimoto T (2012) Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat Commun 3: 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Rempel RE, Maller JL (1996) In vivo regulation of the early embryonic cell cycle in Xenopus. Dev Biol 173: 408–419 [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y et al. (1994) Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370: 68–71 [DOI] [PubMed] [Google Scholar]
- Hörmanseder E, Tischer T, Heubes S, Stemmann O, Mayer TU (2011) Non-proteolytic ubiquitylation counteracts the APC/C-inhibitory function of XErp1. EMBO Rep 12: 436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D, Ohe M, Kanemori Y, Nobui T, Sagata N (2007) A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature 446: 1100–1104 [DOI] [PubMed] [Google Scholar]
- Inoue D, Sagata N (2005) The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J 24: 1057–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Kanemori Y, Nakajo N, Uchida S, Yamashita K, Ueno H, Sagata N (2009) The extracellular signal-regulated kinase-mitogen-activated protein kinase pathway phosphorylates and targets Cdc25A for SCF beta-TrCP-dependent degradation for cell cycle arrest. Mol Biol Cell 20: 2186–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Sako K, Suzuki K, Nishino K, Nakajo N, Ohe M, Ezaki T, Kanemori Y, Inoue D, Ueno H, Sagata N (2011) Dynamic regulation of Emi2 by Emi2-bound Cdk1/Plk1/CK1 and PP2A-B56 in meiotic arrest of Xenopus eggs. Dev Cell 21: 506–519 [DOI] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL (1992) Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell 3: 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT (2007) Intracellular calcium in the fertilization and development of mammalian eggs. Clin Exp Pharmacol Physiol 34: 1084–1089 [DOI] [PubMed] [Google Scholar]
- Katagiri C, Ohsumi K (1994) Remodeling of sperm chromatin induced in egg extracts of amphibians. Int J Dev Biol 38: 209–216 [PubMed] [Google Scholar]
- Kim SH, Li C, Maller JL (1999) A maternal form of the phosphatase Cdc25A regulates early embryonic cell cycles in Xenopus laevis. Dev Biol 212: 381–391 [DOI] [PubMed] [Google Scholar]
- Kimelman D, Kirschner M, Scherson T (1987) The events of the midblastula transition in Xenopus are regulated by changes in the cell cycle. Cell 48: 399–407 [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E (1994) Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J 13: 2131–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM (2003) Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J 22: 6598–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W, Nigg EA (1992) Cell cycle regulation of vertebrate p34cdc2 activity: identification of Thr161 as an essential in vivo phosphorylation site. New Biol 4: 323–329 [PubMed] [Google Scholar]
- Kubiak JZ, Weber M, de Pennart H, Winston NJ, Maro B (1993) The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J 12: 3773–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota HY, Yoshimoto Y, Yoneda M, Hiramoto Y (1987) Free calcium wave upon activation in Xenopus eggs. Dev Biol 119: 129–136 [DOI] [PubMed] [Google Scholar]
- Labit H, Fujimitsu K, Bayin NS, Takaki T, Gannon J, Yamano H (2012) Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C. EMBO J 31: 3351–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence Y, Whitaker M, Swann K (1997) Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development 124: 233–241 [DOI] [PubMed] [Google Scholar]
- Ledan E, Polanski Z, Terret ME, Maro B (2001) Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev Biol 232: 400–413 [DOI] [PubMed] [Google Scholar]
- Liu J, Grimison B, Lewellyn AL, Maller JL (2006) The anaphase-promoting complex/cyclosome inhibitor Emi2 is essential for meiotic but not mitotic cell cycles. J Biol Chem 281: 34736–34741 [DOI] [PubMed] [Google Scholar]
- Liu J, Maller JL (2005) Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol 15: 1458–1468 [DOI] [PubMed] [Google Scholar]
- Longo FJ (1985) Pronuclear events during fertilization. InBiology of Fertilization Metz CB, Monroy A (eds).Vol. 3: pp251–298,New York: Academic Press, [Google Scholar]
- Longo FJ, Anderson E (1968) The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J Cell Biol 39: 339–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Abrieu A, Means A, Doree M (1994) Ca2+ is involved through type II calmodulin-dependent protein kinase in cyclin degradation and exit from metaphase. Biochim Biophys Acta 1223: 325–332 [DOI] [PubMed] [Google Scholar]
- Lorca T, Bernis C, Vigneron S, Burgess A, Brioudes E, Labbe JC, Castro A (2010) Constant regulation of both the MPF amplification loop and the Greatwall-PP2 A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J Cell Sci 123: 2281–2291 [DOI] [PubMed] [Google Scholar]
- Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, Means A, Doree M (1993) Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature 366: 270–273 [DOI] [PubMed] [Google Scholar]
- Luthardt FW, Donahue RP (1973) Pronuclear DNA synthesis in mouse eggs. An autoradiographic study. Exp Cell Res 82: 143–151 [DOI] [PubMed] [Google Scholar]
- Machida YJ, Dutta A (2007) The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev 21: 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT (2006) Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol 174: 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malureanu LA, Jeganathan KB, Hamada M, Wasilewski L, Davenport J, van Deursen JM (2009) BubR1 N terminus acts as a soluble inhibitor of cyclin B degradation by APC/C(Cdc20) in interphase. Dev Cell 16: 118–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoulaki S, Matson S, Abbott AL, Ducibella T (2003) Oscillatory CaMKII activity in mouse egg activation. Dev Biol 258: 464–474 [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL (1971) Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145 [DOI] [PubMed] [Google Scholar]
- Masui Y, Wang P (1998) Cell cycle transition in early embryonic development of Xenopus laevis. Biol Cell 90: 537–548 [PubMed] [Google Scholar]
- Mayer W, Smith A, Fundele R, Haaf T (2000) Spatial separation of parental genomes in preimplantation mouse embryos. J Cell Biol 148: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAulay AD, Wang J, Xu X (1993) Optical perceptron learning for binary classification with spatial light rebroadcasters. Appl Opt 32: 1346–1353 [DOI] [PubMed] [Google Scholar]
- Mendez R, Hake LE, Andresson T, Littlepage LE, Ruderman JV, Richter JD (2000) Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404: 302–307 [DOI] [PubMed] [Google Scholar]
- Miller JJ, Summers MK, Hansen DV, Nachury MV, Lehman NL, Loktev A, Jackson PK (2006) Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev 20: 2410–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagaki Y, Kanemori Y, Baba T (2011) Possible involvement of mitogen- and stress-activated protein kinase 1, MSK1, in metaphase-II arrest through phosphorylation of EMI2 in mouse oocytes. Dev Biol 359: 73–81 [DOI] [PubMed] [Google Scholar]
- Mochida S, Hunt T (2007) Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449: 336–340 [DOI] [PubMed] [Google Scholar]
- Mochida S, Ikeo S, Gannon J, Hunt T (2009) Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J 28: 2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Maslen SL, Skehel M, Hunt T (2010) Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330: 1670–1673 [DOI] [PubMed] [Google Scholar]
- Moshe Y, Boulaire J, Pagano M, Hershko A (2004) A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc Natl Acad Sci USA 101: 7937–7942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshe Y, Bar-On O, Ganoth D, Hershko A (2011) Regulation of the action of early mitotic inhibitor 1 on the anaphase-promoting complex/cyclosome by cyclin-dependent kinases. J Biol Chem 286: 16647–16657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami MS, Copeland TD, Vande Woude GF (1999) Mos positively regulates Xe-Wee1 to lengthen the first mitotic cell cycle of Xenopus. Genes Dev 13: 620–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami MS, Vande Woude GF (1998) Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development 125: 237–248 [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8: 379–393 [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M (1982a) A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30: 675–686 [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M (1982b) A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell 30: 687–696 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Ohsumi K, Kishimoto T (2007a) Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature 446: 1096–1099 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K (2007b) Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature 449: 341–345 [DOI] [PubMed] [Google Scholar]
- Nixon VL, Levasseur M, McDougall A, Jones KT (2002) Ca(2+) oscillations promote APC/C-dependent cyclin B1 degradation during metaphase arrest and completion of meiosis in fertilizing mouse eggs. Curr Biol 12: 746–750 [DOI] [PubMed] [Google Scholar]
- Ohe M, Kawamura Y, Ueno H, Inoue D, Kanemori Y, Senoo C, Isoda M, Nakajo N, Sagata N (2010) Emi2 inhibition of the anaphase-promoting complex/cyclosome absolutely requires Emi2 binding via the C-terminal RL tail. Mol Biol Cell 21: 905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi K, Koyanagi A, Yamamoto TM, Gotoh T, Kishimoto T (2004) Emi1-mediated M-phase arrest in Xenopus eggs is distinct from cytostatic factor arrest. Proc Natl Acad Sci USA 101: 12531–12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapenko D, Burton JL, Wang R, Solomon MJ (2008) Pseudosubstrate inhibition of the anaphase-promoting complex by Acm1: regulation by proteolysis and Cdc28 phosphorylation. Mol Cell Biol 28: 4653–4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7: 644–656 [DOI] [PubMed] [Google Scholar]
- Piko L, Clegg KB (1982) Quantitative changes in total RNA, total poly(A), and ribosomes in early mouse embryos. Dev Biol 89: 362–378 [DOI] [PubMed] [Google Scholar]
- Primorac I, Musacchio A (2013) Panta rhei: the APC/C at steady state. J Cell Biol 201: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Li C, Maller JL (1998) Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol 18: 4262–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU (2005) Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437: 1048–1052 [DOI] [PubMed] [Google Scholar]
- Reber S, Over S, Kronja I, Gruss OJ (2008) CaM kinase II initiates meiotic spindle depolymerization independently of APC/C activation. J Cell Biol 183: 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman TC, Pruslin FH, Hoffmann HP, Allfrey VG (1981) Turnover of basic chromosomal proteins in fertilized eggs: a cytoimmunochemical study of events in vivo. J Cell Biol 90: 351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA (1999) Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCgamma or G(q)-mediated activation of PLCbeta. Dev Biol 214: 399–411 [DOI] [PubMed] [Google Scholar]
- Sagata N, Daar I, Oskarsson M, Showalter SD, Vande Woude GF (1989a) The product of the mos proto-oncogene as a candidate ‘initiator’ for oocyte maturation. Science 245: 643–646 [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF (1988) Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature 335: 519–525 [DOI] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y (1989b) The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 342: 512–518 [DOI] [PubMed] [Google Scholar]
- Santos F, Hendrich B, Reik W, Dean W (2002) Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 241: 172–182 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU (2005) Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev 19: 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab MS, Roberts BT, Gross SD, Tunquist BJ, Taieb FE, Lewellyn AL, Maller JL (2001) Bub1 is activated by the protein kinase p90(Rsk) during Xenopus oocyte maturation. Curr Biol 11: 141–150 [DOI] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC (2006) Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J 25: 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora-Polaczek M, Hupalowska A, Polanski Z, Kubiak JZ, Ciemerych MA (2006) The first mitosis of the mouse embryo is prolonged by transitional metaphase arrest. Biol Rep 74: 734–743 [DOI] [PubMed] [Google Scholar]
- Smythe C, Newport JW (1992) Coupling of mitosis to the completion of S phase in Xenopus occurs via modulation of the tyrosine kinase that phosphorylates p34cdc2. Cell 68: 787–797 [DOI] [PubMed] [Google Scholar]
- Stead E, White J, Faast R, Conn S, Goldstone S, Rathjen J, Dhingra U, Rathjen P, Walker D, Dalton S (2002) Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene 21: 8320–8333 [DOI] [PubMed] [Google Scholar]
- Strathmann RR, Staver JM, Hoffman JR (2002) Risk and the evolution of cell-cycle durations of embryos. Evolution 56: 708–720 [DOI] [PubMed] [Google Scholar]
- Strome S, Wood WB (1983) Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35: 15–25 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Suzuki E, Yoshida N, Kubo A, Li H, Okuda E, Amanai M, Perry AC (2010) Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development 137: 3281–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W, Lipshitz HD (2009) The maternal-to-zygotic transition: a play in two acts. Development 136: 3033–3042 [DOI] [PubMed] [Google Scholar]
- Tang W, Wu JQ, Chen C, Yang CS, Guo JY, Freel CD, Kornbluth S (2010) Emi2-mediated inhibition of E2-substrate ubiquitin transfer by the anaphase-promoting complex/cyclosome through a D-box-independent mechanism. Mol Biol Cell 21: 2589–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan JP, Schultz SJ, Bartek J, Nigg EA (1994) Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase). J Cell Biol 127: 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer T, Hörmanseder E, Mayer TU (2012) The APC/C inhibitor XErp1/Emi2 Is essential for Xenopus early embryonic divisions. Science 338: 520–524 [DOI] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F, Taniguchi E, Nishida E (2002) Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep 3: 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi C, Hoffmann S, Geley S, Graeser R, Polanski Z (2004) The spindle assembly checkpoint is not essential for CSF arrest of mouse oocytes. J Cell Biol 167: 1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunquist BJ, Schwab MS, Chen LG, Maller JL (2002) The spindle checkpoint kinase bub1 and cyclin e/cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr Biol 12: 1027–1033 [DOI] [PubMed] [Google Scholar]
- Ubbels GA, Hara K, Koster CH, Kirschner MW (1983) Evidence for a functional role of the cytoskeleton in determination of the dorsoventral axis in Xenopus laevis eggs. J Embryol Exp Morphol 77: 15–37 [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B (1996) Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 122: 815–822 [DOI] [PubMed] [Google Scholar]
- Vidal A, Koff A (2000) Cell-cycle inhibitors: three families united by a common cause. Gene 247: 1–15 [DOI] [PubMed] [Google Scholar]
- Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A (2009) Greatwall maintains mitosis through regulation of PP2 A. EMBO J 28: 2786–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod PK, Zhou X, Zhang T, Mayer TU, Novak B (2013) The role of APC/C inhibitor Emi2/XErp1 in oscillatory dynamics of early embryonic cell cycles. Biophys Chem 177–178: 1–6 [DOI] [PubMed] [Google Scholar]
- Voets E, Wolthuis RM (2010) MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 9: 3591–3601 [DOI] [PubMed] [Google Scholar]
- Walter SA, Guadagno SN, Ferrell JE Jr. (2000) Activation of Wee1 by p42 MAPK in vitro and in cycling xenopus egg extracts. Mol Biol Cell 11: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kirschner MW (2013) Emi1 preferentially inhibits ubiquitin chain elongation by the anaphase-promoting complex. Nat Cell Biol 15: 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Hansen DV, Guo Y, Wang MZ, Tang W, Freel CD, Tung JJ, Jackson PK, Kornbluth S (2007a) Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc Natl Acad Sci USA 104: 16564–16569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, Freel CD, Tung JJ, Tang W, Margolis SS, Jackson PK, Yamano H, Asano M, Kornbluth S (2007b) A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr Biol 17: 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto TM, Iwabuchi M, Ohsumi K, Kishimoto T (2005) APC/C-Cdc20-mediated degradation of cyclin B participates in CSF arrest in unfertilized Xenopus eggs. Dev Biol 279: 345–355 [DOI] [PubMed] [Google Scholar]
- Yang VS, Carter SA, Hyland SJ, Tachibana-Konwalski K, Laskey RA, Gonzalez MA (2011) Geminin escapes degradation in G1 of mouse pluripotent cells and mediates the expression of Oct4, Sox2, and Nanog. Curr Biol 21: 692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang VS, Carter SA, Ng Y, Hyland SJ, Tachibana-Konwalski K, Fisher RA, Sebire NJ, Seckl MJ, Pedersen RA, Laskey RA, Gonzalez MA (2012) Distinct activities of the anaphase-promoting complex/cyclosome (APC/C) in mouse embryonic cells. Cell Cycle 11: 846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhao Y, Li Z, Galas S, Goldberg ML (2006) Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 22: 83–91 [DOI] [PubMed] [Google Scholar]
- Yudkovsky Y, Shteinberg M, Listovsky T, Brandeis M, Hershko A (2000) Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem Biophys Res Commun 271: 299–304 [DOI] [PubMed] [Google Scholar]
- Zegerman P, Diffley JF (2009) DNA replication as a target of the DNA damage checkpoint. DNA Repair (Amst) 8: 1077–1088 [DOI] [PubMed] [Google Scholar]