Abstract
Dehydroepiandrosterone (DHEA) and its sulfate ester are the most abundant steroids in humans. DHEA levels fall with age in men and women, reaching values sometimes as low as 10%–20% of those encountered in young individuals. This age-related decrease suggests an “adrenopause” phenomenon. Studies point toward several potential roles of DHEA, mainly through its hormonal end products, making this decline clinically relevant. Unfortunately, even if positive effects of DHEA on muscle, bone, cardiovascular disease, and sexual function seem rather robust, extremely few studies are large enough and/or long enough for conclusions regarding its effects on aging. Moreover, because it has been publically presented as a “fountain of youth” equivalent, over-the-counter preparations lacking pharmacokinetic and pharmacodynamic data are widely used worldwide. Conceptually, supplementing a pre-hormone is extremely interesting, because it would permit the human organism to adequately use it throughout long periods, increasing or decreasing end products according to his needs. Nevertheless, data on the safety profile of long-term DHEA supplementation are still lacking. In this article, we examine the potential relation between low DHEA levels and well-known age-related diseases, such as sarcopenia, osteoporosis, dementia, sexual disorders, and cardiovascular disease. We also review risks and benefits of existing protocols of DHEA supplementation.
Introduction
Dehydroepiandrosterone (DHEA) and its sulfate ester (DHEAS) are the most abundant steroids in humans. DHEA is produced from cholesterol mainly in the adrenals but also by the testis, the ovaries, skin, and brain.1–3 DHEAS is exclusively produced from DHEA in the adrenal zona reticularis, rich in sulfuryl transferase activity.1 Adrenocorticotropic hormone (ACTH) acutely stimulates DHEA secretion, but has only a small acute effect on DHEAS levels whose half-life is far longer (10–20 hr compared to 1–3 hr for DHEA)1,4 and provides a stable DHEA reserve.2 DHEAS is thereafter transformed in tissues by sulfotransferases and hydroxysteroid sulfatases back to DHEA.1,5 The greater part of DHEAS is loosely bound to plasma albumin.4 No specific high-affinity plasma binding protein has been identified.5 Sex hormone–binding globulin (SHBG) weakly binds DHEA but not DHEAS.4
DHEA is produced by fetal adrenal glands, and DHEAS levels are high at birth. Following the involution of the adrenal fetal zone, DHEA decreases to almost undetectable levels during the first year of life.6 The production of DHEA starts again at ages 6–8, a phenomenon called “adrenarche.”1 DHEA levels peak during the third decade of life. In premenopausal women, daily production of DHEA is 6–8 mg. A total of 50% is secreted by the adrenals, 1–2 mg by the ovaries, and the rest by peripheral tissues.1 In post-menopausal women, production of estrogens and DHEA from the ovaries is near zero,1 making adrenals the main source of estrogens and testosterone through DHEA.2 DHEA levels range between 1.12 ng/mL and 7.43 ng/mL before and between 0.6 ng/mL and 5.7 ng/mL after menopause with median concentrations of 3 ng/mL and 1.67 ng/mL, respectively.7 In men, DHEA is produced mainly by the adrenals (10% of DHEA is secreted by the testis).8 Median DHEA concentrations are around 2 ng/mL.7
Age-related decrease of DHEA levels suggests an “adrenopause” phenomenon characterized by low DHEA and maintained cortisol levels.9 Indeed, DHEA levels range between 1.33 ng/mL and 7.78 ng/mL between 18 and 40 years, and between 0.63 ng/mL and 4.7 ng/mL after 40 years, for both men and women.7 By the age of 70–80 years, levels may be as low as 10%–20% of those encountered in young adults, even if inter-individual differences are very important.6 Lower concentrations are observed in women compared to men.10 The underlying mechanism is not fully understood, but seems related to a decrease in adrenal production rather than a modification in DHEA metabolism.5 Other conditions related to low DHEA levels are acute stress, severe systemic illness, anorexia nervosa, and adrenal failure. Conversely, levels may be high in patients with hyperprolactinemia.1
Studies point toward several potential roles of DHEA, making its age-related decline clinically relevant. In this article, we will review data on the physiological role of DHEA, as well as the relations between adrenopause and well-known geriatric syndromes.
Physiological Role of DHEA
Activity of DHEA through its end products
DHEA and DHEAS are pre-hormones. DHEAS is hydrophilic and constitutes a circulating stock. Only lipophilic DHEA can be transformed in peripheral tissues to more potent androgens and estrogens.6 Steroid production at a tissue level permits an auto-regulation of the local hormonal environment according to local needs, with less systemic effects.1,2 This phenomenon is called intracrinology. In women before menopause, 50%–75% of estrogens and the majority of androgens are produced through intracrine mechanisms from DHEA. After menopause, practically all androgens and estrogens are synthesized at a tissue level. In men, whose testes continue to secrete androgens throughout life, local hormone production also occurs but is more difficult to assess.4,6
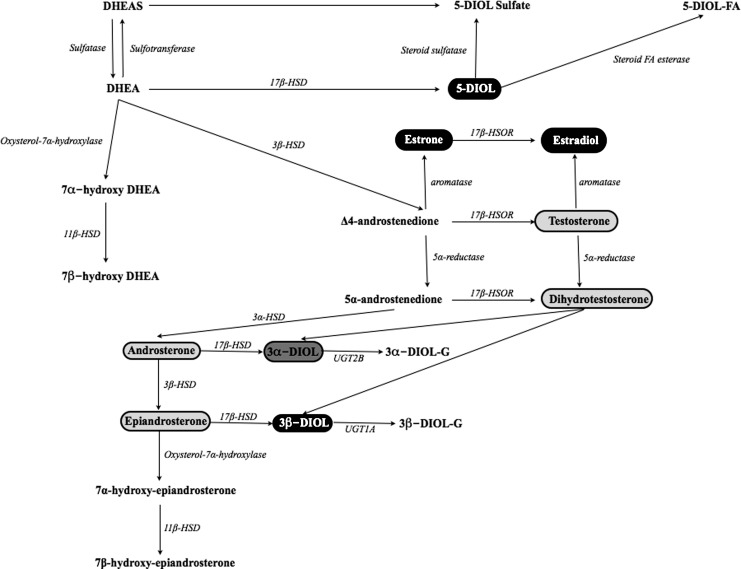
Studies in humans have shown different intracrine patterns in men and women after DHEA administration. Thus, DHEA increases mostly circulating androgens in women and estrogens in men.11–14 Nevertheless, it is practically impossible to measure precisely the hormonal end products of DHEA administration. First, peripheral hormone levels do not always correspond to the tissue hormone levels produced through intracrine mechanisms. Second, other products of steroid metabolism deriving from DHEA may also have estrogenic or androgenic properties, not taken into account when peripheral steroids are measured (Fig. 1).
FIG. 1.
Dehydroepiandrosterone (DHEA) and its hormonal end products. Those with intrinsic estrogenic activity are noted in black, those with androgenic activity in light gray, and those with both in dark gray. DHEAS, DHEA sulfate; HSD, hydroxysteroid dehydrogenase; HSOR, hydroxysteroid oxydoreductase; UGT, uridine glucuronosyl transferase; 5-DIOL-S, androst-5-ene-3α,17β-diol sulfate; 5-DIOL, androst-5-ene-3α,17β-diol; 3α-DIOL-G : androstane-3α,17β-diol glucuronide; 3α-DIOL-G, androstane-3α,17β-diol glucuronide; 5-DIOL-FA, androst-5-ene-3α,17β-diol fatty acid. FA, fatty acid.
Potential activity of DHEA through specific receptors
No specific intra-nuclear receptors of DHEA have been identified yet.5 However, recent evidence supports the existence of membrane receptors interacting with high specificity and affinity with DHEA.15–17 Such receptors have been found in the endothelium, heart, kidney, and liver.18 Studies show that DHEA increases nitric oxide (NO) production from intact endothelial cells in vitro (both animal and human), probably through a G-protein–dependent activation of endothelial NO synthetase (eNOS). This effect was independent from androgens, estrogens, and progesterone, as well as intracellular calcium. Conversely, it seemed influenced by tyrosine and mitogen-activated protein kinases (MAPKs).15–17 A concomitant increase of eNOS activity and intracellular cyclic guanosine monophosphate (cGMP) suggests that DHEA might act partly through the eNOS/cGMP pathway.18 Finally, 7α- and 7β-hydroxylated derivatives of DHEA seem to have a direct effect on nuclear receptors, but their physiological function is not clear yet18 (Fig.1).
DHEA's physiological role is only partly understood. Its role as a steroid hormone stock is crucial because intracrine mechanisms produce a large part of estrogens and androgens. Recent data support also direct actions of DHEA through specific receptors.
Low DHEA and Geriatric Syndromes
Musculoskeletal disorders
One-third of patients over 65 and half of those in nursing homes fall at least once per year.19 The lifetime risk for a fracture after 50 years is 51% for women and 20% for men.20 One of the major risk factors for falls in the elderly is muscle weakness.21 Muscle mass decreases with age at a rate of 6% per decade, beginning around age 45.22 No specific DHEA receptor has been identified in the muscle tissue. Potential effects could be mediated by a DHEA-related increase of insulin-like growth factor-1 (IGF-1) levels11,14 and bioavailability (decrease of insulin growth factor binding protein-1 [IGFBP-1]14) in men and women and an increase of androgen levels mostly in women.11,14,23 Studies in elderly individuals support a positive relation between DHEA blood levels and muscle mass,24 strength,24,25 mobility,26 and a lower risk for falls.27 A positive effect of DHEA administration on body composition,11,28–30 muscle strength,11,31 and physical performance31 has also been reported. Nevertheless, others failed to confirm such an effect.32–36 Disparities between studies could be related to the rather moderate effect of DHEA, but mostly due to great differences between study designs, short durations of treatment, and small population samples. So to date, even if the link between DHEA and musculoskeletal disorders seems clear, the usefulness as well as the modalities of DHEA use in rehabilitation protocols for older patients is not well known.
The risk for fractures depends on the combination of the propensity for falling and bone frailty, today best reflected by the quantitative assessment of bone mineral density (BMD).37 In an in vitro analysis, primary human osteoblasts showed aromatase activity converting DHEA to estrone.38 Another in vitro study showed that DHEA inhibits apoptosis and promotes proliferation of rat osteoblasts through MAPK signaling pathways, independently from androgens and estrogens.39 These findings support a positive effect of DHEA on bone through conversion to estrogens, but also independently from its hormonal end products. In a group of 120 post-menopausal women aged 51–99 years, lumbar spine BMD was related to DHEAS but not to estradiol plasma levels.38 DHEA levels have been positively related to BMD in men40 and post-menopausal women.38
In post-menopausal women, several studies have reported positive effects of DHEA administration on BMD of both the lumbar spine28,41–45 and the hip.9,43,44 Positive effects of DHEA administration on BMD have also been reported in men, for both the lumbar spine28,43,46 and the hip.28,43,44,46,47
To conclude, DHEA has positive effects on BMD in both men and women, even if it is weaker than other treatments of osteoporosis.1 Further studies could help to better define the role of DHEA in osteoporosis prevention and treatment.
Cognitive disorders
Dementia and specifically Alzheimer disease prevalence after the age of 65 are around 6%–8%, and 4.4%, respectively.48,49 Actual estimations put the number of patients suffering from dementia worldwide around 34 million. Numbers could rise three-fold in 2050 if no efficient prevention strategies are implemented.50,51 The related personal, social, and economic burdens are extremely high.
In vitro studies on human neural cells supported neurotrophic and neuroprotective effects of DHEA and its metabolites, mainly through DHEA-dependent neural stem cell stimulation, genomic activity modulation, and up-regulation of androgen receptor levels.52,53 Furthermore, DHEA inhibits the production of pro-inflammatory proteins such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6).18 High levels of cytokines found in aging individuals may affect the metabolism of amyloid precursor protein and increase the production of αβ-40 and αβ-42 peptides forming the amyloid plaques, a hallmark of Alzheimer disease.54 However, to our knowledge, no specific data exist on the role of the immunoregulatory effects of DHEA in prevention of dementia.
In clinical studies, DHEAS levels have been related to better executive function55 and higher scores on the Mini Mental State Examination.56 Surprisingly, an inverse relation between DHEA levels and cognitive performance has also been described.57 Barrett-Connor et al.58 found no relation between DHEA levels and incident dementia 15–20 years later. Concordantly, most studies addressing the effect of DHEA supplementation on cognitive function showed no 59–63 or only a small effect on specific cognitive domains such as verbal fluency.64 However, most studies available were very short (2 weeks to 1 year) and included only small groups of patients. Thus, in our opinion, current data do not appropriately address the potential role of DHEA in dementia prevention and treatment.
Mood disorders
The prevalence of depression increases with age.65 It is related to cognitive and functional decline as well as high morbidity and mortality.66 Animal studies showed that DHEA increased the activity of 5-hydroxytryptamine (5-HT) neurons, offering a physiological basis for a potential anti-depressant effect.67 Other neurotransmitters probably affected by DHEA are dopamine, glutamate, and γ-amino butyric acid.18 Clinical studies have suggested a relation between higher DHEA and psychological well-being in older individuals.1 Low levels of DHEA have been related to the presence of depressive symptoms68,69 and DHEA supplementation improved such symptoms,70–74 even if studies are not unanimous.59 Overall, DHEA treatment has mostly positive effects on mood disorders. Further studies are warranted to better define indications and treatment modalities.
Cardiovascular disease
Cardiovascular disease prevalence increases with age. Thirty percent of all acute myocardial infarctions affect patients over 75 years. More than 60% of patients hospitalized for unstable angina are over 65, and 80% of deaths from coronary disease occur in patients over 60.75
DHEA administration has been related to a decrease in high-density lipoprotein (HDL) levels.47,76,77 This was mostly observed in women, probably due to the more prominent androgenic effects of DHEA compared to male patients.11,14,76,78–80 Conversely, one study reported significantly higher HDL, lower low-density lipoprotein (LDL), and lower triglycerides levels after a daily administration of 25 mg of DHEA for 12 months to 20 post-menopausal women.81 Overall, most studies report no association between DHEA plasma levels82,83 or DHEA administration11,32,61,84,85 and the lipid profile.
Animal studies support a beneficial effect of DHEA administration on obesity and insulin sensitivity.86–88 In humans, actual data are rather inconsistent. Low DHEA levels have been related to higher fasting glucose but not diabetes in post-menopausal women.89 On the contrary, Barrett-Connor et al.90 found a direct relation between higher DHEA levels, higher waist-to-hip ratio, impaired glucose tolerance, and diabetes mellitus in post-menopausal women. A positive effect of DHEA administration on insulin sensitivity has been described in a group of middle-aged hypo-adrenal women91 as well as in groups of elderly men30 and women.30,79,81 Nevertheless, most interventional studies found no effect of DHEA replacement therapy on glucose tolerance and diabetes mellitus.11,14,33,36,47,78,85,92
Animal studies have suggested a protective effect of DHEA against atherosclerosis.93–96 Current opinion consensus supports a lipid profile independent mechanism mediating the anti-atherosclerotic effects of DHEA,18,93–95 partly through transformation to estrogens and testosterone.97 The previously described anti-inflammatory effects of DHEA,18,95 as well as direct stimulation of eNOS could also participate.17,18 Indeed, low DHEAS predicted incident ischemic heart disease in a group of men in a 9-year follow-up study, independently from classic cardiovascular risk factors.98 Others found a significant inverse relation between DHEAS levels and common carotid artery intima media thickness in men and a direct relation with common carotid artery blood flow in women.99 Low DHEA levels were also associated with more severe coronary atherosclerosis on coronary angiography in 206 middle-aged patients.100 In heart transplant patients, cardiac allograft vasculopathy developed more frequently and earlier in patients with low DHEA.101 Finally, actual data support a relation between heart failure and low DHEA levels. In fact, the heart produces DHEA in an autocrine way, which decreases as the disease progresses.102 Low DHEA has been related to a worse prognosis in male patients with chronic heart failure. DHEAS levels were also positively associated with left ventricular ejection fraction, and negatively with amino-terminal pro-brain natriuretic peptide levels.103
Cardiovascular and overall mortality were higher in post-menopausal women with low levels of DHEAS and coronary disease, after a mean follow-up of 6 years.83 Others found no relation between DHEA levels and cardiovascular mortality in post-menopausal women.104 In men, low DHEA has been associated with higher overall and cardiovascular mortality.105 In the same study, an increase of DHEAS levels by 100 μg/dL was associated with a reduction of all cause and cardiovascular mortality by, respectively, 36% and 48%.105
The evidence for an inverse relation between DHEA levels and cardiovascular risk are rather robust. Nevertheless, the effects of DHEA administration have been studied insufficiently.
Sexual function/menopause symptoms
Sexual disorders are most prevalent in aging men. In a group of male patients aged between 75 and 95 years, sexual problems were present in 72% of cases (49% erectile problems, 48% lack of interest, 39% unable to climax, 20% sexual performance-related anxiety).106 Low DHEA levels have been related to a higher risk for erectile dysfunction,107–109 even in people younger than 60.110 DHEA supplementation has been related to an improvement of erectile function, but also of desire, sexual interest, sexual activity, arousal, and fantasy,70,111 even if negative studies also exist.59,112 Positive effects on erectile function were mostly achieved in the absence of any underlying organic pathology.111,113 Indeed, there was no benefit in patients with diabetes or neurological disorders.113
In women, androgens and estrogens are produced from DHEA in the vagina tissue. Androgens are well known for their effects on arousability, pleasure, and intensity of orgasm in women. They are particularly implicated in the neurovascular smooth muscle response of swelling and lubrication, whereas estrogens contribute to vulval and vaginal congestive response and affect mood and sexual responses.6 Pre- and post-menopausal women with lower sexual responsiveness have lower levels of serum DHEAS.1,4,114 However, given the extremely large interval of normal values in women, a threshold for a higher risk of sexual disorders is difficult to define.1
Most studies on DHEA supplementation in post-menopausal women report benefits in various sexual domains such as increased desire, fantasies, lubrication, arousal, activity, interest, sexual drive, satisfaction, and orgasm.9,70,115–117 Intra-vaginal administration of DHEA improved arousability, sensation, lubrication, orgasm, and pain during sexual activity, without increasing serum steroids.118 The positive effects of DHEA on sexual function in post-menopausal women may be related to the concomitant increase of estrogens and androgens and their effects on the central nervous system and the genital tract.115 Nevertheless, others reported no positive effects.14,59,119 Differences in pre-treatment DHEA and testosterone levels could be partly responsible for the discrepancies in results between these studies.18
An improvement in symptoms of menopause in peri-menopausal and early post-menopausal women has also been reported with DHEA.115,120 Intra-vaginal administration of DHEA was effective for reversing vaginal atrophy in post-menopausal women.121 DHEA supplementation in early post-menopausal women is interesting. First, no increase of endometrial thickness has been reported with DHEA, probably because endometrial tissue does not have the necessary enzymes to transform it into estrogens, or because estrogen and progesterone are both its derivatives. Finally, the risk for breast cancer does not seem to increase with DHEA.122
Treatment
Treatment modalities
DHEA is considered as a dietary supplement in the United States but as a hormone in Europe. This difference has no scientific foundation and is mostly a matter of declaration. At present, questionable over-the-counter DHEA preparations lacking pharmacokinetic and pharmacodynamic data are widely used in the United States.
Treatment indications and modalities of DHEA supplementation are not well defined. In one study, after administration of 25–50 mg of DHEA, half-life varied from 18.7±5.5 to 25.1±10.7 hr in men, and from 23.6±8.5 to 26.6±8.6 hr in women.5 Long half-life allows a single take of 25–50 mg/day. Target levels of DHEA are around the middle of normal range for healthy young subjects, controlled by a blood sample 24 hr after the last take.123
End products of DHEA supplementation depend on the patient's sex, with a non-symmetrical transformation of DHEA favoring androgens in women and estrogens in men.2 DHEA administration for 23 months to elderly healthy individuals resulted in higher estradiol and testosterone in women and higher estradiol in men.47 End products may also vary according to the way of administration. Daily oral intake of 50 mg of DHEA for 12 months in post-menopausal women was associated with an increase of estrone, estradiol, androst-5-ene-3α,17β-diol (5-diol), testosterone, dihydrotestosterone, and androstenedione. There was also a four- to five-fold increase of androstane-3α,l7β-diol-3-glucuronide (3α-diol 3G), androstane-3α,17β-diol-17-glucuronide (3α-diol 17G), and androsterone glucuronide.119 In another study, a 12-month transdermal DHEA administration provoked similar increases in estrone, estradiol, and 5-diol, but the sum of androsterone glucuronide, 3α-diol,3G, and 3α-diol,17G increased by only 71%.124 First hepatic pass effect, through hepatic 5α-reductase, could contribute to the difference between oral and transdermal administration1 (Fig. 1). Consequently, depending on the desired effect, less important conversion of DHEA to androgens should be considered when transdermal formulations are used. Moreover, DHEA transformation to DHEAS in the liver after ingestion seems responsible for the longer half-life and better pharmacokinetic profile observed with oral compared to intravenous or transdermal formulations.5,124,125
Adverse effects
DHEA has been very well tolerated in studies using oral or percutaneous administration, with daily doses ranging from 25 mg to 1,600 mg. In women, only minimal adverse effects have been reported such as mild acne, seborrhea, facial hair growth, and ankle swelling.5,18 No adverse effects were observed on endometrium or breast tissue.1 In men, concerns have been expressed about DHEA transformation to androgens and their effects on the prostate tissue. After local transformation, DHEA may be responsible for one-sixth of dihydrotestosterone present in the prostate.126 However, no increases in prostate volume or PSA were noted after 2 years of treatment with 50 mg of DHEA in a group of older men.47
An important metabolite of DHEA is 5-diol (Fig. 1), which appears to bind weakly to the estrogen receptor. The estrogenic effect of 5-diol was supported by a study in breast cancer patients who progressed under aromatase inhibitor treatment but improved with a sulfatase inhibitor. Sulfatase inhibition results in lower levels of DHEAS being transformed to DHEA, and thus to lower or even insignificant levels of 5-diol.127 Nevertheless, studies have reported no increase in cancer risk after DHEA treatment. On the contrary, animal models demonstrated that DHEA administration inhibited experimentally induced tumors of the lymphatic tissue, lung, colon, breast, liver, and skin.128,129 The underlying mechanism is not fully known, but seems to include the inhibition of glucose-6-phosphate dehydrogenase and the pentose phosphate pathways, which are a source of nicotinamide adenosine dinucleotide phosphate hydrogen (NADPH). This results to a decrease of NADPH-dependent reactions generating free radicals, and a decreased oxidative stress.11,81,128,129 Further studies are warranted to better understand the underlying mechanism of DHEA anti-cancer effect and its potential applications in humans.
In general the safety profile of DHEA administration is satisfying. Unfortunately most studies available are too short to assess long-term safety of DHEA supplementation.
Conclusion
Conceptually, supplementing a pre-hormone is extremely interesting, but is very different from supplementing a hormonal end product. The human organism needs the time to adequately use it throughout long periods, increasing or decreasing end products according to his needs that may fluctuate over time. Therefore, DHEA administration is closer to “hormonal optimization” than “hormonal supplementation.” Unfortunately, extremely few studies are large and/or long enough to conclude on the effects of DHEA on aging and age-related diseases, even if positive effects of DHEA on muscle, bone, cardiovascular disease, and sexual function seem rather robust. The safety profile of long-term DHEA supplementation also needs to be better studied.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Davis SR. Panjari M. Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96:1642–1653. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
- 2.Panjari M. Davis SR. DHEA for postmenopausal women: A review of the evidence. Maturitas. 2010;66:172–179. doi: 10.1016/j.maturitas.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Zouboulis CC. Chen WC. Thornton MJ. Qin K. Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007 Feb;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 4.Panjari M. Davis SR. DHEA therapy for women: Effect on sexual function and wellbeing. Hum Reprod Update. 2007;13:239–248. doi: 10.1093/humupd/dml055. [DOI] [PubMed] [Google Scholar]
- 5.Legrain S. Massien C. Lahlou N. Roger M. Debuire B. Diquet B. Chatellier G. Azizi M. Faucounau V. Porchet H. Forette F. Baulieu EE. Dehydroepiandrosterone replacement administration: Pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000;85:3208–3217. doi: 10.1210/jcem.85.9.6805. [DOI] [PubMed] [Google Scholar]
- 6.Genazzani AD. Lanzoni C. Genazzani AR. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging. 2007;24:173–185. doi: 10.2165/00002512-200724030-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kushnir MM. Blamires T. Rockwood AL. Roberts WL. Yue B. Erdogan E. Bunker AM. Meikle AW. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56:1138–1147. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman JM. Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 9.Baulieu EE. Thomas G. Legrain S. Lahlou N. Roger M. Debuire B. Faucounau V. Girard L. Hervy MP. Latour F. Leaud MC. Mokrane A. Pitti-Ferrandi H. Trivalle C. de Lacharrière O. Nouveau S. Rakoto-Arison B. Souberbielle JC. Raison J. Le Bouc Y. Raynaud A. Girerd X. Forette F. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci USA. 2000;97:4279–4284. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebrun CE. van der Schouw YT. de Jong FH. Pols HA. Grobbee DE. Lamberts SW. Relations between body composition, functional and hormonal parameters and quality of life in healthy postmenopausal women. Maturitas. 2006;55:82–92. doi: 10.1016/j.maturitas.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Morales AJ. Haubrich RH. Hwang JY. Asakura H. Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol. 1998;49:421–432. doi: 10.1046/j.1365-2265.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- 12.Arlt W. Justl HG. Callies F. Reincke M. Hübler D. Oettel M. Ernst M. Schulte HM. Allolio B. Oral dehydroepiandrosterone for adrenal androgen replacement: Pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928–1934. doi: 10.1210/jcem.83.6.4850. [DOI] [PubMed] [Google Scholar]
- 13.Arlt W. Haas J. Callies F. Reincke M. Hübler D. Oettel M. Ernst M. Schulte HM. Allolio B. Biotransformation of oral dehydroepiandrosterone in elderly men: Significant increase in circulating estrogens. J Clin Endocrinol Metab. 1999;84:2170–2176. doi: 10.1210/jcem.84.6.5789. [DOI] [PubMed] [Google Scholar]
- 14.Morales AJ. Nolan JJ. Nelson JC. Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- 15.Liu D. Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3) J Biol Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 16.Liu D. Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: Evidence for a cell surface receptor. Steroids. 2004;69:279–289. doi: 10.1016/j.steroids.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Simoncini T. Mannella P. Fornari L. Varone G. Caruso A. Genazzani AR. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology. 2003;144:3449–3455. doi: 10.1210/en.2003-0044. [DOI] [PubMed] [Google Scholar]
- 18.Traish AM. Kang HP. Saad F. Guay AT. Dehydroepiandrosterone (DHEA) a precursor steroid or an active hormone in human physiology. J Sex Med. 2011;8:2960–2982. doi: 10.1111/j.1743-6109.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanders AB. Changing clinical practice in geriatric emergency medicine. Acad Emerg Med. 1999;6:1189–1193. doi: 10.1111/j.1553-2712.1999.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 20.Lippuner K. Johansson H. Kanis JA. Rizzoli R. Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in Swiss men and women. Osteoporos Int. 2009;20:1131–1140. doi: 10.1007/s00198-008-0779-8. [DOI] [PubMed] [Google Scholar]
- 21.Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 22.Janssen I. Ross R. Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging. 2005;9:408–419. [PubMed] [Google Scholar]
- 23.Yen SS. Morales AJ. Khorram O. Replacement of DHEA in aging men and women. Potential remedial effects. Ann NY Acad Sci. 1995;774:128–142. doi: 10.1111/j.1749-6632.1995.tb17377.x. [DOI] [PubMed] [Google Scholar]
- 24.Valenti G. Denti L. Maggio M. Ceda G. Volpato S. Bandinelli S. Ceresini G. Cappola A. Guralnik JM. Ferrucci L. Effect of DHEAS on skeletal muscle over the life span: The InCHIANTI study. J Gerontol Ser A, Biol Sci Med Sci. 2004;59:466–472. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 25.Kostka T. Arsac LM. Patricot MC. Berthouze SE. Lacour JR. Bonnefoy M. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur J Appl Physiol. 2000;82:83–90. doi: 10.1007/s004210050655. [DOI] [PubMed] [Google Scholar]
- 26.Ravaglia G. Forti P. Maioli F. Boschi F. Cicognani A. Bernardi M. Pratelli L. Pizzoferrato A. Porcu S. Gasbarrini G. Determinants of functional status in healthy Italian nonagenarians and centenarians: A comprehensive functional assessment by the instruments of geriatric practice. J Am Geriatr Soc. 1997;45:1196–1202. doi: 10.1111/j.1532-5415.1997.tb03769.x. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari HA. Orav EJ. Dawson-Hughes B. Additive benefit of higher testosterone levels and vitamin D plus calcium supplementation in regard to fall risk reduction among older men and women. Osteoporos Int. 2008;19:1307–1314. doi: 10.1007/s00198-008-0573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villareal DT. Holloszy JO. Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol. 2000;53:561–568. doi: 10.1046/j.1365-2265.2000.01131.x. [DOI] [PubMed] [Google Scholar]
- 29.Kalman DS CC. Swain MA. Torina GC. Shi Q. A randomized, double-blind, placebo-controlled study of 3-acetyl-7-oxo-dehydroepiandrosterone in healthy over- weight adults. Curr Ther Res. 2000;61:435–442. [Google Scholar]
- 30.Villareal DT. Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: A randomized controlled trial. JAMA. 2004;292:2243–2248. doi: 10.1001/jama.292.18.2243. [DOI] [PubMed] [Google Scholar]
- 31.Kenny AM. Boxer RS. Kleppinger A. Brindisi J. Feinn R. Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58:1707–1714. doi: 10.1111/j.1532-5415.2010.03019.x. [DOI] [PubMed] [Google Scholar]
- 32.Jedrzejuk D. Medras M. Milewicz A. Demissie M. Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: Effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male. 2003;6:151–156. [PubMed] [Google Scholar]
- 33.Callies F. Fassnacht M. van Vlijmen JC. Koehler I. Huebler D. Seibel MJ. Arlt W. Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency: Effects on body composition, serum leptin, bone turnover, and exercise capacity. J Clin Endocrinol Metab. 2001;86:1968–1972. doi: 10.1210/jcem.86.5.7483. [DOI] [PubMed] [Google Scholar]
- 34.Flynn MA. Weaver-Osterholtz D. Sharpe-Timms KL. Allen S. Krause G. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999;84:1527–1533. doi: 10.1210/jcem.84.5.5672. [DOI] [PubMed] [Google Scholar]
- 35.Percheron G. Hogrel JY. Denot-Ledunois S. Fayet G. Forette F. Baulieu EE. Fardeau M. Marini JF. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: A double-blind placebo-controlled trial. Arch Int Med. 2003;163:720–727. doi: 10.1001/archinte.163.6.720. [DOI] [PubMed] [Google Scholar]
- 36.Igwebuike A. Irving BA. Bigelow ML. Short KR. McConnell JP. Nair KS. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab. 2008;93:534–538. doi: 10.1210/jc.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzoli R. Management of the oldest old with osteoporosis. Eur Geriatr Med. 2010;1:15–21. [Google Scholar]
- 38.Nawata H. Tanaka S. Tanaka S. Takayanagi R. Sakai Y. Yanase T. Ikuyama S. Haji M. Aromatase in bone cell: Association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol. 1995;53:165–174. doi: 10.1016/0960-0760(95)00031-t. [DOI] [PubMed] [Google Scholar]
- 39.Wang L. Wang YD. Wang WJ. Zhu Y. Li DJ. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J Mol Endocrinol. 2007;38:467–479. doi: 10.1677/jme.1.02173. [DOI] [PubMed] [Google Scholar]
- 40.Clarke BL. Ebeling PR. Jones JD. Wahner HW. O'Fallon WM. Riggs BL. Fitzpatrick LA. Predictors of bone mineral density in aging healthy men varies by skeletal site. Calcified Tiss Int. 2002;70:137–145. doi: 10.1007/s00223-001-1072-4. [DOI] [PubMed] [Google Scholar]
- 41.Weiss EP. Shah K. Fontana L. Lambert CP. Holloszy JO. Villareal DT. Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. Am J Clin Nutr. 2009;89:1459–1467. doi: 10.3945/ajcn.2008.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Muhlen D. Laughlin GA. Kritz-Silverstein D. Bergstrom J. Bettencourt R. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: The DAWN trial. Osteoporosis Int. 2008;19:699–707. doi: 10.1007/s00198-007-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jankowski CM. Gozansky WS. Kittelson JM. Van Pelt RE. Schwartz RS. Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab. 2008;93:4767–4773. doi: 10.1210/jc.2007-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jankowski CM. Gozansky WS. Schwartz RS. Dahl DJ. Kittelson JM. Scott SM. Van Pelt RE. Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: A randomized, controlled trial. J Clin Endocrinol Metab. 2006;91:2986–2993. doi: 10.1210/jc.2005-2484. [DOI] [PubMed] [Google Scholar]
- 45.Labrie F. Diamond P. Cusan L. Gomez JL. Belanger A. Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3498–3505. doi: 10.1210/jcem.82.10.4306. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y. Mao M. Sun L. Feng Y. Yang J. Shen P. Treatment of osteoporosis in men using dehydroepiandrosterone sulfate. Chinese Med J. 2002;115:402–404. [PubMed] [Google Scholar]
- 47.Nair KS. Rizza RA. O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 48.Berr C. Wancata J. Ritchie K. Prevalence of dementia in the elderly in Europe. Eur Neuropsychopharmacol. 2005;15:463–471. doi: 10.1016/j.euroneuro.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Graham JE. Rockwood K. Beattie BL. Eastwood R. Gauthier S. Tuokko H. McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 50.Barnes DE. Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lautenschlager NT. Cox K. Cyarto EV. The influence of exercise on brain aging and dementia. Biochim Biophys Acta. 2012;1822:474–481. doi: 10.1016/j.bbadis.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Lu SF. Mo Q. Hu S. Garippa C. Simon NG. Dehydroepiandrosterone upregulates neural androgen receptor level and transcriptional activity. J Neurobiol. 2003;57:163–71. doi: 10.1002/neu.10260. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M. Wright LS. Marwah P. Lardy HA. Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci USA. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lang PO. Lapenna A. Pitts D. Aspinall R. Immunological pathogenesis of main age-related diseases and frailty: Role of immunosenescence. Eur Ger Med. 2010;1:112–121. [Google Scholar]
- 55.Davis SR. Shah SM. McKenzie DP. Kulkarni J. Davison SL. Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 56.Valenti G. Ferrucci L. Lauretani F. Ceresini G. Bandinelli S. Luci M. Ceda G. Maggio M. Schwartz RS. Dehydroepiandrosterone sulfate and cognitive function in the elderly: The InCHIANTI Study. J Endocrinol Invest. 2009;32:766–772. doi: 10.1007/BF03346534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison MF. Redei E. TenHave T. Parmelee P. Boyce AA. Sinha PS. Katz IR. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry. 2000;47:144–150. doi: 10.1016/s0006-3223(99)00099-2. [DOI] [PubMed] [Google Scholar]
- 58.Barrett-Connor E. Edelstein SL. A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: The Rancho Bernardo Study. J Am Geriatr Soc. 1994;42:420–423. doi: 10.1111/j.1532-5415.1994.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 59.Kritz-Silverstein D. von Muhlen D. Laughlin GA. Bettencourt R. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: The DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc. 2008;56:1292–1298. doi: 10.1111/j.1532-5415.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Niekerk JK. Huppert FA. Herbert J. Salivary cortisol and DHEA: Association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- 61.Barnhart KT. Freeman E. Grisso JA. Rader DJ. Sammel M. Kapoor S. Nestler JE. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab. 1999;84:3896–3902. doi: 10.1210/jcem.84.11.6153. [DOI] [PubMed] [Google Scholar]
- 62.Finckh A. Berner IC. Aubry-Rozier B. So AK. A randomized controlled trial of dehydroepiandrosterone in postmenopausal women with fibromyalgia. J Rheumatol. 2005;32:1336–1340. [PubMed] [Google Scholar]
- 63.Wolf OT. Kudielka BM. Hellhammer DH. Hellhammer J. Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998;23:617–629. doi: 10.1016/s0306-4530(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 64.Yamada S. Akishita M. Fukai S. Ogawa S. Yamaguchi K. Matsuyama J. Kozaki K. Toba K. Ouchi Y. Effects of dehydroepiandrosterone supplementation on cognitive function and activities of daily living in older women with mild to moderate cognitive impairment. Geriatr Gerontol Int. 2010;10:280–287. doi: 10.1111/j.1447-0594.2010.00625.x. [DOI] [PubMed] [Google Scholar]
- 65.Samaras N. Rossi G. Giannakopoulos P. Gold G. Vascular depression. An age-related mood disorder. Eur Ger Med. 2010;4:220–225. [Google Scholar]
- 66.Steffens DC. A multiplicity of approaches to characterize geriatric depression and its outcomes. Curr Opin Psychiatry. 2009;22:522–526. doi: 10.1097/YCO.0b013e32832fcd93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robichaud M. Debonnel G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J Endocrinol. 2004;182:11–21. doi: 10.1677/joe.0.1820011. [DOI] [PubMed] [Google Scholar]
- 68.Barrett-Connor E. von Muhlen D. Laughlin GA. Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: The Rancho Bernardo Study. J Am Geriatr Soc. 1999;47:685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 69.Michael A. Jenaway A. Paykel ES. Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt PJ. Daly RC. Bloch M. Smith MJ. Danaceau MA. St Clair LS. Murphy JH. Haq N. Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- 71.Wolkowitz OM. Reus VI. Roberts E. Manfredi F. Chan T. Raum WJ. Ormiston S. Johnson R. Canick J. Brizendine L. Weingartner H. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry. 1997;41:311–318. doi: 10.1016/s0006-3223(96)00043-1. [DOI] [PubMed] [Google Scholar]
- 72.Wolkowitz OM. Reus VI. Keebler A, et al. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999;156:646–649. doi: 10.1176/ajp.156.4.646. [DOI] [PubMed] [Google Scholar]
- 73.Strous RD. Maayan R. Lapidus R. Stryjer R. Lustig M. Kotler M. Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133–141. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- 74.Bloch M. Schmidt PJ. Danaceau MA. Adams LF. Rubinow DR. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry. 1999;45:1533–1541. doi: 10.1016/s0006-3223(99)00066-9. [DOI] [PubMed] [Google Scholar]
- 75.Samaras N. Chevalley T. Samaras D. Gold G. Older patients in the emergency department: A review. Ann Emerg Med. 2010;56:261–269. doi: 10.1016/j.annemergmed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 76.Srinivasan M. Irving BA. Dhatariya K. Klaus KA. Hartman SJ. McConnell JP. Nair KS. Effect of dehydroepiandrosterone replacement on lipoprotein profile in hypoadrenal women. J Clin Endocrinol Metab. 2009;94:761–764. doi: 10.1210/jc.2008-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rice SP. Agarwal N. Bolusani H. Newcombe R. Scanlon MF. Ludgate M. Rees DA. Effects of dehydroepiandrosterone replacement on vascular function in primary and secondary adrenal insufficiency: A randomized crossover trial. J Clin Endocrinol Metab. 2009;94:1966–1972. doi: 10.1210/jc.2008-2636. [DOI] [PubMed] [Google Scholar]
- 78.Casson PR. Santoro N. Elkind-Hirsch K. Carson SA. Hornsby PJ. Abraham G. Buster JE. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: A six-month trial. Fertil Steril. 1998;70:107–110. doi: 10.1016/s0015-0282(98)00121-6. [DOI] [PubMed] [Google Scholar]
- 79.Diamond P. Cusan L. Gomez JL. Belanger A. Labrie F. Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol. 1996;150(Suppl):S43–S50. [PubMed] [Google Scholar]
- 80.Srinivasan M. Irving BA. Frye RL. O'Brien P. Hartman SJ. McConnell JP. Nair KS. Effects on lipoprotein particles of long-term dehydroepiandrosterone in elderly men and women and testosterone in elderly men. J Clin Endocrinol Metab. 2010;95:1617–1625. doi: 10.1210/jc.2009-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lasco A. Frisina N. Morabito N. Gaudio A. Morini E. Trifiletti A. Basile G. Nicita-Mauro V. Cucinotta D. Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol. 2001;145:457–461. doi: 10.1530/eje.0.1450457. [DOI] [PubMed] [Google Scholar]
- 82.Bell RJ. Davison SL. Papalia MA. McKenzie DP. Davis SR. Endogenous androgen levels and cardiovascular risk profile in women across the adult life span. Menopause. 2007;14:630–638. doi: 10.1097/GME.0b013e31802b6cb1. [DOI] [PubMed] [Google Scholar]
- 83.Shufelt C. Bretsky P. Almeida CM. Johnson BD. Shaw LJ. Azziz R. Braunstein GD. Pepine CJ. Bittner V. Vido DA. Stanczyk FZ. Bairey Merz CN. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: Results from the National Institutes of Health–National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women's Ischemia Syndrome Evaluation (WISE) J Clin Endocrinol Metab. 2010;95:4985–4992. doi: 10.1210/jc.2010-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christiansen JJ. Andersen NH. Sørensen KE. Pedersen EM. Bennett P. Andersen M. Christiansen JS. Jørgensen JO. Gravholt CH. Dehydroepiandrosterone substitution in female adrenal failure: no impact on endothelial function and cardiovascular parameters despite normalization of androgen status. Clin Endocrinol. 2007;66:426–433. doi: 10.1111/j.1365-2265.2007.02750.x. [DOI] [PubMed] [Google Scholar]
- 85.Panjari M. Bell RJ. Jane F. Adams J. Morrow C. Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009;63:240–245. doi: 10.1016/j.maturitas.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 86.Cleary MP. Zisk JF. Anti-obesity effect of two different levels of dehydroepiandrosterone in lean and obese middle-aged female Zucker rats. Int J Obes. 1986;10:193–204. [PubMed] [Google Scholar]
- 87.Mohan PF. Ihnen JS. Levin BE. Cleary MP. Effects of dehydroepiandrosterone treatment in rats with diet-induced obesity. J Nutr. 1990;120:1103–1114. doi: 10.1093/jn/120.9.1103. [DOI] [PubMed] [Google Scholar]
- 88.Hansen PA. Han DH. Nolte LA. Chen M. Holloszy JO. DHEA protects against visceral obesity and muscle insulin resistance in rats fed a high-fat diet. Am J Physiol. 1997;273:R1704–R1708. doi: 10.1152/ajpregu.1997.273.5.R1704. [DOI] [PubMed] [Google Scholar]
- 89.Golden SH. Dobs AS. Vaidya D. Szklo M. Gapstur S. Kopp P. Liu K. Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 90.Barrett-Connor E. Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: The Rancho Bernardo Study. J Clin Endocrinol Metab. 1996;81:59–64. doi: 10.1210/jcem.81.1.8550794. [DOI] [PubMed] [Google Scholar]
- 91.Dhatariya K. Bigelow ML. Nair KS. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes. 2005;54:765–769. doi: 10.2337/diabetes.54.3.765. [DOI] [PubMed] [Google Scholar]
- 92.Gebre-Medhin G. Husebye ES. Mallmin H. Helström L. Berne C. Karlsson FA. Kämpe O. Oral dehydroepiandrosterone (DHEA) replacement therapy in women with Addison's disease. Clin Endocrinol. 2000;52:775–780. doi: 10.1046/j.1365-2265.2000.01017.x. [DOI] [PubMed] [Google Scholar]
- 93.Hayashi T. Esaki T. Muto E. Kano H. Asai Y. Thakur NK. Sumi D. Jayachandran M. Iguchi A. Dehydroepiandrosterone retards atherosclerosis formation through its conversion to estrogen: The possible role of nitric oxide. Arterioscler Thromb Vasc Biol. 2000;20:782–792. doi: 10.1161/01.atv.20.3.782. [DOI] [PubMed] [Google Scholar]
- 94.Gordon GB. Bush DE. Weisman HF Reduction of atherosclerosis by administration of dehydroepiandrosterone. A study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injury. J Clin Invest. 1988;82:712–720. doi: 10.1172/JCI113652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L. Hao Q. Wang YD. Wang WJ. Li DJ. Protective effects of dehydroepiandrosterone on atherosclerosis in ovariectomized rabbits via alleviating inflammatory injury in endothelial cells. Atherosclerosis. 2011;214:47–57. doi: 10.1016/j.atherosclerosis.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 96.Eich DM. Nestler JE. Johnson DE. Dworkin GH. Ko D. Wechsler AS. Hess ML. Inhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantation. Circulation. 1993;87:261–269. doi: 10.1161/01.cir.87.1.261. [DOI] [PubMed] [Google Scholar]
- 97.Samaras N. Samaras D. Lang PO. Forster A. Pichard C. Frangos E. Meyer P. What is the relation between andropause and well-known geriatric syndromes? Maturitas. A view of geriatrics through hormones. 2013;74:213–219. doi: 10.1016/j.maturitas.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Feldman HA. Johannes CB. Araujo AB. Mohr BA. Longcope C. McKinlay JB. Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: Prospective results from the Massachusetts Male Aging Study. Am J Epidemiol. 2001;153:79–89. doi: 10.1093/aje/153.1.79. [DOI] [PubMed] [Google Scholar]
- 99.Yoshida S. Aihara K. Azuma H. Uemoto R. Sumitomo-Ueda Y. Yagi S. Ikeda Y. Iwase T. Nishio S. Kawano H. Miki J. Yamada H. Hirata Y. Akaike M. Sata M. Matsumoto T. Dehydroepiandrosterone sulfate is inversely associated with sex-dependent diverse carotid atherosclerosis regardless of endothelial function. Atherosclerosis. 2010;212:310–315. doi: 10.1016/j.atherosclerosis.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 100.Herrington DM. Dehydroepiandrosterone and coronary atherosclerosis. Ann NY Acad Sci. 1995;774:271–280. doi: 10.1111/j.1749-6632.1995.tb17387.x-i1. [DOI] [PubMed] [Google Scholar]
- 101.Herrington DM. Nanjee N. Achuff SC. Cameron DE. Dobbs B. Baughman KL. Dehydroepiandrosterone and cardiac allograft vasculopathy. J Heart Lung Transplant. 1996;15:88–93. [PubMed] [Google Scholar]
- 102.Nakamura S. Yoshimura M. Nakayama M. Ito T. Mizuno Y. Harada E. Sakamoto T. Saito Y. Nakao K. Yasue H. Ogawa H. Possible association of heart failure status with synthetic balance between aldosterone and dehydroepiandrosterone in human heart. Circulation. 2004;110:1787–1793. doi: 10.1161/01.CIR.0000143072.36782.51. [DOI] [PubMed] [Google Scholar]
- 103.Jankowska EA. Biel B. Majda J. Szklarska A. Lopuszanska M. Medras M. Anker SD. Banasiak W. Poole-Wilson PA. Ponikowski P. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- 104.Barrett-Connor E. Goodman-Gruen D. Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The Rancho Bernardo Study. Circulation. 1995;91:1757–1760. doi: 10.1161/01.cir.91.6.1757. [DOI] [PubMed] [Google Scholar]
- 105.Barrett-Connor E. Khaw KT. Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 106.Hyde Z. Flicker L. Hankey GJ. Almeida OP. McCaul KA. Chubb SA. Yeap BB. Prevalence and predictors of sexual problems in men aged 75–95 years: A population-based study. J Sex Med. 2012;9:442–453. doi: 10.1111/j.1743-6109.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 107.Basar MM. Aydin G. Mert HC. Keles I. Caglayan O. Orkun S. Batislam E. Relationship between serum sex steroids and Aging Male Symptoms score and International Index of Erectile Function. Urology. 2005;66:597–601. doi: 10.1016/j.urology.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 108.Alexopoulou O. Jamart J. Maiter D. Hermans MP. De Hertogh R. De Nayer P. Buysschaert M. Erectile dysfunction and lower androgenicity in type 1 diabetic patients. Diabetes Metab. 2001;27:329–336. [PubMed] [Google Scholar]
- 109.Feldman HA. Goldstein I. Hatzichristou DG. Krane RJ. McKinlay JB. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 110.Reiter WJ. Pycha A. Schatzl G. Klingler HC. Märk I. Auterith A. Marberger M. Serum dehydroepiandrosterone sulfate concentrations in men with erectile dysfunction. Urology. 2000;55:755–758. doi: 10.1016/s0090-4295(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 111.Reiter WJ. Pycha A. Schatzl G. Pokorny A. Gruber DM. Huber JC. Marberger M. Dehydroepiandrosterone in the treatment of erectile dysfunction: A prospective, double-blind, randomized, placebo-controlled study. Urology. 1999;53:590–594. doi: 10.1016/s0090-4295(98)00571-8. [DOI] [PubMed] [Google Scholar]
- 112.Morales A. Black A. Emerson L. Barkin J. Kuzmarov I. Day A. Androgens and sexual function: a placebo-controlled, randomized, double-blind study of testosterone vs. dehydroepiandrosterone in men with sexual dysfunction and androgen deficiency. Aging Male. 2009;12:104–112. doi: 10.3109/13685530903294388. [DOI] [PubMed] [Google Scholar]
- 113.Reiter WJ. Schatzl G. Mark I. Zeiner A. Pycha A. Marberger M. Dehydroepiandrosterone in the treatment of erectile dysfunction in patients with different organic etiologies. Urol Res. 2001;29:278–281. doi: 10.1007/s002400100189. [DOI] [PubMed] [Google Scholar]
- 114.Davis SR. Davison SL. Donath S. Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91–96. doi: 10.1001/jama.294.1.91. [DOI] [PubMed] [Google Scholar]
- 115.Genazzani AR. Stomati M. Valentino V. Pluchino N. Pot E. Casarosa E. Merlini S. Giannini A. Luisi M. Effect of 1-year, low-dose DHEA therapy on climacteric symptoms and female sexuality. Climacteric. 2011;14:661–668. doi: 10.3109/13697137.2011.579649. [DOI] [PubMed] [Google Scholar]
- 116.Arlt W. Callies F. van Vlijmen JC. Koehler I. Reincke M. Bidlingmaier M. Huebler D. Oettel M. Ernst M. Schulte HM. Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341:1013–1020. doi: 10.1056/NEJM199909303411401. [DOI] [PubMed] [Google Scholar]
- 117.Munarriz R. Talakoub L. Flaherty E. Gioia M. Hoag L. Kim NN. Traish A. Goldstein I. Guay A. Spark R. Androgen replacement therapy with dehydroepiandrosterone for androgen insufficiency and female sexual dysfunction: Androgen and questionnaire results. J Sex Marital Ther. 2002;28(Suppl 1):165–173. doi: 10.1080/00926230252851285. [DOI] [PubMed] [Google Scholar]
- 118.Labrie F. Archer D. Bouchard C. Fortier M. Cusan L. Gomez JL. Girard G. Baron M. Ayotte N. Moreau M. Dubé R. Côté I. Labrie C. Lavoie L. Berger L. Gilbert L. Martel C. Balser J. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009;16:923–931. doi: 10.1097/gme.0b013e31819e85c6. [DOI] [PubMed] [Google Scholar]
- 119.Panjari M. Bell RJ. Jane F. Wolfe R. Adams J. Morrow C. Davis SR. A randomized trial of oral DHEA treatment for sexual function, well-being, and menopausal symptoms in postmenopausal women with low libido. J Sex Med. 2009;6:2579–2590. doi: 10.1111/j.1743-6109.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 120.Stomati M. Monteleone P. Casarosa E. Quirici B. Puccetti S. Bernardi F. Genazzani AD. Rovati L. Luisi M. Genazzani AR. Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause. Gynecolog Endocrinol. 2000;14:342–363. doi: 10.3109/09513590009167703. [DOI] [PubMed] [Google Scholar]
- 121.Labrie F. Archer D. Bouchard C. Fortier M. Cusan L. Gomez JL. Girard G. Baron M. Ayotte N. Moreau M. Dubé R. Côté I. Labrie C. Lavoie L. Berger L. Gilbert L. Martel C. Balser J. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907–922. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 122.Labrie F. Luu-The V. Bélanger A. Lin SX. Simard J. Pelletier G. Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 123.Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94:1059–1067. doi: 10.1210/jc.2009-0032. [DOI] [PubMed] [Google Scholar]
- 124.Labrie F. Cusan L. Gomez JL. Martel C. Bérubé R. Bélanger P. Chaussade V. Deloche C. Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008;110:1–9. doi: 10.1016/j.jsbmb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 125.Sulcová J. Hill M. Hampl R. Masek Z. Novácek A. Ceska R. Stárka L. Effects of transdermal application of DHEA on the levels of steroids, gonadotropins and lipids in men. Physiol Res. 2000;49:685–693. [PubMed] [Google Scholar]
- 126.Arnold JT. DHEA metabolism in prostate: For better or worse? Mol Cell Endocrinol. 2009;301:83–88. doi: 10.1016/j.mce.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stanway SJ. Purohit A. Woo LW. Sufi S. Vigushin D. Ward R. Wilson RH. Stanczyk FZ. Dobbs N. Kulinskaya E. Elliott M. Potter BV. Reed MJ. Coombes RC. Phase I study of STX 64 (667 Coumate) in breast cancer patients: The first study of a steroid sulfatase inhibitor. Clin Cancer Res. 2006;12:1585–1592. doi: 10.1158/1078-0432.CCR-05-1996. [DOI] [PubMed] [Google Scholar]
- 128.Schwartz AG. Pashko LL. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res Rev. 2004;3:171–187. doi: 10.1016/j.arr.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 129.Schwartz AG. Pashko LL. Cancer prevention with dehydroepiandrosterone and non-androgenic structural analogs. J Cell Biochem Supplement. 1995;22:210–217. doi: 10.1002/jcb.240590826. [DOI] [PubMed] [Google Scholar]