Abstract
This review synthesizes behavioral research with neuromolecular mechanisms putatively involved with the low-toxicity cognitive enhancing action of Bacopa monnieri (BM), a medicinal Ayurvedic herb. BM is traditionally used for various ailments, but is best known as a neural tonic and memory enhancer. Numerous animal and in vitro studies have been conducted, with many evidencing potential medicinal properties. Several randomized, double-blind, placebo-controlled trials have substantiated BM's nootropic utility in humans. There is also evidence for potential attenuation of dementia, Parkinson's disease, and epilepsy. Current evidence suggests BM acts via the following mechanisms—anti-oxidant neuroprotection (via redox and enzyme induction), acetylcholinesterase inhibition and/or choline acetyltransferase activation, β-amyloid reduction, increased cerebral blood flow, and neurotransmitter modulation (acetylcholine [ACh], 5-hydroxytryptamine [5-HT], dopamine [DA]). BM appears to exhibit low toxicity in model organisms and humans; however, long-term studies of toxicity in humans have yet to be conducted. This review will integrate molecular neuroscience with behavioral research.
Introduction
Cognitive enhancement typically exacts a toxicological and psychological toll.1–4 The milieu of nootropic phytochemicals found within Bacopa monnieri (BM), primarily triperpenoid saponins called bacosides, exhibit minimal observable adverse effects at standard dosages. BM demonstrates anti-oxidant,5 hepatoprotective,6 and neuroprotective7 activity. Emerging research demonstrates several mechanisms of action—acetylcholinesterase inhibition, choline acetyltransferase activation, β-amyloid reduction, increased cerebral blood flow, and monoamine potentiation.
Herbal medicine is regularly used by 80% of the world population and is increasing in popularity in Europe and North America. 8 In 2008, the National Institutes of Health (NIH) found 4 in 10 adults reported using complementary and alternative medicine (CAM) in the last 12 months, 17.7% of such treatments being herbal medicine.9 Those with higher education are most likely to use CAM,10 which may partially reflect the fact that public health insurance used by poor individuals tends not to cover CAM.11 Herbal medicine tends to be cheaper than pharmaceuticals, albeit less standardized.12,13 Western biomedicine is in the midst of investigating the potential value of the Eastern pharmacopeia. Of the 150 most used pharmaceutical drugs in the United States, 118 were derived from plants.14 Traditional medical systems offer a vast library of potentially therapeutic neurological agents,15 BM is only beginning to undergo rigorous experimental research.
Bacopa monnieri (also known as brahmi, water hyssop, Bacopa monniera, and Herpestis monniera), is a creeping perennial with small oblong leaves and purple flowers, found in warm wetlands, and native to Australia and India. Commonly found as a weed in rice fields, BM grows throughout East Asia and the United States.16 The entire plant is used medicinally.
Unlike the potentially addictive and forceful action of widely used psychostimulants, chronic and moderate administration of BM appears to nourish rather than deplete neurons, an action compatible with 1400 years of Ayurvedic study. BM was initially described around the 6th century A.D. in texts such as the Charaka Samhita, Athar-Ved, and Susrutu Samhita as a medhya rasayana–class herb taken to sharpen intellect and attenuate mental deficits. The herb was allegedly used by ancient Vedic scholars to memorize lengthy sacred hymns and scriptures. BM is colloquially called Brahmi, after the Hindu creator-god Brahma, especially when combined with other alleged intellect-sharpening herbs like Centella asiatica (Gotu Kola). BM is consistently found in the many Ayurvedic preparations prescribed for cognitive dysfunction.
An estimated 3.4 million people are affected by dementia in the United States,17 most prevalently in the elderly. The elderly population (aged over 65) is expected to double by 2030, reaching 72 million, or 20% of the total U.S. population.18 BM shows great clinical potential in attenuating dementia via several mechanisms, most notably dose-dependent acetylcholine potentiation and free radical scavenging.
In a 90-day oral administration trial in rats, BM exhibited a no-observed adverse effect level (NOAEL) of 500 mg/kg and a median lethal dose (LD50) of 2400 mg/kg.5 The standard experimental human dose is between 150 and 3000 mg equivalent per day. The most common clinical side effect of BM is mild gastrointestinal upset, but long-term clinical trials are lacking. Several research groups formulate bacoside-standardized BM extract for clinical use, and the herb is widely used in India, the United States, and Australia. BM has been applied in rodents and cell culture for the following uses, which will not be detailed in this review:
Past reviews have focused on the broad therapeutic uses of BM, including the above uses. This review will elucidate the neuropharmacological mechanisms underlying the nootropic effects of the herb, summarize pre-clinical and clinical studies, outline putative mechanisms of action, and address issues of toxicity.
Chemical Constituents
The main nootropic constituents of BM are believed to be dammarane types of triterpenoid saponins known as bacosides, with jujubogenin or pseudo-jujubogenin moieties as aglycone units.38 Bacosides comprise a family of 12 known analogs.39 Novel saponins called bacopasides I–XII have been identified more recently.40–42 The alkaloids brahmine, nicotine, and herpestine have been catalogued, along with D-mannitol, apigenin, hersaponin, monnierasides I–III, cucurbitacins and plantainoside B.43–50 The constituent most studied has been bacoside A, which was found to be a blend of bacoside A3, bacopacide II, bacopasaponin C, and a jujubogenin isomer of bacosaponin C.48 These assays have been conducted using whole plant extract, and bacoside concentrations may vary depending upon the part from which they are extracted.
In one BM sample, Rastogi et al. found this bacoside profile—bacopaside I (5.37%), bacoside A3 (5.59%), bacopaside II (6.9%), bacopasaponin C isomer (7.08%), and bacopasaponin C (4.18%).66 The complete assay of BM is an ongoing effort.
Neuropharmacological Activity
BM has been studied extensively in animal models and in vitro. While BM is implicated in the treatment of anxiety, epilepsy, and other neurodegenerative disorders, this review will concentrate on cognition, learning, and memory. The clinical studies cited focus on memory, omitting other facets of cognition like fluid intelligence or creativity. Past clinical studies were not longitudinal, typically lasting only 12 weeks. The long-term effect of BM on humans is unknown, but animal models suggest considerable protection against age-related neurodegeneration rather than progressive toxicity or tolerance formation.
Putative mechanisms of action
The following mechanisms will be discussed: Anti-oxidant/neuroprotection, acetylcholinesterase inhibition, choline acetyltransferase activation, β-amyloid reduction, increased cerebral blood flow, and monoamine potentiation and modulation.
Anti-oxidant/neuroprotection
Oxidative stress (OS) occurs when free radicals (chemical species with unpaired electrons, produced during normal metabolism) overcome the cell's homeostatic defense mechanisms.49 Protective, free radical–quenching enzymes include superoxide dismutase, catalase, glutathione peroxidase (GPx), glutathione reductase (GSR), and others. Anti-oxidant compounds also play a key protective role, including vitamins A, C, E, and myriad phytonutrients (particularly phenols).50,51 OS plays a role in many diseases, even aging itself,52 by degrading ligands, peroxidizing lipids, disrupting metabolic pathways, denaturing proteins, and breaking DNA strands.53
The brain is especially susceptible to OS because it is metabolically active, possesses high levels of pro-oxidant iron, and is composed of unsaturated lipids (prone to lipid peroxidation).54 Furthermore, the blood–brain barrier prevents many exogenous anti-oxidants from quenching reactive oxygen species (ROS) in the brain.55
Anbarasi et al.56 assessed the neuroprotective role of bacoside A against OS in the brains of rats exposed to cigarette smoke by measuring concentrations of enzymatic and non-enzymatic anti-oxidants as well as trace elements. The researchers administered 10 mg/kg aqueous bacoside A gavage daily and found that BM significantly increased brain levels of glutathione, vitamin C, vitamin E, and vitamin A in rats exposed to cigarette smoke (perhaps an anti-oxidant conservation effect). Bacoside A administration increased the activities of superoxide dismutase (SOD), catalase, GPx, and GSR. As a result, the levels of glutathione (primary endogenous anti-oxidant conjugate) in the brain were significantly increased as well. The researchers found that cigarette smoke depletes zinc and selenium levels in the brain, which is especially problematic because zinc is a SOD co-factor and selenium is a GPx co-factor. Administration of bacoside A also restored zinc and selenium levels.
In a related study, Anbarasi et al.57 also showed that 10 mg/kg aqueous gavage administration of bacoside A inhibited lipid peroxidation, improved the activities of adenosine triphosphatases (ATPases), and maintained ionic equilibrium in the brains of cigarette smoke–exposed rats.
Jadiya et al.58 investigated the effect of BM on aggregation of alpha-synuclein, dopamine (DA) neuron degeneration, lipid profile, and longevity in a transgenic and pharmacologically induced 6-hydroxydopamine (6-OHDA) Parkinson disease (PD) model in Caenorhabditis elegans. The transgenic nematode expressed a “human” version of alpha-synuclein. The researchers found BM extract resulted in a statistically significant 3.5-fold reduction in alpha-synuclein protein aggregation, perhaps due to induction of the stress-buffer protein Hsp-70.59 BM delivered a highly significant 2.7-fold protection against 6-OHDA–induced damage. PD is associated with altered fatty acid levels.60 Accordingly, alpha-synuclein–expressing nematodes exhibited a significant 2.2-fold reduction in total lipids compared to control. Interestingly, in worms expressing human alpha-synuclein who were treated with BM, lipid content was ∼15% higher than the control. These findings indicate BM as a potentially useful PD therapy. Finally, BM was found to produce a statistically significant increase in the life spans of the wild-type nematodes. The transgenic nematodes did not live significantly longer due to BM exposure.
Saini et al.61 found that BM (50 mg/kg per day oral) supplementation reversed memory impairment in colchicine-treated (15 μg in 5 μL artificial cerebrospinal fluid, intracerebroventricularly infused) rat model of Alzheimer disease. Colchicine is a microtubule-distrupting agent that induces cognitive decline via OS and neural death in the subventricular zone, dentate gyrus, and basal forebrain.62 BM significantly diminished colchicine-induced lipid peroxidation and protein carbonyl levels and restored activity of several anti-oxidant enzymes. In the elevated plus maze, colchicine increased transfer latency by 64%, whereas BM co-administration significantly reduced latency by 62%. Colchicine increased lipid peroxidation by 45% in the cortex and 33% in the hippocampus. Protein carbonyl levels were increased by 61% in the cortex and 63% in the hippocampus. Glutathione levels were reduced by 47% in the cortex and 45% in the hippocampus of colchicine-treated rats. All damage was restored to control levels by BM. Colchicine-induced changes in superoxide dismutase, catalase, GPx, GSR, glutathione-S-transferase, acetylcholine esterase (AChE), and Na+K+ATPase activity levels were all restored to levels comparable to controls.
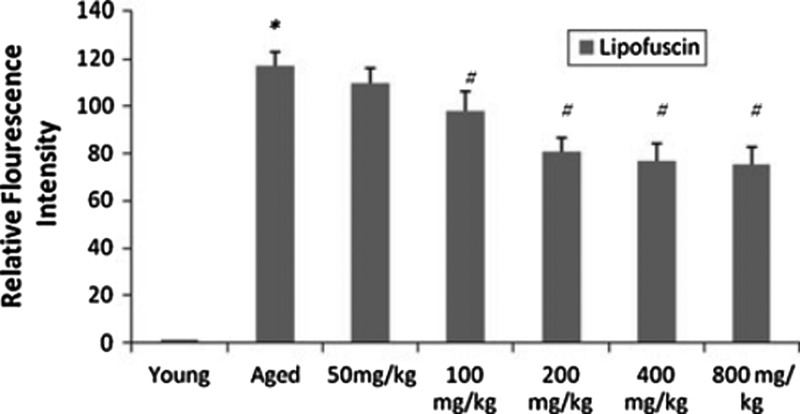
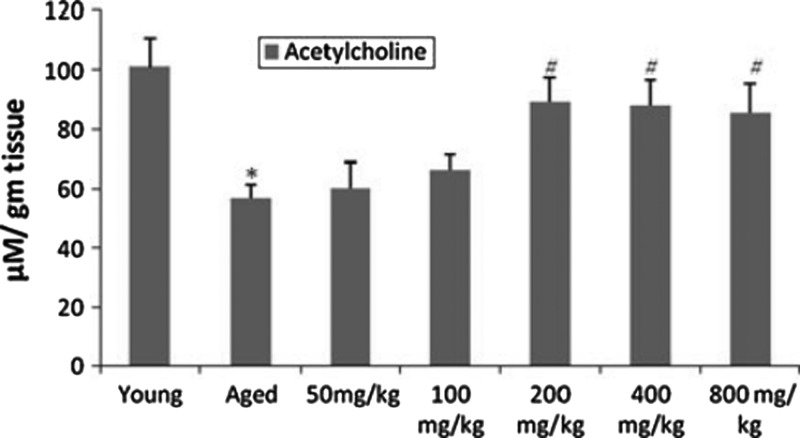
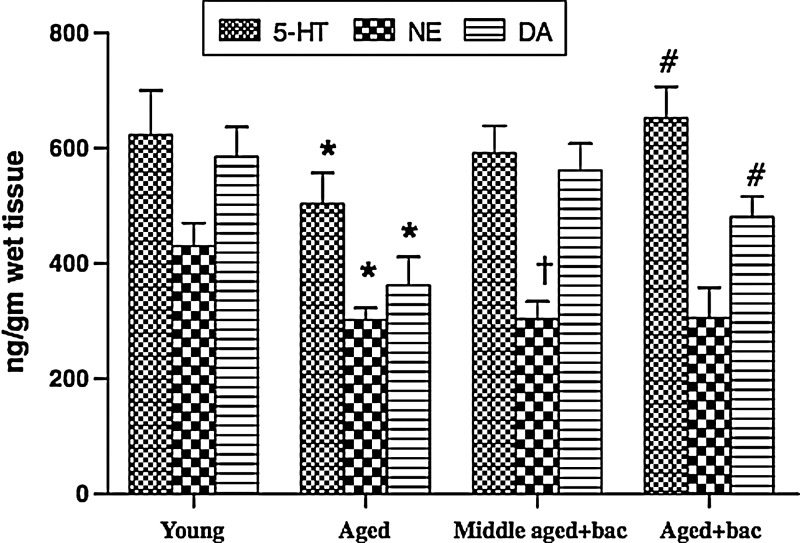
In a comprehensive study, Rastogi et al.7 investigated the neuroprotective mechanisms of purified bacosides (comprised of bacopaside I [5.37%], bacoside A3 [5.59%], bacopaside II [6.9%], bacopasaponin C isomer [7.08%], and bacopasaponin C [4.18%]) at dosages 50, 100, 200, 400, and 800 mg/kg per day orally for 3 months) on the aging biomarker lipofuscin (Fig. 1), oxidative stress, acetylcholine (ACh), (Fig. 2), monoamine levels (Fig. 3) as well as behavioral deficits in the aged rat brain. BM restored ACh and AChE concentrations to those seen in young rats. The authors supported the hypothesis63–65 that the primary ACh-boosting mechanism of BM is not AChE inhibition but choline acetyltransferase activation (synthesis of ACh), and that up-regulated AChE expression is a response to heightened ACh tone. The authors assayed the integrity of CA3 hippocampal neurons, finding that BM “profoundly” protected against age-related structural alterations. SOD and catalase (CAT) activity were not significantly improved, but GPx deficits in middle-aged rats were abolished. The increase in age-dependent protein carbonyl formation was not significantly attenuated by BM. Strong correlations between age-related biomarkers (lipid hydroperoxides and lipofuscin) and behavioral deficits were identified. Lipofuscin and 5-hydroxytryptamine (5-HT) levels were inversely correlated. Transfer latency and ambulation time in the passive avoidance test were inversely correlated with lipid hydroperoxide levels. Monoamine potentiation (5-HT and DA) was a remarkable finding, with concentrations in aged rats significantly restored to levels seen in the young. The behavioral effect was modeled using the tail-suspension depression test, showing an antidepressant effect in accordance with past research.22 This study demonstrated the efficacy of BM in preventing lipofuscin accumulation and enhancing acetylcholine synthesis, monoamine modulation, and inhibition of lipid peroxidation.
FIG. 1.
Dose-dependent changes in relative fluorescence intensity of lipofuscin obtained in chloroform fraction of brain tissue homegenate. Results are expressed as mean±standard deviation of 6 rats in each group. (*) p<0.05 for young versus aged; (#) p<0.05 for aged versus aged treated groups. (Reprinted, with permission, from Rastogi et al.7)
FIG. 2.
Effect of different doses of bacosides on neurotransmitter acetylcholine content in aged rate brain. Results are expressed as mean±standard deviation of 6 rats in each group. (*) p<0.05 for young versus aged; (#) p<0.05 for aged versus aged treated groups. (Reprinted, with permission, from Rastogi et al.7)
FIG. 3.
Amelioration of alterations in monoaminergic neurotransmitters with aging on long-term bacosides treatment. Concentration of serotonin, norepinephrin, and dopamine (ng/gram wet tissue) in the cortex of young, middle-aged, and aged treated or untreated rats. Values are expressed as mean±standard deviation of 6 animals in each group. Level of significance as calculated by one-way analysis of variance (ANOVA) followed by post hoc least significant difference: (*) p<0.05 for young versus aged; (†) p<0.05 for young versus middle-aged treated; and (#) p<0.05 for aged untreated versus aged treated group. 5-HT, 5-hydroxytryptamine; NE, norepinephrine; DA, dopamine. (Reprinted, with permission, from Rastogi et al.7)
In a follow-up study, Rastogi et al. 66 examined the effect of long-term (200 mg/kg orally per day for 3 months) bacoside administration on age-associated neuroinflammation. The researchers found significant decreases in pro-inflammatory cytokines (interleukin-1β, tumor necrosis factor-α but not interferon-γ), significant induction of inducible nitric oxide synthetase (iNOS) expression, and significant reduction of total nitrite and lipofuscin content in the cortex. A correlation between lipofuscin and IL-1β was also illustrated.
Shobana et al.67 examined the protective effects of ethanolic BM extract (orally administered 20 or 40 mg/kg per day for 3 weeks) on 6-OHDA–induced lesions in rats. The neurotoxin 6-OHDA is structurally similar to the catecholamines, which allows for uptake into specified terminals and is therefore useful in modeling Parkinsonian damage. Once inside, 6-OHDA increases lipid peroxidation, generates free radicals, modifies proteins, and diminishes enzymatic activity. On day 21, the authors administered 12 μg of 6-OHDA (suspended in 2 μL 0.1% ascorbic acid–saline solution) into the right striatum. Three weeks later, the rats were tested on the rotarod, radial arm maze, grip, and forced swim tests. The rats were then assayed for glutathione S-transferase (GST), GSR, GPx, SOD, and CAT activity in the brain. The researchers found both neurobehavioral deficits and enzyme activity significantly and dose-dependently restored by BM. Lipid peroxidation and reduced glutathione depletion were also prevented by BM treatment.
Singh et al.68 investigated BM's protective role against the herbicide paraquat (PQ) and 1-methyl-4-phenyl-pyridinium iodide (MPP+)-induced toxicities in a dopaminergic cell line. Some recent epidemiological evidence suggests a link between PQ use and Parkinson's disease onset.69 The authors found that BM pretreatment of 50 μg/mL significantly protected a dopaminergic cell line against both MPP+and PQ-induced toxicities. BM prevented the depletion of glutathione, preserved mitochondrial (MT) membrane potential and maintained MT complex I activity. Lower-concentration BM pretreatment (10.0 μg/ml) also prevented the generation of intracellular reactive oxygen species (ROS) and decreased the MT superoxide level. BM treatment activated the nuclear factor (erythroid-derived) 2 (Nrf2) pathway by modulating the expression of Keap1, effectively up-regulating endogenous glutathione synthesis.
Shinomol and Bharath70 showed that pretreatment with an alcohol extract of BM furnished considerable neuroprotection against 3-nitropropionic acid (3-NPA)-induced oxidative stress in vitro in N27 cells (2, 4, and 6 μg/mL) and pre-pubertal male mice (5 mg/kg orally per day of BM for 10 days). BM completely abolished 3-NPA–induced oxidative stress response in isolated striatal mitochondria in vitro. The authors examined OS and cholinergic function in mouse striatum, cortex, cerebellum, and hippocampus, finding that BM prophylaxis completely prevented 3-NPA damage. Increases of malondialdehyde, hydroperoxide, protein carbonyls, and ROS were abolished in the BM-treated group. BM prophylaxis also prevented reduced glutathione depletions. These findings strongly suggest efficacy as an adjuvant in oxidation-mediated neurodegenerative disorders.
Sumathi et al.71 found that BM extract (40 mg/kg oral for 21 days) “strongly” evidenced protection against methylmercury (MeHg, 5 mg/kg for 21 days) toxicity and behavioral deficits in rats. BM prevented MeHg-induced inhibition of SOD, CAT, and GPx. BM prevented MeHg-induced increases in glutathione reductase activity in the cerebellum. BM-deprived rats showed rotarod deficits, whereas the BM-treated group showed none. BM also significantly reduced serum NO2− and NO3− to nearly the control level.
Tripathi et al.5 conducted an early study on the in vitro anti-oxidant properties of BM, finding it to be a “potent” anti-oxidant in the presence of FeSO4 and cumene hydroperoxide. BM was compared to known anti-oxidants Tris, EDTA, and vitamin E. Alcoholic extract of BM (100 μg) was equivalent to 247 μg of EDTA and 58 μg of vitamin E. BM was suspected to work dose-dependently as a metal chelator and perhaps also a free-radical chain reaction-breaker.
Russo et al.72 investigated the anti-oxidant capacity of a whole plant powder methanol extract (12–25 μg/mL) at the level of in vitro free radical production/formation (using the Paoletti and 2,2-diphenyl-1-picryhydrazyl [DPPH]-radical assays). They found BM quenched free radical reagents dose-dependently and significantly reduced hydrogen peroxide–induced cytotoxicity and DNA damage in human non-immortalized fibroblasts.
Khan et al.73 found BM neuroprotective in an pilocarpine-induced epileptic rat model. Glutamatergic N-methyl-D-aspartate (NMDA) receptor 1 expression was down-regulated in the epilepsy group with no change in glutamate binding affinity. BM treatment of 300 mg fresh plant/kg per day orally for 15 days significantly reversed these changes to near-control levels (n=38). BM further reversed the increase of glutamate dehydrogenase seen in the epileptic group. The authors also employed Morris water maze latency time to evidence highly significant behavioral restoration by BM. These data indicate the broad neuroprotective role of BM in glutamate-mediated excitotoxicity.
George et al.74 found BM neuroprotective against acrylamide-induced neurotoxicity in three models—oxidative damage in the organs of mice (2% wt/wt in food for 30 days, n=6), cultured neuronal cell death (2–10 μg/mL), and locomotive disruption in Drosophila melanogaster (food media containing 0.01%, 0.05%, and 0.1% BM wt/vol for 3 days). Acrylamide (ACR) is a water-soluble vinyl monomer with many industrial and chemical applications, exposure to which results in neuropathy and impaired NT release. In all three models, BM pre- and co-treatment furnished significant protection against ACR toxicity, in some cases effectively restoring function to control levels.
Sandhya et al. 75 found BM (300 mg/kg oral, n=6) to ameliorate behavioral deficits and oxidative stress in a sodium valproate–induced autism model in rats. Hyper-excitability, locomotor activity, social exploration, and other behavioral patterns were significantly normalized. Reduced glutathione and CAT levels were significantly increased (comparable to control), and hippocampal serotonin and total nitrite levels were significantly reduced (near-control levels). The intervention also significantly improved behavioral alterations and restored the histoarchitecture of the cerebellum.
Jyoti et al.76 used the free-radical generator aluminium chloride (AlCl3) to test BM extract (standardized to 50% bacoside A) neuroprotection in the rat hippocampus. Male Wistar rats were administered AlCl3 orally at a dose of 50 mg/kg per day in drinking water for 30 days. Experimental rats were given AlCl3 along with BM extract at a dose of 40 mg/kg per day. They found BM co-administration to significantly prevent the typical aluminum-induced decrease in SOD activity and that BM prevented lipid peroxidation and protein denaturation. Fluorescence and electron microscopy studies revealed inhibition of intra-neuronal lipofuscin accumulation (an indication of lipid oxidation and cellular damage) and necrotic alteration in the hippocampus. BM showed effects comparable to l-deprenyl (1 mg/kg per day oral).
Priyanka et al.77 compared l-deprenyl (l-D) to BM, finding that both agents significantly increased nerve growth factor and tyrosine hydroxylase in the hippocampus and spleen of rats. Three-month-old female Wistar rats were treated with 10 and 40 mg/kg BM and 1 and 2.5 mg/kg l-D for 10 days. The two agents likely work via different mechanisms: Both l-D and BM increased nuclear factor-kB expression, whereas l-D alone augmented extracellular signal-regulated kinase (ERK 1/2) and cAMP response element-binding protein (CREB). Both l-D and BM were found to enhance anti-oxidant/detoxification enzyme activity in the spleen, brain, heart, thymus, and mesenteric lymph nodes.
Some of the same authors found BM to interact with CREB and other synaptic plasticity–related pathways, showing BM significantly improved retention performance in a brightness discrimination task.78 Further research into intracellular signaling molecules is warranted.
According to Liu et al., 79 bacopaside I exhibits neuroprotective, anti-oxidant, and cerebral ATP-increasing effects post-cerebral ischemia in rats (3, 10, and 30 mg/kg orally for 6 days). The singular bacopaside significantly reduced neurological deficits and infarct volume while significantly increasing brain ATP content, energy charge, total adenine nucleotides, nitric oxide, Na+K+ATPase, and Ca2+Mg2+ATPase activity. Bacopaside I treatment also improved anti-oxidant enzyme activities including SOD, CAT, GPx, and markedly inhibited the increase in malondialdehyde (a free radical marker) content of the brain.
Rotenone is a pesticide capable of inducing DA neuron damage in the substantia nigra similar to that seen in PD.80 The compound is thought to operate by exerting oxidative stress. Hosamani et al.81 found that standardized BM powder protected against rotenone-induced oxidative damage and dopamine depletion in Drosophila melanogaster. BM (0.05% and 0.1% wt/wt in food) offered protection against a 500 μM dose of rotenone and inhibited central nervous system (CNS) DA depletion by 33% and peripheral nervous system (PNS) depletion by 44%. Flies dosed with both rotenone and BM exhibited a lower incidence of mortality (40%–66% protection) and 45%–65% better performance on a negative geotaxis assay (a measure of how well flies climb up a vial). BM also conferred 34%–54% resistance to oxidative damage inflicted by paraquat.
In a related study, BM (2–6 μg/mL) significantly attenuated rotenone-induced oxidative stress and N27 cell death. BM (5 mg/k intraperitoneally [i.p.] daily for 7 days) also normalized protein carbonyl content throughout the mouse brain and restored cytosolic anti-oxidant enzyme and dopamine levels in the striatum.82
Rohini et al. 83 investigated the anti-oxidant and anti-neoplastic properties of BM (20 mg/kg, subcutaneous) in 3-methylcholanthrene–induced fibrosarcoma rats. Glutathione, CAT, SOD, and GPx activity were significantly increased by BM, whereas lipid peroxidation and tumor markers like lactate dehydrogenase, creatine kinase, alanine transaminase, aspartate transaminase, and sialic acid were all significantly reduced.
Briefly, BM protects against nitric oxide (NO)-related toxicity in cultured astrocytes,84 and that BM's anti-oxidant potency is greater than ascorbic acid.85 Research on the anti-oxidant and neuroprotective properties of BM is prolific, and necessarily some older or redundant studies must be omitted from this review for the sake of brevity.86–94
Cerebral blood flow and vasodilation
Adequate perfusion of blood to capillary beds within the brain is of utmost importance. Otherwise, deficits of oxygen and nutrients will ensue alongside the buildup of cytotoxic waste. Diminished cerebral blood flow is implicated in various pathologies, including dementia.95
Kamkaew et al.96 compared the effect of daily oral BM (40 mg/kg oral) and Gingko biloba (60 mg/kg oral) on cerebral blood flow (CBF) in rats. In their 8-week trial, rats treated with BM saw a significant 25% increase in CBF, although Gingko biloba increased CBF by 29% (albeit at a 20-mg higher dosage). Chronic oral BM administration had no effect on blood pressure, whereas intravenous infusion decreased diastolic blood pressure ∼31 mmHg with 40 mg/kg of either extract, correspondingly decreasing CBF by 15%. BM appears to act as a vasodilator by releasing NO from the endothelium and inhibiting calcium fluctuations in and out of the sarcoplasmic reticulum.97 More research on this property of BM is warranted.
Neurotransmitter potentiation
Adaptogens enable the body to better cope with the deleterious mental and physical consequences of stress. Eleutherococcus senticosus (Siberian ginseng), Rhodiola rosea, and Panax ginseng are classic adaptogens. Others include Ocimum sanctum (Sweet Holy Basil or Tulsi), Withania somnifera (Ashwaghanda), Astragalus propinquus, Ganoderma lucidum (Reishi mushroom), and many others.98 BM also exhibits adaptogenic qualities. One putative action of the adaptogen is modulation of neurotransmitter production, release, and synaptic concentration.
Sheikh et al. 99 evaluated BM's adaptogenic effect in acute stress and chronic unpredictable stress-induced fluctuations of plasma corticosterone and monoamines in the rat cortex and hippocampus. Panax quinquefolium (PQ) was used as a positive control. Immobilization stress resulted in significant elevation of plasma corticosterone levels, which was significantly reduced by BM at oral doses of 40 and 80 mg/kg, comparable to oral PQ at 100 mg/kg. Treatment with BM attenuated stress-induced changes in levels of 5-HT and DA in the cortex and hippocampus but was ineffective in normalizing noradrenaline (NA) levels in the acute stress model, whereas PQ treatment significantly attenuated all assayed neurochemical effects of acute stress. In the chronic stress model, pretreatment with BM and PQ significantly elevated levels of NA, DA, and 5-HT in the cortex and NA and 5-HT in the hippocampus compared to controls. Prevention of NT depletion is the cornerstone of adaptogenic stamina enhancement, both physical and mental.
Charles et al.100 found BM extract up-regulated tryptophan hydroxylase (TPH2) and serotonin transporter (SERT) expression in rats. The animals were orally administered BM extract (31% bacosides, 40 mg/kg for 15 days) and tested on a Y-maze, hole board, and passive avoidance tasks. The rats' performance dose-dependently and highly significantly improved on seven of eight measures of latency and acquisition. Levels of 5-HT in the BM groups were almost double the control level, which returned to baseline after the treatment period. Glutamate and ACh levels were increased by BM, but not significantly. DA levels were significantly lower (approximately 9%) in BM-treated rats. There were also changes noted in receptor expression. BM elicited highly significant increases in both TPH2 and SERT mRNA levels, almost double the control. These elevated levels returned to baseline 24 days after BM administration ceased. This experiment supports the case that BM enhances learning and memory, but possibly through a novel mechanism involving 5-HT, SERT, and TPH2. The considerable elevation of 5-HT and moderate but significant reduction in DA require further investigation.
Dementia and cognitive dysfunction
Dementia is a global loss of cognitive ability. Aging is a major risk factor for dementia, which includes various types, such as vascular dementia, frontotemporal degenerative dementia, Lewy body dementia, and Alzheimer disease. Dementia results secondarily from many neurodegenerative disorders. The exact etiology of Alzheimer dementia is uncertain and controversial, but there is a general consensus about some of the factors that may be involved. Free radical-induced OS is one such factor.101,102 It is unclear whether OS is primary to the disease process or a secondary by-product, but the presence of OS does appear to play a major role in illness severity.103,104 Cell loss, impaired energy metabolism, dystrophic neurites, DNA damage, β-amyloid plaques, and neurofibrillary tangles are also thought to play key roles.105 Researchers have also put forward the hypothesis that Alzheimer disease is at least partially mediated by insulin resistance, leading some to brand the condition “type 3 diabetes.”106 Deficits in ACh are also often seen in dementia patients, and the dominant therapeutic agents are AChE inhibitors.107
Despite some controversy, cigarette smoking appears to increase dementia risk.108–110 Despite containing nicotine itself, BM protects against nicotine-induced lipid peroxidation and mutagenicity in mice. Aqueous BM extract (50 mg/kg i.p.) restored anti-oxidant enzymes SOD, CAT, and GPx in the liver. BM treatment also significantly decreased the incidence of micro-nucleated polychromatic erythrocytes (micro-nucleation is a product of chromosome damage). Hepatic glutathione, alkaline phosphatase, and glutathione-S-transferase levels were brought to normal values, indicating hepatoprotection.111
Mathew and Subramanian112 examined the effect of BM extract on free β-amyloid aggregation in vitro using fluorescence imaging. They compared a whole plant BM methanol extract (100 μg/μL) to 12 other herb extracts, finding several to prevent aggregation of β-amyloid plaques and to dissociate pre-formed fibrils. Incubating with BM extract was found to “almost completely” inhibit β-amyloid formation. It is unclear whether this activity would take place in vivo.
Scopolamine (SC) is a powerful muscarinic ACh antagonist that impairs long-term potentiation (LTP) and memory.113 Saraf et al.114 found that BM extract (120 mg/kg oral, 55.35% bacosides) effectively reversed SC-induced anterograde and retrograde amnesia (Morris water maze) in mice. Another group of researchers isolated specific triperpenoid saponins from BM and evaluated their reversal of SC-induced amnesia in mice, finding potential in bacopaside I and XI and bacopasaponin C.115
A subsequent study by Saraf et al.116 investigated the effect of SC on downstream signaling molecules involved in LTP and attenuation of changes by BM in mice. The researchers found that SC decreased calmodulin expression and down-regulated protein kinase A, mitogen-activated protein kinase (MAPK), CREB, and pCREB. SC also down-regulated protein kinase C and iNOS without affecting cyclic adenosine monophosphate (cAMP). BM (120 mg/kg i.p., 55.35% bacosides) reversed the SC-induced amnesia by significantly improving calmodulin activity and partially attenuating changes of protein kinase C and pCREB.
Kinshore and Singh117 found BM to attenuate SC, sodium nitrite, and BN52021-induced amnesia, possibly by boosting ACh levels and supporting function under hypoxic conditions. Gingko biloba (15, 30, and 60 mg/kg) and BM (30 mg/kg) were administered for 1 week and compared in a scopolamine-induced mouse amnesia model using the passive avoidance test. Dementia effects were significantly attenuated (40%–80% over control) by both herb extracts separately. Both herbs also showed a dose-dependent AChE inhibitory effect in vitro, with higher potency in Gingko biloba (inhibitory concentration 50 [IC50]=268 μg); BM AChE inhibition did not exceed 50% (an asymptotic effect, perhaps useful in capping ACh excess).118
A recent study by Piyabhan and Wetchateng119 investigated the neuroprotective function of BM (40 mg/kg per day orally for 14 days, n=72) on novel object recognition in a phencyclidine-induced rat model of schizophrenia, finding highly significant protection and improved performance. The same authors conducted another study on vesicular glutamate transporter 1 (VGLUT1), of which schizophrenics have a deficit in the prefrontal cortex, striatum, and hippocampus. Again, a phencyclidine rat model was used. The researchers found significant improvement in all three brain-regions in the BM group.120
Dhanasekaran et al.121 demonstrated that an ethanol extract of BM exhibited neuroprotective effects. BM reduced divalent metals, dose-dependently scavenged ROS, decreased the formation of lipid peroxides, and inhibited lipoxygenase activity in mouse brain homogenate in vitro (100, 250, 500 μg BM extract).
In another study, some of the same researchers found that BM (40 or 160 mg/kg per day orally starting at 2 months of age and lasting 2 or 8 months) reduced β-amyloid plaques in an Alzheimer PSAPP mouse model by up to 60%. BM also reversed Y-maze under-performance and open-field hyperlocomotion.122
A comprehensive in vitro study by Limpeanchob et al.123 on rat cortical neurons found BM to protect against β-amyloid-induced (but not glutamate-induced) neurotoxicity. The mechanism was attributed to greater anti-oxidant activity and AChE inhibition in the BM group (0–500 μg extract verified to contain 5.045%±0.400 bacoside A3, bacopaside II, bacopasaponin C isomer, and bacopasaponin C).
Uabundit et al.124 used a rat Alzheimer model (ethylcholine aziridinium ion-induced) and found that 2 weeks of pre-treatment and 1 week post-inducement oral administration of BM extract (20, 40, 80 mg/kg) significantly reduced latency time in a Morris water maze test and mitigated reduction in cholinergic neuron number and density.
Learning and memory
BM may have a potential application to enhancing cognition in healthy subjects. Singh and Dhawan125 administered rats an ethanolic whole plant BM extract (40 mg/kg orally) for 3 or more days and evaluated cognitive performance using shock-motivated brightness-discrimination reaction, active conditioned flight reaction, and continuous avoidance response tests. The BM-treated group showed significantly better acquisition, improved retention, delayed extinction, and faster reaction times than controls.
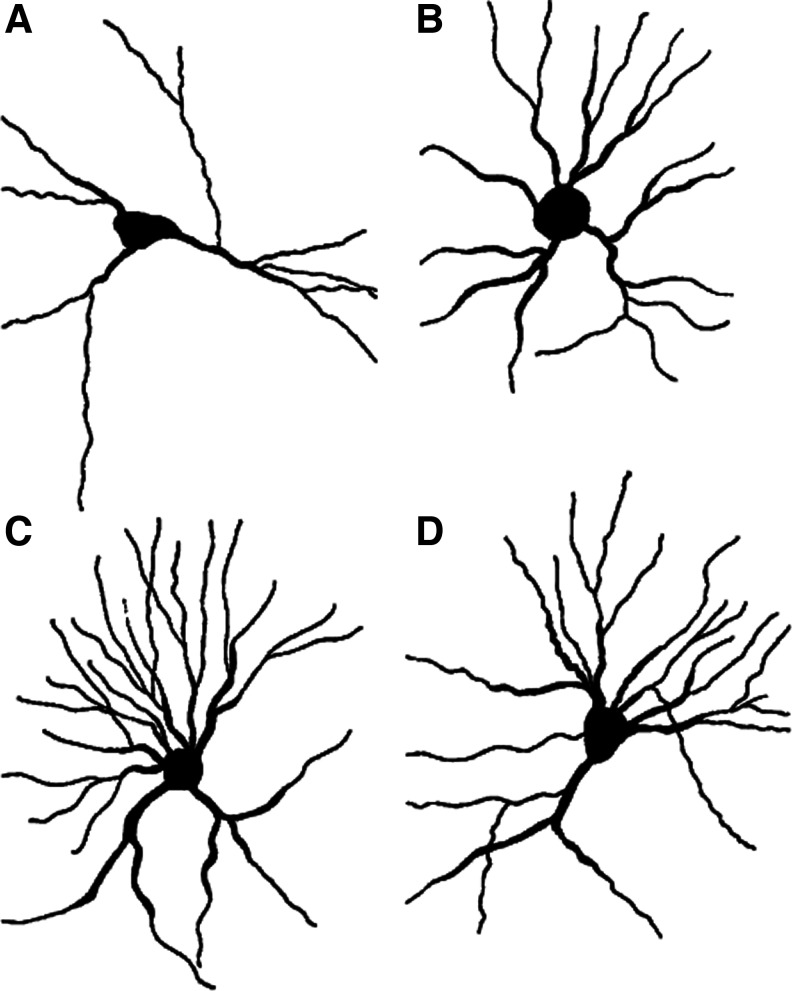
Vollala et al.126 studied the effect of BM on the dendritic morphology of neurons in the basolateral amygdala, a region implicated in learning and memory. Twenty-four rats were divided into 2-, 4-, and 6-week treatment groups, and then further divided into 20, 40, and 80 mg/kg per os (p.o.) dose groups and age matched to controls. All of the rats were tested in spatial learning and passive avoidance tests. They were then decapitated, and their brains were Golgi stained and evaluated for dendritic branching (arborization) and intersections (length). The results indicated an improvement in spatial learning and memory retention and a significant increase in dendritic length and branching points within the basolateral amygdala at the doses 40 and 80 mg/kg at the 4- and 6-week intervals (Fig. 4, Tables 1 and 2).
FIG. 4.
Camera lucida tracings of basolateral amygdaloid neurons from control rats (A) and rats treated with Bacopa monniera for 6 weeks at doses of 20 mg/kg (B), 40 mg/kg (C), and 80 mg/kg (D). (Reprinted, with permission, from Vollala et al.126
Table 1.
Basolateral Amygdaloid Neuronal Dendritic Intersections and the Number of Dendritic Processes Arising from the Somata (6-Week Duration)
| |
|
Distance from soma, μm |
|
||||
|---|---|---|---|---|---|---|---|
| Groups | n | 20 | 40 | 60 | 80 | 100 | Number of processes |
| NC | 8 | 5.34±0.56 | 6.86±1.12 | 6.39±0.50 | 4.58±0.71 | 3.45±0.69 | 4.31±0.64 |
| GAC | 8 | 5.87±0.61 | 7.15±0.75 | 6.25±0.83 | 4.37±0.68 | 3.34±0.48 | 4.39±0.58 |
| BM 20 mg/kg | 8 | 6.12±0.65 | 9.08±1.17## | 8.14±1.25## | 5.18±0.56 | 3.67±0.44 | 4.85±0.70 |
| BM 40 mg/kg | 8 | 7.60±0.73*** | 12.18±1.24*** | 11.81±1.09*** | 8.13±1.04*** | 5.08±0.96** | 5.57±0.62*** |
| BM 80 mg/kg | 8 | 7.38±0.59$$$ | 11.69±1.42$$$ | 11.47±0.94$$$ | 7.79±0.87$$$ | 4.93±1.13$$ | 5.41±0.43$$ |
Note: Each value represents mean±standard deviation. NC vs. BM 20 mg/kg: ##p<0.01; NC vs. BM 40 mg/kg: **p<0.01, ***p<0.001; NC vs. BM 80 mg/kg: $$p<0.01, $$$p<0.001.
BM, Bacopa monniera; GAC, gum acacia control; NC, normal control.
Reprinted with permission from Vollala et al.126
Table 2.
Basolateral Amygdaloid Neuronal Dendritic Branching Points at Different Concentric Zones and Total Number Branching Points (6-Week Duration)
| |
|
Concentric zones, μm |
|
||||
|---|---|---|---|---|---|---|---|
| Groups | n | 0–20 | 20–40 | 40–60 | 60–80 | 80–100 | Total number of branching point |
| NC | 8 | 1.43±0.45 | 2.68±0.56 | 1.12±0.32 | 0.52±0.42 | 0.13±0.10 | 5.91±1.05 |
| GAC | 8 | 1.56±0.42 | 2.76±0.61 | 1.08±0.43 | 0.48±0.39 | 0.11±0.09 | 6.01±1.10 |
| BM 20 mg/kg | 8 | 1.65±0.64 | 3.86±0.67# | 1.93±0.64# | 0.61±0.41 | 0.23±0.13 | 8.15±0.98## |
| BM 40 mg/kg | 8 | 3.16±0.70*** | 5.26±0.75*** | 2.92±0.55*** | 1.40±0.62* | 0.46±0.22* | 13.21±1.20*** |
| BM 80 mg/kg | 8 | 2.92±0.81$$$ | 5.08±0.83$$$ | 2.85±0.61$$$ | 1.28±0.54$ | 0.42±0.29$ | 12.55±1.35$$$ |
Note: Each value represents mean±standard deviation. NC vs. BM 20 mg/kg: #p<0.05, ##p<0.01; NC vs. BM 40 mg/kg: *p<0.05, ***p<0.001; NC vs. 80 mg/kg: $p<0.05, $$$p<0.001.
BM, Bacopa monniera; GAC, gum acacia control; NC, normal control.
Reprinted with permission from Vollala et al.126
In another study, Vollala et al.127 found highly significant improvement in learning and memory in rats administered 40 and 60 mg/kg p.o. on passive avoidance and T-maze tests, with effects increasing the longer the herb was administered (study length of 6 weeks). The lower dosage of 20 mg/kg had significant effects only at the 6-week mark, whereas the two higher dosages began to show significance as early as 2 weeks.
Rajan et al.128 investigated the effect of BM on serotonergic receptor 5-HT3A expression as well as ACh and 5-HT levels during a hippocampal-dependent learning task. Standardized 80 mg/kg p.o. BM extract (55±5% bacosides) was also shown to significantly attenuate 1-(m-chlorophenyl)-biguanide (an 5-HT3A agonist)-induced memory impairment. Compared to the control, BM highly significantly increased 5-HT (39%), ACh (20%), glutamate (20%), and γ-aminobutyric acid (GABA) (20%) levels in the hippocampus. BM highly significantly but more moderately reduced DA levels (16%). This study is particularly notable because it provides additional evidence that constituents of BM interact with the serotonergic system.
The anticonvulsant phenytoin adversely affects cognitive function. Vohora et al.129 combined BM with phenytoin on passive-avoidance, maximal electroshock seizures and locomotor activity in mice. Phenytoin (25 mg/kg p.o. for 14 days) adversely affected cognitive function in the passive avoidance task. BM extract (40 mg/kg p.o. for 7 days) significantly reversed phenytoin-induced memory impairment. Both memory acquisition and retention showed improvement without affecting phenytoin's anti-convulsant activity, supporting BM use as an adjuvant for epileptics and possibly a nootropic for non-epileptics.
Benzodiazepines induce amnesia via GABAergic activation and changes in LTP. Saraf et al.130 used the Morris water maze to examine the effect of downstream LTP markers following diazepam-induced amnesia in mice. They found 120 mg/kg oral BM dosage sufficient to significantly reverse diazepam (1.75 mg/kg i.p.) -induced amnesia. BM also suppressed the diazepam-induced up-regulation of MAP kinase, pCREB, and iNOS.
Prisila et al.131 found that 80 mg/kg p.o. BM extract (55%±5% bacosides) protects against D-galactose (D-gal)-induced brain aging in rats in a contextual-associative learning task. BM-treated individuals showed highly significantly more correct responses and less latency than control and D-gal–treated rats. BM administration significantly decreased advanced glycation end products (AGE) in serum and increased the activity of anti-oxidant response element (ARE) and the anti-oxidant enzymes SOD and GPx. BM increased nuclear transcription factor NF-E2–related factor 2 and the level of hippocampal 5-HT. BM up-regulated expression of the presynaptic proteins synaptotagmin I and synaptophysin. BM also up-regulated calmodulin-dependent protein kinase II and post-synaptic density protein-95 in the hippocampus, involved in neurotransmitter release and synaptic plasticity. Interestingly, rats treated with both D-gal and BM outperformed even the control animals (no D-gal) on several measures. Once again, this study provides compelling evidence that BM protects against age-related neurodegeneration.
Clinical Studies
Although BM clinical research is in its infancy, at least six high-quality (by the Jadad Scale132) randomized, double-blind, placebo-controlled (RDBPC) human trials have been conducted. Pase et al.133 conducted a systematic review, finding some evidence that “Bacopa could potentially be clinically prescribed as a memory enhancer” even in non-demented subjects. BM administration significantly improved 9 out of 17 free recall memory tasks. One out of six of the studies' collective scores evidenced improved memory span; however, BM showed no improvement in memory involving the recall of short passages of text. The analysis of Pace et al. supports previous findings that BM most effectively reduces the rate of forgetting, but not acquisition or other aspects of cognition (though this matter is by no means settled).
In a meta-analysis, Neale et al.134 compared the nootropic effects of BM to Panax ginseng and modafinil (an eugeroic-wakefulness drug). Chronic BM produced the most consistent and largest effect sizes of the three. BM showed small- to medium-effect sizes for attention and information processing tasks. Larger-effect sizes were evident for auditory verbal learning tasks, sizes ranging from d=0.23 for delayed word pair memory to d=0.95 for delayed word recall (on the Auditory Verbal Learning Test) and d=1.01 for protection from proactive interference during delayed memory. These findings evidence the potency of BM, particularly in measures of verbal recall. Remarkably, contemporary findings appear to support the alleged use of BM in Vedic antiquity by scholars memorizing lengthy hymns.
Some of the clinical studies described in detail in Pace et al. will be summarized here. All subjects were administered BM orally. Stough et al.135 conducted a RDBPC study in 46 healthy adults (300 mg/day standardized to 55% bacosides) for 12 weeks. A battery of eight tests was taken, finding significantly improved speed of visual information processing, learning rate, memory consolidation, and state anxiety compared to placebo, with maximal effects evident after 12 weeks.
Roodendrys et al.136 conducted another DBRPC trial wherein 76 healthy adults aged 40–65 were administered 300–450 mg standardized (55% bacosides) extract per day. Reseachers found BM not to increase the rate of learning (apprehension), but the rate of forgetting was significantly attenuated in word pair recall trials after 3 months of chronic use. Morgan and Stevens137 conducted a DBRPC trial with 81 elderly Australians (300 mg/day concentrated extract), finding highly significant improvements in verbal learning, memory acquisition and delayed recall.
No known clinical studies have yet evidenced an acute effect of BM on human cognition. Nathan et al.138 conducted a RDBPC trial using a single administration of 300 mg extract with testing 2 hr post-administration. The researchers found no significant acute cognitive effect in the BM group. The absence of noticeable acute effects may diminish the likelihood of dependency-forming or -reinforcing behavior. More research must be done to firmly establish this case.
To summarize, the clinical research evidences BM to be effective in decreasing the rate of forgetting (especially verbal material), but perhaps only in tandem with chronic use. Although preliminary results are promising, large-scale clinical studies need to be conducted.
Toxicology
Adverse side effects of BM are rarely reported in humans. The most commonly reported and statistically significant symptoms are nausea, increased intestinal motility, and gastrointestinal upset. It is critical that long-term hematological studies be conducted with humans. In animal models, however, toxicity has been more precisely described.
Allen et al. 139 found the rat LD50 to be 2400 mg/kg following a single oral administration. The NOAEL of 500 mg/kg orally for 14 days was established based on a mild lowering of body weight in male rats. At 500 mg/kg, the rats ate less food than controls (−19% males, −16% females). After 90 days at this NOAEL level, rats showed a mild but significant increase in liver weight. Hematological parameters were largely unaffected in the 500-mg/kg group, except for moderate but significant changes in alanine aminotransferase, aspartate aminotransferase, albumin, globulin, urea nitrogen, urea, and sodium, although still within the normal range for controls.
Singh and Dhawan140 conducted a placebo-controlled, double-blind phase I clinical trial using single-dose (20 mg up to 300 mg) bacosides A and B in 31 healthy male adults. No adverse effects were reported. A multiple dosage trial (100 mg or 200 mg oral per day) was conducted for 4 weeks. Clinical, hematological, and biochemical assays revealed no abnormalities. The absence of evidence is not, however, evidence of absence and caution is still warranted.
Singh and Singh141 found BM caused a reduction in motility, viability, morphology, and number of spermatozoa in the mouse epididymis without any reduction in libido. Serum testosterone, alanine aminotransferase, aspartate aminotransferase, and creatinine were all unaffected. Noteworthy histological alternations of seminiferous tubules and epididymis did occur. BM significantly suppressed fertility in male mice. The anti-fertility effect was reversible, with parameters normalizing to control levels 56 days after the drug was withdrawn. A similar reversible anti-fertility effect was also seen during Curcuma longa (turmeric rhizome) administration.142
Rauf et al. 143 found no effect of acute methanolic BM extract (10–30 mg/kg oral) on dopamine and serotonin turnover or behavioral changes in rats,144 indicating diminished risk of adverse dispositional change due to NT imbalance, as seen for instance in selective serotonin reuptake inhibitors.
In comparison to the 500 mg/kg NOAEL established by Allen et al., a sizeable human dosage of 3000 mg BM per day in a 60-kg individual would equal 50 mg/kg. Without accounting for the considerable metabolic differences between rodents and humans, an individual would require 25 grams of BM to reach the NOAEL level, and 120 grams to reach the LD50 in rats. The high therapeutic index for cognitive enhancement furnishes a reasonable buffer, but more research must be done, particularly in longitudinal clinical trials.
Conclusion
BM demonstrates immense potential in the amelioration of cognitive disorders, as well as prophylactic reduction of oxidative damage, NT modulation, and cognitive enhancement in healthy people. Biomedical research on BM is still in its infancy, but preliminary results such as these have begun to open the research floodgates. It is critical that much longer-term studies be conducted BM in combination with other substances, as is prescribed by the Ayurvedic system, may result in synergistic effects and should also be investigated.145–148 The social implications of cognition-enhancing drugs are promising but must be appropriately tempered with ethical consideration as researchers enter the brave new world of neural enhancement.149
Acknowledgments
We thank Clinics (Sao Paulo), Suresh Rattan, and Springer Science for permission to reprint selected figures.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mohamed A. Modafinil has the potential for addiction. AJOB Neuroscience. 2012;3:36–38. [Google Scholar]
- 2.Darke S. Kaye S. McKetin R. Duflou J. Physical and psychological harms of psychostimulant use. Technical Report No. 286 National Drug and Alcohol Research Center. 2007 [Google Scholar]
- 3.Bell S. Lucke J. Hall W. Lessons for enhancement from the history of cocaine and amphetamine use. AJOB Neurosci. 2012;3:24–29. [Google Scholar]
- 4.Blazer DG. Federspiel CF. Ray WA. Schaffner W. The risk of anticholinergic toxicity in the elderly: A study of prescribing practices in two populations. J Gerontol. 1983;38:31–35. doi: 10.1093/geronj/38.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi YB. Chaurasia S. Tripathi E. Upadhyay A. Dubey GP. Bacopa monniera Linn. as an antioxidant: Mechanism of action. Indian J Exp Biol. 1996;34:523–526. [PubMed] [Google Scholar]
- 6.Ghosh T. Maity TK. Das M. Bose A. Dash DK. In vitro antioxidant and hepatoprotective activity of ethanolic extract of Bacopa monnieri. IJPT. 2007;6:77–85. [Google Scholar]
- 7.Rastogi M. Ojha R. Prabu PC. Devi DP. Agrawal A. Dubey GP. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology. 2012;13:183–195. doi: 10.1007/s10522-011-9367-y. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Traditional Medicine, Fact Sheet. WHO. 2003:103. [Google Scholar]
- 9.Barnes PM. Bloom B. Nahin RL. Complementary and alternative medicine use among adults and children: United States. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- 10.Ni H. Simile C. Hardy AM. Utilization of complementary and alternative medicine by United States adults: Results from the 1999 national health interview survey. Med Care. 2002;40:353–358. doi: 10.1097/00005650-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Bodeker G. Kronenberg F. A public health agenda for traditional, complementary, and alternative medicine. Am J Public Health. 2002;92:1582–1591. doi: 10.2105/ajph.92.10.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg DM. Kessler R. Foster C. Norlock F. Calkins D. Delbanco T. Unconventional medicine in the US: Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 13.Patwardhan B. World Health Organization; 2005. Traditional medicine: A novel approach for available, accessible and affordable health care. [Google Scholar]
- 14.Ecological Society of America. Ecosystem Services: Benefits supplied to human societies by natural ecosystems. Issues Ecol. 1997:2. [Google Scholar]
- 15.Howes JR. Houghton PJ. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav. 2003;75:513–527. doi: 10.1016/s0091-3057(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 16.Barrett SC. Strother JL. Taxonomy and natural history of Bacopa in California. Syst Bot. 1978;5:408–419. [Google Scholar]
- 17.Plassman BL. Langa KM. Fisher GG. Heeringa SG. Weir DR. Ofstedal MB. Burke JR. Hurd MD. Potter GG. Rodgers WL. Steffens DC. Willis RJ. Wallace RB. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federal Interagency Forum on Aging-Related Statistics. Older Americans. Key Indicators of Wellbeing 2012 [Google Scholar]
- 19.Mathew J. Gangadharan G. Kuruvilla KP. Paulose CS. Behavioral deficit and decreased GABA receptor functional regulation in the hippocampus of epileptic rats: Effect of Bacopa monnieri. Neurochemical Res. 2011;36:7–16. doi: 10.1007/s11064-010-0253-9. [DOI] [PubMed] [Google Scholar]
- 20.Khan R. Krishnakumar A. Paulose CS. Decreased glutamate receptor binding and NMDA R1 gene expression in hippocampus of pilocarpine-induced epileptic rats: Neuroprotective role of Bacopa monnieri extract. Epilepsy Behav. 2008;12:54–60. doi: 10.1016/j.yebeh.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Mathew J. Paul J. Nandhu MS. Paulose CS. Bacopa monnieri and Bacoside-A for ameliorating epilepsy associated behavioral deficits. Fitoterapia. 2010;81:315–322. doi: 10.1016/j.fitote.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Sairam K. Dorababu M. Goel RK. Bhattacharya SK. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine. 2002;9:207–211. doi: 10.1078/0944-7113-00116. [DOI] [PubMed] [Google Scholar]
- 23.Abbas M. Subhan F. Mohani N. Rauf K. Ali G. Khan M. The involvement of opioidergic mechanisms in the activity of Bacopa monnieri extract and its toxicological studies. Afr J Pharm Pharmacol. 2011;5:1120–1124. [Google Scholar]
- 24.Vohora SB. Khanna T. Athar M. Ahmad B. Analgesic activity of bacosine, a new triterpene isolated from Bacopa monnieri. Fitoterapia. 1997;4:361–365. [Google Scholar]
- 25.Afjalus S. Chakma N. Rahman M. Salahuddin M. Kumar S. Assessment of analgesic, antidiarrhoeal and cytotoxic activity of ethanolic extract of the whole plant of Bacopa monnieri linn. IRJP. 2012;3(10) online publication. [Google Scholar]
- 26.Jain P. Khanna NK. Trehan N. Pendse VK. Godhwani JL. Anti-inflammatory effects of an Ayurvedic preparation, Brahmi Rasayan, in rodents. Indian J Expt Biol. 1994;32:633–636. [PubMed] [Google Scholar]
- 27.Azad A. Awang M. Rahman M. Akter S. Biological and pre-clinical trial evaluation of a local medicinal plant Bacopa monnieri (L.) IJCRR. 2012;4:92–99. [Google Scholar]
- 28.Sairam L. Rao C. Babu M. Goel RK. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine. 2001;8:423–430. doi: 10.1078/S0944-7113(04)70060-4. [DOI] [PubMed] [Google Scholar]
- 29.Goel RK. Sairam K. Babu MD. Tavares IA. Raman A. In vitro evaluation of Bacopa monniera on anti-Helicobacter pylori activity and accumulation of prostaglandins. Phytomedicine. 2003;10:523–527. doi: 10.1078/094471103322331494. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya SK. Ghosal S. Anxiolytic activity of a standardized extract of Bacopa monniera: An experimental study. Phytomedicine. 1998;5:77–82. doi: 10.1016/S0944-7113(98)80001-9. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia G. Palit G. Pal R. Singh S. Singh HK. Adaptogenic effect of Bacopa monniera (Brahmi) Pharmacol Biochem Behav. 2003;75:823–830. doi: 10.1016/s0091-3057(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhuri DK. Parmar D. Kakkar P. Shukla R. Seth PK. Srimal RC. Antistress effects of bacosides of Bacopa monnieri: Modulation of Hsp70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother Res. 2002;16:639–645. doi: 10.1002/ptr.1023. [DOI] [PubMed] [Google Scholar]
- 33.Deb DD. Kapoor D. Dighe DP. Padmaja D. Anand MS. D'Souza P. Deepak M. Murali B. Agarwal A. In vitro safety evaluation and anticlastogenic effect of BacoMind on human lymphocytes. Biomed Environ Sci. 2008;21:7–23. doi: 10.1016/S0895-3988(08)60002-1. [DOI] [PubMed] [Google Scholar]
- 34.Elangovan V. Govindasamy S. Ramamoorthy N. Balasubramanian In vitro studies on the anticancer activity of Bacopa monnieri. Fitoterapia. 1995;66:211–215. [Google Scholar]
- 35.Yamada K. Hung P. Park TK. Park PJ. Lim BO. A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. J Ethnopharmacol. 2011;137:231–235. doi: 10.1016/j.jep.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Samiulla DS. Prashanth D. Amit A. Mast cell stabilising activity of Bacopa monnieri. Fitoterapia. 2001;72:284–285. doi: 10.1016/s0367-326x(00)00309-9. [DOI] [PubMed] [Google Scholar]
- 37.Russo A. Borrelli F. Bacopa monniera, a reputed nootropic plant: An overview. Phytomedicine. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Sivaramakrishna C. Rao CV. Trimurtulu G. Vanisree M. Subbaraju GV. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry. 2005;66:2719–2728. doi: 10.1016/j.phytochem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Garai S. Mahato SB. Ohtani K. Yamasaki K. Dammarane triterpenoid saponins from Bacopa monnieri. Can J Chem. 2009;87:1230–1234. doi: 10.1016/0031-9422(95)00936-1. [DOI] [PubMed] [Google Scholar]
- 40.Chakravarty AK. Sarkar T. Masuda K. Shiojima K. Nakane T. Kawahara N. Bacopaside I and II: two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry. 2001;58:553–556. doi: 10.1016/s0031-9422(01)00275-8. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarty A.K. Garai S. Masuda K. Nakane T. Kawahara N. Bacopasides III–V: Three new triterpenoid glycosides from Bacopa monniera. Chem Pharm Bull. 2003;51:215–217. doi: 10.1248/cpb.51.215. [DOI] [PubMed] [Google Scholar]
- 42.Garai S. Mahato SB. Ohtani K. Yamasaki K. Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry. 1996;42:815–820. doi: 10.1016/0031-9422(95)00936-1. [DOI] [PubMed] [Google Scholar]
- 43.Chatterji N. Rastogi RP. Dhar ML. Chemical examination of Bacopa monniera Wettst: Part II—Isolation of chemical constituents. Ind J Chem. 1965;3:24–29. [Google Scholar]
- 44.Chakravarty AK. Sarkar T. Nakane T. Kawahara N. Masuda K. New phenylethanoid glycosides from Bacopa monniera. Chem Pharm Bull. 2008;50:1616–1618. doi: 10.1248/cpb.50.1616. [DOI] [PubMed] [Google Scholar]
- 45.Kawai KI. Shibata S. Pseudojujubogenin, a new sapogenin from Bacopa monnieri. Phytochemistry. 1978;17:287–289. [Google Scholar]
- 46.Bhandari P. Kumar N. Singh B. Kaul VK. Cucurbitacins from Bacopa monnieri. Phytochemistry. 2007;68:1248–1254. doi: 10.1016/j.phytochem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Phrompittayarat W. Wittaya-areekul S. Jetiyanon K. Putalun W. Tanaka H. Ingkaninan K. Determination of saponin glycosides in Bacopa monnieri by reversed phase high performance liquid chromatography. Thai Pharm Health Sci J. 2007;2:26–32. [Google Scholar]
- 48.Deepak M. Sangli GK. Arun PC. Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem Anal. 2005;16:24–29. doi: 10.1002/pca.805. [DOI] [PubMed] [Google Scholar]
- 49.Kregel CK. Zhang JH. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 50.Valko M. Leibfritz D. Moncol J. Cronin MT. Mazur M. Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Rice-Evans C. Miller N. Paganga G. Antioxidant properties of phenolic compounds. Trends in Plant Science. 1997;2:152–159. [Google Scholar]
- 52.De Grey A. The Mitochondrial Free Radical Theory of Aging. R.G. Landes Company; Austin, TX: 1999. [Google Scholar]
- 53.Maxwell SRJ. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 54.Arivazhagan P. Shila S. Kumaran S. Panneerselvam C. Effect of DL-a-lipoic acid in various brain regions of aged rats. Exp Gerontol. 2002;37:803–811. doi: 10.1016/s0531-5565(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 55.Gilgun-Sherki Y. Melamed E. Offen D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 56.Anbarasi K. Vani G. Balakrishna K. Devi CS. Effect of bacoside-A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006;78:1378–1384. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 57.Anbarasi K. Vani G. Balakrishna K. Devi CS. Effect of bacoside-A on membrane-bound ATPases in the brain of rats exposed to cigarette smoke. J Biochem Mol Toxicol. 2005;19:59–65. doi: 10.1002/jbt.20050. [DOI] [PubMed] [Google Scholar]
- 58.Jadiya P. Khan A. Sammi SR. Kaur S. Mir SS. Nazir A. Anti-Parkinsonian effects of Bacopa monnieri: Insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson's disease. Biochem Biophys Res Commun. 2011;413:605–610. doi: 10.1016/j.bbrc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 59.Chowdhuri DK. Parmar D. Kakkar P. Shukla R. Seth PK. Srimal RC. Antistress effects of bacosides of Bacopa monnieri: Modulation of Hsp70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother Res. 2002;16:639–645. doi: 10.1002/ptr.1023. [DOI] [PubMed] [Google Scholar]
- 60.Sharon R. Bar-Joseph I. Frosch MP. Walsh DM. Hamilton JA. Selkoe DJ. The formation of highly soluble oligomers of α-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron. 2003;37:583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 61.Saini N. Singh D. Sandhir R. Neuroprotective effects of Bacopa monnieri in experimental model of dementia. Neurochem Res. 2012;37:1928–1937. doi: 10.1007/s11064-012-0811-4. [DOI] [PubMed] [Google Scholar]
- 62.Goldschmidt RB. Steward O. Neurotoxic effects of colchicine: differential susceptibility of CNS neuronal populations. Neuroscience. 1982;7:695–714. doi: 10.1016/0306-4522(82)90075-6. [DOI] [PubMed] [Google Scholar]
- 63.Ahirwar S. Tembhre M. Gour S. Namdeo A. Anticholinesterase efficacy of Bacopa monnieri against the brain regions of rat. Asian J Exp Sci. 2012;26:65–70. [Google Scholar]
- 64.Das A. Shanker G. Nath C. Pal R. Singh S. Singh H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba. Pharmacol Biochem Behav. 2002;73:893–900. doi: 10.1016/s0091-3057(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 65.Gray NW. Brimijoin S. Amyloid-beta increases acetylcholinesterase expression in neuroblastoma cells by reducing enzyme degradation. J Neurochem. 2003;86:470–478. doi: 10.1046/j.1471-4159.2003.01855.x. [DOI] [PubMed] [Google Scholar]
- 66.Rastogi M. Ojha R. Prabu PC. Devi DP. Agrawal A. Dubey GP. Amelioration of age associated neuroinflammation on long term bacosides treatment. Neurochem Res. 2012;37:869–874. doi: 10.1007/s11064-011-0681-1. [DOI] [PubMed] [Google Scholar]
- 67.Shobana C. Kumar RR. Sumathi T. Alcoholic extract of Bacopa monniera Linn. protects against 6-hydroxydopamine-induced changes in behavioral and biochemical aspects: A pilot study. Cell Mol Neurobiol. 2012;32:1099–1112. doi: 10.1007/s10571-012-9833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh M. Murthy V. Ramassamy C. Standardized extracts of Bacopa monniera protect against MPP+- and paraquat-induced toxicity by modulating mitochondrial activities, proteasomal functions, and redox pathways. Toxicol Sci. 2012;125:219–232. doi: 10.1093/toxsci/kfr255. [DOI] [PubMed] [Google Scholar]
- 69.Tanner CM. Kamel F. Ross GW. Hoppin JA. Goldman SM. Korell M. Marras C. Bhudhikanok GS. Kasten M. Chade AR. Comyns K. Richards MB. Meng C. Priestley B. Fernandez HH. Cambi F. Umbach DM. Blair A. Sandler DP. Langston JW. Rotenone, Paraquat and Parkinson's xisease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinomol G. Bharath M. Neuromodulatory Propensity of Bacopa monnieri leaf extract against 3-nitropropionic acid-induced oxidative stress: In vitro and in vivo evidences. Neurotox Res. 2012;22:102–114. doi: 10.1007/s12640-011-9303-6. [DOI] [PubMed] [Google Scholar]
- 71.Sumathi T. Shobana C. Christinal J. Anusha C. Protective effect of Bacopa monniera on methyl mercury-induced oxidative stress in cerebellum of rats. Cell Mol Neurobiol. 2012;32:979–987. doi: 10.1007/s10571-012-9813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russo A. Izzo AA. Borrelli F. Renis M. Vanella A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res. 2003;17:870–875. doi: 10.1002/ptr.1061. [DOI] [PubMed] [Google Scholar]
- 73.Khan SR. Krishnakumar A. Paulose CS. Decreased glutamate receptor binding and NMDA R1 gene expression in hippocampus of pilocarpine-induced epileptic rats: Neuroprotective role of Bacopa monnieri extract. Epilepsy Behav. 2008;12:54–60. doi: 10.1016/j.yebeh.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 74.George KS. Raghunath N. Bharath MM. Muralidhara. Prophylaxis with Bacopa monnieri attenuates acrylamide induced neurotoxicity and oxidative damage via elevated antioxidant function. Cent Nerv Syst Agents Med Chem. 2012 doi: 10.2174/1871524911313010003. online publication. [DOI] [PubMed] [Google Scholar]
- 75.Sandhya T. Sowjanya J. Veeresh B. Bacopa monniera (L.) Wettst ameliorates behavioral alterations and oxidative markers in sodium valproate induced autism in rats. Neurochem Res. 2012;37:1121–1131. doi: 10.1007/s11064-012-0717-1. [DOI] [PubMed] [Google Scholar]
- 76.Jyoti A. Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neuro Toxicol. 2006;27:451–457. doi: 10.1016/j.neuro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Priyanka HP. Bala P. Ankisettipalle S. Rajan ST. Bacopa monnieri and L-deprenyl differentially enhance the activities of antioxidant enzymes and the expression of tyrosine hydroxylase and nerve growth factor via ERK 1/2 and NF-jB pathways in the spleen of female Wistar rats. Neurochem Res. 2012 doi: 10.1007/s11064-012-0902-2. online publication. [DOI] [PubMed] [Google Scholar]
- 78.Preethi J. Singh HK. Charles PD. Emmanuvel Rajan K. Participation of microRNA 124-CREB pathway: a parallel memory enhancing mechanism of standardised extract of Bacopa monniera. Neurochem Res. 2012;37:2167–2177. doi: 10.1007/s11064-012-0840-z. [DOI] [PubMed] [Google Scholar]
- 79.Liu X. Yue R. Zhang J. Shan L. Wang R. Zhang W. Neuroprotective effects of bacopaside I in ischemic brain injury. Restor Neurol Neurosci. 2012 doi: 10.3233/RNN-120228. online publication. [DOI] [PubMed] [Google Scholar]
- 80.Gao HM. Liu B. Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hosamani MR. Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology. 2009;30:977–985. doi: 10.1016/j.neuro.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 82.Shinomol GK. Mythri RB. Srinivas Bharath MM. Muralidhara Bacopa monnieri extract offsets rotenone-induced cytotoxicity in dopaminergic cells and oxidative impairments in mice brain. Cell Mol Neurobiol. 2012;32:455–465. doi: 10.1007/s10571-011-9776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rohini G. Sabitha KE. Devi CS. Bacopa monniera Linn. Extract modulates antioxidant and marker enzyme status in fibrosarcoma bearing rats. Indian J Exp Biol. 2004;42:776–780. [PubMed] [Google Scholar]
- 84.Russo A. Borrelli F. Campisi A. Acquaviva R. Raciti G. Vanella A. Nitric oxide-related toxicity in cultured astrocytes: Effect of Bacopa monniera. Life Sci. 2003;73:1517–1526. doi: 10.1016/s0024-3205(03)00476-4. [DOI] [PubMed] [Google Scholar]
- 85.Pawar R. Gopalakrishnan C. Bhutani KK. Dammarane triterpene saponin from Bacopa monniera as the superoxide inhibitor in polymorphonuclear cells. Planta Medica. 2001;67:752–754. doi: 10.1055/s-2001-18351. [DOI] [PubMed] [Google Scholar]
- 86.Sumathy T. Subramanian S. Govindasamy S. Balakrishna K. Veluchamy G. Protective role of Bacopa monniera on morphine induced hepatotoxicity in rats. Phytother Res. 2001;15:643–645. doi: 10.1002/ptr.1007. [DOI] [PubMed] [Google Scholar]
- 87.Shinomol GK. Bharath MM. Muralidhara Pretreatment with Bacopa monnieri extract offsets 3-nitropropionic acid induced mitochondrial oxidative stress and dysfunctions in the striatum of prepubertal mouse brain. Can J Physiol Pharmacol. 2012;90:595–606. doi: 10.1139/y2012-030. [DOI] [PubMed] [Google Scholar]
- 88.Singh L. Eapen S. D'Souza SF. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere. 2006;62:233–246. doi: 10.1016/j.chemosphere.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 89.Sinha S. Saxena R. Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside-A content in medicinal plant Bacopa monnieri L. Chemosphere. 2006;62:1340–1350. doi: 10.1016/j.chemosphere.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 90.Bhattacharya SK. Bhattacharya A. Kumar A. Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14:174–179. doi: 10.1002/(sici)1099-1573(200005)14:3<174::aid-ptr624>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 91.Anbarasi K. Kathirvel G. Vani G. Jayaraman G. Shyamala Devi CS. Cigarette smoking induces heat shock protein 70 kDa expression and apoptosis in rat brain: Modulation by bacoside A. Neuroscience. 2006;138:1127–1135. doi: 10.1016/j.neuroscience.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 92.Mohanty IR. Maheshwari U. Joseph D. Deshmukh Y. Bacopa monniera protects rat heart against ischaemia-reperfusion injury: role of key apoptotic regulatory proteins and enzymes. J Pharm Pharmacol. 2010;62:1175–1184. doi: 10.1111/j.2042-7158.2010.01155.x. [DOI] [PubMed] [Google Scholar]
- 93.Shinomol GK. Muralidhara Bacopa monnieri modulates endogenous cytoplasmic and mitochondrial oxidative markers in prepubertal mice brain. Phytomedicine. 2011;18:317–326. doi: 10.1016/j.phymed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Kapoor R. Srivastava S. Kakkar P. Bacopa monnieri modulates antioxidant responses in brain and kidney of diabetic rats. Environ Toxicol Pharmacol. 2009;27:62–69. doi: 10.1016/j.etap.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 95.de la Torre J. Cerebral hemodynamics and vascular risk factors: Setting the stage for Alzheimer's disease. J Alzheimer Dis. 2012;32:553–567. doi: 10.3233/JAD-2012-120793. [DOI] [PubMed] [Google Scholar]
- 96.Kamkaew N. Scholfield N. Ingkaninan K. Taepavarapruk N. Chootip K. Bacopa monnieri increases cerebral blood flow in rat independent of blood pressure. Phytother Res. 2013;27:135–138. doi: 10.1002/ptr.4685. [DOI] [PubMed] [Google Scholar]
- 97.Kamkaew N. Scholfield CN. Ingkaninan K. Maneesai P. Parkington HC. Tare M. Chootip K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J Ethnopharmacol. 2011;137:790–795. doi: 10.1016/j.jep.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 98.Winston D. Maimes S. Adaptogens: Herbs for Strength, Stamina, and Stress Relief. Healing Arts Press; 2007. [Google Scholar]
- 99.Sheikh N. Ahmad A. Siripurapu KB. Kuchibhotla VK. Singh S. Palit G. Effect of Bacopa monniera on stress induced changes in plasma corticosterone and brain monoamines in rats. J Ethnopharmacol. 2007;111:671–676. doi: 10.1016/j.jep.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 100.Charles PD. Ambigapathy G. Geraldine P. Akbarsha MA. Rajan KE. Bacopa monniera leaf extract up-regulates tryptophan hydroxylase (TPH2) and serotonin transporter (SERT) expression: Implications in memory formation. J Ethnopharmacol. 2011;134:55–61. doi: 10.1016/j.jep.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 101.Markesbery WR. The role of oxidative stress in Alzheimer's disease. Arch Neurol. 1999;56:1449–1452. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 102.Münch G. Deuther-Conrad W. Gasic-Milenkovic J. Glycoxidative stress creates a vicious cycle of neurodegeneration in Alzheimer's disease—a target for neuroprotective treatment strategies? J Neural Transm Suppl. 2002;(62):303–307. doi: 10.1007/978-3-7091-6139-5_28. [DOI] [PubMed] [Google Scholar]
- 103.Pratico D. Delanty N. Oxidative injury in diseases of the central nervous system: Focus on Alzheimer's disease. Am J Med. 2000;109:577–585. doi: 10.1016/s0002-9343(00)00547-7. [DOI] [PubMed] [Google Scholar]
- 104.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer's disease: Strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 105.Shankar G. Li S. Mehta TH. Garcia-Munoz A. Shepardson NE. Smith I. Brett FM. Farrell MA. Rowan MJ. Lemere CA. Regan CM. Walsh DM. Sabatini BL. Selkoe DJ. Amyloid β-protein dimers isolated directly from Alzheimer brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de la Monte S. Wands J. Alzheimer's disease is type 3 diabetes–evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Francis P. Palmer A. Snape M. Wilcock G. The cholinergic hypothesis of Alzheimer's disease: A review of progress. J Neurol Neurosurg Psychiatr. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rusanen M. Kivipelto M. Quesenberry P., Jr Zhou J. Whitmer R. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med. 2011;171:333–339. doi: 10.1001/archinternmed.2010.393. [DOI] [PubMed] [Google Scholar]
- 109.Cataldo J. Prochaska J. Glantz S. Cigarette smoking is a risk factor for Alzheimer's Disease: An analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19:465–480. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirayama T. Large cohort study on the relation between cigarette smoking and senile dementia without cerebrovascular lesions. Tob Control BMJ. 1992;1:176–179. [Google Scholar]
- 111.Vijayan V. Helen A. Protective activity of Bacopa monniera Linn. on nicotine-induced toxicity in mice. Phytotherapy Res. 2007;21:378–381. doi: 10.1002/ptr.2073. [DOI] [PubMed] [Google Scholar]
- 112.Mathew M. Subramanian M. Evaluation of the anti-amyloidogenic potential of nootropic herbal extracts in vitro. IJPSR. 2012;3:4276–4280. [Google Scholar]
- 113.Ovsepian SV. Anwyl R. Rowan MJ. Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: In vivo study. Eur J Neurosci. 2004;20:1267–1275. doi: 10.1111/j.1460-9568.2004.03582.x. [DOI] [PubMed] [Google Scholar]
- 114.Saraf MK. Prabhakar S. Khanduja KL. Anand A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evid Based Complement Alternat Med. 2011 doi: 10.1093/ecam/neq038. electronic publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou Y. Peng L. Zhang WD. Kong DY. Effect of triterpenoid saponins from Bacopa monniera on scopolamine induced memory impairment in mice. Planta Medica. 2009;75:568–574. doi: 10.1055/s-0029-1185339. [DOI] [PubMed] [Google Scholar]
- 116.Saraf MK. Anand A. Prabhakar S. Scopolamine induced amnesia is reversed by Bacopa monniera through participation of kinase-CREB pathway. Neurochem Res. 2010;35:279–287. doi: 10.1007/s11064-009-0051-4. [DOI] [PubMed] [Google Scholar]
- 117.Kinshore K. Singh M. Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (brahmi), on experimental amnesia in mice. Indian J Exp Biol. 2005;43:640–645. [PubMed] [Google Scholar]
- 118.Das A. Shanker G. Nath C. Pal R. Singh S. Singh H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav. 2002;73:893–900. doi: 10.1016/s0091-3057(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 119.Piyabhan P. Wetchateng T. Neuroprotective and cognitive enhancement effects of bacopamonnieri on novel object recognition in schizophrenic rat model. Eur Psychiatry. 2012 online publication. [Google Scholar]
- 120.Piyabhan P. Wetchateng T. Effects of bacopa monnieri on VGLUT1 density in frontal cortex, striatum and hippocampus of schizophrenic rat model. Eur Psychiatry. 2012 online publication. [Google Scholar]
- 121.Dhanasekaran M. Tharakan B. Holcomb LA. Hitt AR. Young KA. Manyam BV. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother Res. 2007;21:965–969. doi: 10.1002/ptr.2195. [DOI] [PubMed] [Google Scholar]
- 122.Holcomb LA. Dhanasekaran M. Hitt AR. Young KA. Riggs M. Manyam BV. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J Alzheimer's Dis. 2006;9:243–251. doi: 10.3233/jad-2006-9303. [DOI] [PubMed] [Google Scholar]
- 123.Limpeanchob N. Jaipan S. Rattanakaruna S. Phrompittayarat W. Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;30(120):112–117. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 124.Uabundit N. Wattanathorn J. Mucimapura S. Ingkaninan K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer's disease model. J Ethnopharmacol. 2010;8(127):26–31. doi: 10.1016/j.jep.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 125.Singh HK. Dhawan BN. Effect of Bacopa monniera Linn. (Brahmi) extract on avoidance responses in rat. J Ethnopharmacol. 1987;5:205–214. doi: 10.1016/0378-8741(82)90044-7. [DOI] [PubMed] [Google Scholar]
- 126.Vollala VR. Upadhya S. Nayak S. Enhancement of basolateral amygdaloid neuronal dendritic arborization following Bacopa monniera extract treatment in adult rats. Clinics (Sao Paulo) 2011;66:663–671. doi: 10.1590/S1807-59322011000400023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vollala VR. Upadhya S. Nayak S. Effect of Bacopa monniera Linn. (brahmi) extract on learning and memory in rats—a behavioral study. J Vet Behavior. 2010;5:69–74. [Google Scholar]
- 128.Emmanuvel Rajan K. Singh HK. Parkavi A. Prisila DC. Attenuation of 1-(m-chlorophenyl) biguanide induced hippocampus-dependent memory impairment by a standardised extract of Bacopa monniera. Neurochem Res. 2011;36:2136–2144. doi: 10.1007/s11064-011-0538-7. [DOI] [PubMed] [Google Scholar]
- 129.Vohora D. Pal SN. Pillai KK. Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J Ethnopharmacol. 2000;71:383–390. doi: 10.1016/s0378-8741(99)00213-5. [DOI] [PubMed] [Google Scholar]
- 130.Saraf MK. Prabhakar S. Pandhi P. Anand A. Bacopa monniera ameliorates amnesic effects of diazepam qualifying behavioral–molecular partitioning. Neuroscience. 2008;155:476–484. doi: 10.1016/j.neuroscience.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 131.Prisila DC. Singh HK. Preethi J. Rajan EK. Standardized extract of Bacopa monniera (BESEB CDRI-08) attenuates contextual associative learning deficits in the aging rat's brain induced by D-galactose. J Neurosci Res. 2012;90:2053–2064. doi: 10.1002/jnr.23080. [DOI] [PubMed] [Google Scholar]
- 132.Jadad AR. Moore RA. Carroll D. Jenkinson C. Reynolds DJ. Gavaghan DJ. McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 133.Pase MP. Kean J. Sarris J. Neale C. Scholey AB. Stough C. The cognitive-enhancing effects of Bacopa monnieri: A systematic review of randomized, controlled human clinical trials. J Altern Complement Med. 2012;18:1–6. doi: 10.1089/acm.2011.0367. [DOI] [PubMed] [Google Scholar]
- 134.Neale C. Camfield D. Reay J. Stough C. Scholey A. Cognitive effects of two nutraceuticals Ginseng and Bacopa benchmarked against modafinil: A review and comparison of effect sizes. Br J Clin Pharmacol. 2012 doi: 10.1111/bcp.12002. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stough C. Lloyd J. Clarke J. Downey LA. Hutchison CW. Rodgers T. Nathan PJ. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology. 2001;156:481–484. doi: 10.1007/s002130100815. [DOI] [PubMed] [Google Scholar]
- 136.Roodenrys S. Booth D. Bulzomi S. Phipps A. Micallef C. Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27:279–281. doi: 10.1016/S0893-133X(01)00419-5. [DOI] [PubMed] [Google Scholar]
- 137.Morgan A. Stevens J. Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. J Altern Complement Med. 2010;16:753–759. doi: 10.1089/acm.2009.0342. [DOI] [PubMed] [Google Scholar]
- 138.Nathan PJ. Clarke J. Lloyd J. Hutchison CW. Downey L. Stough C. The acute effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy normal subjects. Hum Psychopharmacol. 2001;16:345–351. doi: 10.1002/hup.306. [DOI] [PubMed] [Google Scholar]
- 139.Allan J. Damodaran A. Deshmukh NS. Goudar KS. Amit A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monnieri in Sprague–Dawley rats. Food Chem Toxicol. 2007;45:1928–1937. doi: 10.1016/j.fct.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 140.Singh HK. Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi) Indian J Pharmacol. 1997;29:359–365. [Google Scholar]
- 141.Singh A. Singh SK. Evaluation of antifertility potential of Brahmi in male mouse. Contraception. 2009;79:71–79. doi: 10.1016/j.contraception.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 142.Mishra RK. Singh SK. Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception. 2009;79:479–487. doi: 10.1016/j.contraception.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 143.Rauf K. Subhan F. Abbas M. Haq I. Ali G. Ayaz M. Effect of acute and sub-chronic use of Bacopa monnieri on dopamine and serotonin turnover in mice whole brain. AJPP. 2012;6:2767–2774. [Google Scholar]
- 144.Moncrieff J. Kirsch I. Efficacy of antidepressants in adults. Br Med J. 2005;331:155–157. doi: 10.1136/bmj.331.7509.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kumar H. More SV. Han S. Choi J. Choi D. Promising therapeutics with natural bioactive compounds for improving learning and memory—A review of randomized trials. Molecules. 2012;17:10503–10539. doi: 10.3390/molecules170910503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kidd PM. A review of nutrients and botanicals in the integrative management of cognitive dysfunction. Altern Med Rev. 1999;4:144–161. [PubMed] [Google Scholar]
- 147.Spinella M. The Psychopharmacology of Herbal Medicine: Plant Drugs that Alter Mind, Brain, and Behavior. The MIT Press; Cambridge, Massachusetts: 2001. [Google Scholar]
- 148.Kulkarni R. Girish KJ. Kumar A. Nootropic herbs (Medhya Rasayana) in Ayurveda: An update. Pharmacogn Rev. 2012;12:147–153. doi: 10.4103/0973-7847.99949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zonneveld L. Reshaping the Human Condition: Exploring Human Enhancement. Rathenaeu Institute; The Hague: 2008. [Google Scholar]