Abstract
Both nicotine and alcohol addictions are common chronic brain disorders that are of great concern to individuals and society. Although genetics contributes significantly to these disorders, the susceptibility genes and variants underlying them remain largely unknown. Many years of genome-wide linkage and association studies have implicated a number of genes and pathways in the etiology of nicotine and alcohol addictions. In this communication, we focus on current evidence, primarily from human genetic studies, supporting the involvement of genes and variants in the GABAergic signaling system in the etiology of nicotine dependence and alcoholism based on linkage, association, and gene-by-gene interaction studies. Current efforts aim not only to replicate these findings in independent samples, but also to identify which variant contributes to the detected associations and through what molecular mechanisms.
Introduction
Drug addiction is a serious public health concern. According to the World Health Organization (WHO 2002), there are an estimated 2 billion heavy alcohol users, 1.3 billion tobacco users, and 185 million illicit drug users worldwide. There is considerable evidence from family, twin, and adoption studies for the operation of genetic factors in the vulnerability to addiction and that genetics contributes substantially to inter-individual vulnerabilities, with an estimated moderate-to-high heritability for both nicotine dependence (ND) and alcoholism (Goldman et al. 2005; Ho et al. 2010; Li and Burmeister 2009). Estimates of the heritability of alcohol abuse and dependence range from 50 to 70%. Additional studies have revealed a similar high level of heritability across other alcohol-related behaviors, including heavy consumption and “problem” drinking (Gelernter and Kranzler 2009; Goldman et al. 2005; Ho et al. 2010). Similarly, many large twin studies have concluded that genetics contributes significantly to the risk of becoming a regular and dependent smoker. Meta-analysis of a dozen twin studies shows that both genetics and environment play important roles in smoking-related behaviors, with an estimated average heritability for ND of 0.59 in male and 0.46 in female smokers, and an average of 0.56 for the population as a whole (Li et al. 2003a). Nicotine dependence also is influenced by environmental factors, as well as by interaction between genetic and environmental factors (Ho et al. 2010; Lessov-Schlaggar et al. 2008; Li et al. 2003a; Sullivan and Kendler 1999; Swan et al. 2003). Of the important neurotransmitters in the central nervous system (CNS) implicated in ND and alcoholism, GABA is the main inhibitory one, whose modulatory actions are mediated through two types of receptors: the ionotropic GABAA receptor and the metabotropic GABAB receptor (Bettler et al. 2004; Vlachou and Markou 2010). GABAA receptors form ion channels, whereas GABAB receptors activate second-messenger systems through G-protein binding and activation. The GABA neurons are part of the mesolimbic dopamine system, critically important in mediating the reinforcing properties of drugs of abuse. Additionally, the GABA system is diffusely expressed in the brain; therefore, areas other than the mesolimbic system may be partly responsible for these effects. Considering the functional importance of GABAergic signaling in CNS, the genes involved in the system have received great attention in human genetic study on addictions, especially to alcohol and nicotine. The primary objective of this communication is to provide an updated review of what we have learned from the genetic epidemiologic studies on the involvement of genes in GABAergic signaling system in drug addictions. However, given the different levels of understanding of the involvement of GABAergic system in the etiology of ND and alcoholism and in the availability of reviews on this signaling system in its relation to ND and alcoholism, we discuss them separately.
Evidence for the involvement of genes in GABAergic signaling in ND
Evidence from genome-wide linkage analysis
During recent years, a significant number of genome-wide linkage studies have been reported for addiction to nicotine, alcohol, and other abused substances (Li and Burmeister 2009), especially for smoking-related behaviors, in which more than 20 such studies have been reported (Han et al. 2010; Li 2008). By examining those reported linkage regions in each study and applying the rigorous criteria proposed by Lander and Kruglyak (1995), 13 regions, located on chromosomes 3–7, 9–11, 17, 20, and 22, were found to be “suggestive” or “significant” in at least two independent samples (Li 2008). Of them, the regions on chromosomes 9, 10, 11, and 17 have received the strongest support, with the regions on chromosomes 9 and 17 being the most interesting, given the primary objective of this report (Bergen et al. 1999; Bierut et al. 2004; Gelernter et al. 2007; Li et al. 2003b, 2006).
Evidence for association of GABAB receptor subunit 2 (GABBR2) with ND
Based on the linkage results showing a “suggestive” linkage on chromosome 9 (see Fig. 1) with ND, reported initially by our group in the Framingham Heart Study (FHS) sample (Li et al. 2003b) and verified in independent samples by us (Li et al. 2006) and others (Bergen et al. 1999; Bierut et al. 2004; Gelernter et al. 2004), we conducted positional candidate gene-based association studies on this region for several candidate genes in the Mid-South Tobacco Family (MSTF) sample (Beuten et al. 2005, 2007; Li et al. 2009, 2007). The first gene identified from this linkage region was the subunit 2 gene for GABAB receptor (GABBR2) (Beuten et al. 2005). Since this earlier report, we have genotyped more SNPs from GABBR2 in the MSTF study with large sample sizes, in which we not only confirmed our earlier finding that GABBR2 was significantly associated with ND but also showed that genetically determined vulnerability to ND was different in subjects of European and African origin (Li et al. 2009).
Fig. 1.
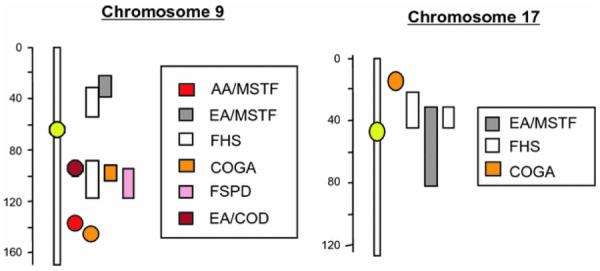
Chromosomal locations of nominated regions on chromosomes 9 and 17 for all smoking-related measures with “significant” or “suggestive” linkage score. The linkage results were obtained from the following studies: AA/MSTF African-American sample of the Mid-South Tobacco Family study (Li et al. 2006), EA/MSTF European-American sample of the Mid-South Tobacco Family study (Li et al. 2008), FHS Framingham Heart Study (Li et al. 2003b; Wang et al. 2005), EA/GCOD European-American sample of Genetics of Cocaine or Opioid Dependence study (Gelernter et al. 2007), COGA Collaborative Studies on the Genetics of Alcoholism (Bergen et al. 1999; Bierut et al. 2004; Duggirala et al. 1999), and FSPD Family Study of Panic Disorder (Gelernter et al. 2004)
The GABAB receptor inhibits neuronal activity through G-protein-coupled second-messenger systems, which regulate the release of neurotransmitters and the activity of ion channels and adenylyl cyclase (Kaupmann et al. 1998; Vlachou and Markou 2010). Although they have not revealed the detailed mechanisms of the involvement of GABAB receptors in ND or alcoholism, preclinical studies have implicated GABAergic receptors in the rewarding effects of drugs of abuse, including nicotine and alcohol (Corrigall et al. 2000; Maccioni and Colombo 2009; Vlachou and Markou 2010). Indeed, GABAB agonists antagonize nicotine-rewarding effects in mice and rats (Fattore et al. 2002) and decrease alcohol consumption and craving in humans and the severity of alcohol-withdrawal symptoms in humans and rats (Colombo et al. 2004; Maccioni and Colombo 2009).
To determine the genetic contribution of GABBR2 variants to the detected linkage signal on chromosome 9, we performed two rounds of linkage analysis, with the first one being considered a regular analysis without correcting for GABBR2 SNPs and the second one a “justified” linkage analysis by including GABBR2 SNPs as covariates (Li 2006). As shown in Fig. 2, we found that the inclusion of GABBR2 SNPs as covariates reduced, but could not completely eliminate, the detected linkage signal on chromosome 9. The inclusion of GABBR2 SNPs decreased the detected linkage signal on chromosome 9 by 36.5, 27.7, and 38.2% for smoking quantity (SQ), the Heaviness of Smoking Index (HSI), and the Fagerstrom test for ND (FTND), respectively. These results indicate that GABBR2 is indeed a candidate gene for ND that contributes to the linkage signal on chromosome 9 for ND detected in our earlier study and there must be other candidate genes within this region that may contribute to our detected linkage signal. This is because GABBR2 SNPs explained only 27.7–38.3% of the detected linkage signal on chromosome 9. Indeed, our further positional candidate gene-based association analyses of this genomic region revealed that neurotropic tyrosine kinase receptor 2 (NTRK2) and Src homology 2 domain-containing transforming protein C3 (SHC3) are significantly associated with ND in the MSTF samples (Beuten et al. 2007; Li et al. 2007).
Fig. 2.
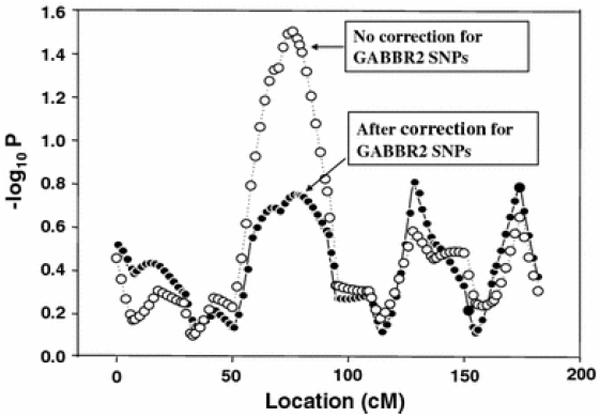
Determination of contribution of GABBR2 SNPs to detected linkage signal on chromosome 9
Interaction of GABBR1 and GABBR2 in affecting ND
Like any other complex trait, addition is controlled by multiple genetic factors, with each having a relatively modest effect, and by environmental factors, as well as by both gene–gene (epistatic) and gene–environment interactions (Flint and Munafo 2008; Ho et al. 2010; van der Zwaluw and Engels 2009). As documented in the literature (Gelernter and Kranzler 2009; Ho and Tyndale 2007; Lessov-Schlaggar et al. 2008; Li 2008; Li and Burmeister 2009), significant efforts have been made in searching for vulnerability genes to addictions. However, these approaches are effective only for genes with moderate-to-major effects. The ability to identify susceptibility genes for addictions and other psychiatric disorders has been improving, but remains considerably limited by the presence of a diverse array of factors such as epistatic interaction, small-modest genetic effects, small sample size, and heterogeneities. Among these factors, detecting gene–gene and gene–environment interactions appears to be more challenging (Flint and Munafo 2008; Ho et al. 2010; van der Zwaluw and Engels 2009).
In the search for determinants of gene–gene and gene–environment interactions, extensive efforts have been expended. Several combinatorial approaches, such as the multifactor dimensionality reduction (MDR) (Ritchie et al. 2001), the combinatorial partitioning method (CPM) (Nelson et al. 2001), and the restricted partition method (RPM) (Culverhouse et al. 2004), are promising tools for detecting gene–gene and gene–environment interactions. Since the original report, MDR has been most widely applied to detect interactions underlying a spectrum of complex disorders. However, these established methods have critical limitations that restrict their practical use. For example, MDR, CPM, and RPM do not allow adjustments for covariates; MDR is applicable only to dichotomous phenotypes; and CPM and RPM cannot handle categorical phenotypes. To overcome the limitations of these existing combinatorial approaches and to meet research needs in determining gene–gene and gene–environment interactions for complex phenotypes, a generalized MDR (GMDR) as well as a pedigree-based GMDR (PGMDR) have been developed for case–control (Lou et al. 2007a) and family-based (Lou et al. 2008) studies, respectively, that permit adjustments for discrete and quantitative covariates and are applicable to both dichotomous and continuous phenotypes.
Specifically, regarding gene–gene interaction for the GABAergic signaling system for ND, using the PGMDR software, we detected significant interactive effects between the variants in GABBR1 and GABBR2 in ND (Li et al. 2009). This is noteworthy in that a relatively weak association of GABBR1 with ND has been detected (Li et al. 2009) and indicates that a significant interaction exists between variants of GABBR1 and GABBR2 in affecting ND, and the involvement of GABBR1 in modulating ND risk is most likely through its interaction with GABBR2, where GABBR2 polymorphisms directly alter the susceptibility to ND (Li et al. 2009). The reason for failing to detect a significant association of GABBR1 with ND by itself may be attributable to a strong dependence of GABBR1 effects on specific GABBR2 variants or a relatively small marginal effect of GABBR1 variants in the samples studied. More importantly, a significant interaction of GABBR1 with GABBR2 in humans confirms previous findings of pharmacologic studies that showed GABAB receptor functions as heterodimers of GABAB1 and GABAB2 subunits (Bettler et al. 2004; Vlachou and Markou 2010).
The involvement of the GABAB receptor in ND has been reported in many studies using animal models (Bettler et al. 2004), including a recently reported genetic study on zebrafish applying a nicotine behavioral assay in a forward screening of genes mutated through a gene-breaking transposon mutagenesis approach (Petzold et al. 2009). This study used transposons in mutant zebrafish and screened for induced changes in the nicotine-induced locomotive response, which generated two mutant fish lines with significantly attenuated nicotine locomotive response: dbav and hbog with the identified mutations in the chaperonin-containing protein 8 (cct8) and a GABAB receptor ortholog, gabbr1.2, respectively. This identification of GABAB receptor involvement in the nicotine response of zebrafish provides further evidence for the role of the GABAergic system in the etiology of ND (Klee et al. 2010). In considering a consistent relation between a reduced reward sensitivity and addiction, these findings point to a potential genetic basis for the involvement of GABAB receptor signaling in the etiology of ND.
Evidence for association of other genes in GABAergic system with ND
In addition, there is another candidate gene, called GABAA receptor-associated protein (GABARAP), located in a “suggestive” linkage region on chromosome 17 (see Fig. 1) for ND or other smoking-related behavior (Duggirala et al. 1999; Li 2008; Li et al. 2003b; Wang et al. 2005). GABARAP belongs to a family of microtubule-associated proteins that includes GABARAP, GABAA receptor-associated protein like 1 (GABARAPL1), GABARAPL2, the yeast protein Apg8p/Aut7, and light chain 3 of microtubule-associated protein 1 (MAP1-LC3) (Kabeya et al. 2000; Lang et al. 1998; Pellerin et al. 1993; Sagiv et al. 2000; Wang et al. 1999). Of the members of the family, GABARAP has been investigated extensively and found to interact with the γ2 subunit of the GABAA receptor. Such interactions among GABAA receptor, GABARAP, and tubulin promote clustering of the receptor, alter its channel kinetics, and enhance its trafficking to the plasma membrane in neurons (Chen et al. 2000; Leil et al. 2004; Wang et al. 1999). Furthermore, our microarray study indicated that GABARAPL2 was highly regulated by nicotine in multiple rat brain regions in a time- and region-dependent manner (Li et al. 2004).
Through a two-stage fine-mapping approach on the basis of linkage analysis findings, we found that two SNPs (i.e., rs222843 and rs17710) in GABARAP are significantly associated with ND in European-American smokers (Lou et al. 2007b). Considering that SNPs rs222843 and rs17710 reside in the promoter and 3′-untranslated region of GABARAP, respectively, we were interested in determining whether they are capable of regulating GABARAP expression. By using a luciferase reporter assay in human embryonic kidney HEK293 cells, we found that the promoter containing the G allele of rs222843 produced a nearly twofold increase in luciferase activity compared with the one containing the A allele (Fig. 3, right panel). In contrast, we detected no difference in expression of the chimeric reporters containing the A and T alleles of rs17710 (Fig. 3, left panel). This indicates that rs222843, and not rs17710, is functional in causing expression divergence of GABARAP. However, whether this differentially allelic-specific expression can be detected in human smokers remains to be further examined for this functional GABARAP variant.
Fig. 3.

Determination of allelic-specific expression of SNPs rs222843 and rs17710 in GABARAP. The SNPs rs222843 (G/A) and rs17710 (A/T) are located in the promoter and 3′ UTR of GABARAP, respectively. Using a luciferase reporter assay, we revealed a significant expression difference between the G and the A alleles of rs222843 (P < 0.0001), but not in the A and the T alleles of rs17710. Data shown as mean ± SD (N = 4). **P < 0.01; paired Student's t test
Evidence for involvement of GABA receptor signaling in ND based on pathway analysis
As mentioned above, both linkage and association analyses have revealed several genes in the GABAergic signaling pathway that are associated with ND or other smoking-related behaviors. However, another study has failed to replicate some of those associations (Agrawal et al. 2008a). Many factors might contribute to difficulty in replicating the findings of linkage and association analysis, which include the presence of substantial heterogenetity, underpowered samples, small genetic effects, inconsistency in defining and assessing the phenotypes of interest, and different study designs and methodologies (Ho et al. 2010; Li 2008; Wang and Li 2010). Generally speaking, a conventional single-gene-based association study reports only the top-ranking SNPs or genes with the smallest statistic and has serious limitations because of functionally critical susceptibility SNPs/genes for a complex trait generally with subtler effects and over-conservative multiple testing correction (Wang et al. 2007). To overcome these limitations, pathway-based association analysis has been proposed (Holmans et al. 2009; Wang et al. 2007), which examines the cumulative impact of a group of genes with modest individual contributions within the same pathway on a phenotype of interest. Compared with single gene-based analysis, pathway-based analysis is thus supposed to reveal more convincing findings, and such findings should be more biologically plausible because a significantly enriched pathway presumably defines a more precise and more specific biological function than a single gene with multiple functions (Holmans et al. 2009). Further, given the fact that Bonferroni correction is considered to be over-conservative for multiple testing and genes with subtler effects could hardly survive such a correction in large-scale association studies, the pathway-based analysis offers an attractive and potentially powerful alternative perspective—a “two-step” testing procedure that first identifies significant clusters of genes, followed by pathway testing within each significant group.
To identify pathways associated with ND and its related behaviors, we recently conducted a comprehensive pathway-based association analysis for three important smoking-related behaviors: smoking initiation, ND, and smoking cessation (Wang and Li 2010). Through searching literature on genetic studies for the behaviors including both candidate gene-based and genome-wide association studies, we identified most, if not all, genes that have been reported to be associated with these phenotypes. We then applied various pathway-based approaches to these associated genes, which revealed 9, 21, and 13 enriched pathways among the genes associated with smoking initiation, ND, and smoking cessation. Of these pathways, we found that GABAergic signaling is significantly associated with ND (Wang and Li 2010). Moreover, we found significant genetic overlap among these three smoking-related phenotypes.
Genetic evidence for the involvement of genes in GABAergic signaling in alcoholism
Alcohol facilitates immediate release of GABA in distinct areas of the human brain (Kelm et al. 2011). The released GABA is then bound to receptors on either side of the synapse, regulating further release of GABA and hyperpolarization of postsynaptic neurons. Because the involvement of GABAergic signaling in alcoholism has been well covered by many reviews (Edenberg and Foroud 2006; Enoch 2008; Gelernter and Kranzler 2009), in this section, we limit our focus primarily to SNPs and haplotypes in GABAA receptor subunit genes associated with AD or its related phenotypes identified in multiple studies. Because genetic associations of presynaptic GABA system with AD and related phenotypes are not yet well characterized, their role is only briefly discussed in this communication.
Genetics of post-synaptic GABAA receptors in alcoholism
Variations in expression of GABAA receptor subunit isoforms have been implicated in developing alcohol tolerance, alcohol dependence (AD), withdrawal, and self-administration (Kohnke 2008; Krystal et al. 2006). At intoxicating doses, alcohol potentiates synaptic pentameric GABAA receptors that exist mainly as α1ß2γ2 (60%), α2ß3γ2 (15%), and α3ß3γ2 (10%) subunit combinations in the adult human brain. At lower doses, alcohol potentiates extrasynaptic GABAA receptors containing α6 and α4 instead of α1–3 and α5 subunits. Molecular studies have indicated increased α4, α6, and γ2 and reduced α1–3, α5, and δ mRNA expression in rat and primate brain after chronic treatment with alcohol (Anderson et al. 2007; Cagetti et al. 2003; Grobin et al. 2000).
The 14 of the 16 genes encoding GABAA receptor subunit isoforms are clustered on four chromosomes: 4p12–q13 (α2, α4, ß1, γ1), 5q31–q35 (α1, α6, ß2, γ2), 15q11–q13 (α5, ß3, γ3), and Xq28 (α3, ß4, ε1) (Darlison et al. 2005; Enoch 2008). The initial link between GABAA loci and alcoholism was identified with genome-wide linkage scans in Native Americans and Caucasians, where a locus on chromosome 4p12 was consistently linked with vulnerability to AD (Long et al. 1998; Reich et al. 1998; Zinn-Justin and Abel 1999). Two later studies revealed a much stronger linkage of 4p12 to the alcoholism-related endophenotype beta frequency band of the human electroencephalogram (EEG) (Edenberg et al. 2004; Porjesz et al. 2002).
Association analyses of GABAA gene cluster on chromosome 4 with alcoholism
The initial fine-mapping of the four genes (GABRA2, GABRA4, GABRB1, and GABRG1) in the chromosome 4p cluster in relation to alcoholism was carried out by Edenberg and colleagues in multiplex families from the Collaborative Study on Genetics of Alcoholism (COGA) sample (Edenberg et al. 2004). Those authors reported significant associations of DSM-IV AD with several SNPs at the individual level and a large, 164-kb, haplotype, all located in GABRA2, within a region starting from intron 3 and expanding over to 58 kb past the 3′ end of the gene. Similar findings were reported in subsequent studies conducted in the COGA sample (Agrawal et al. 2006; Dick et al. 2006a), as well as in several independent samples from populations of European (Covault et al. 2004a; Enoch et al. 2006; Fehr et al. 2006; Lind et al. 2008) and Native American (Enoch 2008) ancestry. Table 1 shows a summary of replicated individual SNPs associated with AD and its related phenotypes. Expanding on these findings in GABRA2, Covault and colleagues demonstrated that the large haplotype in the 3′ end of GABRA2 is in fact a sub-haplotype of a much larger “risk” region that extends across the intergene region to intron 1 of the adjacent GABRG1 (Covault et al. 2008). Conversely, in a later study, Enoch (2008) reported that the GABRA2 and GABRG1 haplotypes appear to be independent. It is clear that more studies are needed for consensus as to whether the risk alleles of the 3′ end of GABRA2 and the 5′ end of GABRG1 are transmitted as a block along generations. When examining those identified haplotypes associated with AD, one of the most interesting findings is that a region around axon 5 (from SNP rs279826 to rs279871) appears to be common among all haplotypes (see most intensely colored part of the bar in Fig. 4). This region has been considered to be the region of GABRA2 with the strongest association with AD (Edenberg and Foroud 2006) and is reported to be associated with AD comorbid with cocaine and marijuana dependence (Agrawal et al. 2006). Moreover, the functional SNP rs279858, which was found to have allele-based mRNA alterations in alcoholic postmortem brain tissue (Haughey et al. 2008), is located on exon 5 in this common haplotype, causing a synonymous change at amino acid residue 132. Perhaps the other function-unknown intronic associations suggest a more prominent role of gene regulation through alternative splicing, mRNA stability, or other post-transcriptional regulatory mechanisms in genetic variability contributing to alcoholism risk, rather than structural changes in GABAA subunit proteins.
Table 1.
Replicated GABRA2 SNP associations for AD and related QTL
| Phenotype | dbSNP ID | Chromosome position | *PMeta value | Design (references) | Associated risk allele/genotype | Race/ethnicity |
|---|---|---|---|---|---|---|
| AD (diagnosed using DSM-IV criteria for AD) | rs490434 | 46,193,279 | **2.45 × 10−4 | Family (Agrawal et al. 2006; Edenberg et al. 2004)‡ | NR | EA (83%), AA (13%), other (4%) |
| rs144013 | 46,201,920 | 7.78 × 10−4 | CC (Bierut et al. 2010; Covault et al. 2008) | A | EA | |
| rs567926 | 46,241,769 | 9.12 × 10−6 | CC (Covault et al. 2008) | C | EA | |
| CC (Fehr et al. 2006) | C | European-German | ||||
| rs572227 | 46,251,393 | 2.00 × 10−2 | CC (Bierut et al. 2010) | A | EA | |
| Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | ||||
| rs534459 | 46,256,805 | 2.21 × 10−5 | CC (Bierut et al. 2010; Covault et al. 2004a; Covault et al. 2008) | C | EA | |
| rs279871 | 46,305,733 | 2.50 × 10−5 | CC (Lydall et al. 2011) | A | European-UK | |
| CC (Fehr et al. 2006) | A | European-German | ||||
| Family (Agrawal et al. 2006; Dick et al. 2006a; Edenberg et al. 2004) | A | EA (83%), AA (13%), other (4%) | ||||
| rs279863 | 46,313,022 | **3.19 × 10−3 | Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | |
| CC (Enoch et al., 2009) | A | European-Finnish | ||||
| rs279858 | 46,314,593 | 2.20 × 10−2 | CC (Bierut et al. 2010; Covault et al. 2004a; Covault et al. 2008) | G | EA | |
| CC (Enoch et al. 2009) | AA and GG | European-Finnish | ||||
| CC (Fehr et al. 2006) | G | European-German | ||||
| CC (Lydall et al. 2011) | G | European-UK | ||||
| CC (Lappalainen et al. 2005) | G | European-Russian | ||||
| Family (Agrawal et al. 2006; Edenberg et al. 2004)‡ | NR | EA (83%), AA (13%), other (4%) | ||||
| Family (Villafuerte et al. 2011) | G | EA (98.2%), Other (1.8%) | ||||
| Family (Lind et al. 2008) | A | European-Australian (>90%) | ||||
| rs279844 | 46,329,655 | 7.71 × 10−3 | CC (Covault et al. 2004a; Covault et al. 2008) | T | EA | |
| rs279826 | 46,334,209 | **3.35 × 10−5 | Family (Agrawal et al. 2006; Edenberg et al. 2004)‡ | NR | EA (83%), AA (13%), other (4%) | |
| rs279837 | 46,339,323 | 8.00 × 10−3 | Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | |
| CC (Covault et al. 2004a) | C | EA | ||||
| rs279841 | 46,340,763 | 2.70 × 10−2 | CC (Bierut et al. 2010) | A | EA | |
| Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | ||||
| Excessive beta EEG fast activity | rs548583 | 46,263,344 | **4.33 × 10−3 | CC (Lydall et al. 2011) | C | European-UK |
| Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | ||||
| rs279871 | 46,305,733 | **1.39 × 10−2 | CC (Lydall et al. 2011) | A | European-UK | |
| Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | ||||
| rs279863 | 46,313,022 | **2.71 × 10−2 | CC (Lydall et al. 2011) | C | European-UK | |
| Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | ||||
| rs279841 | 46,340,763 | **2.93 × 10−3 | CC (Lydall et al. 2011) | G | European-UK | |
| Family (Edenberg et al. 2004) | NR | EA (83%), AA (13%), other (4%) | ||||
| Sensitivity to acute effects of alcohol | rs279858 | 46,314,593 | 3.33 × 10−3 | Human alcohol challenge study (Haughey et al. 2008) | AA | EA (>90%) |
| Human alcohol challenge study (Roh et al. 2010) | A | Japanese | ||||
| Pharmacogenetics (Pierucci-Lagha et al. 2005) | AA | EA (82%), Hispanic (18%) |
All chromosome positions are determined based on the NC_000004.11
EA European-American, AA African-American, NR not reported, CC case–control study design, Family Family-based study design
AD comorbid with other drug dependencies
Meta-analysis was performed with the metal (Wilier et al. 2010) or Fisher's combined probability test (indicated with '**'), where allelic association data were not available; both positive and negative associations were considered for meta-analyses
Fig. 4.

Structures of GABRA2 and GABRG1 and haplotypes associated with alcoholism. The black bars indicate location of haplotypes in Caucasians. The intensity of the bar corresponds to overlapping haplotypes detected in different association analyses. Therefore, the brightest segments correspond to the most frequently replicated haplotype regions. For both genes, main mRNA isoforms reported on NCBI AceView are shown
Association analyses of GABAA gene clusters on chromosomes 5 and 15 with alcoholism
The chromosome 5q34–q35 GABAA cluster, encoding the isoforms of the most abundant type of receptor complex (Barnard et al. 1998; Johnson et al. 1992), is linked to alcohol-related phenotypes, but with mixed results in subsequent replication studies. When linkage scans were performed for DSM-IV AD, no significant linkage was found between AD and the variants in the GABAA cluster in the COGA sample (Reich et al. 1998); however, when more homogeneous intermediate phenotypes such as drinking severity were used, linkage peaks were identified on chromosome 5 (Dick et al. 2006b). Further association studies performed with the COGA sample identified association of AD with GABRG3 (Dick et al. 2004), GABRA5, and GABRB3 (Song et al. 2003). Studies with other samples also found associations of AD with the gene clusters on chromosomes 5 and 15; with GABRB2 and GABRA6 SNPs associated with AD in Finns (Radel et al. 2005), and GABRA6 and GABRB2 SNPs associated with AD in Scottish samples (Loh et al. 1999). In GABRA6, SNP Pro385Ser was found to be associated with AD and a lower response to sedating effects of alcohol, and the 3′ UTR T1221C SNP was associated with physiological response to psychosocial stress (Uhart et al. 2004).
Genetics of pre-synaptic GABA receptors and transporters in alcoholism
Synaptic GABA regulators play an important role by modulating the availability of GABA for post-synaptic GABAergic receptors that relay GABA signaling. At higher concentrations, alcohol induces immediate release of GABA in a number of human brain regions, including the central (CeA) and basolateral amygdala, ventral tegmental area (VTA), and hippocampus (Kelm et al. 2011). A number of studies have demonstrated that the alcohol's ability to induce GABA release is facilitated by activation of Gαs or Gαq-coupled receptors such as CRF1 in CeA (Nie et al. 2004, 2009) and 5-HT2C in VTA; in contrast, activation of Gαi-coupled receptors such as GABAB (Peris et al. 1997; Silberman et al. 2009; Wu et al. 2005), CB1R in the amygdala and hippocampus (Roberto et al. 2010), and OPRD in CeA (Kang-Park et al. 2007) can block GABA release. Thus, it is reasonable to state that the genetic polymorphisms in all of the above-mentioned receptors and their downstream molecules may affect GABA release. However, evidence supporting this hypothesis is limited.
To be consistent with the current theme, we will limit our review to genetic variations in GABAB receptors that affect GABA release. Compared with studies conducted with GABAA receptors, only a few studies have performed association analysis of GABAB receptor subunit polymorphisms with alcoholism, mostly with negative results. An exonic polymorphism T1974C in GABBR1 on chromosome 6p21.3 was shown to be associated with AD, with the T allele being more frequent in alcoholics (Sander et al. 1999).
A number of transmembrane GABA transporters are involved in terminating inhibitory GABAergic signaling through rapid synaptic clearance of GABA by reuptake. The major transmembrane GABA transporter forms present in the human brain are GAT-1, GAT-2 and GAT-3 (Christiansen et al. 2007) encoded by SLC6A1, SLC6A13 and SLC6A11 genes, respectively. Chronic ethanol administration increased GAT-1 and GAT-3 levels in the hypothalamus and hippocampus of male alcohol-dependent rats (Devaud 2001); the authors of this study concluded that enhanced hypothalamic GABA reuptake resulting in greatly reduced synaptic levels of hypothalamic GABA may have a role in the development of tolerance and withdrawal. As with many other pre-synaptic GABA receptors and regulatory molecules, association analyses of GABA transporter polymorphisms with AD are not reported in literature. However, several recently emerged studies have suggested genetic associations of GABA transporter genes with other psychiatric pathology frequently comorbid with AD; for example, genetic associations of SLC6A1 and SLC6A13 with anxiety (Saus et al. 2010; Thoeringer et al. 2009), SLC6A1 with schizophrenia (Hirunsatit et al. 2009), and SLC6A1 with ADHD (Lasky-Su et al. 2008).
Conclusions and future directions
In sum, significant progress has been made in searching for susceptibility loci and genes for ND and alcoholism. On the basis of the identified linkage peaks on chromosomes 9 and 17 and prior knowledge of the biological functions of the products of each gene, variants in GABRA4, GABRA2, GABRE, GABBR2, and GABARAP are significantly associated with ND. Linkage peaks on chromosomes 4 and 5 harboring GABRA2, GABRG1, and GABRA6 genes were identified to be associated with AD in several independent Caucasian populations. Furthermore, the involvement of the GABAergic signaling pathway, to which these genes belong, in the etiology of ND has been confirmed by pathway-based association analysis.
In spite of this progress in molecular genetic studies of addictions, we still have a long way to go, and there are many challenges that remain to be surmounted (Ho et al. 2010; Li 2010; van der Zwaluw and Engels 2009). These challenges include: (1) further identification and replication of known and unknown genes in GABAergic and other signaling pathways and functional variants (including rare variants) for various addictive disorders through high-throughput approaches such as association study and deep sequencing; (2) study of copy number variations (CNVs) and their impact on gene expression in GABAergic and other addiction-related signaling pathways; (3) better understanding of the mechanisms underlying addictions at the molecular and cellular levels using both in vitro and in vivo approaches; and (4) determining appropriate ways of defining environmental factors such that we can assess how gene–environment interaction affects addictions. An improvement of our understanding of the genetic and environmental factors underlying drug addiction has considerable potential to reduce morbidity and death greatly by providing the most suitable methods for prevention and novel medications for treating different addictive disorders.
Acknowledgments
The preparation of this review was provided in part by DA-012844. We thank Dr. David L Bronson for his excellent editing of this manuscript. We also thank Dr. Jinxue Wei for performing part of the experiments reported in Fig. 3.
References
- Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, Nurnberger JI, Crowe R, Kuperman S, Schuckit MA, Begleiter H, Porjesz B, Dick DM. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. PubMed. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Pergadia ML, Saccone SF, Hinrichs AL, Lessov-Schlaggar CN, Saccone NL, Neuman RJ, Breslau N, Johnson E, Hatsukami D, Montgomery GW, Heath AC, Martin NG, Goate AM, Rice JP, Bierut LJ, Madden PA. Gamma-aminobutyric acid receptor genes and nicotine dependence: evidence for association from a case–control study. Addiction. 2008a;103:1027–1038. doi: 10.1111/j.1360-0443.2008.02236.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Pergadia ML, Saccone SF, Lynskey MT, Wang JC, Martin NG, Statham D, Henders A, Campbell M, Garcia R, Broms U, Todd RD, Goate AM, Rice J, Kaprio J, Heath AC, Montgomery GW, Madden PA. An autosomal linkage scan for cannabis use disorders in the nicotine addiction genetics project. Arch Gen Psychiatry. 2008b;65:713–721. doi: 10.1001/archpsyc.65.6.713. PubMed. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International union of pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. PubMed. [PubMed] [Google Scholar]
- Bergen AW, Korczak JF, Weissbecker KA, Goldstein AM. A genome-wide search for loci contributing to smoking and alcoholism. Genet Epidemiol. 1999;17(Suppl 1):S55–S60. doi: 10.1002/gepi.1370170710. PubMed. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. PubMed. [DOI] [PubMed] [Google Scholar]
- Beuten J, Ma JZ, Payne TJ, Dupont RT, Crews KM, Somes G, Williams NJ, Elston RC, Li MD. Single- and multilocus allelic variants within the GABAB receptor subunit 2 (GABAB2) gene are significantly associated with nicotine dependence. Am J Hum Genet. 2005;76:859–864. doi: 10.1086/429839. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuten J, Ma JZ, Payne TJ, Dupont RT, Lou XY, Crews KM, Elston RC, Li MD. Association of specific haplotypes of neurotrophic tyrosine kinase receptor 2 gene (NTRK2) with vulnerability to nicotine dependence in African-Americans and European-Americans. Biol Psychiatry. 2007;61:48–55. doi: 10.1016/j.biopsych.2006.02.023. PubMed. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Rice JP, Goate A, Hinrichs AL, Saccone NL, Foroud T, Edenberg HJ, Cloninger CR, Begleiter H, Conneally PM, Crowe RR, Hesselbrock V, Li TK, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. A genomic scan for habitual smoking in families of alcoholics: common and specific genetic factors in substance dependence. Am J Med Genet. 2004;124A:19–27. doi: 10.1002/ajmg.a.20329. PubMed. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr, Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci USA. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. PubMed. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang H, Vicini S, Olsen RW. The gamma-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc Natl Acad Sci USA. 2000;97:11557–11562. doi: 10.1073/pnas.190133497. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen B, Meinild AK, Jensen AA, Brauner-Osborne H. Cloning and characterization of a functional human gamma-aminobutyric acid (GABA) transporter, human GAT-2. J Biol Chem. 2007;282:19331–19341. doi: 10.1074/jbc.M702111200. PubMed. [DOI] [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, Vacca G, Gessa GL. Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotoxic Res. 2004;6:403–414. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology (Berl) 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004a;129B:104–109. doi: 10.1002/ajmg.b.30091. PubMed. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004b;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5′-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2008;33:837–848. doi: 10.1038/sj.npp.1301456. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culverhouse R, Klein T, Shannon W. Detecting epistatic interactions contributing to quantitative traits. Genet Epidemiol. 2004;27:141–152. doi: 10.1002/gepi.20006. PubMed. [DOI] [PubMed] [Google Scholar]
- Darlison MG, Pahal I, Thode C. Consequences of the evolution of the GABA(A) receptor gene family. Cell Mol Neurobiol. 2005;25:607–624. doi: 10.1007/s10571-005-4004-4. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL. Ethanol dependence has limited effects on GABA or glutamate transporters in rat brain. Alcohol Clin Exp Res. 2001;25:606–611. PubMed. [PubMed] [Google Scholar]
- Dick DM, Edenberg HJ, Xuei X, Goate A, Kuperman S, Schuckit M, Crowe R, Smith TL, Porjesz B, Begleiter H, Foroud T. Association of GABRG3 with alcohol dependence. Alcohol Clin Exp Res. 2004;28:4–9. doi: 10.1097/01.ALC.0000108645.54345.98. PubMed. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Schuckit MA, Bierut L, Hinrichs A, Fox L, Mullaney J, Cloninger CR, Hesselbrock V, Nurnberger JI, Jr, Almasy L, Foroud T, Porjesz B, Edenberg H, Begleiter H. Marital status, alcohol dependence, and GABRA2: evidence for gene–environment correlation and interaction. J Stud Alcohol. 2006a;67:185–194. doi: 10.15288/jsa.2006.67.185. PubMed. [DOI] [PubMed] [Google Scholar]
- Dick DM, Jones K, Saccone N, Hinrichs A, Wang JC, Goate A, Bierut L, Almasy L, Schuckit M, Hesselbrock V, Tischfield J, Foroud T, Edenberg H, Porjesz B, Begleiter H. Endophenotypes successfully lead to gene identification: results from the collaborative study on the genetics of alcoholism. Behav Genet. 2006b;36:112–126. doi: 10.1007/s10519-005-9001-3. PubMed. [DOI] [PubMed] [Google Scholar]
- Drgon T, D'Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:854–860. doi: 10.1002/ajmg.b.30349. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala R, Almasy L, Blangero J. Smoking behavior is under the influence of a major quantitative trait locus on human chromosome 5q. Genet Epidemiol. 1999;17(Suppl 1):S139–S144. doi: 10.1002/gepi.1370170724. PubMed. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006;11:386–396. doi: 10.1111/j.1369-1600.2006.00035.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:599–607. doi: 10.1002/ajmg.b.30336. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan Q, Albaugh B, Virkkunen M, Goldman D. GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology. 2009;34:1245–1254. doi: 10.1038/npp.2008.171. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta MC, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–498. doi: 10.1093/alcalc/37.5.495. PubMed. [DOI] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. PubMed. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo MR. Forum: interactions between gene and environment. Curr Opin Psychiatry. 2008;21:315–317. doi: 10.1097/YCO.0b013e328306a791. PubMed. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126:91–99. doi: 10.1007/s00439-009-0701-2. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Liu X, Hesselbrock V, Page GP, Goddard A, Zhang H. Results of a genomewide linkage scan: support for chromosomes 9 and 11 loci increasing risk for cigarette smoking. Am J Med Genet. 2004;128B:94–101. doi: 10.1002/ajmg.b.30019. PubMed. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, Farrer L, Kranzler HR. Genomewide linkage scan for nicotine dependence: identification of a chromosome 5 risk locus. Biol Psychiatry. 2007;61:119–126. doi: 10.1016/j.biopsych.2006.08.023. PubMed. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. PubMed. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Papadeas ST, Morrow AL. Regional variations in the effects of chronic ethanol administration on GABA(A) receptor expression: potential mechanisms. Neurochem Int. 2000;37:453–461. doi: 10.1016/s0197-0186(00)00058-9. PubMed. [DOI] [PubMed] [Google Scholar]
- Han S, Gelernter J, Luo X, Yang BZ. Meta-analysis of 15 genome-wide linkage scans of smoking behavior. Biol Psychiatry. 2010;67:12–19. doi: 10.1016/j.biopsych.2009.08.028. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Ray LA, Finan P, Villanueva R, Niculescu M, Hutchison KE. Human gamma-aminobutyric acid A receptor alpha2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav. 2008;7:447–454. doi: 10.1111/j.1601-183X.2007.00369.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Hirunsatit R, George ED, Lipska BK, Elwafi HM, Sander L, Yrigollen CM, Gelernter J, Grigorenko EL, Lappalainen J, Mane S, Nairn AC, Kleinman JE, Simen AA. Twenty-one-base-pair insertion polymorphism creates an enhancer element and potentiates SLC6A1 GABA transporter promoter activity. Pharmacogenet Genomics. 2009;19:53–65. doi: 10.1097/FPC.0b013e328318b21a. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Tyndale RF. Overview of the pharmacogenomics of cigarette smoking. Pharmacogenomics J. 2007;7:81–98. doi: 10.1038/sj.tpj.6500436. PubMed. [DOI] [PubMed] [Google Scholar]
- Ho MK, Goldman D, Heinz A, Kaprio J, Kreek MJ, Li MD, Munafo MR, Tyndale RF. Breaking barriers in the genomics and pharmacogenetics of drug addiction. Clin Pharmacol Ther. 2010;88:779–791. doi: 10.1038/clpt.2010.175. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P, Green EK, Pahwa JS, Ferreira MA, Purcell SM, Sklar P, Owen MJ, O'Donovan MC, Craddock N. Gene ontology analysis of GWA study data sets provides insights into the biology of bipolar disorder. Am J Hum Genet. 2009;85:13–24. doi: 10.1016/j.ajhg.2009.05.011. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Sander T, Hicks AA, van Marle A, Janz D, Mullan MJ, Riley BP, Darlison MG. Confirmation of the localization of the human GABAA receptor alpha 1-subunit gene (GABRA1) to distal 5q by linkage analysis. Genomics. 1992;14:745–748. doi: 10.1016/s0888-7543(05)80178-8. PubMed. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Presynaptic delta opioid receptors regulate ethanol actions in central amygdala. J Pharmacol Exp Ther. 2007;320:917–925. doi: 10.1124/jpet.106.112722. PubMed. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. PubMed. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee EW, Ebbert JO, Schneider H, Hurt RD, Ekker SC. Zebrafish for the study of the biological effects of nicotine. Nicotine Tob Res. 2010;13:301–312. doi: 10.1093/ntr/ntr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnke MD. Approach to the genetics of alcoholism: a review based on pathophysiology. Biochem Pharmacol. 2008;75:160–177. doi: 10.1016/j.bcp.2007.06.021. PubMed. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, Gelernter J, Lappalainen J. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. PubMed. [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. PubMed. [DOI] [PubMed] [Google Scholar]
- Lang T, Schaeffeler E, Bernreuther D, Bredschneider M, Wolf DH, Thumm M. Aut2p and Aut7p, two novel microtubule-associated proteins are essential for delivery of autophagic vesicles to the vacuole. EMBO J. 1998;17:3597–3607. doi: 10.1093/emboj/17.13.3597. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. PubMed. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, Vasquez AA, Chen W, Asherson P, Buitelaar J, Banaschewski T, Ebstein R, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Daly M, Laird N, Lange C, Faraone SV. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1345–1354. doi: 10.1002/ajmg.b.30867. PubMed. [DOI] [PubMed] [Google Scholar]
- Leil TA, Chen ZW, Chang CS, Olsen RW. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Pergadia ML, Khroyan TV, Swan GE. Genetics of nicotine dependence and pharmacotherapy. Biochem Pharmacol. 2008;75:178–195. doi: 10.1016/j.bcp.2007.08.018. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD. The genetics of nicotine dependence. Curr Psychiatry Rep. 2006;8:158–164. doi: 10.1007/s11920-006-0016-0. PubMed. [DOI] [PubMed] [Google Scholar]
- Li MD. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Hum Genet. 2008;123:119–131. doi: 10.1007/s00439-008-0473-0. PubMed. [DOI] [PubMed] [Google Scholar]
- Li MD. Grand challenges and opportunities for molecular psychiatry research: a perspective. Front Psychiatry. 2010;1:2. doi: 10.3389/fpsyt.2010.00002. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009;10:225–231. doi: 10.1038/nrg2536. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003a;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Li MD, Ma JZ, Cheng R, Dupont RT, Williams NJ, Crews KM, Payne TJ, Elston RC. A genome-wide scan to identify loci for smoking rate in the Framingham Heart Study population. BMC Genet. 2003b;4(Suppl 1):S103. doi: 10.1186/1471-2156-4-S1-S103. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Kane JK, Wang J, Ma JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment. Brain Res Mol Brain Res. 2004;132:168–180. doi: 10.1016/j.molbrainres.2004.09.009. PubMed. [DOI] [PubMed] [Google Scholar]
- Li MD, Payne TJ, Ma JZ, Lou XY, Zhang D, Dupont RT, Crews KM, Somes G, Williams NJ, Elston RC. A genomewide search finds major susceptibility Loci for nicotine dependence on chromosome 10 in African Americans. Am J Hum Genet. 2006;79:745–751. doi: 10.1086/508208. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Sun D, Lou XY, Beuten J, Payne TJ, Ma JZ. Linkage and association studies in African- and Caucasian-American populations demonstrate that SHC3 is a novel susceptibility locus for nicotine dependence. Mol Psychiatry. 2007;12:462–473. doi: 10.1038/sj.mp.4001933. PubMed. [DOI] [PubMed] [Google Scholar]
- Li MD, Ma JZ, Payne TJ, Lou XY, Zhang D, Dupont RT, Elston RC. Genome-wide linkage scan for nicotine dependence in European Americans and its converging results with African Americans in the Mid-South Tobacco Family sample. Mol Psychiatry. 2008;13:407–416. doi: 10.1038/sj.mp.4002038. PubMed. [DOI] [PubMed] [Google Scholar]
- Li MD, Mangold JE, Seneviratne C, Chen GB, Ma JZ, Lou XY, Payne TJ. Association and interaction analyses of GABBR1 and GABBR2 with nicotine dependence in European- and African-American populations. PLoS One. 2009;4:e7055. doi: 10.1371/journal.pone.0007055. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Agrawal A, Montgomery GW, Heath AC, Martin NG, Whitfield JB. The role of GABRA2 in alcohol dependence, smoking, and illicit drug use in an Australian population sample. Alcohol Clin Exp Res. 2008;32:1721–1731. doi: 10.1111/j.1530-0277.2008.00768.x. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh EW, Smith I, Murray R, McLaughlin M, McNulty S, Ball D. Association between variants at the GABAAbeta2, GABAAalpha6 and GABAAgamma2 gene cluster and alcohol dependence in a Scottish population. Mol Psychiatry. 1999;4:539–544. doi: 10.1038/sj.mp.4000554. PubMed. [DOI] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–221. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. PubMed. [DOI] [PubMed] [Google Scholar]
- Lou XY, Chen GB, Yan L, Ma JZ, Zhu J, Elston RC, Li MD. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. 2007a;80:1125–1137. doi: 10.1086/518312. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou XY, Ma JZ, Sun D, Payne TJ, Li MD. Fine mapping of a linkage region on chromosome 17p13 reveals that GABARAP and DLG4 are associated with vulnerability to nicotine dependence in European-Americans. Hum Mol Genet. 2007b;16:142–153. doi: 10.1093/hmg/ddl450. PubMed. [DOI] [PubMed] [Google Scholar]
- Lou XY, Chen GB, Yan L, Ma JZ, Mangold JE, Zhu J, Elston RC, Li MD. A combinatorial approach to detecting gene–gene and gene–environment interactions in family studies. Am J Hum Genet. 2008;83:457–467. doi: 10.1016/j.ajhg.2008.09.001. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall GJ, Saini J, Ruparelia K, Montagnese S, McQuillin A, Guerrini I, Rao H, Reynolds G, Ball D, Smith I, Thomson AD, Morgan MY, Gurling HM. Genetic association study of GABRA2 single nucleotide polymorphisms and electroencephalography in alcohol dependence. Neurosci Lett. 2011;500:162–166. doi: 10.1016/j.neulet.2011.05.240. PubMed. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Colombo G. Role of the GABA(B) receptor in alcohol-seeking and drinking behavior. Alcohol. 2009;43:555–558. doi: 10.1016/j.alcohol.2009.09.030. PubMed. [DOI] [PubMed] [Google Scholar]
- Nelson MR, Kardia SL, Ferrell RE, Sing CF. A combinatorial partitioning method to identify multilocus genotypic partitions that predict quantitative trait variation. Genome Res. 2001;11:458–470. doi: 10.1101/gr.172901. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. PubMed. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. Sci World J. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin I, Vuillermoz C, Jouvenot M, Ordener C, Royez M, Adessi GL. Identification and characterization of an early estrogen-regulated RNA in cultured guinea-pig endometrial cells. Mol Cell Endocrinol. 1993;90:R17–R21. doi: 10.1016/0303-7207(93)90161-c. PubMed. [DOI] [PubMed] [Google Scholar]
- Peris J, Eppler B, Hu M, Walker DW, Hunter BE, Mason K, Anderson KJ. Effects of chronic ethanol exposure on GABA receptors and GABAB receptor modulation of 3H-GABA release in the hippocampus. Alcohol Clin Exp Res. 1997;21:1047–1052. PubMed. [PubMed] [Google Scholar]
- Petzold AM, Balciunas D, Sivasubbu S, Clark KJ, Bedell VM, Westcot SE, Myers SR, Moulder GL, Thomas MJ, Ekker SC. Nicotine response genetics in the zebrafish. Proc Natl Acad Sci USA. 2009;106:18662–18667. doi: 10.1073/pnas.0908247106. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. PubMed. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O'Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci USA. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radel M, Vallejo RL, Iwata N, Aragon R, Long JC, Virkkunen M, Goldman D. Haplotype-based localization of an alcohol dependence gene to the 5q34 {gamma}-aminobutyric acid type A gene cluster. Arch Gen Psychiatry. 2005;62:47–55. doi: 10.1001/archpsyc.62.1.47. PubMed. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. PubMed. [PubMed] [Google Scholar]
- Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–147. doi: 10.1086/321276. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–1972. doi: 10.1038/npp.2010.70. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, Miyakawa T, Ramchandani VA, Li TK, Higuchi S. Role of GABRA2 in moderating subjective responses to alcohol. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01357.x. doi:10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv Y, Legesse-Miller A, Porat A, Elazar Z. GATE-16, a membrane transport modulator, interacts with NSF and the Golgi v-SNARE GOS-28. EMBO J. 2000;19:1494–1504. doi: 10.1093/emboj/19.7.1494. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Samochowiec J, Ladehoff M, Smolka M, Peters C, Riess O, Rommelspacher H, Schmidt LG. Association analysis of exonic variants of the gene encoding the GABAB receptor and alcohol dependence. Psychiatr Genet. 1999;9:69–73. doi: 10.1097/00041444-199906000-00004. PubMed. [DOI] [PubMed] [Google Scholar]
- Saus E, Brunet A, Armengol L, Alonso P, Crespo JM, Fernandez-Aranda F, Guitart M, Martin-Santos R, Menchon JM, Navines R, Soria V, Torrens M, Urretavizcaya M, Valles V, Gratacos M, Estivill X. Comprehensive copy number variant (CNV) analysis of neuronal pathways genes in psychiatric disorders identifies rare variants within patients. J Psychiatr Res. 2010;44:971–978. doi: 10.1016/j.jpsychires.2010.03.007. PubMed. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Koller DL, Foroud T, Carr K, Zhao J, Rice J, Nurnberger JI, Jr, Begleiter H, Porjesz B, Smith TL, Schuckit MA, Edenberg HJ. Association of GABA(A) receptors and alcohol dependence and the effects of genetic imprinting. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:39–45. doi: 10.1002/ajmg.b.10022. PubMed. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(Suppl 2):S51–57. doi: 10.1080/14622299050011811. discussion S69–70. [DOI] [PubMed] [Google Scholar]
- Swan GE, Hudmon KS, Jack LM, Hemberger K, Carmelli D, Khroyan TV, Ring HZ, Hops H, Andrews JA, Tildesley E, McBride D, Benowitz N, Webster C, Wilhelmsen KC, Feiler HS, Koenig B, Caron L, Illes J, Cheng LS. Environmental and genetic determinants of tobacco use: methodology for a multidisciplinary, longitudinal family-based investigation. Cancer Epidemiol Biomarkers Prev. 2003;12:994–1005. PubMed. [PMC free article] [PubMed] [Google Scholar]
- Thoeringer CK, Ripke S, Unschuld PG, Lucae S, Ising M, Bettecken T, Uhr M, Keck ME, Mueller-Myhsok B, Holsboer F, Binder EB, Erhardt A. The GABA transporter 1 (SLC6A1): a novel candidate gene for anxiety disorders. J Neural Transm. 2009;116:649–657. doi: 10.1007/s00702-008-0075-y. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, McCaul ME, Oswald LM, Choi L, Wand GS. GABRA6 gene polymorphism and an attenuated stress response. Mol Psychiatry. 2004;9:998–1006. doi: 10.1038/sj.mp.4001535. PubMed. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC. Gene–environment interactions and alcohol use and dependence: current status and future challenges. Addiction. 2009;104:907–914. doi: 10.1111/j.1360-0443.2009.02563.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Wendy Yau WY, Majczenko K, Zubieta JK, Zucker RA, Burmeister M. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.33. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Markou A. GABAB receptors in reward processes. Adv Pharmacol. 2010;58:315–371. doi: 10.1016/S1054-3589(10)58013-X. PubMed. [DOI] [PubMed] [Google Scholar]
- Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology. 2010;35:702–719. doi: 10.1038/npp.2009.178. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. PubMed. [DOI] [PubMed] [Google Scholar]
- Wang D, Ma JZ, Li MD. Mapping and verification of susceptibility loci for smoking quantity using permutation linkage analysis. Pharmacogenomics J. 2005;5:166–172. doi: 10.1038/sj.tpj.6500304. PubMed. [DOI] [PubMed] [Google Scholar]
- Wang K, Li M, Bucan M. Pathway-based approaches for analysis of genomewide association studies. Am J Hum Genet. 2007;81:1278–1283. doi: 10.1086/522374. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . The World Health Report 2002. World Health Organization; 2002. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PH, Poelchen W, Proctor WR. Differential GABAB receptor modulation of ethanol effects on GABA(A) synaptic activity in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2005;312:1082–1089. doi: 10.1124/jpet.104.075663. PubMed. [DOI] [PubMed] [Google Scholar]
- Zinn-Justin A, Abel L. Genome search for alcohol dependence using the weighted pairwise correlation linkage method: interesting findings on chromosome 4. Genet Epidemiol. 1999;17(Suppl 1):S421–S426. doi: 10.1002/gepi.1370170771. PubMed. [DOI] [PubMed] [Google Scholar]


