Abstract
Both lithium and valproate are well-established treatments for bipolar disorder. Studies have also found that lithium is effective at reducing suicidal behaviors in patients with mood disorders. Impulsivity is a validated endophenotype of both bipolar disorder and suicidal behavior. We assessed effects of treatment with lithium or valproate on cognitive impulsivity in selectively bred mice previously shown to manifest relatively high levels of cognitive impulsivity. Mice were trained in the delay-discounting paradigm, a measure of cognitive impulsivity reflecting a behavioral bias towards immediacy, and then treated with lithium, valproate, or control chow. After 3 weeks of drug treatment, mice were tested at various delays to a large, delayed reward. Drug treatment continued during this time. Lithium reduced impulsivity, whereas valproate had no effect on choice behavior. Both drugs increased the number of choice trials and reinforcer intake, but effects on choice behavior did not depend on these motivational changes. To our knowledge, this is the first study demonstrating lithium's effects to reduce cognitive impulsivity. Future studies may focus on the ability of putative pharmacotherapies for patients at risk for bipolar disorder or suicide to modify the impulsive choice dimension of this diseases.
Keywords: lithium, valproic acid, suicide, impulsivity, bipolar disorder
INTRODUCTION
For over a century, clinicians treating patients suffering from a wide range of illnesses have prescribed various forms of lithium. Lithium has proven efficacy for the treatment of bipolar disorder (Geddes et al, 2004; Weisler et al, 2011). Extensive evidence also indicates that lithium is effective in reducing the risk of suicidal behaviors—both attempts and completions—in patients with mood disorders (Baldessarini et al, 2006; Cipriani et al, 2005; Guzzetta et al, 2007; Tondo et al, 1997). For example, Baldessarini et al (2006), in a pooled analysis of 31 studies, reported a highly statistically significant, 4.91-fold lower risk of suicidal acts during long-term treatment with vs without lithium, or an 80% sparing of risk associated with lithium treatment. This decreased risk remains highly significant when assessing only suicides or attempted suicides, or when only pooling results from randomized controlled trials (Baldessarini et al, 2006). Importantly, lithium is not only effective in reducing suicidal behavior in patients with bipolar disorder. A meta-analysis including eight studies of patients with major depressive disorder found that the risk of suicidal behavior was nearly 90% lower with lithium treatment (Guzzetta et al, 2007).
The mechanism by which lithium effectively treats bipolar disorder and reduces the risk of suicidal behaviors is unknown. Important insight may arise from understanding the effects of lithium on endophenotypes associated with bipolar disorder and suicidal behaviors, which themselves may be more tractable to dissection in preclinical models or tests. Impulsivity is a prominent component of bipolar disorder and also has a strong correlation with suicidal behaviors (Courtet et al, 2011; Mann et al, 2009; Najt et al, 2007). Studies demonstrate that impulsivity is not only present during a bipolar disorder manic episode, which would indicate state dependence, but also during euthymic states indicating trait-like characteristic. For example, bipolar patients consistently have higher impulsivity ratings compared with healthy controls, regardless of whether they are in a manic or depressive episode (Etain et al, 2012; Lombardo et al, 2012; Peluso et al, 2007; Swann et al, 2001; Swann et al, 2003), supporting the conclusion that impulsivity is a potential endophenotype of bipolar disorder.
Similarly, impulsivity is a well-validated endophenotype associated with suicidal behavior. Data supporting this fact derives from case control, retrospective (psychological autopsy), prospective longitudinal, and family studies (Kovacsics et al, 2009; Melhem et al, 2007; Turecki, 2005). A number of studies have found that trait impulsivity meets criteria for an endophenotype such as being heritable, associated with suicide, state independent, and co-segregated with suicidal behavior in families (Grunebaum et al, 2006; Melhem et al, 2007; Oquendo et al, 2004). Lithium may exert its antisuicidal actions by modifying impulsive, as well as impulsive, aggressive behaviors (Kovacsics et al, 2010). Some clinical evidence suggests that lithium treatment reduces impulsivity in at-risk individuals. The results of randomized, placebo-controlled studies suggest that lithium decreases human impulsivity, although overall, the evidence for such an effect is complicated by concurrent diagnoses, such as pathological gambling, bipolar disorder, and ADHD (Dorrego et al, 2002; Hollander et al, 2005; Kovacsics et al, 2009).
To date, limited research has examined effects of lithium on impulsivity in laboratory animals. A recent study by Ohmura et al (2012) assessed the effects of a single i.p. injection of lithium, valproate, or carbamazepine on impulsivity as assessed by a 3-choice serial reaction time task (3-CSRTT). They reported that lithium significantly reduced premature responses in the task, whereas neither valproate nor carbamazepine had any significant effect. While the 3-CSRTT is considered as a measure of motor impulsivity (Winstanley et al, 2006), there has been little work assessing lithium's effects on a measure of cognitive impulsivity, such as delay discounting (DD). In the DD task, subjects are given a choice between a delayed, large reward and an immediate, smaller reward. Self-defeating choices of the immediate reward are thought to reflect impulsive choice (Rachlin and Green, 1972). Generally, choice of the immediate reward increases with increasing delays to the large reward. The delay-discounting paradigm has been successfully modeled in animals (Green and Snyderman, 1980; Oberlin and Grahame, 2009), and has shown sensitivity to pharmacological manipulation (Oberlin et al, 2010). These factors make DD a good candidate as a translational endophenotype, as it can be assessed in both humans and animals (Gould and Gottesman, 2006). We hypothesized that treatment with lithium would reduce cognitive impulsivity as assessed by the DD task in a strain of mouse previously characterized to manifest relatively high levels of impulsivity.
MATERIALS AND METHODS
Subjects
Experiments were conducted as two studies. Mice in both the studies were 24 female and 24 male High Alcohol-Preferring (HAP) line replicate II mice, aged between 45 and 51 days at the beginning of training. This line was selected because of their relatively high levels of impulsivity in the DD task relative to mice with lower alcohol intake (Oberlin and Grahame, 2009) and sensitivity to the anti-impulsive effects of d-amphetamine (Oberlin et al, 2010). In the lithium study, mice were from the 37th generation of selective breeding, and in the valproate study, the mice were from the 40th generation. Mice were balanced across sex, family, and birth date, and then counter-balanced across squad and run order. The mice were individually housed in polycarbonate cages (27.9 × 9.5 × 12.7 cm) with Cellsorb bedding starting 7 days prior to the study. They were kept on a reversed light–dark cycle with lights out from 0800 to 2000 hours, and were given ad libitum access to food and water, except that they were restricted to 2 h of access to water during the training phase and during the last phase of the DD testing in the lithium study to increase motivation.
Apparatus
The operant chambers, measuring 21.6 × 19.7 × 12.7 cm inside, were contained within sound and light-attenuating cubicles fitted with electric fans to minimize ambient noise and light. Each apparatus contained an illuminated center nosepoke directly above the sipper tube opening. To the left and right of the nosepoke were LED signal lights positioned above response levers. Directly below the nosepoke was the opening for the sipper tube to descend into the chamber. The saccharin solution was dispensed from a 10 ml tube, and intakes were measured to an accuracy of 0.1 ml after each session. The chambers were operated through MED-PC IV software (Med Associates, St Albans, VT).
Procedures
DD task
The mice were trained according to the procedure described in Richards et al, 1997. Briefly, each trial begins with illumination of the center nosepoke light. A nosepoke response results in illumination of the lights above the levers, indicating that the levers are active. A response on the lever designated as the delay lever always results in 2 s of access to reward (0.032 or 0.32% saccharin in tap water) after the predetermined delay. A response on the immediate lever results in 1 s of access at the start of the session, but the duration of access is either increased or decreased by 0.2 s after each response to a ceiling of 2 s and a floor of 0 s. A choice of the delay lever increases the duration of access on the subsequent immediate choice, whereas a response on the immediate lever decreases it by the same amount, thus titrating the value of the immediate reward to the subjective value of the delayed reward at a given delay. There is a maximum of 60 trials per session. The average of this adjusting amount (over the last 18 trials of the session) represents the subjective value of the delayed reward. Higher adjusted amounts indicate lower levels of impulsivity (Richards et al, 1997). During initial training at a 0 s delay to the ‘delayed' lever, mice were required to show magnitude discrimination (preference for the large reward), with criteria for advancement to the testing phase being a minimum of 18 trials completed per 60 min session and a minimum mean adjusted amount of 1.6 s for three out of four consecutive days. These mean adjusted amounts were later used in all analyses to demonstrate magnitude discrimination prior to onset of delayed rewards, and are represented on the graphs as the 0 s time point. These criteria ensured that the subject had acquired the task and was able to correctly choose the larger reinforcer at a 0 s delay. Thereafter, delayed reward trials ensued, with mice being tested for 5 days each at 1, 2, 4, and 8 s delays to the large reward in ascending order. Only sessions in which mice achieved at least 18 trials were counted to the mean adjusted amount at that delay, and mice had to achieve at least 18 trials on at least 2 of the 5 days at each delay. Both experiments in this study used identical training procedures; however, the concentration of the reward in the valproate study was 0.32% saccharin in tap water due to low levels of responding in the absence of water deprivation.
Lithium administration and testing
After completion of training, mice were assigned to either control or lithium groups, balanced across sex, family, date of birth, and mean adjusted amount, and were then returned to their home cages. Their regular chow was replaced with either 4 g/kg LiCl chow or control chow (Custom Animal Diets, Bangor, PA). This dose was used because it results in circulating levels of lithium within the human therapeutic range (0.6–1.3 mM) and consistent behavioral actions of lithium in other mouse behavioral tests (Gould et al, 2008; Kovasics et al, 2010; Can et al, 2011). Mice were given ad libitum access to food and water, and a NaCl solution (9 g/l tap water) while in the home cage and weighed three times weekly. There was no testing at this time. After 3 weeks, the mice were returned to the magnitude discrimination task and run at a 0 s delay for 5 days. DD assessment followed. Because of higher fluid intake in the lithium condition, mice were tested for an additional 5 days at 8 s delay during which time mice in both treatment groups were restricted to 2 h of water access per day (given after daily DD sessions) to increase the fluid intake in both lithium and control groups. Drug treatment continued throughout the delay testing (see Figure 1 for a Timeline). After the conclusion of testing, brains were removed and analyzed for lithium levels. Entire brains were homogenized with a polytron homogenizer (Kinematica AG, Model PT-MR 2100, Littau, Switzerland) in three volumes of 0.5 N trichloroacetic acid, followed by centrifugation as described (Gould et al, 2007; Gould et al, 2008; Hamburger-Bar et al, 1986). Brain lithium levels (mmol/kg, wet weight) were measured with a flame photometer (Cole-Palmer Model 2655-00, Chicago, IL, USA).
Figure 1.
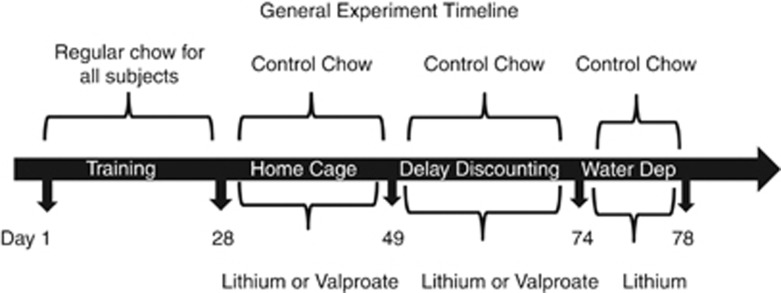
Timeline showing the experimental procedure used in both studies.
Valproate administration and testing
The testing procedure for the valproate study was identical to the lithium study with the exception of drug and the reinforcer saccharin concentration. During training, many of the mice reduced responding once they were no longer being water deprived. In an effort to increase performance and maintain consistency with the lack of water deprivation in the lithium study, the saccharin concentration was increased. Mice in the valproate study received either sodium valproate (20 g/kg) or control chow (both from Custom Animal Diets, Bangor, PA) as previously described (Gould et al, 2004a; Hao et al, 2004). Drug exposure continued throughout the delay testing (Figure 1). After the conclusion of testing, mice were decapitated, and to obtain sufficient serum for testing, blood from two like-treated mice were combined to yield sufficient serum for assessment of valproate levels. Serum was run at Indiana University Hospital Pathology Laboratory using standard procedures for clinical samples.
Statistics
Data were analyzed in SPSS software (SPSS, Version 18, Chicago, IL) and Prism software (Graphpad Prism, v. 5.0 La Jolla, CA). Group X Delay repeated measures ANOVAs were run on mean±SEMs for adjusted amounts, number of trials completed, and volumes consumed during operant sessions. For analysis of stability of adjusted amounts within each delay, we assessed Group X Delay X Day analyses separately, including only days in which at least 70% of the subjects in each group achieved the trials criterion. Number of days used per delay were as follows: 3 days at 1 s delay, 5 days at 2 s delay, 3 days at 4 s delay and 5 days at 8 s delay, with data from a minimum of 16 mice per group per day. An α-value of 0.05 was set to test for significance.
Single values representing the impulsivity level for each mouse, integrating choices over all delays, were calculated according to the formula: mean adjusted amount=delayed reinforcer magnitude/(1+k*delay). Larger values of k indicate steeper discounting. Mice with higher levels of impulsivity will generate steeper discounting curves. The resulting k-values showed a left skew, so they were log-10-transformed values prior to a t-test analysis.
RESULTS
Lithium
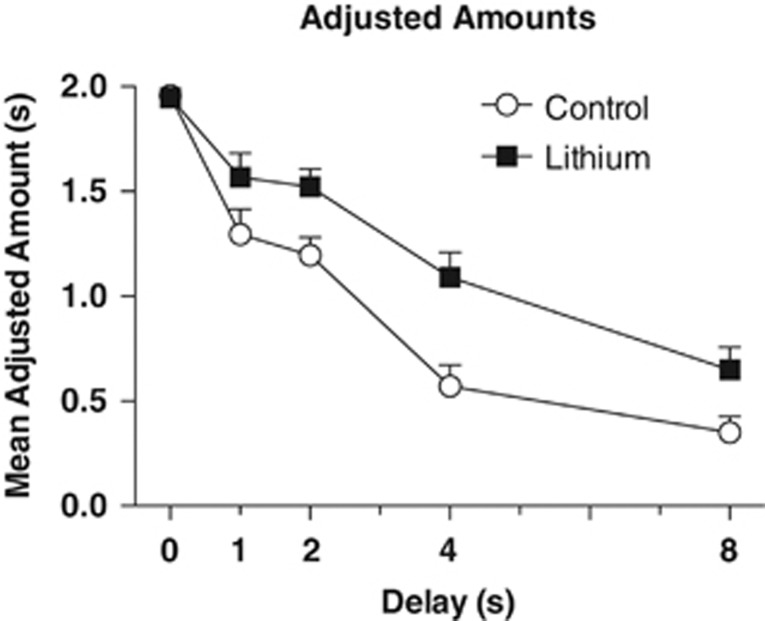
In the lithium study, 9 out of 48 (18.75%) mice were removed for failure to meet initial training criteria prior to lithium or control treatment. Lithium and control mice showed the same adjusted amounts at 0 s delay prior to diet introduction, both averaging (±SEM) 1.95±0.20 s. A mixed ANOVA revealed that treatment with lithium resulted in higher adjusted amounts (ie, lower impulsivity) across all delays (Figure 2). There was an interaction of group X delay, F(4, 148)=2.77, P=0.03, as well as main effects of group, F(1, 37)=11.25, P=0.002, and delay F(4, 148)=97.45, P<0.001. Follow-up t-tests indicated that the interaction probably resulted from no difference at 0 s or the 1 s delay, ts(37)⩽1.62, Ps⩾0.11, whereas at longer delays, lithium increased adjusted amounts, ts(37)⩾2.68, Ps⩽0.05 The mean (±SEM) k-value for the lithium group was 0.301±0.058 and for controls was 0.576±0.094. A t-test on log-10-transformed values indicated a significant difference, t(37)=3.03, P=0.005, which is consistent with lithium-reducing impulsivity.
Figure 2.
Group mean adjusted amounts±SEM as a function of delay (0, 1, 2, 4 and 8 s). The lithium group (n=18) had higher adjusted amounts than the control group (n=20), P<.001 at all delays.
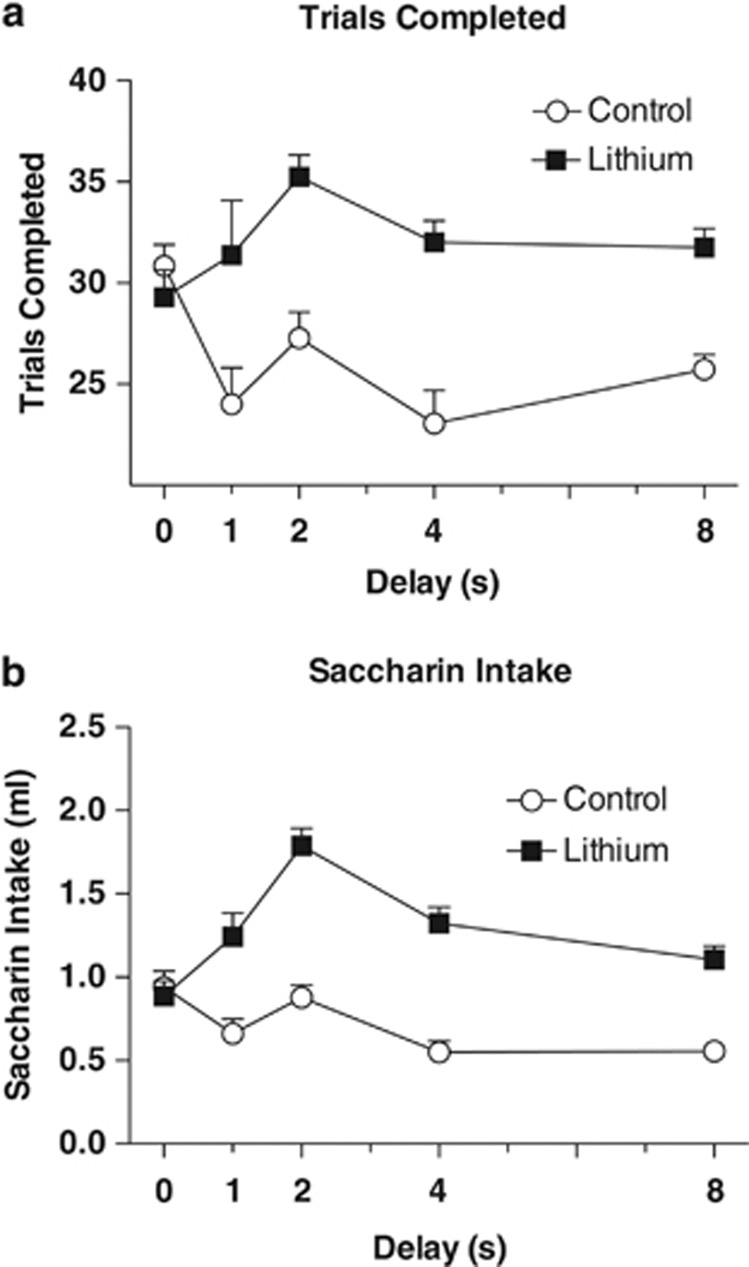
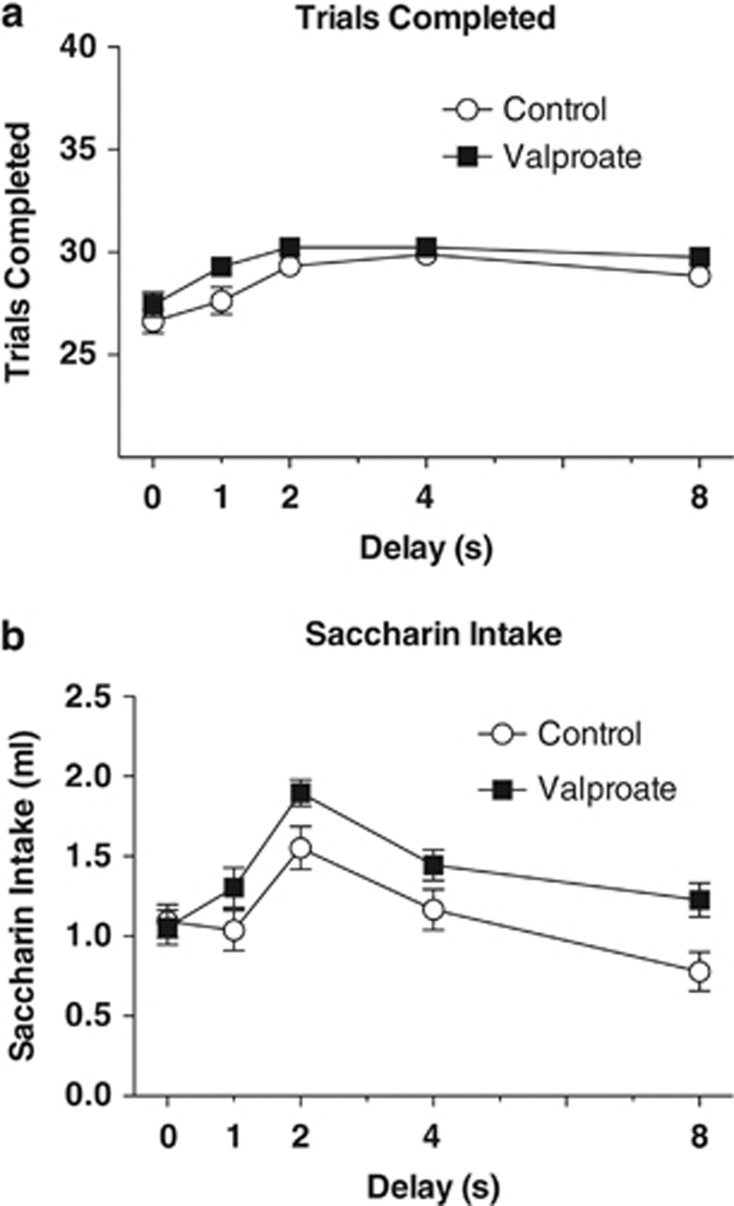
Indices of motivation were increased by lithium, consistent with its known polydipsic effect. A repeated measures ANOVA found a main effect of group on number of trials completed in a session, F(1, 37)=11.97, P<0.001. Lithium also increased fluid intake during the sessions, as indicated by a main effect of group on the volume of reward consumed during each session, F(1, 37)=14.59, P<0.001. Increasing delays were associated with decreasing trials completed and intakes, Fs (4, 148)⩾3.8, Ps⩽0.001, Figure 3.
Figure 3.
(a) Group mean number of trials completed per session±SEM as a function of delay (0, 1, 2, 4, and 8 s). Mice in the lithium group completed a greater number of trials per session than the control group, P<0.001. (b) Volume of saccharin solution consumed per session as a function of delay (0, 1, 2, 4 and 8 s) was also higher in the lithium group, P<0.001.
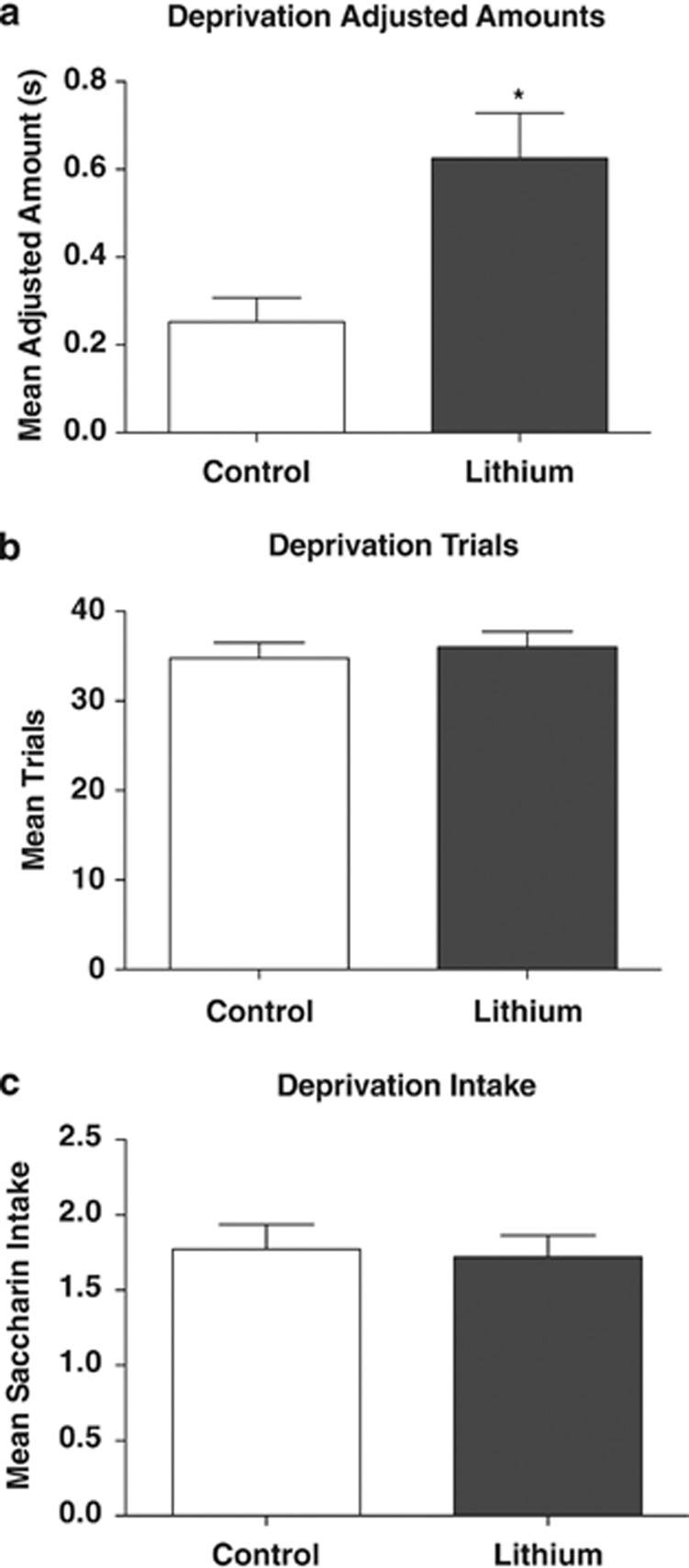
In order to address whether higher adjusted amounts in the lithium group resulted from increased motivation due to thirst, the experiment was extended by 5 days at the 8 s delay. During this time, all mice were restricted to 2 h of water access per day to increase motivation and fluid intake in both groups to an equivalent level. Results from this phase (Figure 4) indicated that there was still a main effect of group on adjusted amount, t(37)=2.40, P=0.02. Importantly, during this period, there was no significant difference in either number of trials completed or volume of reward consumed during each session, ts(37)⩽0.62, Ps⩾0.54. These findings indicate that lithium continued to reduce impulsivity when motivation was equivalently high in both lithium and chow groups. We additionally used a paired t-test to compare adjusted amounts prior to and after water deprivation. Neither the control nor the lithium group was affected by water deprivation, Ps>0.55, consistent with the idea that motivational differences did not drive drug-induced differences in adjusted amounts.
Figure 4.
Mean (±SEM) adjusted amounts (a), trials (b), and intake (c) during the 8 s delay, when both lithium and control mice were water deprived. Adjusted amounts continued to be significantly higher with lithium treatment, (*P<0.005), whereas trials completed and intake did not, demonstrating that the anti-impulsive effects of lithium were not secondary to its effects on thirst motivation during this task.
The analyses for the stability of responding across delays found no main effect of day or group by day interactions (Ps>0.05). This demonstrates that mice showed relatively stable behavior within the days at each delay.
Mean brain lithium levels were 0.74±0.082 mmol/kg wet weight. This is within the recommended human therapeutic range of 0.6–1.3 mM. We and others have reported previously that brain lithium levels in rodents following chronic treatment are similar to serum levels (Ghoshdastidar et al, 1989; Gould et al 2007; Gould et al 2008; Can et al, 2011). There were no significant weight differences between the lithium and control chow-treated groups throughout the duration of the study, F(1, 38), P=0.967.
Valproate
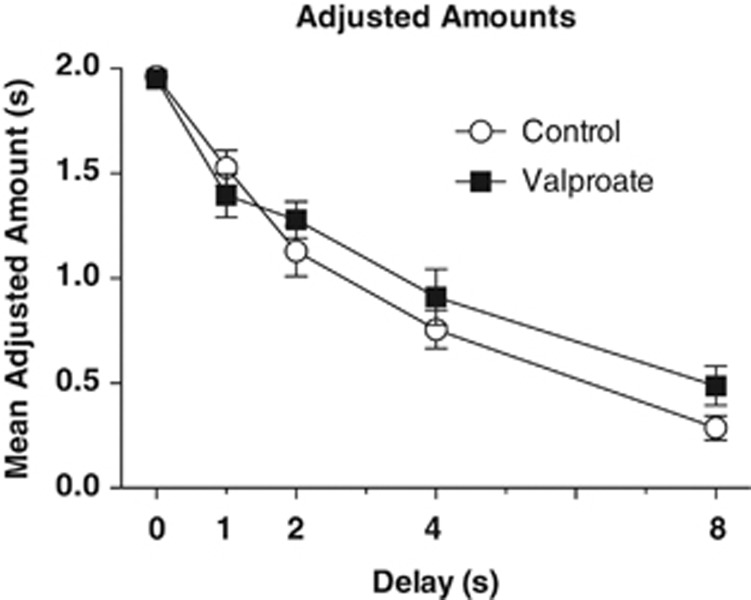
In the valproate study, 11 mice were removed for failure to meet criteria prior to drug treatment, and one mouse in the valproate group died from unknown causes during the study. There was no significant difference in adjusted amounts prior to drug exposure; the valproate group averaged 1.95±0.02 and the control group averaged 1.96±0.01. The repeated measures ANOVA found no effect of group on adjusted amounts, F(1, 34)<1, P=0.393 (Figure 5). Like lithium, though to a lesser degree, valproate significantly increased the number of trials completed during each session, F(1, 34)=5.92 P=0.02, as well as the amount of reward consumed, F(1, 34)=4.32, P=0.045 (Figures 6a). There was also a main effect of delay on adjusted amount, F(4, 136)=125.56, P<0.001, on number of trials completed, F(4, 136)=14.73, P<0.001 and on volume of reward consumed, F(4, 136)=22.26, P<0.001. The k-values derived for these groups were analyzed with an independent sample t-test and, consistent with the adjusted amounts, showed no difference, t(34)=<1.0, P=0.37. The mean k-values for the valproate group and control groups were 0.425±0.074 and 0.486±0.06, respectively.
Figure 5.
Group mean adjusted amounts±SEM as a function of delay (0, 1, 2, 4 and 8 s). Adjusted amounts did not differ between valproate (n=17) and control treatments (n=19), P=0.358.
Figure 6.
(a) Group mean number of trials completed per session±SEM as a function of delay (0, 1, 2, 4 and 8 s). Valproate mice completed modestly more trials per session than the control mice, P=0.02. (b) Valproate mice consumed more saccharin solution than control mice across all delays, P=0.008.
Blood serum was collected from 16 mice from the valproate group and tested for valproate levels. The mean blood serum level was 77.88±11.47 mg/l, which is within the human therapeutic range of 50–100 mg/l. There were significant differences in weight between the valproate and control groups over the course of the study, F(1, 36)=106.0, P<0.001, with mice in the valproate group averaging 27.4±0.01 g and the control group averaging 28.6±0.14 g at completion of the study.
DISCUSSION
To our knowledge, this is the first study to assess the effects of lithium on cognitive impulsivity in rodents. The DD task is representative of a cognitive choice that is affected in many psychopathologies, including bipolar disorder, suicidal behavior, and alcoholism. The main finding from this study is that lithium treatment reduced cognitive impulsivity, whereas another mood stabilizer, valproate, did not. A limitation of our study is that we only used a single dose of lithium or valproate. However, levels of both valproate and lithium were within the human therapeutic range, which is the outcome that guided our choice of drug levels. Similarly, we only addressed a single time point for the effects of lithium, and did not test whether the actions of lithium persisted following withdrawal. Subjects in the lithium group completed a greater numbers of trials per session and consumed more of the saccharin reinforcer, suggesting a possible difference in motivation. We addressed this by water depriving both groups. Water deprivation had no effect on discounting, which is consistent with a prior report (Richards et al, 1997), and lithium continued to exert its effect on mean adjusted amounts during this period, indicating that changes in subjective reward evaluation were not secondary to motivational differences between lithium and control groups. In addition, although valproate treatment also increased the number of trials and volume of reward consumed (albeit more modestly than did lithium treatment), it did not decrease impulsivity. These findings indicate that motivational differences are not primarily responsible for the lower levels of impulsive behavior in lithium-treated mice.
Overall, these findings support our a priori hypothesis that lithium, but not valproate treatment, would decrease impulsivity in relatively impulsive, HAP mice. Impulsivity is a state independent aspect of bipolar disorder, present in manic, depressive and euthymic states, as well as independently associated with suicidal behavior (Kovacsics et al, 2009; Lombardo et al, 2012; Swann et al, 2001; Swann et al, 2003). The ability of the DD task to illustrate changes in cognitive impulsivity due to pharmacological interventions makes it an ideal paradigm in which to test the efficacy of possible new treatments, for such disorders where impulsivity plays a role. Previous studies have shown that treatment with lithium decreases both suicide and suicide attempts in patients with bipolar disorder and major depressive disorder (Baldessarini et al, 2006; Guzzetta et al, 2007). In addition, other effective antidepressants and mood stabilizers may not have similar antisuicidal efficacy (Collins and McFarland, 2008; Goodwin et al, 2003). For example, Goodwin et al (2003) found that the risk of suicide was significantly (2.7 times) higher during the treatment with valproic acid (valproate) than with lithium. Collins and McFarland (2008) reported that suicide attempts were more common in bipolar patients taking valproate than in those taking lithium. In a meta-analysis, Cipriani et al (2005) reported that lithium is more effective in randomized controlled trials in reducing the risk of suicide, deliberate self-harm, and overall mortality than placebo, anticonvulsants, or antidepressants in subjects with mood disorders. However, there are equivocal findings (Oquendo et al, 2011). Both lithium and valproate have been shown to decrease impulsivity in clinical populations (Swann et al, 2002). The mechanism underlying these actions have not been established, but have been speculated to involve decreases in limbic hyper excitability in the case of valproate, and enhancing cortical inhibition in the case of lithium (Terao, 2008). How these putative mechanisms relate to drug action vis-à-vis clinical syndromes remains to be fully elicited.
Impulsivity is a multi-faceted construct, difficult to encapsulate with a single definition or behavioral task (Evenden, 1999). Ohmura et al (2012) recently reported data demonstrating that acute lithium administration, but not treatment with valproate or carbamazepine, decreased impulsivity as measured in a 3-CSRTT. Although this task is reliable for measuring motor impulsivity (Winstanley et al, 2006), it may not be adequate for encompassing all aspects of impulsivity. The DD paradigm is designed to evaluate behavioral choice involving cognitive mechanisms. This argues that converging effects of lithium and valproate in multiple domains of impulsivity may be important to how these pharmacotherapies influence this important endophenotype, as it is unclear which method for measuring impulsivity is more closely associated with suicidal behaviors. The present data add to those of Ohmura et al (2012) in confirming the anti-impulsive actions of lithium, but not valproate.
Our study did not examine molecular targets of lithium that may be responsible for the anti-impulsive effect. Lithium has well-documented effects to increase activity of the 5-HT system (Kovacsics et al, 2008; Shaw and Ratcliffe, 1976; Treiser et al, 1981). The 5-HT system has been implicated in both motor and cognitive impulsivity (Mobini et al, 2000; Winstanley et al, 2004; Wogar et al, 1993). In terms of molecular targets, lithium also has many known effects on intracellular signaling pathways (Gould et al, 2002; Gould et al, 2004b). Of particular importance may be the effects of lithium on the enzyme glycogen synthase kinase-3 (GSK-3) (Gould, 2006; Jope, 2003; Klein and Melton, 1996). Data support a role of GSK-3 in mediating many of the behavioral effects of lithium in rodents related to its antidepressant and antimanic efficacy, as well as modulating serotonin- and dopamine-mediated signaling (Beaulieu et al, 2004, 2008; Li et al, 2004; O'Brien et al, 2004; Gould et al, 2004b). It will be interesting for future studies to assess effects of known direct targets of lithium, such as GSK-3 inhibition, on impulsivity.
Overall, the selectively bred HAP mice used in this study may be useful for modeling aspects of bipolar disorder in addition to identification of novel pharmacotherapies. Approximately 45% of all bipolar patients have a comorbid alcohol addiction and display high levels of impulsivity (McElroy et al, 2001; Swann et al, 2003). HAP mice consistently consume excessive amounts of ethanol, reaching average blood ethanol levels of over 200 mg/dl on a daily basis (Matson and Grahame, 2011) and demonstrate higher levels of impulsivity than selectively bred Low-Alcohol-Preferring (LAP) mice and their outbred progenitor line (Oberlin and Grahame, 2009). In these ways, they parallel the patients with bipolar disorder more closely than LAP mice, which are less impulsive and do not drink alcohol. In addition, we had also previously reported that HAP mice manifest affect-related behavioral changes when compared with LAP mice (Can et al, 2012). In the present study, lithium decreased impulsivity in HAP mice, which suggests that use of this mouse line may be an effective way to test novel bipolar disorder treatments, especially those aimed at reducing comorbid substance abuse. We did not assess effects of either lithium or valproate on the less-impulsive LAP mice. Our rational for choosing HAP mice was due to their high trait impulsivity, and that anti-impulsive effects of d-amphetamine had previously been validated in this line, thus proving its predictive value as a model in which to test anti-impulsive drugs (Oberlin et al., 2010). As LAP mice manifest relatively low levels of impulsivity, there is the possibility of a floor effects in this line, and therefore limited sensitivity to the anti-impulsive effects of drugs. In addition, studying the effects of lithium and valproate on a less-impulsive line of mice would be less appealing from an external validity standpoint because these drugs are typically used in patients with bipolar disorder and the concomitant high trait impulsivity observed in such patients.
In conclusion, the results of this study demonstrate that chronic treatment with lithium reduces cognitive impulsivity in HAP mice. This same effect was not seen with chronic valproate treatment. These findings support the hypothesis that lithium may exert therapeutic action by decreasing impulsivity.
Acknowledgments
This study was supported by the grant MH091816 to TDG. We thank Adem Can for conducting the analysis of brain lithium levels and Amy Buckingham and Nick Villalta for their assistance in technical aspects of conducting these experiments.
The authors declare no conflict of interest.
References
- Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8 (Pt 2:625–639. doi: 10.1111/j.1399-5618.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu J-M, Sotnikova TD, Yao W-D, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Blackwell RA, Piantadosi SC, Dao DT, O'Donnell KC, Gould TD. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes Brain Behav. 2011;10:434–443. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Grahame NJ, Gould TD. Affect-related behaviors in mice selectively bred for high and low voluntary alcohol consumption. Behav Genet. 2012;42:313–322. doi: 10.1007/s10519-011-9505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162:1805–1819. doi: 10.1176/appi.ajp.162.10.1805. [DOI] [PubMed] [Google Scholar]
- Collins JC, McFarland BH. Divalproex, lithium and suicide among Medicaid patients with bipolar disorder. J Affect Dis. 2008;107:23–28. doi: 10.1016/j.jad.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Courtet P, Gottesman II, Jollant F, Gould TD. The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies. Transl Psychiatry. 2011;1:1–7. doi: 10.1038/tp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrego MF, Canevaro L, Kuzis G, Sabe L, Starkstein SE. A randomized, double-blind, crossover study of methylphenidate and lithium in adults with attention-deficit/hyperactivity disorder: preliminary findings. J Neuropsychiatry Clin Neurosci. 2002;14:289–295. doi: 10.1176/jnp.14.3.289. [DOI] [PubMed] [Google Scholar]
- Etain B, Mathieu F, Liquet S, Raust A, Cochet B, Richard JR, et al. Clinical features associated with trait-impulsiveness in euthymic bipolar disorder patients. J Affect Dis. 2012;144:240–247. doi: 10.1016/j.jad.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;161:217–222. doi: 10.1176/appi.ajp.161.2.217. [DOI] [PubMed] [Google Scholar]
- Ghoshdastidar D, Dutta RN, Poddar MK. In vivo distribution of lithium in plasma and brain. Indian J Exp Biol. 1989;27:950–954. [PubMed] [Google Scholar]
- Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290:1467–1473. doi: 10.1001/jama.290.11.1467. [DOI] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. Mood stabilizer psychopharmacology. Clin Neurosci Res. 2002;2:193–212. doi: 10.1016/S1566-2772(02)00044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004a;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004b;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Gould TD. Targeting glycogen synthase kinase-3 as an approach to develop novel mood-stabilising medications. Expert Opin Ther Targets. 2006;10:377–392. doi: 10.1517/14728222.10.3.377. [DOI] [PubMed] [Google Scholar]
- Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Snyderman M. Choice between rewards differing in amount and delay: toward a choice model of self control. J Exp Anal Behav. 1980;34:135–147. doi: 10.1901/jeab.1980.34-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Ramsay SR, Galfalvy HC, Ellis SP, Burke AK, Sher L, et al. Correlates of suicide attempt history in bipolar disorder: a stress-diathesis perspective. Bipolar Disord. 2006;8 (Pt 2:551–557. doi: 10.1111/j.1399-5618.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- Guzzetta F, Tondo L, Centorrino F, Baldessarini RJ. Lithium treatment reduces suicide risk in recurrent major depressive disorder. J Clin Psychiatry. 2007;68:380–383. doi: 10.4088/jcp.v68n0304. [DOI] [PubMed] [Google Scholar]
- Hamburger-Bar R, Robert M, Newman M, Belmaker RH. Interstrain correlation between behavioural effects of lithium and effects on cortical cyclic AMP. Pharmacol Biochem and Behav. 1986;24:9–13. doi: 10.1016/0091-3057(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Pallanti S, Allen A, Sood E, Baldini Rossi N. Does sustained-release lithium reduce impulsive gambling and affective instability versus placebo in pathological gamblers with bipolar spectrum disorders. Am J Psychiatry. 2005;162:137–145. doi: 10.1176/appi.ajp.162.1.137. [DOI] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsics CE, Goyal HK, Thomas KJ, Gould TD. The antisuicidal efficacy of lithium: a review of the clinical literature and underlying pharmacology. Int J Child Health Hum Dev. 2008;1:225–244. [Google Scholar]
- Kovacsics CE, Gottesman II, Gould TD. Lithium's antisuicidal efficacy: elucidation of neurobiological targets using endophenotype strategies. Annu Rev Pharmacol Toxicol. 2009;49:175–198. doi: 10.1146/annurev.pharmtox.011008.145557. [DOI] [PubMed] [Google Scholar]
- Kovacsics CE, Gottesman II, Gould TD. Shock-induced aggression in mice is modified by lithium. Pharmacol Biochem Behav. 2010;94:380–386. doi: 10.1016/j.pbb.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo LE, Bearden CE, Barrett J, Brumbaugh MS, Pittman B, Frangou S, et al. Trait impulsivity as an endophenotype for bipolar I disorder. Bipolar Disord. 2012;14:565–570. doi: 10.1111/j.1399-5618.2012.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P, et al. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. 2009;65:556–563. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biology. 2011. [DOI] [PMC free article] [PubMed]
- McElroy SL, Altshuler LL, Suppes T, Keck JrPE, Frye MA, Denicoff KD, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158:420–426. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- Melhem NM, Brent DA, Ziegler M, Iyengar S, Kolko D, Oquendo M, et al. Familial pathways to early-onset suicidal behavior: familial and individual antecedents of suicidal behavior. Am J Psychiatry. 2007;164:1364–1370. doi: 10.1176/appi.ajp.2007.06091522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Najt P, Perez J, Sanches M, Peluso MA, Glahn D, Soares JC. Impulsivity and bipolar disorder. Eur Neuropsychopharmacol. 2007;17:313–320. doi: 10.1016/j.euroneuro.2006.10.002. [DOI] [PubMed] [Google Scholar]
- O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharmacological dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcohol Clin Exp Res. 2010;34:1363–1375. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Tsutsui-Kimura I, Kumamoto H, Minami M, Izumi T, Yamaguchi T, et al. Lithium, but not valproic acid or carbamazepine, suppresses impulsive-like action in rats. Psychopharmacology. 2012;219:421–432. doi: 10.1007/s00213-011-2496-9. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, et al. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry. 2004;161:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy HC, Currier D, Grunebaum MF, Sher L, Sullivan GM, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168:1050–1056. doi: 10.1176/appi.ajp.2011.11010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MA, Hatch JP, Glahn DC, Monkul ES, Sanches M, Najt P, et al. Trait impulsivity in patients with mood disorders. J Affect Disord. 2007;100:227–231. doi: 10.1016/j.jad.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JP, Ratcliffe F. Effect of lithium on brain 5-hydroxytryptamine metabolism in mice. Arch Int Pharmacodyn Ther. 1976;222:116–124. [PubMed] [Google Scholar]
- Swann AC, Anderson JC, Dougherty DM, Moeller FG. Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Res. 2001;01:195–197. doi: 10.1016/s0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bowden CL, Calabrese JR, Dilsaver SC, Morris DD. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26:530–536. doi: 10.1016/S0893-133X(01)00390-6. [DOI] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Terao T.2008Aggression, suicide, and lithium treatment Am J Psychiatry 1651356–1357,.author reply 1357. [DOI] [PubMed] [Google Scholar]
- Tondo L, Jamison KR, Baldessarini RJ. Effect of lithium maintenance on suicidal behavior in major mood disorders. Ann NY Acad Sci. 1997;836:339–351. doi: 10.1111/j.1749-6632.1997.tb52369.x. [DOI] [PubMed] [Google Scholar]
- Treiser SL, Cascio CS, O'Donohue TL, Thoa NB, Jacobowitz DM, Kellar KJ. Lithium increases serotonin release and decreases serotonin receptors in the hippocampus. Science. 1981;213:1529–1531. doi: 10.1126/science.6269180. [DOI] [PubMed] [Google Scholar]
- Turecki G. Dissecting the suicide phenotype: the role of impulsive-aggressive behaviours. J Psychiatry Neurosci. 2005;30:398–408. [PMC free article] [PubMed] [Google Scholar]
- Weisler RH, Nolen WA, Neijber A, Hellqvist A, Paulsson B. Continuation of quetiapine versus switching to placebo or lithium for maintenance treatment of bipolar I disorder (Trial 144: a randomized controlled study) J Clin Psychiatry. 2011;72:1452–1464. doi: 10.4088/JCP.11m06878. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology. 1993;111:239–243. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]