Abstract
Increased serum levels of IL-15 are reported in type 1 diabetes (T1D). Here we report elevated serum soluble IL-15Rα levels in human T1D. To investigate the role of IL-15/IL-15Rα in the pathogenesis of T1D, we generated double transgenic mice with pancreatic β-cell expression of IL-15 and IL-15Rα. The mice developed hyperglycemia, marked mononuclear cell infiltration, β-cell destruction, and anti-insulin autoantibodies that mimic early human T1D. The diabetes in this model was reversed by inhibiting IL-15 signaling with anti-IL2/IL15Rβ (anti-CD122), which blocks IL-15 transpresentation. Furthermore, the diabetes could be reversed by administration of the Janus kinase 2/3 inhibitor tofacitinib, which blocks IL-15 signaling. In an alternative diabetes model, nonobese diabetic mice, IL15/IL-15Rα expression was increased in islet cells in the prediabetic stage, and inhibition of IL-15 signaling with anti-CD122 at the prediabetic stage delayed diabetes development. In support of the view that these observations reflect the conditions in humans, we demonstrated pancreatic islet expression of both IL-15 and IL-15Rα in human T1D. Taken together our data suggest that disordered IL-15 and IL-15Rα may be involved in T1D pathogenesis and the IL-15/IL15Rα system and its signaling pathway may be rational therapeutic targets for early T1D.
Type 1 diabetes (T1D) is an autoimmune disease in which insulin-producing β cells in pancreatic islets are destroyed by autoreactive T cells. During prolonged lack of insulin, blood glucose increases (hyperglycemia) and tissue damage occurs. Studies in animal models and humans demonstrated that β-cell destruction is usually accompanied by inflammation of pancreatic islets (insulitis), which suggests that activation of inflammatory T cells is important in the development of diabetes (1, 2). What triggers the T-cell infiltrate into the islets and subsequent β-cell destruction? What signaling pathways are important for this process? An understanding of the molecular events and signaling pathways that lead to T-cell activation and subsequent β-cell destruction would be useful in the development of new therapeutics for autoimmune T1D.
Interleukin-15 (IL-15) is a proinflammatory cytokine that promotes the activation and maintenance of natural killer (NK) and CD8 (+) T-effector memory (T-EM) cells (3, 4). IL-15R alpha (IL-15Rα), the high affinity private receptor for IL-15, stabilizes and chaperons IL-15 on dendritic cell membrane and activates neighboring NK and T cells via transpresentation (5–8). Therefore, IL-15 is not secreted; rather, it is a membrane-associated molecule that acts as part of an immunological synapse (5, 6, 8). During an immune response such as viral infection, IL-15 and its private receptor IL-15Rα are coordinately induced (5, 8, 9). As related to T1D, it has been shown that exposure of human pancreatic islets to coxsackie virus, an enterovirus linked to T1D, or directly to IFNγ induced high gene expression of IL-15 and IL-15Rα in the islets in vitro (10).
Abnormal expression of IL-15 has been reported in many autoimmune disorders including rheumatoid arthritis, celiac disease, psoriasis, inflammatory bowel disease, and multiple sclerosis (11). In patients with T1D, elevated serum levels of IL-15 have been reported (12). Using a unique assay we developed for soluble IL-15Rα (sIL-15Rα) (13), we discovered elevated serum levels of sIL-15Rα in T1D.
To investigate whether islet overexpression of IL-15 and IL-15Rα could play a role in the pathogenesis of T1D, we generated double transgenic mice with β-cell–specific expression of both IL-15 and IL-15Rα under a rat insulin promoter (RIP). The mice developed hyperglycemia, marked mononuclear cell infiltration, β-cell destruction, and anti-insulin autoantibodies that mimic the early events of human T1D. Inhibiting IL-15/IL-15Rα signaling either by blocking IL-15 transpresentation using TMβ1, a monoclonal antibody that is directed to IL-2/IL-15Rβ (CD122) or by blocking IL-15 signaling by administration of the Janus kinase 2/3 (Jak2/3) inhibitor tofacitinib reversed the diabetes in the double transgenic mice. Moreover, in another diabetes mouse model, nonobese diabetic (NOD) mice, increased islet cell expression of IL-15 and IL-15Rα were found at the prediabetic stage and the inhibition of IL-15 signaling delayed the diabetes development. Considering viral infection and interferons are often found in the pancreatic islets of patients with T1D (14–16), and they are potent inducers of IL-15/IL15Rα (9, 17–19), we investigated whether IL-15 and IL-15Rα were expressed in the islets of patients with T1D. Our data demonstrated increased expression of both IL-15 and IL-15Rα in the islets of patients with T1D. Taken together, our data suggest that the disordered expression of IL-15/IL-15Rα in islets may play a role in the pathogenesis of T1D and that the IL-15/IL15Rα system and its signaling pathway may be rational therapeutic targets for early T1D.
Results
Generation of IL-15/IL-15Rα Double Transgenic Mice.
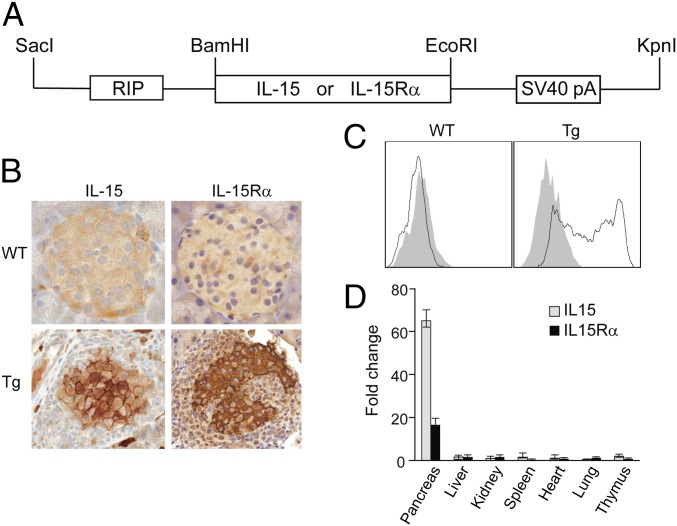
Viral infections and interferons are potent inducers of IL-15 and IL-15Rα (9, 17–19). Both type I (alpha) (17) and type II (gamma) (18) IFN were shown to be able to induce the expression of IL-15/IL-15Rα. In T1D, several reports demonstrated the presence of either enterovirus (14) or viral protein (20) and IFN (15, 16) in the islets. To investigate whether pancreatic islet expression of IL-15 and IL-15Rα could play a role in the pathogenesis of T1D, we generated transgenic mice expressing IL-15 alone, IL-15Rα alone, and both IL-15 and IL-15Rα under the control of a rat insulin promoter (RIP), which restricts IL-15 and/or IL-15Rα expression to the β cells (21) (Fig. 1A). Fourteen different lines were obtained for each transgene and their combination. Four IL-15/IL-15Rα double transgenic lines (J4, J8, BB1, and X5) were maintained either by in vitro fertilization (IVF) or by natural mating. Southern blot analysis confirmed that all transgenic mice carried the transgene/s. IL-15 and IL-15Rα expressions in the islets of double transgenic mice were confirmed by immunohistochemistry (IHC) (Fig. 1B), flow cytometry (Fig. 1C), and real-time RT-PCR (Fig. 1D).
Fig. 1.
Generation of IL-15 and IL-15Rα double transgenic mice. (A) Schematic representation of the IL-15 and IL-15Rα transgene. (B) Immunohistochemistry analysis of IL-15 and IL-15Rα expression in the pancreas of IL-15/IL-15Rα double transgenic mice. (C) Surface expression of IL-15Rα in purified islet cells. (D) Real-time RT-PCR analysis of IL-15 and IL-15Rα mRNA levels. The fold change was calculated compared with IL-15 and IL-15Rα mRNA levels in wild-type C57BL/6 mouse. Open bar, IL-15; solid bar, IL-15Rα.
IL-15/IL-15Rα Double Transgenic Mice Developed Insulin-Dependent Diabetes at a Very Young Age.
Eight IL-15/IL-15Rα double transgenic mouse lines (I1, J4, BB1, E7, G10, J8, X5, and E1) developed hyperglycemia starting from 4 wk of age (both sexes) (Fig. 2A). The blood glucose levels in these mice continued to increase with time, suggesting continued loss of β cells (Fig. 2A). The diabetes onset age among offspring of the same line varied. Approximately 60% of transgene-positive J4 offspring mice developed hyperglycemia within 3 mo of age (Fig. 2B). None of the IL-15 single transgenic mice developed hyperglycemia when euthanized at 12 mo of age. Only one IL-15Rα single transgenic mouse developed hyperglycemia. With this one exception, all of the single transgenic mice (both IL-15 and IL15Rα single transgenic) did not have any abnormal phenotype. This is probably due to the requirement of IL-15Rα to stabilize and chaperon IL-15 on cell membrane and consequently, the necessity of both IL-15 and IL-15Rα expression in the same cell for transpresentation as demonstrated by Mortier et al. (7) and Sandau et al. (8). As we will demonstrate later, IL-15 transpresentation was very important for the induction of diabetes in this model. None of the transgene-negative littermates developed hyperglycemia.
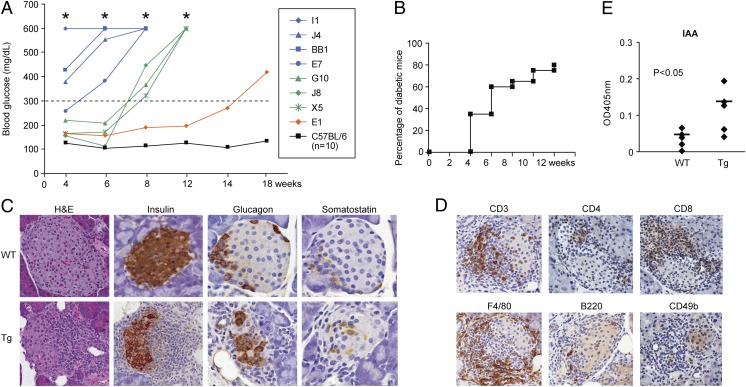
Fig. 2.
IL-15/IL-15Rα double transgenic mice developed insulin-dependent diabetes at a young age. (A) Blood glucose levels in the IL-15/IL-15Rα double transgenic mouse lines (I1, J4, BB1, E7, G10, J8, X5, and E1) that developed diabetes from week 4 to week 14 compared with 10 controls indicated by black line. *Blood glucose levels >600 mg/dL. (B) The percentage of diabetic mice among the offspring of transgene-positive J4 line (10 female, 10 male) 3 mo after birth. (C) Histopathology analysis of pancreas from the IL-15/IL-15Rα double transgenic mice. (D) Infiltrating mononuclear cells in islets of IL-15/IL-15Rα double transgenic mice were visualized by anti-CD3, CD4, CD8a, F4/80, B220, and CD49b antibodies. (E) Increased serum levels of insulin autoantibody (IAA) in the double transgenic mice (n = 5, P < 0.05)
All IL-15/IL-15Rα double transgenic mice with hyperglycemia demonstrated marked mononuclear cell infiltration in the islets and atrophy of islets (Fig. 2C). When stained with an anti-insulin antibody, the loss of insulin-producing β-islet cells was evident in the double transgenic mice, whereas glucagon-producing α cells and somatosatin-producing δ cells were intact at the early stage of the disease (Fig. 2C). The majority of the infiltrating mononuclear cells were T lymphocytes (CD3 positive) and macrophages (F4/80 positive) (Fig. 2D). Both CD4 and CD8 T cells were infiltrated into the islets (Fig. 2D). Few B cells (B220 positive) and much fewer NK cells (CD49b positive) infiltrated into the islets (Fig. 2D). The double transgenic mice had increased serum levels of insulin autoantibody (IAA) (Fig. 2E) and IgA/IgG at the onset of diabetes (Fig. S1). Moreover, increased expression of proinflammatory cytokine genes (TNFα, TNFβ, IFNγ, IL18RAP, etc.) and chemokine genes (CCL3, CXCL9, and CXCL10, etc.) were found in the purified islet cells from the double transgenic mice before diabetes onset (Fig. S2, genes with fold induction ≥3). Increased cell surface expression of MHC class I, class II, and ICAM-1 were also found on the purified islet cells from the double transgenic mice before diabetes onset (Fig. S3).
Inhibiting IL-15 Signaling at the Onset of Diabetes Reversed the Diabetes in IL-15/IL-15Rα Double Transgenic Mice.
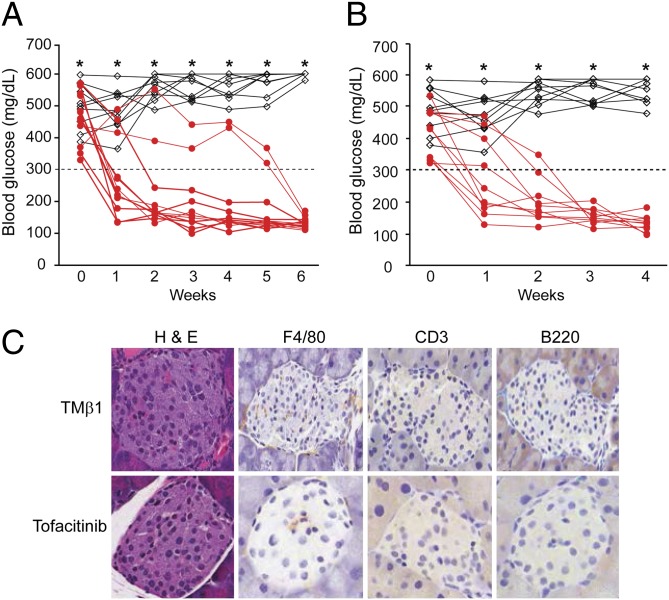
To investigate whether the diabetes in IL-15/IL-15Rα double transgenic mice was IL-15/IL-15Rα dependent, we inhibited IL-15–mediated signaling in the IL-15/IL-15Rα double transgenic mice with new onset of diabetes. Inhibiting IL-15 signaling by blocking IL-15 transpresentation using a monoclonal antibody, TMβ1, which is directed to IL-2/IL-15Rβ (CD122), reversed the hyperglycemia in all 12 mice examined (Fig. 3A). Furthermore, a 4-wk continuous infusion of the Jak2/3 inhibitor tofacitinib (CP-690,550), which blocks the IL-15 signaling pathway, also reversed the hyperglycemia in all 10 mice examined (Fig. 3B). Mice treated with TMβ1 had minimal mononuclear cell infiltration in the pancreatic islets (Fig. 3C). The majority of the mice treated with tofacitinib had no mononuclear cell infiltration in the pancreas (Fig. 3C). These data suggested that blockade of IL-15 signaling in the double transgenic mice not only reversed the hyperglycemia but also eliminated mononuclear cell infiltration. Therefore, the diabetes observed in the IL-15/IL-15Rα double transgenic mice was IL-15/IL-15Rα dependent.
Fig. 3.
Inhibiting IL-15 signaling reversed the diabetes in IL-15/IL-15Rα double transgenic mice. (A) The blood glucose levels in the double transgenic mice with new onset of diabetes untreated (black, n = 10) or treated with monoclonal anti–IL-2/IL-15Rβ (anti-CD122) antibody TMβ1 for 6 wk (red, n = 12). (B) The blood glucose levels in the IL-15/IL-15Rα transgenic mice with new onset of diabetes untreated (black, n = 10) or treated with the Jak2/3 inhibitor tofacinitib for 4 wk (red, n = 10). (C) Immunohistochemistry staining of F4/80 (macrophage), CD3 (T cells), and B220 (B cells) on the pancreas of mice treated with TMβ1 and the Jak2/3 inhibitor tofacitinb. The data represent five individual mice.
Depletion of CD4 Cells at the Onset of Diabetes Reversed Hyperglycemia in IL-15/IL15Rα Double Transgenic Mice.
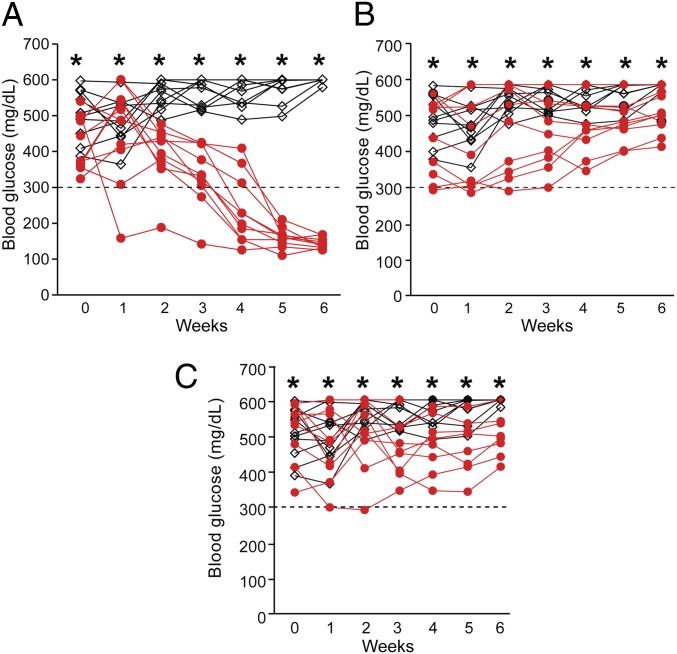
Autoreactive T cells can play a major role in β-cell destruction (22, 23). However, recent studies suggested other cell types such as NK or B cells may also be involved in β-cell autoimmunity (24, 25). IL-15 activates both T and NK cells (26). To investigate what cells are required for the autoimmunity in the double transgenic mice, we depleted CD4, CD8, and NK cells from the double transgenic mice with new onset of diabetes. Depletion of CD4 T cells with a monoclonal anti–CD4-depleting antibody (clone GK1.5) slowly reversed the hyperglycemia in the double transgenic mice in all 10 mice examined (Fig. 4A). Histological examination of the mice with CD4 cell depletion showed persistent infiltration of CD3 cells, B cells, and macrophages (F4/80) (Fig. S4). However, CD8 cell depletion and NK cell depletion had no effect on the hyperglycemia in the double transgenic mice (n = 10, Fig. 4 B and C). The depletion of CD4, CD8, and NK cells in the peripheral blood was confirmed by flow cytometry. These data suggested that CD4 cells were required for the development of the disease after diabetes onset in this model. Interestingly, CD4 cells (or peripheral blood mononuclear cells) from the double transgenic mice could respond to wild-type islets in vitro and wild-type islets were rejected when transplanted into the diabetic double transgenic mice in vivo (Fig. S5). This suggested that an immune response toward wild-type islets that have no exogenously induced IL-15/IL-15Rα expression was generated in the double transgenic mice. However, transfer of CD4 or total T cells into congenic wild-type or Rag1-deficient mice did not induce diabetes in the recipient mice. This suggested that CD4 cells (or T cells) alone may not be sufficient to induce diabetes.
Fig. 4.
Depletion of CD4 cells reversed hyperglycemia in IL-15/IL-15Rα double transgenic mice. (A) The blood glucose levels in untreated (black, n = 10) or treated (with CD4 cell depletion antibody) mice (red, n = 10). (B) The blood glucose levels in untreated (black, n = 10) or treated (with CD8 cell depletion antibody) mice (red, n = 10). (C) The blood glucose levels in untreated (black, n = 10) or treated (with NK cell depletion antibody anti-asialo GM1) mice (red, n = 10).
Inhibiting IL-15 Signaling at the Prediabetic Stage Delayed the Diabetes Development in NOD Mice.
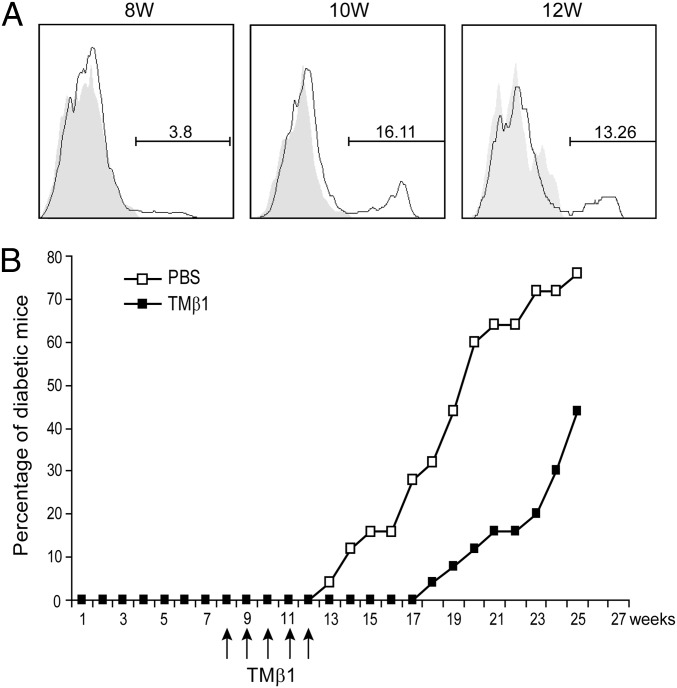
NOD mice are widely used for the study of T1D where multiple gene loci have been shown to participate in the diabetes development. In NOD mice, IL-15 expression was increased in purified islet cells at the prediabetic stage (27). We investigated whether IL-15Rα was also induced at this stage. Flow cytometry showed an increase of IL-15Rα expression in purified islet cells (CD45 negative population) at week 10 (16.11%) and week 12 (13.26%) compared with week 8 (3.8%) (Fig. 5A). We inhibited IL-15 signaling at the prediabetic stage (from week 8 to week 12) using the monoclonal antibody TMβ1, which blocks IL-15 transpresentation. TMβ1 treatment at the prediabetic stage delayed diabetes development in NOD mice (Fig. 5B, n = 25, P < 0.001). This suggested that IL-15 signaling was also involved in the diabetes development in NOD mice.
Fig. 5.
Inhibiting IL-15 signaling with TMβ1 delayed the diabetes development in NOD mice. (A) IL-15Rα expression on the purified islet cells from NOD mice. The histograms were gated on CD45− population. Gray, isotype control; solid, IL-15Rα. (B) The percentage of diabetic mice in NOD mice treated with PBS (n = 25) or monoclonal anti-CD122 antibody TMβ1 (n = 25) (P < 0.0001).
Increased Expression of IL-15 and IL-15Rα in the Islets of Patients with T1D.
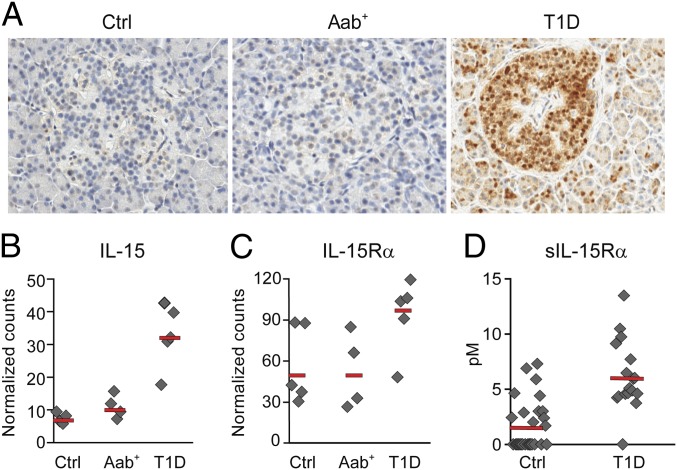
We next investigated whether IL-15 and IL-15Rα were expressed in the islets of patients with T1D. The pancreatic tissue samples were obtained from Network for Pancreatic Organ Donors (nPOD) (www.jdrfnpod.org). Immunohistochemistry demonstrated IL-15 expression in the islets in all T1D patients examined (four out of four, nPOD-6038, nPOD-6046, nPOD-6050, and nPOD-6084), whereas IL-15 was detected only in the pancreatic islets in one out of four normal donors and two out of five autoantibody positive donors (Fig. 6A). Interestingly, in two cases where IL-15 was positive, we also detected islet expression of MxA, a GTPase induced by type 1 IFN (α/β), that has been reported to interfere with virus multiplication and spread (28) (Fig. S6). Furthermore, we analyzed IL-15 and IL-15Rα mRNA levels in the islets from T1D patients, autoantibody positive, and normal donors. The islets were microdissected based on insulin positivity using laser capture microscopy (LCM) (29). Consistent with the immunohistochemistry results, we found higher IL-15 (Fig. 6B) and IL-15Rα (Fig. 6C) mRNA levels in T1D compared with normal or autoantibody positive donors (T1D vs. normal: P < 0.01 IL-15; P = 0.07 IL-15Rα. T1D vs. autoantibody positive donors: P < 0.01 IL-15; P = 0.06 IL-15Rα). Moreover, because IL-15 and IL-15Rα are usually coordinately induced and elevated serum levels of IL-15 have been found in T1D (14), we measured the serum levels of soluble IL-15Rα in T1D using a unique assay we developed (13). The serum sIL-15Rα levels were significantly higher in patients with T1D compared with those of normal controls (Fig. 6D, P < 0.001). The average sIL-15Rα level in patients with T1D was 6.18 pM compared with 1.7 pM in normal donors (Fig. 6D). Taken together, our data suggest that disordered IL-15 and IL-15Rα may be involved in the pathogenesis of T1D.
Fig. 6.
Increased expression of IL-15 and IL-15Rα in patients with T1D. (A) Immunohistochemistry staining of IL-15 in pancreatic tissues from T1D, autoantibody positive (Aab), and normal donors (Ctrl). (B) IL-15 mRNA levels in microdissected islets analyzed by Nanostring. The counts were normalized to three housekeeping genes. T1D vs. ctrl: P < 0.01. (C) IL-15Rα mRNA levels in microdissected islets analyzed by Nanostring. The counts were normalized to three housekeeping genes. T1D vs. ctrl: P = 0.07. (D) sIL-15Rα levels in the serum from 29 controls and 20 T1D patients (P < 0.001).
Discussion
In the present study, we demonstrated islet expression of both IL-15 and IL-15Rα and elevated serum levels of sIL-15Rα in patients with T1D. Furthermore, we demonstrated β-cell expression of IL-15– and IL-15Rα–induced insulin-dependent diabetes in mice with many characteristics similar to human T1D. Inhibiting IL-15 signaling by blocking IL-15 transpresentation with TMβ1 or by Jak2/3 inhibitor tofacitinib completely reversed the diabetes in the mice. Moreover, in NOD mice, IL-15 and IL-15Rα up-regulations were demonstrated in the islets at the prediabetic stage and the inhibition of IL-15 signaling at the prediabetic stage delayed diabetes development. Collectively, these data suggest islet expression of IL-15 and IL-15Rα may be involved in the pathogenesis of human T1D.
The pathogenesis of T1D remains elusive even after decades of study. Increasing evidence suggests that both genetic susceptibility and environmental influences are likely to contribute to the disease development. Although the precise etiology of T1D is still unknown, viruses have long been suggested as a potential environmental trigger for the disease. A recent meta-analysis by Yeung et al. showed a clinically significant association between enterovirus infection and T1D (30). Furthermore, rare variants of IFIH1, a gene implicated in antiviral responses, were shown to protect against T1D (31). Moreover, recent studies suggested a causative role for enteroviruses in T1D (30, 32, 33). Oikarinen et al. showed that enterovirus RNA positivity during the 6-mo period preceding the first autoantibody-positive sample was associated with increased risk for the development of islet autoimmunity (33). The study published by Diabetes and Autoimmunity Study in the Young (DAISY) consortium concluded that the rate of progression from islet autoimmunity (islet autoantibody positive) to T1D was significantly increased following detection of enterovirus RNA in the serum in genetically susceptible children (32). Together, these studies suggested enteroviruses may be involved in both the initiation and progression of T1D. In support of these association studies, clinically, coxsackie B4 virus, an entrovirus that belongs to Picornaviridae family, was found in the β cells in 3 out of 6 recent onset T1D patients (9). Furthermore, enteroviral capsid protein VP1 was found in islets from 44 out of 72 recent onset patients versus 3 out of 50 controls (20). As indirect evidence, viral signatures such as IFN, MHC hyperexpression, and CD8 infiltrations were found in T1D (15, 34–36). All of these studies suggested an important role for viral infection in T1D. Interestingly, viral infection induced the expression of interferon, which in turn acts on the upstream promoters of IL-15 and IL-15Rα and induces their expressions (17). Exposure of human pancreatic islets to coxsackie virus, an enterovirus linked to T1D or directly to IFNγ, induced the expression of both IL-15 and IL-15Rα in vitro (10). Based on these observations, we hypothesized that IL-15 and IL-15Rα may be expressed in the islets in patients with T1D. As predicted by this hypothesis, we detected islet expression of both IL-15 and IL-15Rα in 4 out of 4 patients with T1D (Fig. 6 A–C). Moreover, higher levels of serum IL-15 and IL-15Rα (Fig. 6D) were also demonstrated in T1D. Interestingly, in 2 cases where IL-15 was positive, we also detected islet expression of MxA (Fig. S6), a GTPase that is induced during viral infection exclusively and in a dose-dependent manner by IFNα and IFNβ, but not by IFNγ, IL-1, TNFα, or other cytokines. MxA has been used clinically as a marker for viral infection and has antiviral activity by interfering with virus multiplication and spread (28).
To investigate if islet expression of IL-15 and IL-15Rα could play a role in the pathogenesis of T1D, we generated double transgenic mice with beta cell expression of IL-15 and IL-15Rα. The mice developed insulin-dependent diabetes with many characteristics similar to the human disease (Fig. 2). Furthermore, we demonstrated that the diabetes in the mice was specifically IL-15/IL-15Rα dependent because inhibiting IL-15 signaling with TMβ1, which blocks IL-15 transpresentation or by addition of the Jak2/3 inhibitor tofacitinib, which blocks IL-15 signaling, reversed the diabetes in the mice (Fig. 3). IL-15 is a proinflammatory cytokine that has been described as being at the apex of a proinflammatory cytokine cascade (37). It has been suggested that IL-15 expression might precede expression of tumor necrosis factor and the downstream cytokines IL-1, IL-6, and granulocyte/macrophage colony-stimulating factor, as well as the proinflammatory chemokines CCL3 (MIP-1α), CCL4 (MIP-1β), and CXCL8 (IL-8). We tried to define the molecular mechanisms that underlie the IL-15/IL-15Rα–induced autoimmune diabetes in the double transgenic mice. We found increased gene expression of both proinflammatory cytokines and proinflammatory chemokines in the islets before the diabetes onset, which suggested ongoing inflammation long before the disease onset (Fig. S2). Moreover, there were hyperexpressions of both MHC class I, class II, and the adhesion molecule ICAM-1 on the islet cells, which could greatly enhance islet antigen presentation to the infiltrating T cells (Fig. S3). These events could eventually lead to autoimmune diabetes. Collectively, our data suggested a causative role of islet expression of IL-15 and IL-15Rα and T1D development in mice. Although what exactly induced the islet expression of IL-15 and IL-15Rα is still unknown in human T1D, the murine data would suggest that IL-15 and IL-15Rα may act as downstream mediators to induce diabetes development in humans.
Studies in human and in animal models suggest that T cells are the primary effector cells in T1D (22, 23). Recently, a number of studies suggested that B cells and NK cells might also contribute to the beta cell loss (24, 25, 38). IL-15 is a growth factor for both T cells and NK cells. In vivo administration of IL-15 induced expansion of circulating NK and CD8 central and effector-memory T cells (39). It would be logical to suggest that both NK and T cells might contribute to the beta cell autoimmunity in the IL-15/IL-15Rα double transgenic mice. However, we did not observe NK cell expansion in the peripheral and pancreatic lymph nodes in the double transgenic mice. Only very few NK cells were found in the infiltrating lymphocytes in the islets (Fig. 2D). Moreover, depletion of NK cells with asialo-GM1, which impacted both NK and CD8 T cells, had no effect on the diabetes development in the double transgenic mice (Fig. 4C). Therefore, NK cells were not likely to play a major role in the beta-cell autoimmunity in the double transgenic mice. On the other hand, T cells and macrophages were the dominant cells that infiltrated into the pancreas in the double transgenic mice (Fig. 2D). To determine which cell types are required for the disease development after the diabetes onset, we depleted CD4, CD8, and macrophages in the double transgenic mice with new onset of diabetes. CD8 T-cell depletion did not reverse the hyperglycemia in these mice (Fig. 4B). Interestingly, we noticed that there was an increase of CD8CD44high effector memory T cells in the pancreatic lymph node in the double transgenic mice at the onset of diabetes (Fig. S7). Therefore, it is possible that CD8 T cells may be needed for the disease development before the diabetes onset and became dispensable after the diabetes onset. Macrophage depletion with clodronate liposomes resulted in a 50% reversal of the diabetes in treated mice. However, further analysis suggested that clodronate liposomes treatment not only depleted macrophages, but also significantly reduced the number of CD4 and CD8 cells. Therefore, it remains elusive whether macrophages were required for the disease development after the diabetes onset. CD4 T-cell depletion slowly reversed the hyperglycemia in all treated mice (Fig. 4A). Persistent mononuclear cell infiltration in the islets was found in the mice depleted of CD4 T cells (Fig. S4). The reversal of diabetes would suggest that those infiltrating cells were unable to mount further beta-cell destruction. These data suggested that CD4 T cells were very important for the disease development after diabetes onset. Interestingly, we noticed the double transgenic mice had lower CD4 cell percentages (and total cell numbers) both in the spleen and in the pancreatic lymph node (PLN) at the diabetes onset (Fig. S7). This may be due to the increased B-cell number in circulation that dilute out CD4 cells as we noticed higher B-cell percentages (and total cell numbers) were observed both in the spleen and in the PLN in the mice (Fig. S7). Furthermore, the CD4 cells from the double transgenic mice were more prone to Th1 priming in vitro (Fig. S8). However, transfer of CD4 or total T cells into congenic wild-type or Rag1-deficient mice did not induce diabetes in the recipient mice. This suggested that CD4 (or T cells) alone may not be sufficient to induce diabetes. It will be interesting to investigate whether B cells and their antibodies are also required in diabetes development in the double transgenic mice because higher numbers of B cells were observed both in the spleen and in the pancreatic lymph nodes at the diabetes onset.
In summary, we demonstrated islet expression of both IL-15 and IL-15Rα in patients with T1D. Coexpression of IL-15 and IL-15Rα on beta islet cells induced insulin-dependent diabetes in mice. Inhibiting IL-15 signaling with TMβ1 or the Jak2/3 inhibitor tofacitinib completely reversed the diabetes in the mice. These preclinical data in combination with human data suggested that disordered IL-15 and IL-15Rα expression may be involved in the pathogenesis of type I diabetes in humans and that the IL-15/IL-15Ra pathway may be a rational therapeutic target for the prevention of the development of T1D.
Materials and Methods
Pancreatic Tissues and Sera.
Pancreatic tissue samples from patients with T1D, autoantibody positive donors, and normal donors were obtained from the Network for Pancreatic Organ Donors (nPOD) (www.jdrfnpod.org). Islets from pancreatic tissues were microdissected on Arcturus PixCell IIe LCM instrument (Arcturus Engineering) using a series of cut slides that were stained with insulin as a reference. Sera from 20 patients with T1D were acquired from Promeddx, Inc. Sera from 29 normal donors were collected from the National Institutes of Health blood bank. Patient informed consent was obtained. The study was approved by the institutional review board of the National Cancer Institute (NCI), Bethesda, MD.
Mice.
The transgenic mice were generated in NCI-Frederick (accredited by American Association for the Accreditation of Laboratory Animal Care International). C57BL/6, B6.Rag1−/−, and NOD/ShiLtJ mice were purchased from The Jackson Laboratory. The animal study was approved by the Animal Care and Use Committee of the National Institutes of Health, Bethesda, MD.
Plasmid Construction and Generation of Transgenic Mice.
RIP-IL15 and RIP-IL15Rα transgene constructs were generated by cloning mouse IL-15 and IL-15Rα coding regions into BamHI and EcoRI sites of pKS-RIP (provided by Jun-Li Liu, McGill University Health Center, Montreal). Mouse oocytes from inbred C57BL/6 mice were used to establish transgenic mice. RIP-IL15/IL15Rα double transgenic mice were established by coinjection of RIP-IL15 and RIP-IL15Rα gene fragments. Genotyping was performed on genomic DNA extracted from tail-tip biopsies by Southern blotting analysis.
Assessment of Hyperglycemia and Diabetes.
Blood glucose levels were measured using the test strips and the Contour monitoring system (Bayer). Mice were considered hyperglycemic when their blood glucose constantly rose above 300 mg/dL.
Antibody Treatment and Jak2/3 Inhibitor Tofacitinib Treatment.
The double transgenic mice with blood glucose higher than 300 mg/dL in two consecutive weeks were treated with (i) i.p. 200 μg of TMβ1 twice per week; (ii) s.c. 4-wk pump infusion of Jak2/3 inhibitor tofacitinib (CP-690,550) 30 mg/kg per d; (iii) i.p. anti-CD4 (clone GK1.5) 200 μg on days 1, 2, and 3, then twice per week; (iv) i.p. anti-CD8 (clone 2.43) 200 μg on days 1, 2, and 3, then twice per week; (v) i.p. anti-asialo GM1 50 μL on days 1, 2, and 3, then twice per week; and (vi) i.p. clodronate liposome 100 μL every 2 d. TMβ-1, anti-CD4, and anti-CD8 monoclonal antibodies were obtained from Bio X Cell and were administered for 6 wk. Anti-asialo GM1 was obtained from Wako Pure Chemical Industries and was administered for 6 wk. Clodronate liposomes were obtained from www.clodronateliposomes.org and were administered for 2 wk.
Statistical Analyses.
Statistical significance between two means was determined by unpaired t tests. Statistical significance was two-tailed and P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Oksana Gavrilova (National Institute of Diabetes and Digestive and Kidney Diseases) for the technical support of islet isolation and Dr. Jun-Li Liu (McGill University) for kindly providing us the rat insulin promoter construct. This work was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH) and in part by federal funds from NCI, NIH, under Contract HHSN261200800001E.
Footnotes
Conflict of interest statement: The National Cancer Institute (NCI) and T.A.W. hold patent 5,833,983 to monoclonal antibody to IL-2/IL-15Rβ. The sIL-15Rα assay is under the patent applications 61/241,265 and 61/242,595 filed by NCI, National Institutes of Health. The authors have no additional financial interests.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312911110/-/DCSupplemental.
References
- 1.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: Immune dysregulation gets the NOD. Immunity. 1997;7(6):727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 2.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 3.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 4.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168(10):4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 5.Burkett PR, et al. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17(5):537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 7.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205(5):1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: Transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173(11):6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 9.Verbist KC, Rose DL, Cole CJ, Field MB, Klonowski KD. IL-15 participates in the respiratory innate immune response to influenza virus infection. PLoS ONE. 2012;7(5):e37539. doi: 10.1371/journal.pone.0037539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ylipaasto P, et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia. 2005;48(8):1510–1522. doi: 10.1007/s00125-005-1839-7. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann TA. Targeting the interleukin-15/interleukin-15 receptor system in inflammatory autoimmune diseases. Arthritis Res Ther. 2004;6(4):174–177. doi: 10.1186/ar1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczyński S, et al. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;69(3):231–236. doi: 10.1016/j.diabres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, et al. Increased serum soluble interleukin-15 receptor alpha (sIL-15Rα) levels in T cell large granular lymphocyte leukemia. Blood. 2011;119(1):137–143. doi: 10.1182/blood-2011-04-346759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dotta F, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104(12):5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987;2(8573):1423–1427. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44(6):658–664. doi: 10.2337/diab.44.6.658. [DOI] [PubMed] [Google Scholar]
- 17.Dubois SP, Waldmann TA, Müller JR. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci USA. 2005;102(24):8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 19.Azimi N, Shiramizu KM, Tagaya Y, Mariner J, Waldmann TA. Viral activation of interleukin-15 (IL-15): Characterization of a virus-inducible element in the IL-15 promoter region. J Virol. 2000;74(16):7338–7348. doi: 10.1128/jvi.74.16.7338-7348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52(6):1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 21.Smith SJ, et al. In vivo monitoring of pancreatic beta-cells in a transgenic mouse model. Mol Imaging. 2006;5(2):65–75. [PubMed] [Google Scholar]
- 22.Miller BJ, Appel MC, O’Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140(1):52–58. [PubMed] [Google Scholar]
- 23.Herold KC, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 24.Gur C, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol. 2010;11(2):121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 25.Pescovitz MD, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamford RN, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91(11):4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardozo AK, et al. IL-1β and IFN-γ induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46(2):255–266. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 28.Kochs G, Haller O. GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae) J Biol Chem. 1999;274(7):4370–4376. doi: 10.1074/jbc.274.7.4370. [DOI] [PubMed] [Google Scholar]
- 29.Golubeva Y, Salcedo R, Mueller C, Liotta LA, Espina V. Laser capture microdissection for protein and NanoString RNA analysis. Methods Mol Biol. 2013;931:213–257. doi: 10.1007/978-1-62703-056-4_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324(5925):387–389. doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stene LC, et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: The Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59(12):3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oikarinen S, et al. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes. 2011;60(1):276–279. doi: 10.2337/db10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh N, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92(5):2313–2322. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottazzo GF, et al. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 36.Coppieters KT, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209(1):51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 38.Flodström M, Shi FD, Sarvetnick N, Ljunggren HG. The natural killer cell—friend or foe in autoimmune disease? Scand J Immunol. 2002;55(5):432–441. doi: 10.1046/j.1365-3083.2002.01084.x. [DOI] [PubMed] [Google Scholar]
- 39.Waldmann TA, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.