Abstract
The pain mediator prostaglandin E2 (PGE2) sensitizes nociceptive pathways through EP2 and EP4 receptors, which are coupled to Gs proteins and increase cAMP. However, PGE2 also activates EP3 receptors, and the major signaling pathway of the EP3 receptor splice variants uses inhibition of cAMP synthesis via Gi proteins. This opposite effect raises the intriguing question of whether the Gi-protein–coupled EP3 receptor may counteract the EP2 and EP4 receptor-mediated pronociceptive effects of PGE2. We found extensive localization of the EP3 receptor in primary sensory neurons and the spinal cord. The selective activation of the EP3 receptor at these sites did not sensitize nociceptive neurons in healthy animals. In contrast, it produced profound analgesia and reduced responses of peripheral and spinal nociceptive neurons to noxious stimuli but only when the joint was inflamed. In isolated dorsal root ganglion neurons, EP3 receptor activation counteracted the sensitizing effect of PGE2, and stimulation of excitatory EP receptors promoted the expression of membrane-associated inhibitory EP3 receptor. We propose, therefore, that the EP3 receptor provides endogenous pain control and that selective activation of EP3 receptors may be a unique approach to reverse inflammatory pain. Importantly, we identified the EP3 receptor in the joint nerves of patients with painful osteoarthritis.
Keywords: mechanical hyperalgesia, sodium currents
Prostaglandins regulate immune responses (1), and they are key mediators of pain and other sickness symptoms such as fever, sleepiness, and anorexia (2). In particular, prostaglandin E2 (PGE2) is a key mediator of pain because it sensitizes peripheral and spinal nociceptive pathways (3–7). Hence the most common pain treatment is the inhibition of prostaglandin synthesis by cyclooxygenase inhibitors. PGE2 activates neuronal EP1–4 receptors (8). In this context, it is noteworthy that different EP receptors are coupled to different, partly even opposing intracellular signaling pathways. EP2 and EP4 receptors, which sensitize neurons (9–11), are coupled to Gs proteins and increase cAMP (12, 13). In contrast, the major signaling pathway of the EP3 receptor splice variants uses inhibition of cAMP synthesis via Gi proteins (12, 13). The functional significance of such opposite effects raises the intriguing question of whether the Gi-protein–coupled EP3 receptor may counteract the EP2 and EP4 receptor-mediated pronociceptive effects of PGE2 (9). Thus, the role of PGE2 may not be just pronociceptive as usually assumed but it may be rather more diverse and depend on the biological context, as during inflammation (1). In the present experiments, we addressed the hypothesis that EP3 receptor activation is rather antinociceptive than pronociceptive. We found that the EP3 receptor is heavily expressed in rat sensory dorsal root ganglia (DRG) and spinal cord as well as in peripheral nerves including nerve fibers of osteoarthritic knees of humans. Selective activation of the EP3 receptor did not sensitize nociceptive neurons but caused striking antinociception when the joint was inflamed. This sheds light on the neuronal EP receptors in pain pathways and offers the opportunity to explore a unique approach to treat inflammatory pain.
Results
Localization of EP3 Receptor-Like Immunoreactivity in Rat DRG and Spinal Cord.
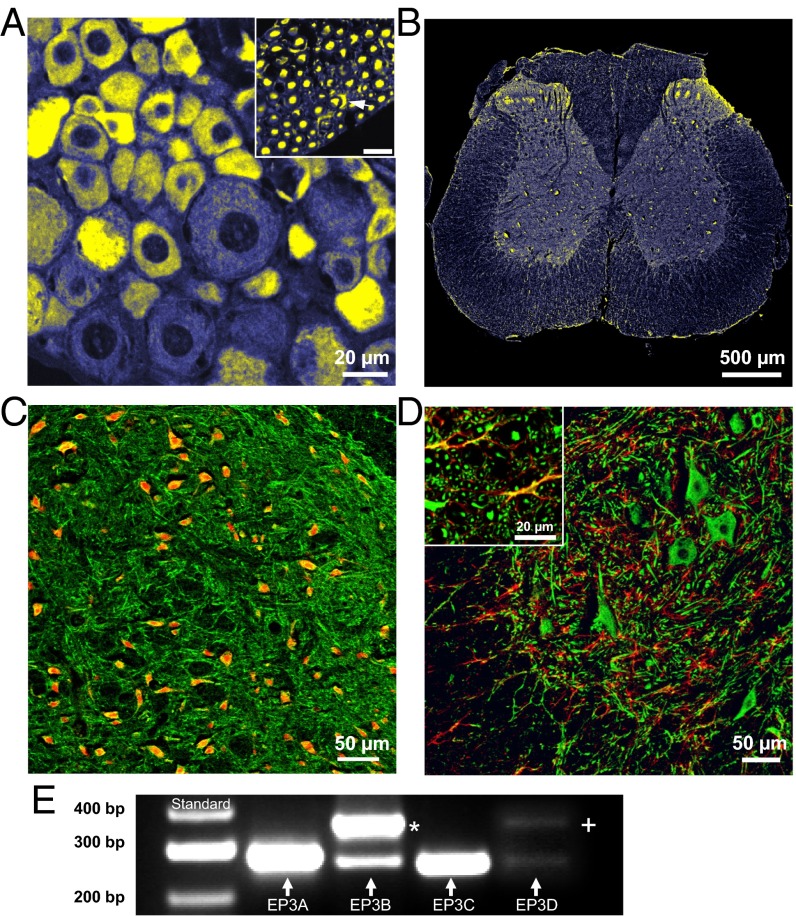
EP3 receptor-like immunoreactivity (IR) (yellow) in the rat was visualized in DRG neurons (mainly small- and medium sized, Fig. 1A), in dorsal root axons and some Schwann cells (Fig. 1A, Inset), in peripheral axons, and in the spinal cord, with most intense staining in the dorsal horn (Fig. 1B). We found EP3 receptor-like IR in 86 ± 16% (mean ± SD) of lumbar DRG neurons. Furthermore we identified EP1 receptor-like IR in 87 ± 7%, EP2 receptor-like IR in 87 ± 12%, and EP4 receptor-like IR in 53 ± 12% of the DRG neurons (Fig. S1). The large proportions of labeled neurons indicate coexpression of the EP3 with the other EP receptors. EP3 receptor-like IR was identified in different types of DRG neurons such as peptidergic calcitonin gene-related peptide (CGRP)-positive, nonpeptidergic I isolectin B4 (IB4)-positive, and also in myelinated neurofilament-positive DRG neurons. Retrograde labeling of knee joint afferents by FAST DiI showed EP3 receptor-like IR in DRG neurons supplying the knee joint (Fig. S2E).
Fig. 1.
Localization of EP3 receptor-like immunoreactivity (IR) in dorsal root ganglia and the spinal cord. (A) EP3 receptor-like IR (yellow) in a DRG section. (Inset) EP3 receptor-like IR in DR axons in a cross-section. The arrowhead points to a labeled Schwann cell. (B) EP3 receptor-like IR (yellow) in a lumbar spinal cord section. (C) EP3 receptor-like IR (now green) in the dorsal horn. Red shows NeuN labeling for neuronal cell bodies; yellow-orange, neuronal cell bodies with EP3 receptor-like IR. (D) EP3 receptor-like IR (green) in the ventral horn in the gray matter as well as in the white matter (Inset). Red shows GFAP staining of glia. Yellow shows double labeling of radial glia and EP3 receptor-like IR. (E) Agarose gel showing subtype-specific PCR products of the EP3A, EP3B, EP3C, and EP3D isoforms from rat DRGs (Lower lane). * in E: EP3A-PCR byproduct with the EP3 forward and EP3B reverse primer combination. + in E: EP3A-PCR byproduct with the EP3 forward and the EP3D reverse primer combination.
In the spinal cord, the EP3 receptor (now green) was localized in nerve fibers and neurons of the gray matter throughout the dorsal (Fig. 1C) and the ventral horn including large motoneurons (Fig. 1D). In Fig. 1C, neuronal cell bodies were labeled with neuronal nuclei (NeuN) (red) and yellow-orange labeling shows neuronal cell bodies with EP3 receptor-like IR. In Fig. 1D, glial cells were stained with glial fibrillary acidic protein (GFAP) (red), and yellow-orange labeling shows EP3 receptor-like IR in the radial glia of the white matter (Fig. 1D, Inset) but not in the gray matter.
PCR analysis of total DRGs (Fig. 1E) yielded PCR products of the EP3A, the EP3B, and the EP3C receptor and a faint signal of the EP3D receptor (lower lane). The upper lane shows different EP3A-PCR products resulting from different primer combinations (Materials and Methods and Fig. S3).
Lumbar DRGs from rats with antigen-induced arthritis (AIA) in the knee joints (harvested at days 1, 3, 7, and 21, five rats at each time point) showed similar high proportions of DRG neurons expressing EP1, EP2, and EP3 receptor-like IR as in normal rats (see above). The proportion of DRG neurons with EP4 receptor-like IR increased from 53% (see above) to about 90%.
Localization of EP3 Receptor-Like IR in Peripheral Nerve Bundles.
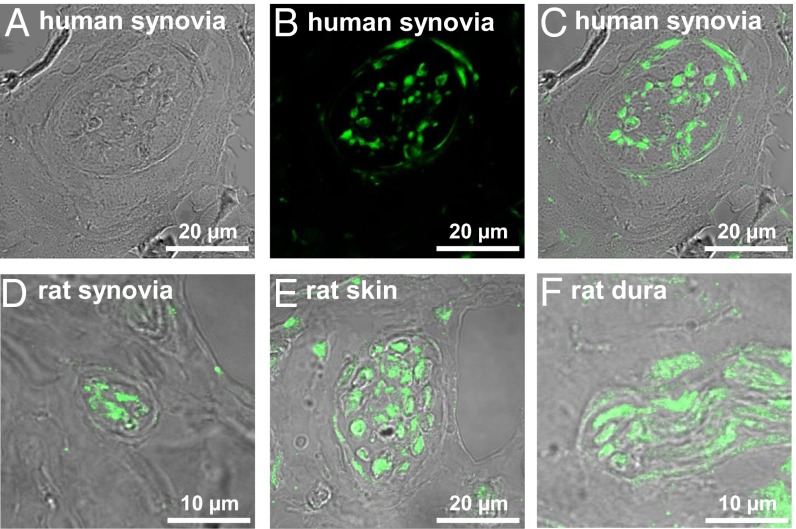
EP3 receptor-like IR was also visualized in peripheral nerve bundles. Fig. 2A displays a nerve fiber bundle in the excised fibrous joint capsule close to the synovial layer from a human osteoarthritic (OA) knee joint in the transmission mode; Fig. 2B shows EP3 receptor-like IR in the same section; and the overlay in Fig. 2C shows that EP3 receptor-like IR was localized in nerve fibers and in some fibroblasts. Such nerve fiber bundles were observed at similar locations in tissue from eight patients with OA with primary idiopathic osteoarthritis who underwent surgical replacement of knee joints. Patients had radiological signs of OA, pain, and loss of function and mobility. In rats, nerve fiber bundles with EP3 receptor-like IR were not only found in the knee joint but also, e.g., in skin and dura mater (Fig. 2 D–F).
Fig. 2.
Localization of EP3 receptor-like IR in nerve fiber bundles. (A) Nerve fiber bundle in a section of the excised human fibrous joint capsule in the transmission mode. (B) EP3 receptor-like IR (green) in the section displayed in A. (C) Overlay of A and B, showing EP3 receptor-like IR in nerve fibers and some fibroblasts (arrows). (D–F) Overlay pictures of nerve fiber bundles in rat knee joint, rat skin over the knee joint, and dura mater.
Behavioral Antinociceptive Effect of the EP3 Receptor Agonist ONO-AE-248.
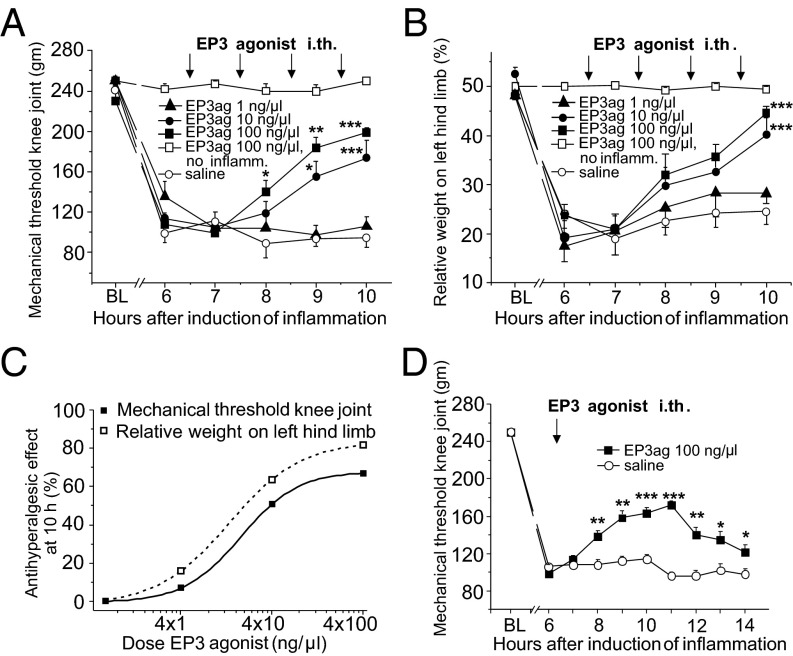
Because EP3 receptor-like IR was extensively localized in both the DRGs and the spinal cord we tested in awake rats whether intrathecal application of the selective EP3 receptor agonist ONO-AE-248 changes pain-related behavior. In contrast to previously available agonists, ONO-AE-248 is a highly selective EP3 receptor agonist (13, 14), and responses to ONO-AE-248 are absent in EP3-deficient mice (15). We focused on nociception in the joint. In rats without inflammation the repeated application of the agonist (single doses of 100 ng/µL, arrows) neither changed the mechanical withdrawal threshold at the knee (Fig. 3A, squares) nor the symmetry of weight bearing on hind limbs (Fig. 3B, squares). However, in rats with an acute inflammation in the knee, the EP3 agonist dose-dependently reversed hyperalgesia. During development of inflammation, the mechanical withdrawal threshold at the inflamed knee dropped and weight bearing became asymmetric, and these symptoms were significantly reversed by repeated injections of 10 and 100 ng/µL EP3 agonist (Fig. 3 A and B). The maximal antihyperalgesic effect in these experiments is shown in Fig. 3C. Further experiments revealed that already a single application of the EP3 agonist at 100 ng/µL significantly attenuated inflammatory hyperalgesia (Fig. 3D). The EP3 agonist also improved walking and exploration behavior. Thus, EP3 receptor activation had no effect in control rats but significantly reduced pain-related behavior under inflammatory conditions.
Fig. 3.
Antihyperalgesic effect of the intrathecal (i.th.) application of the EP3 receptor agonist ONO-AE-248 in behavioral experiments. (A) Reduction of mechanical threshold at the knee during development of joint inflammation and reversal of mechanical hyperalgesia at the knee by repeated i.th. applications of ONO-AE-248 at 10 and 100 ng/µL but not at 1 ng/µL or with saline (each group n = 8; arrows show injections, each injection had a volume of 10 µL). BL: preinflammation baseline. In rats without inflammation (n = 11, open squares), there was no change of threshold, no effect of ONO-AE-248. (B) In parallel generation of inflammation-evoked asymmetric weight bearing and reversal by ONO-AE-248. (C) Maximal antihyperalgesic effect of different doses of ONO-AE-248 in the knee pressure and incapacitance tests in rats with inflammation at hour 10. The antihyperalgesic effect = 0 represents the values after i.th. saline injection instead of ONO-AE-248. (D) Attenuation of inflammation-evoked mechanical hyperalgesia by one i.th. injection of 100 ng/µL ONO-AE-248 (n = 6) compared with i.th. application of saline (n = 5). *P < 0.05; **P < 0.01; ***P < 0.001 (repeated measures ANOVA, followed by post hoc t tests).
Antinociceptive Effects of the EP3 Receptor Agonist in Joint Nociceptors and Spinal Cord Neurons.
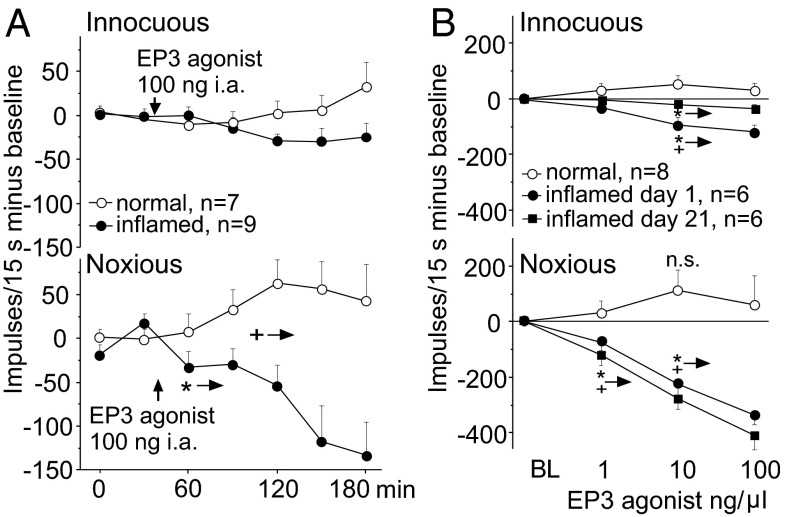
Electrophysiological recordings from peripheral joint nociceptors (Fig. 4A) and from spinal cord neurons with joint input (Fig. 4B) in anesthetized rats showed that the EP3 agonist reduced the neuronal responses under inflammatory conditions at both the peripheral and the spinal levels. During the repetitive testing of the responses of C-fiber nociceptors to innocuous rotation of the normal or acutely inflamed knee joint (7–11 h after induction of inflammation), the small responses were not altered by the injection of the EP3 agonist into the knee joint (Fig. 4A, Upper). However, the EP3 agonist slightly increased the responses of these C fibers to noxious rotation of the normal joint but significantly reduced the responses to noxious rotation of the inflamed knee joint (Fig. 4A, Lower). The difference between the experimental groups (normal versus inflamed joints) was significant from 120 min onwards after injection of ONO-AE-248 (+ →: P < 0.05, from this interval differences between groups were significant, Mann–Whitney U test).
Fig. 4.
Antinociceptive effects of the EP3 receptor agonist ONO-AE-248 on joint nociceptors and spinal cord neurons with knee joint input in vivo. (A, Upper) Upon intraarticular ONO-AE-248 no change of responses of C fibers to innocuous outward rotation of the normal joint (baseline 42 ± 11 impulses (imp)/15 s) and the inflamed joint (baseline 92 ± 36 imp/15 s). (Lower) Differential effect on responses of C fibers to noxious rotation of the normal knee joint (baseline 166 ± 37 imp/15 s) and the inflamed knee joint (baseline 264 ± 33 imp/15 s) [for display baseline responses set 0, each symbol: average of three values (±SEM) within 15-min intervals]. (B) Upon spinally applied ONO-AE-248 no reduction of responses of spinal cord neurons to innocuous pressure onto the normal joint (baseline 61 ± 29 imp/15 s) and to noxious pressure onto the normal joint (baseline 345 ± 72 imp/15 s) but dose-dependent reduction of responses to innocuous and noxious pressure during acute inflammation (baseline innocuous pressure 145 ± 28 imp/15 s, baseline noxious pressure 506 ± 35 imp/15 s) and chronic knee inflammation (baseline innocuous pressure 46 ± 17 imp/15 s, baseline noxious pressure 465 ± 72 imp/15 s). Symbols: average reduction 25–50 min after ONO-AE-248. In A and B: *→ from here onwards: significant difference at P < 0.05 compared with intragroup BL, Wilcoxon matched pairs signed rank test; +→ from here onwards: significant difference at P < 0.05 between inflammatory groups and the control group, Mann-Whitney U test.
A similar pattern of effect was observed when neurons of the deep dorsal horn of the spinal cord (968 ± 148 µm from the dorsal spinal surface) with knee joint input were recorded and the EP3 agonist was applied spinally (Fig. 4B). In rats with noninflamed knee joints spinal administration of the EP3 agonist at three different doses slightly enhanced responses to innocuous and noxious stimulation of the knee joint (Fig. 4B, circles) but the effect was not significant (Wilcoxon matched pairs signed rank test). By contrast, when the knee joint was acutely at day 1 (1 d) or chronically (21 d) inflamed and spinal hyperexcitability was established, the responses to innocuous and noxious pressure applied to the knee were significantly reduced (Fig. 4B, dots and filled squares).
Effect of the EP3 Receptor Agonist in Isolated DRG Neurons.
In further experiments we explored whether activation of the inhibitory EP3 receptor counteracts the pronociceptive effects of EP2 and EP4 receptor activation in the same neuron. We performed patch clamp recordings from isolated and cultured DRG neurons, the vast majority of which showed all EP receptor subtypes. As readout parameter, we recorded tetrodotoxin-resistant (TTX-R) Na+ currents because the TTX-R Na+ channels Nav1.8 and Nav1.9 are expressed in small- to medium-sized nociceptive sensory neurons where they are involved in the excitation of neurons by noxious mechanical stimuli (16–18), and because PGE2 increases TTX-R Na+ currents in a cAMP-dependent manner (19, 20).
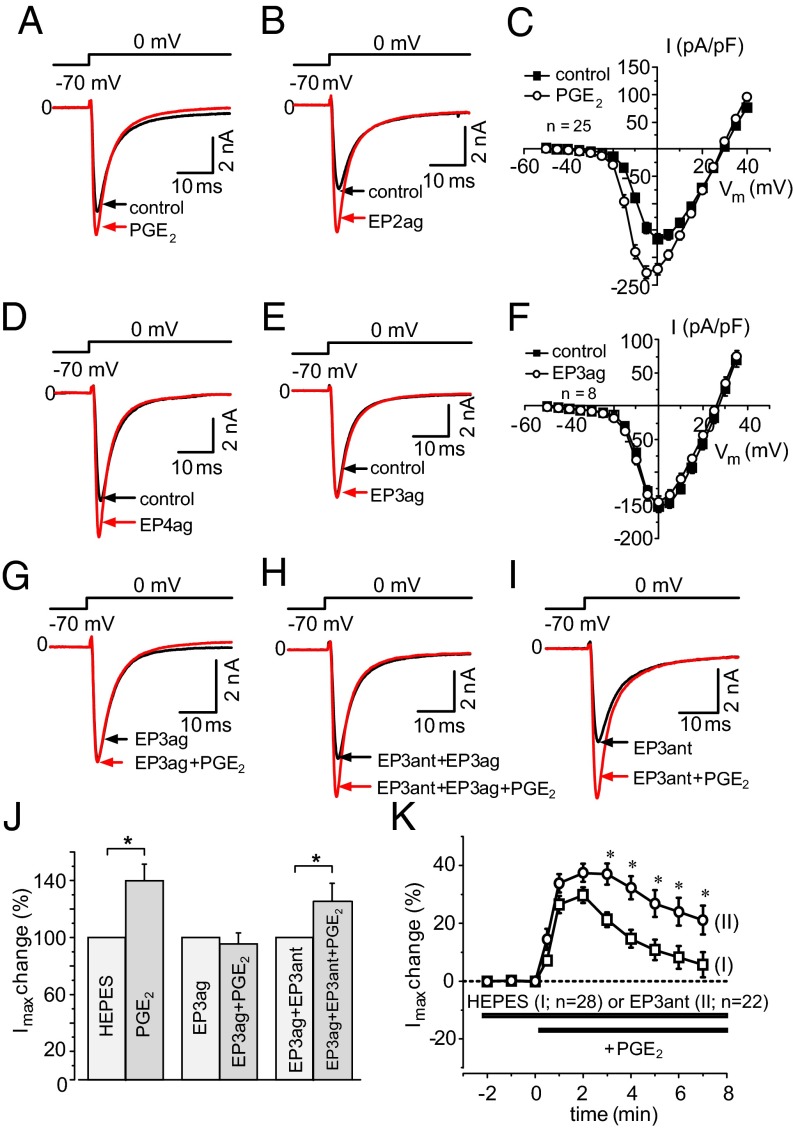
PGE2 (Fig. S4) and the highly selective EP2 and EP4 agonists, ONO-AE1-259–01 and ONO-AE1-329 (13, 14), increased TTX-R Na+ currents in DRG neurons (Fig. S5) but the EP3 agonist did not mimic this effect (Fig. S4). Specimens in Fig. 5 A, B, D, and E show Na+ currents elicited by voltage steps from −70 mV to 0 mV. The difference between the effects of PGE2 and the EP3 agonist on Na+ currents is also displayed in the current–voltage (I/V) curves in Fig. 5 C and F. Maximal peak current densities were enhanced by 2.5 µM PGE2 from −168.3 ± 9.4 pA/pF to −233.1 ± 11.7 pA/pF (P < 0.05, paired t test) but they remained unaltered after 2.0 µM EP3 agonist (−143.0 ± 9.0 pA/pF before and −134.8 ± 9.4 pA/pF after the EP3 agonist). Furthermore, when the EP3 agonist was administered 2 min before PGE2, the increase of Na+ currents by PGE2 was prevented (Fig. 5G, for all neurons see Fig. 5J). The response to PGE2 was restored upon coadministration of both the EP3 agonist and the EP3 receptor antagonist ONO-AE3-240 (21) (Fig. 5H, for all neurons see Fig. 5J).
Fig. 5.
Effects of PGE2 and selective EP receptor agonists on voltage-gated TTX-R Na+ currents of cultured DRG neurons. (A) Increase of current of a neuron 3 min after 2.5 µM PGE2. (B) Increase of current 3 min after 1.0 µM EP2 agonist ONO-AE1-259–01. (C) I/V curves of Na+ currents before and 3–5 min after 2.5 µM PGE2. (D) Increase of current 3 min after 1.0 µM EP4 agonist ONO-AE1-329. (E) No increase of current 5 min after 2.0 µM EP3 agonist ONO-AE-248. (F) I/V curves of Na+ currents before and 3–5 min after 2.0 µM EP3 agonist. (G) No increase of current after coadministration of 1.0 µM PGE2 and 2.0 µM EP3 agonist. (H) Increase of current by PGE2 (1.0 µM) during coadministration of 2.0 µM EP3 agonist and EP3 antagonist ONO-AE3-240 (4 nM). (I) Increase of current by PGE2 (0.5 µM) in the presence of the EP3 antagonist (4 nM). (J) Changes of maximal currents (Imax) evoked by 1.0 µM PGE2 after Hepes buffer (n = 10), in the presence of 2.0 µM EP3 receptor agonist (n = 8) and in presence of both 2.0 µM EP3 agonist and 4 nM EP3 antagonist (n = 7). (K) Changes of Imax either after application of PGE2 (0.5 µM) alone (I, open squares) or after application of PGE2 (0.5 µM) in the presence of 4 nM EP3 receptor antagonist ONO-AE3-240 (II, circles). *Significantly higher maximal current amplitudes in the presence of the EP3 receptor antagonist; P < 0.01, Fisher's exact test.
Because PGE2 may coactivate the excitatory and inhibitory EP receptors at the same time, we hypothesized that the PGE2 effect on Na+ currents is increased when EP3 receptors are blocked by the antagonist. PGE2 (0.5 µM) alone increased TTX-R Na+ currents transiently (I in Fig. 5K) and shifted conductance toward hyperpolarization (from −5.3 ± 0.7 mV to −9.7 ± 0.9 mV). Upon pre- and coadministration of the EP3 receptor antagonist (specimen in Fig. 5I and Fig. S6 A and B), the effect of 0.5 µM PGE2 was more sustained and overall significantly increased (Fig. 5K, II; asterisks show significantly higher currents in the presence of the EP3 receptor antagonist; P < 0.01, Fisher's exact test), and conductance was further shifted toward hyperpolarization (from −6.7 ± 0.7 mV to −10.7 ± 0.7 mV) (Fig. S6). The EP3 receptor antagonist itself did not influence TTX-R Na+ currents (Fig. S7). Thus, blockade of the inhibitory EP3 receptor increases the excitatory PGE2 effect.
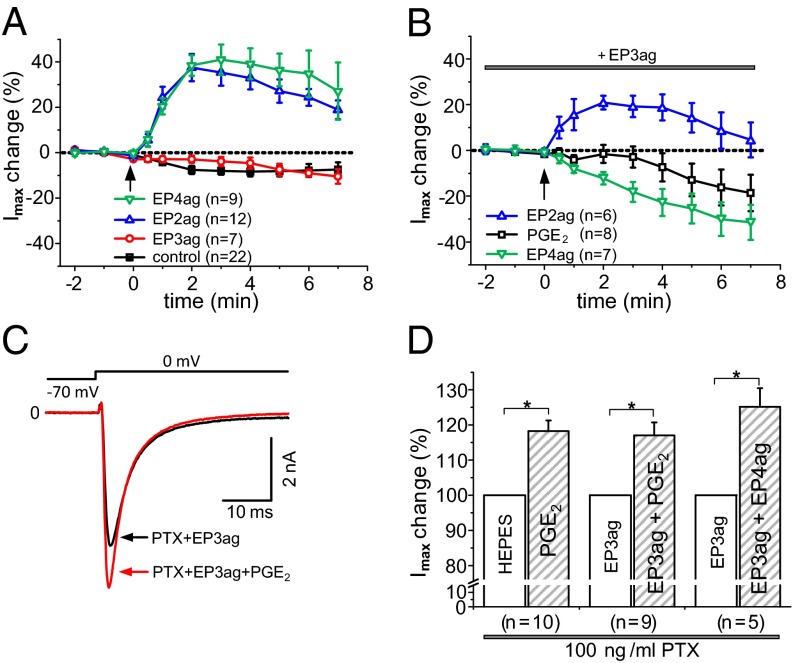
The inhibitory effect of EP3 receptor activation on the PGE2 response is mainly due to an interference with EP4 receptor activation. Fig. 6A shows the increases of TTX-R Na+ currents by either the EP2 agonist ONO-AE1-259–01 (1.0 µM) or the EP4 agonist ONO-AE1-329 (1.0 µM), whereas the EP3 agonist (2.0 µM) had the same effect as the buffer control. Coapplication of the EP2 and EP4 agonist caused similar effects as each agonist alone (Fig. S5). In the presence of the EP3 agonist (2.0 µM) the current increase by the EP2 agonist was smaller, the EP4 agonist even reduced the currents, and PGE2 slightly reduced the current (Fig. 6B). Pertussis toxin (PTX), which inhibits Gi-protein–mediated actions, largely prevented the inhibitory EP3 effect. Upon pretreatment with PTX, PGE2 (specimen in Fig. 6C), or the EP4 agonist enhanced TTX-R Na+ currents similarly whether the bath contained either Hepes buffer or the EP3 agonist (Fig. 6D).
Fig. 6.
Interaction of the EP3 agonist with PGE2, the EP2, and the EP4 agonist. (A) Increases of maximal Na+ currents (Imax) by the EP2 receptor agonist ONO-AE1-259–01 (1.0 µM) or the EP4 receptor agonist ONO-AE1-329 (1.0 µM), no increase of Imax by the EP3 agonist or buffer control. The arrow marks the application of the substance. (B) Changes of Imax by either the EP2 (1.0 µM), or the EP4 agonist (1.0 µM), or PGE2 (1.0 µM) during pre- and coadministration of the EP3 agonist (2.0 µM). The arrow marks application of the EP2 agonist, of the EP4 agonist, or of PGE2. (C) Increase of current by PGE2 (1.0 µM) in the presence of both the EP3 agonist (2.0 µM) and PTX (100 ng/mL). (D) Similar increases of Imax by PGE2 (1.0 µM) in the presence of Hepes or the EP3 agonist upon PTX pretreatment, and increase of Imax by the EP4 agonist upon coapplication of the EP3 agonist and PTX. *P < 0.05 (paired t test).
Membrane-Associated Expression of EP Receptors in DRG Neurons.
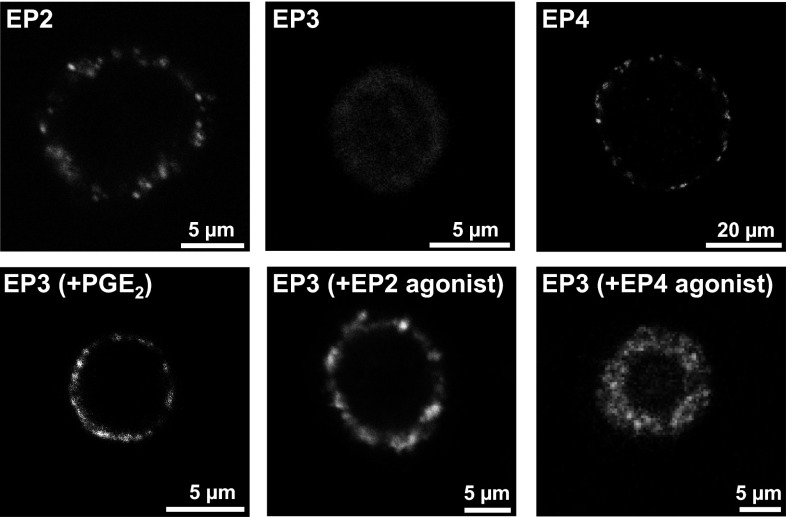
Finally, we explored whether EP2, EP3, and EP4 receptors exhibit different expression patterns in the membrane of DRG neurons. Cultured native unfixed DRG neurons were incubated in medium containing antibodies to either the EP2, EP3, and EP4 receptor for 4 h at 4 °C (to prevent endocytosis). Examples of single cells are displayed in Fig. 7, Upper panels. Under these conditions both EP2 and EP4 receptor-like IR were detected as randomly distributed clusters in the membrane of neurons, but EP3 receptor-like IR was not visualized. However, when the medium contained either PGE2 (500 nM), the EP2 agonist (500 nM), or the EP4 agonist (500 nM), membrane-associated clusters of EP3 receptor-like IR were visualized (Fig. 7, Lower panels). These data suggest that inflammatory stimulation of DRG neurons promotes the availability of membrane-associated EP3 receptor sites.
Fig. 7.
Membrane-associated expression of EP receptors in DRG neurons at 4 °C. (Upper panels) Localization of clusters of EP2 (Left) and EP4 receptor-like IR (Right) but not of EP3 receptor-like IR (Center) in the membrane of single cultured DRG neurons. (Lower panels) Localization of clusters of EP3 receptor-like IR in the membrane of single cultured neurons after preincubation of cells with either PGE2 [EP3 (+PGE2)], EP2 receptor agonist [EP3 (+EP2 agonist)], or EP4 receptor agonist [EP3 (+EP4 agonist)].
Discussion
The data show a striking difference between EP2/EP4 and EP3 receptor activation and provide evidence that the opposite effects of these receptors on signaling pathways are functionally important. Whereas EP2 and EP4 receptors sensitize nociceptive neurons (9–11), selective EP3 receptor activation in the present experiments caused antinociception. Importantly, in vivo this antinociceptive effect was only observed after the sensitization of neurons, and therefore this particular EP receptor may rather provide endogenous pain control than pronociceptive sensitization. Because endogenous EP3 receptor activation is not fully used under inflammatory conditions (the administration of the EP3 agonist evoked pronounced antinociception), the application of EP3 agonists may offer a unique strategy to treat pain.
The localization of all four EP receptors in the majority of DRG neurons indicates that the EP3 receptor is coexpressed with the other EP receptors, and it is reasonable to assume that it has a particular function. In fact, the patch clamp recordings from single neurons show directly that EP3 receptor activation counteracts the excitatory effects of PGE2 and the EP2 and EP4 receptor agonists. The application of pertussis toxin inhibited the inhibitory effect of EP3 receptor activation (EP3 receptor activation decreases cAMP) (21, 22) consistent with an involvement of Gi proteins in this effect. Thus, different EP receptors can provide a balance between excitatory and inhibitory PGE2 effects within single nerve cells.
The increased responses of DRG neurons to PGE2 in the presence of the EP3 receptor antagonist indicate that the coactivation of EP3 receptors limits the pronociceptive effects of PGE2. In addition, the membrane-associated staining of EP3 receptor-like IR after stimulation of living DRG neurons with either PGE2 or the EP2 or EP4 receptor agonist suggests that the EP3 receptor availability in the membrane is enhanced in the course of PGE2 stimulation. At present we cannot differentiate whether under the conditions of these experiments (4 °C to prevent endocytosis of antibodies), initially no EP3 receptor was in the membrane or whether the EP3 receptor in the membrane was in a configuration that did not allow binding of the antibody from the outside. Because of the low temperature the time course of membrane-associated EP3 receptor expression cannot be directly related to the patch clamping experiments, which suggest rapid availability of EP3 receptors after EP3 receptor agonist application.
Both the behavioral experiments and the recordings clearly show the antinociceptive effects of the EP3 receptor agonist under inflammatory conditions in vivo. Intrathecal application of ONO-AE-248 significantly reduced inflammation-evoked mechanical hyperalgesia, and application of ONO-AE-248 either to the inflamed knee joint or into the spinal trough elicited antinociception. This is consistent with the localization of EP3 receptor-like IR in joint nerves, DRGs, and spinal cord. The localization of EP3 receptor-like IR in peptidergic and nonpeptidergic DRG neurons, in nerves of joint, skin, and dura suggests that activation of EP3 receptors may have an impact at many sites. In the periphery, EP3 receptor activation is likely to interfere with the sensitization process of sensory neurons, and in the spinal cord the EP3 receptor may modify the release of mediators from primary sensory neurons (23, 24) as well as exert direct effects on spinal (inter)neurons (25, 26). The recordings from spinal cord neurons showed in addition that the nociceptive processing was reduced by the EP3 agonist during both acute and chronic inflammation. The persistent expression of EP3 receptors in DRGs of rats with acute and chronic AIA and in nerves from human OA joints indicates that this target remains available in long-lasting pain states.
We identified in the DRGs the EP3A, EP3B, EP3C, and EP3D splice variant. EP3A and EP3B receptor isoforms are coupled to Gi proteins, which reduce cAMP synthesis, whereas EP3C receptors either reduce or increase intracellular cAMP (13). Because the splice variants differ in their intracellular molecule chains and because the EP3 receptor agonist activates all splice variants, the small and nonsignificant increase of responses of afferent fibers and spinal cord neurons under normal conditions may be due to an increase of cAMP via the EP3C receptor. Importantly, under inflammatory conditions only inhibition of neuronal activity was observed.
Interestingly, compared with wild-type mice EP3-deficient mice showed increased experimental allergic conjunctivitis, and treatment of wild-type mice with ONO-AE-248 reduced eosinophil infiltration (15), showing that EP3 receptor activation reduces inflammation after the allergic challenge. Thus, EP3 receptors may have an important role in body protection by keeping inflammation and pain under control. From this point of view, selective EP3 receptor activation may be a unique approach to treat pain, e.g., in the musculoskeletal system. Both rheumatoid arthritis and osteoarthritis are characterized by inflammation and central sensitization, which renders pain and hyperalgesia widespread beyond the afflicted joint (27–30). Thus, peripheral as well as spinal targets are important for pain treatment. Currently mainly cyclooxygenase inhibitors are used to treat musculoskeletal pain. However, general inhibition of prostaglandin synthesis may also reduce beneficial prostaglandin effects such as for prostaglandin D2 (PGD2), which is anti-inflammatory (31), antinociceptive under inflammatory conditions (32), and/or neuroprotective (33). Selective activation of EP3 receptors may be an alternative, in particular because this pain inhibitory mechanism is only functional under pathophysiological conditions such as inflammation. It is also an alternative concept to the blockade of PGE2 actions at excitatory EP receptors (34), which might be difficult due to the involvement of multiple excitatory EP receptors in sensitization (9–11, 25, 26, 35).
Materials and Methods
Immunohistochemistry in Rat and Human Tissue and PCR.
Rats were perfused intracardially. DRGs and spinal cords were postfixed in 4% (wt/vol) paraformaldehyde. Peripheral tissues from knee joint, skin, and dura of rat and from human knee joint were resected and fixed in 4% (wt/vol) paraformaldehyde for at least 48 h. The tissues were embedded in paraffin and cut into sections (4 or 6 µm). After brief heating, sections were incubated overnight at 4 °C with polyclonal antibodies (rabbit, all from Cayman Chemical) against the EP1 receptor (1:50 or 1:3,000; different batches), the EP2 receptor (1:200), the EP3 receptor (1:1,000), or the EP4 receptor (1:100). Antibodies were raised against human EP receptors (EP1: amino acids 380–402, EP2: amino acids 335–358, EP3: amino acids 308–327, EP4: amino acids 459–488) and had cross-reactivity with respective rat EP receptors. For detection of EP receptors, sections were incubated in biotinylated secondary antibody (1:200), then an avidin-biotin peroxidase complex was applied which was visualized using the fluorochrome (E)-2-[2-(4-hydroxystyryl)]quinoline. For microscopy, confocal laser scanning microscopes were used. Specificity of antibodies was confirmed by preabsorption experiments with the corresponding blocking peptides (Fig. S8). Glial cells were stained with monoclonal antibody against GFAP (1:100), neurons with monoclonal NeuN antibody (1:100). For double labeling of DRG sections antibodies against CGRP or neurofilament, and IB4 were used. In some rats the tracer FAST DiI was injected into the knee joint. For details see SI Materials and Methods.
For joint tissue sampling during knee replacement surgery, all patients gave written informed consent (approval by the Ethical Committee for Clinical Trials of the Friedrich Schiller University, Jena, Germany 1714–01/06 and 2443–12/08).
Membrane-associated EP receptor expression was studied in unfixed cultured rat DRG neurons. They were incubated 4 h at 4 °C with either EP2, EP3, or EP4 receptor primary antibody (1:100) and then incubated with secondary anti-rabbit antibodies labeled with Alexa Fluor 488 (1:400; Invitrogen) for 2 h at 4 °C. In some cultures, the EP3 receptor antibody was incubated together with PGE2 or EP2 or EP4 receptor agonist.
To identify EP3 isoforms (EP3A, EP3B, EP3C, and EP3D) in rat DRGs with PCR, the mRNA from total DRGs was transcribed into cDNA. For PCR of the EP3B splice variant different numbers of base pairs were used to differentiate it from EP3A and EP3D splice variants. The specificity of all PCR products was confirmed by restriction analysis using the endonucleases BamHI and TaqI. For primers and reaction conditions see SI Materials and Methods.
Experiments in Vivo.
All experiments were approved by the Thuringian government commission for animal protection (permission nos. 02–07/04, 02–039/06, and 02–088/13). The animals were treated according to the declaration of Helsinki and the guiding principles in the care and use of animals.
Acute inflammation in the knee joint was induced by injection of 4% (wt/vol) kaolin and 2% (wt/vol) lambda-carrageenan into the joint cavity (36). Chronic unilateral AIA in the knee joint of Lewis rats was induced by prior immunization against the antigen methylated bovine serum albumin (mBSA) and the subsequent injection of mBSA into the left knee joint cavity (37). For details see SI Materials and Methods.
In behavioral experiments in Lewis rats the effect of the EP3 receptor agonist ONO-AE-248 on joint nociception was assessed. Intrathecal catheters for application of the EP3 agonist were implanted 8–12 d before induction of acute kaolin/carrageenan inflammation. Mechanical pain thresholds at the knee joints were assessed using a dynamometer. Static motor behavior was assessed using the incapacitance test. In rats without inflammation and rats with inflammation either saline or ONO-AE-248 were intrathecally applied, either repeatedly or one time only. For details of protocol see SI Materials and Methods.
In male Wistar rats anesthetized with sodium thiopentone i.p. (100 mg/kg initially, supplemental doses 20 mg/kg) and spontaneously breathing, the medial aspect of the knee joint was exposed. The femur was fixed allowing rotation of the lower leg. Extracellular recordings from filaments of the saphenous nerve identified single C fibers with receptive fields in the knee joint by mechanical stimulation of the joint and electrical stimulation of the receptive field. Responses of fibers were repeatedly tested with application of innocuous (20 mNm, 15 s each) and noxious (40 mNm, 15 s each) outward rotation of the lower leg against the fixed femur before and after injection of the EP3 agonist into the joint. Knee joints were normal or acutely inflamed with kaolin/carrageenan 7–11 h before recordings.
After laminectomy, extracellular recordings from spinal cord neurons with knee joint input were performed in segments L1–L4 using glass-insulated carbon filaments. The EP3 agonist was spinally applied to a trough over the recording site at increasing concentrations (1, 10, 100 ng/µL, each for 50 min). For testing calibrated innocuous pressure (1.9 N/40 mm2) and noxious pressure (7.8 or 5.9 N/40 mm2) (each 15 s) were applied to the knee. The knee joint was normal or acutely or chronically inflamed.
Whole-Cell Patch Clamp Recordings from Cultured DRG Neurons.
In single cultured DRG neurons from all spinal levels (12–48 h in culture) TTX resistant Na+ currents were measured in the whole-cell configuration. Na+ currents were elicited by 40-ms pulses in increments of 5 mV to potentials between −40 mV and +40 mV from a holding potential of −70 mV (interpulse interval 2.0 s). The voltage protocol was applied before and after application of the test compound(s) every minute for about 10 min after compound application. Applied compounds were the EP2 agonist ONO-AE1-259–01, the EP3 agonist ONO-AE-248, the EP4 agonist ONO-AE1-329, the EP3 antagonist ONO-AE3-240, PGE2, and in some experiments cells were preincubated with pertussis toxin (100 ng/mL) for 30 min to 2 h. The Ki values (in nanomoles) of compounds obtained by competition-binding isotherms to displace [3H]PGE2 binding to the EP1, EP2, EP3, and EP4 receptors were: EP 2 agonist ONO-AE1-259-01: >104 for EP1, 3 for EP2, >104 for EP3, 600 for EP4; EP3 agonist ONO-AE-248: >104 for EP1, 3,700 for EP2, 8 for EP3, 4,200 for EP4; EP4 agonist ONO-AE1-329: >104 for EP1, 2,100 for EP2, 1,200 for EP3, 10 for EP4; EP3 antagonist ONO-AE3-240: 590 for EP1, >104 for EP2, 0.23 for EP3, 58 for EP4 (13). The response to ONO-AE-248 is absent in mice deficient in EP3 receptor (15). For further details see SI Materials and Methods.
Statistics.
Behavioral groups were compared with multivariate ANOVA and post hoc t tests with Bonferroni correction. In in vivo recordings, the Wilcoxon matched pairs signed rank test and the Mann–Whitney U test were used. Patch clamp data were analyzed with paired t test and Fisher's exact test. Significance was accepted at P < 0.05.
Supplementary Material
Acknowledgments
The authors thank Mrs. Wallner, Mrs. Ernst, Mrs. Bernhardt, and Mrs. Hitschke for excellent technical help. The work was supported by the Deutsche Forschungsgemeinschaft (SCHA 404/13-2). All EP agonists and antagonists were provided by Ono Pharmaceutical.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300820110/-/DCSupplemental.
References
- 1.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012;15(8):1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinold H, et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115(3):673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samad TA, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410(6827):471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 6.Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–583. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- 7.Vanegas H, Schaible H-G. Prostaglandins and cyclooxygenases in the spinal cord. Prog Neurobiol. 2001;64:327–363. doi: 10.1016/s0301-0082(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 8.Popp L, et al. Comparison of nociceptive behavior in prostaglandin E, F, D, prostacyclin and thromboxane receptor knockout mice. Eur J Pain. 2009;13(7):691–703. doi: 10.1016/j.ejpain.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Bär K-J, et al. Changes in the effect of spinal prostaglandin E2 during inflammation: Prostaglandin E (EP1-EP4) receptors in spinal nociceptive processing of input from the normal or inflamed knee joint. J Neurosci. 2004;24(3):642–651. doi: 10.1523/JNEUROSCI.0882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CR, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther. 2006;319(3):1096–1103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, et al. Prostaglandin E2-induced modification of tetrodotoxin-resistant Na+ currents involves activation of both EP2 and EP4 receptors in neonatal rat nodose ganglion neurones. Br J Pharmacol. 2005;145(4):503–513. doi: 10.1038/sj.bjp.0706212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta. 1995;1259(1):109–119. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto H, et al. Novel four selective agonists for prostaglandin E receptor subtypes. Prostaglandins Other Lipid Mediat. 1999;59:150. [Google Scholar]
- 15.Ueta M, Matsuoka T, Narumiya S, Kinoshita S. Prostaglandin E receptor subtype EP3 in conjunctival epithelium regulates late-phase reaction of experimental allergic conjunctivitis. J Allergy Clin Immunol. 2009;123(2):466–471. doi: 10.1016/j.jaci.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Abrahamsen B, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321(5889):702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 17.Akopian AN, et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2(6):541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 18.Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA. 1998;95(15):8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495(Pt 2):429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18(24):10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amano H, et al. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197(2):221–232. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacharowski K, et al. Selective activation of the prostanoid EP(3) receptor reduces myocardial infarct size in rodents. Arterioscler Thromb Vasc Biol. 1999;19(9):2141–2147. doi: 10.1161/01.atv.19.9.2141. [DOI] [PubMed] [Google Scholar]
- 23.Vasko MR, Campbell WB, Waite KJ. Prostaglandin E2 enhances bradykinin-stimulated release of neuropeptides from rat sensory neurons in culture. J Neurosci. 1994;14(8):4987–4997. doi: 10.1523/JNEUROSCI.14-08-04987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasko MR, Zirkelbach SL, Waite KJ. Prostaglandins stimulate the release of substance P from rat spinal cord slices. Prog Pharmacol Clin Pharmacol. 1993;10:69–89. [Google Scholar]
- 25.Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5(1):34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- 26.Baba H, Kohno T, Moore KA, Woolf CJ. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J Neurosci. 2001;21(5):1750–1756. doi: 10.1523/JNEUROSCI.21-05-01750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: Future directions. Arthritis Rheum. 2013;65(2):291–302. doi: 10.1002/art.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arendt-Nielsen L, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Schaible H-G. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep. 2012;14(6):549–556. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]
- 31.Zayed N, et al. Inhibition of interleukin-1beta-induced matrix metalloproteinases 1 and 13 production in human osteoarthritic chondrocytes by prostaglandin D2. Arthritis Rheum. 2008;58(11):3530–3540. doi: 10.1002/art.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telleria-Diaz A, et al. Different effects of spinally applied prostaglandin D2 on responses of dorsal horn neurons with knee input in normal rats and in rats with acute knee inflammation. Neuroscience. 2008;156(1):184–192. doi: 10.1016/j.neuroscience.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Grill M, Heinemann A, Hoefler G, Peskar BA, Schuligoi R. Effect of endotoxin treatment on the expression and localization of spinal cyclooxygenase, prostaglandin synthases, and PGD2 receptors. J Neurochem. 2008;104(5):1345–1357. doi: 10.1111/j.1471-4159.2007.05078.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones RL, Giembycz MA, Woodward DF. Prostanoid receptor antagonists: Development strategies and therapeutic applications. Br J Pharmacol. 2009;158(1):104–145. doi: 10.1111/j.1476-5381.2009.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama Y, Omote K, Kawamata T, Namiki A. Role of prostaglandin receptor subtype EP1 in prostaglandin E2-induced nociceptive transmission in the rat spinal dorsal horn. Brain Res. 2004;1010(1–2):62–68. doi: 10.1016/j.brainres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Neugebauer V, Lücke T, Schaible H-G. N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat’s knee joint. J Neurophysiol. 1993;70(4):1365–1377. doi: 10.1152/jn.1993.70.4.1365. [DOI] [PubMed] [Google Scholar]
- 37.Boettger MK, et al. Antinociceptive effects of TNF-α neutralization in a rat model of antigen-induced arthritis. Arthritis Rheum. 2008;58:2368–2378. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.