Pannexin-1 hemichannels as key regulators of HIV infection and replication in human T lymphocytes.
Keywords: AIDS, channels, disease
Abstract
HIV is a major public health issue, and infection of CD4+ T lymphocytes is one of its key features. Whereas several cellular proteins have been identified that facilitate viral infection and replication, the role of hemichannels in these processes has not been fully characterized. We now show that the HIV isolates, R5 and X4, induced a transient-early (5–30 min) and a later, persistent (48–120 h) opening of Panx1 hemichannels, which was dependent on the binding of HIV to CD4 and CCR5/CXCR4 receptors. Blocking Panx1 hemichannels by reducing their opening or protein expression inhibited HIV replication in CD4+ T lymphocytes. Thus, our findings demonstrate that Panx1 hemichannels play an essential role in HIV infection.
Introduction
HIV infects mostly immune cells by binding the viral env protein gp120 to the host cellular proteins, CD4 and CCR5 and/or CXCR4, resulting in fusion of the viral env with the cellular membrane. To date, in addition to CD4, CCR5, and/or CXCR4, no other plasma membrane proteins have been identified to participate directly in the process of viral entry [1]. However, a few studies indicated that upon binding of the virus to its cellular receptors, intracellular-free Ca2+ levels rise [2–4], and opening of nonselective cation channels, as well as Ca2+-activated K+ channels, occurs [5], suggesting that signaling and activation of other proteins may be required for infection and replication. Recently, studies in cell lines, PBMCs, and human macrophages indicated that ATP release through pannexin-1 (Panx1) hemichannels is required for HIV replication [6, 7], supporting the hypothesis that additional host proteins are required for infection/replication.
Hemichannels are plasma membrane channels that can be opened at the unapposed cell surface, forming aqueous conduits permeable to ions and small molecules (e.g., ATP, glutamate, NAD+, and PGE2). They allow diffusional exchange between the intra- and extracellular compartments, constituting a route for autocrine/paracrine cellular communication [8]. Hemichannels are constituted by the oligomerization of six protein subunits, termed Cxs or Panxs, both highly conserved protein families encoded by 21 or three genes in humans, respectively [9, 10]. Panx1 hemichannels, in concert with purinergic receptors, have been described to be important in different immune functions, including cellular activation [11–13], apoptosis [14], stress signals [15], secretion of inflammatory cytokines [16], and HIV replication [7]. However, how HIV infection changes the opening of these channels in primary human CD4+ T lymphocytes—one of the main targets of HIV—remains to be elucidated. Here, we show that depending on the HIV isolate, the interaction of CD4 and CCR5 and/or CXCR4 is crucial for the biphasic Panx1 hemichannel opening induced by HIV in human primary CD4+ T lymphocytes. We also found that chemokines that bind CCR5 and CXCR4 increase Panx1 hemichannel activity, but only early, and more transiently, as compared with the biphasic opening of Panx1 hemichannels in response to HIV infection. Down-regulation or pharmacological blockade of Panx1 hemichannels inhibited HIV replication in CD4+ T lymphocytes, demonstrating that the opening of these channels is essential for HIV replication.
MATERIALS AND METHODS
Materials
RPMI medium, FBS, penicillin/streptomycin, and trypsin-EDTA were purchased from Gibco-BRL/Invitrogen (Carlsbad, CA, USA). The HIV isolates, CXCR4 and CCR5 blockers, and blocking antibodies were obtained from NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID (Germantown, MD, USA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise designated. Cx43E2, an antibody that blocks Cx43 hemichannels, was kindly provided by Dr. Jean Jiang (Department of Biochemistry, University of Texas Health Science Center at San Antonio, TX, USA).
Isolation of human PBMCs and CD4+ T lymphocytes
Anticoagulated blood was obtained from leukopacks obtained from the New York and New Jersey Blood Centers, as described previously [17]. PBMCs were isolated by underlayering with Ficoll-Paque (Amersham Bioscience, Uppsala, Sweden), according to the procedure described by the manufacturer. Purified PBMCs used in these studies were ∼10% monocytes and ∼90% lymphocytes and other hematopoietic cells, as determined by incubation with premixed human CD45 and CD14 mAb conjugated to FITC and PE, respectively (1:50; Caltag Laboratories, Burlingame, CA, USA), followed by FACS, as we described previously [18]. To obtain a pure population of CD4+ T lymphocytes (92–99% CD4+ T lymphocytes), monocytes were removed from PBMCs by using CD14-coupled magnetic beads, and subsequently, CD4+ T lymphocytes were isolated with CD4-coupled magnetic beads (Stemcell Technologies, Vancouver, BC, Canada, or Miltenyi Biotec, Germany). We also isolated CD4 cells by negative selection and CD3 cells by positive selection to discount any effects of the isolation process on infectivity or viral replication. No differences were found as compared with positive selection.
HIV infection and quantification of replication
Cell-free viral inocula were obtained from NIH AIDS Research and Reference Reagent Program. Three isolates were used: HIVADA and HIVBal, two R5 isolates, and HIVLAI, an X4 isolate. PBMCs and purified CD4+ T lymphocytes were isolated and activated with PHA (5 μg/ml) plus IL (IL-2; 10 U/ml) in RPMI 1640 for 48 h in polypropylene tubes at a density of 2 × 106 cells/ml. PBMCs and CD4+ T lymphocytes were then infected by incubation for 2 or 24 h with viral stocks of HIVADA (20 ng p24/ml/1×106 cells), HIVLAI (20 ng p24/ml/1×106 cells), HIVBal (12 ng/ml p24/ml/1×106 cells) or pseudotyped with env protein from R5 JRFL (20 ng p24/ml/1×106 cells), washed thoroughly, resuspended in fresh medium, and maintained in polypropylene tubes. No differences in the Panx1 hemichannel opening induced by the virus were detected when infection was performed for 2 or 24 h (data not shown). Uninfected control cells were activated with PHA plus IL-2 for 48 h, washed, and maintained in polypropylene tubes in fresh media without any virus. Experiments using chemokines did not show any differences in the Panx-1 hemichannel opening in the presence or absence of PHA or IL-2 (data not shown). To determine the levels of HIV infection, the amount of HIV-p24 released into the medium was determined by ELISA (PerkinElmer, Boston, MA, USA).
Dye uptake and time-lapse fluorescence imaging
To characterize the functional state of Panx1 hemichannels, dye-uptake experiments using ethidium (Etd) bromide were performed as described previously [19, 20]. Cells were washed twice in HBSS and then exposed to Locke's solution (containing 154 mM NaCl, 5.4 mM KCl, 2.3 mM CaCl2, 5 mM HEPES, and pH 7.4) with 5 μM Etd, and time-lapse microscopy was performed. Phase-contrast and fluorescence microscopy with time-lapse imaging were used to record cell appearance and fluorescence-intensity changes in each condition. Fluorescence was recorded every 30 s. The NIH ImageJ program was used for off-line image analysis and fluorescence quantification. For data representation and calculation of Etd uptake slopes, the average of two independent FBs (expressed as AU) was subtracted from F1. Results of this calculation (F1−FB), for at least 20 cells, were averaged and plotted against time (expressed in minutes). Slopes were calculated using Microsoft Excel software and expressed as AU/min. Microscope and camera settings remained the same in all experiments. Dead cells or cells with a damaged plasma membrane were clearly identified during the time-lapse microscopy as a result of their nonspecific Etd uptake, determined by lack of time dependency and stability in dye uptake (not inhibited by hemichannel blockers), and were not quantified.
Flow cytometry
To measure Etd uptake in CD4+ T cell populations, FACS analysis was performed in cells under control conditions or after HIV exposure at several time-points. Etd uptake experiments were performed in CD4+ lymphocytes (3–5×105), and after the respective time of Etd uptake, cells were fixed with 2% paraformaldehyde for 30 min at room temperature, incubated with 1% BSA in PBS for 30 min, and stained with CD4 antibody for 30 min at 4°C. For analyses of Etd-positive populations, 10,000 events were acquired on a FACSCanto II or Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo (TreeStar, Ashland, OR, USA) software, as we described previously [18, 21].
siRNA and peptides
Three unique 27mer siRNA duplexes against human Panx1 were predesigned and obtained from Origene (Rockville, MD, USA). siRNA (10 nM) transfection was performed with Oligofectamine (Invitrogen, Life Technologies, Carlsbad, CA, USA), according to the Origene application guide for Trilencer-27 siRNA, and minimal cell death was detected after transfection. Experiments were carried out 2 days post-transfection. In addition, we used a siRNA to Cx43 that targeted an irrelevant protein not involved in HIV infection of immune cells. siRNA to Cx43 did not change HIV infection or replication (data not shown). The sequences of the siRNA are from Origene. The Panx1 mimetic blocking peptide 10Panx1 (WRQAAFVDSY) and the scramble peptide (FADRYWAQVS) were synthesized by PeproTech (Rocky Hill, NJ, USA).
qRT-PCR
Cultured CD4+ T lymphocytes (2×106 cells/ml in 5 ml) were transfected with oligofectamine (Invitrogen, Life Technologies) containing siRNA A, B, and C (Origene) for 2 days, as described above. Total RNA was extracted using TRIzol (Invitrogen, Life Technologies) and the phase-lock system (Eppendorf, Hauppauge, NY, USA), following the manufacturer's instructions. cDNA synthesis was performed using 2 μg total RNA using the iSript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), according to the manufacturer's instructions. The amplified cDNA was used to amplify and quantify GAPDH and Panx1 mRNA expression by qPCR using ABsolute Blue qPCR SYBR low ROX mix in a StepOnePlus thermocycler (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). The primers used correspond to GAPDH forward: 5′-GAGAAGTATGACAACAGCCTCAA-3′, GAPDH reverse: 5′-AGTCCTTCCACGATACCAAAG-3′; Panx1 forward: 5′-AGAAGAATGCCCGACAGAGA-3′, Panx 1 reverse: 5′-TTGCAAACCAGCTGTGAAAC-3′. The program used was denaturation for 15 min at 95°C and 40 cycles of denaturation, 15 s at 95°C; anneal, 30 s at 60°C; and amplification, 30 s at 72°C. Expression was determined using the ΔΔCT method (Applied Biosystems, Life Technologies), according to the CT values.
Statistical analysis
Student's two-tailed, paired t-test was used to compare the different groups. A value of P < 0.005 was considered significant.
RESULTS
HIV infection of primary human PBMCs and CD4+ T lymphocytes induces opening of Panx1 hemichannels but not of Cx43 hemichannels
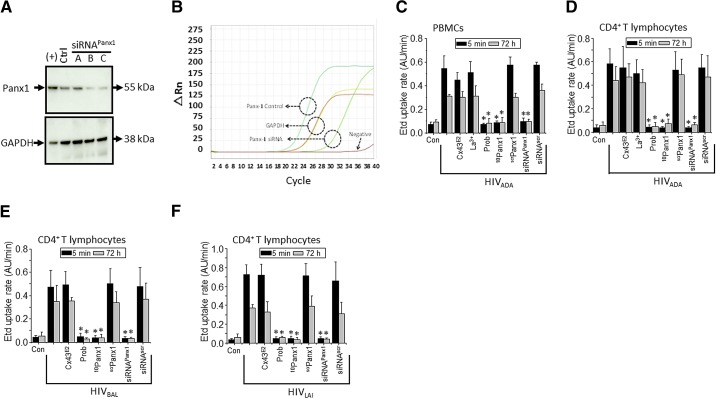
To identify the HIV-induced hemichannel opening in PBMCs and CD4+ T lymphocytes, we evaluated the effect of two R5 viruses—HIVADA and HIVBal, 20 ng/ml and 12 ng/ml, respectively—and one X4 virus—HIVLAI, 20 ng/ml—by measuring the rate of uptake of Etd (5 μM). Etd only crosses the plasma membrane in healthy cells by passing through specific large channels, such as Cx and Panx hemichannels, and its intracellular fluorescence is reflective of channel opening [22–24]. Our results indicated that HIVADA induced an early and transient (5–30 min) increase in the Etd uptake rate that returned to near-baseline levels at ∼30 min and remained at that level for the following 24 h in PBMCs and CD4+ T lymphocytes (Fig. 1A and B). At 48 h, the Etd uptake rate increased, reaching maximal values at 72 h, which was subsequently maintained or gradually decreased to control values up to 120 h, the last time-point assayed, depending on the viral isolate used (Fig. 1A and B). In addition, HIVBal and HIVLAI induced similar responses of Etd uptake in response to HIV infection in PBMCs, CD3+ (not shown), and CD4+ T lymphocytes (Fig. 1C and D). HIVBal is a purified virus that lacks cytokines and other factors released that may be present in HIV stock-culture supernatants. We also purified the LAI virus, an X4-dependent virus, by ultracentrifugation and had similar results in Etd uptake compared with the unpurified virus (data not shown). Thus, our data indicated that HIV and no contaminant from the virus isolation process induced the hemichannel opening. No significant changes in Etd uptake were detected in uninfected cells (Fig. 1A–D). Representative examples of Etd uptake in single experiments at 5 min (Fig. 1E) or after 72 h (Fig. 1F) of HIVADA exposure are shown. We detected a minimal opening of the Panx1 hemichannel in untreated conditions, but the mechanisms involved in this basal opening require further examination.
Figure 1. HIV increases Etd uptake in a biphasic manner in human primary PBMCs and CD4+ T lymphocytes.
(A–D) Time course of Etd uptake rate obtained from PBMCs or CD4+ T lymphocytes under control conditions (squares) or after infection with HIV (circles), 0–120 h. PBMCs infected with HIVADA (A) and CD4+ T lymphocytes infected with HIVADA (B), HIVBal (C), or HIVLAI (D). (E and F) Representative time-lapse measurements of Etd uptake in CD4+ T lymphocytes under control conditions (squares) or after 5 min (E) or 72 h (F) of exposure to HIVADA (circles). No differences were observed between R5 and X4 isolates. *P < 0.005; **P < 0.002 ; ***P = 0.0001 denote significance as compared with control conditions. Each value corresponds to the mean ± sd of the Etd intracellular intensity, present in at least 20 cells/time-point (n=4).
To identify whether Panx1 hemichannels were involved in the HIV-induced Etd uptake in PBMCs and CD4+ T lymphocytes, we down-regulated Panx1 protein expression using siRNA. Three siRNAs (siRNA A, B, and C) to Panx1 were tested by transfection of CD4+ T lymphocytes, as described in Materials and Methods. Each siRNA reduced Panx1 protein expression, as determined by Western blot (Fig. 2A). The siRNA B and C to Panx1 were the most effective in reducing Panx1 protein expression in CD4+ T lymphocytes (Fig. 2A). Thus, siRNA B or C to Panx1 was used in the subsequent experiments. qRT-PCR experiments confirmed that transfection of siRNA C into CD4+ T lymphocytes reduced Panx1 mRNA expression from a CT value of 12.3 ± 0.7 cycles in control conditions to a CT of 23.5 ± 2.5 cycles after transfection of siRNA C (Fig. 2B). Amplification of GAPDH as a loading control did not show any significant alterations in CT values, as values for control conditions were 16.7 ± 0.2 and after Panx1 siRNA transfection, were 16.9 ± 0.2 (Fig. 2B; n=3). Based on the kinetics of the Panx1 hemichannel opening in response to HIV, we selected two representative time-points of the early and late events of opening to perform blocking experiments (5 min and 72 h). Knockdown of Panx1 using siRNA in PBMCs and CD4+ T lymphocytes reduced the Etd uptake rate induced by HIVADA (Fig. 2C and D), HIVBal (Fig. 2E), and HIVLAI (Fig. 2F) to almost undetectable control levels (uninfected cells) at the two representative time-points: 5 min and 72 h (Fig. 2C–F). scrPanx1 siRNA or siRNA to Cx43 (data not shown), a protein not involved in HIV infection or replication, did not alter the HIV-induced increase in Etd uptake rate (Fig. 2C–F; siRNAscr). To support further the involvement of Panx1 hemichannels in the HIV-induced Etd uptake rate, specific blockers against these channels were used. Two Panx1 hemichannel blockers—the mimetic peptide 10Panx1 (200 μM) and Prob (500 μM) [25, 26]—completely reduced the HIVADA-induced Etd uptake rate in PBMCs (Fig. 2C) and CD4+ T lymphocytes (Fig. 2D). Similar results were obtained when these blockers were used to inhibit the Etd uptake rate induced by HIVBal and HIVLAI in CD4+ T lymphocytes (Fig. 2E and F). No toxic or nonspecific effects of these blockers alone were detected at all time-points, as determined by trypan blue and TUNEL staining (data not shown). All of these results indicate that Panx1 hemichannels are responsible for the HIV-induced Etd uptake in PBMCs and CD4+ T lymphocytes. In contrast, La3+, a general Cx hemichannel blocker, or Cx43E2, an antibody that blocks Cx43 hemichannels at several concentrations, as described previously [23, 27, 28], did not affect the HIV-induced Etd uptake rate (Fig. 2C–F), suggesting that Cx43 hemichannels do not participate in this process.
Figure 2. HIV increases the activity of Panx1 hemichannels in human primary CD4+ T lymphocytes.
(A) Total levels of Panx1 in CD4+ T lymphocytes under control conditions (Ctrl) or after transfection for 48 h with three different siRNAs (siRNA A, B, and C) to Panx1 hemichannels were analyzed by Western blot. HeLa cells transfected with Panx1 were used as a positive control of Panx1 protein expression [(+)]. Loading controls, analyzed by probing for GAPDH, are also shown. (B) Representative example of qRT-PCR curves of Panx1 mRNA expression in control (cyan line) and after transfection of siRNA to Panx1 (light green line). GAPDH was used as a control (yellow and red lines). The negative control without enzyme did not show any amplification (dark red line). (C and D) Etd uptake rate for PBMCs exposed to HIVADA (C); CD4+ T lymphocytes exposed to HIVADA (D), HIVBal (E), or HIVLAI (F) after 5 min (black bars) or 72 h (gray bars). (C–F) Control (Con), uninfected conditions are shown. Addition of Cx43 hemichannel blockers, Cx43E2 (1:500 dilution) and La3+ (200 μM), did not affect the Etd uptake rate induced by the virus. In contrast, Panx1 hemichannel blockers, Prob (500 μM) and 10Panx1 (200 μM), completely blocked Etd uptake rate induced by the virus. The negative control using a scrPanx1 peptide (200 μM) did not affect Etd uptake rate induced by the virus. Cells were transfected with siRNA C for Panx1 or with the appropriate siRNAscr (both 10 nM), and Etd uptake rate was analyzed. siRNA for Panx1, but not scramble control, reduced Etd uptake rate induced by the virus. Each value corresponds to the mean ± sd of the Etd intracellular intensity present in at least 20 cells/time-point (n=4; *P<0.005).
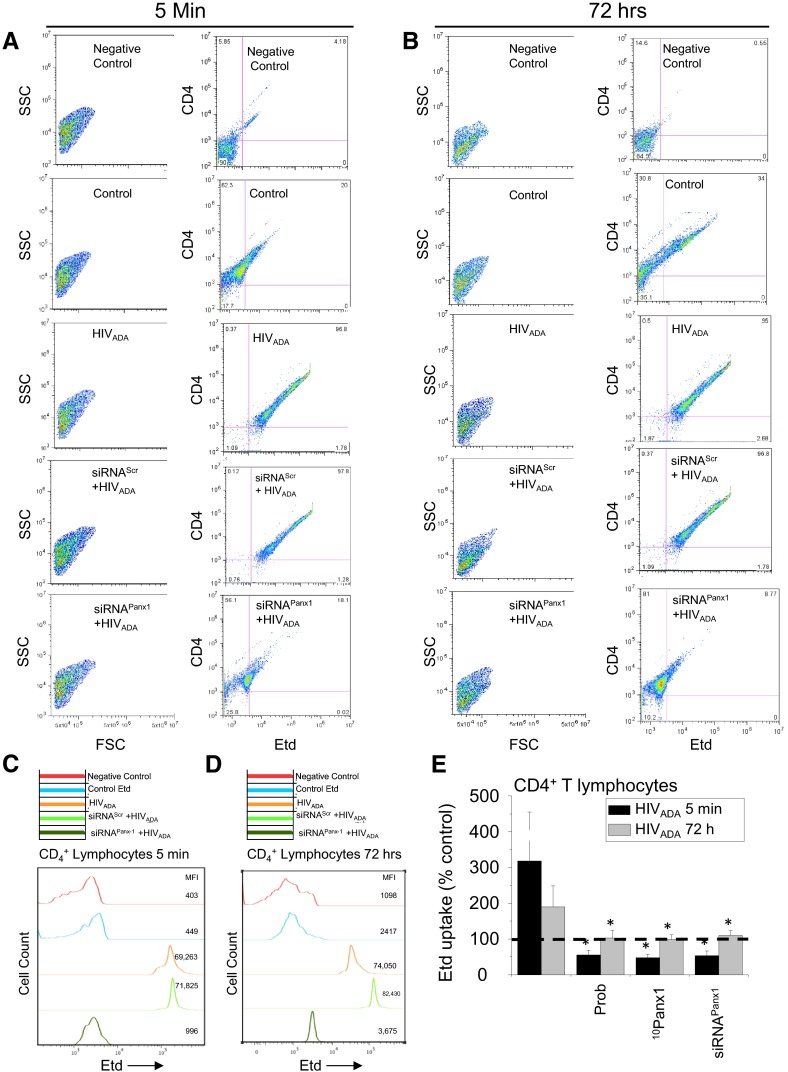
We also used FACS analyses to demonstrate that HIV induces Panx1 hemichannel activity in CD4+ T lymphocytes. FACS analyses were performed on CD4+ T lymphocytes used for Etd uptake and live cell imaging for 5 min and 72 h post-HIVADA exposure. The first column of Fig. 3A and B corresponds to dot plots representing the purity and populations of cells analyzed at both time-points (Fig. 3A and B). The second column represents the CD4 and Etd staining at both time-points. HIVADA infection of CD4+ T lymphocytes showed a prominent Etd uptake in all cells at 5 min (Fig. 3A; HIVADA) and 72 h (Fig. 3B; HIVADA) compared with uninfected cells (Fig. 3A and B; control cells) or non-Etd-treated cells (Fig. 3A and B; negative control). Moreover, the increase in Etd uptake induced by the exposure to HIVADA (Fig. 3A and B; HIVADA) was blocked by Panx1 siRNA (Fig. 3A and B). Histogram analyses after 5 min (Fig. 3C) and 72 h (Fig. 3D) postinfection summarize the data showed in the dot plots. Figure 3E summarizes all of the data obtained using Etd uptake analyzed by FACS. HIV infection increased Etd uptake (representative time-points are shown, 5 min and 72 h) in a Panx1 hemichannel-dependent manner in CD4+ T lymphocytes. Blockers of Panx1 hemichannels, including Prob, 10Panx1, and siRNA, reduced to control conditions (100%) Etd uptake induced by the virus at both time-points (Fig. 3E).
Figure 3. HIV exposure/infection of CD4+ T lymphocytes results in opening of Panx1 hemichannels as quantified by FACS.
(A and B) FACS analysis showing Etd uptake in CD4+ T lymphocytes after 5 min (A) or 72 h (B) of HIV exposure. (A and B, left) Side-scatter (SSC) versus forward-scatter (FSC) of the cells to demonstrate the purity and selection of the CD4+ population of lymphocytes; (right) CD4 staining versus Etd uptake for each condition. Unstained Etd conditions (Negative Control) showed no staining for Etd, whereas untreated cells (Control) showed minimal Etd uptake compared with HIVADA-treated cultures (HIVADA) for 5 min (A) or 72 h (B). Cells transfected with siRNA for Panx1 abolished the Etd-uptake increase induced by the virus at both time-points (A and B; siRNAPanx1+HIVADA). (C and D) Representative histograms of FACS staining using unstained cells (Negative Control, red lines), control conditions with Etd (blue lines), after exposure to HIVADA (orange lines), after siRNAscr + HIVADA (light green lines), or after siRNAPanx1 + HIVADA (dark green lines). MFI, Mean fluorescence intensity. (E) Quantification of data from five independent experiments using FACS analysis showed that blocking of Panx1 hemichannels with Prob, 10Panx1, or siRNAPanx1 reduced Etd uptake induced by HIVADA in CD4+ T lymphocytes. Each value corresponds to the mean ± sd of the Etd intracellular intensity, present in at least 20 cells/time-point (n=4 independent CD4+ T lymphocyte isolations; *P<0.005).
Binding of HIV to its cellular receptors—CD4 and CCR5 or CXCR4—results in opening of Panx1 hemichannels in CD4+ T lymphocytes
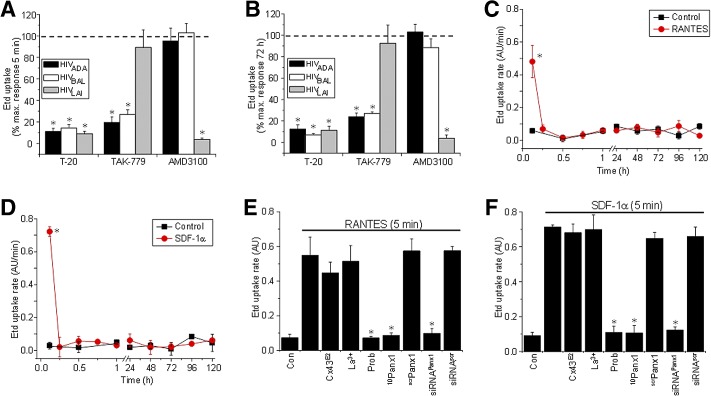
HIV entry into immune cells is dependent on viral tropism mediated by interaction of the viral envelope with CD4 and CCR5 and/or CXCR4. To evaluate whether binding of the virus to its receptors was required for opening of Panx1 hemichannels, Etd uptake experiments were performed in human CD4+ T lymphocytes exposed to HIVADA (CCR5-dependent), HIVBal (CCR5-dependent), or HIVLAI (CXCR4-dependent) in the presence or absence of CD4 or CCR5 receptor blockers. Trimeris/T-20 (Roche, Basel, Switzerland; obtained from the AIDS NIH Repository; 1 μg/ml), a blocker of viral fusion mediated by CD4 receptors in CD4+ T lymphocytes, inhibited the Etd uptake induced by all three viruses tested at 5 min and 72 h postinfection (Fig. 4A and B). TAK-779 (100 ng/ml; [29]), a potent inhibitor of the binding of HIV to its coreceptor CCR5 [30], or AMD3100 [31], a potent inhibitor of the binding of HIV to its coreceptor CXCR4 (300 ng/ml; [29]), reduced the Etd uptake, according to the viral isolate used (Fig. 4A and B). In addition, experiments using a pseudotyped virus with env protein from R5 JRFL only recapitulated the early events of the Panx1 hemichannel opening and not the latter events (Supplemental Fig. 1C). These results demonstrate that the HIV-induced Panx1 hemichannel activity requires binding of the virus to CD4, as well as to its corresponding chemokine receptor.
Figure 4. The HIV-induced Panx1 hemichannel opening occurs by a CD4 and a CCR5/CXCR4-binding/fusion-dependent mechanism.
A fusion inhibitor (T-20), CCR5 blocker (TAK-779), or CXCR4 blocker (AMD3100) on Etd uptake, induced by HIVADA (black bars), HIVBal (white bars), and HIVLAI (gray bars) infection at 5 min (A) and 72 h (B) of exposure. Data are presented as percent of maximal response of Etd uptake (% max. response). (C and D) Etd uptake rate obtained from CD4+ T lymphocytes under control conditions (squares) or after a single addition of the following chemokines (circles): RANTES/CCL5 (C) or SDF-1α/CXCL12 (D). (E and F) The effects on Etd uptake of the Cx43 hemichannel blockers, Cx43E2 (1:500 dilution) and La3+ ion (200 μM), or Panx1 hemichannel blockers, Prob (500 μM), 10Panx1 (200 μM), siRNA to Panx1, as well as negative control scrPanx1 (200 μM) or siRNAscr, are shown (E and F; represent 5 min after chemokine treatment). *P < 0.005. Each value corresponds to the mean ± sd of Etd intracellular intensity in at least 20 cells/time-point (n=4 for all experiments).
Binding of chemokines to the receptor CCR5 or CXCR4 results in a transient Panx1 hemichannel opening
To examine whether chemokines that bind CCR5 or CXCR4 also induced opening of Panx1 hemichannels, a time course of Etd uptake, as described in Fig. 1, was performed. Addition of RANTES/CCL5 (100 ng/ml), a physiological ligand for CCR5, resulted in a fast and extremely transient increase (only 5 min) of Etd uptake in CD4+ T lymphocytes (Fig. 4C) compared with HIV exposure. However, HIV maintained the opening of the Panx1 hemichannel for a longer time, up to 30 min, compared with the chemokines (compare with Fig. 1), suggesting a different mechanism of opening. Similar results were obtained for MIP-1α/CCL3 (100 ng/ml) and MIP-1β/CCL4 (100 ng/ml), both ligands for CCR5 (data not shown). In addition, the chemokine ligand for CXCR4, SDF-1α/CXCL12 (100 ng/ml), induced a similar transient increase in Etd uptake (Fig. 4D), as occurred with RANTES (Fig. 4C). However, no chemokine treatment reproduced the later stages of Etd uptake induced by HIV infection (Fig. 4C and D; compared with Fig. 1). The increase in Etd uptake induced by chemokines in CD4+ T lymphocytes was reduced by Panx1 hemichannel blockers, but not by Cx43 hemichannel blockers, in a similar manner as seen for HIV infection (Fig. 4E and F). Negative controls using scrambled peptides or siRNAscr did not alter the Etd uptake induced by these chemokines (Fig. 4E and F). In addition, we found that the purified viral env protein gp120 (1 μg/ml) also induced a transient Etd uptake at 5 min (Fig. 1A and Supplemental Fig. 1). The gp120-induced Etd uptake was inhibited by using Prob, 10Panx1 and siRNAPanx1, demonstrating that Panx1 hemichannels are opened in response to gp120s binding (Fig. 1B and Supplemental Fig. 1A and B). No effects of scramble peptides (scrPanx1) or siRNAscr were detected (Fig. 1B and Supplemental Fig. 1A and B). These data indicate that all known physiological ligands for CCR5 and CXCR4, as well as a viral env protein, increase the Panx1 hemichannel opening in CD4+ T lymphocytes. However, our results using functional virus, and the fusion blocker T-20 indicated that there are at least three mechanisms by which Panx1 hemichannels can be open. The first mechanism is dependent of Panx1 channel opening mediated by binding chemokines to their receptors. A second mechanism mediated by binding gp120 to CD4 and to the respective chemokine receptor. Lastly, the Panx1 hemichannel opening mediated by the virus in a long-lasting and biphasic manner, suggesting that other stages of the viral life cycle, such as fusion, are also required for opening of these channels.
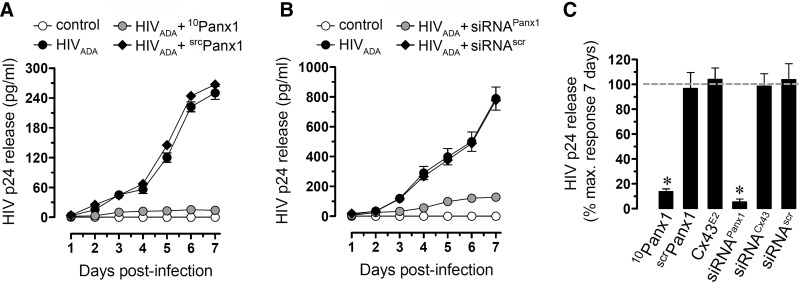
Opening of Panx1 hemichannels in response to HIV infection is required for efficient HIV replication in CD4+ T lymphocytes
To examine whether opening of Panx1 hemichannels induced by HIV was required for viral replication in CD4+ T lymphocytes, we used the mimetic peptides 10Panx1 and siRNA to Panx1. These treated cells were then infected with HIVADA, and viral replication was determined by HIV-p24 ELISA of supernatants collected each day for 7 days, as we described previously [18, 21, 32, 33]. 10Panx1 (200 μM) and the Panx1 siRNA dramatically reduced HIV replication in CD4+ T lymphocytes at all times tested (Fig. 5A–C). Negative controls using scrPanx1, siRNA to Cx43, or siRNAscr did not alter HIV replication (Fig. 5C, and data not shown). Thus, activation of Panx1 hemichannels in CD4+ T lymphocytes is essential for HIV infection and replication.
Figure 5. Blockade of Panx1 hemichannels abolishes HIV replication in CD4+ T lymphocytes.
(A and B) ELISA for HIV-p24 release in uninfected (squares) or HIVADA-infected (circles) CD4+ T lymphocytes. (A) Blockade of Panx1 hemichannels by using mimetic peptides (10Panx1, 200 μM, upright triangles) or (B) knockdown of Panx1 by using siRNA (upright triangles, 10 nM) abolished HIV replication to almost control, undetectable levels (squares). Scrambled peptides and siRNAscr (downward triangles) did not alter viral replication. (C) Summary of three independent experiments after 7 days postinfection of CD4+ T lymphocytes. HIV-p24 release into the media in the presence or absence of the Cx43 hemichannel blocker Cx43E2 (1:500 dilution), Panx1 hemichannel blocker, 10Panx1 (200 μM), and scrPanx1 (200 μM) are shown (*P<0.005). No differences in viability were observed among the different conditions. Each value corresponds to the mean ± sd of the Etd uptake intensity of at least 20 cells/time-point (n=4 for all experiments).
DISCUSSION
Our results demonstrate that HIV causes opening of Panx1 hemichannels in a biphasic manner during infection of PBMCs and CD4+ T lymphocytes. The mechanism of the Panx1 hemichannel opening in response to HIV involved binding/fusion of the virus to its receptors CD4 and CCR5/CXCR4. However, opening of Panx1 hemichannels was not dependent on differences in viruses using X4 and R5 viral strains. T cells express higher amounts of CXCR4; thus, other proteins, including CD4, may also be playing a role in promoting opening of Panx1 hemichannels. In addition, we found that chemokines that bind CCR5 and CXCR4, as well as the recombinant viral env protein gp120, resulted in a more transient opening of Panx1 hemichannels (only 5 min) compared with the early opening of the channels in response to HIV infection (5–30 min). Chemokines, recombinant viral env protein gp120, or a single round-replication virus did not mimic the later events of Panx1 hemichannel opening (48–120 h). We also demonstrated that the Panx1 hemichannel opening was required for HIV replication in CD4+ T lymphocytes. Thus, we propose that Panx1 hemichannels are critical host proteins required for HIV infection and replication in CD4+ T lymphocytes.
HIV infects immune cells by binding its env protein gp120 to host CD4 and then CCR5 and/or CXCR4 receptors, depending on the viral tropism [34–37]. In addition to the well-described binding of HIV to its receptors and viral fusion, signaling in response to binding of gp120 occurs, such as increased intracellular-free Ca2+ and G-protein signaling [2–4] and opening of nonselective cation channels and Ca2+-activated K+ channels [5]. The identity of these nonselective cation channels remained unknown. We now propose that the Panx1 hemichannel is one of these channels.
It was estimated that four to six CCR5 receptors and several CD4 receptors need to cluster together to bind several HIV env proteins to form a fusion pore [38, 39]. The probability of several CD4 and CCR5 and/or CXCR4 molecules coming together naturally in the membrane is low, so there must be a cellular response that facilitates the formation of this fusion pore [40], likely by a mechanism that involves lipid rafts and actin rearrangements [41–43]. We propose that some of these functions are controlled by opening of Panx1 hemichannels in response to HIV exposure. Opening of Panx1 hemichannels results in changes in ionic gradients, as well as release of several factors, including ATP [12–14, 19, 44], which is a regulator of the channel but also is a signaling molecule that can activate purinergic and adenosine receptors in an autocrine and paracrine manner [11, 24, 45, 46]. In agreement, P2Y2 receptors have been implicated recently in HIV replication in T cell lines and PBMCs [7]. Our results in human macrophages demonstrated that ATP and purinergic receptors are essential for HIV infection and replication [6, 47]. All chemokines that bind CCR5 or CXCR4 opened Panx1 hemichannels transiently (only 5 min), whereas the time course of opening induced by the virus was longer, suggesting key differences in signaling and regulation of Panx1 hemichannel gating activated by HIV and chemokines. In agreement, calcium imaging studies demonstrated that HIV gp120 protein as well as chemokines that bind CCR5 and CXCR4 elicit different calcium signaling, which likely depends on binding characteristics to the respective chemokine receptors [5]. The later events of activation of Panx1 hemichannels and the molecules that induce this constant opening from 24 h to 72 h postinfection are unknown. These differences in signaling are unknown and warrant further investigation to dissect both mechanisms. However, we propose that part of this Panx1 hemichannel opening is mediated by the synthesis of new viral particles and subsequent infection of new and superinfection of previously infected cells. In agreement, infection of CD4+ lymphocytes with a single-round replicating virus only recapitulated the initial opening of Panx1 hemichannels but not the second, later events.
Our proposed mechanism of how and when Panx1 hemichannels participate in HIV infection and replication in CD4+ T lymphocytes is that upon binding/fusion of the virus to its host cellular receptors, signaling occurs, allowing the virus to infect the target cell. These signals result in opening of Panx1 hemichannels, resulting in changes in ionic gradients and release of second messengers, including ATP and its subproducts that activate extracellular ATP, ADP, and adenosine receptors, which ultimately signal to facilitate HIV infection of CD4+ T lymphocytes. However, we cannot rule out, as described above, that Panx1 hemichannels, also participate in recruitment of cellular receptors to areas of viral fusion, especially based on our results obtained using the fusion inhibitor, T-20, which inhibits viral–cell fusion and still can affect other stages of the viral fusion process.
Thus, we propose that Panx1 hemichannels are additional host proteins required for HIV infection and replication. Further studies on the mechanisms that control the opening, closing, and potential changes in expression of Panx1 hemichannels during infection could provide a valuable basis for the design of new, therapeutic interventions to reduce HIV infection of immune cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health grants MH075679, MH090958, and DA025567 to J.W.B. and MH096625 to E.A.E. NIAID Training Grant T32A1070117 funded D.W.W. Chilean funding was supported by the following grants: MECESUP-PUC0708 (to J.A.O.), CONICYT 79090028 (to J.A.O.), FONDECYT 11121133 (to J.A.O.), Anillo ACT-71 (to J.C.S.), and FONDEF D07I1086 (to J.C.S.).
We thank Dr. Jean X. Jiang from the Department of Biochemistry, University of Texas, for providing the Cx43 hemichannel blocker antibody (Cx43E2).
SEE CORRESPONDING EDITORIAL ON PAGE 390
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- AU
- arbitrary unit(s)
- CT
- comparative threshold
- Cx
- connexin
- env
- envelope
- Etd
- ethidium
- F1
- fluorescence intensity in each cell
- FB
- background fluorescence-intensity measurement
- La3+
- lanthanum ion
- NIAID
- National Institute of Allergy and Infectious Diseases
- NIMH
- National Institute of Mental Health
- Panx1
- pannexin1
- Prob
- probenecid
- qRT-PCR
- quantitative RT-PCR
- scr
- scrambled
- SDF-1α
- stroma cell-derived factor-1α
- siRNA
- small interfering RNA
AUTHORSHIP
J.A.O., S.V., D.W.W., J.C.S., J.W.B., and E.A.E. conceived of and designed the experiments as well as wrote and edited the manuscript.
REFERENCES
- 1. Melikyan G. B. (2011) Membrane fusion mediated by human immunodeficiency virus envelope glycoprotein. Curr. Top. Membr. 68, 81–106 [DOI] [PubMed] [Google Scholar]
- 2. Harmon B., Ratner L. (2008) Induction of the Gα(q) signaling cascade by the human immunodeficiency virus envelope is required for virus entry. J. Virol. 82, 9191–9205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melar M., Ott D. E., Hope T. J. (2007) Physiological levels of virion-associated human immunodeficiency virus type 1 envelope induce coreceptor-dependent calcium flux. J. Virol. 81, 1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weissman D., Rabin R. L., Arthos J., Rubbert A., Dybul M., Swofford R., Venkatesan S., Farber J. M., Fauci A. S. (1997) Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature 389, 981–985 [DOI] [PubMed] [Google Scholar]
- 5. Liu Q. H., Williams D. A., McManus C., Baribaud F., Doms R. W., Schols D., De Clercq E., Kotlikoff M. I., Collman R. G., Freedman B. D. (2000) HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc. Natl. Acad. Sci. USA 97, 4832–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hazleton J. E., Berman J. W., Eugenin E. A. (2012) Purinergic receptors are required for HIV-1 infection of primary human macrophages. J. Immunol. 188, 4488–4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seror C., Melki M. T., Subra F., Raza S. Q., Bras M., Saidi H., Nardacci R., Voisin L., Paoletti A., Law F., Martins I., Amendola A., Abdul-Sater A. A., Ciccosanti F., Delelis O., Niedergang F., Thierry S., Said-Sadier N., Lamaze C., Metivier D., Estaquier J., Fimia G. M., Falasca L., Casetti R., Modjtahedi N., Kanellopoulos J., Mouscadet J. F., Ojcius D. M., Piacentini M., Gougeon M. L., Kroemer G., Perfettini J. L. (2011) Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J. Exp. Med. 208, 1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sáez J. C., Schalper K. A., Retamal M. A., Orellana J. A., Shoji K. F., Bennett M. V. (2010) Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp. Cell. Res. 316, 2377–2389 [DOI] [PubMed] [Google Scholar]
- 9. MacVicar B. A., Thompson R. J. (2010) Non-junction functions of pannexin-1 channels. Trends Neurosci. 33, 93–102 [DOI] [PubMed] [Google Scholar]
- 10. Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Guldenagel M., Deutsch U., Sohl G. (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725–737 [DOI] [PubMed] [Google Scholar]
- 11. Schenk U., Westendorf A. M., Radaelli E., Casati A., Ferro M., Fumagalli M., Verderio C., Buer J., Scanziani E., Grassi F. (2008) Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 1, ra6. [DOI] [PubMed] [Google Scholar]
- 12. Woehrle T., Yip L., Elkhal A., Sumi Y., Chen Y., Yao Y., Insel P. A., Junger W. G. (2010) Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116, 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woehrle T., Yip L., Manohar M., Sumi Y., Yao Y., Chen Y., Junger W. G. (2010) Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J. Leukoc. Biol. 88, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qu Y., Misaghi S., Newton K., Gilmour L. L., Louie S., Cupp J. E., Dubyak G. R., Hackos D., Dixit V. M. (2011) Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186, 6553–6561 [DOI] [PubMed] [Google Scholar]
- 15. Chekeni F. B., Elliott M. R., Sandilos J. K., Walk S. F., Kinchen J. M., Lazarowski E. R., Armstrong A. J., Penuela S., Laird D. W., Salvesen G. S., Isakson B. E., Bayliss D. A., Ravichandran K. S. (2010) Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis. Nature 467, 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pelegrin P. (2008) Targeting interleukin-1 signaling in chronic inflammation: focus on P2X(7) receptor and pannexin-1. Drug News Perspect. 21, 424–433 [DOI] [PubMed] [Google Scholar]
- 17. Buckner C. M., Calderon T. M., Willams D. W., Belbin T. J., Berman J. W. (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell. Immunol. 267, 109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eugenin E. A., Osiecki K., Lopez L., Goldstein H., Calderon T. M., Berman J. W. (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 26, 1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orellana J. A., Froger N., Ezan P., Jiang J. X., Bennett M. V., Naus C. C., Giaume C., Sáez J. C. (2011) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 118, 826–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orellana J. A., Sáez P. J., Cortes-Campos C., Elizondo R. J., Shoji K. F., Contreras-Duarte S., Figueroa V., Velarde V., Jiang J. X., Nualart F., Sáez J. C., García M. A. (2012) Glucose increases intracellular free Ca2+ in tanycytes via ATP released through connexin 43 hemichannels. Glia 60, 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eugenin E. A., Berman J. W. (2003) Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods 29, 351–361 [DOI] [PubMed] [Google Scholar]
- 22. Sánchez H. A., Orellana J. A., Verselis V. K., Sáez J. C. (2009) Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am. J. Physiol. Cell. Physiol. 297, C665–C678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orellana J. A., Shoji K. F., Abudara V., Ezan P., Amigou E., Sáez P. J., Jiang J. X., Naus C. C., Sáez J. C., Giaume C. (2011) Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci. 31, 4962–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Contreras J. E., Sanchez H. A., Eugenin E. A., Speidel D., Theis M., Willecke K., Bukauskas F. F., Bennett M. V., Sáez J. C. (2002) Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. USA 99, 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silverman W., Locovei S., Dahl G. (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell. Physiol. 295, C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pelegrin P., Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siller-Jackson A. J., Burra S., Gu S., Xia X., Bonewald L. F., Sprague E., Jiang J. X. (2008) Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J. Biol. Chem. 283, 26374–26382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans W. H., De Vuyst E., Leybaert L. (2006) The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem. J. 397, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eugenin E. A., Morgello S., Klotman M. E., Mosoian A., Lento P. A., Berman J. W., Schecter A. D. (2008) Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular disease. Am. J. Pathol. 172, 1100–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baba M., Nishimura O., Kanzaki N., Okamoto M., Sawada H., Iizawa Y., Shiraishi M., Aramaki Y., Okonogi K., Ogawa Y., Meguro K., Fujino M. (1999) A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96, 5698–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donzella G. A., Schols D., Lin S. W., Este J. A., Nagashima K. A., Maddon P. J., Allaway G. P., Sakmar T. P., Henson G., De Clercq E., Moore J. P. (1998) AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4, 72–77 [DOI] [PubMed] [Google Scholar]
- 32. Eugenin E. A., Berman J. W. (2007) Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J. Neurosci. 27, 12844–12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eugenin E. A., Clements J. E., Zink M. C., Berman J. W. (2011) Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J. Neurosci. 31, 9456–9465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu L., LaRosa G., Kassam N., Gordon C. J., Heath H., Ruffing N., Chen H., Humblias J., Samson M., Parmentier M., Moore J. P., Mackay C. R. (1997) Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 186, 1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu L., Gerard N. P., Wyatt R., Choe H., Parolin C., Ruffing N., Borsetti A., Cardoso A. A., Desjardin E., Newman W., Gerard C., Sodroski J. (1996) CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384, 179–183 [DOI] [PubMed] [Google Scholar]
- 36. Dragic T., Litwin V., Allaway G. P., Martin S. R., Huang Y., Nagashima K. A., Cayanan C., Maddon P. J., Koup R. A., Moore J. P., Paxton W. A. (1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381, 667–673 [DOI] [PubMed] [Google Scholar]
- 37. Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P. D., Wu L., Mackay C. R., LaRosa G., Newman W., Gerard N., Gerard C., Sodroski J. (1996) The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85, 1135–1148 [DOI] [PubMed] [Google Scholar]
- 38. Layne S. P., Merges M. J., Dembo M., Spouge J. L., Nara P. L. (1990) HIV requires multiple gp120 molecules for CD4-mediated infection. Nature 346, 277–279 [DOI] [PubMed] [Google Scholar]
- 39. Kuhmann S. E., Platt E. J., Kozak S. L., Kabat D. (2000) Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74, 7005–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gallo S. A., Finnegan C. M., Viard M., Raviv Y., Dimitrov A., Rawat S. S., Puri A., Durell S., Blumenthal R. (2003) The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 1614, 36–50 [DOI] [PubMed] [Google Scholar]
- 41. Viard M., Parolini I., Sargiacomo M., Fecchi K., Ramoni C., Ablan S., Ruscetti F. W., Wang J. M., Blumenthal R. (2002) Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76, 11584–11595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manes S., del Real G., Lacalle R. A., Lucas P., Gomez-Mouton C., Sanchez-Palomino S., Delgado R., Alcami J., Mira E., Martinez A. C. (2000) Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1, 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carter G. C., Bernstone L., Sangani D., Bee J. W., Harder T., James W. (2009) HIV entry in macrophages is dependent on intact lipid rafts. Virology 386, 192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., Scemes E. (2008) P2X7 receptor-pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell. Physiol. 295, C752–C760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garre J. M., Retamal M. A., Cassina P., Barbeito L., Bukauskas F. F., Sáez J. C., Bennett M. V., Abudara V. (2010) FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc. Natl. Acad. Sci. USA 107, 22659–22664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khakh B. S., North R. A. (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527–532 [DOI] [PubMed] [Google Scholar]
- 47. Hazleton J. E., Berman J. W., Eugenin E. A. (2010) Novel mechanisms of central nervous system damage in HIV infection. HIV AIDS (Auckl) 2, 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.