Abstract
Although phosphorus is an essential factor for proper plant growth in natural environments, an excess of phosphate in water sources causes serious pollution. In this paper we describe transgenic plants which hyperaccumulate inorganic phosphate (Pi) and which may be used to reduce environmental water pollution by phytoremediation. AtPHR1, a transcription factor for a key regulator of the Pi starvation response in Arabidopsis thaliana, was overexpressed in the ornamental garden plants Torenia, Petunia, and Verbena. The transgenic plants showed hyperaccumulation of Pi in leaves and accelerated Pi absorption rates from hydroponic solutions. Large-scale hydroponic experiments indicated that the enhanced ability to absorb Pi in transgenic torenia (AtPHR1) was comparable to water hyacinth a plant that though is used for phytoremediation causes overgrowth problems.
1. Introduction
Water pollution has become a serious problem around the world. Contamination by toxic substances such as endocrine disruptors and heavy metals and excessive inflows of phosphorus, nitrogen and other elements all contribute to water pollution. Eutrophication is one of the major problems associated with water pollution and is caused by inflow of excess amounts of nutrients (especially phosphorus and nitrogen) [1]. The sources of excessive amounts of phosphorus and nitrogen are agricultural run-off, sewage, industrial effluents, and natural erosion from soil and rocks. Eutrophication is due to rapid growth of phytoplankton causing algal blooms or “red tides,” the result of which are serious environmental problems such as bad odor and fish death as a result of oxygen depletion and accumulation of toxic cyanotoxins [2].
Phosphorus can be removed by physical, chemical, and biological methods [3–6]. Physical and chemical methods (e.g., electrolytic, crystallization, filtration, and aggregation/separation methods) are superior in terms of removal efficiency and throughput capacity. However, these methods require complicated equipment and large quantities of chemicals, resulting in high cost and environmental burdens. A biological method, the anaerobic-anoxic-oxic method (A2O), is one of the advanced activated sludge methods and has been widely examined in sewage plants. However this method is also very expensive [7], and presently, there are no practically useable technologies to remove inorganic ions such as phosphorus and nitrogen during sewage treatment using activated sludge methods. Thus, though various types of water purification systems have been developed for water and sewage plants [8], these technologies are often difficult to apply directly to aquatic environments due to cost and the need for special equipment. Eutrophication therefore remains a problem.
Concurrently with improving sewage treatment technology, a low-cost and highly efficient method is still needed for sustainable water purification in aquatic environments. A treatment for environmental pollution using plants (phytoremediation) is a possible solution [9, 10]. Since phosphorus is an essential and often limiting nutritive substance for plants, plants actively absorb it from environments through the roots. Phytoremediation of aquatic systems has been attempted using water plants such as water hyacinth and Phragmites, as these plants absorb phosphorus relatively efficiently in comparison to terrestrial plants, and they also grow rapidly [11]. However, the high cost of collection and disposal of water plants (especially water hyacinth) presents difficulties in habitat management, and the impact of the plants on preexisting ecosystems hamper their wide application. In addition, the ability of these water plants to eliminate phosphorus in aquatic ecosystems is still inadequate as an even higher efficiency is needed for effective phytoremediation.
Inorganic phosphate (Pi) transporter is a key component in Pi absorption by plant roots. In Arabidopsis thaliana, 9 high-affinity transporters are known [12]. One of these, AtPHT1, encodes a cell membrane-located Pi transporter with high affinity for Pi. It has been reported that overexpression of AtPHT1 in cultured cells of Nicotiana leads to an acceleration of Pi absorption and an increase in cell growth rate [13]. In contrast, when the same Pi transporter was overexpressed in Hordeum vulgare, an increase in absorption of Pi was not observed [14]. These two contradicting reports suggest that merely increasing the number of Pi transporters does not necessarily lead to enhanced Pi absorption.
Several Pi starvation-related genes have been identified in A. thaliana mutants [15]. One of the known control factors which function when plants enter a state of Pi starvation is the AtPHR1 gene. AtPHR1 gene encodes a transcription factor which activates the transcription of genes in response to states of Pi starvation [16]. Recently, it is reported that overexpression of AtPHR1 in A. thaliana increases the Pi concentration in aerial plant parts [17].
In this study, we introduced the AtPHR1 gene into the garden plants Torenia, Petunia, and Verbena, in order to enhance Pi absorption. Small and large-scale hydroponic trials with transgenic torenia plants expressing the AtPHR1 gene were performed. We demonstrate for the first time that over expression of the AtPHR1 gene results in enhanced Pi absorption rate in different plant species. The AtPHR1 transgenic plants can possibly facilitate effective phytoremediation in polluted aquatic environments.
2. Materials and Methods
2.1. Plant Materials
Plants of Torenia hybrida cv. Summer Wave blue, Petunia hybrida cv. Surfinia purple mini, and Verbena hybrida cv. Temari scarlet (Suntory Flowers, Ltd.) were grown in soil and supplied with full nutrients every week in a green house or a growth chamber in controlled conditions (22–25°C, 12 hours light).
2.2. Constructs for Expression in Plants and Plant Transformation
Molecular biology techniques were employed according to the methods described by Sambrook et al. [18], unless otherwise specified.
The AtPHR1 gene was amplified by PCR using primers PHRf (5′-ATGGAGGCTCGTCCAGTTCAT-3′) and PHRr (5′-TCAATTATCGATTTTGGGACGC-3′) and subcloned into the pCR2.1 vector using a TOPO-TA cloning kit (Life Technologies) according to the manufacturer's instructions. A fragment of the AtPHR1 gene was inserted into binary vector pBinPLUS [19] which contains an enhanced cauliflower mosaic virus 35S promoter [20] and a nopaline synthase (nos) terminator. This plasmid was named pSPB1898.
Transformation with transformation vector pSPB1898 was carried out as described previously for Torenia [21], Petunia [22], and Verbena [23] using Agrobacterium tumefaciens strain AGL0 [24].
RNAs were extracted from leaves of the obtained recombinant plants using the RNeasy Plant Mini Kit (Qiagen). Positive strains were selected by RT-PCR.
2.3. Method for Measuring Phosphorus Concentration
Phosphorus concentration was measured according to a modified method of Ames [25]. Leaves were weighed (approximately 100 mg per sample) and inserted into a 2 mL tube for crushing with zirconia beads (4 mm diameter), at −80°C. The frozen sample was taken to room temperature, and 500 µL of 1% (v/v) acetic acid was added to each tube. The mixture was then shaken and crushed for 6 minutes using a TissueLyser (Qiagen). After crushing, the mixture was centrifuged at 15,000 rpm for 5 minutes using a desktop centrifuge to obtain 500 µL of supernatant. This Pi extract was diluted with distilled water (from 10 to 100-fold dilution) to a final concentration of 800 µL. To this solution, 160 µL of measuring buffer (1.25 M sulfuric acid, 30 mM ascorbic acid, 0.405 mg/mL antimony potassium tartrate, and 24 mg/mL ammonium molybdate) was added, and the mixture was stirred well and left for 10 minutes. The absorbance was measured at 880 nm using a spectrophotometer BioSpec-mini (Shimadzu, Japan). The amount of phosphorus in 1 g of leaf was calculated from phosphorus concentration and weight of the sample. For calculations on a dry weight basis, samples were dried at 80°C for about 2 days.
An independent Student's t-test was used to compare differences between host and transgenic plants. All tests were two-sided, and P < 0.05 was considered statistically significant. Data are the mean ± SD from at least three different samples.
2.4. Hydroponic Experiment
Wild-type torenia or transgenic torenia was grown on a support made of polystyrene foam with holes to allow the root systems of the plants to grow into the hydroponic solution. Plants were floated on 5 liter of hydroponic solution (0.5 mM KNO3, 0.2 mM MgSO4, 0.2 mM Ca(NO3)2, 0.161 mM KPO4, 5 µM Fe-EDTA, 7 µM H3BO3, 1.4 µM MnCl2, 0.05 µM CuSO4, 0.1 mM ZnSo4, 0.02 µM Na2MoO4, 1 µM NaCl, and 0.001 µM CoCl2). The initial phosphorus concentration in the hydroponic solution was 5 mg/L. Four plants were used in each support. The Pi concentration in the hydroponic solution was measured each day. Since the fluid volume of the hydroponic solution decreased due to transpiration and evaporation, on every fourth day, deionised water was added to the solution. For large container experiments, the same solution was used, but the volume of hydroponic solution was 400 liter, and 13 plants were used per container. The volume of each container was adjusted with deionised water on a weekly basis.
3. Results and Discussion
3.1. Overexpression of AtPHR1 Enhances Pi Accumulation and Absorption in Transgenic Plants
It has been shown in A. thaliana that over expression of AtPHR1 causes enhanced Pi accumulation in aerial parts [17]. To examine whether AtPHR1 is effective in otherplant species, we transformed torenia, petunia, and verbena with AtPHR1. These plants were transformed with the plasmid pSPB1898, which contains the AtPHR1 gene under the control of the constitutive 35S promoter. We screened over 30 transgenic plants for each species for the presence of the transgene with RT-PCR and for leaf Pi concentration 4 weeks after potting up from tissue culture. Concentration of phosphorus per fresh leaf weight was then measured for selected lines. In each of the 3 plant species, phosphorus concentration in the leaves of the transgenic plants was 2 to 3-fold higher than that of control host plants (Figure 1).
Figure 1.
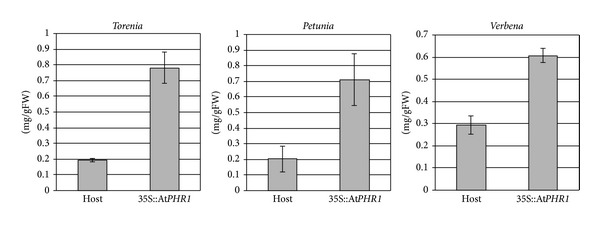
Phosphorus measurements of AtPHR1 transgenic plants. Phosphorus concentrations in the leaves of AtPHR1 transgenic plants of potted torenia, petunia, and verbena were measured. The longitudinal axis shows the phosphorus amounts per gram fresh weight (mg/gFW). Significant differences in means between host and transgenic plants were detected for all three species.
We examined other Pi starvation-related genes (AtPHT1;1, AtPHT1;2, AtIPS1, and AtPHO1) from A. thaliana by constitutively overexpressing them in transgenic torenia and petunia (data not shown). None of these transgenic plants showed enhanced Pi accumulation. This result is consistent with the observation that over-expression of the Pi transporter did not cause any change to Pi accumulation in H. vulgare [14]. Thus, we focused on AtPHR1 in the following experiments.
To confirm that introduction of the AtPHR1 gene accelerates Pi absorption rates, we grew plants of a transgenic torenia line in a hydroponic system. Torenia was chosen as this plant grows luxuriantly and roots tolerate being submerged in water. The torenia plants were grown in 5 liters of hydroponic solution containing 5 mg/L phosphorus for 1 to 2 months in a green house or a growth chamber. The phosphorus concentration of the hydroponic solutions was measured daily. The superior transgenic line expressing AtPHR1 (35S::AtPHR1) showed enhanced Pi absorption from the hydroponic solution (Figure 2(a)). Enhanced accumulation of Pi in the transgenic leaves was also confirmed by measurements of leaf phosphorus concentration (Figure 2(b)). The phosphorus concentration of the hydroponic solution in which 35S::AtPHR1 was grown decreased during the two weeks of the experiment. The Pi absorption rate observed for 35S::AtPHR1 was up to 0.091 mgP/day/plant in this experiment compared to 0.056 mgP/day/plant for the host (Figure 2(a)). This result suggests that the enhanced Pi accumulation observed in the potted AtPHR1 transgenic torenia plants is mainly due to enhanced Pi absorption rate.
Figure 2.
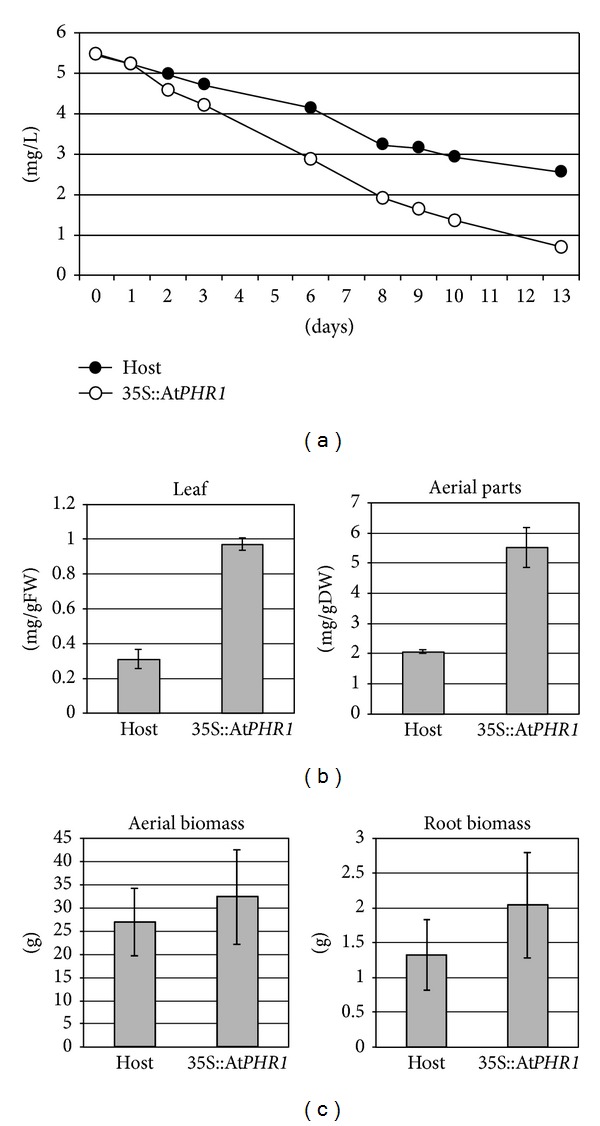
Pi accumulation and growth properties of AtPHR1 transgenic torenia. (a) Changes of Pi concentration in hydroponic solutions. The phosphorus concentration in a hydroponic solution in which host (filled circle) and AtPHR1 transgenic torenia (empty circle) were cultured was measured. The longitudinal axis shows the phosphorus concentration (mg/L), and the horizontal axis shows the number of days after exchange of the hydroponic solution. (b) Pi concentration in the leaves and aerial parts of hydroponically-cultivated torenia. The longitudinal axis shows the phosphorus concentration per gram fresh weight of samples (mg/gFW) (left) and the phosphorus concentration per gram dry weight of samples (mg/gDW) (right). There were significant differences in means between host and transgenic plants. (c) Comparison of growth rate. Weights of aerial parts and root parts of the torenia plants were measured at the end of hydroponic experiments. There was no statistically significant difference between transgenic and host.
To see if the decrease of Pi concentration in the hydroponic solution was also reflected in an increase in Pi accumulation in the plant, Pi accumulation in the aerial parts of the plants was measured. Three plants each of the transgenic and the host torenia were hydroponically cultivated in the solution containing 5 mg/L phosphorus for about 2 months. The aerial parts of those plants were collected and dried on the phosphorus concentration measured (Figure 2(b)). The Pi concentration in the transgenic plants was approximately 2.5-fold that of the host.
We weighed aerial and root parts of the tested plants after each hydroponic experiment. Even though slightly less weight was measured in the host, there was no statistically significant difference between the transgenic and host (Figure 2(c)). This suggests that excessively absorbed Pi is not used for plant growth but is accumulated and stored in the aerial part of the plants. As a result, overexpression of AtPHR1 does not retard plant growth. Since the transgenic plants did not show any morphological or reproductive abnormalities, over-expression of the AtPHR1 gene can enhance Pi accumulation with no negative effects on plant growth.
3.2. Limitation of Pi Capacity
Sections of dead tissues in the leaves were often observed in transgenic torenia during the 4 weeks of the hydroponic experiments (Figures 3(a)–3(c)). We collected the dead sections and compared them to the unaffected areas of the leaves from the same plants. The harvested leaves were dried and then measured for phosphorus concentration. The phosphorus concentration in the dead sections was slightly higher than that of unaffected portions of leaves (Figure 3(d)). Since excess Pi may cause cell toxicity [26], the death may have been the result of exceeding a critical limit of Pi concentration in the torenia leaf cells. It thus appears that the critical limit of Pi accumulation level in AtPHR1 transgenic torenia is approximately 20 mg/gDW. One possible way to overcome the death of leaf tissues due to high Pi accumulation is to convert Pi to a nontoxic form of phosphorus that is phytic acid. Genetic modification could be used to achieve this, resulting in transgenic plants accumulating even more Pi than reported here.
Figure 3.
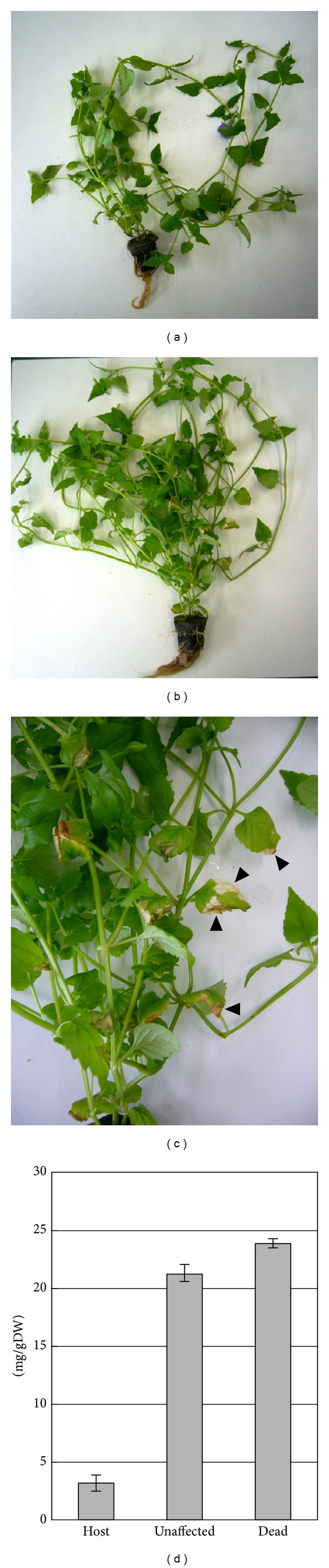
Dead tissue in AtPHR1 transgenic torenia. (a) Host plant at the end of hydroponic experiment. (b) AtPHR1 transgenic plant after 4 weeks of hydroponic experiment. (c) Magnified image of (b). Arrowheads indicate partially dead sections. (d) Phosphorus concentration in unaffected and dead areas from leaves of host and AtPHR1 transgenic plants. The longitudinal axis shows the phosphorus concentration per gram dry weight of sample (mg/gDW).
3.3. Large-Scale Hydroponic Experiment
To access the potential for phytoremediation using the transgenic torenia at a larger scale, we performed longer term hydroponic experiments. Thirteen torenia plants were put each into 400-liter tub and incubated for approximately 2 months (Figure 4). There was no significant difference in average biomass between transgenic and host plants after 65 days incubation (Figure 4 and Table 1). However, approximately 3-fold more Pi accumulation was seen in the transgenic plant when compared to the host. This confirmed that transgenic torenia shows the accelerated absorption as well as accumulation of Pi in the leaves when grown on a larger scale. From the daily calculation of Pi accumulation of the transgenic torenia plant, Pi accumulation rates were able to be compared to water hyacinth (Table 1). The AtPHR1 transgenic torenia showed an equivalent efficiency of Pi accumulation to that of water hyacinth [27, 28].
Figure 4.

Large-scale hydroponic experiments. (a) Changes in Pi concentration in hydroponic solutions. The phosphorus concentration in a hydroponic solution in which host (filled circle) and AtPHR1 transgenic torenia (empty circle) were cultured was measured. The longitudinal axis shows the phosphorus concentration (mg/L), and the horizontal axis shows the number of days. Hydroponic solutions were fully exchanged 30 days after starting the experiment. (b) Large-scale experiment (0 day). (c) Large-scale experiment (65 days).
Table 1.
Comparison of phosphate absorption performances. Phosphorus content, total biomass, and absorption rate after 65 days of the hydroponic experiment are indicated. Data are the mean ± SD from 13 plants. Values of water hyacinth were calculated from values listed in [27, 28].
| Phosphorus in leaf (mg/gFW) |
Total biomass (g/plant) | Absorption rate (mg/plant/day) |
|
|---|---|---|---|
| Host | 0.18 ± 0.11 | 396.34 ± 146.06 | 1.08 |
| 35S::AtPHR1 | 0.69 ± 0.20 | 382.95 ± 178.85 | 4.15 |
| Water hyacinth | 0.38 | 1.79 |
Overexpression of AtPHR1 gene might drive a Pi starvation response in the transgenic plants. As a result, excessive amounts of Pi accumulated in transgenic leaves. In A. thaliana, AtPHR1 gene is not transcriptionally regulated even under Pi starvation condition [17]. Since the key mechanism of the Pi starvation response is still debatable in Arabidopsis thalinana [17, 29], it is difficult to postulate why overexpression of AtPHR1 is effective for Pi uptake in other species. We have isolated orthologous Pi starvation-related genes (AtPHR1, AtIPS1, AtPHT1;1, and AtPHO2) in torenia and examined expression pattern of these genes (data not shown). We could not detect any differences between transgenic torenia and host plants. Overexpression of AtPHR1 may interfere with the proper posttranscriptional modification of the endogenous AtPHR1 counterpart, possibly through competitive inhibition.
Since phosphorus is expected to be exhausted as a natural resource within a hundred year [30], it is necessary to recover phosphorus from the environment, especially in polluted areas. Currently, over 90% of the produced phosphorus in the world is used as fertilizers. Therefore, it is most reasonable to recover phosphorus from fertilized soils and agricultural run-offs. Phytoremediation is a suitable method for such a recycling process, in addition to cleaning up phosphorus from the aquatic environment. One of the critical problems of phytoremediation is the cost of the disposal of the plant [31]. The plant used for phytoremediation was in many cases simply discarded without being used as a source of Pi. Ideally, plants containing high accumulation of Pi can be returned to soils of agricultural land without processing and can be directly used as fertilizer. However, at present, absorbing ability of the existing plants used for phytoremediation is not efficient enough to be used as Pi sources for agriculture in this way. In this study, the AtPHR1 transgenic plants accumulated a high level of Pi. Therefore, applications of AtPHR1 transgenic plants for phytoremediation of water could be cost-effective. Moreover, the Pi recycling ability of flowers and ornamental plants for gardening can be increased by means of AtPHR1 gene introduction, and thereby purifying water with plants having both ornamental beauty and high purification ability.
4. Conclusions
In this study, we prove the feasibility of using AtPHR1 as an enhancer of Pi uptake in transgenic plants. By introducing AtPHR1 to garden plants, amounts of Pi accumulation and absorption of Pi were increased to rates approximately 3-fold higher than host plant. There was no significant reduction in biomass or morphology of the transgenic plant expressing AtPHR1. Taken together, these observations indicate that the AtPHR1 gene will be valuable for production of hyperaccumulator plants for the purification of waters polluted with Pi. In addition, an improved appearance of purification sites can be provided by using ornamental plants with many flowers, as shown in Figure 4(c).
Conflict of Interests
The authors have no conflict of interests to declare.
Acknowledgments
The authors thank Mses. Keiko Takeda, Masumi Taniguchi, and Sarah Parsons for producing the transgenic plants and Mses. Chika Shimadzu, Kumi Takemura, Miyuki Ogawa, and Kim Stevenson for their technical assistance. The authors thank Dr. Robert A. Ludwig for providing A. tumefaciens Agl0 and appreciate Mr. Masayasu Yoshikawa for his critical reading of the paper.
References
- 1.Smith VH, Tilman GD, Nekola JC. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution. 1998;100(1–3):179–196. doi: 10.1016/s0269-7491(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 2.Smith VH, Schindler DW. Eutrophication science: where do we go from here? Trends in Ecology and Evolution. 2009;24(4):201–207. doi: 10.1016/j.tree.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Vohlaa C, Koiva M, Bavor HJ, et al. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecological Engineering. 2011;37(1):70–89. [Google Scholar]
- 4.QU J. Research progress of novel adsorption processes in water purification: a review. Journal of Environmental Sciences. 2008;20(1):1–13. doi: 10.1016/s1001-0742(08)60001-7. [DOI] [PubMed] [Google Scholar]
- 5.de Haas DW, Wentzel MC, Ekama GA. The use of simultaneous chemical precipitation in modified activated sludge systems exhibiting biological excess phosphate removal part 1: literature review. Water SA. 2000;26(4):439–452. [Google Scholar]
- 6.Wang FY, Rudolph V, Zhu ZH. Sewage Sludge technologies. Encyclopedia of Ecology. 2008:3227–3242. [Google Scholar]
- 7.Peng Y, Wang X, Wu W, Li J, Fan J. Optimisation of anaerobic/anoxic/oxic process to improve performance and reduce operating costs. Journal of Chemical Technology and Biotechnology. 2006;81(8):1391–1397. [Google Scholar]
- 8.Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marĩas BJ, Mayes AM. Science and technology for water purification in the coming decades. Nature. 2008;452(7185):301–310. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]
- 9.Pilon-Smits E. Phytoremediation. Annual Review of Plant Biology. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- 10.Luqman M, Batt TM, Tanvir A, et al. Phytoremediation of polluted water by trees: a review. African Journal of Agricultural Research. 2013;8(17):1591–1595. [Google Scholar]
- 11.Gupta P, Roy S, Mahindrakar AB. Treatment of water using water hyacinth, water lettuce and vetiver grass—a review. Resources and Environment. 2012;2(5):202–215. [Google Scholar]
- 12.Rausch C, Bucher M. Molecular mechanisms of phosphate transport in plants. Planta. 2002;216(1):23–37. doi: 10.1007/s00425-002-0921-3. [DOI] [PubMed] [Google Scholar]
- 13.Mitsukawa N, Okumura S, Shirano Y, et al. Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(13):7098–7102. doi: 10.1073/pnas.94.13.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rae AL, Jarmey JM, Mudge SR, Smith FW. Over-expression of a high-affinity phosphate transporter in transgenic barley plants does not enhance phosphate uptake rates. Functional Plant Biology. 2004;31(2):141–148. doi: 10.1071/FP03159. [DOI] [PubMed] [Google Scholar]
- 15.Ticconi CA, Abel S. Short on phosphate: plant surveillance and countermeasures. Trends in Plant Science. 2004;9(11):548–555. doi: 10.1016/j.tplants.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Rubio V, Linhares F, Solano R, et al. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes and Development. 2001;15(16):2122–2133. doi: 10.1101/gad.204401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson L, MÜller R, Nielsen TH. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana . Plant, Cell and Environment. 2007;30(12):1499–1512. doi: 10.1111/j.1365-3040.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.van Engelen FA, Molthoff JW, Conner AJ, Nap JP, Pereira A, Stiekema WJ. pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Research. 1995;4(4):288–290. doi: 10.1007/BF01969123. [DOI] [PubMed] [Google Scholar]
- 20.Mitsuhara I, Ugaki M, Hirochika H, et al. Efficient promoter cassettes or enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant and Cell Physiology. 1996;37(1):49–59. doi: 10.1093/oxfordjournals.pcp.a028913. [DOI] [PubMed] [Google Scholar]
- 21.Aida R, Shibata M. Agrobacterium-mediated transformation of torenia (Torenia fournieri) Breeding Science. 1995;45(1):71–74. [Google Scholar]
- 22.Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- 23.Tamura M, Togami J, Ishiguro K, et al. Regeneration of transformed verbena (Verbena × hybrida) by Agrobacterium tumefaciens . Plant Cell Reports. 2003;21(5):459–466. doi: 10.1007/s00299-002-0541-1. [DOI] [PubMed] [Google Scholar]
- 24.Lazo GR, Stein PA, Ludwig RA. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium . Bio/Technology. 1991;9(10):963–967. doi: 10.1038/nbt1091-963. [DOI] [PubMed] [Google Scholar]
- 25.Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymology. 1966;8:115–118. [Google Scholar]
- 26.Clarkson DT, Scattergood CB. Growth and phosphate transport in barley and tomato plants during the development of, and recovery from, phosphate-stress. Journal of Experimental Botany. 1982;33(5):865–875. [Google Scholar]
- 27.Haller WT, Sutton DL. Effect of pH and high phosphorus concentrations on growth of water hyacinth. Hyacinth Control Journal. 1973:59–61. [Google Scholar]
- 28.Morii M, Doyama Y, Katayama J. On the absorption of nitrogen and phosphorus from water by water hyacinth, Eichhornia crassipes (Mart.) Solms. Bulletin of the Osaka Agricultural Research Center. 1990;26:11–15. [Google Scholar]
- 29.Bustos R, Castrillo G, Linhares F, et al. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in arabidopsis. PLoS Genetics. 2010;6(9) doi: 10.1371/journal.pgen.1001102.e1001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggio A, Malingreau J-P, Bock A-K, et al. NPK: Will there be enough plant nutrients to feed a world of 9 billion in 2050? Publication Office of the European Union, 2012, http://publications.jrc.ec.europa.eu/repository/handle/111111111/25770.
- 31.Sas-Nowosielska A, Kucharski R, Małkowski E, Pogrzeba M, Kuperberg JM, Kryński K. Phytoextraction crop disposal—an unsolved problem. Environmental Pollution. 2004;128(3):373–379. doi: 10.1016/j.envpol.2003.09.012. [DOI] [PubMed] [Google Scholar]


