Abstract
Objective
To compare the effects of transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) on aortic valve haemodynamics, ventricular reverse remodelling and myocardial fibrosis (MF) by cardiovascular magnetic resonance (CMR) imaging.
Design
A 1.5 T CMR scan was performed preoperatively and 6 months postoperatively.
Setting
University hospitals of Leeds and Leicester, UK.
Patients
50 (25 TAVI, 25 SAVR; age 77±8 years) high-risk severe symptomatic aortic stenosis (AS) patients.
Main outcome measures
Valve haemodynamics, ventricular volumes, ejection fraction (EF), mass and MF.
Results
Patients were matched for gender and AS severity but not for age (80±6 vs 73±7 years, p=0.001) or EuroSCORE (22±14 vs 7±3, p<0.001). Aortic valve mean pressure gradient decreased to a greater degree post-TAVI compared to SAVR (21±8 mm Hg vs 35±13 mm Hg, p=0.017). Aortic regurgitation reduced by 8% in both groups, only reaching statistical significance for TAVI (p=0.003). TAVI and SAVR improved (p<0.05) left ventricular (LV) end-systolic volumes (46±18 ml/m2 vs 41±17 ml/m2; 44±22 ml/m2 vs32±6 ml/m2) and mass (83±20 g/m2 vs 65±15 g/m2; 74±11 g/m2 vs 59±8 g/m2). SAVR reduced end-diastolic volumes (92±19 ml/m2 vs 74±12 ml/m2, p<0.001) and TAVI increased EF (52±12% vs 56±10%, p=0.01). MF reduced post-TAVI (10.9±6% vs 8.5±5%, p=0.03) but not post-SAVR (4.2±2% vs 4.1±2%, p=0.98). Myocardial scar (p≤0.01) and baseline ventricular volumes (p<0.001) were the major predictors of reverse remodelling.
Conclusions
TAVI was comparable to SAVR at LV reverse remodelling and superior at reducing the valvular pressure gradient and MF. Future work should assess the prognostic importance of reverse remodelling and fibrosis post-TAVI to aid patient selection.
Introduction
Aortic stenosis (AS) is the most common valve disease in the western world,1 and the onset of symptoms predicts a substantially reduced life expectancy.2 Restricted aortic valve leaflets cause a pressure overloaded left ventricle (LV) to compensate by altering its wall geometry in order to maintain wall stress. This hypertrophic remodelling process is pathological, with myocyte degeneration and replacement myocardial fibrosis (MF), leading to ventricular dysfunction. Aortic valve replacement removes this aorto-valvular impedance, resulting in geometric changes (mass regression, volume reduction and improved function) known as ‘reverse remodelling’. This process has been shown to be the essential factor in improving symptoms and prognosis following surgical aortic valve replacement (SAVR).3–6
Transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment option for severe AS patients who are unsuitable or too high-risk for SAVR. Randomised trials have shown the 2-year mortality following TAVI to be superior to standard medical therapy and non-inferior to SAVR,7–10 with good registry outcomes at 5 years. TAVI studies have used transthoracic echocardiography (TTE) to demonstrate an improvement in aortic valve haemodynamics and LV function.11 However, TTE has limited reproducibility and relies on mathematical assumptions of LV geometry and cavity size, which may not apply in the remodelled ventricle. In addition, paravalvular aortic regurgitation (AR) is difficult to quantify using TTE, yet is common post-TAVI.8 Finally, MF has been shown to adversely affect prognosis and functional outcomes following SAVR,12 but as yet has not been assessed in a TAVI population.
Cardiovascular magnetic resonance (CMR) is the reference standard for the assessment of right ventricular (RV) and LV mass, volumes and ejection fraction (EF). AS severity can be determined comparably to echocardiography and regurgitant volume assessed with greater precision and reproducibility.13 14 CMR can also determine the presence, distribution and quantity of MF.15
The primary aim of this study was to use CMR to accurately assess and compare the postoperative changes in aortic valve haemodynamics, reverse ventricular remodelling and MF, at 6 months following TAVI and SAVR. Secondary aims were to identify clinical predictors of impaired ‘reverse ventricular remodelling’ and to establish the importance of preoperative MF on clinical outcomes.
Methods
Study population
This study prospectively recruited 77 patients with severe AS who were referred for either TAVI (n=50) or SAVR (n=27) at the University Hospitals of Leeds and Leicester, UK, between July 2008 and December 2010. Severe AS was classified by TTE as an aortic valve area of ≤0.8 cm2 or peak velocity >4 m/s. Decision for TAVI was taken by a multidisciplinary heart team in accordance with international guidance (Logistic EuroSCORE >20 or inoperable co-morbidities). Higher-risk (higher EuroSCORE) SAVR patients were recruited so that their baseline demographics were more comparable to the TAVI group. Exclusion criteria included any contraindication to CMR. The study was approved by the institutional ethics committee, complied with the Declaration of Helsinki and all patients provided written informed consent.
Transcatheter aortic valve implantation
TAVI was performed under general anaesthesia using an 18F CoreValve Revalving system (CVR, Medtronic, Minneapolis, Minnesota, USA) as previously described.16 A percutaneous femoral route was used when vascular access was suitable. In the presence of significant peripheral vascular disease, a subclavian artery approach was performed.
Surgical aortic valve replacement
SAVR was performed by standard midline sternotomy with cardiopulmonary bypass and mild hypothermia. Biological or mechanical prostheses of varying sizes were used according to surgical preference. The procedural details of both SAVR and TAVI techniques are reported in online supplementary appendix 1.
CMR protocol
Identical baseline preoperative and 6-month postoperative scans were performed on the same 1.5 T MRI system (Intera, Phillips Healthcare, Best, Netherlands or Avanto, Siemens Medical Systems, Erlangen, Germany). Multi-slice, multi-phase cine imaging was performed using a standard steady-state free procession pulse sequence in the short axis (8 mm thickness, 0 mm gap, 30 phases, typical field of view (FOV) 340 mm) to cover the entire left and right ventricle. Through-plane velocity encoded (VENC) phase contrast imaging was performed perpendicular to the aortic valve jet at the aortic sinotubular junction (VENC 250–500 cm/s, retrospective gating, slice thickness 6 mm, 40 phases, FOV 340 mm). Late gadolinium enhancement (LGE) imaging (10–12 short axis slices, 10 mm thickness, matrix 240×240, typical FOV 340 mm) was performed following a Look-Locker sequence (inversion time scout), 10 min after the administration of 0.2 mmol/kg of gadoteric acid (Dotarem, Guerbet, Villepinte) or gadolinium-DTPA (Magnevist, Schering, Germany). Identical contrast agent was used at both study time-points.
CMR analysis
Endocardial and epicardial borders were manually contoured at end-diastole and end-systole to allow the calculation of ventricular volumes (summation of discs methodology) and mass (epicardial volume – endocardial volume multiplied by myocardial density (1.05 g/cm3)); values were indexed to body surface area. Geometric remodelling was defined by LV mass/end diastolic volume ratio as previously described.17 Aortic flow was quantified using cross-sectional phase contrast images with contouring of the aortic lumen to provide a peak forward flow velocity (m/s), forward flow volume (ml), backward flow volume (ml) for the calculation of trans-valvular pressure gradient (Bernoulli equation) and regurgitant fraction (%). Mitral regurgitant fraction (%) was calculated as (LV stroke volume – aortic stroke volume)/LV stroke volume × 100. Focal MF and scarring (secondary to infarction) were differentiated, then reported qualitatively as either present or absent. Quantitative assessment was performed by semi-automated signal intensity analysis according to the full width half maximum technique. All analyses were performed using QMass or QFlow (V.7.2, Medis, Leiden, The Netherlands) by two experienced observers, blinded to the clinical details.
Sample size and statistical analysis
Based on published data, 20 patients per group were required to detect a 10 ml change in LV end-diastolic volume ( LVEDV) or 10 g difference in LV mass regression between the two treatments (90% power and an α error of 0.05); 30 per group would be sufficient to detect a clinically meaningful 10% absolute difference in aortic peak forward flow velocity or regurgitant fraction (85% power and an α error of 0.05).18 Data are presented as mean±SD (continuous) or median (IQR). Normality was determined by the Shapiro–Wilks test. Frequencies are reported as number (%). The Student t test and Wilcoxon signed rank test were used for continuous variables, and χ2 or Fisher's exact test for categorical comparisons. Changes over time were assessed for differences between the treatment groups and clinical variables by two-way repeated measures analysis of variance (ANOVA). Predictors of functional change were calculated by a stepwise multiple linear regression model with baseline measurements entered as covariate factors. Variables with a univariate p<0.1 were entered into the multivariable analysis. All statistical analyses were performed using the PASW software package (V.17.0 SPSS, IBM, Chicago, Illinois, USA), with a two-sided significance level of p<0.05 considered statistically significant.
Results
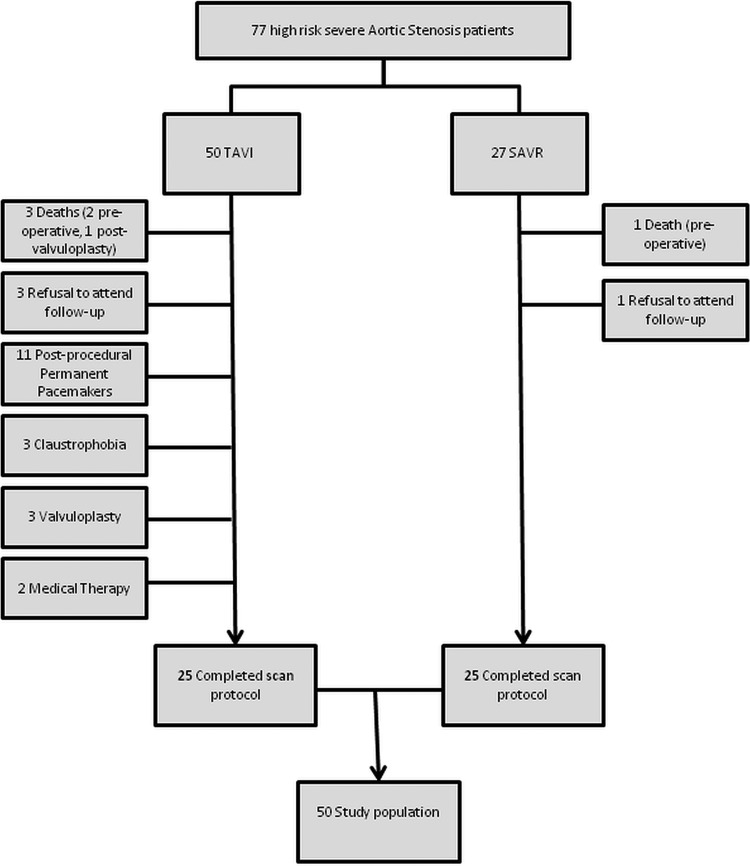
Fifty patients (25 TAVI and 25 SAVR) completed both preoperative and 6-month postoperative scans. Reasons for non-completion of the CMR protocol were varied and are depicted in figure 1. The baseline characteristics of the final study population are reported in table 1. The TAVI group were older but comparable in terms of gender and body mass index to the SAVR group. Co-morbidities were equally prevalent in the two groups, except for a greater frequency of atrial fibrillation, and coronary and peripheral artery disease in the TAVI group. No major adverse cardiac events or percutaneous coronary interventions occurred during the study period.
Figure 1.
Patient recruitment pathway.
Table 1.
Patient characteristics and baseline echocardiographic data
| Characteristics | Total (n=50) | TAVI (n=25) | SAVR (n=25) | p Value* |
|---|---|---|---|---|
| Age | 77±8 | 80±6 | 73±7 | 0.001 |
| Male gender, n (%) | 31 (62) | 14 (56) | 17 (68) | 0.56 |
| Body mass index (kg/m2) | 27±4 | 27±3 | 27±5 | 0.77 |
| Body surface area (m2) | 1.86±0.2 | 1.84±0.2 | 1.89±2 | 0.33 |
| Systolic blood pressure (mm Hg) | 139±24 | 136±28 | 142±20 | 0.48 |
| Diastolic blood pressure (mm Hg) | 73±11 | 67±10 | 77±9 | 0.002 |
| Valvuloarterial impedance (Zva) | 4.0±1 | 3.98±1 | 4.01±1 | 0.94 |
| EuroSCORE (%) | 16±15 | 22±14 | 7±3 | <0.001 |
| CV history | ||||
| NYHA class | 2.38±0.7 | 2.48±0.7 | 2.28±7 | 0.30 |
| Coronary artery disease (%DS) | ||||
| < 50% | 29 (58) | 9 (36) | 20 (80) | 0.004 |
| 50–70% | 8 (16) | 5 (20) | 3 (12) | |
| >70% | 13 (26) | 11 (44) | 2 (8) | |
| Prior PCI, n (%) | 5 (10) | 4 (16) | 1 (4) | 0.17 |
| Prior CABG, n (%) | 9 (18) | 8 (32) | 1 (4) | 0.01 |
| Prior MI, n (%) | 8 (16) | 5 (20) | 3 (12) | 0.70 |
| Co-morbidities | ||||
| Hypertension, n (%) | 35 (70) | 15 (60) | 20 (80) | 0.22 |
| Hypercholesterolemia, n (%) | 26 (52) | 16 (64) | 10 (40) | 0.16 |
| Diabetes, n (%) | 13 (26) | 7 (28) | 6 (24) | 0.75 |
| CKD, n (%) | 2 (4) | 2 (8) | 0 (0) | 0.49 |
| Atrial fibrillation, n (%) | 7 (14) | 6 (24) | 1 (4) | 0.04 |
| Previous stroke, n (%) | 6 (12) | 4 (16) | 2 (8) | 0.39 |
| Peripheral vascular disease, n (%) | 7 (14) | 6 (24) | 1 (4) | 0.04 |
| COPD, n (%) | 7 (14) | 5 (20) | 2 (8) | 0.23 |
| Echocardiographic data | ||||
| AVA (cm2) | 0.62±0.3 | 0.58±0.2 | 0.68±0.4 | 0.24 |
| MPG (mm Hg) | 51 (41–61) | 57±22 | 47±13 | 0.05 |
| LV function, ejection fraction, n (%) | ||||
| Good (>50%) | 34 (68) | 15 (60) | 19 (76) | 0.48 |
| Fair (30–50%) | 11 (22) | 7 (28) | 4 (16) | |
| Poor (<30%) | 5 (10) | 3 (12) | 2 (8) | |
Values are mean±SD or n (%)
*p Value for comparison between procedure types.
AVA, aortic valve area; CABG, coronary artery bypass graft; CKD, chronic kidney disease (eGFR<30); COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; MPG, mean pressure gradient; NYHA, New York Health Association; PCI, percutaneous intervention; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; Zva, valvuloarterial impedance (systolic arterial pressure+mean transvalvular gradient/stroke volume index).
Aortic and mitral valve haemodynamics
The severity of preoperative aortic valve stenosis was similar between the TAVI and SAVR groups. Postoperatively the trans-valvular pressure gradient at 6 months was significantly lower in both groups; compared to SAVR the TAVI group had a significantly greater reduction in their pressure gradient. The baseline AR fraction (%) was similar between the groups. Valve replacement resulted in an absolute 8% reduction of AR following both procedures, reaching statistical significance in the TAVI (p=0.003) but not in the SAVR group (p=0.09). ANOVA comparison of the two techniques showed no difference in the efficacy of the two procedures to reduce AR.
Mitral regurgitation (MR) preoperatively was greater in the TAVI (mild) compared to the SAVR (trivial) group. At follow-up, mitral regurgitant fraction (%) had significantly reduced post-TAVI and remained unaltered post-SAVR (table 2).
Table 2.
Preoperative baseline measurements and postoperative changes in the separate procedural groups
| TAVI | SAVR | ANOVA | |||
|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | p Value | |
| Left ventricle | |||||
| LVEDVI (ml/m2) | 94±18 | 90±20 | 92±19 | 74±12** | 0.04 |
| LVESVI (ml/m2) | 46±18 | 41±17* | 44±22 | 32±6* | 0.19 |
| LVSVI (ml/m2) | 48±10 | 50±10 | 49±8 | 42±7* | 0.14 |
| LVEF (%) | 52±12 | 56±10* | 55±11 | 57±8 | 0.57 |
| LVM (g) | 153±48 | 120±38*** | 143±57 | 114±42*** | 0.53 |
| LVMI (g/m2) | 83±20 | 65±17** | 74±11 | 59±8** | 0.35 |
| LVM/LVEDV (g/ml) | 0.88±0.2 | 0.73±0.2*** | 0.80±0.1 | 0.81±0.2 | 0.001 |
| Right ventricle | |||||
| RVEDVI (ml/m2) | 77±19 | 74±13 | 78±14 | 76±17 | 0.60 |
| RVESVI (ml/m2) | 38±13 | 35±10* | 31±7 | 34±10 | 0.80 |
| RVSVI (ml/m2) | 39±9 | 39±9 | 47±11 | 41±14 | 0.37 |
| RVEF (%) | 51±9 | 53±10 | 60±8 | 54±11* | 0.63 |
| RVMI (g/m2) | 19±4 | 16±3** | 18±4 | 17±4 | 0.17 |
| Aortic valve | |||||
| Mean PG (mm Hg) | 58 (43–73) | 21±8*** | 51 (37–66) | 35±13** | 0.017 |
| AR fraction (%) | 16±11 | 8±6* | 18±7 | 10±11 | 0.46 |
| Mitral valve | |||||
| MR fraction (%) | 20±16 | 14±23 | 2±8 | 2±6 | 0.007 |
| Late gadolinium enhancement | |||||
| Focal myocardial fibrosis | |||||
| Mass (g) | 14.1±8 | 8.6±5*** | 5.9±3 | 5.1±3 | 0.005 |
| Percentage myocardium (%) | 10.9±6 | 8.5±5* | 4.2±2 | 4.1±2 | 0.02 |
| Myocardial scar (infarction) | |||||
| Mass (g) | 20.6±12 | 13.8±11* | 22.7 | 18.0 | 0.80 |
| Percentage myocardium (%) | 15.6±10 | 11.2±9 | 10.0 | 10.0 | 0.12 |
Values are mean±SD or median (interquartile range).
ANOVA repeated measure overtime with procedure as covariate. Paired t test vs baseline: *p<0.05, **p<0.01, ***p<0.001.
AR, aortic regurgitation; EDVI, end diastolic volume indexed to body surface area; EF, ejection fraction; ESVI, end systolic volume indexed; LV, left ventricle; LVM, left ventricular mass; mean PG, peak pressure gradient; MR, mitral regurgitation; RV, right ventricle; SVI, stroke volume indexed; TAVI, transcatheter aortic valve implantation.
LV reverse remodelling
Results of the baseline and follow-up CMR scans are shown in table 2. No difference existed between the groups’ preoperative indexed measurements of end-diastolic volume (EDVI), end-systolic volume (ESVI), stroke volume (SVI), mass (LVMI), mass to volume ratio (LVM/LVEDV) and EF. Postoperatively, the TAVI group significantly decreased their ESVI, LVMI and LVM/LVEDV ratio and increased their EF. EDVI had reduced and SVI increased but did not reach statistical significance. The SAVR group experienced significant reductions in EDVI, ESVI and LVMI, postoperative SVI decreased and no significant change occurred in EF or LVM/LVEDV ratio. ANOVA showed that the greater reduction in EDVI post-SAVR compared to post-TAVI was statistically significant, yet TAVI appeared superior at reversing the geometric LV remodelling.
RV reverse remodelling
Baseline RV volumes and mass were similar between the groups (table 2). Postoperatively, TAVI resulted in a significant decrease in RV ESVI, RV mass index (RVMI) and a trend towards an increase in RV ejection fraction (RVEF) (p=0.07). SAVR resulted in non-significant reductions in EDVI and RVMI, an increase in ESVI (p=0.09) and an overall significant reduction in RVEF (p=0.04) at 6 months.
MF and infarction
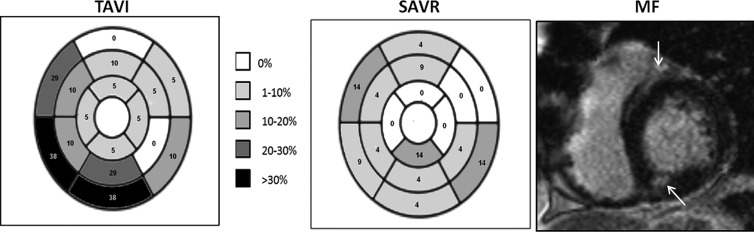
MF was assessed by LGE in 47 patients (three TAVI patients were not given contrast agent due to severe chronic kidney disease). Preoperative MF was detected in 25 (53%) patients: 13 (59%) TAVI and 12 (48%) SAVR (p=0.38). Fibrosis was predominantly distributed in the basal region and the septal segments for both groups (figure 2). The MF percentage of myocardial mass was greater in the TAVI group compared to the SAVR group (10.9±6% vs 4.2±2%, p=0.003) at baseline. The severity of AS (aortic valve area and pressure gradient) had no relationship to the amount of MF, but increased valvuloarterial impedance was associated with greater mass (g) of MF (β6.4, p=0.019). Postoperatively, MF decreased post-TAVI (10.9±6% vs 8.5±5%, p=0.03) but not post-SAVR (4.2±2% vs 4.1±2%, p=0.98) (table 2).
Figure 2.
The distribution and frequency (%) of focal myocardial fibrosis (MF) for transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) groups as represented on a 16 segment AHA model. MF was greatest in the basal and septal regions and was significantly higher in the TAVI group. A typical example of MF (as highlighted by the white arrows) is shown on a single mid-ventricular late gadolinium enhancement image.
Sub-endocardial LGE consistent with previous MI (scar) was observed in five TAVI patients compared to one SAVR patient at baseline (p<0.001). Myocardial scar (g) appeared to reduce following TAVI, but the actual scar percentage did not decrease. Post-SAVR, scar mass (g) and percentage showed no difference from baseline (table 2). New postoperative sub-endocardial infarction was evident in six individuals (one TAVI and five SAVR, p=0.11). No variable (including procedure type, p=0.09) increased the risk of new postoperative myocardial infarction.
Predictors of LV reverse remodelling
Clinical variables including patient demographics, co-morbidities and preoperative cardiac measurements were analysed to determine predictors of reverse remodelling (table 3). Worse individual baseline LV parameters (EDVI, ESVI, EF and LVMI) were independent predictors of reduced reverse remodelling following valve replacement. MF (mass and %) at baseline had no association to the degree of subsequent reverse remodelling, but increased myocardial scar (%) did, resulting in higher EDVI, ESVI and reduced EF postoperatively. The TAVI procedure, AR, mean pressure gradient and peripheral vascular disease were also predictors of adverse reverse remodelling (table 3).
Table 3.
Individual and multivariable regression analysis of clinical and CMR variables for the prediction of left ventricular reverse remodelling
| Variables | Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| B Coefficient±SD | R2 | 95% CI | p Value | B Coefficient±SD | t | 95% CI | p Value | |
| EDVI (ml/m2) | ||||||||
| EDVI | 0.57±0.12 | 0.32 | 0.33 to 0.81 | <0.001 | 0.53±0.1 | 4.56 | 0.29 to 0.76 | <0.001 |
| TAVI procedure | 15.5±3.8 | 0.50 | 7.9 to 23.1 | <0.001 | 10.43±3.7 | 2.78 | 2.8 to 18 | 0.008 |
| CAD | 11.5±4.3 | 0.42 | 3.0 to 20.1 | 0.009 | 1.87±4.1 | 0.45 | −6.5 to 10.2 | 0.65 |
| Hypercholesterolaemia | 11.9±4.1 | 0.43 | 3.7 to 20.2 | 0.005 | 6.41±3.7 | 1.73 | −1.1 to 13.9 | 0.09 |
| PVD | 14.4±6.2 | 0.39 | 1.8 to 26.9 | 0.03 | 6.31±4.9 | 1.28 | −3.6 to 16.3 | 0.20 |
| AR (%) | 0.34±0.19 | 0.38 | 0.39 to 0.71 | 0.08 | 0.3±0.1 | 2.18 | 0.02 to 0.57 | 0.04 |
| Scar (%) | 2.01±0.34 | 0.68 | 1.31 to 2.70 | <0.001 | 1.25±0.3 | 3.79 | 0.58 to 1.91 | 0.001 |
| ESVI (ml/m2) | ||||||||
| ESVI | 0.44±0.08 | 0.63 | 0.28 to 0.59 | <0.001 | 0.21±0.06 | 3.20 | 0.07 to 0.34 | 0.003 |
| TAVI procedure | 8.12±2.8 | 0.49 | 2.4 to 13.8 | 0.006 | 3.50±2.5 | 1.37 | −1.6 to 8.6 | 0.17 |
| CAD | 5.23±3.1 | 0.44 | −0.97 to 11.4 | 0.09 | 1.66±2.6 | 0.65 | −3.5 to 6.8 | 0.52 |
| AF | 8.97±4.3 | 0.45 | 0.25 to 17.7 | 0.04 | 5.29±3.3 | 1.59 | −1.4 to 11.9 | 0.12 |
| PVD | 10.6±4.2 | 0.47 | 2.1 to 19.2 | 0.02 | 7.05±3.3 | 2.12 | 0.35 to 13.7 | 0.04 |
| Scar (%) | 1.61±0.3 | 0.71 | 1.0 to 2.20 | <0.001 | 1.30±0.2 | 5.43 | 0.81 to 1.78 | <0.001 |
| EF (%) | ||||||||
| EF | 0.49±0.8 | 0.66 | 0.33 to 0.65 | <0.001 | 0.51±0.08 | 6.78 | 0.36 to 0.66 | <0.001 |
| MPG | 0.14±0.05 | 0.53 | 0.05 to 0.23 | 0.005 | 0.14±0.05 | 2.98 | 0.05 to 0.23 | 0.005 |
| LVMI (g/m2) | ||||||||
| LVMI | 0.64±0.07 | 0.67 | 0.51 to 0.77 | <0.001 | 0.69±0.07 | 9.88 | 0.55 to 0.83 | <0.001 |
| NYHA | 4.66±2.5 | 0.74 | 0.48 to 9.81 | 0.07 | 4.3±2.5 | 1.74 | −0.73 to 9.34 | 0.09 |
| CVA | 3.77±2.2 | 0.69 | −0.71 to 8.24 | 0.09 | 3.66±2.2 | 1.69 | −0.72 to 8.05 | 0.1 |
Individual variables with a significance level of p<0.1 were entered in to the multivariable model. Each parameter of change had a separate multiple regression analysis performed.
AR, aortic regurgitation; CAD, coronary artery disease; CMR, cardiovascular magnetic resonance; CVA, cerebrovascular accident; EDVI, end diastolic volume indexed to body surface area; EF, ejection fraction; ESVI, end systolic volume indexed; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; MF, myocardial fibrosis; MPG, mean pressure gradient; NYHA, New York Health Association; PVD, peripheral vascular disease; SAVR, surgical aortic valve replacement; SVI, stroke volume indexed; TAVI, transcatheter aortic valve implantation.
Discussion
This study is the first using the reference standard of CMR to show that in an older, higher risk AS population, TAVI when compared to SAVR resulted in similar levels of overall LV reverse remodelling by 6 months post-procedure. This was associated with a greater postoperative reduction in trans-aortic pressure gradient and MF in the TAVI group and an equivalent reduction in AR compared to SAVR.
Our study population demonstrated baseline concentric and eccentric structural LV remodelling processes consistent with severe AS.17 No significant difference existed between the two treatment groups’ baseline LV parameters, aortic valve haemodynamics or the majority of co-morbidities. An ‘afterload mismatch’ process is known to alter ventricular geometry, raise LVM and progress to diastolic and systolic dysfunction. These factors are recognised adverse prognostic markers pre- and post-SAVR.5 6 19 Removing the valvular impedance allows the ventricle to ‘reverse remodel’ and thus improve patient symptoms and prognosis.20 This study used CMR with its greater accuracy and reproducibility to assess these factors in a TAVI population. While comparisons of reverse remodelling between TAVI and SAVR have been previously conducted using echocardiography,21 22 the limitations of this technique in remodelled ventricles restricts their conclusions.
Improved valvular haemodynamics are markers of procedural success and influence ventricular remodelling. The superior reduction of trans-valvular pressure gradient post-TAVI has been previously noted at 6 months using echocardiography. This can be partially explained by a lower incidence of patient prosthesis mismatch compared to SAVR and may reflect smaller valve sizes inserted at surgery compared to those implanted during TAVI.11
AR is an important complication following TAVI and has been identified as an independent predictor of mortality.23 Quantifying valvular and paravalvular regurgitation is however difficult using echocardiography. CMR assesses total (paravalvular and valvular) regurgitation with high levels of accuracy. In this study TAVI actually improved ‘total’ AR from baseline and was comparable to SAVR at 6 months. As may be expected in a pressure overloaded ventricle, MR decreased post-TAVI, although any comparison to SAVR remains difficult due to differences in the severity of baseline regurgitation.
The reverse remodelling changes observed following TAVI are consistent with the current SAVR literature4 24 and include new important observations. Similar levels of LV reverse remodelling occurred between transcatheter and surgical procedures except for small differences in end-diastolic volume (EDV) and EF. The smaller reduction in EDV post-TAVI could be secondary to the greater burden of coronary artery disease and myocardial infarction in this sub-group, as both were predictors of reduced EDV reverse remodelling. The significant increase in EF following TAVI but not SAVR may be attributed to the greater reduction in aortic valvular impedance and wall stress post-TAVI.11 This greater reduction in ventricular workload could also explain the differing geometric pattern of reverse remodelling (mass/volume) which only reduced post-TAVI. However, the smaller EDVI reduction in the TAVI group (secondary to greater myocardial infarction and coronary disease) is a more probable explanation for this observation. Therefore geometric remodelling, which is an important predictor of stroke, myocardial infarction, heart failure and all cause mortality,17 is unlikely to differ between the procedures. Global ventricular work is dependent not just on valvular but also on vascular load. Valvuloarterial impedance (Zva) has been shown to adversely effect outcomes in AS patients. Our groups had similar levels of baseline Zva, indicating that this was not a confounding factor in influencing reverse remodelling between the groups.
RV reverse remodelling appeared more favourable post-TAVI, as volumes and mass reduced and function improved compared to an actual decline in RV function following SAVR. This may reflect a post-bypass phenomenon but does require further research to establish if there is a specific role for TAVI in individuals with significant right heart disease. The high pacemaker implantation rate post-TAVI meant some of our TAVI cohort could not undergo follow-up scans. As a consequence the impact of pacing on left and RV reverse remodelling could not be established.
Focal MF secondary to AS has been reported in a similar frequency and distribution to the MF in our study.25–27 MF is an adverse prognostic indicator and is associated with reduced reverse remodelling post-SAVR.12 28 In this study it was the quantity of baseline scar (infarction), not focal MF that was associated with worse postoperative ventricular volumes and function. Following multivariate analysis the baseline LV parameters also remained significant predictors of reverse remodelling. This observation supports the theory that MF does not predict reduced reverse remodelling,29 but is associated with poor baseline volumes and function, which are the true independent predictors of adverse reverse remodelling. Fibrosis has been found not to regress substantially post-AVR using histological29 and diffuse equilibrium measurements.30 Our study, using a less specific but well validated technique of LGE, found similar results post-SAVR but evidence of regression post-TAVI. This finding needs to be validated using a more sensitive technique such as T1 mapping, as it may reflect greater reverse remodelling post-TAVI at the cellular level rather than a true reduction in fibrosis.
Post-procedural subendocardial myocardial infarction occurred more frequently in the SAVR group compared to the TAVI group. This has not been previously described and its clinical significance is limited by the small patient numbers involved. However, concerns related to covering the coronary ostia with the CoreValve device and crushing the calcified native aortic valve leaflets do not seem to result in myocardial infarction as detectable on CMR. Equally it may suggest that perioperative myocardial protection in severely hypertrophied ventricles is suboptimal in surgically treated patients.
Limitations
Although this was a small study population, comparisons between the two groups using the highly reproducible technique of CMR meant it was appropriately powered. Patients in the two treatment groups had similar risk factor profiles, but due to the nature of current guidelines for TAVI patient selection, they could not be matched for age or EuroSCORE. Despite the positive selection of higher risk SAVR candidates, this hampers our direct comparison of SAVR versus TAVI. Finally, quantification of fibrosis on LGE images was analysed using a semi-automatic, signal intensity threshold method rather than the newer T1 mapping techniques, as the latter were not widely employed at the time of patient recruitment. However, as of yet there is no consensus as to which of the multitude of T1 mapping techniques should be employed in myocardial interstitial disease.
Conclusions
This study provides evidence of significant reverse remodelling post-TAVI in a high-risk AS population with multiple adverse prognostic factors such as old age, high LVM, reduced LV systolic function and substantial MF. TAVI was comparable to SAVR in terms of global LV reverse remodelling. Baseline LV measurements and myocardial scar (infarction) were the dominant factors predicting change in reverse remodelling for both TAVI and SAVR. TAVI significantly reduced the trans-aortic pressure gradient and AR at 6 months, and when compared to SAVR produced a greater reduction in focal MF.
Acknowledgments
The authors acknowledge Petra Bijsterveld and Fiona Richards for patient recruitment.
Footnotes
Contributors: TAF: design, collection, analysis and interpretation of data, drafting and revision of manuscript. CDS: collection, analysis and interpretation of data and revision of manuscript. ANM, MM, DJB, SP: analysis and interpretation of data and revision of manuscript. DJB: conception, design, collection and interpretation of data; revision of manuscript. GPM: design, collection, analysis and interpretation of data and revision of manuscript. JPG: conception, design, collection, interpretation and analysis of data; drafting and revision of manuscript.
Funding: This study was part-funded by the British Heart Foundation (BHF) (PG/11/126/29321). GPM and CDS have received support from the NIHR Leicester Cardiovascular BRU.
Competing interests: DB is a proctor for Medtronic CoreValve.
Ethics approval: Leeds West Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11 [DOI] [PubMed] [Google Scholar]
- 2.Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol 2006;47:2141–51 [DOI] [PubMed] [Google Scholar]
- 3.Sandstede JJ, Beer M, Hofmann S, et al. Changes in left and right ventricular cardiac function after valve replacement for aortic stenosis determined by cine MR imaging. J Magn Reson Imaging 2000;12:240–6 [DOI] [PubMed] [Google Scholar]
- 4.Sandstede JJ, Johnson T, Harre K, et al. Cardiac systolic rotation and contraction before and after valve replacement for aortic stenosis: a myocardial tagging study using MR imaging. AJR Am J Roentgenol 2002;178:953–8 [DOI] [PubMed] [Google Scholar]
- 5.Gaudino M. Survival after aortic valve replacement for aortic stenosis: does left ventricular mass regression have a clinical correlate? Eur Heart J 2004;26:51–7 [DOI] [PubMed] [Google Scholar]
- 6.Lund O, Flo C, Jensen FT, et al. Left ventricular systolic and diastolic function in aortic stenosis. Prognostic value after valve replacement and underlying mechanisms. Eur Heart J 1997;18:1977–87 [DOI] [PubMed] [Google Scholar]
- 7.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366(18):1696–704 [DOI] [PubMed] [Google Scholar]
- 8.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98 [DOI] [PubMed] [Google Scholar]
- 9.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607 [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1056/NEJMoa1200384. Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366(18):1686–95. [DOI] [PubMed] [Google Scholar]
- 11.Clavel M-A, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009;53:1883–91 [DOI] [PubMed] [Google Scholar]
- 12.Azevedo CF, Nigri M, Higuchi ML, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278–87 [DOI] [PubMed] [Google Scholar]
- 13.Caruthers SD, Lin SJ, Brown P, et al. Practical value of cardiac magnetic resonance imaging for clinical quantification of aortic valve stenosis: comparison with echocardiography. Circulation 2003;108:2236–43 [DOI] [PubMed] [Google Scholar]
- 14.Cawley PJ, Hamilton-Craig C, Owens DS, et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging 2013;6:48–57 [DOI] [PubMed] [Google Scholar]
- 15.Kwong RY, Farzaneh-Far A. Measuring myocardial scar by CMR. JACC Cardiovasc Imaging 2011;4:157–60 [DOI] [PubMed] [Google Scholar]
- 16.Fairbairn TA, Mather AN, Bijsterveld P, et al. Diffusion-weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart 2012;98:18–23 [DOI] [PubMed] [Google Scholar]
- 17.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 2011;58:1733–40 [DOI] [PubMed] [Google Scholar]
- 18.Bellenger NG, Davies LC, Francis JM, et al. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2000;2:271–8 [DOI] [PubMed] [Google Scholar]
- 19.Lund O. Regression of left ventricular hypertrophy during 10 years after valve replacement for aortic stenosis is related to the preoperative risk profile. Eur Heart J 2003;24:1437–46 [DOI] [PubMed] [Google Scholar]
- 20.Biederman RW, Doyle M, Yamrozik J, et al. Physiologic compensation is supranormal in compensated aortic stenosis: does it return to normal after aortic valve replacement or is it blunted by coexistent coronary artery disease? An intramyocardial magnetic resonance imaging study. Circulation 2005;112(9 Suppl):I429–36 [DOI] [PubMed] [Google Scholar]
- 21.Clavel MA, Webb JG, Rodes-Cabau J, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 2010;122:1928–36 [DOI] [PubMed] [Google Scholar]
- 22.Forsberg LM, Tamas E, Vanky F, et al. Left and right ventricular function in aortic stenosis patients 8 weeks post-transcatheter aortic valve implantation or surgical aortic valve replacement. Eur J Echocardiogr 2011;12:603–11 [DOI] [PubMed] [Google Scholar]
- 23.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299–308 [DOI] [PubMed] [Google Scholar]
- 24.Biederman RWW, Magovern JA, Grant SB, et al. LV reverse remodeling imparted by aortic valve replacement for severe aortic stenosis; is it durable? A cardiovascular MRI study sponsored by the American Heart Association. J Cardiothoracic Surg 2011;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudolph A, Abdel-Aty H, Bohl S, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol 2009;53:284–91 [DOI] [PubMed] [Google Scholar]
- 26.Weidemann F, Herrmann S, Stork S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577–84 [DOI] [PubMed] [Google Scholar]
- 27.Nigri M, Azevedo CF, Rochitte CE, et al. Contrast-enhanced magnetic resonance imaging identifies focal regions of intramyocardial fibrosis in patients with severe aortic valve disease: correlation with quantitative histopathology. Am Heart J 2009;157:361–8 [DOI] [PubMed] [Google Scholar]
- 28.Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271–9 [DOI] [PubMed] [Google Scholar]
- 29.Krayenbuehl HP, Hess OM, Monrad ES, et al. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 1989;79:744–55 [DOI] [PubMed] [Google Scholar]
- 30. doi: 10.1093/ehjci/jes102. Flett AS, Sado DM, Quarta G, et al. Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2012;13(10):819–26. [DOI] [PubMed] [Google Scholar]