Abstract
Based on recent clinical practice guidelines, imaging is largely replacing pathology as the preferred diagnostic method for determination of hepatocellular carcinoma (HCC). A variety of imaging modalities, including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), nuclear medicine, and angiography, are currently used to examine patients with chronic liver disease and suspected HCC. Advancements in imaging techniques such as perfusion imaging, diffusion imaging, and elastography along with the development of new contrast media will further improve the ability to detect and characterize HCC.
Early diagnosis of HCC is essential for prompt treatment, which may in turn improve prognosis. Considering the process of hepatocarcinogenesis, it is important to evaluate sequential changes via imaging which would help to differentiate HCC from premalignant or benign lesions. Recent innovations including multiphasic examinations, high-resolution imaging, and the increased functional capabilities available with contrast-enhanced US, multidetector row CT, and MRI have raised the standards for HCC diagnosis. Although hemodynamic features of nodules in the cirrhotic liver remain the main diagnostic criterion, newly developed cellspecific contrast agents have shown great possibilities for improved HCC diagnosis and may overcome the diagnostic dilemma associated with small or borderline hepatocellular lesions. In the 20th century paradigm of medical imaging, radiological diagnosis was based on morphological characteristics, but in the 21st century, a paradigm shift to include biomedical, physiological, functional, and genetic imaging is needed. A multidisciplinary team approach is necessary to foster an integrated approach to HCC imaging. By developing and combining new imaging modalities, all phases of HCC patient care, including screening, diagnosis, treatment, and therapy, can be dramatically improved.
Key Words: CT, Hepatocarcinogenesis, Hepatocellular carcinoma, MRI, Ultrasonography
Introduction
Hepatocellular carcinoma (HCC) is the 6th most common malignancy worldwide, representing 6% of all cancers. It is highly prevalent in Asia and Sub-Saharan Africa and is currently increasing in Western countries [1, 2, 3, 4, 5, 6, 7]. Majority of HCCs develop in patients with risk factors such as chronic hepatitis B or C and non-viral liver cirrhosis, which may be associated with alcoholic liver disease or non-alcoholic fatty liver disease [5, 8, 9]. Unfortunately, HCC is a devastating cancer with a five-year survival rate of <5% when diagnosed at an advanced stage [10]. Because early diagnosis of HCC followed by prompt treatment can increase patient survival, HCC surveillance is important, particularly in high-risk populations [10, 11, 12].
Imaging studies play a key role in HCC diagnosis. According to recent clinical practice guidelines for HCC, use of imaging techniques is increasing and the importance of biopsy is decreasing [13, 14, 15, 16, 17]. Classically, HCC diagnosis with imaging techniques is based on enhancing patterns according to the time sequence or phase, experienced as high attenuation or signal intensity in the arterial phase and a washout pattern in the portal venous and equilibrium phases.
Imaging tools for HCC include ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), angiography, and fusion imaging. These techniques have continuously evolved during recent decades, driving a paradigm shift in HCC imaging. Herein we present a review of imaging techniques for HCC with a focus on recent progress, diagnosis of hepatocarcinogenesis using these methods, current guidelines, and future perspectives.
Recent Imaging Techniques
Ultrasound
Contrast-enhanced US
Contrast-enhanced US (CEUS) is useful for the characterization of focal liver lesions. Using microbubble contrast agents, it is possible to obtain hemodynamic information from hepatic nodules with multiphasic US images on a real-time basis, making it feasible to characterize HCC and to differentiate it from other hepatocellular nodules related to cirrhosis [18, 19, 20, 21, 22]. Second-generation contrast agents, such as SonoVue® or Definity®, are useful for the assessment of tumor vascularity because these agents can be used in continuous bubble imaging at a low mechanical index. With CEUS, typical findings related to HCC are hypervascularity of the lesion relative to the liver parenchyma in the arterial phase and washout in the portal venous or equilibrium phase, which are similar to those obtained with CT and MRI [20, 22, 23, 24].
A new contrast agent, Sonazoid™, has recently been introduced in Japan. Because Sonazoid is taken up by Kupffer cells, it allows for the evaluation of hepatic nodules in the vascular phase as well as the Kupffer (post-vascular) phase. HCC shows hypervascularity in the vascular phase and defects in the Kupffer phase with Sonazoid CEUS; therefore, this agent is useful in the diagnosis and estimation of the histological grade of HCC [25, 26, 27, 28]. Recently, Kudo et al. reported innovative defect reperfusion US imaging as a very useful method for the detection and characterization of HCC [29].
US Elastography
US elastography is a technique for studying the stiffness of tissue. While the concept is similar to that of manual palpation, elastography, a virtual palpation technique, can provide more quantitative and objective information than manual palpation.
Recently, shear wave elasticity imaging (SWEI) was introduced for use with deep organs including the liver [30]. There are currently three SWEI techniques: transient elastography (Fibroscan®), acoustic radiation force impulse imaging, and supersonic shear imaging. Because the degree of liver fibrosis is a predictive factor for HCC development [31, 32], identification of the presence and severity of liver fibrosis is important. Many studies have reported the efficacy and usefulness of US elastography for the evaluation of liver fibrosis by measuring the stiffness of the liver [33, 34, 35, 36, 37, 38, 39, 40, 41, 42]. Therefore, US elastography is a promising non-invasive surrogate marker for evaluating liver fibrosis and can be used as an alternative to liver biopsy.
Volumetric US
Volumetric US has progressed because of the development of the transducer, which performs volume acquisition via freehand acquisition through mechanical or electronic scanning [43, 44]. Nowadays, the number of transducer elements currently used is greater than 9,000.
Volumetric US provides three-dimensional (3D) anatomic information, which is useful in clinical practice. It can measure the size of organs and lesions more precisely than conventional two-dimensional US, which helps in diagnosis and monitoring of treatment response [45, 46, 47, 48]. This technique also facilitates needle localization for local-regional HCC treatment and biopsies of indeterminate hepatic nodules [49].
3D visualization of tumor vessels including feeding arteries is possible with the 3D power Doppler US imaging technique; thus this technique is helpful in HCC diagnosis and is a possible alternative to angiography [50, 51, 52]. In addition, 3D CEUS may be a useful method for the evaluation of therapeutic efficacy of local-regional HCC treatment [53, 54, 55].
CT
Dual energy CT
The clinical incentive to use dual energy CT (DECT) is that DECT can measure chemical composition by the dual energy index. This index characterizes the spectral behavior of material. The potential clinical applications of this technology include virtual non-contrast imaging, determination of biliary stone composition, estimation of average iron or fat content in the liver, and perfusion of the liver [56, 57]. Given that iodinated contrast material provides greater X-ray attenuation at low tube voltage settings, low kVp images of dual energy datasets might be more sensitive for the detection of hypervascular lesions such as HCC than high kVp images, but may result in an increase in high image noise [58, 59, 60, 61]. Using blending techniques of dual energy datasets, images with the contrast of the low kVp images and the noise characteristics of the high kVp images can be created [62, 63, 64].
Perfusion CT
Perfusion CT is an in vivo functional imaging. It provides quantitative data regarding perfusion parameters and differentiates diverse tumor tissues based on perfusion behavior [65]. Because perfusion parameters reflect tumor vascularity, this is regarded as a useful tool for monitoring the response to anti-angiogenic drug treatment [66, 67, 68, 69] and local-regional treatment in HCC patients [70, 71, 72, 73]. However, a major problem with perfusion CT is high radiation exposure, making it difficult to use this technique for HCC surveillance or serial examinations for the evaluation of treatment response [74, 75].
MRI
Diffusion-weighted MRI
MR diffusion-weighted imaging (DWI) is a technique that obtains image contrasts based on differences in the motion of water molecules between tissues [76]. Because recent advances in MRI have overcome motion-related problems, DWI is widely used for abdominal imaging.
DWI does not require contrast agents and has a short acquisition time [77, 78]. Therefore, many recent studies have examined its clinical applications, especially for oncologic imaging. In terms of HCC, DWI can improve lesion detection [79, 80, 81], predict the histological grade of HCC [82, 83, 84, 85], and assess treatment response and recurrence [86, 87, 88, 89, 90].
MR elastography
MR elastography (MRE] is an emerging technique that allows for the quantitative assessment of the mechanical properties of tissues. In the field of HCC surveillance, as mentioned previously, detection and quantification of liver fibrosis is quite important. Based on the results of recent studies, MRE is a non-invasive, reproducible, and accurate method for the quantitative assessment of liver fibrosis. It can be used to differentiate normal liver from fibrotic liver and evaluate the stage of fibrosis [91, 92, 93, 94, 95, 96, 97, 98]. Venkatesh et al. reported that MRE would be a promising tool for assessing solid liver tumors by differentiating them from benign and malignant liver tumors [99]. Further investigations are needed to clarify the value of MRE for focal liver lesions.
MRI using new contrast media
Recently, hepatocyte-specific contrast agents such as gadoxetic acid (Primovist; Bayer Healthcare, Berlin, Germany) and gadobenate dimeglumine (MultiHance; Bracco, Milan, Italy) have become commercially available. These agents are taken up by normally functioning hepatocytes and are excreted into the biliary system. Because hepatocyte-specific contrast agents have a biphasic nature, the perfusion function in the vascular phase and the hepatocyte function in the hepatobiliary phase can be evaluated [100, 101, 102]. Dynamic MRI using extracellular contrast agents provides sufficient information to make a confident diagnosis of typical enhancing HCC. However, there are hypovascular HCCs and hypervascular HCCs without washout. Thus, in addition to the enhancement pattern, more information is needed to diagnose indeterminate nodules. Hepatocyte-specific contrast agents (so-called dual functional agents) may provide additional functional information that can improve the detection and characterization of HCCs [103, 104, 105, 106, 107, 108, 109, 110].
Hybrid Imaging
Hybrid imaging such as positron emission tomography (PET)-CT, single photon emission computed tomography (SPECT)-CT, MR-PET, MR-optical imaging (OI), and virtual US can be used for HCC diagnosis and treatment monitoring.
Hybrid imaging with MR-PET is an emerging technique providing high soft tissue contrast as well as functional information for the evaluation of tissue microenvironment and cellular and molecular processes. There have been several reports concerning the usefulness of MR-PET for liver tumors [111, 112].
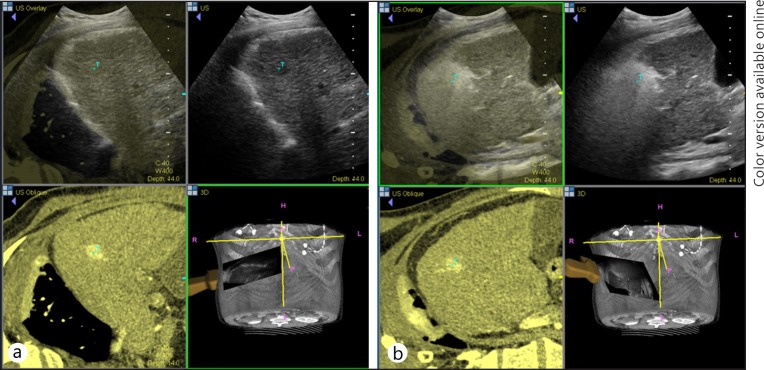
Virtual US, a fusion imaging technique that combines US with other imaging modalities such as CT or MRI, may be helpful in HCC diagnosis [113, 114] and can be applied for local treatment or biopsy of hepatic lesions, particularly those lesions that are poorly visualized with US alone [115, 116] (fig. 1).
Fig. 1.
Real-time virtual US with a simultaneous display of US and contrast-enhanced CT images. A 1.5-cm HCC located in segment seven of the liver in a patient with liver cirrhosis. a Using a hybrid imaging technique, the CT-detected hypervascular nodule can be found on US. b After radiofrequency ablation of the tumor, the safety margin can be assessed by registering pre-procedural images with post-procedural images.
Imaging Diagnosis of Hepatocarcinogenesis
The role of imaging for HCC surveillance is early detection and characterization; therefore, an adequate understanding of hepatocarcinogenesis is necessary. There are two pathways involved: one is the de novo pathway and the other is a multistep pathway. The de novo pathway involves the development of HCC without a background of chronic liver disease or liver cirrhosis. The multistep pathway involves the development of HCC with a background of liver cirrhosis from regenerating nodules (RN) going through low-grade (LG) dysplastic nodules (DN), high-grade (HG) DN, early HCC, and finally advanced HCC [117, 118]. In terms of histopathological changes during hepatocarcinogenesis, hemodynamic and molecular profiles are altered progressively [119, 120, 121, 122]. Imaging tools for evaluating hepatocarcinogenesis include contrast-enhanced US, CT, MR, angio-CT for assessing hemodynamic changes and liver-specific imaging using a reticuloendothelial system (RES) agent or a hepatocyte-specific contrast agent for assessing cellular and functional changes.
Hemodynamic Changes
When a nodule becomes DN during hepatocarcinogenesis, normal hepatic arterial flow is decreased while portal venous flow is maintained. In cases of early HCC, abnormal hepatic arterial flow increases and portal venous flow decreases. Finally, in cases of advanced HCC, the tumor is supplied only by the abnormal hepatic artery and is usually seen as a hypervascular lesion on imaging studies [119, 123]. Such intranodular hemodynamic changes can be well visualized with CT hepatic angiography and CT arterial portography [118, 121, 124, 125].
Kupffer Cells
We can evaluate Kupffer cells in the liver by immunohistochemical staining with the anti-human macrophage antibody anti-CD68. According to previous studies, a decrease in the number of Kupffer cells may play an important role in hepatocarcinogenesis [126, 127]. Superparamagnetic iron oxide (SPIO) particles are a MR contrast media that are sequestered by phagocytic Kupffer cells in the normal RES. Because the degree of enhancement of SPIO-MR is correlated with the number of Kupffer cells, SPIO-MRI might be helpful in differentiating HCC from DN and predicting the histological grade of HCC [128, 129, 130, 131, 132].
Bile Duct
In hepatocarcinogenesis, normal bile canaliculi progressively decrease and are replaced by tumor cells. Carcinoembryonic antigen (CEA) immunostaining is useful for the demonstration of bile canaliculi in pathological specimens. The presence of many bile canaliculi in RN indicates normal biliary function, whereas the presence of sparse bile canaliculi in HCC indicates deficient biliary function [133].
Dual Function Agent-Perfusion and Hepatocyte Function
The benefit of hepatobiliary phase imaging using hepatocyte-specific contrast agents, such as Sonazoid in US or gadoxetic acid in MRI, is that it enables homogeneous, strong, and prolonged enhancement of the liver parenchyma, which permits better detection of small HCCs. In addition, knowledge regarding the functional status of hepatocytes makes it possible to differentiate between DN and HCC and between HCC and arterioportal shunts [134, 135, 136, 137, 138] and to evaluate hepatic function [139, 140, 141]. In terms of differential diagnosis of DN and HCC, LGDN shows high signal intensity on hepatobiliary phase images, indicating the presence of functional hepatocytes. HGDN shows decreased signal intensity, and HCC usually shows a clear defect on hepatobiliary phase images.
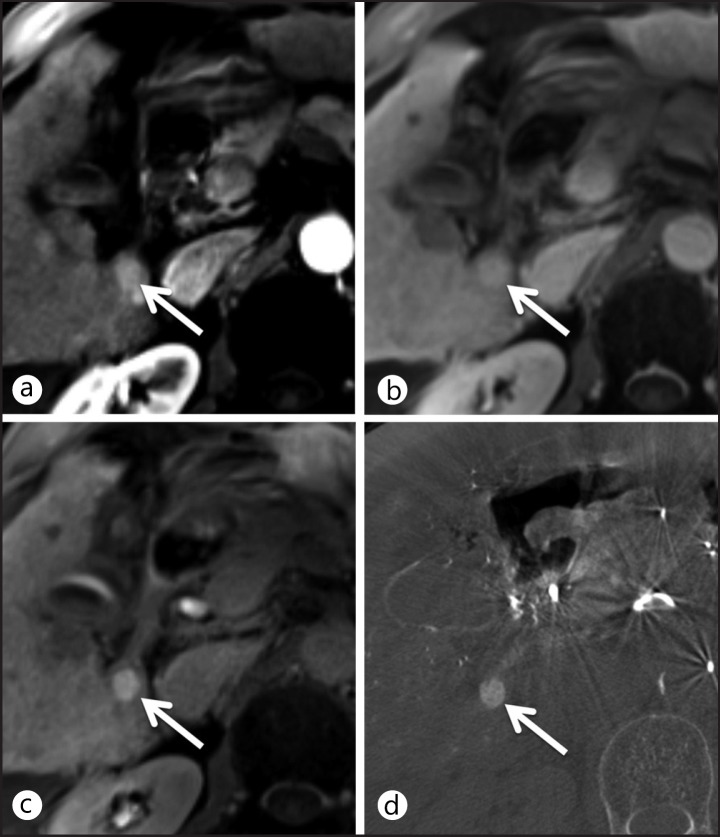
Recently, investigators reported on the transport mechanism of gadoxetic acid in HCC. Gadoxetic acid is taken up into hepatocytes by organic anion-transporting polypeptide 8 (OATP8) and excreted into the biliary system by multidrug resistance-associated protein 2 (MRP2) [142, 143, 144]. As hepatic nodules become more malignant, OATP8 expression usually decreases. Therefore, uptake of gadoxetic acid decreases, resulting in low signal intensity of HGDN and HCC in the hepatobiliary phase whereas high signal intensity of LGDN. However, approximately 10% of overt HCCs also show iso or high signal intensity in the hepatobiliary phase (fig. 2). This phenomenon can be explained by a genetic alteration that results in the overexpression of OATP8 and MRP2 [142, 145, 146].
Fig. 2.
Transverse MR images obtained in the arterial (a) and portal-venous (b) phases show an arterial enhancing nodule without washout. This nodule shows high signal intensity in the hepatobiliary phase (c), which is an uncommon finding of HCC. (d) Angio-CT and transarterial chemoembolization confirm HCC diagnosis.
Histopathology and Functional Imaging
The International Pathology Consensus Group for Hepatocellular Neoplasia has published an interesting, evolving concept, i.e., pathological and imaging features define the phases in the evolution of neoplasia in the cirrhotic liver [147]. According to this idea, we must consider not only pathological features but also imaging findings in the evaluation of hepatocarcinogenesis.
Molecular pathological tools for hepatocarcinogenesis are mainly immunohistochemical stains. An antibody against CD34 is used for sinusoidal capillarization, α-smooth muscle actin for unpaired artery, CD68 for Kupffer cells, and CEA for bile canaliculi [126, 133, 148]. For imaging evaluation, various contrast agents, such as extracellular contrast agents, RES agents, and hepatocyte-specific agents, can be used to obtain functional images that reflect molecular pathological features of hepatocarcinogenesis [100, 149]. As molecular and imaging techniques advance and develop, further studies are needed to correlate pathological and imaging features in hepatocarcinogenesis and document their usefulness in clinical practice.
Current HCC Imaging Guidelines
Since the announcement of European Association for the Study of the Liver guidelines in 2000 [150], imaging diagnosis of HCC has become more significant. Therefore, use of dynamic US, CT, and MRI for HCC diagnoses has increased while use of biopsies has decreased. According to the American Association for the Study of Liver Diseases (AASLD) guidelines in 2005 and the updated guidelines in 2010, the first step to diagnosing HCC in liver cirrhosis is US. If a nodule is detected on US examination, the next step depends on its size. If the nodule is <1 cm, follow-up US is recommended, whereas if it is >1 cm, further contrast-enhanced imaging evaluation such as CT or MRI with typical imaging findings is required for HCC diagnosis [151, 152]. Typical findings for confirming HCC are high attenuation or signal intensity in the arterial phase and a washout pattern in the portal venous and equilibrium phases. If a nodule does not show a characteristic enhancement pattern, a second contrast-enhanced study with another imaging modality (CT or MRI) should be conducted (fig. 3). According to the Asia Pacific Association for the Study of the Liver (APASL) consensus guidelines in 2010, when a nodule shows hypervascularity in the arterial phase on dynamic CT and/or MRI and washout in the portal venous or delayed phase, a non-invasive diagnosis of HCC can be made, regardless of the size of the lesion. Moreover, when a nodule shows no washout in the portal venous or delayed phase on initial diagnostic tests with dynamic CT and/or MRI, secondary imaging studies such as Sonazoid CEUS or SPIO-MRI can be used instead of biopsy [13, 16]. In summary, there are two differences between AASLD and APASL guidelines. First, there are no size criteria in APASL guidelines. Second, in case of hypovascular nodules on dynamic CT or MRI, secondary diagnostic tests using Kupffer-specific agents instead of biopsy are recommended by APASL guidelines.
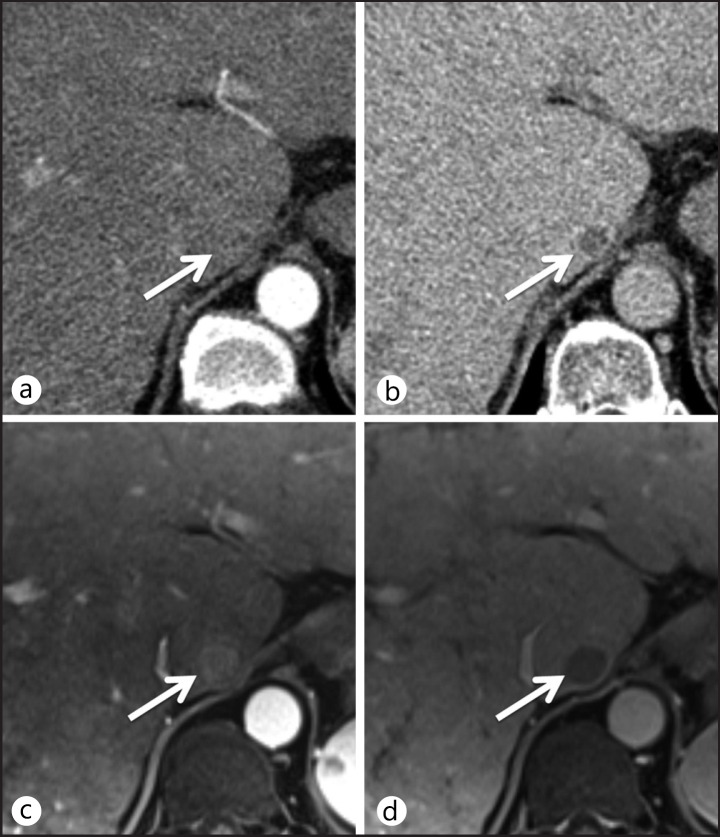
Fig. 3.
HCC with atypical enhancing pattern on dynamic CT and typical enhancing pattern on MRI. On dynamic CT images, a 1.5-cm nodule is seen in the caudate lobe of the liver without hypervascularity in the arterial phase (a) and with washout in the delayed phase (b). For this hypovascular nodule, an imaging diagnosis of HCC based on CT findings cannot be made. Dynamic MRI performed as a secondary imaging test, shows nodule enhancement in the arterial phase (c) and washout in the portal-venous phase (d). MRI revealed a typical enhancement pattern of HCC, permitting diagnosis as HCC without biopsy according to the AASLD 2010 guidelines.
There are several problems associated with the current guidelines. Although US is widely used as a screening test for HCC, it has shown limited sensitivity for detecting early-stage HCC. Recent studies revealed that surveillance with US in patients with cirrhosis detected early-stage HCC with a sensitivity of approximately 60% [153, 154]. Dynamic CT or MRI may be used as a primary imaging test, but HCC with an atypical enhancement pattern is not rare, making it difficult to differentiate between HCC and other mimickers [155, 156]. Therefore, guidelines should be continuously re-evaluated and updated.
There are many guidelines from different regions and countries, such as Barcelona Clinic Liver Cancer, APASL, Korean Liver Cancer Study Group, and Japan Society of Hepatology guidelines that address HCC treatment [15, 16, 157, 158]. Current treatment methods for HCC include surgery, intervention, and systemic chemotherapy. Recently, local-regional HCC treatment has been progressing rapidly. Local ablation therapies include chemical ablation via ethanol injection and thermal ablation such as radiofrequency ablation (RFA), microwave ablation, and high-intensity focused US [159, 160, 161, 162, 163]. For regional (intravascular) therapies, in addition to conventional transarterial chemoembolization (TACE), TACE with drug-eluting beads or radioembolization has now been intensively investigated [164, 165, 166, 167].
Summary and Future Perspectives of HCC Imaging
Imaging technology is continuously evolving and becoming more important in HCC diagnosis. In the early 20th century, no useful imaging modality for liver imaging existed. Only simple abdominal radiography was available. However, currently, we have several powerful imaging modalities for HCC. Among these modalities, US is used as a screening technique, and CT is a standard technique widely accepted by clinicians because of its fast speed, wide availability, and good capability for tumor depiction and characterization. The role of MRI is rapidly expanding as a tool complementary to US and CT and as an analytical tool for hepatic nodules.
The paradigm for HCC imaging in the 20th century consisted of gross morphological imaging-pathology correlation. However, the new 21st century paradigm is biochemical, physiological, and functional imaging correlated with molecular diagnostics, in other words, correlation of radiophenotype and molecular phenotype.
In carcinogenesis, from the conversion of a normal cell to invasive cancer, the hallmarks of cancer are manifested from metabolic reprogramming [168, 169]. Therefore, functional imaging would depict these metabolic processes and hallmarks at the tumor level [170].
Radiogenomics was recently introduced as an emerging technology in the field of radiology. Radiogenomics is an integration of in vivo imaging with large-scale gene expression profiles, in other words, an integration of radiophenotypes with molecular phenotypes [171, 172]. As a surrogate for gene expression, radiophenotypes can be used for the molecular assessment of tumors for diagnosis and staging, prediction of prognosis, and determination of HCC treatment [173]. Kuo et al. reported that radiophenotypes of HCC showed an association with drug response gene expression programs [174].
Multiparametric imaging is now being actively investigated. By combining the information derived from multiple imaging techniques and modalities, we can obtain more detailed information about tumor biology, thus multiparametric imaging would be useful for drug development and predicting therapeutic efficacy [170, 175].
Personalized medicine is the key to future drug development, providing individualized care and treatment based on personal and genetic variations. This new concept of personalized medicine will be wildly applied to HCC [176, 177, 178, 179]. The risk of HCC development can be predicted by gene expression or DNA sequencing, and early diagnoses can be made based on various imaging techniques, thereby providing customized treatment for each patient.
In conclusion, future imaging of HCC will include gross morphological imaging, microimaging such as micro-CT/MR/PET, functional imaging, and molecular imaging. The information obtained will be evaluated on the basis of anatomy via dynamic functional imaging, molecular imaging, and genetic imaging with collaboration of physiology, biochemistry, and biology. Therefore, a multidisciplinary, multimodality team approach is mandatory for the diagnosis and treatment of HCC in the future.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.El-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/s1089-3261(05)70155-0. vi. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Tabor E. Hepatocellular carcinoma: global epidemiology. Dig Liver Dis. 2001;33:115–117. doi: 10.1016/s1590-8658(01)80062-1. [DOI] [PubMed] [Google Scholar]
- 4.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl 2):S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 8.Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346–353. doi: 10.1111/j.1440-1746.2009.05784.x. [DOI] [PubMed] [Google Scholar]
- 9.Nagaoki Y, Hyogo H, Aikata H, Tanaka M, Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S, Hiramatsu A, Waki K, Imamura M, Kawakami Y, Takahashi S, Chayama K. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. DOI:10.1111/j.1872-034X.2011.00929.x. [DOI] [PubMed]
- 10.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Kim JW, Kuromatsu R, Ahn SH, Torimura T, Sherman M. Controversies in surveillance and early diagnosis of hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):56–60. doi: 10.1159/000333261. [DOI] [PubMed] [Google Scholar]
- 12.Amarapurkar D, Han KH, Chan HL, Ueno Y. Application of surveillance programs for hepatocellular carcinoma in the Asia-Pacific Region. J Gastroenterol Hepatol. 2009;24:955–961. doi: 10.1111/j.1440-1746.2009.05805.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan CH, Low SC, Thng CH. APASL and AASLD Consensus Guidelines on Imaging Diagnosis of Hepatocellular Carcinoma: A Review. Int J Hepatol. DOI:10.4061/2011/519783. [DOI] [PMC free article] [PubMed]
- 14.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 15.Practice guidelines for management of hepatocellular carcinoma. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 16.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, Shiina S, Cheng AL, Jia JD, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica MI, Davila R, Ensminger WD, Gibbs JF, Laheru D, Malafa MP, Marrero J, Meranze SG, Mulvihill SJ, Park JO, Posey JA, Sachdev J, Salem R, Sigurdson ER, Sofocleous C, Vauthey JN, Venook AP, Goff LW, Yen Y, Zhu AX. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgio A, Calisti G, di Sarno A, Farella N, de Stefano G, Scognamiglio U, Giorgio V. Characterization of dysplastic nodules, early hepatocellular carcinoma and progressed hepatocellular carcinoma in cirrhosis with contrast-enhanced ultrasound. Anticancer Res. 2011;31:3977–3982. [PubMed] [Google Scholar]
- 19.Giorgio A, De Stefano G, Coppola C, Ferraioli G, Esposito V, Di Sarno A, Giorgio V, De Stefano M, Sangiovanni V, Liorre G, Del Viscovo L. Contrast-enhanced sonography in the characterization of small hepatocellular carcinomas in cirrhotic patients: comparison with contrast-enhanced ultrafast magnetic resonance imaging. Anticancer Res. 2007;27:4263–4269. [PubMed] [Google Scholar]
- 20.Inoue T, Kudo M, Maenishi O, Komuta M, Nakashima O, Kojiro M, Maekawa K. Value of liver parenchymal phase contrast-enhanced sonography to diagnose premalignant and borderline lesions and overt hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:698–705. doi: 10.2214/AJR.07.3282. [DOI] [PubMed] [Google Scholar]
- 21.Kim TK, Lee KH, Khalili K, Jang HJ. Hepatocellular nodules in liver cirrhosis: contrast-enhanced ultrasound. Abdom Imaging. 2011;36:244–263. doi: 10.1007/s00261-011-9686-0. [DOI] [PubMed] [Google Scholar]
- 22.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349–361. doi: 10.7863/jum.2006.25.3.349. [DOI] [PubMed] [Google Scholar]
- 23.Jang HJ, Kim TK, Wilson SR. Small nodules (1-2 cm) in liver cirrhosis: characterization with contrast-enhanced ultrasound. Eur J Radiol. 2009;72:418–424. doi: 10.1016/j.ejrad.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Jang HJ, Yu H, Kim TK. Contrast-enhanced ultrasound in the detection and characterization of liver tumors. Cancer Imaging. 2009;9:96–103. doi: 10.1102/1470-7330.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arita J, Hasegawa K, Takahashi M, Hata S, Shindoh J, Sugawara Y, Kokudo N. Correlation between contrast-enhanced intraoperative ultrasound using Sonazoid and histologic grade of resected hepatocellular carcinoma. AJR Am J Roentgenol. 2011;196:1314–1321. doi: 10.2214/AJR.10.4310. [DOI] [PubMed] [Google Scholar]
- 26.Arita J, Takahashi M, Hata S, Shindoh J, Beck Y, Sugawara Y, Hasegawa K, Kokudo N. Usefulness of contrast-enhanced intraoperative ultrasound using Sonazoid in patients with hepatocellular carcinoma. Ann Surg. 2011;254:992–999. doi: 10.1097/SLA.0b013e31822518be. [DOI] [PubMed] [Google Scholar]
- 27.Hatanaka K, Chung H, Kudo M, Haji S, Minami Y, Maekawa K, Hayaishi S, Nagai T, Takita M, Kudo K, Ueda T, Tatsumi C, Kitai S, Ishikawa E, Yada N, Inoue T, Hagiwara S, Ueshima K. Usefulness of the post-vascular phase of contrast-enhanced ultrasonography with sonazoid in the evaluation of gross types of hepatocellular carcinoma. Oncology. 2010;78(Suppl 1):53–59. doi: 10.1159/000315231. [DOI] [PubMed] [Google Scholar]
- 28.Korenaga K, Korenaga M, Furukawa M, Yamasaki T, Sakaida I. Usefulness of Sonazoid contrast-enhanced ultrasonography for hepatocellular carcinoma: comparison with pathological diagnosis and superparamagnetic iron oxide magnetic resonance images. J Gastroenterol. 2009;44:733–741. doi: 10.1007/s00535-009-0053-7. [DOI] [PubMed] [Google Scholar]
- 29.Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010;78(Suppl 1):40–45. doi: 10.1159/000315229. [DOI] [PubMed] [Google Scholar]
- 30.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 31.Akima T, Tamano M, Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. Hepatol Res. 2011;41:965–970. doi: 10.1111/j.1872-034X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 32.Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N, Ikeda H, Shiina S, Kawabe T, Omata M. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954–1961. doi: 10.1002/hep.22870. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, Herrmann E. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 34.Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666–672. doi: 10.1016/j.jhep.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakao H, Yoneda M. Liver stiffness measurement using transient elastography and hepatocellular carcinoma. Hepatol Res. 2011;41:921–924. doi: 10.1111/j.1872-034X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 36.Koizumi Y, Hirooka M, Kisaka Y, Konishi I, Abe M, Murakami H, Matsuura B, Hiasa Y, Onji M. Liver fibrosis in patients with chronic hepatitis C: noninvasive diagnosis by means of real-time tissue elastography-establishment of the method for measurement. Radiology. 2011;258:610–617. doi: 10.1148/radiol.10100319. [DOI] [PubMed] [Google Scholar]
- 37.Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M, Pol S. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361–1373. doi: 10.1016/j.ultrasmedbio.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Malik R, Lai M, Sadiq A, Farnan R, Mehta S, Nasser I, Challies T, Schuppan D, Afdhal N. Comparison of transient elastography, serum markers and clinical signs for the diagnosis of compensated cirrhosis. J Gastroenterol Hepatol. 2010;25:1562–1568. doi: 10.1111/j.1440-1746.2010.06371.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee MH, Cheong JY, Um SH, Seo YS, Kim DJ, Hwang SG, Yang JM, Han KH, Cho SW. Comparison of surrogate serum markers and transient elastography (Fibroscan) for assessing cirrhosis in patients with chronic viral hepatitis. Dig Dis Sci. 2010;55:3552–3560. doi: 10.1007/s10620-010-1219-0. [DOI] [PubMed] [Google Scholar]
- 40.Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol. 2009;35:219–229. doi: 10.1016/j.ultrasmedbio.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758–764. doi: 10.2214/AJR.06.0322. [DOI] [PubMed] [Google Scholar]
- 42.Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Ledinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott ST. Volume ultrasound: the next big thing? Br J Radiol. 2008;81:8–9. doi: 10.1259/bjr/13475432. [DOI] [PubMed] [Google Scholar]
- 44.Wilson SR, Gupta C, Eliasziw M, Andrew A. Volume imaging in the abdomen with ultrasound: how we do it. AJR Am J Roentgenol. 2009;193:79–85. doi: 10.2214/AJR.08.2273. [DOI] [PubMed] [Google Scholar]
- 45.Boito SM, Laudy JA, Struijk PC, Stijnen T, Wladimiroff JW. Three-dimensional US assessment of hepatic volume, head circumference, and abdominal circumference in healthy and growth-restricted fetuses. Radiology. 2002;223:661–665. doi: 10.1148/radiol.2233010656. [DOI] [PubMed] [Google Scholar]
- 46.Xu HX, Yin XY, Lu MD, Liu GJ, Xu ZF. Estimation of liver tumor volume using a three-dimensional ultrasound volumetric system. Ultrasound Med Biol. 2003;29:839–846. doi: 10.1016/s0301-5629(02)00775-5. [DOI] [PubMed] [Google Scholar]
- 47.Park SH, Choi BI, Han JK, Yoon CJ, Lee JW, Kim SS, Han H. Volumetric tumor measurement using three-dimensional ultrasound: in vitro phantom study on measurement accuracy under various scanning conditions. Ultrasound Med Biol. 2004;30:27–34. doi: 10.1016/j.ultrasmedbio.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Kim SJ, Choi BI, Kim SH, Lee JY. Three-dimensional imaging for hepatobiliary and pancreatic diseases: Emphasis on clinical utility. Indian J Radiol Imaging. 2009;19:7–15. doi: 10.4103/0971-3026.45336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Downey DB, Fenster A, Williams JC. Clinical utility of three-dimensional US. Radiographics. 2000;20:559–571. doi: 10.1148/radiographics.20.2.g00mc19559. [DOI] [PubMed] [Google Scholar]
- 50.Xu HX, Liu L, Lu MD, Li HP, Liu GJ, Li JP. Three-dimensional power Doppler imaging in depicting vascularity in hepatocellular carcinoma. J Ultrasound Med. 2003;22:1147–1154. doi: 10.7863/jum.2003.22.11.1147. [DOI] [PubMed] [Google Scholar]
- 51.Liang JD, Yang PM, Liang PC, Huang GT, Sheu JC, Chen DS. Three-dimensional power Doppler ultrasonography for demonstrating associated arteries of hepatocellular carcinoma. J Formos Med Assoc. 2003;102:367–374. [PubMed] [Google Scholar]
- 52.Sato S, Yoshida H, Teratani T, Obi S, Koike Y, Shiina S, Omata M. Three-dimensional power Doppler ultrasonography for hepatocellular carcinoma: a comparison with angiography? Hepatogastroenterology. 2005;52:72–75. [PubMed] [Google Scholar]
- 53.Numata K, Fukuda H, Ohto M, Itou R, Nozaki A, Kondou M, Morimoto M, Karasawa E, Tanaka K. Evaluation of the therapeutic efficacy of high-intensity focused ultrasound ablation of hepatocellular carcinoma by three-dimensional sonography with a perflubutane-based contrast agent. Eur J Radiol. 2010;75:e67–e75. doi: 10.1016/j.ejrad.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Luo W, Numata K, Morimoto M, Oshima T, Ueda M, Okada M, Takebayashi S, Zhou X, Tanaka K. Role of Sonazoid-enhanced three-dimensional ultrasonography in the evaluation of percutaneous radiofrequency ablation of hepatocellular carcinoma. Eur J Radiol. 2010;75:91–97. doi: 10.1016/j.ejrad.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Xu HX, Lu MD, Xie XH, Xie XY, Kuang M, Xu ZF, Liu GJ, Wang Z, Chen LD, Lin MX. Treatment response evaluation with three-dimensional contrast-enhanced ultrasound for liver cancer after local therapies. Eur J Radiol. 2010;76:81–88. doi: 10.1016/j.ejrad.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Graser A, Johnson TR, Chandarana H, Macari M. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol. 2009;19:13–23. doi: 10.1007/s00330-008-1122-7. [DOI] [PubMed] [Google Scholar]
- 57.Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol. 2009;193:47–54. doi: 10.2214/AJR.09.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altenbernd J, Heusner TA, Ringelstein A, Ladd SC, Forsting M, Antoch G. Dual-energy-CT of hypervascular liver lesions in patients with HCC: investigation of image quality and sensitivity. Eur Radiol. 2011;21:738–743. doi: 10.1007/s00330-010-1964-7. [DOI] [PubMed] [Google Scholar]
- 59.Okada M, Kim T, Murakami T. Hepatocellular nodules in liver cirrhosis: state of the art CT evaluation (perfusion CT/volume helical shuttle scan/dual-energy CT, etc.) Abdom Imaging. 2011;36:273–281. doi: 10.1007/s00261-011-9684-2. [DOI] [PubMed] [Google Scholar]
- 60.Murakami T, Imai Y, Okada M, Hyodo T, Lee WJ, Kim MJ, Kim T, Choi BI. Ultrasonography, computed tomography and magnetic resonance imaging of hepatocellular carcinoma: toward improved treatment decisions. Oncology. 2011;81(Suppl 1):86–99. doi: 10.1159/000333267. [DOI] [PubMed] [Google Scholar]
- 61.Schindera ST, Nelson RC, Mukundan S, Jr, Paulson EK, Jaffe TA, Miller CM, DeLong DM, Kawaji K, Yoshizumi TT, Samei E. Hypervascular liver tumors: low tube voltage, high tube current multi-detector row CT for enhanced detection-phantom study. Radiology. 2008;246:125–132. doi: 10.1148/radiol.2461070307. [DOI] [PubMed] [Google Scholar]
- 62.Kim KS, Lee JM, Kim SH, Kim KW, Kim SJ, Cho SH, Han JK, Choi BI. Image fusion in dual energy computed tomography for detection of hypervascular liver hepatocellular carcinoma: phantom and preliminary studies. Invest Radiol. 2010;45:149–157. doi: 10.1097/RLI.0b013e3181d32119. [DOI] [PubMed] [Google Scholar]
- 63.Ehman EC, Guimaraes LS, Fidler JL, Takahashi N, Ramirez-Giraldo JC, Yu L, Manduca A, Huprich JE, McCollough CH, Holmes D, 3rd, Harmsen WS, Fletcher JG. Noise reduction to decrease radiation dose and improve conspicuity of hepatic lesions at contrast-enhanced 80-kV hepatic CT using projection space denoising. AJR Am J Roentgenol. 2012;198:405–411. doi: 10.2214/AJR.11.6987. [DOI] [PubMed] [Google Scholar]
- 64.Holmes DR, 3rd, Fletcher JG, Apel A, Huprich JE, Siddiki H, Hough DM, Schmidt B, Flohr TG, Robb R, McCollough C, Wittmer M, Eusemann C. Evaluation of non-linear blending in dual-energy computed tomography. Eur J Radiol. 2008;68:409–413. doi: 10.1016/j.ejrad.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661–673. doi: 10.1148/radiol.2343031362. [DOI] [PubMed] [Google Scholar]
- 66.Zhu AX, Holalkere NS, Muzikansky A, Horgan K, Sahani DV. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120–125. doi: 10.1634/theoncologist.2007-0174. [DOI] [PubMed] [Google Scholar]
- 67.Petralia G, Fazio N, Bonello L, D'Andrea G, Radice D, Bellomi M. Perfusion computed tomography in patients with hepatocellular carcinoma treated with thalidomide: initial experience. J Comput Assist Tomogr. 2011;35:195–201. doi: 10.1097/RCT.0b013e31820ccf51. [DOI] [PubMed] [Google Scholar]
- 68.Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Sahani DV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST) Invest Radiol. 2012;47:11–17. doi: 10.1097/RLI.0b013e3182199bb5. [DOI] [PubMed] [Google Scholar]
- 69.Maksimovic O, Schraml C, Hartmann JT, Bitzer M, Claussen CD, Pintoffl J, Horger M. Evaluation of response in malignant tumors treated with the multitargeted tyrosine kinase inhibitor sorafenib: a multitechnique imaging assessment. AJR Am J Roentgenol. 2010;194:5–14. doi: 10.2214/AJR.09.2744. [DOI] [PubMed] [Google Scholar]
- 70.Meijerink MR, van Waesberghe JH, van der Weide L, van den Tol P, Meijer S, Comans EF, Golding RP, van Kuijk C. Early detection of local RFA site recurrence using total liver volume perfusion CT initial experience. Acad Radiol. 2009;16:1215–1222. doi: 10.1016/j.acra.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 71.Choi SH, Chung JW, Kim HC, Baek JH, Park CM, Jun S, Kim MU, Lee ES, Cho HR, Jae HJ, Lee W, Park JH. The role of perfusion CT as a follow-up modality after transcatheter arterial chemoembolization: an experimental study in a rabbit model. Invest Radiol. 2010;45:427–436. doi: 10.1097/RLI.0b013e3181e07516. [DOI] [PubMed] [Google Scholar]
- 72.Yang L, Zhang XM, Zhou XP, Tang W, Guan YS, Zhai ZH, Dong GL. Correlation between tumor perfusion and lipiodol deposition in hepatocellular carcinoma after transarterial chemoembolization. J Vasc Interv Radiol. 2010;21:1841–1846. doi: 10.1016/j.jvir.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Ippolito D, Bonaffini PA, Ratti L, Antolini L, Corso R, Fazio F, Sironi S. Hepatocellular carcinoma treated with transarterial chemoembolization: dynamic perfusion-CT in the assessment of residual tumor. World J Gastroenterol. 2010;16:5993–6000. doi: 10.3748/wjg.v16.i47.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goh V, Padhani AR. Imaging tumor angiogenesis: functional assessment using MDCT or MRI? Abdom Imaging. 2006;31:194–199. doi: 10.1007/s00261-005-0387-4. [DOI] [PubMed] [Google Scholar]
- 75.Choi BI. Advances of imaging for hepatocellular carcinoma. Oncology. 2010;78(Suppl 1):46–52. doi: 10.1159/000315230. [DOI] [PubMed] [Google Scholar]
- 76.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 77.Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009;193:14–27. doi: 10.2214/AJR.09.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JM, Choi BI. Hepatocellular nodules in liver cirrhosis: MR evaluation. Abdom Imaging. 2011;36:282–289. doi: 10.1007/s00261-011-9692-2. [DOI] [PubMed] [Google Scholar]
- 79.Park MS, Kim S, Patel J, Hajdu CH, Do RK, Mannelli L, Babb JS, Taouli B. Hepatocellular carcinoma: Detection with diffusion-weighted vs. contrast-enhanced MRI in pre-transplant patients. Hepatology. DOI: 10.1002/hep.25681. [DOI] [PubMed]
- 80.Xu PJ, Yan FH, Wang JH, Lin J, Ji Y. Added value of breathhold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrast-enhanced MRI alone using receiver operating characteristic curve analysis. J Magn Reson Imaging. 2009;29:341–349. doi: 10.1002/jmri.21650. [DOI] [PubMed] [Google Scholar]
- 81.Yu JS, Chung JJ, Kim JH, Cho ES, Kim DJ, Ahn JH, Kim KW. Detection of small intrahepatic metastases of hepatocellular carcinomas using diffusion-weighted imaging: comparison with conventional dynamic MRI. Magn Reson Imaging. 2011;29:985–992. doi: 10.1016/j.mri.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 82.An C, Park MS, Jeon HM, Kim YE, Chung WS, Chung YE, Kim MJ, Kim KW. Prediction of the histopathological grade of hepatocellular carcinoma using qualitative diffusion-weighted, dynamic, and hepatobiliary phase MRI. Eur Radiol. DOI: 10.1007/s00330-012-2421-6. [DOI] [PubMed]
- 83.Lee MH, Kim SH, Park MJ, Park CK, Rhim H. Gadoxetic acid-enhanced hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am J Roentgenol. 2011;197:W868–875. doi: 10.2214/AJR.10.6237. [DOI] [PubMed] [Google Scholar]
- 84.Nakanishi M, Chuma M, Hige S, Omatsu T, Yokoo H, Nakanishi K, Kamiyama T, Kubota K, Haga H, Matsuno Y, Onodera Y, Kato M, Asaka M. Relationship between diffusion-weighted magnetic resonance imaging and histological tumor grading of hepatocellular carcinoma. Ann Surg Oncol. 2012;19:1302–1309. doi: 10.1245/s10434-011-2066-8. [DOI] [PubMed] [Google Scholar]
- 85.Nishie A, Tajima T, Asayama Y, Ishigami K, Kakihara D, Nakayama T, Takayama Y, Okamoto D, Fujita N, Taketomi A, Yoshimitsu K, Honda H. Diagnostic performance of apparent diffusion coefficient for predicting histological grade of hepatocellular carcinoma. Eur J Radiol. 2011;80:e29–e33. doi: 10.1016/j.ejrad.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 86.Bonekamp S, Jolepalem P, Lazo M, Gulsun MA, Kiraly AP, Kamel IR. Hepatocellular carcinoma: response to TACE assessed with semiautomated volumetric and functional analysis of diffusion-weighted and contrast-enhanced MR imaging data. Radiology. 2011;260:752–761. doi: 10.1148/radiol.11102330. [DOI] [PubMed] [Google Scholar]
- 87.Babsky AM, Ju S, George B, Bennett S, Huang M, Jayaram HN, McLennan G, Bansal N. Predicting response to benzamide riboside chemotherapy in hepatocellular carcinoma using apparent diffusion coefficient of water. Anticancer Res. 2011;31:2045–2051. [PubMed] [Google Scholar]
- 88.Kubota K, Yamanishi T, Itoh S, Murata Y, Miyatake K, Yasunami H, Morio K, Hamada N, Nishioka A, Ogawa Y. Role of diffusion-weighted imaging in evaluating therapeutic efficacy after transcatheter arterial chemoembolization for hepatocellular carcinoma. Oncol Rep. 2010;24:727–732. doi: 10.3892/or_00000914. [DOI] [PubMed] [Google Scholar]
- 89.Goshima S, Kanematsu M, Kondo H, Yokoyama R, Tsuge Y, Shiratori Y, Onozuka M, Moriyama N. Evaluating local hepatocellular carcinoma recurrence post-transcatheter arterial chemoembolization: is diffusion-weighted MRI reliable as an indicator? J Magn Reson Imaging. 2008;27:834–839. doi: 10.1002/jmri.21316. [DOI] [PubMed] [Google Scholar]
- 90.Schraml C, Schwenzer NF, Martirosian P, Bitzer M, Lauer U, Claussen CD, Horger M. Diffusion-weighted MRI of advanced hepatocellular carcinoma during sorafenib treatment: initial results. AJR Am J Roentgenol. 2009;193:W301–7. doi: 10.2214/AJR.08.2289. [DOI] [PubMed] [Google Scholar]
- 91.Asbach P, Klatt D, Schlosser B, Biermer M, Muche M, Rieger A, Loddenkemper C, Somasundaram R, Berg T, Hamm B, Braun J, Sack I. Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography. Radiology. 2010;257:80–86. doi: 10.1148/radiol.10092489. [DOI] [PubMed] [Google Scholar]
- 92.Rustogi R, Horowitz J, Harmath C, Wang Y, Chalian H, Ganger DR, Chen ZE, Bolster BD, Jr, Shah S, Miller FH. Accuracy of MR elastography and anatomic MR imaging features in the diagnosis of severe hepatic fibrosis and cirrhosis. J Magn Reson Imaging. DOI: 10.1002/jmri.23585. [DOI] [PMC free article] [PubMed]
- 93.Wang Y, Ganger DR, Levitsky J, Sternick LA, McCarthy RJ, Chen ZE, Fasanati CW, Bolster B, Shah S, Zuehlsdorff S, Omary RA, Ehman RL, Miller FH. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:553–561. doi: 10.2214/AJR.10.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–1213. doi: 10.1016/j.cgh.2007.06.012. e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hines CD, Bley TA, Lindstrom MJ, Reeder SB. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010;31:725–731. doi: 10.1002/jmri.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huwart L, Sempoux C, Salameh N, Jamart J, Annet L, Sinkus R, Peeters F, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 97.Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 98.Kim BH, Lee JM, Lee YJ, Lee KB, Suh KS, Han JK, Choi BI. MR elastography for noninvasive assessment of hepatic fibrosis: experience from a tertiary center in Asia. J Magn Reson Imaging. 2011;34:1110–1116. doi: 10.1002/jmri.22723. [DOI] [PubMed] [Google Scholar]
- 99.Venkatesh SK, Yin M, Glockner JF, Takahashi N, Araoz PA, Talwalkar JA, Ehman RL. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008;190:1534–1540. doi: 10.2214/AJR.07.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goodwin MD, Dobson JE, Sirlin CB, Lim BG, Stella DL. Diagnostic challenges and pitfalls in MR imaging with hepatocyte-specific contrast agents. Radiographics. 2011;31:1547–1568. doi: 10.1148/rg.316115528. [DOI] [PubMed] [Google Scholar]
- 101.Lee JM, Zech CJ, Bolondi L, Jonas E, Kim MJ, Matsui O, Merkle EM, Sakamoto M, Choi BI. Consensus report of the 4th International Forum for Gadolinium-Ethoxybenzyl-Diethylenetriamine Pentaacetic Acid Magnetic Resonance Imaging. Korean J Radiol. 2011;12:403–415. doi: 10.3348/kjr.2011.12.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fidler J, Hough D. Hepatocyte-specific magnetic resonance imaging contrast agents. Hepatology. 2011;53:678–682. doi: 10.1002/hep.24158. [DOI] [PubMed] [Google Scholar]
- 103.Kim TK, Lee KH, Jang HJ, Haider MA, Jacks LM, Menezes RJ, Park SH, Yazdi L, Sherman M, Khalili K. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011;259:730–738. doi: 10.1148/radiol.11101549. [DOI] [PubMed] [Google Scholar]
- 104.Park Y, Kim SH, Jeon YH, Lee J, Kim MJ, Choi D, Lee WJ, Kim H, Koo JH, Lim HK. Gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI versus gadobenate dimeglumine (Gd-BOPTA)-enhanced MRI for preoperatively detecting hepatocellular carcinoma: an initial experience. Korean J Radiol. 2010;11:433–440. doi: 10.3348/kjr.2010.11.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marin D, Di Martino M, Guerrisi A, De Filippis G, Rossi M, Ginanni Corradini S, Masciangelo R, Catalano C, Passariello R. Hepatocellular carcinoma in patients with cirrhosis: qualitative comparison of gadobenate dimeglumine-enhanced MR imaging and multiphasic 64-section CT. Radiology. 2009;251:85–95. doi: 10.1148/radiol.2511080400. [DOI] [PubMed] [Google Scholar]
- 106.Kim YK, Kim CS, Chung GH, Han YM, Lee SY, Chon SB, Lee JM. Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186:149–157. doi: 10.2214/ajr.04.1206. [DOI] [PubMed] [Google Scholar]
- 107.Vogl TJ, Stupavsky A, Pegios W, Hammerstingl R, Mack M, Diebold T, Lodemann KP, Neuhaus P, Felix R. Hepatocellular carcinoma: evaluation with dynamic and static gadobenate dimeglumine-enhanced MR imaging and histopathologic correlation. Radiology. 1997;205:721–728. doi: 10.1148/radiology.205.3.9393527. [DOI] [PubMed] [Google Scholar]
- 108.Hwang J, Kim SH, Lee MW, Lee JY. Small (<=2 cm) hepatocellular carcinoma in patients with chronic liver disease: comparison of gadoxetic acid-enhanced 3.0 T MRI and multiphasic 64-MDCT. Br J Radiol. DOI: 10.1259/bjr/27727228. [DOI] [PMC free article] [PubMed]
- 109.Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, Choi D, Lee WJ, Lim HK. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:1675–1681. doi: 10.2214/AJR.08.1262. [DOI] [PubMed] [Google Scholar]
- 110.Rhee H, Kim MJ, Park YN, Choi JS, Kim KS. Gadoxetic acid-enhanced MRI findings of early hepatocellular carcinoma as defined by new histologic criteria. J Magn Reson Imaging. 2012;35:393–398. doi: 10.1002/jmri.22828. [DOI] [PubMed] [Google Scholar]
- 111.Yong TW, Yuan ZZ, Jun Z, Lin Z, He WZ, Juanqi Z. Sensitivity of PET/MR images in liver metastases from colorectal carcinoma. Hell J Nucl Med. 2011;14:264–268. [PubMed] [Google Scholar]
- 112.Wissmeyer M, Heinzer S, Majno P, Buchegger F, Zaidi H, Garibotto V, Viallon M, Becker CD, Ratib O, Terraz S. Y Time-of-flight PET/MR on a hybrid scanner following liver radioembolisation (SIRT) Eur J Nucl Med Mol Imaging. 2011;38:1744–1745. doi: 10.1007/s00259-011-1792-2. [DOI] [PubMed] [Google Scholar]
- 113.Kunishi Y, Numata K, Morimoto M, Okada M, Kaneko T, Maeda S, Tanaka K. Efficacy of fusion imaging combining sonography and hepatobiliary phase MRI with Gd-EOB-DTPA to detect small hepatocellular carcinoma. AJR Am J Roentgenol. 2012;198:106–114. doi: 10.2214/AJR.10.6039. [DOI] [PubMed] [Google Scholar]
- 114.Sandulescu DL, Dumitrescu D, Rogoveanu I, Saftoiu A. Hybrid ultrasound imaging techniques (fusion imaging) World J Gastroenterol. 2011;17:49–52. doi: 10.3748/wjg.v17.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fukuda H, Numata K, Nozaki A, Morimoto M, Kondo M, Tanaka K, Maeda S, Yamagata J, Ohto M, Ito R, Sakamoto A, Zhu H, Wang ZB. Usefulness of US-CT 3D dual imaging for the planning and monitoring of hepatocellular carcinoma treatment using HIFU. Eur J Radiol. 2011;80:e306–e310. doi: 10.1016/j.ejrad.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 116.Krücker J, Xu S, Venkatesan A, Locklin JK, Amalou H, Glossop N, Wood BJ. Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol. 2011;22:515–524. doi: 10.1016/j.jvir.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi BI, Takayasu K, Han MC. Small hepatocellular carcinomas and associated nodular lesions of the liver: pathology, pathogenesis, and imaging findings. AJR Am J Roentgenol. 1993;160:1177–1187. doi: 10.2214/ajr.160.6.8388618. [DOI] [PubMed] [Google Scholar]
- 118.Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44(Suppl 19):112–118. doi: 10.1007/s00535-008-2274-6. [DOI] [PubMed] [Google Scholar]
- 119.Park YN, Yang CP, Fernandez GJ, Cubukcu O, Thung SN, Theise ND. Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol. 1998;22:656–662. doi: 10.1097/00000478-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 120.Roskams T, Kojiro M. Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis. 2010;30:17–25. doi: 10.1055/s-0030-1247129. [DOI] [PubMed] [Google Scholar]
- 121.Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885–897. doi: 10.2214/ajr.178.4.1780885. [DOI] [PubMed] [Google Scholar]
- 122.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 123.Matsui O, Kobayashi S, Sanada J, Kouda W, Ryu Y, Kozaka K, Kitao A, Nakamura K, Gabata T. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging. 2011;36:264–272. doi: 10.1007/s00261-011-9685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Honda H, Tajima T, Kajiyama K, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Vascular changes in hepatocellular carcinoma: correlation of radiologic and pathologic findings. AJR Am J Roentgenol. 1999;173:1213–1217. doi: 10.2214/ajr.173.5.10541091. [DOI] [PubMed] [Google Scholar]
- 125.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Nonomura A, Nakanuma Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999;172:969–976. doi: 10.2214/ajr.172.4.10587130. [DOI] [PubMed] [Google Scholar]
- 126.Liu K, He X, Lei XZ, Zhao LS, Tang H, Liu L, Lei BJ. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J Gastroenterol. 2003;9:1946–1949. doi: 10.3748/wjg.v9.i9.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanaka M, Nakashima O, Wada Y, Kage M, Kojiro M. Pathomorphological study of Kupffer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology. 1996;24:807–812. doi: 10.1053/jhep.1996.v24.pm0008855180. [DOI] [PubMed] [Google Scholar]
- 128.Park HS, Lee JM, Kim SH, Chang S, Kim SJ, Han JK, Choi BI. Differentiation of well-differentiated hepatocellular carcinomas from other hepatocellular nodules in cirrhotic liver: value of SPIO-enhanced MR imaging at 3.0 Tesla. J Magn Reson Imaging. 2009;29:328–335. doi: 10.1002/jmri.21615. [DOI] [PubMed] [Google Scholar]
- 129.Tanimoto A, Kuribayashi S. Application of superparamagnetic iron oxide to imaging of hepatocellular carcinoma. Eur J Radiol. 2006;58:200–216. doi: 10.1016/j.ejrad.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 130.Yoo HJ, Lee JM, Lee JY, Kim SH, Kim SJ, Han JK, Choi BI. Additional value of SPIO-enhanced MR imaging for the noninvasive imaging diagnosis of hepatocellular carcinoma in cirrhotic liver. Invest Radiol. 2009;44:800–807. doi: 10.1097/RLI.0b013e3181bc271d. [DOI] [PubMed] [Google Scholar]
- 131.Yoon MA, Kim SH, Park HS, Lee DH, Lee JY, Han JK, Choi BI. Value of dual contrast liver MRI at 3.0 T in differentiating well-differentiated hepatocellular carcinomas from dysplastic nodules: preliminary results of multivariate analysis. Invest Radiol. 2009;44:641–649. doi: 10.1097/RLI.0b013e3181ab6e57. [DOI] [PubMed] [Google Scholar]
- 132.Lim JH, Choi D, Cho SK, Kim SH, Lee WJ, Lim HK, Park CK, Paik SW, Kim YI. Conspicuity of hepatocellular nodular lesions in cirrhotic livers at ferumoxides-enhanced MR imaging: importance of Kupffer cell number. Radiology. 2001;220:669–676. doi: 10.1148/radiol.2203001777. [DOI] [PubMed] [Google Scholar]
- 133.Bartolozzi C, Crocetti L, Lencioni R, Cioni D, Della Pina C, Campani D. Biliary and reticuloendothelial impairment in hepatocarcinogenesis: the diagnostic role of tissue-specific MR contrast media. Eur Radiol. 2007;17:2519–2530. doi: 10.1007/s00330-007-0602-5. [DOI] [PubMed] [Google Scholar]
- 134.Sano K, Ichikawa T, Motosugi U, Sou H, Muhi AM, Matsuda M, Nakano M, Sakamoto M, Nakazawa T, Asakawa M, Fujii H, Kitamura T, Enomoto N, Araki T. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011;261:834–844. doi: 10.1148/radiol.11101840. [DOI] [PubMed] [Google Scholar]
- 135.Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Muhi A, Araki T. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010;256:151–158. doi: 10.1148/radiol.10091885. [DOI] [PubMed] [Google Scholar]
- 136.Sun HY, Lee JM, Shin CI, Lee DH, Moon SK, Kim KW, Han JK, Choi BI. Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (< or =2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol. 2010;45:96–103. doi: 10.1097/RLI.0b013e3181c5faf7. [DOI] [PubMed] [Google Scholar]
- 137.Inoue T, Kudo M, Hatanaka K, Takahashi S, Kitai S, Ueda T, Ishikawa E, Hagiwara S, Minami Y, Chung H, Ueshima K, Maekawa K. Imaging of hepatocellular carcinoma: qualitative and quantitative analysis of postvascular phase contrast-enhanced ultrasonography with sonazoid. Comparison with superparamagnetic iron oxide magnetic resonance images. Oncology. 2008;75(Suppl 1):48–54. doi: 10.1159/000173424. [DOI] [PubMed] [Google Scholar]
- 138.Sugimoto K, Moriyasu F, Saito K, Taira J, Saguchi T, Yoshimura N, Oshiro H, Imai Y, Shiraishi J. Comparison of kupffer-phase sonazoid-enhanced sonography and hepatobiliary-phase gadoxetic Acid-enhanced magnetic resonance imaging of hepatocellular carcinoma and correlation with histologic grading. J Ultrasound Med. 2012;31:529–538. doi: 10.7863/jum.2012.31.4.529. [DOI] [PubMed] [Google Scholar]
- 139.Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Kitamura T, Araki T. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging. 2009;30:1042–1046. doi: 10.1002/jmri.21956. [DOI] [PubMed] [Google Scholar]
- 140.Motosugi U, Ichikawa T, Oguri M, Sano K, Sou H, Muhi A, Matsuda M, Fujii H, Enomoto N, Araki T. Staging liver fibrosis by using liver-enhancement ratio of gadoxetic acid-enhanced MR imaging: comparison with aspartate aminotransferase-to-platelet ratio index. Magn Reson Imaging. 2011;29:1047–1052. doi: 10.1016/j.mri.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 141.Katsube T, Okada M, Kumano S, Hori M, Imaoka I, Ishii K, Kudo M, Kitagaki H, Murakami T. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol. 2011;46:277–283. doi: 10.1097/RLI.0b013e318200f67d. [DOI] [PubMed] [Google Scholar]
- 142.Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S, Nakanuma Y. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging-correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817–826. doi: 10.1148/radiol.10092214. [DOI] [PubMed] [Google Scholar]
- 143.Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, Koda W, Kobayashi S, Gabata T, Zen Y, Yamashita T, Kaneko S, Nakanuma Y. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol. 2011;21:2056–2066. doi: 10.1007/s00330-011-2165-8. [DOI] [PubMed] [Google Scholar]
- 144.Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, Taura K, Yasuchika K, Nitta T, Ikai I, Uemoto S. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol. 2009;44:793–798. doi: 10.1007/s00535-009-0056-4. [DOI] [PubMed] [Google Scholar]
- 145.Pastor CM. Gadoxetic acid-enhanced hepatobiliary phase MR imaging: cellular insight. Radiology. 2010;257:589. doi: 10.1148/radiol.101172. [DOI] [PubMed] [Google Scholar]
- 146.Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K, Tomoda K. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging-correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824–833. doi: 10.1148/radiol.10091557. [DOI] [PubMed] [Google Scholar]
- 147.Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 148.Nakamura K, Zen Y, Sato Y, Kozaka K, Matsui O, Harada K, Nakanuma Y. Vascular endothelial growth factor, its receptor Flk-1, and hypoxia inducible factor-1alpha are involved in malignant transformation in dysplastic nodules of the liver. Hum Pathol. 2007;38:1532–1546. doi: 10.1016/j.humpath.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 149.Weinmann HJ, Ebert W, Misselwitz B, Schmitt-Willich H. Tissue-specific MR contrast agents. Eur J Radiol. 2003;46:33–44. doi: 10.1016/s0720-048x(02)00332-7. [DOI] [PubMed] [Google Scholar]
- 150.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 151.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 152.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 155.Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, Choi BI. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009;193:W482–9. doi: 10.2214/AJR.08.1818. [DOI] [PubMed] [Google Scholar]
- 156.Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (</= 2 cm) HCC in cirrhosis. Eur Radiol. 2011;21:1233–1242. doi: 10.1007/s00330-010-2030-1. [DOI] [PubMed] [Google Scholar]
- 157.Kudo M, Han KH, Kokudo N, Cheng AL, Choi BI, Furuse J, Izumi N, Park JW, Poon RT, Sakamoto M. Liver Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i19–i27. doi: 10.1093/jjco/hyq123. [DOI] [PubMed] [Google Scholar]
- 158.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 159.Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol. DOI: 10.4061/2011/104685. [DOI] [PMC free article] [PubMed]
- 160.Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 161.Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, Hamada E, Takahashi M, Shiratori Y, Terano A, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023–1028. doi: 10.2214/ajr.160.5.7682378. [DOI] [PubMed] [Google Scholar]
- 162.Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol. 2011;26:1354–1360. doi: 10.1111/j.1440-1746.2011.06812.x. [DOI] [PubMed] [Google Scholar]
- 163.Zhang L, Zhu H, Jin C, Zhou K, Li K, Su H, Chen W, Bai J, Wang Z. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19:437–445. doi: 10.1007/s00330-008-1137-0. [DOI] [PubMed] [Google Scholar]
- 164.Song MJ, Park CH, Kim JD, Kim HY, Bae SH, Choi JY, Yoon SK, Chun HJ, Choi BG, Lee HG. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011;23:521–527. doi: 10.1097/MEG.0b013e328346d505. [DOI] [PubMed] [Google Scholar]
- 165.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 166.Sangro B, Inarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 167.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 168.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 169.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 170.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256:348–364. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 171.Rutman AM, Kuo MD. Radiogenomics: creating a link between molecular diagnostics and diagnostic imaging. Eur J Radiol. 2009;70:232–241. doi: 10.1016/j.ejrad.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 172.Zinn PO, Mahajan B, Sathyan P, Singh SK, Majumder S, Jolesz FA, Colen RR. Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in glioblastoma multiforme. PLoS ONE. 2011;6:e25451. doi: 10.1371/journal.pone.0025451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, Chan BK, Matcuk GR, Barry CT, Chang HY, Kuo MD. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007;25:675–680. doi: 10.1038/nbt1306. [DOI] [PubMed] [Google Scholar]
- 174.Kuo MD, Gollub J, Sirlin CB, Ooi C, Chen X. Radiogenomic analysis to identify imaging phenotypes associated with drug response gene expression programs in hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:821–831. doi: 10.1016/j.jvir.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 175.Braren R, Altomonte J, Settles M, Neff F, Esposito I, Ebert O, Schwaiger M, Rummeny E, Steingoetter A. Validation of preclinical multiparametric imaging for prediction of necrosis in hepatocellular carcinoma after embolization. J Hepatol. 2011;55:1034–1040. doi: 10.1016/j.jhep.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 176.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mínguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol. 2009;25:186–194. doi: 10.1097/MOG.0b013e32832962a1. [DOI] [PubMed] [Google Scholar]
- 178.Olsen SK, Brown RS, Siegel AB. Hepatocellular carcinoma: review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol. 2010;3:55–66. doi: 10.1177/1756283X09346669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tremosini S, Reig M, de Lope CR, Forner A, Bruix J. Treatment of early hepatocellular carcinoma: Towards personalized therapy. Dig Liver Dis. 2010;42(Suppl 3):S242–S248. doi: 10.1016/S1590-8658(10)60512-9. [DOI] [PubMed] [Google Scholar]