Abstract
Objective
To assess the relationship of timing of hormone therapy (HT) use with angiographic coronary artery disease (CAD) and cardiovascular disease (CVD) events in women with natural versus surgical menopause.
Methods
We studied 654 postmenopausal women undergoing coronary angiography for evaluation of suspected ischemia. Timing and type of menopause, HT use, and quantitative angiographic evaluations were obtained at baseline, and the women were followed for a median of 6 years for CVD events.
Results
Ever users of HT had a significantly lower prevalence of obstructive CAD compared to never users (age-adjusted OR=0.41 [0.28, 0.60]). Naturally menopausal women initiating HT at age <55 years had lower CAD severity compared to never users (age-adjusted beta [SE] = −6.23 [1.50], p<0.0001) while those initiating HT age ≥55 years did not differ statistically from never users (−3.34 [2.13], p=0.12). HT use remained a significant predictor of obstructive CAD when adjusting for a “healthy user” model OR 0.44 [0.30, 0.73] (p=0.002). An association between HT and fewer CVD events was observed only in the natural menopause group (HR [95%CI] = 0.60[0.41, 0.88], p=0.009) but became non-significant when adjusting for presence or severity of obstructive CAD.
Conclusions
Using quantitative measurements of timing and type of menopause and HT use, earlier initiation of HT was associated with less angiographic CAD in women with natural but not surgical menopause. Our data suggest that the effect of HT use on reduced cardiovascular event rates is mediated by the presence or absence of angiographic obstructive atherosclerosis.
Keywords: cardiovascular disease, menopause, hormone therapy, atherosclerosis
Introduction
Women have a relatively lower risk of cardiovascular disease (CVD) compared to age matched men1, suggesting that endogenous reproductive hormones play a protective role against coronary artery disease(CAD). Indeed, animal models2 and human epidemiological studies3 suggest that oophorectomy is a risk factor for accelerated CAD. Exogenous reproductive hormones have also been proposed to play a role in CAD.4 In animal oophorectomy models, hormone therapy (HT) has anti-atherosclerotic effects when initiated early after oophorectomy,5 and observational epidemiological studies in humans have demonstrated protective effects for CAD among postmenopausal HT users.6–7 Clinical trials, however, have demonstrated overall no benefit and early adverse effects in women following menopause randomized to a variety of forms of HT, using angiographic8–9 or clinical outcomes.10–12 This discrepancy has called into question the validity of the endogenous estrogen protection hypothesis, as well as the prior animal and human epidemiological exogenous HT studies.
Recent data have shed light on this controversy. In the observational Nurses Health Study, younger women without coronary heart disease had a reduction of adverse events with HT6 but older women with known disease had an initial increase in adverse events followed by a reduction after several years of treatment,13 which was similar to what was seen with older women in the Women’s Health Initiative (WHI) trials. In the pooled WHI trials, women who started HT within 10 years of menopause had less relative risk for adverse events compared with those who started HT greater than 10 years from menopause.14 Pooled data from randomized trials including the WHI found a statistical trend in lower relative risk, suggesting that HT when started closer to the age of menopause may reduce adverse events compared to HT started at an older age.14–15 Prior studies have also not been able to assess directly the putative mechanism of HT benefit, e.g. coronary atherosclerosis.
The Women’s Ischemia Syndrome Evaluation (WISE) is a prospective, multi-center NHLBI-sponsored study designed to explore female-specific CVD pathophysiology in a large sample of women undergoing coronary angiography for suspected ischemic heart disease.16 We undertook a detailed study to examine the relationship between timing and type of menopause and the timing of HT use with CAD, measured by quantitative coronary angiography and prospective adverse CVD events, to shed additional light on this controversy.
Methods
Women undergoing coronary angiography due to suspected ischemia underwent a one time baseline evaluation that included collection of demographic, medical history, detailed reproductive history and exogenous hormone use, psychosocial and symptom data from 1998–2002, as described previously16 (cross-sectional phase). These women have been followed annually for major adverse CVD events for a median of six years (prospective phase). Blood for reproductive hormone determinations was drawn at baseline following an overnight fast in close temporal proximity to the WISE testing. The study was approved by all involved sites’ Institutional Review Boards.
Reproductive Status Questionnaire
The WISE reproductive status algorithm has been validated to be an accurate assessment of menopausal status and current HT use using both a detailed questionnaire and blood reproductive hormone levels.17 The WISE reproductive status questionnaire includes a detailed history of menarche, date of last menstrual period, current and prior menstrual cycling patterns, prior reproductive events (pregnancy, hysterectomy, uni- and bilateral oophorectomy), current and prior perimenopausal symptoms, and current and prior oral contraceptive or HT use.17 Reproductive hormone levels were assessed using validated steroid and protein assay methods for total estradiol, bioavailable estradiol, estrone, progesterone, follicle-stimulating hormone (FSH) and luteinizing hormone (LH).18 Documentation of type of gynecological surgery was obtained when the blood reproductive hormone levels were not consistent with the reported status during expert consensus review for menopausal status determination.
A total of 654 postmenopausal WISE women were included in the current analysis. Among those without a history of hysterectomy or oophorectomy, the mean age of the last menstrual cycle was 49 years in smokers compared to 51 years in non-smokers, thus we imputed these menopausal ages for cessation of ovarian cycling in women with a pre-menopausal hysterectomy (with and without unilateral oophorectomy)(n=27). Because 95% of women are postmenopausal by age 55 years,19 we used a conservative threshold of 55 years to define initiation of HT use before versus after menopause. Surgical menopause was defined as bilateral oophorectomy performed within less than one year after last menstrual period.
Measurement of Coronary Artery Disease
Coronary angiography was assessed at baseline by a core laboratory (blinded to historical or clinical data) used in previous NHLBI-sponsored multi-center trials.20 Measurements included quantitative assessment of the presence of obstructive CAD, defined as ≥ 70% luminal diameter stenosis in ≥1 epicardial coronary artery and the WISE CAD severity score, using previously published methods.20 The WISE CAD severity score was based on percent stenosis adjusted for any complete collaterals with the possible range of score being 5 (no detectable stenosis) to 100 (multiple severe lesions), and the actual range in the WISE was 5–78.
Adverse Cardiovascular Events
Major adverse CVD events were defined as CVD-related mortality, myocardial infarction, congestive heart failure, or stroke. Patients were contacted by telephone annually by experienced study coordinators completing a scripted interview about adverse CVD events or hospitalizations up to 9 years (median 6.0 [IQR 3.8–7.1]). If a patient was no longer living, we obtained a death certificate where available, records of any related CVD hospitalizations during the preceding follow-up time period, and/or description by a primary relative regarding the circumstances surrounding the death. The cause of death was reviewed by two cardiologist investigators blinded to the clinical and angiographic data; a third reviewer was used in discrepant death classification. For non-fatal major events (myocardial infarction, stroke, or heart failure), one WISE clinical site examined hospital and clinic records from 113 WISE women and only 1.8% of the self-reported events required reclassification, suggesting high accuracy in self-reported morbidity rates.
Statistical Methods
Values are expressed as raw means ± standard deviations or percentages as indicated. Because of the strong association of age with predictors and outcomes, we age-adjusted all p-values, odds ratios (OR) and 95% confidence intervals (CI), as well as beta coefficients and standard errors throughout this report. We used logistic regression when comparing women with obstructive CAD versus those without obstructive CAD (Tables 2 and 3) or women with natural versus surgical menopause (Table 4). We used linear regression when the dependent variable was the continuous CAD severity score rather than the dichotomous variable of obstructive CAD presence. For Table 5, we performed a two-factor ANOVA that assessed demographic and health risk variables according to HT use and menopause type (surgical versus natural), as well as their interaction terms, with p-values again adjusted for age (Table 5).
Table 2.
Menarche, Menopause, Gynecological Surgery and Coronary Artery Disease
| No Coronary Disease (n=487) | Coronary Disease (n=167) | OR (95% CI) | p | |
|---|---|---|---|---|
| Age (yrs±SD) | 61±9 | 66±9 | 1.05 (1.03,1.08) | <0.0001 |
| Menopausal age (yrs±SD) | 44±9 | 46±8 | 1.02 (0.99,1.04) | 0.24 |
| “ (No hysterectomy, yrs±SD) | 48±5 | 49±5 | 1.02 (0.97,1.07) | 0.49 |
| “ (Natural menopause, yrs±SD) | 46±8 | 48±6 | 1.02 (0.99,1.05) | 0.25 |
| “ (Surgical menopause, yrs±SD) | 38±8 | 37±10 | 0.96 (0.90,1.02) | 0.22 |
| Menopausal symptoms (%) | 71 | 55 | 0.58 (0.40,0.84) | 0.004 |
| Any gynecological surgery (%) | 70 | 47 | 0.44 (0.30,0.63) | <0.0001 |
| Surgical menopause (%) | 23 | 13 | 0.62 (0.38,1.04) | 0.07 |
| Bilateral oophorectomy (BO) (%) | 41 | 23 | 0.53 (0.35,0.80) | 0.003 |
| BO before age 55 (%) | 39 | 22 | 0.57 (0.37,0.86) | 0.008 |
| Hysterectomy only (%) | 19 | 17 | 0.80 (0.55,1.28) | 0.35 |
| Hysterectomy before age 55 (%) | 17 | 16 | 0.84 (0.51,1.36) | 0.47 |
| Unilateral oophorectomy (UO) (%) | 10 | 6 | 0.64 (0.32,1.28) | 0.64 |
| Hysterectomy with UO (%) | 8 | 5 | 0.66 (0.31,1.40) | 0.28 |
| Hysterectomy with BO (%) | 41 | 23 | 0.53 (0.35,0.80) | 0.003 |
| Hysterectomy with or without UO/BO before age 55 (%) | 64 | 42 | 0.49 (0.34,0.72) | 0.0002 |
BO=bilateral oophorectomy, SD=standard deviation, UO, unilateral oophorectomy, OR=odds ratio, CI=confidence interval.
CAD defined as ≥70% luminal diameter stenosis in ≥ one epicardial coronary artery. Surgical menopause defined as bilateral oophorectomy performed less than one year after last menstrual period. All ORs, CIs, and p values are age-adjusted except age.
Table 3.
Hormone Therapy Use and Angiographic Coronary Artery Disease
| No Coronary Disease (n=487) | Coronary Disease (n=167) | OR (95% CI) | P | |
|---|---|---|---|---|
| Overall: | ||||
| History of HT use (%) | 66 | 39 | 0.41 (0.28,0.60) | <0.0001 |
| Current HT use (%) | 49 | 29 | 0.51 (0.34,0.75) | 0.0007 |
| Among Ever HT Users: | (n=319) | (n=65) | ||
| Age onset of HT (yrs±SD) | 46±11 | 55±11 | 1.03 (1.00,1.06) | 0.052 |
| Duration of HT use (yrs±SD) | 10.4±10.0 | 8.7±10.2 | 0.98 (0.95,1.003) | 0.08 |
| Among Current HT Users: | (n=241) | (n=48) | ||
| Unopposed estrogen (%) | 74 | 75 | 1.03 (0.55,2.11) | 0.93 |
Abbreviations and definitions as previously.
All ORs, CIs, and p values are age-adjusted.
Table 4.
HT Use in Women with Natural versus Surgical Menopause
| Natural Menopause (n=520) | Surgical Menopause (n=134) | OR (95% CI) | p | |
|---|---|---|---|---|
|
Overall: | ||||
| Age (yrs±SD) | 63 ± 9 | 58 ± 10 | 0.95 (0.93,0.97) | <0.0001 |
| History of HT use (%) | 54 | 78 | 2.52 (1.59,4.01) | <0.0001 |
| Current HT use (%) | 42 | 53 | 1.25 (0.84,1.86) | 0.27 |
| Among Ever HT Users: | (n=279) | (n=105) | ||
| Age onset of HT (yrs±SD) | 48±11 | 42±10 | 0.96 (0.93,0.98) | 0.0003 |
| Duration of HT use (yrs±SD) | 10±10 | 11±10 | 1.02 (0.997,1.04) | 0.09 |
| Among Current HT Users: | (n=218) | (n=71) | ||
| Unopposed estrogen (%) | 68 | 93 | 1.03 (0.55,2.11) | 0.93 |
Abbreviations and definitions as previously.
All ORs, CIs, and p values are age-adjusted.
Table 5.
Demographic and Health Risk Profiles in Surgical vs Natural Menopause According to HT Use
| Natural Menopause | Surgical Menopause | p(HT) | p(MP) | |||||
|---|---|---|---|---|---|---|---|---|
| HT≤55 yrs HT>55 yrs No HT | HT≤55 yrs HT>55 yrs No HT | |||||||
| (n=218) | (n=62) | (n=234) | (n=97) | (n=8) | (n=28) | |||
| Age (±SD)(yrs) | 59(8) | 66(5) | 66(9) | 57(10) | 65(7) | 60(12) | <.0001 | 0.001 |
| Income ≥35K (%) | 44 | 34 | 29 | 43 | 38 | 35 | 0.02 | 0.98 |
| High School+ Ed. (%) | 84 | 71 | 73 | 81 | 75 | 78 | 0.03 | 0.84 |
| Non-White Race (%) | 16 | 23 | 18 | 16 | 25 | 11 | 0.27 | 0.56 |
| Hx Diabetes (%) | 19 | 29 | 34 | 27 | 0 | 18 | 0.10 | 0.94 |
| Chronic Disease (%) | 39 | 55 | 52 | 42 | 0 | 56 | 0.11 | 0.92 |
| Current Smoke (%) | 16 | 13 | 18 | 26 | 12 | 32 | 0.005 | 0.07 |
| BMI (±SD) | 29(6) | 29(6) | 30(6) | 30(8) | 29(6) | 31(6) | 0.03 | 0.26 |
| Metabolic Synd (%) | 23 | 31 | 32 | 28 | 12 | 48 | 0.02 | 0.36 |
| ATP III Risk (±SD) | 4(4) | 5(4) | 7(6) | 4(4) | 4(2) | 6(7) | 0.0006 | 0.06 |
| DASI (±SD) | 20(15) | 22(15) | 18(12) | 19(16) | 26(10) | 14(14) | 0.15 | 0.17 |
| Hx Depression (%) | 33 | 18 | 12 | 34 | 25 | 36 | 0.04 | 0.22 |
| Hx Cancer (%) | 12 | 10 | 17 | 14 | 0 | 32 | 0.03 | 0.22 |
| Antidepressants (%) | 26 | 18 | 10 | 24 | 0 | 18 | 0.004 | 0.65 |
| Vitamins C, E, A (%) | 32 | 35 | 26 | 28 | 38 | 11 | 0.02 | 0.29 |
| Aspirin Use (%) | 58 | 68 | 71 | 61 | 100 | 71 | 0.15 | 0.17 |
| Age at menopause | 45(8) | 49(5) | 47(6) | 39(8) | 35(8) | 35(9) | 0.008 | <.0001 |
| Years since menopause | 14(10) | 17(7) | 19(10) | 19(9) | 30(7) | 25(11) | <.0001 | <.0001 |
| Yrs since initiating HT | 16(11) | 4(4) | - | 17(10) | 3(2) | - | <.0001 | 0.44 |
| Duration of HT use | 12(10) | 3(4) | - | 12(10) | 2(1) | - | <.0001 | 0.99 |
p(HT)=p values for HT group across the entire sample after adjusting for natural vs. surgical menopause and age. p(MP)=p value for type of menopause from the same model, after adjusting for HT group and age.
BMI=body mass index, DASI=Duke Activity Status Inventory, a measure of functional capacity and physical activity habits, Hx=history of, ATP III Risk=National Cholesterol Education Program, Adult Treatment Panel III Global Coronary Heart Disease Risk Score (see Methods), Chronic Disease includes diabetes, COPD, renal disease, autoimmune disease, anorexia, or >25 drinks/week.
Non-significant variables included: marital status, Hx hypertension, Hx dyslipidemia, Hx smoking, Family Hx CAD, systolic and diastolic blood pressure, waist circumference, waist/hip ratio, prior CAD, lipid lowering therapy, antihypertensive therapy, anxiolytics, ACE inhibitors and nitrates. There were significant menopause type * HT group interaction terms for diabetes (p=0.01), Vitamins C, E, A (p=0.04), other chronic disease (p=0.01), age at menopause (p<0.0001), years since menopause (p=0.04). All other interactions were non-significant.
To assess the degree to which HT use provided incremental prediction of the presence versus absence of obstructive CAD over and above available healthy user variables, we performed logistic regression modeling in three steps. The first step evaluated the unadjusted odds ratios (OR) and 95% confidence intervals (CI) of history of HT use in predicting the presence of obstructive CAD. In the second step we developed a multivariate model of selected lifestyle and risk factor variables. These variables included traditional risk factors (diabetes, chronic disease, functional limitation [DASI], aspirin use) as well as those that were associated with either menopausal type or HT use with a p≤0.05. To avoid over-fitting, we included metabolic syndrome and the ATP III risk score that combine several traditional risk factors. This model included all variables listed in Table 5 with the exception of depression (which was collinear with antidepressant use), menopause, or HT use. In the third step we added HT use to this model. We then used the log rank test to estimate the incremental predictive power of HT use over and above that provided by the multivariate model. The same sequence of steps was repeated using linear regression when the outcome was the continuous log-transformed CAD severity score. In this latter case we used the F-test to estimate the significance of the incremental R2 obtained when HT was added to the multivariate model.
Unadjusted Kaplan-Meier curves were used to compare event-free survival from CVD events among women with no history of HT use, those who initiated their HT use prior to age 55, and those initiating HT use at age 55 or older. We then used Cox proportional hazards regression to adjust for important covariates. The proportional hazards assumption of invariant relative risk was tested and found to be satisfactory for all models constructed. For all analyses a p-value of <0.05 was considered statistically significant. All analyses were performed using the SAS 9.1 software (Cary, N.C.).
The study was funded by National Institutes of Health (NIH)-National Heart, Lung and Blood Institute (NHLBI) which was involved in the WISE study design, conduct and reporting.
Results
The demographics and clinical profile for the 654 postmenopausal women with complete reproductive hormone variables and coronary angiography results are shown in Table 1. The women represent a broad age range (36–86 yrs), 17% were non-white (primarily African American), and the majority had at least one cardiac risk factor. A total of 134 (20%) had undergone surgical menopause. These women were younger than the naturally menopausal women (58 vs. 63 years, p<0.0001) and were more than twice as likely to use HT (age-adjusted OR 2.52 [1.59, 4.01]).
Table 1.
Baseline Demographic and Clinical Variables (n=654)
| Age (yrs)(±SD) | 62±10 |
| Race (% white) | 83% |
| Hypertension (%) | 61% |
| Diabetes Mellitus (%) | 26% |
| Current Smoking (%) | 18% |
| Lipid Lowering Rx (%) | 33% |
| Current Postmenopausal HT Use (%) | 44% |
| Coronary Artery Disease (% ≥1 coronary ≥70%) | 26% |
| CAD Severity Score (± SD) | 15.8±14.8 |
HT=hormone therapy, Rx=therapy, SD=standard deviation
Menopause, Gynecological Surgery and Angiographic Coronary Artery Disease
Women with angiographic obstructive CAD (n=167) were older compared to those without obstructive CAD (n=487). Following age-adjustment, there were no significant differences between the two groups in age of menopause (Table 2). There was an inverse relationship between presence of obstructive CAD and history of menopausal symptoms (OR [95% CI] 0.58 [0.40, 0.84]), and bilateral oophorectomy before the age of 55 (0.57 [0.37, 0.86])(Table 2). Sensitivity analyses using the CAD severity score as the dependent variable demonstrated similar differences (age-adjusted beta coefficient [standard error] for menopausal symptoms = −4.08 [1.21], p=0.0008; for any gynecological surgery = −3.67 [1.18], p=0.002). Repeat age-adjusted analyses excluding the women with imputed menopausal age attenuated the effect of menopausal symptoms (p=0.19) but not of any gynecological surgery (OR [95% CI] = 0.40 [0.26, 0.61], p<0.0001).
Hormone Therapy (HT) Use and Obstructive Coronary Artery Disease
We evaluated relationships between HT use and obstructive CAD in the total population. After adjusting for age, historical ever users of HT had a significantly lower prevalence of CAD compared to never users (0.41 [0.28, 0.60]) (Table 3). Similarly, women with current HT use were less likely to have obstructive CAD (0.51 [0.34, 0.75]). Initiation of HT use at a younger age was marginally associated with lower prevalence of angiographic CAD in the overall population. A majority of the HT users were taking unopposed estrogen (Table 3).
HT Use in Surgical and Natural Menopause
Women who had undergone surgical menopause were significantly younger than naturally menopausal women (Table 4). Compared to women with natural menopause, those with surgical menopause were more than twice as likely to use HT (age-adjusted OR 2.52 [1.59, 4.01]), consisting almost exclusively of unopposed estrogen (Table 4). Notably, a relatively high proportion of naturally menopausal women also used unopposed estrogen, likely related to the relatively high hysterectomy rate (Table 2).
Association of HT Use with Coronary Artery Disease in Surgical and Natural Menopause
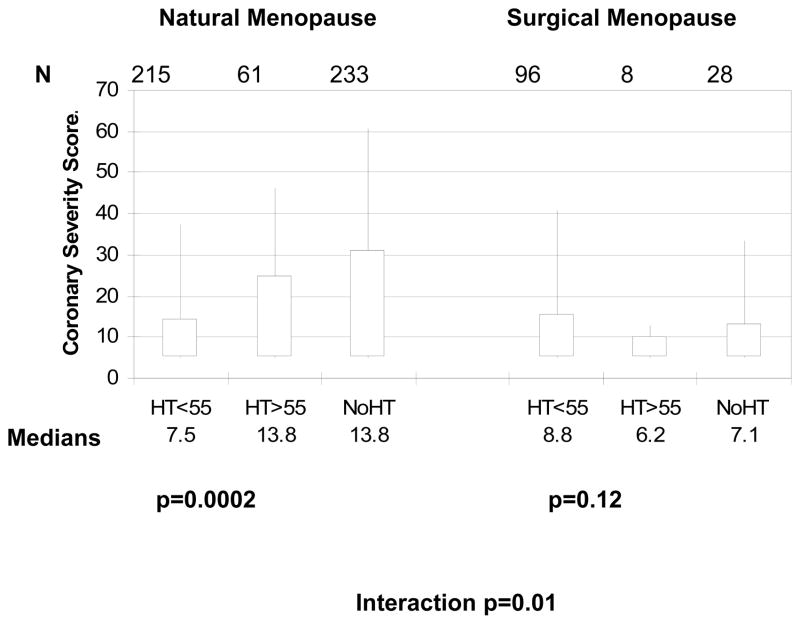
We compared the angiographic CAD severity score across HT subgroups stratified by natural versus surgical menopause (Figure 1). Among the surgical menopause women, there was no statistical difference among those who never used HT, those who initiated HT prior to age 55, and those initiating HT after age 55 (age-adjusted p=0.12), although there were very few women in the latter group. In contrast, among the naturally menopausal women the overall difference among the three HT subgroups was statistically significant (age-adjusted p=0.0002). Among the natural menopause subgroups, women initiating HT prior to age 55 had significantly lower CAD severity compared to never HT users (age-adjusted beta [SE] = −6.23 [1.50], p<0.0001) while those initiating HT after age 55 did not differ statistically from never HT users (−3.34 [2.13], p=0.12). The interaction term between type of menopause and HT subgroup was significant (p=0.010), suggesting a differential HT effect by surgical vs natural menopause.
FIG. 1. Surgical versus natural menopause, HT, and angiographic CAD severity.
CAD severity score box plots according to HT use in surgical versus natural menopause and age of HT use onset. The upper and lower edges of the boxes represent the interquartile range, and the whiskers represent the 95th percentile. P values are age adjusted. CAD, coronary artery disease; HT, hormone therapy.
In further sensitivity analyses, we stratified the obstructive CAD results according to no HT use, initiation of HT use before 2 years following menopause, and initiation after 2 years of reaching menopause. This was repeated for 5 years and 10 years, according to more recently published thresholds (14). The p-values for the menopause type by HT group interaction term were 0.025, 0.026, and 0.036 for 2, 5 and 10 year thresholds, respectively, when the outcome was presence vs. absence of obstructive CAD, and 0.06, 0.07, and 0.07, respectively, when the outcome was the log transformed CAD severity score. While naturally menopausal women showed the same relations in angiographic CAD severity across HT groups as shown in Figure 1 (all p-values <0.0001), the differences were non-significant in surgically menopausal women (p-value range = 0.72 to 0.91).
Exploration of “Healthy User” Effects
Initiating HT use before and after the age of 55 years in women with natural and surgical menopause differed from non-HT users by both traditional and non-traditional risk factors including physical activity, nutritional and psychosocial variables (Table 5). When pooling the women with natural and surgical menopause, the HT subgroups (no HT, initiating HT before age 55, and after age 55), showed significant differences in both traditional and non-traditional risk factors that included physical activity and psychosocial variables (Table 5). At the time of WISE enrollment, HT users were younger, had a higher income, were less likely to smoke, were less obese and had a lower Framingham Risk Score, however had more frequent depression, lower usage of aspirin, and an earlier onset of menopause (Table 5). We used a “healthy user” risk model that included all the factors listed in Table 5, with the exception of history of depression, which was collinear with antidepressant use, or the menopausal status or HT variables such as age at menopause, duration of HT use, etc. Unadjusted, a history of HT use was strongly associated with a lower incidence of CAD (OR [95% CI] = 0.34 [0.24, 0.48], p<0.0001) and explained 8% of the total variance in CAD. When HT use was added to the full “healthy user” model, the OR [95% CI] for HT use was attenuated to 0.44 [0.30, 0.73] (p=0.002) and the contribution of HT use to the total variance dropped to 2% which still provided a statistically significant increment (p=0.0003) over and above the healthy user model for predicting presence or absence of CAD. When the outcome was the CAD severity score, history of HT use became non-significant when added to the healthy user model (from beta [SE] = −6.46 [1.16] p<0.0001 to −2.35 [1.36], p=0.08).
Association of HT Use with Adverse CVD Events in Surgical and Natural Menopause
After adjusting for age, historical ever users of HT had a trend toward lower incidence of adverse CVD events compared to never users (hazard ratio [95% CI]=0.72 [0.50, 1.04)], p<0.08). When stratified by surgical versus natural menopause, there was no difference in event rates among the surgical menopause subgroups (p=0.51), but a significant difference among the natural menopause subgroups (p=0.012) (Figure 2). Parallel to the finding for angiographic CAD, HT use was associated with fewer CVD events in women with natural menopause, with the women initiating HT prior to 55 years having the lowest CVD event rate.
FIG. 2. Surgical versus natural menopause, HT, and freedom from major adverse cardiovascular events.
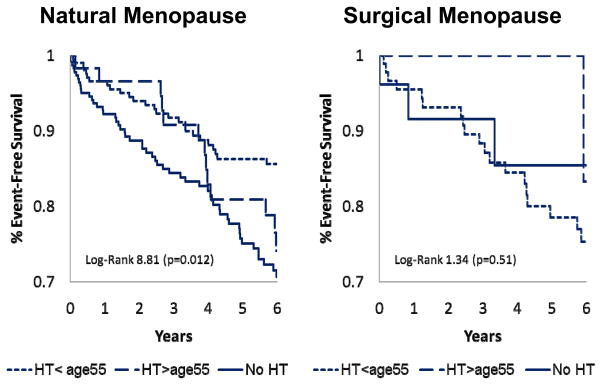
Unadjusted Kaplan-Meier curves and P values for natural menopause HT less than 55 years (n = 215), HT 55 years or more (n = 61), and no HT (n = 233) as well as surgical menopause HT less than 55 years (n = 96), HT 55 years or more (n = 8), and no HT (n = 28). Major events defined as CVD mortality or nonfatal myocardial infarction, heart failure, or stroke. HT, hormone therapy; CVD, cardiovascular disease.
Because the number of women in the surgical menopause subgroups was relatively low thus limiting statistical power to detect event differences within this stratum, we collapsed the two HT groups into type of menopause (natural/surgical) and HT history (no HT vs. HT use). This strengthened the association between HT and adverse CVD events in the natural menopause group (HR [95%CI] = 0.60[0.41, 0.88], p=0.009) but not in the surgical menopause group (1.55 [0.46, 5.17], p=0.48). When the relationships between HT use and adverse CVD events were adjusted for angiographic CAD, these findings became non-significant (0.76 [0.51, 1.14], p=0.18 in the natural menopause group and 1.52 [0.46, 5.10] in the surgical menopause group).
Discussion
To our knowledge, our findings represent the first observation evaluating HT use to core-laboratory assessment of angiographic CAD, and adverse CVD events in women. The current results link HT use with less angiographic CAD events particularly with an earlier onset of HT use. A significant but small residual beneficial association remained even after statistical adjustment for a multitude of variables measured in the WISE study attributable to a “healthy user” effect. HT use, and particularly earlier initiation of HT use was also associated with a lower incidence of adverse CVD events. However, this effect became non-significant after adjusting for the presence or severity of obstructive CAD. This type of a pattern is suggestive of a mediating effect, such that the beneficial association between HT use and reduced CVD event rates appears to be mediated via the mechanism of less atherosclerosis.
The current results further suggest that HT initiated at a relatively early age (<55 years) in natural menopause is associated with a benefit, consistent with recent studies using an intermediate cardiovascular disease marker in relatively young women,21–22 as well as subgroup analyses of the Women’s Health Initiative.14, 23 The current results are also consistent with recent clinical trials that have failed to show benefit either for angiographic coronary disease,8–9 or cardiac events10–12 in older women, and suggest the anti-atherosclerotic HT effect, if present, may be age-dependent.
Early prior observational human studies3, 18, 21–22, 24–25 failed to distinguish premature surgical menopause (removal of ovaries prior to the time natural menopause would have occurred) from other forms of surgical menopause (hysterectomy or postmenopausal oophorectomy), or separate hysterectomy (cessation of menstrual periods with persistent ovulatory function) from bilateral oophorectomy (cessation of ovulatory function), while contemporary studies do not have this failing. 24, 26 Stampfer and colleagues indicated that the magnitude of observed HT protection was greater among those with surgical menopause compared to natural menopause in 5 of the 7 studies that addressed this,27 however these data are similarly limited by the failure to accurately define type of menopause. Notably, our current results, using a specific definition of surgical menopause that includes the age of surgery relative to the age of menopause, are consistent with more recent studies using carotid intimal-medial thickness (IMT), which found no association with HT in surgically menopausal women.28 Additional studies also failed to find an adverse association between surgical menopause and carotid IMT,29 or hysterectomy and future cardiovascular events.30 Finally, our results are consistent with a prior study that evaluated arteriosclerotic heart disease in women defined as documented myocardial infarction, positive ischemic stress test or angina and demonstrated the same prevalence of arteriosclerosis among 267 women with bilateral oophorectomy and 385 hysterectomized women.31
The mechanism(s) behind this apparent differential association between HT use and CAD by surgical versus natural menopause are unknown. Because HT use following surgical menopause was routinely prescribed, we had very few women in the surgical menopause group with either HT initiation after 55 years, or non-HT use. Thus limited statistical power is potentially a leading explanation for the lack of association of HT use in surgically menopausal women. It is also possible that the residual beneficial HT association observed in the natural menopause women can be explained by unadjusted additional healthy user variables (residual confounding), for example prescribing physician decision-making. Alternatively, physiological differences between surgical and natural menopause may account for these findings. Surgical oophorectomy, for example, reduces both blood estrogens and androgens, compared to natural menopause in which the ovaries continue to produce androgens that are converted to estrogens in peripheral tissue,32 with a resulting endogenous estrogen and androgen hormonal milieu that may shape a differential HT response. Bilateral oophorectomy is performed for a variety of reasons in humans, including polycystic ovary syndrome where oophorectomy might theoretically lower subsequent CAD risk. Our results demonstrating a lower angiography CAD severity score in our surgical menopause non-HT users compared to the natural menopause non-HT users supports this possibility. Finally, these results suggest that animal oophorectomy-HT models may not accurately reflect human physiology.2, 5, 33–34
Limitations
The current study results are limited by the observational design that precludes determination of causality. Our results obtained from a patient population of women undergoing coronary angiography for suspected ischemia may not be generalizable to a healthy population with regard to referral bias due to cardiac risk factor status. Statistical power to detect relations between HT use, CAD and adverse CVD events in our surgical menopause women was limited, due to the low prevalence of no HT use in this group. Moreover, it is inherently difficult to pinpoint age of onset of HT use and duration of HT therapy that rely on patient recollection. A larger dataset with a greater distribution of HT duration in early and late users could help further evaluate this. Also, the cross-sectional component of our study design is unable to detect early adverse cardiac events due to HT, as have been observed in prospective, randomized trials.10, 12 Finally, our choice of the age cut-point for initiating HT use of 55 years may be questioned. This age was chosen because this is the age at which 95% of the population has reached menopause. In subsequent sensitivity analyses (reported above), we looked at other possible cut-points which yielded very similar results, suggesting that the distinction between earlier vs. later HT initiation is robust and not dependent on the specific age selected.
Conclusion
Recent HT trials8–12 have consistently demonstrated overall no benefit and early adverse effects in older postmenopausal women, and have questioned the validity of the prior animal2, 5 and human epidemiological results.5–7 The current study results combined with recent publications evaluating younger women and early onset of HT use13–15 suggest that the beneficial association between HT and CAD in natural menopause women observed in prior studies is attributable to both “healthy user” effect and a residual anti-atherosclerotic effect, particularly among those starting HT at a relatively earlier age (<55 years). Because menopausal symptoms requiring treatment remain common,35 further investigation to document the presence or absence of these putative anti-atherosclerotic benefits, as well as improve the safety of HT regimens is clearly warranted. The current analysis sheds light on prior data discrepancies, and may be useful for prospective HT clinical trial planning.
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, a GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, and The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
Footnotes
Conflicts of Interest: Dr. Berga has served as a medical advisor for Bayer Schering, Noven, and Watson Pharmaceuticals. Dr. C. Noel Bairey Merz has the following disclosures: lecture honorarium: Washington University of St. Louis, Society for Women’s Health Research, Brentwood Country Club, Rush-Copley Medical Center, Scienta Healthcare Education, SCS Healthcare and Mayo Foundation for Medical Education. Consulting: Pollock Communications, Medical Education Speakers Net, University of Oklahoma Health, Navvis Healthcare, Axis Healthcare Comm LLC, Itamar Medical Inc, Gilead Sciences, Practice Point Commu Inc, Bristol-Myers Squibb, Curtis Green LLP.
References
- 1.DeStefano F, Merritt RK, Anda RF, Casper ML, Eaker ED. Trends in nonfatal coronary heart disease in the United States, 1980 through 1989. Arch Intern Med. 1993 Nov 8;153(21):2489–2494. [PubMed] [Google Scholar]
- 2.Adams MR, Kaplan JR, Manuck SB, et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990 Nov-Dec;10(6):1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987 Apr 30;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991 Apr 10;265(14):1861–1867. [PubMed] [Google Scholar]
- 5.Wagner JD, Clarkson TB, St Clair RW, Schwenke DC, Shively CA, Adams MR. Estrogen and progesterone replacement therapy reduces low density lipoprotein accumulation in the coronary arteries of surgically postmenopausal cynomolgus monkeys. J Clin Invest. 1991 Dec;88(6):1995–2002. doi: 10.1172/JCI115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000 Dec 19;133(12):933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 7.Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiovasc Dis. 1995 Nov-Dec;38(3):199–210. doi: 10.1016/s0033-0620(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 8.Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000 Aug 24;343(8):522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 9.Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002 Nov 20;288(19):2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 10.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998 Aug 19;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 11.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001 Oct 25;345(17):1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 12.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann Intern Med. 2001 Jul 3;135(1):1–8. doi: 10.7326/0003-4819-135-1-200107030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007 Apr 4;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 15.Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med. 2006 Apr;21(4):363–366. doi: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999 May;33(6):1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BD, Merz CN, Braunstein GD, et al. Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 2004 Oct;13(8):872–887. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976 Aug;28(2):179–196. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 19.Burger HG, Dudley EC, Hopper JL, et al. The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab. 1995 Dec;80(12):3537–3545. doi: 10.1210/jcem.80.12.8530596. [DOI] [PubMed] [Google Scholar]
- 20.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001 Apr 15;87(8):937–941. A933. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 21.Hodis HN, Mack WJ, Azen SP, et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003 Aug 7;349(6):535–545. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan JM, Vander Zwaag R, Lemp GF, et al. Postmenopausal estrogen use and coronary atherosclerosis. Ann Intern Med. 1988 Mar;108(3):358–363. doi: 10.7326/0003-4819-108-3-358. [DOI] [PubMed] [Google Scholar]
- 23.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006 Feb 13;166(3):357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 24.Bain C, Willett W, Hennekens CH, Rosner B, Belanger C, Speizer FE. Use of postmenopausal hormones and risk of myocardial infarction. Circulation. 1981 Jul;64(1):42–46. doi: 10.1161/01.cir.64.1.42. [DOI] [PubMed] [Google Scholar]
- 25.Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996 Aug 15;335(7):453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007 Jun 21;356(25):2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 27.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991 Jan;20(1):47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 28.Dwyer KM, Nordstrom CK, Bairey Merz CN, Dwyer JH. Carotid wall thickness and years since bilateral oophorectomy: the Los Angeles Atherosclerosis Study. Am J Epidemiol. 2002 Sep 1;156(5):438–444. doi: 10.1093/aje/kwf051. [DOI] [PubMed] [Google Scholar]
- 29.Mack WJ, Slater CC, Xiang M, Shoupe D, Lobo RA, Hodis HN. Elevated subclinical atherosclerosis associated with oophorectomy is related to time since menopause rather than type of menopause. Fertil Steril. 2004 Aug;82(2):391–397. doi: 10.1016/j.fertnstert.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 30.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005 Mar 29;111(12):1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 31.Ritterband AB, Jaffe IA, Densen PM, Magagna JF, Reed E. GONADAL FUNCTION AND THE DEVELOPMENT OF CORONARY HEART DISEASE. Circulation. 1963 Feb;27:237–251. doi: 10.1161/01.cir.27.2.237. [DOI] [PubMed] [Google Scholar]
- 32.Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril. 1994 Jul;62(1):20–27. doi: 10.1016/s0015-0282(16)56810-1. [DOI] [PubMed] [Google Scholar]
- 33.Clarkson TB, Anthony MS, Wagner JD. A comparison of tibolone and conjugated equine estrogens effects on coronary artery atherosclerosis and bone density of postmenopausal monkeys. J Clin Endocrinol Metab. 2001 Nov;86(11):5396–5404. doi: 10.1210/jcem.86.11.8021. [DOI] [PubMed] [Google Scholar]
- 34.Suparto IH, Williams JK, Cline JM, Anthony MS, Fox JL. Contrasting effects of two hormone replacement therapies on the cardiovascular and mammary gland outcomes in surgically postmenopausal monkeys. Am J Obstet Gynecol. 2003 May;188(5):1132–1140. doi: 10.1067/mob.2003.237. [DOI] [PubMed] [Google Scholar]
- 35.Kritz-Silverstein D, Goldani Von Muhlen D, Barrett-Connor E. Prevalence and clustering of menopausal symptoms in older women by hysterectomy and oophorectomy status. J Womens Health Gend Based Med. 2000 Sep;9(7):747–755. doi: 10.1089/15246090050147727. [DOI] [PubMed] [Google Scholar]