Abstract
Background and Aims
Leaf hydraulic properties are strongly linked with transpiration and photosynthesis in many species. However, it is not known if gas exchange and hydraulics will have co-ordinated responses to climate change. The objective of this study was to investigate the responses of leaf hydraulic conductance (Kleaf) in Glycine max (soybean) to growth at elevated [CO2] and increased temperature compared with the responses of leaf gas exchange and leaf water status.
Methods
Two controlled-environment growth chamber experiments were conducted with soybean to measure Kleaf, stomatal conductance (gs) and photosynthesis (A) during growth at elevated [CO2] and temperature relative to ambient levels. These results were validated with field experiments on soybean grown under free-air elevated [CO2] (FACE) and canopy warming.
Key results
In chamber studies, Kleaf did not acclimate to growth at elevated [CO2], even though stomatal conductance decreased and photosynthesis increased. Growth at elevated temperature also did not affect Kleaf, although gs and A showed significant but inconsistent decreases. The lack of response of Kleaf to growth at increased [CO2] and temperature in chamber-grown plants was confirmed with field-grown soybean at a FACE facility.
Conclusions
Leaf hydraulic and leaf gas exchange responses to these two climate change factors were not strongly linked in soybean, although gs responded to [CO2] and increased temperature as previously reported. This differential behaviour could lead to an imbalance between hydraulic supply and transpiration demand under extreme environmental conditions likely to become more common as global climate continues to change.
Keywords: Leaf hydraulic conductance, elevated CO2, temperature, climate change, water potential, soybean, Glycine max
INTRODUCTION
A strong interdependency of leaf water transport capacity and photosynthetic capacity is expected from the principles of leaf gas exchange. Under natural mesophytic conditions, leaves lose several orders of magnitude more water to the atmosphere than they acquire CO2 from the atmosphere. Thus, leaves must resupply water to the sites of evaporation within the leaf mesophyll to enable the maintenance of open stomata for photosynthetic CO2 acquisition without desiccating the leaf. Leaf hydraulic conductance (Kleaf) is a measure of water flow efficiency through the leaf and is defined as the water flux through the leaf per unit water potential driving force (Sack and Holbrook, 2006). Across sets of angiosperm species, Kleaf was found to correlate positively with maximum stomatal pore area per leaf area, mid-day stomatal conductance, photosynthetic electron transport rate and light-saturated CO2 assimilation (Sack et al., 2003; Brodribb et al., 2007), suggesting evolutionary co-ordination between hydraulic and photosynthetic capacities of the leaf. Indeed, dynamic co-ordination of leaf and plant hydraulic conductance with gas exchange and photosynthesis has been observed in numerous species in response to environmental perturbation. Leaf net photosynthetic rates (A) were limited by whole-plant hydraulics under sufficient soil moisture conditions in Pinus ponderosa (Hubbard et al., 1999), and stomatal conductance (gs) was limited by whole-plant and shoot hydraulics in several different deciduous and evergreen tree species (Salleo et al., 2000; Nardini and Salleo, 2000). Within given species, photosystem II quantum yield correlated with Kleaf on a diurnal cycle and also during leaf senescence (Brodribb and Holbrook, 2003, 2004). Kleaf plasticity has also been observed in response to dynamic changes in temperature and light (Sack et al., 2004; Scoffoni et al., 2008; Nardini et al., 2010), in some cases in co-ordination with A during growth under different environmental conditions (Brodribb and Jordan, 2011).
Atmospheric [CO2] is expected to exceed 550 µmol mol−1 (ppm) by mid-century and to drive increases of global temperature by 1–6 °C (Meehl et al., 2007). Elevated [CO2] almost always leads to a reduction of gs, lowering leaf- and canopy-level transpiration (Ainsworth and Long, 2005; Bernacchi et al., 2007). A lower transpiration rate should permit the reduction of Kleaf with no penalty to photosynthetic rate at elevated [CO2]. Consistent with this expectation, whole-plant hydraulic conductance was reported to decrease in response to short-term exposure to elevated [CO2] in chamber-grown Amaranthus hypochondriacus and Zea mays, as well as to long-term growth at elevated [CO2] for chamber-grown Glycine max (soybean) and Medicago sativa (Bunce, 1996; Bunce and Ziska, 1998). Further, Kleaf decreased in Pinus taeda needles grown at elevated [CO2] with free-air concentration enrichment (FACE) (Domec et al., 2009).
The effects of elevated [CO2] on soybean have been studied extensively because of its importance as the world's third most economically valuable agricultural crop (Food and Agriculture Organization of the United Nations, 2010). With approx. 75 million acres of soybean planted annually in the USA, a thorough understanding of how soybean water relations respond to climate change is crucial to predicting how climate change will affect environmental processes and global food security. A generally increases at elevated [CO2]. However, in field-grown soybean, the maximum velocity of carboxylation by ribulose-1,5-bisphosphate carboxylasae/oxygenase (Rubisco, Vc,max) tends to decrease during acclimation to growth at elevated [CO2], thus restricting the degree to which elevated [CO2] increases A (D. M. Rosenthal, USDA-ARS, Urbana, IL, USA, unpubl. res.). Leaf gs consistently decreases at elevated [CO2], but no acclimation of gs to elevated [CO2] has been observed across a decade of study at the SoyFACE field site (Leakey et al., 2006). The consistent decrease in upper canopy leaf gs during growth at elevated [CO2] is accompanied by decreased canopy evapotranspiration for field-grown soybean (Bernacchi et al., 2007). Despite the larger canopy leaf area for elevated [CO2]-grown soybean, the reduction in gs significantly decreased water flow through the canopy, coupled with only a slight and inconsistent water potential (Ψleaf) decrease, suggesting that Kleaf could be lower for soybean grown at elevated [CO2] without limiting A (Bernacchi et al., 2007). That hypothesis was also supported by a recent field study in which intrinsic water use efficiency (A/gs) was observed to increase for soybeans grown at elevated [CO2] (Ruiz-Vera et al., 2013).
Temperature and vapour pressure deficit (VPD) are major determinants of evapotranspiration. Global temperature increases throughout the 21st century will result in increased evaporative demand, but projections for VPD are less certain (Meehl et al., 2007). As temperatures rise with climate change, evapotranspiration (E) is likely to increase on a leaf area basis, as has been measured in soybean and Z. mays (Yang et al., 2012). With higher transpiration demand, Kleaf could become limiting to A if it has insufficient capacity to adjust, thereby causing reduction of gs. A recent study with soybean grown at increased temperature in the field indeed found that gs declined at higher temperatures, but intrinsic water use efficiency increased at higher temperatures due to biochemical properties of Rubisco (Ruiz-Vera et al., 2013). However, the stimulatory effect of temperature on photosynthesis declines at peak summer temperatures in many soybean-growing regions (D. M. Rosenthal, USDA-ARS, Urbana, IL, USA, unpubl. res.), and A will decline even as E continues to rise. There is evidence of a fast, reversible increase of Kleaf with rising temperature in Acer saccharum, Aesculus hippocastanum and Quercus rubra relating to changes in the viscosity of water and of membrane properties and/or aquaporin activity (Sack et al., 2004; Nardini et al., 2010). It is not known, however, if long-term growth at elevated temperature induces a more permanent increase in Kleaf.
Our objective in this study was to investigate the responses of Kleaf to growth at elevated [CO2] and temperature in soybean. We hypothesized that for leaves grown at elevated [CO2], Kleaf will decrease to reduce costly investment in water transport capacity while maintaining Ψleaf, gs and A. Furthermore, we hypothesized that for leaves grown at elevated temperature, Kleaf will increase to match higher E driven by an increase in VPD. These hypotheses were tested in a two growth chamber experiments, one a factorial CO2 × temperature experiment, and the second focused on temperature responses alone. Gas exchange parameters were measured along with Kleaf in these experiments. These chamber experiments were validated using soybean grown under FACE for [CO2] and open-air, infrared temperature elevation in the field. Because the leaf is a critical component in the transpiration pathway, knowledge of leaf hydraulic responses and limits is necessary to be able to predict the extent to which gas exchange can adjust under increasingly extreme environmental conditions.
MATERIALS AND METHODS
Plant material and growth conditions
For both chamber experiments, seeds of soybean (Glycine max) cultivar ‘93B15’ (Pioneer Hi-Bred, Johnston, IA, USA), a variety with indeterminate growth, were planted in 14·5 L pots with LC-1 Sunshine mix (SunGro Horticulture Canada Ltd, Bellevue, WA, USA). All seeds emerged from the soil within 4 d of planting. For the CO2 × temperature factorial experiment, soybeans were germinated and grown in eight temperature- and CO2-controlled growth chambers inside a greenhouse; four plants were grown per chamber. The chambers were constructed with aluminium frames and enclosed with clear acetate to allow entry of greenhouse light (Maherali and DeLucia, 2000). Natural light was supplemented with overhead lighting to reach approx. 750 µmol m−2 s−1 of photosynthetically active radiation (PAR) at plant level inside the growth chambers. The experiment was conducted from January to May 2010, with two separate sets of plants grown in succession. Measurements were taken on the youngest fully expanded leaves at 31, 36 and 43 d after planting in the first run of the experiment, and at 36, 38 and 43 d after planting in the second run of the experiment, so leaf developmental stage and leaf age were consistent for all measurements while plant age varies. Daylength varied greatly over the course of the experiment, but supplemental lighting was provided for 12 h d−1. [CO2] was continuously monitored in the centre of each chamber and fumigation was automatically adjusted to sustain the target. Treatments were applied in a 2 × 2 factorial design with [CO2] treatments of 400 (ambient) and 700 µmol mol−1 (ppm) (elevated) and daytime temperatures of 27 °C (ambient) and 31 °C (elevated). Elevated [CO2] and temperature treatments began at seed planting, so plants experienced their assigned treatment conditions for their entire life span. Plants were randomly rotated among chambers weekly to reduce chamber effects, watered every other day, and fertilized twice weekly with 50 % Long Ashton solution supplemented with 10 mM NH4NO3 (Hewitt, 1966). In the CO2 × temperature experiment, we were unable to control humidity, but it was high enough in all treatments to produce condensation on the walls of the growth chambers occasionally. High humidity may have obscured a possible response to temperature via VPD in the experiment.
For the temperature-only experiment, plants were grown in eight controlled environment chambers (GC-15, Environmental Growth Chambers, Chagrin Falls, OH, USA). Twelve plants were grown in each chamber, and ambient and elevated temperature treatments were replicated in four chambers each. Daytime temperatures were 25° C for ambient plants and 30° C for the elevated treatment; night-time temperature was 22° C and [CO2] was 400 ppm for all plants. Elevated temperature treatment began at seed planting, so plants experienced their assigned treatment temperature for their entire life span. Measurements were taken on the youngest fully expanded leaves at 32, 39 and 41 d after planting. Plants were randomly rotated within chambers every 2 d and among chambers every 4 d to reduce chamber effects. Light levels were approx. 1200 µmol m−2 s−1 PAR at plant height. Plants were fertilized every other day with 50 % Long Ashton solution supplemented with 10 mm NH4NO3. Dataloggers were placed inside each chamber to record temperature and humidity at 15 min intervals (HOBO U12-012, Onset Computer Corporation, Inc., Pocasset, MA, USA). In this chamber experiment, relative humidity was carefully controlled at 60 % for both temperature treatments and continuously recorded, with an average VPD increase of 0·4 kPa for plants grown at elevated temperature (Fig. 1).
Fig. 1.
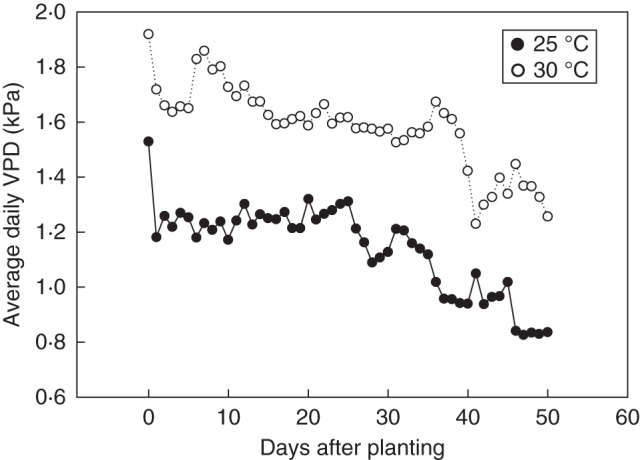
Average vapour pressure deficit (VPD) calculated from the four control and four elevated temperature chambers on every day of the experiment, beginning at planting. Temperature and relative humidity were logged at 15 min intervals; VPD was calculated for each of those intervals and then averaged over each 14 h daylight period. Ambient temperature chambers (25 °C) and elevated temperature chambers (30 °C) are as indicated. Relative humidity for all chambers was set at 60 %, although chamber dehumidification was insufficient to maintain this level as plants grew. Thus, relative humidity increased slightly and VPD decreased slightly over time.
In 2010 and 2012, the same soybean cultivar ‘93B15’ (Pioneer Hi-Bred) was planted at the SoyFACE facility in Champaign, IL, USA in 0·38 m row spacing. Planting occurred on 27 May in 2010 and 15 May in 2012. A detailed description of the field site and SoyFACE CO2 fumigation method can be found in Rogers et al. (2004). The [CO2] experiment was conducted in a completely randomized block design. Each block consisted of two 20 m diameter rings, one at ambient [CO2] and one fumigated with pure CO2 to an elevated target [CO2]. In 2010, ambient [CO2] was 385 ppm and target elevated [CO2] was 585 ppm; in elevated plots, [CO2] was within 10 % of the target 75 % of the time. In 2012, ambient [CO2] was 390 ppm. Elevated [CO2] treatment began by the time seedlings emerged from the soil and continued for the full growing season, so all leaves developed entirely under their assigned treatment conditions. Measurements were taken at 32 and 50 d after planting.
In 2012, temperature elevation of 3·5 °C above ambient was achieved by placing infrared heaters above the canopy as detailed in Ruiz-Vera et al. (2013). Temperature treatment was applied continuously from 7 d after planting, with two exceptions from 10–15 July and 12–16 August, when electrical power was not available. After power was restored, measurements were not taken until at least one new leaf had fully developed under the elevated temperature treatment. Measurements were taken at 64 and 86 d after planting.
Gas exchange
Photosynthesis (A) and stomatal conductance (gs) were measured on the uppermost, fully expanded leaf on a plant using an open-path gas analysers equipped with a leaf chamber fluorometer (LI-6400, LI-COR Biosciences, Lincoln, NE, USA). Gas exchange was measured between 1200 h and 1400 h central standard time, as this typically corresponds to daily peak photosynthetic rates. Plants were briefly removed from growth chambers for gas exchange measurement, but [CO2], temperature and PAR were set equal to growth conditions for each plant. Relative humidity in the gas exchange cuvette was maintained between 60 and 70 %. Different LI-6400s were used for the CO2 × temperature chamber experiment and the temperature-only chamber experiment, but comparisons are only made between measurements taken with the same instrument.
Leaf water potential
Discs of 1·5 cm diameter were cut from uppermost mature leaves and sealed inside a stainless steel thermocouple psychrometer chamber within 15 s of cutting (Wescor C-30, Wescor, Inc., Logan, UT, USA). The psychrometer temperature was maintained at 22 °C in a controlled-environment chamber for 3 h until equilibrium temperature was achieved, and then the water potential of the leaf discs was recorded using a datalogger (Campbell CF-1000, Campbell Scientific, Logan, UT, USA). When pre-dawn Ψleaf was measured in the CO2 × temperature experiment, leaves were collected between 0500 h and 0700 h. In this experiment, 12 leaf discs were sampled from the youngest, fully expanded leaf on each of two plants in each growth chamber. These discs were divided evenly among four psychrometer chambers for water potential measurement. Pre-dawn Ψleaf is assumed to be equal to soil water potential and was used to confirm that the treatments did not result in large soil moisture differences. There were only small differences in pre-dawn Ψleaf, with values ranging from –0·44 to –0·73 MPa across both experiments. When mid-day Ψleaf was measured in the temperature-only chamber experiment, three leaves were sampled per growth chamber, and three leaf discs were punched per leaf and measured in one psychrometer chamber. In this experiment, discs were sampled between 1300 h and 1400 h.
Leaf hydraulic conductance
Kleaf was measured using the evaporative flux method (Sack et al., 2002), with modifications as described below. Leaves were cut at the base of the petiole immediately prior to turning on supplemental lighting for the growth chamber experiments or just prior to sunrise for the field experiments. Sampling at that time ensured that all leaves were fully hydrated and any emboli acquired during the previous day were refilled, so maximum Kleaf could be measured for each treatment. The youngest, fully expanded leaves were sampled for all measurements, so leaf age is consistent among all measurements, while plant age varied with the repeated sampling within experiments. Leaves of this soybean cultivar fully mature in approx. 8 d, so sampled leaf ages were within 4 d of each other, between the time the leaf becomes fully expanded and the time when it becomes shaded by a younger leaf above.
Evaporative flux measurements were made in the lab at ambient [CO2] and about 25 °C air temperature, so persistent but not quickly reversible effects of treatments were measured. Cut petioles were submerged immediately in water and re-cut under water. Crevices in the soybean petioles were blocked with petroleum jelly, and petioles were wrapped in Parafilm (Pechiney Plastic Packaging Company, Chicago, IL, USA) to ensure a tight seal with tubing that supplied water to the leaf (Tygon R-3603, Saint-Groban Performance Plastics Corporation, Paris, France). This tubing was connected to a reservoir of ultrapure, partially degassed water situated on a high-precision balance (XS 250, Mettler Toledo, Columbus, OH, USA). The leaf was then placed under halogen lighting, with a glass tray of water placed between the lamp and the leaf to absorb infrared radiation, allowing approx. 700 µmol m−2 s−1 PAR to reach the leaf. A box fan was used to disrupt the leaf boundary layer, and water flow through the leaf was allowed to stabilize for at least 30 min. When water flow reached steady state, top and bottom leaf surface temperatures were measured (Fluke 574 Precision Infrared Thermometer, Fluke Corporation, Everett, WA, USA; calibrated using a black body calibrator, BB701, Omega Engineering, Inc., Stamford, CT, USA). Leaf discs were subsequently removed for Ψleaf determination using thermocouple psychrometers as described above; 12 discs were measured per leaf in four psychrometer chambers. Leaves were then photographed and leaf area was measured with ImageJ software (NIH, http://rsbweb.nih.gov/ij/). Kleaf was calculated as flow rate/leaf water potential and normalized for leaf area and the effect of temperature on water viscosity (Yang and Tyree, 1993). To increase throughput, four identical balances were connected to a 4-channel serial I/O interface (SDM-SIO4, Campbell Scientific, Inc.) which was linked to a datalogger (CR1000, Campbell Scientific, Inc.). This allowed water flow data from four leaves on four balances to be recorded in a single file and viewed simultaneously on a computer in real-time. Approximately 15 % of leaves wilted during evaporative flux measurement, and these values were not included in the analyses.
Statistical analyses
The SAS MIXED procedure was used for all statistical analyses (SAS 9·1, SAS Institute, Cary, NC, USA). For each experiment, data from multiple measurements days were pooled and analysed by repeated measures. This accounted for variation due to measurement day, as measurements were taken from the same replicate chambers or plots on multiple days in each experiment. [CO2] and temperature were always considered as fixed effects. In chamber experiments, each group of plants within a chamber was a replicate, and plants within these groups were treated as sub-samples. Because groups of plants were randomly rotated among chambers every 4 d, chamber effects were considered to be evenly distributed and were not included in the model. In field experiments, each treatment plot was a replicate, and plants within plots were considered as sub-samples. Plots were spatially blocked in the field, with one replicate of each treatment per block.
Optimizing α
To avoid unnecessarily high rates of Type II error, α values were optimized for hypothesis testing. Instead of minimizing only Type I or Type II error, this approach minimizes the average of Type I and Type II error, and therefore the overall error rate, which is optimal for a study in which Type I and type II errors are considered to have equal consequence (Mudge et al., 2012). Degrees of freedom and Cohen's f2 were inputs for R code provided by Mudge et al. (2012). Degrees of freedom were taken from the data sets, and Cohen's f2 of 0·35 was chosen a priori as representing a large effect size and corresponding 26 % of variance explained (Cohen, 1988). Based on the degrees of freedom in our study, the α values generated for hypothesis testing are higher than the standard α = 0·05 that is used to interpret most plant physiological data (Table 1).
Table 1.
Optimal α and β values used for hypothesis testing
| Degrees of freedom | Optimal α | Optimal β |
|---|---|---|
| 2 | 0·38 | 0·40 |
| 7 | 0·25 | 0·30 |
| 12 | 0·18 | 0·21 |
| 39 | 0·04 | 0·05 |
Values were calculated according to Mudge et al. (2012); inputs were degrees of freedom from the data set and Cohen's f2 of 0·35, chosen a priori. Degrees of freedom for each data set can be found in the figures.
RESULTS
Kleaf did not acclimate to growth at elevated [CO2]
The Kleaf values obtained using the evaporative flux method were comparable with values observed in past studies with pot-grown soybeans and other herbaceous crop species (Sober, 1997; Tsuda and Tyree, 2000; Sack and Holbrook, 2006). In the CO2 × temperature experiment, growth at elevated [CO2] did not significantly affect Kleaf (P = 0·5466, d.f. = 39) (Fig. 2A). Measurements with field-grown soybean showed similar results to the chamber experiment. Elevated [CO2] did not lead to a significant change in Kleaf for field-grown soybean under free-air CO2 enrichment (P = 0·9852, d.f. = 3) (Fig. 3).
Fig. 2.
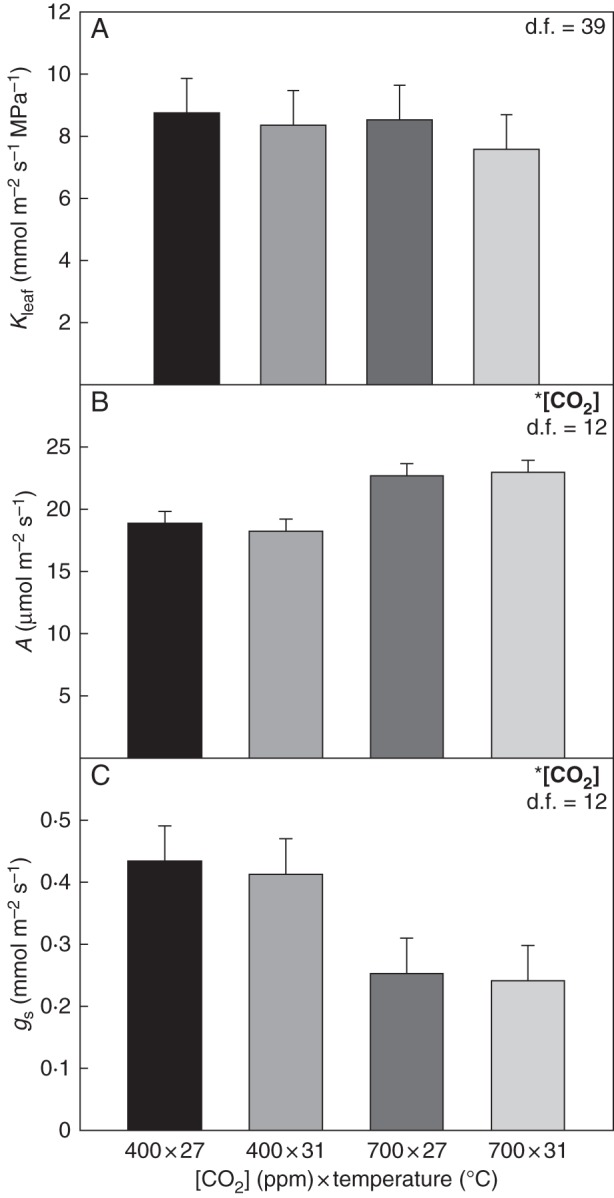
Leaf hydraulic conductance (Kleaf), photosynthesis (A) and stomatal conductance (gs) for the CO2 × temperature chamber experiment. Measurements were taken with the youngest, fully expanded leaves. Growth [CO2] was 400 ppm (ambient) or 700 ppm (elevated), and growth temperature was 27 °C (ambient) or 31 °C (elevated). Kleaf was measured for leaves sampled before sunrise, while A and gs were measured at mid-day on the previous day. Asterisks denote significant treatments effects, and degrees of freedom for each data set are noted in the upper right corner of the graphs. Alpha levels for each hypothesis test can be found in Table 1.
Fig. 3.
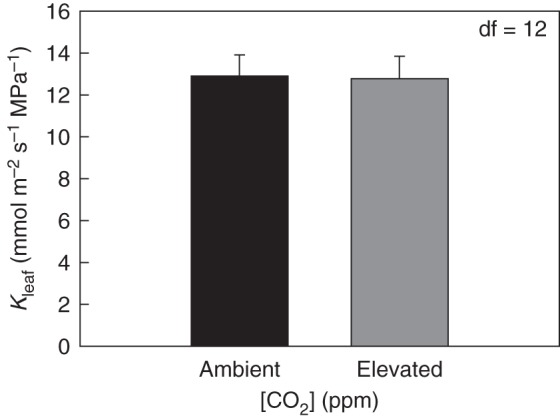
Leaf hydraulic conductance (Kleaf) for field-grown soybean under free-air [CO2] enrichment (FACE). The youngest, fully expanded leaves were sampled pre-sunrise from ambient and elevated [CO2] plots. [CO2] did not affect Kleaf. Alpha levels for each hypothesis test can be found in Table 1.
Elevated [CO2] increased carbon gain at both ambient and elevated temperature
Elevated [CO2] increased A by 23 % in the CO2 × temperature experiment (P = 0·0009, d.f. = 12) (Fig. 2C). Elevated [CO2] decreased gs by 42 % compared with ambient [CO2] (P = 0·0093, d.f. = 12) (Fig. 2D).
Growth at elevated temperature did not alter Kleaf
In the CO2 × temperature experiment, increased temperature did not change Kleaf (P = 0·4213, d.f. = 39) (Fig. 2A). Similarly, in the temperature-only chamber experiment, growth temperature did not alter Kleaf (P = 0·9542, d.f. = 4) (Fig. 3). Elevated temperature also did not affect Kleaf for field-grown plants in 2012 (P = 0·8002, d.f. = 7) (Fig. 5). Additionally, in the growth chamber experiment, mid-day Ψleaf was not significantly altered by elevated temperature (P = 0·6731, d.f. = 2) (Fig. 4B).
Fig. 5.
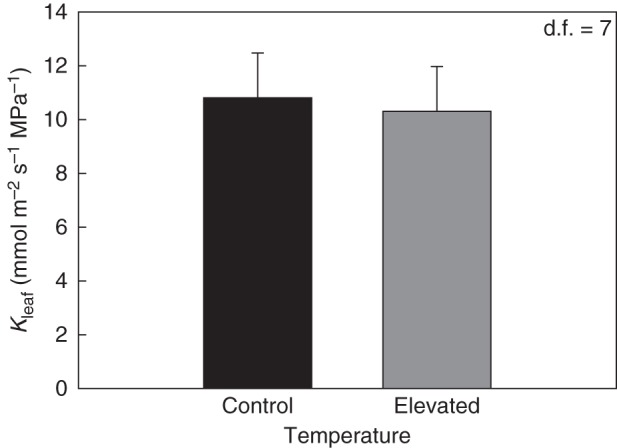
Leaf hydraulic conductance (Kleaf) for field-grown soybean. Temperature was ambient or elevated to 3·5 °C over ambient with infrared heaters. The youngest, fully expanded leaves were sampled pre-sunrise from control plots and heated plots. Temperature did not affect Kleaf. Alpha levels for each hypothesis test can be found in Table 1.
Fig. 4.
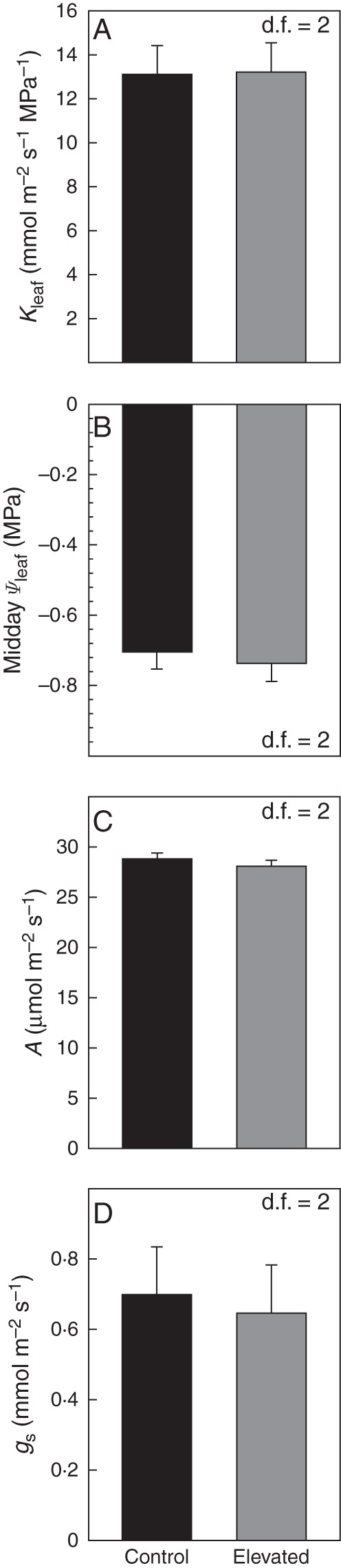
Leaf hydraulic conductance (Kleaf), mid-day leaf water potential (Ψleaf), photosynthesis (A) and stomatal conductance (gs) for the temperature-only chamber experiment. Measurements were taken with the youngest, fully expanded leaves. Daytime growth temperature was 25 °C (ambient) or 30 °C (elevated). Kleaf was measured for leaves sampled before daytime growth lights turned on, while Ψleaf, A and gs were measured at mid-day on the previous day. Degrees of freedom for each data set are given in the corner of each panel. Alpha levels for each hypothesis test can be found in Table 1.
Temperature did not consistently affect gas exchange
In the CO2 × temperature experiment, temperature did not have a significant effect on A (P = 0·8691, df = 12) or gs (P = 0·7828, d.f. = 12) (Fig. 2B, C). Growth at elevated temperature also did not affect A (P = 0·4589, d.f. = 2) or gs (P = 0·8128, d.f. = 2) in the temperature-only chamber experiment (Fig. 4).
For each experiment, data for individual measurement days were also analysed individually. Kleaf across treatments was statistically different for measurement days in the temperature-only chamber experiment (P = 0·01, d.f. = 2) and in the [CO2] field experiment (P = 0·06, d.f. = 13). Some variation was present in these data which was not consistent or significant across experiments; these results are presented separately (Supplementary Data Tables S1, S2, Figs S1–S4).
DISCUSSION
To our knowledge, we present the first measurements of Kleaf and its co-ordination with gas exchange in response to temperature and [CO2] for field-grown soybean. Kleaf in field-grown soybean was maintained within a stable range of values for plants grown under open-air CO2 fumigation or temperature elevation (Figs 3 and 5). Although there was some variation between measurement days for pooled data sets in the temperature chamber experiment and the [CO2] field experiment, this variation could not be attributed to any known factor in these experiments, and these differences were accounted for in the statistical analyses.
Because Kleaf measurements were made at ambient indoor [CO2] while leaves developed at either ambient (385 ppm) or elevated (585 ppm) [CO2], our inferences are restricted to acclimation which would have occurred over the course of leaf development rather than instantaneous, rapidly reversible effects of [CO2]. The findings of a consistent Kleaf across growth [CO2] levels in growth chambers and in the field did not support the hypothesis that Kleaf would be reduced at elevated [CO2] to match the decline of gs and transpiration (Figs 2 and 3). Our data also suggested that the 46 % decrease in whole-plant hydraulic conductance at elevated [CO2] previously reported from a chamber experiment with soybean (Bunce, 1996) probably did not involve a contribution from the leaves. Although that previous study did not measure Kleaf, it reported a much greater [CO2] effect on stem hydraulic conductance than on root hydraulic conductance. In our study, gas exchange parameters responded as expected to elevated [CO2], with gs decreasing and A increasing. That these responses were not reflected in Kleaf measurements indicates that soybean leaf gas exchange and leaf hydraulics are not closely coupled in their ability to acclimate to environmental conditions, and that Kleaf itself was not mechanistically influenced by growth [CO2] in the ways that have been recently hypothesized (Flexas et al., 2012), for example, due to developmental acclimation of aquaporin/CO2-porin activity. Notably, the high Kleaf relative to gs in plants grown at high [CO2] could contribute to drought tolerance. Plants undergoing the onset of soil drying, or increases in VPD, can better maintain open stomata given high Kleaf relative to gs (Brodribb and Jordan, 2008; Osborne and Sack, 2012). Furthermore, this insensitivity of Kleaf to growth [CO2] suggests that Kleaf was not limiting gas exchange under either the ambient or elevated [CO2] conditions tested in this study, which included field conditions.
Kleaf was similarly unresponsive to growth at elevated temperature (Figs 4A and 5). Kleaf has consistently been observed to increase with temperature in other studies, both at the time scale of minutes during measurement for A. hippocastanum, A. saccharum and Q. rubrum (Sack et al., 2004; Nardini et al., 2010) and with varying in situ leaf temperatures in Tilia cordata (Sellin and Kupper, 2007). While Kleaf did not show acclimation to growth at increased temperature in this study, evapotranspiration has been observed to increase for soybeans grown at elevated temperature in a chamber study (Allen et al., 2003). This finding suggests a lack of co-ordination of hydraulic and stomatal plasticity in soybean leaves.
Since the CO2 and temperature treatment differentials were not maintained during Kleaf measurements in this study, it is possible that soybean in a high temperature environment does have a higher Kleaf, but that this effect is transient and fully reversed when steady state was reached during the evaporative flux measurement. If so, this would suggest a lack of phenotypic plasticity for response to temperature in the structural components of the leaf which influence Kleaf, such as vein density (Sack and Frole, 2006; Brodribb et al., 2007). A previous study of the effect of growth [CO2] on Quercus petraea (350 ppm vs. 700 ppm) found no effects on vein density although stomatal density was reduced at high CO2 (Uhl and Mosbrugger, 1999), results analogous to our findings for Kleaf and gs in soybean. In contrast, elevated growth temperature was reported to increase Kleaf in Populus tremula when measurements were taken at a constant temperature (Aasamaa et al., 2005), and vein density is often found to increase in leaves grown under higher temperatures (Uhl and Mosbrugger, 1999; Sack and Scoffoni, 2013). Beyond vein density, the limited capability to adjust realized leaf hydraulic capacity observed in soybean may relate to its being a herbaceous annual bred under strong artificial selection. Greater Kleaf plasticity could also be more adaptive in tall plants than in short, herbaceous species. In Sclerobium paniculatum, taller individuals had lower maximum Kleaf and lower Kleaf vulnerability than shorter individuals (Zhang et al., 2009). As cultivated soybean is short with ancestors that were vines, there may be less penalty for overall lack of hydraulic plasticity in the shoot.
In summary, our data suggest a lack of phenotypic plasticity in soybean Kleaf during growth at elevated [CO2] and temperature. The responses of Kleaf and gas exchange to [CO2] and temperature do not appear to be mechanistically co-ordinated in soybean. This independence allows a shift in hydraulic supply relative to demand, such that plants grown at high [CO2] have high Kleaf relative to gs, and thus would be able to sustain higher gs and A during declines in soil water potential or high VPD. As gas exchange and leaf hydraulic conductance appear to be acting independently of each other in these studies, it is likely that Kleaf is not limiting to gas exchange under the conditions tested in these experiments. However, if Kleaf cannot be increased under elevated temperature, then it is possible that Kleaf could limit the delivery of water to points of evaporation within the leaf and thereby lead to a decline in leaf water potential, and a reduction of gs, that would limit A under such extreme weather conditions as are projected to become more frequent during this century (Meehl et al., 2007). Such a hydraulic limitation could be responsible for an increase in stomatal limitation to A at high temperatures, as had been previously observed in a study with field-grown soybean (D. M. Rosenthal, USDA-ARS, Urbana, IL, USA, unpubl. res.).
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Nathan Couch, Adrian Zimbelman, Bob Koester, Sharon Gray, Katie Richter, Bob Koester, Courtney Leisner, Becky Slattery and Colleen Cahill for assistance with data collection and plant care, John Drake for assistance with setting up dataloggers, and David Drag, Charlie Mitsdarfer and Kannan Puthuval for maintaining growth chambers and managing operations at SoyFACE. This research was funded by the United States Department of Agriculture (USDA) Agriculture Research Service (ARS) and by the Office of Science (BER), US Department of Energy, through the Midwestern Center of the National Institute for Climate Change Research (NICCR).
LITERATURE CITED
- Aasamaa K, Niinemets U, Sõber A. Leaf hydraulic conductance in relation to anatomical and functional traits during Populus tremula leaf ontogeny. Tree Physiology. 2005;25:1409–1418. doi: 10.1093/treephys/25.11.1409. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Allen LH, Jr, Pan D, Boote KJ, Pickering NB, Jones JW. Carbon dioxide and temperature effects on evapotranspiration and water use efficiency of soybean. Agronomy Journal. 2003;95:1071–1981. [Google Scholar]
- Bernacchi CJ, Kimball BA, Quarles DR, Long SP, Ort DR. Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiology. 2007;143:134–144. doi: 10.1104/pp.106.089557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytologist. 2003;158:295–303. [Google Scholar]
- Brodribb TJ, Holbrook NM. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant, Cell and Environment. 2004;27:820–827. [Google Scholar]
- Brodribb TJ, Jordan GJ. Internal coordination between hydraulics and stomatal control in leaves. Plant, Cell and Environment. 2008;31:1557–1564. doi: 10.1111/j.1365-3040.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Jordan GJ. Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytologist. 2011;192:437–448. doi: 10.1111/j.1469-8137.2011.03795.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology. 2007;144:1890–1898. doi: 10.1104/pp.107.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JA. Growth at elevated carbon dioxide concentration reduces hydraulic conductance in alfalfa and soybean. Global Change Biology. 1996;2:155–158. [Google Scholar]
- Bunce JA, Ziska LH. Decreased hydraulic conductance in plants at elevated carbon dioxide. Plant, Cell and Environment. 1998;21:121–126. [Google Scholar]
- Cohen JE. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Domec J-C, Palmroth S, Ward E, Maier CA, Thérézien M, Oren R. Acclimation of leaf hydraulic conductance and stomatal conductance of Pinus taeda (loblolly pine) to long-term growth in elevated CO2 (free-air CO2 enrichment) and N-fertilization. Plant, Cell and Environment. 2009;32:1500–1512. doi: 10.1111/j.1365-3040.2009.02014.x. [DOI] [PubMed] [Google Scholar]
- Flexas J, Barbour MM, Brendel O, et al. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science. 2012;193–194:70–84. doi: 10.1016/j.plantsci.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations. Food and agricultural commodities production. Rome: FAO; 2010. [Google Scholar]
- Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. London: Commonwealth Agricultural Bureau; 1966. [Google Scholar]
- Hubbard RM, Bond BJ, Ryan MG. Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiology. 1999;19:165–172. doi: 10.1093/treephys/19.3.165. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Bernacchi CJ, Ort DR, Long SP. Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant, Cell and Environment. 2006;29:1794–1800. doi: 10.1111/j.1365-3040.2006.01556.x. [DOI] [PubMed] [Google Scholar]
- Maherali H, DeLucia EH. Interactive effects of elevated CO2 and temperature on water transport in ponderosa pine. American Journal of Botany. 2000;87:243–249. [PubMed] [Google Scholar]
- Meehl GA, Stocker TF, Collins WD, et al. Global climate projections. In: Solomon S, Quin D, Manning M, et al., editors. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Mudge JF, Baker LF, Edge CB, Houlahan JE. Setting an optimal α that minimizes errors in null hypothesis significance tests. PLoS One. 2012;7:e32734. doi: 10.1371/journal.pone.0032734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Salleo S. Limitation of stomatal conductance by hydraulic traits: sensing or preventing xylem cavitation? Trees. 2000;15:14–24. [Google Scholar]
- Nardini A, Raimondo F, Lo Gullo MA, Salleo S. Leafminers help us understand leaf hydraulic design. Plant, Cell and Environment. 2010;33:1091–1100. doi: 10.1111/j.1365-3040.2010.02131.x. [DOI] [PubMed] [Google Scholar]
- Osborne CP, Sack L. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:583–600. doi: 10.1098/rstb.2011.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Allen DJ, Davey PA, et al. Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under Free-Air Carbon dioxide Enrichment. Plant, Cell and Environment. 2004;27:449–458. [Google Scholar]
- Ruiz-Vera UM, Siebers M, Gray SB, et al. Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwest United States. Plant Physiology. 2013;162:410–423. doi: 10.1104/pp.112.211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology. 2006;87:483–491. doi: 10.1890/05-0710. [DOI] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C. Leaf venation: structure, function, development, evolution, ecology, and applications in past, present, and future. New Phytologist. 2013;198:983–1000. doi: 10.1111/nph.12253. [DOI] [PubMed] [Google Scholar]
- Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM. The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany. 2002;53:2177–2184. doi: 10.1093/jxb/erf069. [DOI] [PubMed] [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell and Environment. 2003;26:1343–1356. [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology. 2004;134:1824–1833. doi: 10.1104/pp.103.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, Nardini A, Pitt F, Lo Gullo MA. Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.) Plant, Cell and Environment. 2000;23:71–79. [Google Scholar]
- Scoffoni C, Pou A, Aasamaa K, Sack L. The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell and Environment. 2008;31:1803–1812. doi: 10.1111/j.1365-3040.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- Sellin A, Kupper P. Temperature, light and leaf hydraulic conductance of little-leaf linden (Tilia cordata) in a mixed forest canopy. Tree Physiology. 2007;27:679–688. doi: 10.1093/treephys/27.5.679. [DOI] [PubMed] [Google Scholar]
- Sober A. Hydraulic conductance, stomatal conductance, and maximal photosynthetic rate in bean leaves. Photosynthetica. 1997;34:599–603. [Google Scholar]
- Tsuda M, Tyree MT. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. Journal of Experimental Botany. 2000;51:823–828. [PubMed] [Google Scholar]
- Uhl D, Mosbrugger V. Leaf venation density as a climate and environmental proxy: a critical review and new data. Palaeogeography, Palaeoclimatology, Palaeoecology. 1999;149:15–26. [Google Scholar]
- Yang S, Tyree MT. Hydraulic resistance in Acer saccharum shoots and its influence on leaf water potential and transpiration. Tree Physiology. 1993;12:231–242. doi: 10.1093/treephys/12.3.231. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sinclair TR, Zhu M, Messina CD, Cooper M, Hammer GL. Temperature effect on transpiration response of maize plants to vapour pressure deficit. Environmental and Experimental Botany. 2012;78:157–162. [Google Scholar]
- Zhang Y-J, Meinzer FC, Hao G-Y, et al. Size-dependent mortality in a Neotropical savanna tree: the role of height-related adjustments in hydraulic architecture and carbon allocation. Plant, Cell and Environment. 2009;32:1456–1466. doi: 10.1111/j.1365-3040.2009.02012.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


