Abstract
The docking protein FRS2α has been implicated as a mediator of signaling via fibroblast growth factor receptors (FGFRs). We have demonstrated that targeted disruption of FRS2α gene causes severe impairment in mouse development resulting in embryonal lethality at E7.0–E7.5. Experiments with FRS2α-deficient fibroblasts demonstrate that FRS2α plays a critical role in FGF-induced mitogen-activated protein (MAP) kinase stimulation, phosphatidylinositol-3 (PI-3) kinase activation, chemotactic response, and cell proliferation. Following FGF stimulation, tyrosine phosphorylated FRS2α functions as a site for coordinated assembly of a multiprotein complex that includes Gab1 and the effector proteins that are recruited by this docking protein. Furthermore, we demonstrate that different tyrosine phosphorylation sites on FRS2α are responsible for mediating different FGF-induced biological responses. These experiments establish the central role of FRS2α in signaling via FGFRs and demonstrate that FRS2α mediates multiple FGFR-dependent signaling pathways critical for embryonic development.
Receptor tyrosine kinases (RTK) play an important role in the control of a variety of cellular processes including regulation of the cell cycle, cell metabolism, and migration, as well as cell proliferation and differentiation (1). Upon ligand-induced activation, RTKs undergo rapid autophosphorylation on numerous tyrosine residues. Autophosphorylation sites located within the catalytic protein tyrosine kinase (PTK) core are involved in regulation of enzymatic activity, whereas autophosphorylation sites in other regions of the cytoplasmic domain serve as binding sites for SH2 (Src homology 2) or PTB (phosphotyrosine-binding) domains of signaling proteins (2). It has been shown that upon activation, tyrosine autophosphorylated RTKs function as platforms for the assembly of a variety of signaling proteins. However, recruitment of signaling proteins in response to RTK stimulation can be mediated by an alternative, indirect mechanism involving a family of membrane-linked docking proteins. For instance, stimulation of insulin receptor leads to tyrosine phosphorylation of the docking protein IRS1, enabling recruitment and activation of phosphatidylinositol-3 (PI-3) kinase (3). Fibroblast growth factor (FGF) or nerve growth factor (NGF) stimulation leads to tyrosine phosphorylation of the docking proteins FRS2α and -β, resulting in recruitment of multiple Grb2/Sos complexes and leading to activation of the Ras/mitogen-activated protein (MAP) kinase signaling pathway (4–6).
FRS2 proteins contain myristyl anchors and PTB domains in their N termini and large regions with multiple tyrosine phosphorylation sites at their C termini (4). FRS2α contains four binding sites for the adaptor protein Grb2 and two binding sites for the protein tyrosine phosphatase Shp2. FGF stimulation leads to phosphorylation of Shp2 on a tyrosine residue that forms a complex with an additional molecule of Grb2. Grb2/Sos complexes are thus recruited directly and indirectly via Shp2 upon tyrosine phosphorylation of FRS2α in response to growth factor stimulation (5).
FGFs represent a large family of at least 22 different growth factors. FGFs play a critical role in the control of multiple cellular processes, including cell migration and survival, cell proliferation, and differentiation, among others (7). FGFs mediate their biological responses by binding to and activating a family of four receptor tyrosine kinases designated FGFR1–4 (8, 9).
To understand the biological role of FRS2α in signaling via FGF receptors (FGFRs), we introduced a targeted mutation into the murine Frs2α gene. In this report, we describe the characterization of mouse embryo fibroblasts deficient in FRS2α to demonstrate that FRS2α plays a critical role in signaling via FGFRs. Deficiency in FRS2α results in impairment in cell migration, MAP kinase stimulation, PI-3 kinase activity, and cell proliferation most evident at low concentrations of FGF. FGF-induced tyrosine phosphorylation of FRS2α results in a coordinated assembly of the docking protein Gab1 and effector proteins crucial for the activation of the Ras/MAP kinase and PI-3 kinase signaling cascades.
Methods
FRS2α Gene Targeting.
A library of 129sv/EV mouse genomic DNA was screened with a probe derived from the 5′ portion of Frs2α. A 0.5-kb fragment containing the first coding exon was replaced by IRES β-galactosidase neomycin resistance cassette (β-geo) (10). NotI linearized targeting vector was electroporated into W4 embryonic stem (ES) cell line, and resistant clones were screened by Southern blotting. Germline transmitting chimeras of two independent Frs2α+/− cell lines were obtained by aggregating ES cells with morula-staged Swiss–Webster embryos. The genotypes of the embryos were determined by Southern blotting using the 3′ external probe. The 5′ probe was generated by PCR using the following set of oligonucleotides: 5′-GACAAAACACATGAACATACATTAAC-3′ and 5′-TCTGATGCTGCATC AGG AG-3′. The 3′ probe was generated by PCR using the following set of oligonucleotides: 5′-TCTGACCATAGAGAGGTGCAGCTG-3′ and 5′-AACTGTTACTCATAAATGATCATG-3′. In both cases Frs2α genomic clone was used as template. The embryos were also genotype by PCR with the nucleotides 5′-GATCTAGTGCTCACATGGTCTTCTGAAGAAGCC-3′ and 5′-CAACCACCCATAATGAGATCTGATGCCCTC-3′ to detect a 440-bp fragment from the wild-type allele. The nucleotides 5′-CAATGTATCTTATCATGTCTGGATCCGGGG-3′ and 5′-CAGTAACAGAAAGCACACAAGTGCTAAGC-3′ were used to detect a 910-bp fragment from the mutant allele.
Generation of FRS2α-Deficient ES Cells and Mouse Embryo Fibroblasts.
Frs2α+/− ES cells were subjected to selection in medium containing G418 (0.3–1 mg/ml) to yield a homozygous mutant line. Two independent Frs2α-deficient cells were identified and used in the experiments. To generate Frs2α-deficient mouse embryo fibroblasts, Frs2α-deficient ES cells were aggregated with morula-stage Swiss–Webster embryos. Females were dissected at day E12.5, and mouse embryo fibroblasts were infected with a virus encoding for polyoma large T antigen. Cell populations were subjected to selection in medium containing 0.4 mg/ml G418 for 2 weeks.
PCR Reactions.
Reverse transcriptase (RT)–PCR was performed according to the manufacturers instructions (CLONTECH). The RT-PCR reaction was performed on total RNA extracted from wild-type or Frs2α-deficient ES cells. The following primer was used: 5′-GGAGCTAGTGTAGACATGAA-3′. The product of the RT-PCR reaction was then used as a template for PCR reaction, using the primers set: 5′-GGGGGATCCAACAATATTAATGGCCAG-3′ and 5′-GGGAAGCTTTCACATGGGCAGGTCAGT-3′. The PCR products from wild-type or Frs2α-deficient ES cells were subcloned into pRK5 vector and sequenced.
Immunoprecipitation and Immunoblot Analysis.
A mammalian vector, pBABE, containing puromycin resistance gene was used to express wild-type FRS2α and FRS2α mutant cDNAs in mouse embryo fibroblasts. Cells were lysed in 25 mM Hepes, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 20 mM β-glycerophosphate, 10 mM pyrophosphate, 1 mM NaVO3, 1 mM PMSF, 5 μg/ml aprotinin, and 5 μg/ml leupeptin (pH 7.5) (Buffer I). Cells were serum-starved overnight and stimulated with FGF1 (100 ng/ml) and heparin (5 μg/ml) for 5 min at 37°C. Lysates from solubilized cells were subjected to immunoprecipitation followed by immunoblotting with different antibodies according to published procedures (11, 12). FRS2, FGFR, Shc, Shp2, and Grb2 antibodies were described (4). PLCγ and Gab1 antibodies have been described (12, 13). Phospho-specific MAP kinase antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Assays for MAP Kinase Activation, Cell Migration, Cell Proliferation, and PI-3 Kinase Activity.
Mouse embryo fibroblasts were lysed in Buffer I and immunoblotted with phospho-MAP kinase antibodies. The migration of wild-type or mutant fibroblasts in Boyden chambers (Costar; 12-μm pore) was determined as described (14). Cell proliferation assays of mouse embryo fibroblasts were performed according to a published procedure (15). The PI-3 kinase assay was performed as described (13).
Results
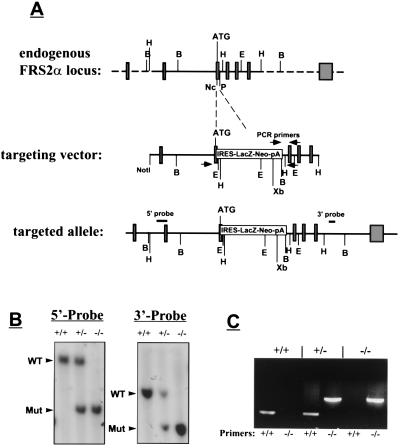
A cDNA probe derived from the 5′ coding region of murine Frs2α was used for screening of a genomic library prepared from DNA isolated from 129sv/EV mice. The sequence within the first coding exon of the Frs2α gene, encoding for 22 amino acids following the ATG codon, was deleted and replaced with the β-galactosidase neomycin-resistant cassette by homologous recombination in W4 embryonic stem cells (Fig. 1A). Probes specific for Frs2α were used to reveal the existence of the targeted gene in DNA of drug-resistant ES cells. Five independent ES cell lines positive for the targeted Frs2α gene were isolated and used for generation of chimeric male mice from embryo aggregates. Germline transmission was obtained with two of the ES cell clones. The chimeric mice were crossed with 129sv/EV and Swiss–Webster mice to generate heterozygous mice, and homozygous mice were obtained by heterozygous intercrosses. Animals were genotyped by Southern blot analysis (Fig. 1B) or by PCR probes specific for Frs2α (Fig. 1C). No abnormality was detected in heterozygous mice for more than 21 months. Screening of more than 400 offspring from heterozygous intercrosses failed to identify viable homozygous mice, indicating a recessive lethal phenotype.
Figure 1.
Targeted disruption of the Frs2α locus. (A) Strategy for generation of Frs2α mutation. The targeting vector contains a β-galactosidase neomycin resistance cassette in place of a 0.5-kb region of Frs2α gene that includes the first coding exon. Open boxes mark noncoding exons, and shaded boxes mark coding regions. Arrowheads mark the primers for genotyping by PCR, and the locations of the internal 5′ and external 3′ probes that were used for Southern blotting are also indicated. Restriction sites for several restriction enzymes are indicated. B, BamHI; E, EcoRI; H, HindIII; Nc, NcoI; P, PstI; Xb, XbaI. (B) Southern blot analysis of genomic DNA isolated from ES cell lines. For hybridization with the 5′ internal probe, DNA was digested with XbaI; for hybridization with the 3′ external probe, DNA was digested with BamHI. Arrows mark the positions of wild-type or mutant fragments. The sizes of these fragments are: 5′ probe: wild type 12 kb, mutant 7 kb; 3′ probe: wild type 8 kb, mutant 5 kb. (C) PCR analysis of Frs2α mutation. DNA was isolated from ES cell lines and PCR was performed as described in Methods. Two sets of primers were used, one for the wild-type allele (+/+) and the other for the Frs2α mutant allele (−/−).
We have demonstrated that FRS2α−/− embryos die at E7.0–E7.5 as a result of severe impairment in embryonic development (Table 1). Full details about the phenotype of FRS2α-deficient embryos will be published elsewhere. To clarify the role played by FRS2α in signaling via FGFRs, we have generated Frs2α−/− ES cells and used them to produce chimeric embryos from which mutant fibroblasts were isolated (see Methods).
Table 1.
Genotype analysis of the progeny from Frs2α +/− intercrosses
| Embryonic day | Total | +/+ | +/− | −/− | −/− Resorptions | Empty Deciduas |
|---|---|---|---|---|---|---|
| E7.5 | 79 | 19 | 44 | 16 | — | — |
| E8.5 | 90 | 22 | 48 | 1 | 19 | — |
| E9.5 | 81 | 21 | 43 | — | — | 17 |
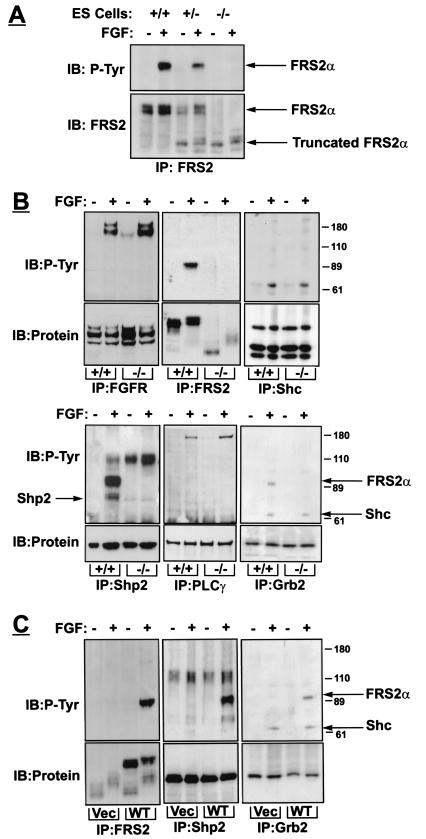
Wild-type or Frs2α−/− ES cells were stimulated with FGF1, and lysates from stimulated or unstimulated cells were subjected to immunoprecipitation with anti-FRS2α antibodies followed by immunoblotting with anti-pTyr antibodies. FGF1-induced tyrosine phosphorylation of FRS2α is detected in wild-type ES cells, but not in mutant ES cells (Fig. 2A). Whereas wild-type ES cells express a typical FRS2α protein with apparent molecular weight of 90 kDa, a weak band of a protein with apparent molecular weight of 60 kDa was detected with anti-FRS2α antibodies in Frs2α−/− ES cells (Fig. 2A). A similar low molecular weight species was detected with anti-FRS2α antibodies in lysates from Frs2α−/− fibroblasts and in lysates from Frs2α+/− ES cells in addition to the 90 kDa form. Using Northern blot analysis, we were able to demonstrate that this band is not FRS2β, because these cells do not express the FRS2β homologue (data not shown).
Figure 2.
Biochemical characterization of FRS2α-deficient fibroblasts. (A) Expression and tyrosine phosphorylation of FRS2α in wild-type, heterozygous, and homozygous FRS2α mutant cells. ES cells grown on gelatin in the presence of 1 μM retinoic acid for 24 h, starved overnight, treated with FGF1 (100 ng/ml) and heparin (5 μg/ml) for 5 min, lysed, and subjected to immunoprecipitation with anti-FRS2 antibodies followed by immunoblotting with anti-FRS2 or anti-pTyr antibodies. (B) Mouse embryo fibroblasts were serum-starved overnight, treated with FGF1 (100 ng/ml) and heparin (5 μg/ml) for 5 min, lysed, and immunoprecipitated with antibodies as indicated. (C) FRS2α mutant fibroblasts were infected with retroviral expression vector for wild-type FRS2α or with vector alone. Fibroblasts were treated with FGF1, and cell lysates were immunoprecipitated and immunoblotted with different antibodies as indicated.
The cDNA of the protein fragment was cloned by RT-PCR and shown to encode a truncated form of FRS2α lacking 37 N-terminal amino acids. The truncated form lacks the myristyl anchor and part of the PTB domain of FRS2α, and therefore is not tethered to the cell membrane, does not form a complex with FGFRs, and is not tyrosine phosphorylated in response to FGF stimulation. We have considered the possibility that the truncated FRS2α protein may mediate certain FGF responses or may function as a dominant negative inhibitor of signaling via FGFRs. Because membrane attachment and tyrosine phosphorylation of FRS2α are critical for FGF-induced recruitment of effector proteins, it is unlikely that this deletion mutant will mediate any biological response crucial for signaling via FGFRs. We have examined the possibility of whether a nonmyristylated FRS2α mutant with an impaired PTB domain could exert a dominant-negative inhibition of signaling via FGFRs. Elimination of the myristyl anchor alone with or without the PTB domain prevents membrane attachment and impairs tyrosine phosphorylation of mutant FRS2α protein. However, neither these nor any other FRS2α mutants exerted dominant-negative inhibition of signaling via FGFRs (refs. 4 and 5 and data not shown). It is therefore likely that this mutation is effectively a null mutation.
To clarify the molecular basis behind the critical role of FRS2α in embryonal development and signaling via FGF receptors, we have analyzed cellular signaling pathways that are activated in response to FGF stimulation in mouse embryo fibroblasts derived from FRS2α-deficient embryos (see Methods). We have first compared FGF-induced tyrosine phosphorylation of proteins that have been previously shown to play a role in signaling via FGFRs. A similar FGFR autophosphorylation as well as tyrosine phosphorylation of phospholipase-Cγ (PLCγ) and Shc were detected in wild-type or FRS2α-deficient fibroblasts (Fig. 2B), demonstrating that FRS2α is not required for activation of FGFR or for tyrosine phosphorylation of these two effector proteins.
We have used the FRS2α-deficient cells for exploring potential alternative mechanisms for FGF-induced recruitment of Grb2 that may lead to alternative mechanisms for activation of the Ras/MAP kinase cascade. This experiment demonstrated that Grb2 forms a complex with tyrosine-phosphorylated Shc in FGF-stimulated cells in an FRS2α-independent manner (Fig. 2). Because the level of expression and tyrosine phosphorylation of Shc remain unchanged in FRS2α-deficient cells, it is clear that signaling via Shc does not compensate for the loss of FRS2α in these cells. Next we determined the phosphorylation level of Shp2 in both wild-type and FRS2α-deficient fibroblasts. Tyrosine-phosphorylated Shp2 molecules have not been detected in FRS2α mutant cell lysates, indicating that FRS2α is required for tyrosine phosphorylation of Shp2 leading to complex formation between Shp2 and Grb2. FGF-induced tyrosine phosphorylation of Shp2 as well as FRS2α/Grb2 complex formation were completely restored by ectopic expression of FRS2α in mutant cells. These findings demonstrate that expression of functional FRS2α is indeed crucial for the recruitment and tyrosine phosphorylation of these two effector proteins (Fig. 2C).
FRS2α Is Required for Recruitment of PI-3 Kinase via the Docking Protein Gab1.
It has been reported that Grb2 forms a complex with the docking protein Gab1 by means of its SH3 domains (16). Indeed, we have recently found that FGF-induced tyrosine phosphorylation of FRS2α leads to formation of a ternary complex composed of tyrosine phosphorylated FRS2α, Grb2, and Gab1 (17). Moreover, we have demonstrated that the SH2 domain of Grb2 is bound to tyrosine-phosphorylated FRS2α and that the C-terminal SH3 domain of Grb2 forms a complex with the proline-rich region of Gab1 to provide a link between these two docking proteins. On the basis of these observation we have considered the possibility that recruitment of Gab1 in response to FGF stimulation depends on FRS2α. Because PI-3 kinase is one of the effectors of Gab1 (13, 18), this could provide a mechanism for FGF-induced activation of PI-3 kinase. Moreover, it raises an intriguing possibility that FRS2α functions not only as a platform for recruitment of signaling proteins, but also as a signal for coordinated assembly of docking proteins that recruit an additional complement of effector proteins (Fig. 3).
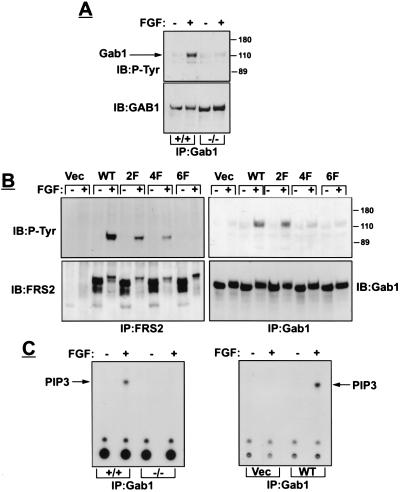
Figure 3.
FRS2α is essential for Gab1 recruitment and activation of PI-3 kinase. (A) Fibroblasts were stimulated with FGF1 and heparin. Cell lysates were immunoprecipitated with anti-Gab1 antibodies followed by immunoblotting with the indicated antibodies. (B) FRS2α-deficient fibroblasts were infected with expression vector for wild-type or FRS2α mutants or with vector alone. Following FGF1 stimulation, cell lysates were subjected to immunoprecipitation with anti-FRS2 or anti-Gab1 antibodies, followed by immunoblotting with antibodies as indicated. (C) PI3-kinase assay was performed on Gab1 immunoprecipitates derived from wild-type or mutant fibroblasts (Left) or from mutant fibroblasts transfected with expression vector encoding for FRS2α or vector alone (Right).
We tested this hypothesis by comparing FGF-induced tyrosine phosphorylation of Gab1 in wild-type and FRS2α-deficient fibroblasts (Fig. 3A). Although FGF-induced tyrosine phosphorylation of Gab1 was detected in lysates from wild-type fibroblasts, tyrosine phosphorylation of Gab1 could not be detected in FRS2α-deficient fibroblasts. However, FGF-induced tyrosine phosphorylation of Gab1 was fully restored in FRS2α-deficient cells upon ectopic expression of FRS2α cDNA in these cells (Fig. 3B). These experiments demonstrate that FGF-induced tyrosine phosphorylation of Gab1 is mediated via FRS2α.
To further define the mechanisms of recruitment of Gab1, we have expressed in the FRS2α-deficient fibroblasts different FRS2α mutants and examined their capacity to rescue FGF-induced tyrosine phosphorylation of Gab1 (Fig. 3B). We have previously demonstrated that complex formation between the SH2 domain of Grb2 and FRS2α is mediated via Y196-, Y306-, Y349-, and Y392-designated Grb2 binding sites (4, 5). In addition, FRS2α recruits Grb2 indirectly by means of two binding sites for the SH2 domains of the protein tyrosine phosphatase Shp2 at Y436- and Y471-designated Shp2 binding sites (5). Site-directed mutagenesis was used to generate the following FRS2α mutants. An FRS2α mutant protein in which all six tyrosine residues were replaced by phenylalanine residues (6F), a mutant FRS2α in which Grb2 binding sites at Y196, Y306, Y349, and Y392 were substituted by phenylalanine residues (4F), and a mutant FRS2α in which Shp2 binding sites (Y436 and Y471) were replaced by phenylalanine residues (2F). We next examined the capacity of wild type, 6F, 4F, or the 2F mutant to rescue FGF-induced tyrosine phosphorylation of Gab1. The experiment presented in Fig. 3B shows that FGF-induced tyrosine phosphorylation of Gab1 was completely restored in FRS2α-deficient fibroblasts expressing wild type or the 2F mutant of FRS2α. However, tyrosine phosphorylation of Gab1 could not be detected in FRS2α-deficient fibroblasts expressing the 6F, the 4F FRS2α mutants, or transfected with vector alone. This experiment shows that tyrosine phosphorylation of Gab1 in response to FGF stimulation takes place only in cells expressing FRS2α and that tyrosine phosphorylation of Gab1 depends on Grb2 binding sites and not on Shp2 binding sites of FRS2α. FGF-induced tyrosine phosphorylation of FRS2α results in recruitment of Grb2/Gab1 complex; a process mediated by binding of the SH2 domain of Grb2 to tyrosine-phosphorylated FRS2α in the vicinity of FGFR, enabling tyrosine phosphorylation of Gab1. It is not clear yet whether a particular combination of all four Grb2 binding sites on FRS2α are responsible for recruitment of Grb2/Gab1 complexes.
If FRS2α is required for tyrosine phosphorylation of Gab1, it is expected that FRS2α might play a role in activation of PI-3 kinase, because PI-3 kinase is one of the effectors that is recruited by Gab1 (13, 18). To test this hypothesis, wild-type or FRS2α-deficient fibroblasts were stimulated with FGF, and lysates from stimulated or unstimulated cells were subjected to immunoprecipitation with anti-Gab1 antibodies, followed by analysis of PI-3 kinase activity in anti-Gab1 immunoprecipitates (see Methods). Fig. 3C shows FGF-induced stimulation of PI-3 kinase activity in Gab1 immunoprecipitates from wild-type fibroblasts. By contrast, PI-3 kinase activity was not detected in Gab1 immunoprecipitates from FGF-stimulated FRS2α-deficient fibroblasts. However, stimulation of PI-3 kinase was completely restored by ectopic expression of FRS2α cDNA in FRS2α-deficient fibroblasts (Fig. 3C). These experiments demonstrate that FRS2α functions as a site of assembly of an additional docking protein that brings to the complex its own effectors in addition to the effectors that bind directly to FRS2α.
FRS2α-Dependent and -Independent MAP Kinase (ERK) Stimulation.
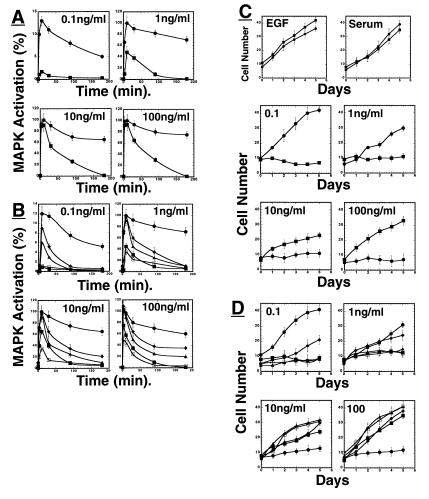
FRS2α has been implicated as a critical link between FGF stimulation and the Ras/MAP kinase signaling pathway (4, 5). We have tested this hypothesis by exploring FGF-induced MAP kinase stimulation in wild-type or FRS2α-deficient fibroblasts (Fig. 4A). These cells were stimulated with increasing concentrations of FGF, and lysates of unstimulated or stimulated cells were analyzed by immunoblotting with anti-phospho-specific MAP kinase antibodies; that recognize specifically the activated form of MAP kinase. Stimulation of wild-type fibroblasts with a low concentration of FGF (0.1 ng/ml) resulted in rapid MAP kinase response with maximal activation at 10 min post stimulation followed by a gradual decline lasting for several hours (Fig. 4A). By contrast, very weak stimulation of MAP kinase was detected in FRS2α-deficient cells in response to 0.1 ng/ml of FGF. Stimulation of wild-type fibroblast with high (1 ng/ml) or oversaturating concentrations of FGF (10 or 100 ng/ml) resulted in rapid, robust, and sustained MAP kinase response (Fig. 4A). A reduced MAP kinase response was detected in FRS2α-deficient cells in response to stimulation with 1 ng/ml of FGF. At this dose maximal stimulation occurred with similar kinetics and was ≈50% of the maximal response detected in wild-type cells in response to stimulation with 1 ng/ml of FGF. However, unlike the sustained MAP kinase response seen in wild-type fibroblasts, the response detected in FRS2α-deficient cells was transient; MAP kinase activation declined gradually to the baseline after ≈90 min. A similar pattern was seen in FRS2α-deficient cells stimulated with 10 or 100 ng/ml of FGF (Fig. 4A). Interestingly, at this oversaturating dose of FGF, the onset of the response in FRS2α-deficient cells was virtually identical to the initial phase of MAP kinase stimulation seen in wild-type cells. However, unlike the sustained MAP kinase response in FGF-stimulated wild-type cells, MAP kinase stimulation in mutant cells rapidly declined. Fig. 4B shows that the pattern of MAP kinase response was completely restored by ectopic expression of FRS2α in the FRS2α-deficient cells resulting in normal FGF-induced MAP kinase stimulation similar to the response seen in wild-type fibroblasts. However, a partial restoration of MAP kinase stimulation was achieved by ectopic expression of the 4F mutant, a weak restoration by the 2F mutant, and no rescue by expression of the 6F mutant in mutant fibroblasts (Fig. 4B).
Figure 4.
Impaired FGF-induced MAP kinase (ERK) stimulation and cell proliferation of FRS2α-deficient fibroblasts. (A) Comparison of MAP kinase activation in wild-type or FRS2α-deficient fibroblasts in response to FGF1 stimulation. Fibroblasts were starved, treated with different concentrations of FGF1, lysed, and immunoblotted with phospho-specific MAP kinase antibodies or with anti-Erk1 antibodies, and the intensity of the protein bands was quantitated by densitometry. ●, Wild-type fibroblasts; ■, FRS2α-deficient fibroblasts. (B) FRS2α mutant fibroblasts were infected with expression vector encoding for wild-type FRS2α or mutant FRS2α, or with vector alone. MAP kinase stimulation was revealed by immunoblotting with anti-phospho-MAP-kinase antibodies followed by quantitation by densitometry. ●, Wild-type FRS2α; ▴, ♦, or □, the 2F, 4F, or 6F FRS2α mutants; ■, vector alone. Similar results were obtained in three different experiments. (C) Growth curves of wild-type and FRS2α-deficient fibroblasts in response to growth factor stimulation. Wild-type or FRS2α mutant fibroblasts were grown in medium containing 10 ng/ml EGF, 10% serum, or different concentrations of FGF. The cells were trypsinized at the indicated time points and counted. ● Wild-type fibroblasts; ■ FRS2α-deficient fibroblasts. (D) FRS2α-deficient fibroblasts were infected with expression vector for wild-type FRS2α, FRS2α mutants, or vector alone. The cell lines were stimulated with different concentrations of FGF and growth curves were determined. ●, Wild-type FRS2α; ▴, ♦, or □, the 2F, 4F, or 6F FRS2α mutants; ■, vector alone.
These experiments demonstrate that FRS2α is essential for FGF-induced MAP kinase stimulation in response to low concentrations of FGF that exert maximal mitogenic response (see below). However, at high concentrations of FGF there is evidence for FRS2α-dependent and FRS2α-independent mechanisms for MAP kinase stimulation. In the absence of FRS2α, even oversaturating concentrations of FGF stimulate a transient MAP kinase response different from the sustained response seen in FGF-stimulated wild-type fibroblasts.
FRS2α Is Required for FGF-Induced Cell Proliferation.
We next examined the role of FRS2α in FGF-induced cell proliferation. Wild-type or FRS2α-deficient cells were stimulated with 0.1 ng/ml of FGF and their rate of proliferation was quantitated for a period of 5 days. The experiment presented in Fig. 4C shows that both wild-type or FRS2α-deficient fibroblasts exhibit similar growth curves in response to stimulation with serum or EGF. By contrast, the mitogenic response of FGF (0.1 ng/ml) was totally impaired in the mutant fibroblasts (Fig. 4C). The mitogenic response to FGF was restored in mutant fibroblasts by ectopic expression of FRS2α cDNA in these cells; the growth curves of the transfected mutant cells were similar to the growth curves of wild-type fibroblasts stimulated with FGF (Fig. 4D). Whereas a partial restoration of the mitogenic response was obtained by ectopic expression of the 4F mutant, no rescue of the mitogenic response was achieved following ectopic expression of the 6F or 2F mutants in mutant fibroblasts (Fig. 4D). This experiment shows that Shp2 binding sites on FRS2α play an important role in mediating the mitogenic response. However, a complete restoration of the mitogenic response was achieved only by expression of wild-type FRS2α cDNA in mutant cells.
The experiment presented in Fig. 4C shows the effect of 1, 10, and 100 ng/ml of FGF on the proliferation of wild-type or FRS2α-deficient fibroblasts. Consistent with previous studies, maximal stimulation of proliferation of wild-type fibroblasts was induced by 0.1 ng/ml of FGF and higher concentrations of FGF induced a gradual decrease in cell proliferation (Fig. 4C). By contrast, mutant fibroblasts responded only to high nonphysiological concentrations of FGF. Fig. 4 C and D show that more than three orders of magnitude of FGF are required for stimulation of a similar mitogenic response in mutant fibroblasts. These results suggest that in response to high FGF concentrations, activation of MAP kinase by the alternative mechanism can also lead to cell proliferation.
FRS2α Is Required for FGF-Induced Cell Migration.
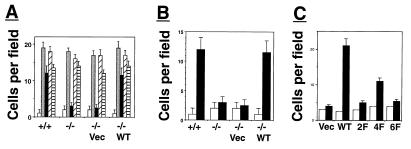
Because cell migration plays a critical role during embryonal development (14, 19–21), a cell migration assay was used to compare wild-type and FRS2α-deficient fibroblasts for their response to growth factors as chemoattractants. Wild-type or FRS2α-deficient fibroblasts were tested by using a Boyden-chamber migration assay for chemotactic response toward FGF, EGF, PDGF, or serum (Fig. 5A). Both wild-type and mutant fibroblasts migrate normally toward PDGF, EGF, or serum, demonstrating that the intracellular signaling pathways that control cell migration and the chemotactic response to EGF, PDGF, and serum are intact in FRS2α-deficient fibroblasts. However, when these cells were stimulated with FGF, only wild-type fibroblasts were able to migrate through the chamber's membrane (Fig. 5 A and B). FRS2α mutant fibroblasts failed to migrate in response to FGF stimulation. The chemotactic response of FRS2α-deficient fibroblasts toward FGF was completely restored by ectoptic expression of FRS2α cDNA in FRS2α-deficient cells (Fig. 5 A and B). However, the chemotactic response was partially restored by ectopic expression of the 4F mutant and a very weak restoration was achieved by expression of the 2F or the 6F mutants (Fig. 5 B and C). These experiments demonstrate that FRS2α is required for linking FGFR signaling with the intracellular machinery responsible for cell migration, and that Shp2 binding sites and Grb2 binding sites play a primary and secondary role, respectively, in this process.
Figure 5.
FRS2α is essential for FGF-induced cell migration. (A) Migration assay using Boyden chamber was performed with wild-type fibroblasts, FRS2α-deficient fibroblasts, or FRS2α-deficient fibroblasts infected with expression vectors for FRS2α or vector alone. The fibroblasts were stimulated with 10% serum ░⃞, FGF1 (100 ng/ml) and heparin (5 μg/ml) ■, PDGF (50 ng/ml) ▨, or EGF (100 ng/ml) ▤ or medium alone □. (B) Fibroblasts were stimulated with FGF1 (100 ng/ml) and heparin (5 mg/ml) ■ or medium alone □. (C) Migration analysis of unstimulated □ or FGF-stimulated ■ FRS2α-deficient fibroblasts that were transfected with expression vectors for FRS2α (WT), for the 2F, 4F, or 6F mutants, or with vector alone.
Discussion
To reveal the biological role of FRS2α, we have introduced germ-line mutation in the gene and generated Frs2α-deficient mice and mouse embryo fibroblasts deficient in FRS2α. Deficiency in FRS2α results in impairment in recruitment of Grb2 and Shp2. However, proteins such as Shc or PLCγ are equally well tyrosine phosphorylated in wild-type or FRS2α-deficient fibroblasts, demonstrating that signaling pathways that are dependent on PLCγ and the adaptor protein Shc are likely to be intact in mutant cells. Experiments presented in this report reveal an unexpected mechanism for coordinated coupling between two docking proteins in response to growth factor stimulation. It is demonstrated that FRS2α is essential for FGF-induced recruitment and tyrosine phosphorylation of Gab1, a docking protein that functions as a platform for the assembly of an additional complement of effector proteins including PI-3 kinase. FRS2α thus functions as a focus of assembly of a multiprotein complex critical for signaling via FGF receptors.
We have demonstrated that FRS2α has a critical role in FGF-induced MAP kinase (ERK) stimulation, cell migration, and cell proliferation. These experiments demonstrate that FRS2α is required for MAP kinase stimulation in response to a low concentration of FGF, whereas at high nonphysiological concentrations, a strong but transient response was induced in mutant cells different from the sustained MAP kinase response in wild-type fibroblasts. FGF-induced stimulation of MAP kinase in fibroblasts is therefore mediated by FRS2α-dependent and FRS2α-independent mechanisms. The FRS2α-independent response becomes operational only upon strong activation of FGFR and is probably mediated at least in part by Shc; tyrosine phosphorylation of Shc and its complex formation with Grb2 are normal in FRS2α-deficient cells. Similarly, whereas FGF-induced cell proliferation is strongly impaired in FRS2α-deficient cells in response to low physiological concentrations of FGF, high concentrations of FGF stimulate mutant cell proliferation. However, at least a 1,000-fold higher concentration of FGF is required for achieving a similar level of mitogenic response in FRS2α-deficient cells as compared with FGF-induced proliferation of normal fibroblasts.
We have demonstrated that the chemotactic response to FGF is impaired in FRS2α-deficient fibroblasts. However, mutant fibroblasts migrate normally in response to chemoattractants such as PDGF or EGF, demonstrating that the cellular machinery responsible for cell migration is intact in mutant cells. Moreover, expression of FRS2α cDNA in mutant fibroblasts results in complete restoration of FGF-induced cell migration. The rescue experiments with FRS2α phosphorylation site mutants show that Shp2 binding sites on FRS2α are crucial for FGF-induced MAP kinase stimulation, cell migration, and cell proliferation. Grb2 binding sites on FRS2α probably play a secondary role in these responses because a complete restoration of MAP kinase stimulation, cell migration, and cell proliferation is achieved only by ectopic expression of wild-type FRS2α in mutant cells. The good correlation seen in the rescue of these responses by the FRS2α mutants may indicate that MAP kinases play a role in mediating the other two responses. Although it is not surprising that MAP kinases play a critical role in FGF-induced cell proliferation, these results raise the possibility that MAP kinases play a role in the chemotactic response (22, 23). The experiments described in this report establish the central role of FRS2α in mediating multiple FGFRs—dependent signaling pathways crucial for cell proliferation, survival, and early embryonic development.
Acknowledgments
Y.R.H. and N.G. were supported by a Long Term Fellowship from the International Human Frontier Science Program Organization (HFSPO).
Abbreviations
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- ES
embryonic stem
- RT
reverse transcriptase
- SH2
Src homology 2
- PI-3
phosphatidylinositol-3
- PTB
phosphotyrosine-binding
- MAP
mitogen-activated protein
References
- 1.Hunter T. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 2.Pawson T, Schlessinger J. Curr Biol. 1993;3:434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- 3.Baker J M, Myers M G, Shoelson S E, Chin D J, Sun X J, Miralpeix M, Hu P, Margolis B, Skolnik E, Schlessinger J, White M F. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Lax I, Schlessinger J. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 5.Hadari Y R, Kouhara H, Lax I, Schlessinger J. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong S H, Guy G R, Hadari Y R, Laks S, Gotoh N, Schlessinger J, Lax I. Mol Cell Biol. 2000;20:979–989. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naski M C, Ornitz D M. Front Biosci. 1998;3:781–794. doi: 10.2741/a321. [DOI] [PubMed] [Google Scholar]
- 8.Jaye M, Schlessinger J, Dionne C A. Biochim Biophys Acta. 1992;1135:185–199. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- 9.Johnson D F, Williams L T. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 10.Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batzer A G, Rotin D, Urena J M, Skolnik E Y, Schlessinger J. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi M, Dikic I, Sorokin A, Burgess W H, Jaye M, Schlessinger J. Mol Cell Biol. 1996;16:977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues G A, Falasca M, Zhang Z, Ong S H, Schlessinger J. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxton T M, Pawson T. Proc Natl Acad Sci USA. 1999;96:3790–3795. doi: 10.1073/pnas.96.7.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spivak-Kroizman T, Lemmon M A, Dikic I, Ladbury J E, Pinchasi D, Huang J, Jaye M, Crumley G, Schlessinger J, Lax I. Cell. 1994;79:1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 16.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. Nature (London) 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 17.Ong S H, Hadari Y R, Gotoh N, Guy G R, Schlessinger J, Lax I. Proc Natl Acad Sci USA. 2001;98:6074–6079. doi: 10.1073/pnas.111114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H H, Vijapurkar U, Hellyer N J, Bravo D, Koland J G. Biochem J. 1998;334:189–195. doi: 10.1042/bj3340189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam P P, Behringer R R. Mech Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 20.Ciruna B G, Schwartz L, Harpal K, Yamaguchi T P, Rossant J. Development (Cambridge, UK) 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Meyers E N, Lewandoski M, Martin G R. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen D H, Catling A D, Webb D J, Sankovic M, Walker L A, Somlyo A V, Weber M J, Gonias S L. J Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]