Abstract
Nuclear factor-κB (NF-κB) is a key transcriptional factor family that consists of five members in mammalian cells, including NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB and c-Rel. NF-κB is implicated in multiple physiological and pathological processes, including cell proliferation and differentiation, inflammatory and immune response, cell survival and apoptosis, cellular stress reactions and tumorigenesis. Recent studies by our group and others have highlighted the novel functions of the p50 protein. In this review, we will focus on the regulation and functions of NF-κB p50.
INTRODUCTION
NF-κB was first discovered as a transcription factor by demonstration of its DNA-binding activity for the enhancer of the immunoglobulin κ light-chain in activated B cells by Baltimore in 1986. The NF-κB family consists of p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), c-Rel (Rel), and RelB. Studies using mice deficient in NF-κB p65 demonstrate that NF-κB plays roles in many different compartments of the immune system during lymphoid cell and organ differentiation and immune activation, as well as multiple physiological and pathological processes including inflammatory and immune response, cellular stress reactions, carcinogenesis, cell survival and apoptosis [1, 2]. Growing evidence shows that NF-κB activation can enhance specific anti-apoptotic gene expression, which then induces cell survival in lymphocytes and many other cell types in various experimental systems.
Of the NF-κB family members, the function of NF-κB1 (p50), a member without transcriptional activity, is far from understood. The nf-kb1 gene that is located at human chromosome 4q24, encodes protein p105. p50 corresponds to the N terminus of p105, which is called the Rel-homology domain (RHD). There is evidence indicating that p50 is generated by the 26S proteasome-mediated removal of C terminal consensus sequence of p105 [3]. As a cleaved product of p105, p50 only has the DNA binding domain and must form a heterodimer with RelA, RelB or C-Rel to act as a transcription factor to regulate its target gene transcription. The p50 homodimer may also recruit the coactivator Bcl-3, one member of IκB family [4, 5]. Previous report shows that bcl-3, with novel binding property, can inhibit the DNA binding of both the homodimeric NF-κB p50 subunit and a closely related homolog p52. Bcl-3 phosphorylation partially inactivates its inhibitory property [6].
Since p50 lacks a transactivation domain, the homodimer can only translocate into the nucleus and bind with the NF-κB binding sites of target genes. It cannot act as transcription factor to regulate NF-κB downstream genes expression alone. For this reason, its biological functions are much less studied in comparison with other members of NF-κB family. This review will focus on the progresses made in regard to this particular issue.
p50 AND ITS ACTIVATION
p50 dimers are confined to cytoplasm through interaction with inhibitor proteins termed I Bs or its precursor p105 in resting cells [7]. Stimulated by LPS, mitogens or inflammatory cytokines, IκB proteins can become phosphorylated by IκB kinase (IKK) and degraded by the 26S proteasome [8]. Activated IKKβ can phosphorylate p105 on serine residues 927 and 932, and resulting in the degradation of p105 to generate p50 [9]. Other studies show that p50 homodimer can bind to DNA in unstimulated cells. p50 homodimers can associate with histone deacetylase-1 (HDAC1) or be phosphorylated by the protein kinase A catalytic subunit (PKAc) at serine residue 337; then it can bind to NF-κB-dependent genes and repress their expression [10, 11] (Fig. 2B). Activated p50 homodimers or heterodimers can translocate into the nucleus by their nuclear localization sequence (NLS) in C terminus and bind to the promoter region of its target genes [12]. Recent studies show that phosphorylation of serine residues is very important for the activation of the NF-κB family. p50 phosphorylation at residues serine 65, 337 and 342 is critical for its DNA binding function but not for its dimerization [13]. Further studies show that the p50 phosphorylation at serine 337 is mediated by PKA [10, 13].
Fig. (2). The activity of p50 homodimer and its functions.
The p50 can be generated from p105 with removal of C-terminal by 26S proteasome. The heterodimer of p105/p50 can be cleaved by 26S proteasome to active p50/p50 homodimer. This homodimer become an inactive complex by binding to p105, and this complex also can be cleaved to p50 homodimer by 26S proteasome. A. p50 homodimer can activate MAPKs or invoved in the up-regulation of Cyclin D1 expression and in turn promotes cell proliferation. B. p50 homodimer associated with HDAC1 can bind to target gene promoter and represses its target gene expression. C. Exposed to special chemical materials such as asenite, activated p50 homodimer can increase in GADD45α protein de-ubiquitination, and protect it from degradation, subsequently leads to JNKs activation and in turn results in cell apoptosis. D. p50 homodimer can also bind with Bcl3 and up-regulates the expression of its target gene expression, and protects cell from undergo to apoptosis.
p50 MEDIATES GADD45α PROTEIN ACCUMULATION AND CELL APOPTOSIS
Recent studies from our laboratory demonstrate that p50 can mediate the accumulation of growth arrest and DNA damage 45α (GADD45α) protein and in turn induces cell apoptosis in cellular the response to arsenite exposure, suggesting a novel function of the p50 protein [14]. We found that arsenite exposure can activate the MEKK4/JNK signal pathway through GADD45α protein accumulation in mouse embryonic fibroblast (MEFs). Further studies indicate that the GADD45α protein accumulation upon arsenite exposure is dependent on p50 protein, which mediates the inhibition of proteasome-dependent GADD45α degradation [14]. GADD45α is one member of the GADD45 family whose expression could be induced via either p53-dependent or - independent mechanisms. Exposure of organisms or cells to environmental stress such as UV and γ-irradiation can up-regulate GADD45α expression [15]. The oligomerization of GADD45α plays a central role in the process of MEKK4 activation [16]. GADD45α can bind to the N terminus of MEKK4 and induce N-C dissociation, which leads to MEKK4 dimerization and autophosphorylation at threonine 1493, and subsequent activation [17, 18]. Activated MEKK4 can regulate MAPKK through phosphorylation of its conserved threonine and/or serine residues, and subsequently activate MAPKs such as JNKs and p38 [17]. MEKK4 binding with other proteins can also specifically activate the JNK signaling pathway which is involved in environmental stress-induced cell apoptosis [19, 20]. The JNK signal pathway may have a feedback mechanism to regulate the expression of GADD45α. Yin et al. [21] reported that JNK is involved in the up-regulation of GADD45α expression induced by arsenite. Our subsequent study also demonstrates that coordination of JNK1 and JNK2 is critical for GADD45α induction at late phase in cell responses to arsenite exposure, and that this process is regulated by c-Jun dependent pathways [22] (Figs. 1 and 2C). Considering the crucial role of p50 in the regulation of cell apoptosis, we anticipate that p50 itself could serve as a target for cancer prevention and therapy.
Fig. (1). Molecular mechanisms involved in p50-mediated apoptosis by arsenite.
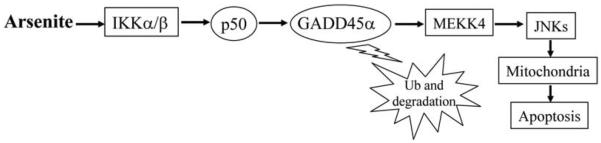
Arsenite exposure leads to IKK activated, which will interact with p50 to form IKK/p50 complex. IKK/p50 complex mediates GADD45α de-ubiquitination and protein accumulation. Accumulated GADD45α protein activates MEKK4/JNKs pathway and in turn results in cell apoptosis.
p50 AND ITS ANTI-APOPTOSIS
The major anti-apoptotic effect of p50 is mediated by its function as a component of transcription factor NF-κB. NF-κB is one of the major signaling pathways involved in cellular response to environmental stress. It plays a central role in the inhibition of cell apoptosis through modulation of several survival gene expressions [23, 24]. It has been reported that p50 can selectively protect the small intestine from irradiation damage. p50−/− mice exhibit elevated levels of apoptosis in intestinal epithelial cells compared with that in wild-type mice [25]. There are reports demonstrating that TNF-α also induce cell anti-apoptosis associated with NF-κB activation in human endothelial cells [26], prostate cancer cells [27], and pancreatic cancer cells [28]. p50 protein has been reported as an important component for NF-κB complexes induced by ionizing irradiation [29]. There are also reports indicating that some anti-apoptosis proteins such as Bcl-xL, Fas-associated death domain-like IL-1-converting enzyme inhibitor protein (FLIP), cellular inhibitor of apoptosis (CIAP), and X chromosome-linked inhibitors of apoptosis (XIAP), are up-regulated through NF-κB signalling pathway, and play role in the protection of cells from apoptosis in malignant lymphoid cells, prostate and colon cancer cells [30–32]. Bcl-3 is over-expressed in keratinocytes and human hepatocellular carcinomas and could act as a co-activator binding with the p50 homodimer to form a Bcl-3/p50 complex, and it plays an important role in anti-apoptosis and carcinogenesis [5, 33] (Fig. 2D).
INVOLVEMENT OF p50 IN CELL PROLIFERATION
Accumulating evidence shows that the NF-κB p50 signalling pathways play a role in cellular proliferation. Studies using p50−/− mice demonstrate that p50 deficiency represses the expression of IL-5 that is crucial for cell proliferation in human eosinophil cells [34–36]. The investigation also demonstrates that p50 can regulate survival of cells in G0 phase through regulation of c-myc expression in B lymphocytes. p50 knockout results in the reduction of B lymphocyte proliferation induced by mitogens [18, 37]. The studies using p50−/− mice also show that p50 is critical for IL-15-induced bone marrow (BM) cell proliferation [38]. Moreover, the increase in the regeneration of liver observed in p50−/− mice with partial hepatectomy has been associated with decreased transcription factor STAT3 DNA binding activities and increased NF-κB p65 translocation [39]. p50 has been shown to be implicated in the regulation of MAPK activation. For example, dendritic cells (DC) from p50−/− mice show the reduction of MAPKs phosphorylation [40]. The extracellular adenine nucleotide inhibitory effect on cell proliferation of human lung adenocarcinoma cell line LXF-289 has also been reported to be mediated by p50 [41]. On the other hand, many studies support p50 as a positive regulatory component of NF-κB on cell proliferation by mediation of cyclin D1 expression. Two NF-κB binding sites have been identified in human cyclin D1 promoter region [42, 43]. It has been found that obovatol, an active compound isolated from M. obovata, can inhibit the growth of prostate and colon cells by inhibition of cyclin D1 expression through targeting p50 and p65 [30]. In the autochthonous transgenic mouse model, increases in p50 and p65 DNA binding activity are associated with prostate cancer progression and up-regulation of cyclin D1 expression [44]. In contrast, the up-regulation of cyclin D1 transcription is not involved in the increase in cell proliferation and liver tumor promotion in p50−/− mice exposed to polychlorinated biphenyls, a group of synthetic chemicals [45].
p50, INFLAMMATION AND CARCINOGENESIS
The link between inflammation and cancers was observed almost 150 years ago and has been supported by accumulated epidemiological studies [46–49]. The sustained existence of a chronic inflammatory microenvironment is thought to be a major driving force for cancer development [50]. It has been found that many cancers arise from sites of infection, chronic irritation and inflammation [49]. The generation of p105−/−/p50−/− mice and p105−/−/p50+/+ mice has provided the opportunity to detect the biological functions of p50 in vivo. p105−/−/p50+/+ mice were generated through mutant mice lacking the COOH terminal half of the precursor p105, but expressing the p50 product. These mice developed spontaneous lymphocytic inflammation in the lung and liver, indicating the important role of p105 as a suppressor of inflammation [51]. Gene deletion of NF-κB p50 did not alter the hepatic inflammatory response to ischemia/reperfusion [52]. It has been found that the neutrophilic inflammation and fibrosis of liver in the p50−/− mice is more severe than that in wild-type mice. Other reports show that the p50 subunit can inhibit inflammation through regulating cytokine expression. p50 recruits its coactivators cAMP response element-binding (CREB) protein or Bcl-3, to form the complexes and promotes IL-10 expression, which is an anti-inflammatory cytokine [53, 54]. Macrophages taken from p50−/− mice decrease the secretion of IL-10 and increase the production of pro-inflammatory cytokines such as TNF and IL-12. The lack of p50 reduces the expression of histone deacetylase-1 (HDAC1), which can repress TNF-α expression [54, 55]. Thus, p50 dimer may act as an active repressor of inflammation. However, there are also many reports about p50 involvement in the process of inflammation and carcinogenesis in some experimental systems. For example, it is well-known that NF-κB is critical for the development of inflammation-associated cancers, including hepatoma, some breast cancer and colitis-associated cancers [56–58]. Wang, et al. reported that a peptide designed to bind with p50 can inhibit NF-κB activation and attenuate local acute inflammation [59]. Tumor associated macrophage (TAM) has been demonstrated to be an important player in carcinogenesis and it represents a major component of the lymphoreticular infiltrate of tumors [60]. It polarizes to M1 or M2 phenotypes induced by different stimuli. M2 TAM has a characterization of highly IL-10 expression and defective expression of IL-12 [60, 61]. M2 TAM can infiltrate tumor tissues driven by tumor-derived and T cell-derived cytokines [60, 61]. Previous studies indicate that the decreased expression of IL-12 in TAMs has been found to be associated with the inhibition of p50/p65 activation [62]. It has also been shown that p50 over-expression in normal macrophages reduced their IL-12 expression, while over-expression of p50 in TAMs resulted in inhibition of their M1 inflammation response and lost their anti-tumor activities [63].
Involvement of p50 in carcinogenesis has also been observed in clinical studies. It has been reported that p50 promoter polymorphisms could be valuable for assessment of risk of oral squamous cell carcinoma [46, 64]. The complexes of p50 homodimers and Bcl-3 are activated in nasopharyngeal carcinoma [6, 65, 66]. p50 overexpression is also detected in mouse skin cancer and non-small cell lung carcinoma [67, 68]. Since p50 over-expression is frequently observed in various tumor tissues, it may be feasible to be the target for chemoprevention and therapy.
CONCLUSION REMARKS
Although p50 is understood to exert its effects predominantly as a regulatory subunit of the NF-kB complex, our recent studies have demonstrated a novel function for p50 whereby it directly modulates GADD45α protein expression and function. Therefore, p50 can potentially regulate important mechanisms such as apoptosis and cell death independent of the NF-κB complex. Since arsenite is a well-known environmental human carcinogen and is widely used for cancer therapy, further studies on the mechanisms involved in the p50-mediated the increases in GADD45α de-ubiquitination will not only shed insight into the molecular basis of this novel function, but may also provide the key information for research of p50 as a potential target for cancer prevention and therapy.
ACKNOWLEDGEMENTS
We thank Ms. Nedda Tichi for her editing assistance. This work was supported in part by grants from NIH/NCI (CA094964 and CA112557), and NIH/NIEHS (ES012451, ES000260 and ES-010344).
ABBREVIATIONS
- Bcl
B cell lymphoma/leukemia
- CREB
cAMP response element-binding
- CIAP
cellular inhibitor of apoptosis
- FLIP
Fas-associated death domain-like IL-1-converting enzyme inhibitor protein
- GADD45α
growth arrest and DNA damage 45α
- HDAC1
histone deacetylase-1
- IKK
IκB kinase
- IL
interleukin
- JNK
c-Jun NH2-terminal kinase
- LPS
lipopolysacharides
- MAPKKK
mitogen- activated protein kinase kinase kinase
- MAPKK
mitogen-activated protein kinase kinase
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- MEKK
MAP kinase kinase kinase
- NF-κB
nuclear factor-kappa B
- TAM
tumor associated macrophages
- TNF
tumor necrosis factor
- UV
ultraviolet
- XIAP
X-chromosome-linked inhibitor of apoptosis
Footnotes
Copyright of Current Cancer Drug Targets is the property of Bentham Science Publishers Ltd. and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
REFERENCES
- [1].Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol. Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- [2].Chariot A. 20 years of NF-kappaB. Biochem. Pharmacol. 2006;7:1051–1053. doi: 10.1016/j.bcp.2006.08.023. [DOI] [PubMed] [Google Scholar]
- [3].Lin L, DeMartino GN, Greene WC. Cotranslational biogenesis of NF-kappaB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- [4].Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- [5].Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- [6].Nolan GP, Fujita T, Bhatia K, Huppi C, Liou HC, Scott ML, Baltimore D. The bcl-3 proto-oncogene encodes a nuclear I kappa B-like molecule that preferentially interacts with NF-kappa B p50 and p52 in a phosphorylation-dependent manner. Mol. Cell Biol. 1993;13:3557–3566. doi: 10.1128/mcb.13.6.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beg AA, Baldwin AS., Jr. The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- [8].Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lang V, Janzen J, Fischer GZ, Soneji Y, Beinke S, Salmeron A, Allen H, Hay RT, Ben-Neriah Y, Ley SC. betaTrCP-mediated proteolysis of NF-kappaB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol. Cell Biol. 2003;23:402–413. doi: 10.1128/MCB.23.1.402-413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guan H, Hou S, Ricciardi RP. DNA binding of repressor nuclear factor-kappaB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. J. Biol. Chem. 2005;280:9957–9962. doi: 10.1074/jbc.M412180200. [DOI] [PubMed] [Google Scholar]
- [11].Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol. Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- [12].Blank V, Kourilsky P, Israel A. Cytoplasmic retention, DNA binding and processing of the NF-kappa B p50 precursor are controlled by a small region in its C-terminus. EMBO J. 1991;10:4159–4167. doi: 10.1002/j.1460-2075.1991.tb04994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hou S, Guan H, Ricciardi RP. Phosphorylation of serine 337 of NF-kappaB p50 is critical for DNA binding. J. Biol. Chem. 2003;278:45994–45998. doi: 10.1074/jbc.M307971200. [DOI] [PubMed] [Google Scholar]
- [14].Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, Shen HM, Whiteman M, Huang C. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J. Cell Biol. 2006;175:607–617. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95:521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- [16].Kovalsky O, Lung FD, Roller PP, Fornace AJ., Jr. Oligomerization of human Gadd45a protein. J. Biol. Chem. 2002;276:39330–39339. doi: 10.1074/jbc.M105115200. [DOI] [PubMed] [Google Scholar]
- [17].Mita H, Tsutsui J, Takekawa M, Witten EA, Saito H. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell Biol. 2002;22:4544–4555. doi: 10.1128/MCB.22.13.4544-4555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grumont RJ, Rourke IJ, O'Reilly LA, Strasser A, Miyake K, Sha W, Gerondakis S. B lymphocytes differentially use the Rel and nuclear factor kappaB1 (NF-kappaB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J. Exp. Med. 1998;187:663–674. doi: 10.1084/jem.187.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J. Biol. Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- [20].Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- [21].Yin F, Bruemmer D, Blaschke F, Hsueh WA, Law RE, Herle AJ. Signaling pathways involved in induction of GADD45 gene expression and apoptosis by troglitazone in human MCF-7 breast carcinoma cells. Oncogene. 2004;23:4614–4623. doi: 10.1038/sj.onc.1207598. [DOI] [PubMed] [Google Scholar]
- [22].Zhang D, Song L, Li J, Wu K, Huang C. Coordination of JNK1 and JNK2 is critical for GADD45alpha induction and its mediated cell apoptosis in arsenite responses. J. Biol. Chem. 2006;281:34113–34123. doi: 10.1074/jbc.M602821200. [DOI] [PubMed] [Google Scholar]
- [23].de Martin R, Schmid JA, Hofer-Warbinek R. The NF-kappaB/Rel family of transcription factors in oncogenic transformation and apoptosis. Mutat. Res. 1999;437:231–243. doi: 10.1016/s1383-5742(99)00089-7. [DOI] [PubMed] [Google Scholar]
- [24].Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- [25].Xie DH, Tang XD, Xia SJ, Tan JM, Wang XH, Cai Y. Expression of NF-kappa B in human bladder cancer and its clinical significance. Ai Zheng. 2002;21:663–667. [PubMed] [Google Scholar]
- [26].Zen K, Karsan A, Stempien-Otero A, Yee E, Tupper J, Li X, Eunson T, Kay MA, Wilson CB, Winn RK, Harlan JM. NF-kappaB activation is required for human endothelial survival during exposure to tumor necrosis factor-alpha but not to interleukin-1beta or lipopolysaccharide. J. Biol. Chem. 1999;274:28808–28815. doi: 10.1074/jbc.274.40.28808. [DOI] [PubMed] [Google Scholar]
- [27].Sumitomo M, Tachibana M, Nakashima J, Murai M, Miyajima A, Kimura F, Hayakawa M, Nakamura H. An essential role for nuclear factor kappa B in preventing TNF-alpha-induced cell death in prostate cancer cells. J. Urol. 1999;161:674–679. [PubMed] [Google Scholar]
- [28].McDade TP, Perugini RA, Vittimberga FJ, Jr., Carrigan RC, Callery MP. Salicylates inhibit NF-kappaB activation and enhance TNF-alpha-induced apoptosis in human pancreatic cancer cells. J. Surg. Res. 1999;83:56–61. doi: 10.1006/jsre.1998.5560. [DOI] [PubMed] [Google Scholar]
- [29].Zhou D, Yu T, Chen G, Brown SA, Yu Z, Mattson MP, Thompson JS. Effects of NF-kappaB1 (p50) targeted gene disruption on ionizing radiation-induced NF-kappaB activation and TNFalpha, IL-1alpha, IL-1beta and IL-6 mRNA expression in vivo. Int. J. Radiat. Biol. 2001;77:763–772. doi: 10.1080/09553000110050047. [DOI] [PubMed] [Google Scholar]
- [30].Glauert HP, Tharappel JC, Banerjee S, Chan LS, Kania-Korwel I, Lehmler HJ, Lee EY, Robertson LW, Spear BT. Inhibition of the promotion of hepatocarcinogenesis by 2,2',4,4',5,5'-hexachlorobiphenyl (PCB-153) by the deletion of the p50 subunit of NF-kappaB in mice. Toxicol. Appl. Pharmacol. 2008;232:302–308. doi: 10.1016/j.taap.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cahir-McFarland ED, Davidson DM, Schauer SL, Duong J, Kieff E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA. 2000;97:6055–6060. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bernal-Mizrachi L, Lovly CM, Ratner L. The role of NF-{kappa}B-1 and NF-{kappa}B-2-mediated resistance to apoptosis in lymphomas. Proc. Natl. Acad. Sci. USA. 2006;103:9220–9225. doi: 10.1073/pnas.0507809103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].O'Neil BH, Buzkova P, Farrah H, Kashatus D, Sanoff H, Goldberg RM, Baldwin AS, Funkhouser WK. Expression of nuclear factor-kappaB family proteins in hepatocellular carcinomas. Oncology. 2007;72:97–104. doi: 10.1159/000111116. [DOI] [PubMed] [Google Scholar]
- [34].Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J. Exp. Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J. Exp. Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J. Exp. Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Snapper CM, Zelazowski P, Rosas FR, Kehry MR, Tian M, Baltimore D, Sha WC. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 1996;156:183–191. [PubMed] [Google Scholar]
- [38].Pelletier M, Girard D. Differential effects of IL-15 and IL-21 in myeloid (CD11b+) and lymphoid (CD11b-) bone marrow cells. J. Immunol. 2006;177:100–108. doi: 10.4049/jimmunol.177.1.100. [DOI] [PubMed] [Google Scholar]
- [39].DeAngelis RA, Kovalovich K, Cressman DE, Taub R. Normal liver regeneration in p50/nuclear factor kappaB1 knockout mice. Hepatology. 2001;33:915–924. doi: 10.1053/jhep.2001.23192. [DOI] [PubMed] [Google Scholar]
- [40].Artis D, Kane CM, Fiore J, Zaph C, Shapira S, Joyce K, Macdonald A, Hunter C, Scott P, Pearce EJ. Dendritic cell-intrinsic expression of NF-kappa B1 is required to promote optimal Th2 cell differentiation. J. Immunol. 2005;174:7154–7159. doi: 10.4049/jimmunol.174.11.7154. [DOI] [PubMed] [Google Scholar]
- [41].Schafer R, Hartig R, Sedehizade F, Welte T, Reiser G. Ade-nine nucleotides inhibit proliferation of the human lung adenocarcinoma cell line LXF-289 by activation of nuclear factor kappaB1 and mitogen-activated protein kinase pathways. FEBS J. 2006;273:3756–3767. doi: 10.1111/j.1742-4658.2006.05384.x. [DOI] [PubMed] [Google Scholar]
- [42].Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol. Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of PI3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224–239. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- [45].Lu Z, Lee EY, Robertson LW, Glauert HP, Spear BT. Effect of 2,2',4,4',5,5'-hexachlorobiphenyl (PCB-153) on hepatocyte proliferation and apoptosis in mice deficient in the p50 subunit of the transcription factor NF-kappaB. Toxicol. Sci. 2004;81:35–42. doi: 10.1093/toxsci/kfh193. [DOI] [PubMed] [Google Scholar]
- [46].Lin SC, Liu CJ, Yeh WI, Lui MT, Chang KW, Chang CS. Functional polymorphism in NFKB1 promoter is related to the risks of oral squamous cell carcinoma occurring on older male areca (betel) chewers. Cancer Lett. 2006;243:47–54. doi: 10.1016/j.canlet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- [47].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- [48].Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- [49].Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peebles KA, Lee JM, Mao JT, Hazra S, Reckamp KL, Krysan K, Dohadwala M, Heinrich EL, Walser TC, Cui X, Baratelli FE, Garon E, Sharma S, Dubinett SM. Inflammation and lung carcinogenesis: applying findings in prevention and treatment. Expert Rev. Anticancer Ther. 2007;7:1405–1421. doi: 10.1586/14737140.7.10.1405. [DOI] [PubMed] [Google Scholar]
- [51].Ishikawa H, Claudio E, Dambach D, Raventos-Suarez C, Ryan C, Bravo R. Chronic inflammation and susceptibility to bacterial infections in mice lacking the polypeptide (p)105 precursor (NF-kappaB1) but expressing p50. J. Exp. Med. 1998;187:985–996. doi: 10.1084/jem.187.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kato A, Edwards MJ, Lentsch AB. Gene deletion of NF-kappa B p50 does not alter the hepatic inflammatory response to ischemia/reperfusion. J. Hepatol. 2002;37:48–55. doi: 10.1016/s0168-8278(02)00068-5. [DOI] [PubMed] [Google Scholar]
- [53].Wessells J, Baer M, Young HA, Claudio E, Brown K, Siebenlist U, Johnson PF. BCL-3 and NF-kappaB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J. Biol. Chem. 2004;279:49995–50003. doi: 10.1074/jbc.M404246200. [DOI] [PubMed] [Google Scholar]
- [54].Cao S, Zhang X, Edwards JP, Mosser DM. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 2006;281:26041–26050. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Oakley F, Mann J, Nailard S, Smart DE, Mungalsingh N, Constandinou C, Ali S, Wilson SJ, Millward-Sadler H, Iredale JP, Mann DA. Nuclear factor-kappaB1 (p50) limits the inflammatory and fibrogenic responses to chronic injury. Am. J. Pathol. 2005;166:695–708. doi: 10.1016/s0002-9440(10)62291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- [57].Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R, Bieche I. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer. 2008;8:41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- [59].Wang ZP, Cai SX, Liu DB, Xu X, Liang HP. Anti-inflammatory effects of a novel peptide designed to bind with NF-kappaB p50 subunit. Acta. Pharmacol. Sin. 2006;27:1474–1478. doi: 10.1111/j.1745-7254.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- [60].Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol. Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- [61].Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- [62].Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J. Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- [63].Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- [64].Lin SC, Lu SY, Lee SY, Lin CY, Chen CH, Chang KW. Areca (betel) nut extract activates mitogen-activated protein kinases and NF-kappaB in oral keratinocytes. Int. J. Cancer. 2005;116:526–535. doi: 10.1002/ijc.21104. [DOI] [PubMed] [Google Scholar]
- [65].Kurland JF, Kodym R, Story MD, Spurgers KB, McDonnell TJ, Meyn RE. NF-kappaB1 (p50) homodimers contribute to transcription of the bcl-2 oncogene. J. Biol. Chem. 2001;276:45380–45386. doi: 10.1074/jbc.M108294200. [DOI] [PubMed] [Google Scholar]
- [66].Thornburg NJ, Pathmanathan R, Raab-Traub N. Activation of nuclear factor-kappaB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res. 2003;63:8293–8301. [PubMed] [Google Scholar]
- [67].Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- [68].Budunova IV, Perez P, Vaden VR, Spiegelman VS, Slaga TJ, Jorcano JL. Increased expression of p50-NF-kappaB and constitutive activation of NF-kappaB transcription factors during mouse skin carcinogenesis. Oncogene. 1999;18:7423–7431. doi: 10.1038/sj.onc.1203104. [DOI] [PubMed] [Google Scholar]



