Abstract
OBJECTIVE
The purpose of this study was to determine the efficacy of two Internet-based psycho-educational programs designed to improve outcomes for youth with type 1 diabetes transitioning to adolescence.
RESEARCH DESIGN AND METHODS
The study was a multisite clinical trial of 320 youth (aged 11–14 years; 37% minority; 55% female) randomized to one of two Internet-based interventions: TeenCope or Managing Diabetes. Primary outcomes were HbA1c and quality of life (QOL). Secondary outcomes included coping, self-efficacy, social competence, self-management, and family conflict. Data were collected at baseline and after 3, 6, and 12 months online. Youth were invited to cross over to the other program after 12 months, and follow-up data were collected at 18 months. Analyses were based on mixed models using intent-to-treat and per-protocol procedures.
RESULTS
Youth in both groups had stable QOL and minimal increases in HbA1c levels over 12 months, but there were no significant differences between the groups in primary outcomes. After 18 months, youth who completed both programs had lower HbA1c (P = 0.04); higher QOL (P = 0.02), social acceptance (P = 0.01), and self-efficacy (P = 0.03) and lower perceived stress (P = 0.02) and diabetes family conflict (P = 0.02) compared with those who completed only one program.
CONCLUSIONS
Internet interventions for youth with type 1 diabetes transitioning to adolescence result in improved outcomes, but completion of both programs was better than only one, suggesting that these youth need both diabetes management education and behavioral interventions. Delivering these programs via the Internet represents an efficient way to reach youth and improve outcomes.
Youth with type 1 diabetes in transition to adolescence are a vulnerable population. They exhibit deteriorating metabolic control (1,2), poorer self-management, and increased social stressors and psychosocial distress, as well as lower quality of life (QOL) compared with children with type 1 diabetes at other ages (2). Resolving independence/dependence issues and acquiring positive acceptance by peers within the context of a complex treatment regimen are particularly challenging for these youth. Having diabetes involves fear of hypoglycemia, fear of future complications, feelings of guilt for possible wrongdoing, and feelings of stress associated with challenging self-management tasks (3). In addition, these stressors occur within the broader context of increased expectations for adolescents to maintain excellent metabolic control (4), despite the insulin resistance associated with puberty (5).
As youth transition to adolescence and take on greater responsibility for their diabetes self-management, educational needs are higher. Standards of care for youth with type 1 diabetes identify the importance of education; yet, the provision of education in the clinical setting is mostly informal and inconsistent. Considerable evidence indicates that in-person psycho-educational interventions, such as Coping Skills Training (CST), improve metabolic control of type 1 diabetes as well as psychosocial adjustment and QOL in youth (6–8). However, implementing these evidence-based programs in clinical care is challenging because of provider and organizational barriers, such as lack of time, resources, and expertise (9). Rapid advances in technology and access to the Internet have made it not only a viable mode for the delivery of psycho-educational interventions but also a platform that can be widely disseminated and implemented. Internet interventions allow for standardization of program content, can be targeted to specific ages and developmental phases, allow for social interaction, and can be easily updated. Access to the Internet is increasingly available nationwide and has risen to its highest level ever, with 93% of youth using the Internet regularly for school assignments, hobbies or special interests, entertainment, and connection with others (10,11). The Internet, therefore, represents an efficient way to deliver psycho-educational interventions to youth with type 1 diabetes.
Psycho-educational interventions delivered via the Internet have demonstrated efficacy in improving symptoms and health behaviors in youth of different ages and illness experiences (12–14). In a pilot study, youth with type 1 diabetes who completed an Internet program with a focus on problem solving and social networking demonstrated improved self-management and problem solving compared with a control group (15). Internet-based interventions that can reach large numbers of youth with diabetes have the potential to result in significant improvements in long-term health, as well as reductions in the costs of care for diabetes-related complications. They also have the potential to improve access for diverse youth with type 1 diabetes. While there is ongoing evidence of the digital divide, this has been decreasing over time, particularly with English-speaking youth in the U.S. (10).
TeenCope, a new Internet-based version of CST, was developed by our group. It is based on social cognitive theory and posits that improving coping skills will lead to improved self-efficacy and self-management of diabetes that result in better outcomes, as has been demonstrated in studies of CST delivered in a group-based in-person format (16). Managing Diabetes was developed to serve as the control condition and was a diabetes education and problem-solving program.
Thus, the purpose of this multisite randomized clinical trial was to compare the efficacy of two Internet-based programs on the primary outcomes of HbA1c and QOL and on the secondary outcomes of stress, coping, self-efficacy, self-management, social competence, and family conflict at 12 months. At 12-month follow-up, youth were invited to participate in the alternate program, allowing us to explore the effect of participating in two programs compared with participating in only one. We hypothesized that youth who participated in TeenCope would have lower HbA1c levels and better QOL after 12 months than those who participated in Managing Diabetes.
RESEARCH DESIGN AND METHODS
The study was a multisite, randomized, parallel-group trial designed to evaluate the comparative efficacy and combined effect of two Internet psycho-educational programs for youth with type 1 diabetes transitioning to adolescence (17). The two programs were an Internet CST program (TeenCope) and an Internet diabetes health education program (Managing Diabetes) (18). Each program consisted of five sessions with content tailored to transitioning adolescents with type 1 diabetes that were released once per week for 5 weeks. TeenCope used a cast of ethnically diverse characters with type 1 diabetes and a graphic novel video format to model common problematic social situations (i.e., parent conflict) and different coping skills to solve the problems. Content of CST was based on our previous studies and included communication skills, social problem solving, stress management, positive self-talk, and conflict resolution (16,19). A monitored discussion board allowed TeenCope participants to communicate with youth from the other participating clinical sites. Managing Diabetes, the control condition, was designed as a diabetes education and problem-solving program to be delivered via the Internet. It used visuals and an interactive interface that allowed youth to learn about healthy eating, physical activity, glucose control, sick days, and diabetes technology. Interactivity consisted of active links to more detailed information, polling about diabetes care issues, and problem-solving exercises with tailored feedback to participant responses. Content of Managing Diabetes was based on standards of care for diabetes management in youth (8), with an emphasis on decision making for optimal outcomes.
A convenience sample was recruited from four university-affiliated clinical sites (Children’s Hospital of Philadelphia, University of Arizona, University of Miami, and Yale University). Inclusion criteria were as follows: diagnosis with type 1 diabetes for at least 6 months, age 11–14 years, no other significant medical problem, school grade appropriate to age within 1 year, ability to speak and write English, and access to high-speed Internet at home or school or in the community. The sample size was determined by a power analysis of the primary outcomes as well as a mediator analysis that will be reported in a future article. For the primary analyses, the sample of 320 youth yielded a power of 90% for HbA1c and 82% for QOL. Institutional review boards at all clinical sites reviewed and approved the study. Trained research personnel approached youth and parents/guardians in the clinic setting and obtained informed consent/assent. Demographic data were supplied by parents or guardians at enrollment, and e-mail communication was subsequently established with youth. If online data collection was not completed within 3 months of enrollment, youth were considered to be passively refusing study participation. Escalating incentives (20–50 USD) were provided for completion of each round of data collection. No incentives were provided for completing the programs.
After completion of baseline data collection, an automated e-mail was sent to youth and their parents/guardians to identify their group assignment and provide a link to the randomly assigned program. A unique password was provided to each participant, and they were instructed to change this password the first time they logged onto the program. Each program had five sessions that were released weekly and took ~30 min to complete. The average time to complete all sessions was 40 ± 23.2 days. Research staff at each site sent e-reminders if youth did not complete a session within 2 weeks. After completion of 12-month data, participants were invited to complete the alternate program.
A framework developed by our group guided our measurement design (20). Data were collected at baseline and 3, 6, 12, and 18 months. The primary outcomes were HbA1c levels and QOL. Secondary outcomes included stress, coping, self-efficacy, self-management, social competence, and family conflict. Data were collected by online survey with the exception of HbA1c and other clinical data that were collected by chart review and demographic data that were provided at consent by parents/guardians. All measures were thoroughly evaluated for reliability and validity.
Primary outcomes
HbA1c levels were determined using the DCA2000 (Bayer, Tarrytown, NY) at each of the sites. Very few (3%) of the results were done by outside laboratories, and these results were not significantly different from those from the DCA, so these data were combined. QOL was measured by the Pediatric Quality of Life Inventory (PedsQL) (teen version)-Core (21), a 23-item measure of global QOL. Higher scores reflect better QOL. High reliability and validity have been established in clinical and community samples. Cronbach α for our sample was 0.87.
Secondary outcomes
Stress/coping was measured with the Perceived Stress Scale, a 14-item scale that measures the degree to which situations in one’s life are appraised as stressful (22). Items assess feelings of stress, hassles, and coping during the past month. Respondents rate items on a 5-point Likert scale ranging from 0 (never) to 4 (very often), with higher scores indicative of greater perceived stress and less effective coping. The reliability estimate in our data was 0.80.
Coping style in response to diabetes-related stresses was assessed with the Responses to Stress Questionnaire (23). The first 10 items of the measure list stressors specific to adolescents with diabetes (24), followed by 57 items asking how the individual responds to these stressors. There are three coping factors: primary engagement coping (problem solving, emotional modulation, and emotional expression), secondary control engagement coping (positive thinking, cognitive restructuring, acceptance, and distraction), and disengagement coping (avoidance, denial, and wishful thinking). Proportion scores were used in the analyses to control for response bias and base rates of item endorsement. Cronbach α ranged from 0.77 to 0.87 in our sample.
The Self-Efficacy for Diabetes Scale measures self-perceptions or expectations held by people with diabetes about their personal competence, power, and resourcefulness for successfully managing their diabetes (25). The diabetes-specific self-efficacy subscale (24 items) was used in this study, with lower scores indicative of higher self-efficacy. The reliability coefficient was 0.88 in our sample.
Self-management was assessed with Self-Management of Diabetes - Adolescents, a new self-report measure of self-management for adolescents with type 1 diabetes (26) that was developed to encompass a view of self-management that moves beyond adherence to treatment regimens. There are five subscales (Collaboration with Parents, Diabetes Care Activities, Diabetes Problem Solving, Diabetes Goals, and Diabetes Communication) with reliability estimates in our sample ranging from 0.62 to 0.80.
Social competence was measured with the five-item social acceptance subscale of the Self-Perception Profile for Adolescents (27). Statements are scored on a 4-point rating scale, such that high scores reflect greater perceived competence. Cronbach α for this sample was 0.75.
The revised Diabetes Family Conflict Scale was used to evaluate diabetes-related treatment conflict (28). The scale is a 19-item questionnaire adapted from the Diabetes Responsibility and Conflict Checklist (29) and is used to measure the degree of conflict between family members on diabetes management activities. Diabetes conflict is rated on a 3-point scale with higher scores indicative of greater conflict. Cronbach α for this sample was 0.87.
Data were also collected on sociodemographic data (i.e., ethnicity, socioeconomic status, number of children, birth order, and sex of child with diabetes) at baseline from the consenting parent/guardian. Pubertal status was assessed with the Pubertal Development Scale (30) to control for the level of pubertal development, which has been shown to correlate well with clinical observations. Diabetes clinical variables, such as length of time since diagnosis and treatment type (injections or pump), were collected by research staff from the medical record. Satisfaction was evaluated by youth with a 6-item survey on how helpful, enjoyable, easy to use, and worthwhile the program was. Items were rated on a 5-point Likert-type scale from not at all to very satisfied, with higher scores indicative of higher satisfaction. Scale reliability was 0.73 in our sample.
Data analyses
The sample and each of the variables were described using frequency distributions and appropriate summary statistics. Group differences at baseline were tested with t tests or χ2. The main hypotheses tested were that youth who participated in the TeenCope program would demonstrate better HbA1c and QOL than those who participated in the Managing Diabetes program. For testing of these hypotheses, a series of mixed-effects models (repeated-measures linear regression with arbitrary within-subject correlation structures) in the SAS procedure MIXED was conducted using an intent-to-treat approach and a per-protocol analysis (completion of ≥4 lessons), controlling for sex, age, race/ethnicity, duration, income, therapy type, and site. The moderation effect of puberty was examined by testing the interaction between time and puberty level. Our second and exploratory hypothesis was that youth who participated in both programs would demonstrate better outcomes compared with youth who participated in only one program. For this analysis, youth who completed both programs were compared with youth who completed one program in an intent-to-treat analysis and on a per-protocol analysis (≥4 lessons for initial program) in a series of mixed-effects models, controlling for the same variables.
RESULTS
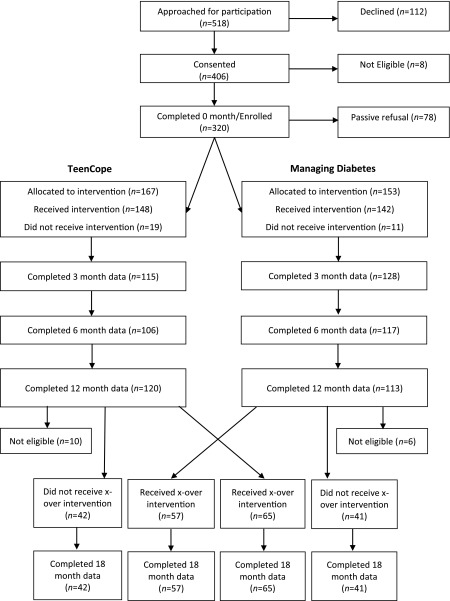
The final sample of 320 youth had a mean age of 12.3 ± 1.1 years with diabetes duration of 6.1 ± 3.5 years. Mean HbA1c was 8.46 (69.0 mmol/mol) (±1.42%). The sample was 55% female, 62.2% non-Hispanic white, and 37.8% black/Hispanic/other. Approximately 50% of families had incomes ≥80,000 USD. Nearly 59% of the youth used pump therapy, and 53% began the study with HbA1c >8%. At baseline, 97 (30%) of the subjects had not yet entered puberty. Figure 1 shows the CONSORT flow diagram.
Figure 1.
CONSORT flow diagram. x-over, crossover.
The two groups were comparable at baseline, with the exception of years of parental education, with those in Managing Diabetes having 0.7 years more education. There were, however, differences among the four sites in race/ethnicity, income, therapy type (pump or injections), parent education, and HbA1c, and these were controlled in the analyses.
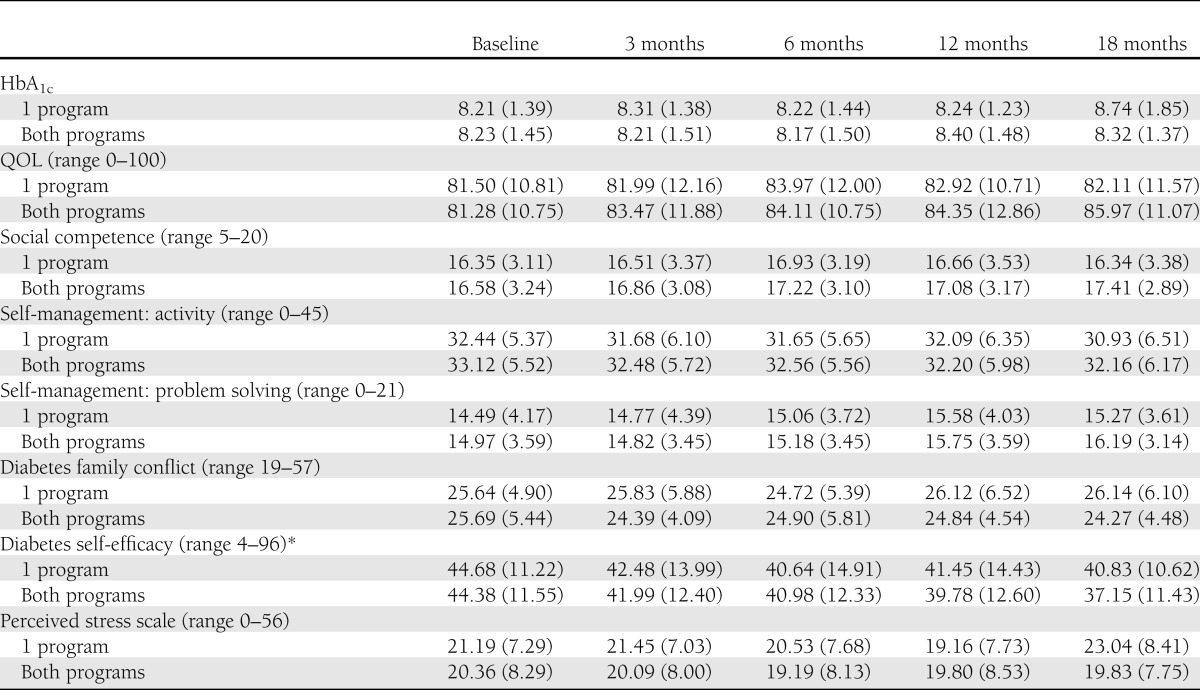
Participation in sessions was high, with 78% of all youth completing four of five sessions, and 90% of youth completed at least one session. TeenCope participants completed 82% of sessions and Managing Diabetes participants completed 74% of sessions, and these differences were not statistically different. More than one-half (52%) of TeenCope participants participated in the discussion boards. Satisfaction was high with both programs, with no significant difference between groups. The mean satisfaction score was 3.97 ± 0.71 (median = 4) for TeenCope and was 3.89 ± 0.56 (median = 4) for Managing Diabetes. Both groups had slight increases in HbA1c levels (P = 0.05) and improved QOL (P < 0.001) (Table 1) over time, but there were no significant differences between the two groups on either of these primary outcomes over 12 months of follow-up in the intent-to-treat analyses (Table 2). Thus, the primary hypotheses were not supported. Mean HbA1c levels increased slightly (mean 0.12%) (TeenCope 8.43 ± 1.5% [68.6 mmol/mol] vs. Managing Diabetes 8.25 ± 1.3% [66.7 mmol/mol]) and stayed >8% in both groups. Those with baseline HbA1c <8% at baseline had worsened control at 12 months of follow-up (mean change = 0.06%), whereas those who had HbA1c ≥8% at baseline improved over 12 months by 0.5%. This difference was significant but not affected by group assignment. Pubertal level did not moderate HbA1c levels.
Table 1.
Means (SD) of primary and secondary outcomes over 18 months: intent-to-treat analyses, n = 320
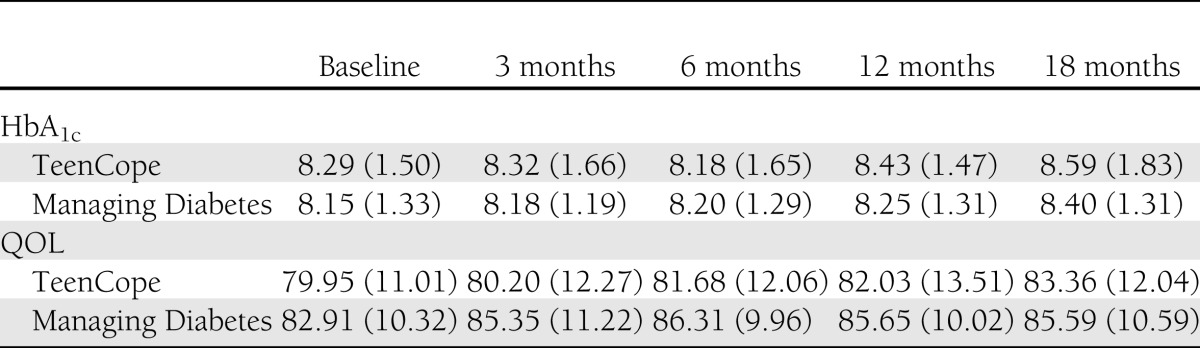
Table 2.
Mixed-effects model of HbA1c and QOL controlling for covariates at 12 months: intent-to-treat analysis, n = 320
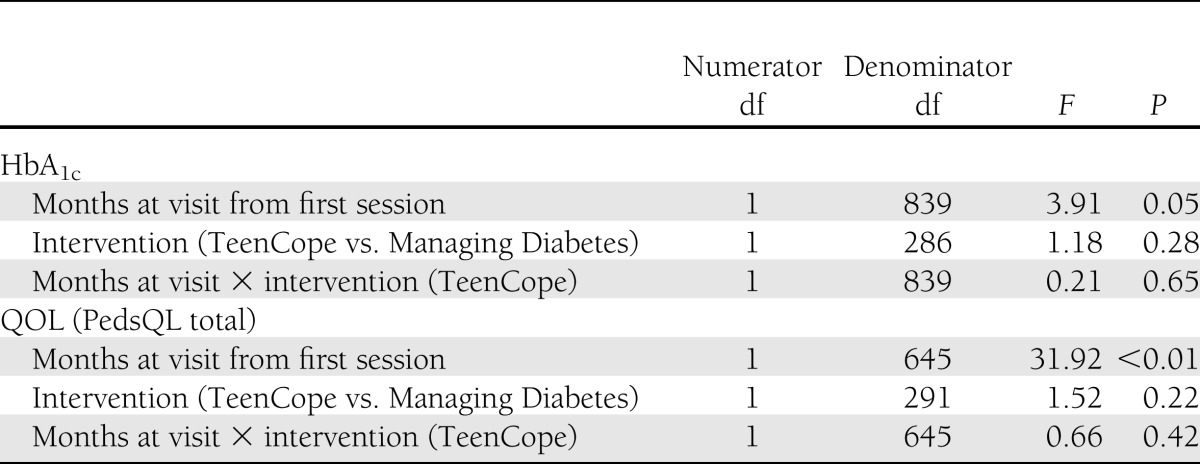
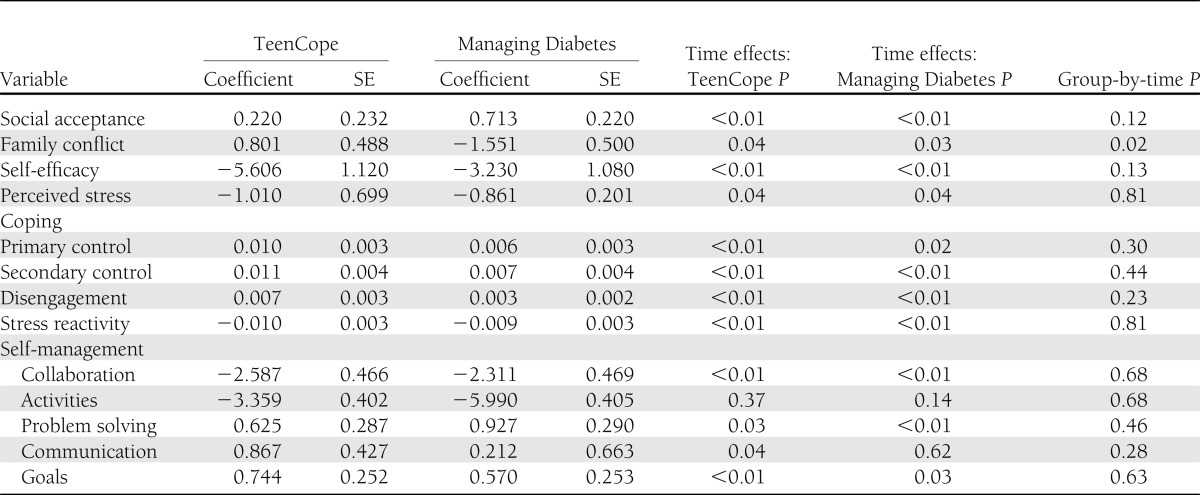
There were few differences in secondary outcomes after 12 months (Table 3). Group-by-time analyses showed that youth in Managing Diabetes had significantly less (P = 0.02) diabetes family conflict than those in TeenCope. There were no other differences in secondary outcomes in the group-by-time analyses, although there were a number of time effects in each group that indicated improvement over time. We then analyzed these outcomes using only those who completed at least four sessions of the respective program. The results of this per-protocol analysis were similar to those of the intent-to-treat analysis.
Table 3.
Coefficients of change in secondary outcomes at 12 months, controlling for covariates: intent-to-treat analysis, n = 320
After 12 months, youth were invited to cross over and do the other program. To examine whether participating in both programs was significantly better than participating in one program, we compared the 128 youth who completed both programs with those who completed only their initially assigned program (n = 122). Those who did two programs were not different in demographics or clinical data than those who did only one program. With baseline scores controlled for, significant improvements in HbA1c levels were found for those who completed both programs compared with those who completed only one (P = 0.04; one program 8.74 ± 1.8% [72.0 mmol/mol] vs. both programs 8.32 ± 1.4% [67.3 mmol/mol]) and QOL (P = 0.02) (Table 4). Social acceptance (P = 0.01), diabetes family conflict (P = 0.02), self-efficacy (P = 0.03), and perceived stress (P = 0.02) were also improved in those who did the two programs compared with those who did only one. After controlling for race, therapy type, income, and site in addition to baseline score, participation in both programs resulted in significantly improved QOL (P = 0.045), social acceptance (P = 0.023), and diabetes family conflict (P = 0.044) and trends in HbA1c (P = 0.16; mean difference 0.3%), self-efficacy (P = 0.07), and perceived stress (P = 0.08) compared with participating in only one. Results using intent-to-treat procedures were similar.
Table 4.
Means (SD) of outcomes in youth who participated in one program versus both programs (n = 250)
CONCLUSIONS
While both study groups experienced improvement over the 12 months of follow-up in QOL as well as slightly higher HbA1c levels, the hypothesis that TeenCope would yield superior outcomes after 12 months was not supported. This was unexpected and may be the result of several factors. First, in this comparative effectiveness trial, we compared two new relatively sophisticated Internet programs aimed at different needs of youth transitioning to adolescence. TeenCope, built on a highly successful group-based model designed for a wider range of adolescent ages, focused on teaching a series of coping skills shown previously to improve both QOL and HbA1c (16). Managing Diabetes was designed as a program to teach advanced diabetes problem solving and healthy lifestyles using an interactive format. The results of the 18-month analyses suggest that youth in this age-group require both sets of skills to transition successfully to adolescence without the risk of poorer outcomes. Secondly, most previous studies of behavioral interventions for youth with type 1 diabetes compared the new intervention with usual care or a minimally active control condition. In this comparative effectiveness trial, the control condition was a very active condition. Such work is extremely important in examining the relative impact of established interventions. Further, with regard to HbA1c levels, the mean HbA1c for the sample at baseline was just over 8% (63.9 mmol/mol), creating the potential for a floor effect.
The results after 18 months were more in line with our primary hypotheses than those at 12 months. One intriguing possibility is that youth who actually completed the second program were different in some way from those who only did one program, even though analyses of clinical and demographic data showed no differences between these groups. Youth who did the second program may have been more motivated and engaged in the process of taking responsibility for self-management because the timing was right for them. In addition, it may be that just longer contact, regardless of content, led to the improved longer-term outcomes. These results may also suggest that these youth need ongoing support and encouragement from nonparents and nonproviders to support this transition. Further studies comparing such programs are necessary.
Nonetheless, both interventions resulted in minimal deterioration in metabolic control over months during the transition period to adolescence. The onset of puberty is associated with deterioration in metabolic control, which is usually associated with increases in levels of growth hormone associated with sexual development (5). Thus, in this group of youth ages 11–14 years, who were entering (30% of the sample) or progressing through puberty (70%), it would be expected that metabolic control would worsen over the 12 months of follow-up coupled with an increase in family conflict (31). Both programs resulted in minimal worsening of HbA1c levels over 12 months as well as improvement in HbA1c, better QOL, and less family conflict with participation in both programs, suggesting that both programs are useful during this period of transition for youth. Nonetheless, HbA1c levels increased by ~0.3% over the course of the study in both groups. It is not possible to know with the design of this study what would be the likely increase over the same period without the interventions, but in several previous studies it was reported that increases of 2% in HbA1c are common during adolescence (2,32).
Previous reports have suggested that youth from minority and underserved groups may participate less in diabetes care and behavioral interventions, which has been partially explained by perceptions of greater perceived risks for short-term versus long-term complications and greater risks to others with diabetes than to self (33). Sites for this study were selected to purposefully oversample minority youth. In a recent article, our group reported that there was no difference in participation by sex or race/ethnicity but that lower-income youth were less likely to participate (34). Race/ethnicity and income did not moderate outcomes in this study, however.
An important contribution of this study is the use of a well-developed conceptual framework that guided intervention development and measurement. While secondary outcomes do not appear to be changed by participation in one program alone, further analyses of mediation will allow for examination of model testing. It may be that different forms of coping are facilitated by the two programs that would support the similar outcomes. For example, Managing Diabetes may help with primary engagement coping, which includes problem solving, but TeenCope enhances secondary control engagement coping (positive thinking, emotional modulation, etc.), and both contribute to improved outcomes in adolescents transitioning to independent diabetes self-management.
As with any study, there are limitations. Despite efforts to recruit a highly diverse sample, low-income youth were more likely to passively refuse to participate after consent than those from higher-income families, probably as a result of more limited access to the Internet. Since previous studies led to the conclusion that low-income youth have poorer metabolic control (35), our sample is biased toward youth with better metabolic control. Thus, our findings cannot be generalized to all youth with type 1 diabetes who are transitioning to adolescence. Attrition over 12 months was 28%, even with multiple approaches taken to retain subjects. Nonetheless, intent-to-treat procedures were used for analyses, which mostly likely resulted in conservative results. In addition, the sample was limited in age range.
In summary, the results of this study indicate that youth with type 1 diabetes transitioning to adolescence will participate in Internet programs and were highly satisfied. Such programs result in relatively stable HbA1c levels and improvement in QOL and perception of social acceptance, along with a decrease in family conflict—critical outcomes at this developmental phase. Research is needed on how to use such programs in the routine care of youth with type 1 diabetes.
Acknowledgments
This research was supported by grant R01NR04009 from the National Institute of Nursing Research.
No potential conflicts of interest relevant to this study were reported.
M.G. wrote the manuscript, researched data, and was a principal investigator. R.W. reviewed and edited the manuscript, contributed to the discussion, and was a principal investigator. S.J. researched data and reviewed and edited the manuscript. K.M., M.S.F., and A.D. reviewed and edited the manuscript and contributed to the discussion. M.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes, Miami Beach, Florida, 19–22 October 2011.
APPENDIX
The following individuals and institutions constitute the TeenCope Study Group (1R01NR004009) (*principal investigator or director): Yale University School of Nursing, M.G.*, R.W.*, W. Tamborlane, L. Liberti, S. Jaser, N. Hunter, S.J., and T. Ma; Children’s Hospital of Philadelphia, K. Murphy* and S. Dumser; University of Arizona College of Nursing, M.S.F.*, S. Michaliszyn, and E. Crockett; and University of Miami Department of Pediatrics, A.D.*, J. Hernandez, and D. Wile.
Footnotes
Clinical trial reg. no. NCT00684658, clinicaltrials.gov.
A complete list of the TeenCope Study Group can be found in the Appendix.
References
- 1.Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistence of puberty: A defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 1991;72:277–282 [DOI] [PubMed] [Google Scholar]
- 2.Insabella G, Grey M, Knafl GK, Tamborlane WV. Transition from adolescence to young adulthood in youth with T1D. Pediatr Diabetes 2007;8:228–234 [DOI] [PubMed] [Google Scholar]
- 3.Karlsson A, Arman M, Wikblad K. Teenagers with type 1 diabetes—a phenomenological study of the transition towards autonomy in self-management. Int J Nurs Stud 2008;45:562–570 [DOI] [PubMed] [Google Scholar]
- 4.Schilling LS, Knafl KA, Grey M. Changing patterns of self-management in youth with type I diabetes. J Pediatr Nurs 2006;21:412–424 [DOI] [PubMed] [Google Scholar]
- 5.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 1986;315:215–219 [DOI] [PubMed] [Google Scholar]
- 6.Grey M. Interventions for children and adolescents with diabetes. Annu Rev Nurs Res 2000;22:149–170 [PubMed] [Google Scholar]
- 7.Anderson B, Loughlin C, Goldberg E, Laffel L. Comprehensive, family-focused outpatient care for very young children living with chronic disease: Lessons from a program in pediatric diabetes. Child Serv (Mahwah NJ) 2001;4:235–240 [Google Scholar]
- 8.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr 2003;142:409–416 [DOI] [PubMed] [Google Scholar]
- 9.Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005;28:186–212 [DOI] [PubMed] [Google Scholar]
- 10.Pew Research Center. Trend data for teens [article online], 2009. Available from http://www.pewinternet.org/static-pages/trend-data-for-teens/whos-online.aspx Accessed 3 June 2011
- 11.Nielsen J. Usability of websites for teenagers (Jakob Nielsen's Alertbox) [article online], 2005. Available from http://www.useit.com/alertbox/20050131.html Accessed 8 August 2009
- 12.Siemer CP, Fogel J, Van Voorhees BW. Telemental health and web-based applications in children and adolescents. Child Adolesc Psychiatr Clin N Am 2011;20:135–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stinson J, Wilson R, Gill N, Yamada J, Holt J. A systematic review of internet-based self-management interventions for youth with health conditions. J Pediatr Psychol 2009;34:495–510 [DOI] [PubMed] [Google Scholar]
- 14.Ritterband LM, Tate DF. The science of internet interventions. Introduction. Ann Behav Med 2009;38:1–3 [DOI] [PubMed] [Google Scholar]
- 15.Mulvaney SA, Rothman RL, Wallston KA, Lybarger C, Dietrich MS. An internet-based program to improve self-management in adolescents with type 1 diabetes. Diabetes Care 2010;33:602–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr 2000;137:107–113 [DOI] [PubMed] [Google Scholar]
- 17.Grey M, Whittemore R, Liberti L, Delamater A, Murphy K, Faulkner MS. A comparison of two internet programs for adolescents with type 1 diabetes: design and methods. Contemp Clin Trials 2012;33:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whittemore R, Grey M, Lindemann E, Ambrosino J, Jaser S. Development of an Internet coping skills training program for teenagers with type 1 diabetes. Comput Inform Nurs 2010;28:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grey M, Whittemore R, Jaser S, et al. Effects of coping skills training in school-age children with type 1 diabetes. Res Nurs Health 2009;32:405–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittemore R, Jaser SS, Guo J, Grey M. A conceptual model of childhood adaptation to type 1 diabetes. Nurs Outlook 2010;58:242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126–139 [DOI] [PubMed] [Google Scholar]
- 22.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396 [PubMed] [Google Scholar]
- 23.Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H. Responses to stress in adolescence: measurement of coping and involuntary stress responses. J Consult Clin Psychol 2000;68:976–992 [PubMed] [Google Scholar]
- 24.Davidson M, Penney ED, Muller B, Grey M. Stressors and self-care challenges faced by adolescents living with type 1 diabetes. Appl Nurs Res 2004;17:72–80 [DOI] [PubMed] [Google Scholar]
- 25.Grossman HY, Brink S, Hauser ST. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabetes Care 1987;10:324–329 [DOI] [PubMed] [Google Scholar]
- 26.Schilling LS, Dixon JK, Knafl KA, et al. A new self-report measure of self-management of type 1 diabetes for adolescents. Nurs Res 2009;58:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harter S. Manual for the Self-Perception Profile for Adolescents. Denver, CO, University of Denver, 1988 [Google Scholar]
- 28.Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised Diabetes Family Conflict Scale. Diabetes Care 2007;30:1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin R, Young-Hyman D, Peyrot M. Parent-child responsibility and conflict in diabetes care. Diabetes 1989;38(Suppl. 1):28 [Google Scholar]
- 30.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity and initial norms. J Youth Adolesc 1988;17:117–133 [DOI] [PubMed] [Google Scholar]
- 31.Johnson SB, Pollak RT, Silverstein JH, et al. Cognitive and behavioral knowledge about insulin-dependent diabetes among children and parents. Pediatrics 1982;69:708–713 [PubMed] [Google Scholar]
- 32.Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood: a longitudinal cohort study. Diabetes Care 2001;24:1536–1540 [DOI] [PubMed] [Google Scholar]
- 33.Patino AM, Sanchez J, Eidson M, Delamater AM. Health beliefs and regimen adherence in minority adolescents with type 1 diabetes. J Pediatr Psychol 2005;30:503–512 [DOI] [PubMed] [Google Scholar]
- 34.Whittemore R, Jaser S, Faulkner M, Murphy K, Delamater A, Grey M. Recruitment, participation, and satisfaction with an eHealth psycho-educational program for youth with type 1 diabetes. J Med Internet Res 2013;15:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delamater AM, Shaw KH, Applegate EB, et al. Risk for metabolic control problems in minority youth with diabetes. Diabetes Care 1999;22:700–705 [DOI] [PubMed] [Google Scholar]