Abstract
Autophagy activity is essential for the survival of neural cells. Impairment of autophagy has been implicated in the pathogenesis of neurodegenerative disorders. Unlike the massive neuron loss in mice deficient for autophagy genes essential for autophagosome formation, we demonstrated that mice deficient for the metazoan-specific autophagy gene Epg5 develop selective neuronal damage and exhibit key characteristics of amyotrophic lateral sclerosis. Epg5 deficiency blocks the maturation of autophagosomes into degradative autolysosomes, slows endocytic degradation and also impairs endocytic recycling. Recessive mutations in human EPG5 have recently been causally associated with the multisystem disorder Vici syndrome. Here we show that while Epg5 knockout mice display some features of Vici syndrome, many phenotypes are absent.
Keywords: autophagy, autophagosome, Epg5, Vici syndrome, neurodegeneration
Mice Deficient for Genes Essential for Autophagosome Formation Exhibit Massive Neuronal Death
Autophagy is an evolutionarily conserved lysosome-mediated degradation process, involving formation of an enclosed double-membrane structure, called the autophagosome, and its subsequent delivery to lysosomes for degradation.1,2 In higher eukaryotes, autophagosomes undergo a maturation process before fusing with lysosomes to form functional autolysosomes.3 Autophagy serves as a cell survival mechanism by degrading a portion of the cytosol in response to various stresses. Basal autophagy also acts as a quality control system to selectively remove misfolded or aggregate-prone proteins, and damaged or superfluous organelles. Dysfunction of autophagy has been linked to various pathological diseases in mammals, including tumorigenesis and neurodegeneration.4
Neural-specific knockouts of mouse genes essential for autophagosome formation, including Atg5, Atg7 and Rb1cc1/Fip200, reveal that loss of autophagy activity causes accumulation of protein aggregates in neurons and leads to axonal degeneration and massive neuronal death.5-7 However, many neurodegenerative diseases exhibit selective age-dependent vulnerability of certain neuronal populations. For example, in Huntington disease, cell death in the caudate causes chaotic movement, and in Parkinson disease, loss of substantia nigra neurons results in slowness of movement, tremor and rigidity.8 Neurodegenerative diseases such as Huntington disease, Parkinson disease and amyotrophic lateral sclerosis (ALS) exhibit impaired autophagic flux, and accumulation of nondegradative autophagic vacuoles in affected neurons.9 Therefore, investigating the physiological function of genes that act downstream of autophagosome formation is essential to understand whether loss of autophagy activity causes degeneration of specific neuronal populations.
Epg5-Deficient Mice Show Selective Neuron Loss
Genetic screens in C. elegans identified a metazoan-specific autophagy gene, epg-5, which is required for formation of degradative autolysosomes.10 We generated conventional Epg5 knockout mice to investigate the physiological function of Epg5. Mice with neural-specific Atg5, Atg7 or Ei24 deficiency exhibit behavioral and neurological deficits at the age of ~2–3 weeks, show extensive neuron loss in various cerebral and cerebellar regions, and suffer early death at the age of ~3–4 mo.5,6,11 In contrast, Epg5-deficient mice start to show abnormal neural symptoms at 4 mo of age, and gradually develop complete hind limb paralysis, eventually dying by 10–12 mo. Autophagy flux is systematically impaired and SQSTM1/p62 aggregates accumulate in many regions of the brain and spinal cord in Epg5 knockout mice. However, histological analysis demonstrates that only certain neuronal populations have degenerated. For example, neuron loss is evident in the 5th layer of the cerebral cortices and the anterior horn of the spinal cord, but not in the Purkinje layer of the cerebellum or the spinal cord interneurons. Cytoplasmic aggregates of TARDBP/TDP-43 (TAR DNA binding-domain protein) also accumulate in vulnerable motor neurons in the spinal cord in Epg5 mutant mice. Severe muscle atrophy and muscle denervation are detected in epg5−/− mice. Specific motor neuron damage, muscle atrophy and cytoplasmic TARDBP accumulation are key features of ALS,8,12 indicating that Epg5 knockout mice recapitulate the characteristic pathogenesis of ALS.
Why do Epg5-deficient mice have distinct neuropathological defects? It is unlikely that loss of Epg5 activity causes less severe autophagic defects than other essential autophagy genes. Mice that have reduced activity of Atg16l or are deficient in Atg4b, both of which cause systematic reduction of autophagic activity, do not show obvious histopathological alterations.13,14 Furthermore, selective neuronal damage in Epg5-deficient mice is not caused by increased vulnerability of motor neurons to autophagic inhibition. Interneurons in the spinal cord of mice with neural-specific Ei24 deficiency are much more dramatically affected than motor neurons. Eosinophilic spheroids, suggestive of axonal degeneration, accumulate in the dorsal corticospinal tract of the spinal cord of Epg5-, but not Ei24-deficient mice.11 Mice deficient in Ei24 also lack cytoplasmic TARDBP aggregates. A recent study shows that impairment of the ubiquitin-proteasome system in motor neurons causes ALS in mice, while motor neuron-specific knockout of Atg7 results in accumulation of ubiquitin and SQSTM1, but not TARDBP and causes no motor dysfunction.15 Proteasome activity in Epg5-deficient mice appears to be normal. Epg5 deficiency impairs autophagic flux by blocking the maturation of autophagosomes into degradative autolysosomes. Epg5-deficient MEFs contain numerous autophagic vacuoles at all stages, including autophagosomes, possible amphisomes and early autolysosomes. Loss of Epg5 function also impairs endosomal trafficking. Epg5 knockdown slows endocytic degradation and delays endocytic recycling. Functional impairment of the ESCRT complex or VCP (valosin containing protein), which causes accumulation of nondegradative autophagic vacuoles and also defective endocytic trafficking, has been linked with familial ALS.16-19 We favor the possibility that accumulation of nondegradative autophagic vacuoles, together with defective endocytic trafficking, in Epg5-deficient mice contributes to selective degeneration of certain neuronal populations.
Mutations in EPG5 Cause Vici Syndrome
A recent study by Cullup et al. shows that recessive EPG5 mutations have a causative role in Vici syndrome.20 Among 18 patients from 15 families, 16 individuals show homozygosity or compound heterozygosity for truncating mutations, missense mutations and splice mutations in the EPG5 gene.20 Vici syndrome is a recessively inherited multisystem disorder, characterized by agenesis of the corpus callosum, cutaneous hypopigmentation, bilateral cataracts, cleft lip and palate, hypotonia and combined immunodeficiency.21 Since the original report, nine other papers have described patients with Vici syndrome,22-30 confirming it as a distinct clinical entity.
Epg5-Deficient Mice Display Only Some Features of Vici Syndrome
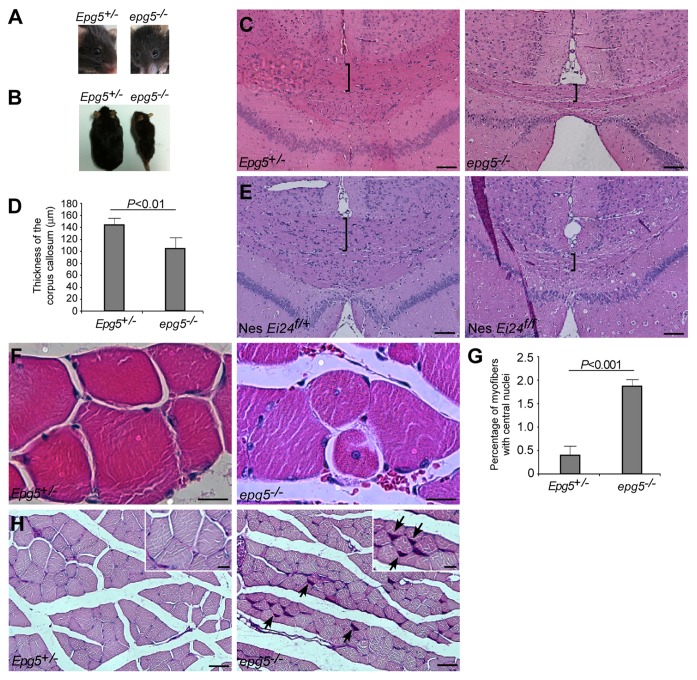
We investigated whether Epg5 knockout mice possess core features of Vici syndrome. All reported patients have postnatal growth retardation, developmental delay and severe psychomotor delay, and most of them die before the age of 3.21-30 In contrast, Epg5 knockout mice grow normally into sexually mature adults. Neurological defects gradually worsen from 4 mo of age, but the animals still survive to 10–12 mo. Facial dysmorphism, including cleft lip and palate, high-arched palate, and micrognathia have been reported in most cases of Vici syndrome,21-24,26-30 but these abnormalities are absent in Epg5 knockout mice. Cataract is another common trait of Vici syndrome that is not obviously detected in Epg5 knockout mice (Fig. 1A).21-23,25-30 Hypopigmentation has been reported in all Vici syndrome cases, variably involving the skin, hair and/or retina,21-30 but epg5−/− mice displayed the same skin and fur color as their control littermates (Fig. 1B).
Figure 1. Neurological and muscular defects in epg5−/− mice. (A) epg5−/− mice do not show obvious cataracts. (B) epg5−/− mice have the same fur color as controls. (C) H&E staining of cerebra shows decreased thickness of the corpus callosum (brackets) in epg5−/− mice. Scale bar: 100 µm. (D) The thickness of the corpus callosum in mutant and control Epg5 mice. Mean ± SEM of 5 mice is shown. (E) H&E staining of cerebra shows decreased thickness of the corpus callosum (brackets) in Ei24flox/flox; nestin-Cre mice. Scale bar: 100 µm. (F) H&E staining of gastrocnemius muscles shows muscle atrophy and centrally nucleated fibers in epg5−/− mice. Scale bar: 10 µm. (G) The percentages of myofibers with central nuclei. Mean ± SEM of 3 mice is shown. (H) PAS staining of gastrocnemius muscles shows glycogen accumulation (arrows) in epg5−/− mice. Scale bar: 50 µm in main panels, 20 µm in insets.
One invariable characteristic of Vici syndrome is agenesis of the corpus callosum, a failure to develop the large bundle of fibers that connect the cerebral hemispheres.21-30 When we performed hematoxylin and eosin (H&E) staining of cerebral sections from 10 pairs of Epg5+/− and epg5−/− mice, we discovered that the corpus callosum was significantly thinner in mutant mice compared with controls, and was partially absent in three and completely absent in one of the knockout mice (Fig. 1C and D). A reduced number of pyramidal neurons in layer 5 of the motor and sensory cortices may contribute to the loss of white matter. Similarly, Ei24flox/flox; nestin-Cre mice also showed a reduced thickness of the corpus callosum compared with Ei24flox/wt; nestin-Cre mice (Fig. 1E), suggesting that this change is not unique to Epg5 knockout mice. Environmental factors, such as fetal alcohol syndrome, hypothyroidism and enrichment or deprivation of experience, may also contribute to postnatal and prenatal callosal development.31
Muscle biopsy was not performed in the initially reported Vici syndrome cases, but neurogenic and myopathic abnormalities were discovered in muscle biopsies from five of seven patients reported more recently.22,25-30 The pathological changes comprise increased variability in fiber size, fiber atrophy and prominent central nuclei in atrophic fibers.22,25,26,28,29 Accumulation of mitochondria and abnormal glycogen deposits in subsarcolemmal locations suggests an unidentified metabolic abnormality with structural manifestations and possible mitochondrial cytopathy.22,25,26,28,29 Muscle atrophy is evident in Epg5 knockout mice and the percentage of myofibers with centrally located nuclei was significantly increased compared with controls, as shown in Figure 1F and 1G. There is a marked variation in fiber size in the knockout mice, and atrophic fibers are scattered or clustered. Some of the small fibers are angular. We previously reported that electromyographs from epg5−/− mice show fibrillation and positive sharp waves, indicative of active denervation of muscle fibers. The duration of the motor unit action potential during moderate contractions is markedly increased in end-stage epg5−/− mice compared with controls, indicating reinnervation of denervated muscle fibers by sprouting from relatively normal axons. These results indicate that Epg5 knockout mice show neurogenic muscle damage. On the other hand, periodic acid-Schiff (PAS) staining of muscle sections shows higher glycogen content in Epg5 knockout mice (Fig. 1H). Ultrastructural analysis also shows that epg5−/− muscles exhibit accumulation of abnormally enlarged mitochondria. Thus, the pathological muscle changes in epg5−/− mice suggest a combination of neurogenic and metabolic etiologies.
Our findings indicate some phenotypic similarities between epg5−/− mice and Vici syndrome patients including corpus callosum changes and myopathy. However, many features of Vici syndrome, including facial dysmorphism, cataracts and hypopigmentation, are not evident in epg5−/− mice. Nearly all Vici syndrome patients suffer recurrent viral, bacterial, and fungal infections and immunodeficiency.21-23,25-30 epg5−/− mice showed no sign of infection, but they were raised under specific pathogen-free conditions and benefit from a low-stress environment with minimal immunological challenges, so it remains to be determined whether their immune systems are compromised. Alternatively, Vici syndrome may be a polygenic disease, caused by simultaneous mutation in EPG5 and one or more other genes. Another possibility is that EPG5 plays a more important function in humans.
Acknowledgments
We are grateful to Dr. Isabel Hanson for editing work, and the animal facility at the Institute of Biophysics, Chinese Academy of Sciences for mice maintenance. This work was supported by the National Basic Research Program of China (2013CB910100, 2011CB910100) and also a grant from the National Natural Science Foundation of China (31225018) to H.Z. The research of H.Z. was supported in part by an International Early Career Scientist grant from the Howard Hughes Medical Institute.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/24856
References
- 1.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 2.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–67. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 3.Longatti A, Tooze SA. Vesicular trafficking and autophagosome formation. Cell Death Differ. 2009;16:956–65. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–30. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 6.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 7.Liang CC, Wang C, Peng X, Gan B, Guan JL. Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. J Biol Chem. 2010;285:3499–509. doi: 10.1074/jbc.M109.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy J, Gwinn-Hardy K. Genetic classification of primary neurodegenerative disease. Science. 1998;282:1075–9. doi: 10.1126/science.282.5391.1075. [DOI] [PubMed] [Google Scholar]
- 9.Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 10.Tian Y, Li ZP, Hu WQ, Ren HY, Tian E, Zhao Y, et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell. 2010;141:1042–55. doi: 10.1016/j.cell.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YG, Zhao H, Miao L, Wang L, Sun F, Zhang H. The p53-induced gene Ei24 is an essential component of the basal autophagy pathway. J Biol Chem. 2012;287:42053–63. doi: 10.1074/jbc.M112.415968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 13.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–63. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariño G, Fernández AF, Cabrera S, Lundberg YW, Cabanillas R, Rodríguez F, et al. Autophagy is essential for mouse sense of balance. J Clin Invest. 2010;120:2331–44. doi: 10.1172/JCI42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tashiro Y, Urushitani M, Inoue H, Koike M, Uchiyama Y, Komatsu M, et al. Motor neuron-specific disruption of proteasomes, but not autophagy, replicates amyotrophic lateral sclerosis. J Biol Chem. 2012;287:42984–94. doi: 10.1074/jbc.M112.417600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EM, et al. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, et al. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol. 2009;187:875–88. doi: 10.1083/jcb.200908115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, et al. ITALSGEN Consortium Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–64. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tresse E, Salomons FA, Vesa J, Bott LC, Kimonis V, Yao TP, et al. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–27. doi: 10.4161/auto.6.2.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullup T, Kho AL, Dionisi-Vici C, Brandmeier B, Smith F, Urry Z, et al. Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat Genet. 2013;45:83–7. doi: 10.1038/ng.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vici CD, Sabetta G, Gambarara M, Vigevano F, Bertini E, Boldrini R, et al. Agenesis of the corpus callosum, combined immunodeficiency, bilateral cataract, and hypopigmentation in two brothers. Am J Med Genet. 1998;29:1–8. doi: 10.1002/ajmg.1320290102. [DOI] [PubMed] [Google Scholar]
- 22.del Campo M, Hall BD, Aeby A, Nassogne MC, Verloes A, Roche C, et al. Albinism and agenesis of the corpus callosum with profound developmental delay: Vici syndrome, evidence for autosomal recessive inheritance. Am J Med Genet. 1999;85:479–85. doi: 10.1002/(SICI)1096-8628(19990827)85:5<479::AID-AJMG9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Chiyonobu T, Yoshihara T, Fukushima Y, Yamamoto Y, Tsunamoto K, Nishimura Y, et al. Sister and brother with Vici syndrome: agenesis of the corpus callosum, albinism, and recurrent infections. Am J Med Genet. 2002;109:61–6. doi: 10.1002/ajmg.10298. [DOI] [PubMed] [Google Scholar]
- 24.Miyata R, Hayashi M, Sato H, Sugawara Y, Yui T, Araki S, et al. Sibling cases of Vici syndrome: sleep abnormalities and complications of renal tubular acidosis. Am J Med Genet A. 2007;143:189–94. doi: 10.1002/ajmg.a.31584. [DOI] [PubMed] [Google Scholar]
- 25.McClelland V, Cullup T, Bodi I, Ruddy D, Buj-Bello A, Biancalana V, et al. Vici syndrome associated with sensorineural hearing loss and evidence of neuromuscular involvement on muscle biopsy. Am J Med Genet A. 2010;152A:741–7. doi: 10.1002/ajmg.a.33296. [DOI] [PubMed] [Google Scholar]
- 26.Al-Owain M, Al-Hashem A, Al-Muhaizea M, Humaidan H, Al-Hindi H, Al-Homoud I, et al. Vici syndrome associated with unilateral lung hypoplasia and myopathy. Am J Med Genet A. 2010;152A:1849–53. doi: 10.1002/ajmg.a.33421. [DOI] [PubMed] [Google Scholar]
- 27.Rogers RC, Aufmuth B, Monesson S. Vici syndrome: a rare autosomal recessive syndrome with brain anomalies, cardiomyopathy, and severe intellectual disability. Case Rep Genet. 2011;2011:421582. doi: 10.1155/2011/421582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Said E, Soler D, Sewry C. Vici syndrome--a rapidly progressive neurodegenerative disorder with hypopigmentation, immunodeficiency and myopathic changes on muscle biopsy. Am J Med Genet A. 2012;158A:440–4. doi: 10.1002/ajmg.a.34273. [DOI] [PubMed] [Google Scholar]
- 29.Finocchi A, Angelino G, Cantarutti N, Corbari M, Bevivino E, Cascioli S, et al. Immunodeficiency in Vici syndrome: a heterogeneous phenotype. Am J Med Genet A. 2012;158A:434–9. doi: 10.1002/ajmg.a.34244. [DOI] [PubMed] [Google Scholar]
- 30.Ozkale M, Erol I, Gümüş A, Ozkale Y, Alehan F. Vici syndrome associated with sensorineural hearing loss and laryngomalacia. Pediatr Neurol. 2012;47:375–8. doi: 10.1016/j.pediatrneurol.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–99. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]